Abstract
Objective
This study aimed to determine which bariatric procedure allows patients to obtain the best weight‐loss outcomes and a remission of type 2 diabetes.
Methods
Databases were searched for randomized‐controlled trials comparing Roux‐en‐Y gastric bypass (RYGB) with sleeve gastrectomy (SG) or one‐anastomosis gastric bypass (OAGB). The mean difference (MD) or the relative risk was determined.
Results
Twenty‐five randomized‐controlled trials were analyzed. Excess weight loss (EWL, percentage) was greater for RYGB patients at 3 years (MD: 11.93, p < 0.00001) and 5 years (MD: 13.11, p = 0.0004). Higher excess BMI loss (percentage) was found in RYGB at 1 year (MD: 11.66, p = 0.01). Total weight loss (percentage) was greater for RYGB patients after 3 months (MD: 2.41, p = 0.02), 6 months (MD: 3.83, p < 0.00001), 1 year (MD: 6.35, p < 0.00001), and 5 years (MD: 3.90, p = 0.005). No difference in terms of remission of type 2 diabetes was seen between RYGB and SG. EWL was significantly more important after OAGB than after RYGB after 1 year (MD: −10.82, p = 0.003).
Conclusions
RYGB is more efficient than SG in the midterm. OAGB offers greater EWL than RYGB after 1 year, but further evidence is needed to confirm this result.
Study Importance.
What is already known?
-
►
Roux‐en‐Y gastric bypass (RYGB) is the gold standard in bariatric surgery.
-
►
However, RYGB is getting challenged by the increasingly popular sleeve gastrectomy.
-
►
The one‐anastomosis gastric bypass (OAGB) is showing encouraging results.
What does this study add?
-
►
RYGB is more efficient than sleeve gastrectomy in terms of total weight loss after 3 months and, in terms of excess weight loss, after 3 years of follow‐up.
-
►
OAGB seems to offer greater excess weight loss than RYGB after 12 months.
How might these results change the direction of research or the focus of clinical practice?
-
►
RYGB should remain the gold standard in bariatric surgery.
-
►
More high‐quality evidence is needed regarding the effect of OAGB.
INTRODUCTION
Obesity represents a serious medical and public health challenge and carries a global economic burden estimated to be of $1.72 trillion (1, 2, 3). Over the past decade, the prevalence has not stopped increasing worldwide, contributing to the development of numerous diseases such as diabetes, cardiovascular disease, sleep apnea, and joint diseases (4). By 2050, it is estimated that 50% of the female population and 60% of the male population will suffer from obesity (5, 6).
Therapeutic management of obesity includes lifestyle changes such as the adoption of an appropriate balanced diet, physical exercise, and the use of pharmacological drugs such as orlistat (7). For refractory cases, bariatric surgery can be proposed (8, 9). However, patients have to meet some criteria established by the National Health System (NHS) or other equivalent supervising organizations: BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 potentially associated with an obesity‐related comorbidity, a failure of conservative management, a willingness to be followed over the long term after the procedure, and no health problems contraindicating general anesthesia (9, 10). Many surgical procedures have been developed to obtain adequate weight loss: gastric banding, Roux‐en‐Y gastric bypass (RYGB), sleeve gastrectomy (SG), biliopancreatic diversion with duodenal switch, and one‐anastomosis gastric bypass (OAGB). Cesar Roux, a Swiss surgeon, was the first to perform a Y loop in 1892 to treat gastric outlet obstruction due to carcinoma or peptic ulcer disease (11). The procedure was adapted in 1967 by Mason, who associated the restrictive effect of the gastric pouch to the reduced bowel absorption of the Roux‐en‐Y reconstruction and created the first surgical procedure to treat obesity (11, 12, 13, 14). RYGB is nowadays considered to be the gold standard of bariatric procedures (12, 13, 14), allowing patients to reach efficient weight loss and a resolution of obesity‐related comorbidities.
Nevertheless, the past decades have seen the emergence of alternative and promising techniques. For instance, SG, proposed by Gagner in 1998, has rapidly gained in popularity. Its technical simplicity, the lower incidence of surgical complications, and the fact that the procedure can later be converted to RYGB in case of insufficient weight loss ensures its attractiveness (15, 16). Second, OAGB, developed by Rutledge in 1997, consists of the creation of a gastric pouch with a single gastro‐jejunal latero‐lateral anastomosis. It is considered as efficient as RYGB, easier to perform, and less likely to cause morbidity and mortality (17, 18, 19). However, numerous authors have suggested that this technique is responsible for more bile reflux (20, 21) and, owing to the lack of available high‐quality evidence as of yet, this procedure is not yet widely accepted (22, 23).
Weight‐loss performance is one of the key decisional factors in the choice of the primary surgical procedure, although several other variables must be considered, such as the risk of complications, metabolic comorbidities, and gastroesophageal reflux disease (GERD). The objective of this study was to perform a systematic review and meta‐analysis to summarize the most recent randomized‐controlled trials (RCTs) comparing RYGB with OAGB and SG in terms of weight‐loss outcomes and remission of type 2 diabetes, which constitute the main outcomes of interest for decision‐making when choosing a surgical procedure.
METHODS
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Supporting Information Table S1 [PRISMA checklist]) (24). MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched on April 30, 2020, and May 10, 2020, for RCTs comparing, respectively, RYGB with either SG or OAGB (Supporting Information Table S2). Additional studies were identified through Google search and manual search of the reference lists of existing systematic reviews in the field. To be included, studies had to be an RCT, written in French or English, and comparing RYGB with either SG and/or OAGB. Abstracts of RCTs with preliminary results reporting weight loss or number of patients with resolution of comorbidities were also included in the case of OAGB (owing to the paucity of the literature in the field). Systematic reviews, meta‐analyses, observational studies, protocols, and letters to the editor were excluded after title and abstract screening. RCTs reporting duplicate patients; those comparing SG or OAGB with another bariatric procedure other than RYGB; and those not reporting excess weight loss (EWL, percentage), excess BMI loss (EBMIL, percentage), or total weight loss (TWL percentage) were excluded. RCTs not reporting enough weight‐outcome data for meta‐analysis (such as not reporting SEM or SD or not reporting exact values) were also excluded. Of note, authors of publications potentially eligible for inclusion were contacted to provide missing values. Two independent reviewers (IU, JM) carried out the systematic review. Discrepancies were solved by a third author (JD).
Risk of bias assessment
Studies were ranked using the modified Cochrane Collaboration tool and classified as low risk, unclear risk, or high risk.
Statistical analysis
A meta‐analysis was performed if two or more trials reported the same outcome for the same time point. The mean difference (MD) in terms of weight‐loss outcomes (percentage; TWL, EWL, and EBMIL) or the relative risk (RR) for type 2 diabetes remission was determined using a model with random effects. Heterogeneity was assessed using the Q test and reported using the I 2 value. The software Review Manager 5 was used for the meta‐analysis. A p < 0.05 was considered to be statistically significant.
In the network meta‐analysis, the pooled MD between RYGB and OAGB was based on direct comparisons as well as the comparison between RYGB and SG. In contrast, the pooled MD between SG and OAGB was based on indirect comparisons (25). The network meta‐analysis was conducted with the R package netmeta (R Foundation) with random effects.
RESULTS
Inclusion process
The literature search based on the comparison of RYGB versus SG identified 42 publications from MEDLINE, 5 from Embase, 52 from the Cochrane Central Register of Controlled Trials, and 21 from other sources. Fifteen duplicates were removed. Of the 105 publications left, 57 were not retained after title and abstract screening, leaving 48 publications for full text screening. Of those, we excluded 28 publications for meeting at least one of the exclusion criteria (details for exclusion are provided in Supporting Information Table S3). Ultimately, 20 publications were retained for the quantitative analysis (Supporting Information Figure S1, Supporting Information Table S4). To note, authors were contacted to provide missing values such as EBMIL, EWL, TWL, and remission of type 2 diabetes, which allowed us to include three more RCTs (26, 27, 28).
The literature search based on the comparison of RYGB versus OAGB identified 32 studies from MEDLINE, 52 from Embase, 162 from the Cochrane Central Register of Controlled Trials, and 13 from other sources. Seventy‐four duplicates were removed. Of the 185 publications left, 167 were not retained after title and abstract screening, leaving 18 publications for full text screening. Because they did not meet our inclusion criteria, 13 publications were excluded (details of excluded trials are reported in Supporting Information Table S3), leaving 5 articles for the meta‐analysis (Supporting Information Figure S2, Supporting Information Table S4). Several trials reported their outcomes at different time points of follow‐up in several articles. In this case, we avoided pooling duplicate patients and kept only one reporting per time point in our meta‐analysis (Supporting Information Table S3).
Characteristics of included studies and patients
In total, 25 RCTs were included, representing a total of 2,715 patients, a total of 2,273 of which were in the RYGB versus SG comparison and 442 of which were in the RYGB versus OAGB comparison. The largest trial included 238 participants, and the smallest trial included 20 patients. Except for one study that did not report information regarding the surgical technique used for RYGB (29), all procedures were performed laparoscopically (Supporting Information Tables S4‐S5).
Among studies comparing RYGB versus SG, 14 were performed in Europe (26, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40), 3 in Asia (41, 42, 43), 2 in the United States (44, 45), and 1 in Brazil (46). Twelve studies were monocentric (28, 29, 30, 31, 32, 34, 41, 42, 43, 44, 45, 46), and eight were multicentric (26, 33, 35, 36, 37, 38, 39, 40). Eleven studies were designed with weight loss as primary outcome (either reported as EWL [percentage], EBMIL [percentage], or TWL [percentage]), and eight studies assessed EWL (percentage) or EBMIL (percentage) as secondary outcomes. Each study reported one weight outcome except for Yang et al., who reported both EWL (percentage) and TWL (percentage) (41) (Supporting Information Table S4). Ten publications reported results of the same trials but with different follow‐ups. For instance, Schauer et al. released two publications: one in 2012 and one in 2017, with follow‐up time points at, respectively, 1 and 5 years (44, 45). Salminen et al. also published two articles based on the same trial, one in 2014 and one in 2018 (33, 39). Peterli et al. published six articles, with follow‐up time points at 3 months and 1, 2, 3, 4, and 5 years (26, 36, 37, 38, 40) (Supporting Information Table S4). Regarding the comparison between RYGB and OAGB, three trials were performed in Europe (13, 16, 18), one in Taiwan (47), and one in Venezuela (48). There were four monocentric studies (16, 47, 48, 49) and one multicentric study (18). All five studies assessed weight loss as their primary outcome (13, 18, 47, 48, 50) (Supporting Information Table S4).
Quality ranking of included studies
The risk of bias of included RCTs was determined using the Cochrane Collaboration’s tool for assessing risk of bias and is reported in Supporting Information Table S6 (51). Two studies were classified as low risk (28, 34), fifteen as unclear risk (18, 26, 29, 31, 32, 33, 36, 37, 38, 39, 40, 41, 42, 46, 47), and eight as high risk (13, 27, 30, 43, 44, 45, 48, 50).
TWL
TWL (percentage) between RYGB and SG
TWL (percentage) after RYGB and SG was reported in six RCTs (27, 28, 40, 41, 44, 45). One of them did not document standard deviation (45), leaving five articles for the meta‐analysis (Supporting Information Table S7). TWL (percentage) was similar between both procedures at 1 month (2 studies, 158 patients, MD: 0.74, 95% CI: −1.39 to 2.88, I 2: 72%, p = 0.5; Table 1, Figure 1A) (27, 28). However, RYGB showed a better TWL (percentage) after 3 months (2 studies, 131 patients, MD: 2.41, 95% CI: 0.46 to 4.36, I 2: 76%, p = 0.02; Table 1, Figure 1B) (28, 40), 6 months (2 studies, 69 patients, MD: 3.83, 95% CI: 2.46 to 5.21, I 2: 5%, p < 0.00001; Table 1, Figure 1C) (27, 40), 1 year (3 studies, 180 patients, MD: 6.35, 95% CI: 4.69 to 8.01, I 2: 0%, p < 0.00001; Table 1, Figure 1D) (27, 28, 40), and 5 years (2 studies, 128 patients, MD: 3.90, 95% CI: 1.21 to 6.59, I 2: 0%, p = 0.005; Table 1, Figure 1E) (27, 44).
TABLE 1.
Summary of weight outcomes
| n | MD (95% CI) | I 2 | p value | Interpretation | |
|---|---|---|---|---|---|
| RYGB versus SG | |||||
| EWL (percentage) | |||||
| At 1 month | 4 | 3.30 (−0.80 to 7.40) | 33% | 0.12 | No difference between groups |
| At 3 months | 2 | 0.89 (−3.51 to 5.29) | 60% | 0.69 | No difference between groups |
| At 6 months | 4 | 0.11 (−5.52 to 5.73) | 71% | 0.97 | No difference between groups |
| At 1 year | 6 | 1.77(−5.11 to 8.64) | 72% | 0.61 | No difference between groups |
| At 2 years | 3 | 5.06 (−7.27 to 17.38) | 76% | 0.42 | No difference between groups |
| At 3 years | 3 | 11.93 (6.90 to 16.95) | 0% | <0.00001 | Favors RYGB |
| At 5 years | 3 | 13.11 (5.83 to 20.39) | 0% | 0.0004 | Favors RYGB |
| TWL (percentage) | |||||
| At 1 month | 2 | 0.74 (−1.39 to 2.88) | 72% | 0.5 | No difference between groups |
| At 3 months | 2 | 2.41 (0.46 to 4.36) | 76% | 0.02 | Favors RYGB |
| At 6 months | 2 | 3.83 (2.46 to 5.21) | 5% | <0.00001 | Favors RYGB |
| At 1 years | 3 | 6.35 (4.69 to 8.01) | 0% | <0.00001 | Favors RYGB |
| At 5 years | 2 | 3.90 (1.21 to 6.59) | 0% | 0.005 | Favors RYGB |
| EBMIL (percentage) | |||||
| At 1 month | 2 | 2.20 (−4.30 to 8.71) | 73% | 0.51 | No difference between groups |
| At 3 months | 2 | −6.64 (−27.02 to 13.74) | 94% | 0.52 | No difference between groups |
| At 1 year | 3 | 11.66 (2.33 to 21.00) | 70% | 0.01 | Favors RYGB |
| At 2 years | 2 | 10.26 (−4.69 to −25.21) | 77% | 0.18 | No difference between groups |
| At 5 years | 2 | 11.57 (−2.51 to 25.64) | 61% | 0.11 | No difference between groups |
| RYGB versus OAGB | |||||
| EWL (percentage) | |||||
| At 6 months | 2 | −8.59 (−33.57 to 16.38) | 91% | 0.5 | No difference between groups |
| At 1 year | 4 | −10.82 (−18.02 to −3.62) | 68% | 0.003 | Favors OAGB |
Abbreviations: EBMIL, excess BMI loss; EWL, excess weight loss; MD, mean difference; OAGB, one‐anastomosis gastric bypass; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy; TWL, total weight loss.
FIGURE 1.
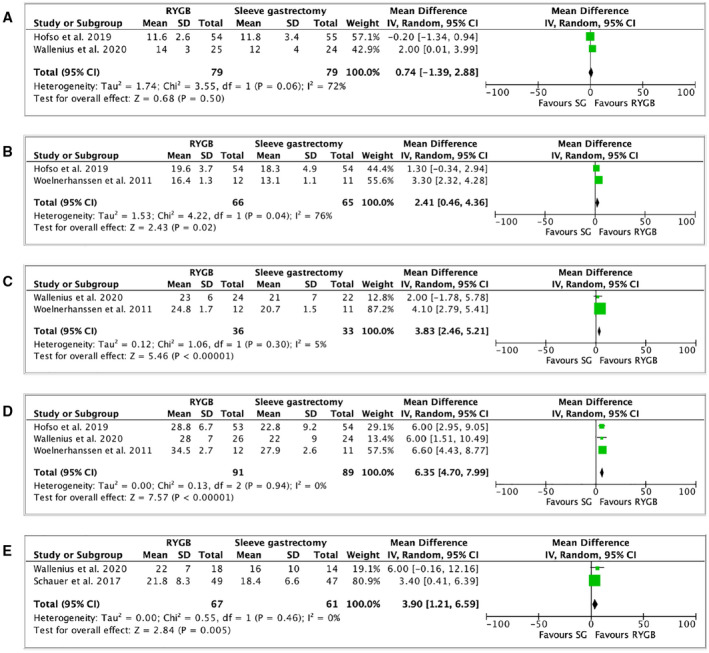
Forest plot comparing TWL (percentage) between RYGB and SG at (A) 1 month after surgery; (B) 3 months after surgery; (C) 6 months after surgery; (D) 1 year after surgery; and (E) 5 years after surgery. The vertical line represents the null effect. Each horizontal line represents the 95% CI of one study. The size of the green box is related to the weight of each study. The diamond symbolizes the overall effect of all the studies. RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy; TWL, total weight loss [Colour figure can be viewed at wileyonlinelibrary.com]
TWL (percentage) between RYGB and OAGB
Out of the five RCTs comparing RYGB and OAGB, none reported TWL (percentage).
Network meta‐analysis of TWL (percentage) between RYGB, SG, and OAGB
TWL (percentage) was not reported by RCTs comparing weight‐loss outcomes between RYGB and OAGB, which prevented any network meta‐analysis.
EWL
EWL (percentage) between RYGB and SG
Eleven RCTs compared EWL (percentage) between RYGB and SG (27, 29, 30, 31, 32, 33, 34, 41, 42, 43, 46). Two studies did not document SD and could not be pooled in the meta‐analysis (34, 46). After bariatric surgery procedure, EWL (percentage) was similar between RYGB and SG at 1 month (4 studies, 254 patients, MD: 3.30, 95% CI: −0.80 to 7.40, I 2: 33%, p = 0.12; Table 1, Figure 2A) (27, 29, 31, 42), 3 months (2 studies, 262 patients, MD: 0.89, 95% CI: −3.51 to 5.29, I 2: 60%, p = 0.69; Table 1, Figure 2B) (32, 33), 6 months (4 studies, 380 patients, MD: 0.11, 95% CI: −5.52 to 5.73, I 2: 71%, p = 0.97; Table 1, Figure 2C) (27, 29, 32, 33), 1 year (6 studies, 440 patients, MD: 1.77, 95% CI: −5.11 to 8.64, I 2: 72%, p = 0.61; Table 1, Figure 2D) (29, 30, 31, 32, 35, 42), and 2 years (3 studies, 250 patients, MD: 5.06, 95% CI: −7.27 to 17.38, I 2: 76%, p = 0.42; Table 1, Figure 2E) (27, 31, 42, 43). However, EWL (percentage) was greater in patients who underwent RYGB than SG at 3 years (3 studies, 170 patients, MD: 11.93, 95% CI: 6.90 to 16.95, I 2: 0%, p < 0.00001; Table 1, Figure 2F) (31, 41, 42) and at 5 years (3 studies, 156 patients, MD: 13.11, 95% CI: 5.83 to 20.39, I 2: 0%, p = 0.0004) (Table 1, Figure 2G) (27, 31, 42) after surgery. Detailed EWL (percentage) per study and per time point are reported in Supporting Information Table S8.
FIGURE 2.
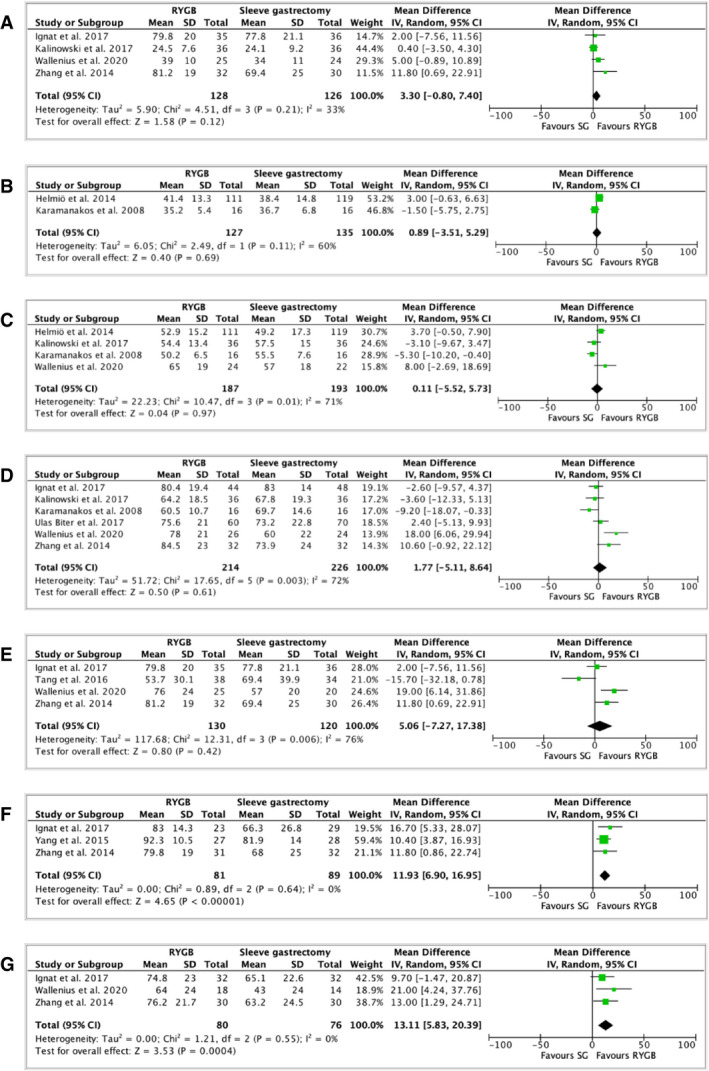
Forest plot comparing EWL (percentage) between RYGB and SG at (A) 1 month after surgery; (B) 3 months after surgery; (C) 6 months after surgery; (D) 1 year after surgery; (E) 2 years after surgery; (F) 3 years after surgery; and (G) 5 years after surgery. The vertical line represents the null effect. Each horizontal line represents the 95% CI of one study. The size of the green box is related to the weight of each study. The diamond symbolizes the overall effect of all the studies. EWL, excess weight loss; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy [Colour figure can be viewed at wileyonlinelibrary.com]
EWL (percentage) between RYGB and OAGB
Four RCTs compared EWL (percentage) between RYGB and OAGB (13, 47, 48, 50) at different time points, as reported on Supporting Information Table S9. Results at 1 month (48), 3 months (49), 9 months (50), 24 months (47), and 60 months (48) were reported by one study each; therefore, a quantitative analysis could not be performed. After bariatric surgery procedure, EWL (percentage) did not differ between RYGB and OAGB at 6 months (2 studies, 38 patients, MD: −8.59, 95% CI: −33.57 to 16.38, I 2: 91%, p = 0.50; Table 1, Figure 3A) (48, 50). At 1 year, EWL (percentage) was significantly more important after OAGB than after RYGB (4 studies, 208 patients, MD: −10.82, 95% CI: −18.02 to −3.62, I 2: 68%, p = 0.003; Table 1, Figure 3B) (13, 47, 48, 50). Two studies reported longer follow‐up periods (47, 48). However, both studies did not find a statistically significant difference in EWL between RYGB and OAGB at 2 years (59.2% [15.1%] vs. 64.4% [8.8%], respectively, p = 0.154) (47) and 5 years (75.6% [4.25%] vs. 77.2% [3.48%], respectively, p = 0.46) (48) (Supporting Information Table S9). One RCT did not report EWL (percentage) (18).
FIGURE 3.
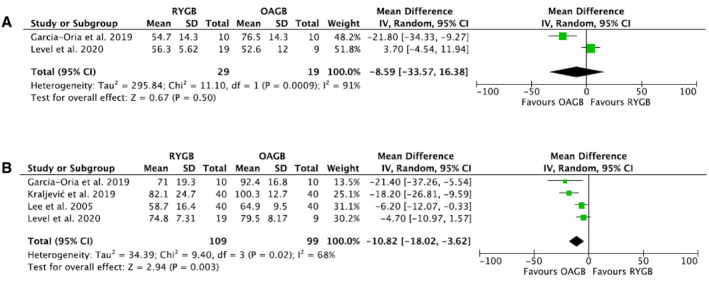
Forest plot comparing EWL (percentage) between RYGB and OAGB at (A) 6 months after surgery; and (B) 1 year after surgery. The vertical line represents the null effect. Each horizontal line represents the 95% CI of one study. The size of the green box is related to the weight of each study. The diamond symbolizes the overall effect of all the studies. EWL, excess weight loss; OAGB, one‐anastomosis gastric bypass; RYGB, Roux‐en‐Y gastric bypass [Colour figure can be viewed at wileyonlinelibrary.com]
Network meta‐analysis of EWL (percentage) between RYGB, SG, and OAGB
EWL (percentage) was reported at 1 year by 11 RCTs: 7 comparing weight‐loss outcomes between RYGB versus SG and 4 comparing RYGB versus OAGB (Supporting Information Table S10). SD from the trial by Zhang et al. (42) were extracted from the article iconography using the WebPlotDigitizer software (52). SD were not reported by Kehagis et al. (34). However, the standard error of the MD could be reconstructed using the reported p value, which allowed inclusion of the RCTs in the network meta‐analysis. With RYGB and SG, EWL is slightly more important for RYGB patients (pooled MD: −0.3, 95% CI: −6.7 to 6.0). However, the difference is not statistically significant (p = 0.92; Supporting Information Table S11, Figure 4). For RYGB versus OAGB, EWL is significantly more important for OAGB patients (pooled MD: −11.2, 95% CI: −19.6 to −2.7, p = 0.010; Supporting Information Table S11, Figure 4). The comparison between SG and OAGB shows that EWL is more important for OAGB patients, with a statistically significant effect (pooled MD: −11.5, 95% CI: −22.1 to −0.9, p = 0.033; Supporting Information Table S11, Figure 4). The I 2 statistic for the overall heterogeneity/inconsistency is 70.8% (Q = 30.77, p < 0.001; Supporting Information Table S11, Figure 4). Overall, by ranking the interventions using the p value, OAGB offers the best weight‐loss results at 1 year, with p = 0.24 (Supporting Information Table S12).
FIGURE 4.
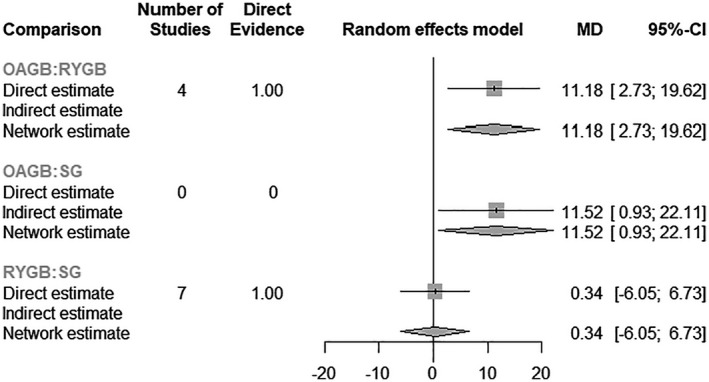
Forest plot of the network meta‐analysis for EWL (percentage) at 12 months, MD, mean difference; OAGB, one‐anastomosis gastric bypass; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy
EMBIL
EBMIL (percentage) between RYGB and SG
EBMIL (percentage) between RYGB and SG was reported in four studies (26, 27, 28, 36) (Supporting Information Table S13). Similar EMBIL were observed at 1 month (2 studies, 154 patients, MD: 2.20, 95% CI: −4.30 to 8.71, I 2: 73%, p = 0.51; Table 1, Figure 5A) (27, 28), 3 months (2 studies, 134 patients, MD: −6.64, 95% CI:−27.02 to 13.74, I 2: 94%, p = 0.52; Table 1, Figure 5B) (26, 28), 2 years (2 studies, 262 patients, MD: 10.26, 95% CI: −4.69 to −25.21, I 2: 77%, p = 0.18; Table 1, Figure 5D) (26, 27), and 5 years (2 studies, 237 patients, MD: 11.57, 95% CI: −2.51 to 25.64, I 2: 61%, p = 0.11; Table 1, Figure 5E) (26, 27). However, patients who underwent RYGB had higher EBMIL (percentage) at 1 year when compared with patients who underwent SG (3 studies, 364 patients, MD: 11.66, 95% CI: 2.33 to 21.00, I 2: 70%, p = 0.01) (Table 1, Figure 5C) (26, 27, 28).
FIGURE 5.
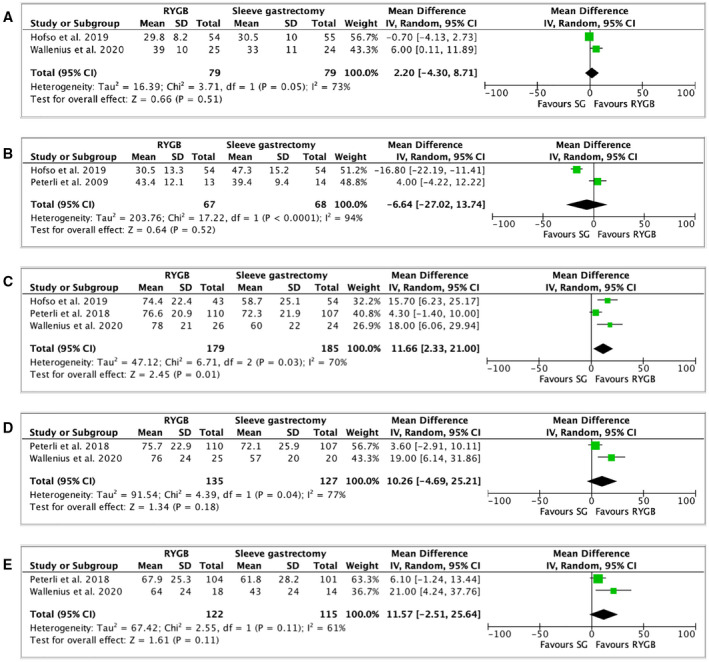
Forest plot comparing excess body mass index loss (percentage) at (A) 1 month after surgery; (B) 3 months after surgery; (C) 1 year after surgery; (D) 2 years after surgery; and (E) 5 years after surgery. The vertical line represents the null effect. Each horizontal line represents the 95% CI of one study. The size of the green box is related to the weight of each study. The diamond symbolizes the overall effect of all the studies. RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy [Colour figure can be viewed at wileyonlinelibrary.com]
EBMIL (percentage) between RYGB and OAGB
EBMIL (percentage) between RYGB and OAGB was only mentioned in the YOMEGA trial (18). After 2 years, the OAGB group showed an EBMIL (percentage) of 87.9% (23.6%) when compared with RYGB with an EBMIL (percentage) of 85.8% (23.1%), with an MD of −3.3% (95% CI: −9.1 to 2.6; Supporting Information Table S14). The authors concluded that OAGB was not inferior to RYGB in terms of EBMIL (p = 0.0024).
Network meta‐analysis of EBMIL (percentage) between RYGB, SG, and OAGB
Only one RCT compared EBMIL (percentage) between RYGB and OAGB at 1 year. At this time point, two RCTs compared RYGB with SG. Owing to the low number of trials, a network meta‐analysis could not be performed.
Remission of type 2 diabetes
Remission of type 2 diabetes between RYGB and SG
Fourteen RCTs comparing RYGB with SG reported the remission of type 2 diabetes after bariatric surgery (26, 27, 28, 29, 33, 34, 37, 38, 39, 41, 43, 44, 46). To note, seven trials were issued from the same group of patients, but no duplicate patients were used for the same time point. Except for the trial of de Barros et al. (46), all studies defined complete diabetes remission as a hemoglobin A1c level of <6% and/or fasting blood glucose of <100 mg/dL, without antidiabetic medication (Supporting Information Table S15). No difference in terms of remission of type 2 diabetes was put in evidence between RYGB and SG at 1 month (3 studies, 184 patients, RR: 0.67, 95% CI: 0.41 to 1.12, I 2: 0%, p = 0.13; Table 2, Figure 6A) (27, 28, 29), 3 months (3 studies, 385 patients, RR: 0.86, 95% CI: 0.47 to 1.58, I 2: 77%, p = 0.63; Table 2, Figure 6B) (28, 33, 46), 6 months (3 studies, 305 patients, RR: 1.21, 95% CI: 0.95 to 1.55, I 2: 0%, p = 0.13; Table 2, Figure 6C) (27, 29, 33), 1 year (6 studies, 483 patients, RR: 1.18, 95% CI: 0.90 to 1.54, I 2: 62%, p = 0.22; Table 2, Figure 6D) (27, 28, 29, 37, 39, 45), 2 years (2 studies, 121 patients, RR: 1.03, 95% CI: 0.52 to 2.06, I 2: 65%, p = 0.93; Table 2, Figure 6E) (27, 43), 3 years (4 studies, 208 patients, RR: 1.14, 95% CI: 0.94 to 1.38, I 2: 0%, p = 0.18; Table 2, Figure 6F) (34, 38, 39, 41), and 5 years (3 studies, 231 patients, RR: 1.21, 95% CI: 0.87 to 1.67, I 2: 0%, p = 0.25; Table 2, Figure 6G) (33, 39, 46).
TABLE 2.
Summary of type 2 diabetes remission
| n | RR (95% CI) | I 2 | p value | Interpretation | |
|---|---|---|---|---|---|
| RYGB versus SG | |||||
| Type 2 diabetes resolution | |||||
| At 1 month | 3 | 0.67 (0.41‐1.12) | 0% | 0.13 | No difference between groups |
| At 3 months | 3 | 0.86 (0.47‐1.58) | 77% | 0.63 | No difference between groups |
| At 6 months | 3 | 1.21 (0.95‐1.55) | 0% | 0.13 | No difference between groups |
| At 1 year | 6 | 1.18 (0.92‐1.51) | 62% | 0.22 | No difference between groups |
| At 2 years | 2 | 1.03 (0.52‐2.06) | 65% | 0.93 | No difference between groups |
| At 3 years | 4 | 1.14 (0.94‐1.38) | 0% | 0.18 | No difference between groups |
| At 5 years | 3 | 1.21 (0.87‐1.67) | 0% | 0.25 | No difference between groups |
Abbreviations: RR, relative risk; RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy.
FIGURE 6.
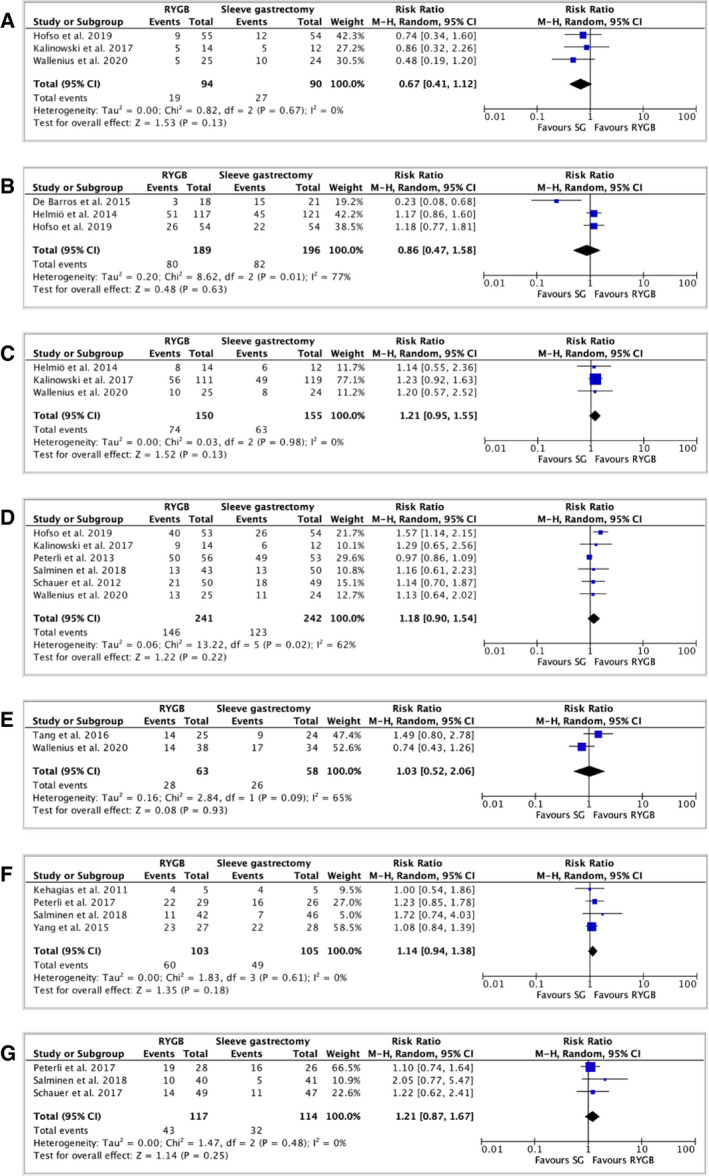
Forest plot comparing remission of type 2 diabetes between RYGB and SG at (A) 1 month after surgery; (B) 3 months after surgery; (C) 6 months after surgery; (D) 1 year after surgery; (E) 2 years after surgery; (F) 3 years after surgery; and (G) 5 years after surgery. The vertical line represents the null effect. Each horizontal line represents the 95% CI of one study. The size of the green box is related to the weight of each study. The diamond symbolizes the overall effect of all the studies. RYGB, Roux‐en‐Y gastric bypass; SG, sleeve gastrectomy [Colour figure can be viewed at wileyonlinelibrary.com]
Remission of type 2 diabetes between RYGB and OAGB
Two RCTs (18, 48) reported the remission rate of type 2 diabetes after RYGB and OAGB. Both studies defined the complete remission as a hemoglobin A1c level of <6% and/or fasting blood glucose of <100 mg/dL, without antidiabetic medications. In the study by Level et al. (48), complete remission of type 2 diabetes was achieved in 100% of the cases at 5 years for both OAGB (1 of 1 patient) and RYGB (2 of 2 patients; Supporting Information Table S16). Robert et al. (18) did not find any significant difference between the remission rate of RYGB patients and OAGB patients at 2 years; for RYGB, complete remission was seen in six out of sixteen patients (38%) versus twelve out of twenty patients (60%) for OAGB, and partial remission was described for one out of sixteen patients (6%) for RYGB versus two out of twenty patients (10%) for OAGB. The p value was equal to 0.28. No meta‐analysis could be performed.
Network meta‐analysis of type 2 diabetes remission between RYGB, SG, and OAGB
Type 2 diabetes remission was reported only by two RCTs comparing RYGB with OAGB, one at a 2‐year time point and one at a 5‐year time point, therefore preventing a network meta‐analysis.
DISCUSSION
Several systematic reviews and meta‐analyses of RCTs have recently compared bariatric surgery interventions in terms of weight loss and/or resolution of obesity‐related comorbidities (53, 54, 55, 56). However, an updated meta‐analysis reporting all weight‐outcome measures as recommended by the American Society for Metabolic and Bariatric Surgery (ASMBS), including OAGB in its pooled analysis, was lacking. Therefore, we conducted a systematic review and meta‐analysis of RCTs comparing RYGB with either SG or OAGB in order to determine which one of the surgical procedures is the most appropriate in terms of weight loss and remission of type 2 diabetes.
Briefly, we pooled 20 RCTs comparing RYGB and SG and found no statistical difference in terms of EWL (percentage) between SG and RYGB at 3 and 6 months and at 1 and 2 years after bariatric surgery. However, a statistical difference emerged in favor of RYGB 3 years after the surgical procedure. Indeed, EWL (percentage) was greater after RYGB than after SG by 11.93 percentage units and by 13.11 percentage units at 3 and 5 years, respectively. Therefore, it appears that RYGB tends to provide greater weight loss than SG on longer follow‐ups when considering EWL (percentage). In a systematic review and meta‐analysis including RCTs and observational studies, Gu et al. also showed that EWL (percentage) was greater after RYGB at 3 years compared with SG (MD: −4.37, 95% CI: −8.10 to −0.64) (57). This finding was confirmed when performing subgroup analysis pooling only RCTs. However, this difference was not statistically significant at 5 years (57). When analyzing TWL (percentage), RYGB showed a better weight loss at 3 months (MD: 2.41, p = 0.02), 6 months (MD: 3.83, p < 0.00001), 1 year (MD: 6.35, p < 0.00001), and 5 years (MD: 3.90, p = 0.005). In a systematic review and meta‐analysis including both RCTs and observational studies, Guraya et al. also observed a higher TWL (percentage) after RYGB than SG at 1 year (MD: 6.47, 95% CI: 1.22 to 11.72, z statistics = 2.42, p < 0.05), 3 years (MD: −0.23, 95% CI: 0.39 to 0.06, z statistics = 2.65, p < 0.05), and 5 years (MD: 1.87, 95% CI: 0.27 to 3.48, z statistics = 2.28, p < 0.05 respectively) (58). If we compare weight loss in terms of BMI change, a recent meta‐analysis of RCTs by Lee et al. concluded that patients who benefit from RYGB had significant higher decrease in BMI than those with SG at 1 year (16 studies, 1,673 patients, 95% CI −2.01 to −0.49, I 2 = 88%, p < 0.001), 3 years (5 studies, 595 patients, 95% CI −2.68 to −0.74, I 2 = 47%, p < 0.001), and 5 years (3 studies, 353 patients, 95% CI −2.36 to −2.04, I 2 = 0%, p < 0.001) (53). In our analysis, we did not find any difference in EBMIL in the midterm. A recent published article by Wölnerhanssen et al. combining outcome values of the finished SLEEVEPASS and Swiss SM‐BOSS trials notably found higher EBMIL (percentage) in the RYGB group compared with the SG group, with a mean estimate of EBMIL (percentage) of 7 (95% CI: 3.5 to 10.5, p < 0.001) (59). To note, exact values of the SLEEVEPASS trial were not in our possession when writing the manuscript. Nevertheless, these findings confirm the better efficiency of RYGB in obtaining weight loss when compared with SG.
These results should be weighted by several limitations. First, the follow‐up periods of included RCTs are short, and only two studies could be pooled at 5 years. It appears that the greater weight loss achieved with RYGB is maintained for longer follow‐ups. For instance, the SLEEVEPASS trial reported that EWL (percentage) was more important after RYGB than after SG by 8.7 percentage units (95% CI: 3.5 to 13.9 percentage units) after 7 years (60). Moreover, the incidence of weight regain seems to be important after SG and was reported to be of 27.8% (95% CI: 22.8% to 32.7%, I 2: 60%) after 7 years (61). However, longer follow‐up periods such as 10 or 20 years are not yet available. Second, during screening of eligible RCTs, an important heterogeneity in weight outcomes was found, which precluded meta‐analysis of several publications. Indeed, in some publications in which weight outcomes were reported, SD or SEM were missing, which did not allow us to assess repartition of the data and proceed to a meta‐analysis. Therefore, as suggested by the ASMBS, systematic reporting of recommended weight outcomes in bariatric surgery trials should become the rule, even if outcomes have to be reported using different methods in the same paper (62, 63). Third, even if longer follow‐up periods were not available for all included RCTs, earlier results were shown to be predictive of maximal weight loss (64, 65).
It must be emphasized that the choice of the primary bariatric procedure should not be driven by weight‐loss performance alone. Several other factors must be considered to establish a patient‐centered tailored surgical strategy and, notably, the presence of type 2 diabetes. In 1995, Pories et al. claimed that bariatric surgery was the most effective long‐term treatment for type 2 diabetes (66). Schauer et al. later showed in the STAMPEDE trial that bariatric surgery (RYGB or SG) led to a greater mean percentage reduction in glycated hemoglobin level than medical treatment alone (2.1% vs. 0.3%, p = 0.003) (44). However, the type of bariatric surgery procedure does not seem to matter. When comparing RYGB with SG, our meta‐analysis demonstrated no difference in terms of remission of type 2 diabetes between both procedures for follow‐up time points until 5 years. This was also shown by Li et al. in a systematic review and meta‐analysis pooling 1,160 patients (67). Therefore, despite being more efficient than SG in terms of weight loss, RYGB does not offer any advantage over SG when considering type 2 diabetes remission.
Our analysis regarding remission of type 2 diabetes has several limitations. First, according to the American Diabetes Association (ADA), type 2 diabetes remission should be considered after maintaining normal blood sugar levels for 3 months or more (68). However, we can notice that the definition varies among the studies, as reported in Supporting Information Table S15. Second, there is a heterogeneity in patients’ populations (patients with less‐severe diabetes are more likely to achieve a remission).
Other factors should be considered when choosing the optimal bariatric surgical technique, such as GERD. Owing to heterogeneity in reporting, we did not perform any meta‐analysis of gastrointestinal symptoms. Nevertheless, two previous systematic reviews and meta‐analyses have suggested a better improvement of GERD with RYGB than SG (53, 67). In the SM‐BOSS trial, not only was the remission rate of GERD higher in the RYGB group at 5 years (absolute difference: −0.36%, 95% CI: −0.57% to −0.15%, p = 0.002), but worsening of symptoms or newly GERD was more often observed in SG patients at 5 years (26). In a narrative review comparing RYGB, SG, and laparoscopic adjustable banding, results also demonstrated an improvement of GERD for patients undergoing RYGB (69). A worsening of the GERD symptoms was noted in patients who underwent SG, which was hypothesized to be caused by the increased intraluminal pressure, the tunnel‐shaped stomach, and the disruption of the angle of His (69, 70). However, several other elements tend to be in favor of SG. SG has, notably, the advantage of being associated with a lower incidence of short‐term complications (e.g., 1% 30‐day mortality for SG vs. 2% for RYGB, and 1.3% admission to the intensive care unit for SG vs. 6% for RYGB) and might be suggested for patients at higher operative risk (71).
The second part of our meta‐analysis aimed at comparing RYGB with the emerging OAGB. To this end, we pooled five RCTs comparing OAGB with RYGB. Owing to the low number of RCTs, only EWL (percentage) could be pooled for the weight outcome. We showed that EWL (percentage) did not differ between OAGB and RYGB at 6 months. However, OAGB allowed for a statistically significant, more important EWL (percentage) after 1 year (4 RCTs, MD: −10.82, 95% CI: −18.02 to −3.62, I 2: 68%, p = 0.003). This finding was confirmed by a recent systematic review and meta‐analysis of observational and interventional studies totalizing 12,445 patients. Magouliotis et al. found greater EWL (percentage) after OAGB at 1 year (weighted MD [WMD]: −6.02, 95% CI: −8.84 to −3.20, p < 0.0001), 2 years (WMD: −7.33, 95% CI: −10.08 to −4.58, p < 0.0001), and 5 years (WMD: −12.82, 95% CI: −20.27 to −5.37, p = 0.0007) (72). Another systematic review and meta‐analysis also documented greater EWL (percentage) after OAGB at 1 and 2 years (73). The network meta‐analysis also demonstrated that OAGB offers the greater weight loss compared with RYGB or SG. This finding may be explained by the longer bypass limb created in the OAGB procedure (74). However, this may also lead to nutritional deficiencies, and future trials comparing the two techniques should assess this outcome in longer follow‐up periods. We would like to also highlight the low number of RCTs in the field.
Regarding the remission of type 2 diabetes, the paucity of available RCTs did not allow us to proceed to a meta‐analysis. We note that, in the YOMEGA trial, the proportion of patients who obtained remission of type 2 diabetes was higher in the OAGB group than in the RYGB group (60% vs. 38%, respectively). The difference was, however, not statistically significant (p = 0.28) (18). Other observational studies indicated that patients benefiting from OAGB have a higher remission rate of type 2 diabetes than RYGB, as shown in the meta‐analysis of Magouliotis et al. (odds ratio: 0.41, 95% CI: 0.25 to 0.69, p = 0.0006) (72, 73). Therefore, future analyses are required before concluding on the superiority of one procedure over the other in terms of diabetes remission. Furthermore, existing observational studies showed that OAGB tends to be associated with a higher incidence of malnutrition, GERD, diarrhea, and steatorrhea, whereas RYGB shows a greater incidence of bowel obstruction and internal hernia (18, 72, 74, 75). Notably, malnutrition can be explained by the length of the biliopancreatic limb; several studies have demonstrated that a limb length of more than 250 cm, particularly created in the case of patients with severe obesity, was associated with a higher risk of malnutrition, whereas a 150‐cm length led to minimal nutritional deficiencies (76, 77, 78). However, a shorter biliopancreatic limb, especially if associated with a small gastric pouch of less than 9 cm, has the disadvantage to promote the development of GERD in OAGB (18, 79, 80).
CONCLUSION
RYGB is more efficient than SG in terms of TWL (percentage) and in terms of EWL (percentage), notably in the midterm. However, no advantage emerged regarding type 2 diabetes remission. OAGB seems to be a procedure offering greater EWL (percentage) than RYGB after 1 year, but further evidence is needed to confirm this result.O
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
IU, JD, and JM conceived and designed the study; IU, JD, and JM acquired the data; IU and JM analyzed the data; IU, JM, JD, MC, MP, CT, and MJ interpreted the data; IU, JM, JD, MC, MP, CT, and MJ contributed to the writing of the manuscript and to its critical revision; and IU, JM, JD, MC, MP, CT, and MJ approved the final version of the manuscript.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Table S10
Table S11
Table S12
Table S13
Table S14
Table S15
Table S16
ACKNOWLEDGMENTS
We would like to thank Christophe Combescure, biostatistician at the University of Geneva, who helped us in the elaboration of the network meta‐analysis. Open Access Funding provided by Universite de Geneve. [Correction added on 13 April 2022, after first online publication: CSAL funding statement has been added.]
Uhe I, Douissard J, Podetta M, et al. Roux‐en‐Y gastric bypass, sleeve gastrectomy, or one‐anastomosis gastric bypass? A systematic review and meta‐analysis of randomized‐controlled trials. Obesity (Silver Spring). 2022;30:614–627. doi: 10.1002/oby.23338
REFERENCES
- 1. Munich RE. Obesity – a challenge for global healthcare systems. Published June 3, 2008. Accessed October 22, 2020. https://www.munichre.com/en/company/media‐relations/media‐information‐and‐corporate‐news/media‐information/2008/2008‐06‐03‐obesity‐a‐challenge‐for‐global‐healthcare‐systems.html
- 2. Waters H, Graf M. America’s Obesity Crisis: The Health and Costs of Excess Weight. Milken Institute; 2018. [Google Scholar]
- 3. Mitchell N, Catenacci V, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34:717‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr. 1998;67(3 suppl):563S‐572S. [DOI] [PubMed] [Google Scholar]
- 5. Lobstein T, Leach RJ. Part 1: Adult obesity. In: Foresight. Tackling Obesities: Future Choices – International Comparisons of Obesity Trends, Determinants and Responses – Evidence Review. UK Government Office for Science; 2007. [Google Scholar]
- 6. Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol (N Y). 2017;2:e17. doi: 10.1097/IJ9.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruban A, Stoenchev K, Ashrafian H, Teare J. Current treatments for obesity. Clin Med (Lond). 2019;19:205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bour ES. Evidence supporting the need for bariatric surgery to address the obesity epidemic in the United States. Curr Sports Med Rep. 2015;14:100‐103. [DOI] [PubMed] [Google Scholar]
- 9. Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118:1844‐1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singhal R. Can I get a gastric band on the NHS? Healthier Weight website. Published February 14, 2020. Accessed October 22, 2020. https://www.healthierweight.co.uk/blog/can‐i‐get‐a‐gastric‐band‐on‐the‐nhs/
- 11. Tham E, Ang SM, Cowan SW, Yeo CJ, Isenberg GA. Cesar Roux—the mind behind the Roux‐en‐Y. Am Surg. 2019;85:14‐17. [PubMed] [Google Scholar]
- 12. Torgersen Z, Osmolak A, Forse RA. Sleeve gastrectomy and Roux En Y gastric bypass: current state of metabolic surgery. Curr Opin Endocrinol Diabetes Obes. 2014;21:352‐357. [DOI] [PubMed] [Google Scholar]
- 13. Kraljević M, Delko T, Köstler T, et al. Laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic mini gastric bypass in the treatment of obesity: study protocol for a randomized controlled trial. Trials. 2017;18:226. doi: 10.1186/s13063-017-1957-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.History of RouxenY gastric bypass. Accessed October 22, 2020. https://www.plasticsurgeons.com/article/weight‐loss‐surgery/gastric‐bypass/history‐of‐rouxeny‐gastric‐bypass
- 15. Kehagias I, Zygomalas A, Karavias D, Karamanakos S. Sleeve gastrectomy: have we finally found the holy grail of bariatric surgery? A review of the literature. Eur Rev Med Pharmacol Sci. 2016;20:4930‐4942. [PubMed] [Google Scholar]
- 16. 24th IFSO World Congress. Obes Surg. 2019;29(suppl 5):347‐1720. [DOI] [PubMed] [Google Scholar]
- 17. Aleman R, Lo Menzo E, Szomstein S, Rosenthal RJ. Efficiency and risks of one‐anastomosis gastric bypass. Ann Transl Med. 2020;8(suppl 1):S7. doi: 10.21037/atm.2020.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert M, Espalieu P, Pelascini E, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux‐en‐Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open‐label, non‐inferiority trial. Lancet. 2019;393:1299‐1309. [DOI] [PubMed] [Google Scholar]
- 19. Carbajo MA, Luque‐de‐León E, Jiménez JM, Ortiz‐de‐Solórzano J, Pérez‐Miranda M, Castro‐Alija MJ. Laparoscopic one‐anastomosis gastric bypass: technique, results, and long‐term follow‐up in 1200 patients. Obes Surg. 2017;27:1153‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braghetto I, Csendes A. SINGLE anastomosis gastric bypass (one anastomosis gastric bypass or mini gastric bypass): the experience with Billroth II must be considered and is a challenge for the next years. Arq Bras Cir Dig. 2017;30:267‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saarinen T, Pietiläinen KH, Loimaala A, et al. Bile reflux is a common finding in the gastric pouch after one anastomosis gastric bypass. Obes Surg. 2020;30:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gero D, Schneider MA, Suter M, et al. Sleeve gastrectomy or gastric bypass: a “post‐code” lottery? A comprehensive national analysis of the utilization of bariatric surgery in Switzerland between 2011–2017. Surg Obes Relat Dis. 2021;17:563‐574. [DOI] [PubMed] [Google Scholar]
- 23. Parikh M, Eisenberg D, Johnson J, El‐Chaar M, Kothari S. American Society for Metabolic and Bariatric Surgery review of the literature on one‐anastomosis gastric bypass. Surg Obes Relat Dis. 2018;14:1088‐1092. [DOI] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rucker G, Krahn U, Konig U, et al. Package 'netmeta': Network meta‐analysis using frequentist methods. Published January 20, 2022. https://cran.r‐project.org/web/packages/netmeta/netmeta.pdf
- 26. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss in patients with morbid obesity: the SM‐BOSS randomized clinical trial. JAMA. 2018;319:255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallenius V, Alaraj A, Björnfot N, et al. Sleeve gastrectomy and Roux‐en‐Y gastric bypass in the treatment of type 2 diabetes. Two‐year results from a Swedish multicenter randomized controlled trial. Surg Obes Relat Dis. 2020;16:1035‐1044. [DOI] [PubMed] [Google Scholar]
- 28. Hofsø D, Fatima F, Borgeraas H, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single‐centre, triple‐blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:912‐924. [DOI] [PubMed] [Google Scholar]
- 29. Kalinowski P, Paluszkiewicz R, Wróblewski T, et al. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux‐en‐Y gastric bypass‐results of a randomized clinical trial. Surg Obes Relat Dis. 2017;13:181‐188. [DOI] [PubMed] [Google Scholar]
- 30. Biter LU, van Buuren MMA, Mannaerts GHH, Apers JA, Dunkelgrün M, Vijgen GHEJ. Quality of Life 1 year after laparoscopic sleeve gastrectomy versus laparoscopic Roux‐en‐Y gastric bypass: a randomized controlled trial focusing on gastroesophageal reflux disease. Obes Surg. 2017;27:2557‐2565. [DOI] [PubMed] [Google Scholar]
- 31. Ignat M, Vix M, Imad I, et al. Randomized trial of Roux‐en‐Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg. 2017;104:248‐256. [DOI] [PubMed] [Google Scholar]
- 32. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide‐YY levels after Roux‐en‐Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401‐407. [DOI] [PubMed] [Google Scholar]
- 33. Helmiö M, Victorzon M, Ovaska J, et al. Comparison of short‐term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6‐month follow‐up. Scand J Surg. 2014;103:175‐181. [DOI] [PubMed] [Google Scholar]
- 34. Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21:1650‐1656. [DOI] [PubMed] [Google Scholar]
- 35. Wallenius V, Dirinck E, Fändriks L, Maleckas A, le Roux CW, Thorell A. glycemic control after sleeve gastrectomy and Roux‐en‐Y gastric bypass in obese subjects with type 2 diabetes mellitus. Obes Surg. 2018;28:1461‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234‐241. [DOI] [PubMed] [Google Scholar]
- 37. Peterli R, Borbély Y, Kern B, et al. Early results of the Swiss multicentre bypass or sleeve study (SM‐BOSS). Ann Surg. 2013;258:690‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peterli R, Wölnerhanssen BK, Vetter D, et al. Laparoscopic sleeve gastrectomy versus Roux‐Y‐gastric bypass for morbid obesity‐3‐year outcomes of the prospective randomized Swiss multicenter bypass or sleeve study (SM‐BOSS). Ann Surg. 2017;265:466‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbély Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy—a prospective randomized trial. Surg Obes Relat Dis. 2011;7:561‐568. [DOI] [PubMed] [Google Scholar]
- 41. Yang J, Wang C, Cao G, et al. Long‐term effects of laparoscopic sleeve gastrectomy versus roux‐en‐Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28–35 kg/m(2). BMC Surg. 2015;15:88. doi: 10.1186/s12893-015-0074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux‐en‐Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5‐year outcome. Obes Surg. 2014;24:1617‐1624. [DOI] [PubMed] [Google Scholar]
- 43. Tang QI, Sun Z, Zhang N, et al. Cost‐effectiveness of bariatric surgery for type 2 diabetes mellitus. Medicine (Baltimore). 2016;95:e3522. doi: 10.1097/MD.0000000000003522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes — 5‐year outcomes. N Engl J Med. 2017;376:641‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Barros F, Setúbal S, Martinho JM, Monteiro ABS. Early endocrine and metabolic changes after bariatric surgery in grade III morbidly obese patients: a randomized clinical trial comparing sleeve gastrectomy and gastric bypass. Metab Syndr Relat Disord. 2015;13:264‐271. [DOI] [PubMed] [Google Scholar]
- 47. Lee W‐J, Yu P‐J, Wang W, Chen T‐C, Wei P‐L, Huang M‐T. Laparoscopic Roux‐en‐Y versus mini‐gastric bypass for the treatment of morbid obesity. Ann Surg. 2005;242:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Level L, Rojas A, Piñango S, Avariano Y. One anastomosis gastric bypass vs. Roux‐en‐Y gastric bypass: a 5‐year follow‐up prospective randomized trial. Langenbecks Arch Surg. 2021;406:171‐179. [DOI] [PubMed] [Google Scholar]
- 49. Ruiz‐Tovar J, Carbajo MA, Jimenez JM, et al. Long‐term follow‐up after sleeve gastrectomy versus Roux‐en‐Y gastric bypass versus one‐anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc. 2019;33:401‐410. [DOI] [PubMed] [Google Scholar]
- 50. Garcia‐Oria MJ, Rivera JA, Alvarez J, et al. Prospective randomized trial of OAGB vs. LRYGB, weight results in the first 18 months after surgery [IFSO abstract]. Obesity Surg. 2019;29(5):639‐639. doi: 10.1007/s11695-019-04101-1 [DOI] [Google Scholar]
- 51.Table 8.5.a: The Cochrane Collaboration tool for assessing risk of bias. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. version 5.1.0. Updated March 2011. Accessed June 21, 2021. https://handbook‐5‐1.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm
- 52. WebPlotDigitizer Accessed July 2, 2021. https://automeris.io/WebPlotDigitizer/
- 53. Lee Y, Doumouras AG, Yu J, et al. Laparoscopic sleeve gastrectomy versus laparoscopic Roux‐en‐Y gastric bypass: a systematic review and meta‐analysis of weight loss, comorbidities, and biochemical outcomes from randomized controlled trials. Ann Surg. 2021;273:66‐74. [DOI] [PubMed] [Google Scholar]
- 54. Sharples AJ, Mahawar K. Systematic review and meta‐analysis of randomised controlled trials comparing long‐term outcomes of Roux‐en‐Y gastric bypass and sleeve gastrectomy. Obes Surg. 2020;30:664‐672. [DOI] [PubMed] [Google Scholar]
- 55. Hayoz C, Hermann T, Raptis DA, Brönnimann A, Peterli R, Zuber M. Comparison of metabolic outcomes in patients undergoing laparoscopic Roux‐en‐Y gastric bypass versus sleeve gastrectomy – a systematic review and meta‐analysis of randomised controlled trials. Swiss Med Wkly. 2018;148:w14633. doi: 10.4414/smw.2018.14633 [DOI] [PubMed] [Google Scholar]
- 56. Zhao K, Liu J, Wang M, Yang H, Wu A. Safety and efficacy of laparoscopic sleeve gastrectomy versus laparoscopic Roux‐en‐Y gastric bypass: a systematic review and meta‐analysis. J Eval Clin Pract. 2020;26:290‐298. [DOI] [PubMed] [Google Scholar]
- 57. Gu L, Huang X, Li S, et al. A meta‐analysis of the medium‐ and long‐term effects of laparoscopic sleeve gastrectomy and laparoscopic Roux‐en‐Y gastric bypass. BMC Surg. 2020;20:30. doi: 10.1186/s12893-020-00695-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guraya SY, Strate T. Effectiveness of laparoscopic Roux‐en‐Y gastric bypass and sleeve gastrectomy for morbid obesity in achieving weight loss outcomes. Int J Surg. 2019;70:35‐43. [DOI] [PubMed] [Google Scholar]
- 59. Wölnerhanssen BK, Peterli R, Hurme S, et al. Laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy: 5‐year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM‐BOSS). Br J Surg. 2021;108:49‐57. [DOI] [PubMed] [Google Scholar]
- 60. Grönroos S, Helmiö M, Juuti A, et al. Effect of laparoscopic sleeve gastrectomy vs Roux‐en‐Y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2021;156:137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta‐analysis. Surg Obes Relat Dis. 2018;14:741‐747. [DOI] [PubMed] [Google Scholar]
- 62. Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11:489‐506. [DOI] [PubMed] [Google Scholar]
- 63. Grover BT, Morell MC, Kothari SN, Borgert AJ, Kallies KJ, Baker MT. Defining weight loss after bariatric surgery: a call for standardization. Obes Surg. 2019;29:3493‐3499. [DOI] [PubMed] [Google Scholar]
- 64. Manning S, Pucci A, Carter NC, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux‐en‐Y gastric bypass. Surg Endosc. 2015;29:1484‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mor A, Sharp L, Portenier D, Sudan R, Torquati A. Weight loss at the first postoperative visit predicts long term outcome of Roux‐en‐Y gastric bypass using the Duke Weight Loss Surgery Chart. Surg Obes Relat Dis. 2012;8:556‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult‐onset diabetes mellitus. Ann Surg. 1995;222:339‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li J, Lai D, Wu D. Laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity‐related comorbidities: a systematic review and meta‐analysis. Obes Surg. 2016;26:429‐442. [DOI] [PubMed] [Google Scholar]
- 68. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44:2438‐2444. doi: 10.2337/dci21-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. El‐Hadi M, Birch DW, Gill RS, Karmali S. The effect of bariatric surgery on gastroesophageal reflux disease. Can J Surg. 2014;57:139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015;21:10348‐10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chaar ME, Lundberg P, Stoltzfus J. Thirty‐day outcomes of sleeve gastrectomy versus Roux‐en‐Y gastric bypass: first report based on Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database. Surg Obes Relat Dis. 2018;14:545‐551. [DOI] [PubMed] [Google Scholar]
- 72. Magouliotis DE, Tasiopoulou VS, Tzovaras G. One anastomosis gastric bypass versus roux‐en‐Y gastric bypass for morbid obesity: an updated meta‐analysis. Obes Surg. 2019;29:2721‐2730. [DOI] [PubMed] [Google Scholar]
- 73. Wang F‐G, Yan W‐M, Yan M, Song M‐M. Outcomes of mini vs Roux‐en‐Y gastric bypass: a meta‐analysis and systematic review. Int J Surg. 2018;56:7‐14. [DOI] [PubMed] [Google Scholar]
- 74. Lee W‐J, Ser K‐H, Lee Y‐C, Tsou J‐J, Chen S‐C, Chen J‐C. Laparoscopic Roux‐en‐Y vs. mini‐gastric bypass for the treatment of morbid obesity: a 10‐year experience. Obes Surg. 2012;22:1827‐1834. [DOI] [PubMed] [Google Scholar]
- 75. Mustafa A, Rizkallah NNH, Samuel N, Balupuri S. Laparoscopic Roux‐En‐Y gastric bypass versus one anastomosis (loop) gastric bypass for obesity: a prospective comparative study of weight loss and complications. Ann Med Surg (Lond). 2020;55:143‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ahuja A, Tantia OM, Goyal G, et al. MGB‐OAGB: effect of biliopancreatic limb length on nutritional deficiency, weight loss, and comorbidity resolution. Obes Surg. 2018;28:3439‐3445. [DOI] [PubMed] [Google Scholar]
- 77. Mahawar KK, Parmar C, Carr WRJ, Jennings N, Schroeder N, Small PK. Impact of biliopancreatic limb length on severe protein–calorie malnutrition requiring revisional surgery after one anastomosis (mini) gastric bypass. J Minim Access Surg. 2018;14:37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pizza F, Lucido FS, D’Antonio D, et al. Biliopancreatic limb length in one anastomosis gastric bypass: which is the best? Obes Surg. 2020;30:3685‐3694. [DOI] [PubMed] [Google Scholar]
- 79. Kermansaravi M, Mahawar KK, Davarpanah Jazi AH, Eghbali F, Kabir A, Pazouki A. Revisional surgery after one anastomosis/mini gastric bypass: a narrative review. J Res Med Sci. 2020;25:62. doi: 10.4103/jrms.JRMS_727_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Musella M, Susa A, Manno E, et al. Complications following the mini/one anastomosis gastric bypass (MGB/OAGB): a multi‐institutional survey on 2678 patients with a mid‐term (5 years) follow‐up. Obes Surg. 2017;27:2956‐2967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Table S10
Table S11
Table S12
Table S13
Table S14
Table S15
Table S16


