Abstract
Background
Autologous haematopoietic stem cell transplantation (AHSCT) is a highly effective treatment for multiple sclerosis (MS). The impact of previous long-lasting disease-modifying treatments (DMT) for safety and efficacy of AHSCT is unknown.
Objective
To explore whether previous DMTs with long-lasting effects on the immune system (anti-CD20 therapy, alemtuzumab and cladribine) affect treatment-related complications, long-term outcome and risk of new MS disease activity in patients treated with AHSCT.
Methods
Retrospective observational study of 104 relapsing remitting patients with MS treated by AHSCT in Sweden and Norway from 2011 to 2021, grouped according to the last DMT used ≤6 months prior to AHSCT. The primary outcomes were early AHSCT-related complications (mortality, neutropenic fever and hospitalisation length), long-term complications (secondary autoimmunity) and proportion of patients with No Evidence of Disease Activity (NEDA-3 status): no new relapses, no MRI activity and no disease progression during the follow-up.
Results
The mean follow-up time was 39.5 months (range 1–95). Neutropenic fever was a common AHSCT-related complication affecting 69 (66%) patients. There was no treatment-related mortality. During the follow-up period, 20 patients (19%) were diagnosed with autoimmunity. Occurrence of neutropenic fever, hospitalisation length or secondary autoimmunity did not vary dependent on the last DMT used prior to AHSCT. A total of 84 patients (81%) achieved NEDA-3 status, including all patients (100%) using rituximab, alemtuzumab or cladribine before AHSCT.
Conclusion
This study provides level 4 evidence that AHSCT in patients previously treated with alemtuzumab, cladribine or rituximab is safe and efficacious.
Keywords: multiple sclerosis, haematology
Key messages.
What is already known on this topic
Autologous haematopoietic stem cell transplantation (AHSCT) is a highly effective treatment for patients with aggressive relapsing remitting multiple sclerosis (RRMS), most often used in patients failing other disease-modifying treatments (DMTs).
Newer DMTs (like anti-CD20 therapies, alemtuzumab and cladribine) have a long-term effect on the immune system.
Whether AHSCT is safe and effective for patients that have previously been treated with these medications is unknown, and most studies have excluded these patients from AHSCT.
What this study adds
In our study of 104 RRMS patients, we found that patients who previously used alemtuzumab, cladribine or rituximab had the same rate of treatment-related complications and long-term complications as patients previously treated with other DMTs.
All these patients attained a sustained No Evidence of Disease Activity-3 status after AHSCT.
How this study might affect research, practice and/or policy
This study provides level 4 evidence that AHSCT in patients previously treated with alemtuzumab, cladribine or rituximab is safe and efficacious.
Introduction
Autologous haematopoietic stem cell transplantation (AHSCT) is a treatment option for patients with aggressive relapsing remitting multiple sclerosis (RRMS). The therapeutic rationale is based on giving immunoablative treatment causing depletion of autoreactive cells, followed by infusion of cryopreserved autologous haematopoietic stem cells to support immune reconstitution.1 AHSCT is primarily offered to patients with highly aggressive MS, mostly after failing other disease-modifying treatments (DMTs). Available DMTs vary according to potency, side effects and the route and frequency of administration. Rituximab, cladribine and alemtuzumab cause depletion of immune cells, and also have the potential to induce long-term drug-free remissions.2 3 The preferred first-line treatment choice and sequence of DMTs vary between treatment centres, but DMTs with high potencies are recommended for patients with aggressive disease.4 Most centres have strict criteria for AHSCT treatment, preserving it as a second-line or third-line therapy for patients with highly aggressive MS, but sometimes it is offered as a first treatment choice.5 The results published after AHSCT in RRMS patients demonstrate excellent outcomes, with 60%–80% of patients attaining No Evidence of Disease Activity (NEDA-3)-status for >5 years.6–8
Whether AHSCT should be offered to patients previously treated with DMTs with long-lasting effects on the immune system is debated, and currently no consensus has been reached. Some treatment centres and studies have excluded patients due to preceding use of DMTs, especially alemtuzumab.6 The reasons have been fear of prolonged aplasia and risk of infections after AHSCT in patients with pre-AHSCT lymphopenia due to DMTs, and development of secondary autoimmune diseases.9 However, a recently published case report of three patients indicated that AHSCT may be safe after previous alemtuzumab treatment in patients with MS.10 So far, no reports are available on the efficacy and safety after AHSCT following cladribine or anti-CD20 therapy. The use of DMTs with long-lasting effects on the immune system is increasing. Accordingly, it is of importance to evaluate the effect and safety of AHSCT for patients previously exposed to these treatment options. In this study, data regarding the previous use of DMTs were collected from patients treated at Haukeland University Hospital in Norway and at Uppsala University Hospital in Sweden between 2011 and -2021 to determine whether various DMTs had an impact on AHSCT-related complications and long-term prognosis in RRMS, with the main aim to clarify the safety of previous use of rituximab, alemtuzumab and cladribine.
Material and methods
Study population, design and data sources
This is a retrospective observational cohort study of 104 RRMS (35 Norwegian and 69 Swedish) patients. The Swedish patients consisted of RRMS patients treated with AHSCT from 1 January 2011 to 31 December 2018 at Uppsala University Hospital. All patients with MS in Sweden are recorded in the Swedish Multiple Sclerosis Register, and the patients were identified through a register search and originally asked to participate in a comparative study of AHSCT and alemtuzumab.11 In Norway, all patients with MS treated with AHSCT at Haukeland University Hospital from January 2015 to February 2021 before or outside an ongoing randomised trial (RAM-MS=Randomised controlled trial comparing autologous haematopoietic stem cell transplantation versus alemtuzumab, cladribine or ocrelizumab in MS), were asked to participate, and 95% accepted. In both countries, AHSCT is offered to patients with highly active MS, most frequently after detection of new disease activity in spite of ongoing standard DMT. In Sweden, some patients who present with aggressive MS are offered AHSCT as their first treatment option.5 All patients had clinical follow-ups at least annually, including assessment of Expanded Disability Status Scale (EDSS), MRI and registration of adverse events. Medical records of all patients were assembled and evaluated.
Procedure
The treatment protocols were the same at both treatment centres and consisted of an intermediate intensity, lymphoablative/not-myeloablative conditioning regimen.12 13 Peripheral haematopoietic stem cells were mobilised by a single dose of cyclophosphamide (2 g/m2), followed by daily granulocyte colony-stimulating factor, 5–10 µg/kg × 1 per day for 5–7 days. Patients were conditioned with a combination of cyclophosphamide and rabbit antithymocyte globulin (cyclophosphamide 200 mg/kg; rATG 6 mg/kg). The cryopreserved autologous stem cells (a minimum amount of 3×106 CD34+cells/kg) were reinfused without any graft manipulation. All patients received prophylactic antibiotic regimens with ciproxine, valaciclovir and fluconazole.
Measures of disease activity
NEDA-3 was defined as a composite score comprising absence of clinical relapses, sustained disability progression and new MRI disease activity (new T1 gadolinium enhancing lesions or new/enlarging T2-lesions) on MRI examinations for the given period.14 A relapse was defined as the appearance of new symptoms or signs that lasted for more than 24 hours without concurrent fever or illness. Progression was defined as an increase in EDSS score of at least one point from baseline sustained between two follow-up visits separated in time by no less than 6 months (1.5 point if EDSS at baseline was 0, 0.5 points if the baseline EDSS ≥5.5).
Statistics
Baseline data and demographic results were assessed using descriptive statistics. For illustration of safety outcomes and long-term treatment results, we used Kaplan-Meier survival curves. The differences between groups were assessed by one-way analysis of variance. A p<0.05 was considered statistically significant. All analyses were assessed by SPSS Statistics V.26 (IBM). Data are described as means and percentages.
Results
Patient characteristics
The cohort consisted of 104 RRMS patients (69 Swedish and 35 Norwegian) with a mean age of 30.8 years (10.2–58.8), and a female-male ratio of 2.7:1. All patients had active disease, and the mean ARR 1 year prior to AHSCT were 1.7. Baseline demographics are shown in table 1. Most patients had a history of suboptimal treatment responses to other DMTs, due to either side effects or clinical relapses, but 12 patients had no previous MS treatment. The mean number of previous DMTs was 2.1, while 17 patients (16.3 %) had used ≥4 previous DMTs. A total of 79 (76 %) of the patients had been exposed to a DMT the last 6 months prior to AHSCT. A total of 26 patients (25 %) had used DMTs with a long-term effect on the immune system; rituximab,15 alemtuzumab6 and cladribine.2 The rest of the patients had been treated with natalizumab,16 fingolimod,17 interferons,6 dimethyl fumarate,5 glatiramer acetate4 and teriflunomide.3 The mean follow-up time was 39.5 months (range 1–95).
Table 1.
Demographic and clinical data at baseline
| Patients, n Swedish/Norwegian | 104 (69/35) |
| Gender, female/male | 76/28 |
| Age, years (mean/range) | 30 /(10-58) |
| Disease duration, years (mean) | 5.8 |
| EDSS at baseline (median/range) | 3/(0–6.5) |
| No of previous treatments | 2.1 (0–6) |
| Last treatment (≤6 months prior to HSCT) | |
| No treatment | 25 (24%) |
| Standard DMT | |
| Interferons | 6 (6%) |
| Glatiramer acetate | 4 (4%) |
| Fingolimod | 15 (14%) |
| Natalizumab | 20 (19%) |
| Dimethyl fumarate | 5 (5%) |
| Teriflunomide | 3 (3%) |
| DMT with long-lasting effect | |
| Alemtuzumab | 6 (6%) |
| Cladribine | 2 (2%) |
| Rituximab | 18 (17 %) |
DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; HSCT, haematopoietic stem cell transplantation.
Early adverse events
The patients had a mean 13 days of inpatient care (range 10–23). Time to engraftment did not vary according to the last DMT given prior to AHSCT. A total of 69 patients (66 %) had neutropenic fever, and were treated by intravenous antibiotics, of these 20 (29 %) patients had used DMTs with long-term effects on the immune system. There was no significant difference between the groups (p=0.3). One patient (1%) treated with natalizumab 3 months before AHSCT, had septic febrile neutropenia with hypotonia and Epstein-Barr virus reactivation, and was observed in an intensive care unit for 1 day. One patient without previous DMT developed fever and psychosis, was treated with intravenous antibiotics and steroids, and had a good recovery. One patient previously treated with fingolimod had a thoracic venous thrombosis during hospitalisation. There was no treatment-related mortality. All patients were discharged from the hospital within 23 days.
Late adverse events
During the follow-up period, 20 patients (19 %) had a secondary autoimmune disease. A total of 11 acquired hyperthyroidism (10 %), seven hypothyroidism (7 %), one patient hypothyroidism and psoriasis vulgaris (1 %), and one patient (1 %) autoimmune thrombocytopenic purpura. Among the patients treated with DMTs with a long-lasting effect (N=26), four patients (15 %) developed secondary autoimmunity. Two patients previously treated with alemtuzumab were diagnosed with hyperthyroidism. A total of seven patients (7 %) with secondary autoimmunity was not exposed to DMT the last 6 months prior to AHSCT. There was no statistically significant difference in the number of patients developing secondary autoimmunity between the different pre-AHSCT DMTs (table 2).
Table 2.
Demographics and results according to previous treatment (<6 months prior to AHSCT)
| Whole cohort | None | INTF | GA | FTY | NTZ | DMF | TFM | ALEM | CLD | RTX | |
| Patients, no | 104 | 25 | 6 | 4 | 15 | 20 | 5 | 3 | 6 | 2 | 18 |
| Age, mean | 30.8 | 29.7 | 24.4 | 37.5 | 28 | 29 | 28.5 | 34.8 | 32.5 | 23.5 | 25 |
| Gender, no (F/M) | 76/28 | 19/6 | 4/2 | 2/2 | 12/3 | 13/7 | 4/1 | 2/1 | 6/0 | 1/1 | 13/5 |
| Baseline EDSS, median | 3.0 | 3.5 | 2.7 | 3.0 | 3.0 | 3.3 | 4.0 | 2.5 | 4.0 | 2.3 | 2.0 |
| ARR, 1 year prior to treatment, mean | 1.7 | 1.6 | 1.8 | 1.3 | 1.7 | 1.6 | 2.2 | 0.7 | 1.5 | 0.5 | 1.9 |
| Washout duration last DMT (months, mean) | – | – | 1.9 | 2.5* | 3.4 | 3.6 | 5.3* | 3.5 | – | – | – |
| Follow-up, months, mean | 39.5 | 38.3 | 59.3 | 73.3 | 37.7 | 41.9 | 44.8 | 16.6 | 23 | 24.5 | 30.1 |
| Neutropenic fever, n (%) | 69 (66) | 14 (56) | 4 (66) | 3 (75) | 12 (80) | 10 (50) | 4 (80) | 2 (66) | 6 (100) | 1 (50) | 13 (72) |
| Hospitalisation, days, mean | 13.1 | 12.8 | 13 | 12 | 12.9 | 13.2 | 12.6 | 14.3 | 14 | 12.5 | 13.9 |
| Secondary autoimmunity, n (%) | 20 (19) | 7 (28) | 1 (17) | 1 (25) | 2 (13) | 2 (10) | 2 (40) | 1 (33) | 2 (33) | 0 (0) | 2 (11) |
| New disease activity, n (%) | 20 (19) | 6 (24) | 3 (50) | 1 (25) | 5 (33) | 4 (20) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
*1 missing.
AHSCT, autologous haematopoietic stem cell transplantation; ALEM, alemtuzumab (Lemtrada); ARR, annualised relapse rate; CLD, cladribine (Mavenclad); DMF, dimethyl fumarate (Tecfidera); DMT, disease-modifying treatments; FTY, fingolimod (Gilenya); GA, glatiramer acetate (Copaxone); INTF, interferons (Pledigry/Betaferon/Avonex); NTZ, natalizumab (Tysabri); RTX, rituximab (MabThera); TFM, teriflunomide (Aubagio).
Efficacy
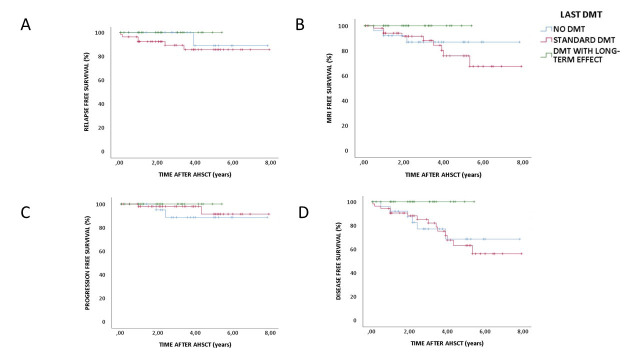
A total of 20 patients (19 %) had evidence of disease activity during the follow-up period; 9 patients had new relapses and MRI activity, 8 had only new MRI activity and 3 had sustained disease progression. The patients with disease progression had a baseline EDSS of 4–5.5 that increased from 1 to 3 points. The time span before new disease activity varied from 1 to 64 months after AHSCT. The number of patients attaining sustained NEDA-3 status differed according to the last pre-AHSCT MS treatment (p<0.01) as illustrated in figure 1. A total of 14 (70 %) of the patients with new disease activity after AHSCT had used standard DMTs the last 6 months prior to AHSCT. All patients using rituximab (N=18), alemtuzumab (N=6) or cladribine (N=2) before AHSCT attained NEDA-3 status during the follow-up period (table 2).
Figure 1.
Kaplan-Meier survival curve of relapse-free survival (A), MRI event-free survival (B), EDSS progression-free survival (C) and disease-free survival (D) (patients with achieved NEDA-3 status) according to last DMT. AHSCT, autologous haematopoietic stem cell transplantation; DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; NEDA, no evidence of disease activity.
Discussion
In this retrospective study of 104 RRMS patients, former treatment with rituximab, alemtuzumab or cladribine were associated with the same frequency of early and late adverse events as pre-AHSCT treatment with other DMTs. Neutropenic fever was a common complication, but was not associated with specific DMTs. None of the three patients with the most serious early adverse events had been treated with rituximab, alemtuzumab or cladribine before AHSCT.
Rituximab, cladribine and alemtuzumab cause immunosuppression by lymphocyte depletion.2 Rituximab treatment is particularly associated with increased risk of infections, but this has also been shown for alemtuzumab.17–19 Since alemtuzumab increases the risk of opportunistic infections, such as Listeria meningitis, cautious and restricted use are recommended.4 15 Interestingly, we did not find any increase in AHSCT-related infections in patients previously treated with rituximab, cladribine or alemtuzumab. Alemtuzumab has previously been shown to increase the risk of secondary autoimmune diseases, with thyroid autoimmune disease being the most frequent, affecting nearly half of the patients treated.20 A total of 20 patients were diagnosed with a secondary autoimmune disease during the follow-up period, two of which had used alemtuzumab. The impact of DMTs on the safety and efficacy of a subsequent AHSCT has not been previously explored in patients with MS, except for a case report of three AHSCT patients treated with alemtuzumab.10 The results were consistent with our findings, indicating that the risk of AHSCT is not relatively increased after previous treatment with alemtuzumab.
We found that all patients previously treated with rituximab, alemtuzumab and cladribine attained a sustained NEDA-3 status throughout the follow-up period. For patients with other DMTs or no treatment the last 6 months prior to AHSCT, the proportion with new MS disease activity varied. The impact of previous fingolimod use on disease activity after AHSCT has so far not been examined. However, a number of reports have explored the effects of treatment shifts for various sequences of DMTs. An observational study found that fingolimod treatment was less effective in patients discontinuing natalizumab.16 For fingolimod, there is a known risk of a rebound effect after discontinuation,21–23 and several reports have described a suboptimal disease control for treatment with alemtuzumab, rituximab and ocrelizumab in patients previously treated with fingolimod.24–29 This may be explained by the mode of action of fingolimod, that is, sequestering the immune cells in the lymph nodes. Hence, autoreactive pathogenic B cells may be sequestered in secondary lymph nodes and return to the circulation after new treatment is started. In this study, a total of 15 patients used fingolimod as the last treatment before AHSCT, with a wash-out period ranging from 1.5 to 5.5 months. Interestingly, no impact of fingolimod was registered regarding the clinical effects or adverse events after AHSCT.
We found a significant difference in MS disease activity after AHSCT depending on the patients pre-AHSCT medication, in favour of alemtuzumab, rituximab and cladribine. One possible reason may be that previous DMTs with long-lasting effects could influence the amount of remaining autoreactive lymphocytes in the circulation and in the autologous stem cell product.
This study examined a significant cohort of RRMS patients treated with AHSCT, constituting most patients treated with AHSCT in Sweden and Norway from 2011 to 2021, and with an average follow-up period of more than 3 years. All patients were treated with the same intermediate intensity conditioning regime consisting of cyclophosphamide and ATG. The patients studied had been treated by most of the available DMTs prior to AHSCT. Treatment with rituximab, alemtuzumab or cladribine was used for 26 patients before AHSCT, making this a cohort suitable to explore potential differences in AHSCT treatment effect and side effects between pre-AHSCT-treatments with long-lasting and short-lasting immunomodulatory effects. There are, however, some limitations. The groups of patients treated with each individual DMT was small, and data indicating a higher chance of obtaining NEDA-3 among patients given pre-AHSCT DMTs with long-term effects on the immune system should accordingly be interpreted with caution. Furthermore, we cannot rule out that a longer observation time could have yielded somewhat different results regarding the occurrence of secondary autoimmunity or the post-AHSCT MS disease activities.
DMTs with long-term effects on the immune system have pronounced effects on disease activity,17 19 30–33 and are increasingly preferred treatment choices for MS. In the upcoming years, a higher proportion of AHSCT patient candidates will have a medical history of prior usage of such DMTs. Our data indicate that previous treatment with alemtuzumab, cladribine or rituximab is safe, and associated with a high likelihood of sustained NEDA-3 after transplantation.
Conclusion
This study provides level 4 evidence that AHSCT in patients previously treated with alemtuzumab, cladribine or rituximab is safe and efficacious.
Footnotes
Contributors: SASK: study concept and design, acquisition of data. JB: acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. AKL: acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. AT: acquisition of data, critical revision of the manuscript for important intellectual content. CZ: acquisition of data, critical revision of the manuscript for important intellectual content. GKM: critical revision of the manuscript for important intellectual content. LB: acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. ØT: study concept and design, analysis and interpretation, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. SASK acts as guarantor and is eesponsible for the overall content
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SASK has received unrestricted grants from Novartis and Biogen Idec. LB has received speaker honoraria from Novartis. ØT has received speaker honoraria from and served on scientific advisory boards for Biogen, Sanofi-Aventis, Merck and Novartis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Regional Ethical Committee (REK 2018/377) of Norway.
References
- 1. Scolding NJ, Pasquini M, Reingold SC, et al. Cell-Based therapeutic strategies for multiple sclerosis. Brain 2017;140:2776–96. 10.1093/brain/awx154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lünemann JD, Ruck T, Muraro PA, et al. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol 2020;16:56–62. 10.1038/s41582-019-0268-z [DOI] [PubMed] [Google Scholar]
- 3. Sellner J, Rommer PS. Immunological consequences of "immune reconstitution therapy" in multiple sclerosis: A systematic review. Autoimmun Rev 2020;19:102492. 10.1016/j.autrev.2020.102492 [DOI] [PubMed] [Google Scholar]
- 4. Yamout B, Sahraian M, Bohlega S, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord 2020;37:101459. 10.1016/j.msard.2019.101459 [DOI] [PubMed] [Google Scholar]
- 5. Das J, Snowden JA, Burman J, et al. Autologous haematopoietic stem cell transplantation as a first-line disease-modifying therapy in patients with 'aggressive' multiple sclerosis. Mult Scler 2021;27:1198–204. 10.1177/1352458520985238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA 2019;321:165–74. 10.1001/jama.2018.18743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nash RA, Hutton GJ, Racke MK, et al. High-Dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology 2017;88:842–52. 10.1212/WNL.0000000000003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burman J, Iacobaeus E, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry 2014;85:1116–21. 10.1136/jnnp-2013-307207 [DOI] [PubMed] [Google Scholar]
- 9. Burt RK, Balabanov R, Han X, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 2015;313:275–84. 10.1001/jama.2014.17986 [DOI] [PubMed] [Google Scholar]
- 10. Boffa G, Sbragia E, Raiola AM, et al. Autologous hematopoietic stem cell transplantation following alemtuzumab therapy in aggressive multiple sclerosis: a report of three cases. Mult Scler 2021;27:1145–8. 10.1177/1352458520914818 [DOI] [PubMed] [Google Scholar]
- 11. Zhukovsky C, Sandgren S, Silfverberg T, et al. Autologous haematopoietic stem cell transplantation compared with alemtuzumab for relapsing-remitting multiple sclerosis: an observational study. J Neurol Neurosurg Psychiatry 2021;92:189–94. 10.1136/jnnp-2020-323992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharrack B, Saccardi R, Alexander T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT autoimmune diseases Working Party (ADWP) and the joint accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant 2020;55:283–306. 10.1038/s41409-019-0684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kvistad SAS, Lehmann AK, Trovik LH, et al. Safety and efficacy of autologous hematopoietic stem cell transplantation for multiple sclerosis in Norway. Mult Scler 2020;26:1889–97. 10.1177/1352458519893926 [DOI] [PubMed] [Google Scholar]
- 14. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015;72:152–8. 10.1001/jamaneurol.2014.3537 [DOI] [PubMed] [Google Scholar]
- 15. Buonomo AR, Zappulo E, Viceconte G, et al. Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis. Expert Opin Drug Saf 2018;17:709–17. 10.1080/14740338.2018.1483330 [DOI] [PubMed] [Google Scholar]
- 16. Baldi E, Guareschi A, Vitetta F, et al. Previous treatment influences fingolimod efficacy in relapsing-remitting multiple sclerosis: results from an observational study. Curr Med Res Opin 2014;30:1849–55. 10.1185/03007995.2014.921144 [DOI] [PubMed] [Google Scholar]
- 17. Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology 2016;87:2074–81. 10.1212/WNL.0000000000003331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Flon P, Gunnarsson M, Laurell K, et al. Reduced inflammation in relapsing-remitting multiple sclerosis after therapy switch to rituximab. Neurology 2016;87:141–7. 10.1212/WNL.0000000000002832 [DOI] [PubMed] [Google Scholar]
- 19. CAMMS223 Trial Investigators, Coles AJ, Compston DAS, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–801. 10.1056/NEJMoa0802670 [DOI] [PubMed] [Google Scholar]
- 20. Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015;86:208–15. 10.1136/jnnp-2014-307721 [DOI] [PubMed] [Google Scholar]
- 21. Frau J, Sormani MP, Signori A, et al. Clinical activity after fingolimod cessation: disease reactivation or rebound? Eur J Neurol 2018;25:1270–5. 10.1111/ene.13694 [DOI] [PubMed] [Google Scholar]
- 22. Hatcher SE, Waubant E, Nourbakhsh B, et al. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 2016;73:790–4. 10.1001/jamaneurol.2016.0826 [DOI] [PubMed] [Google Scholar]
- 23. Barry B, Erwin AA, Stevens J, et al. Fingolimod rebound: a review of the clinical experience and management considerations. Neurol Ther 2019;8:241–50. 10.1007/s40120-019-00160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pfeuffer S, Ruck T, Pul R, et al. Impact of previous disease-modifying treatment on effectiveness and safety outcomes, among patients with multiple sclerosis treated with alemtuzumab. J Neurol Neurosurg Psychiatry 2021;92:1007–13. 10.1136/jnnp-2020-325304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boudot de la Motte M, Louapre C, Papeix C, et al. Challenges of switching towards anti-CD20 monoclonal antibodies in RR-MS: a monocentric study. Mult Scler Relat Disord 2021;52:102981. 10.1016/j.msard.2021.102981 [DOI] [PubMed] [Google Scholar]
- 26. Zhong M, van der Walt A, Stankovich J, et al. Prediction of multiple sclerosis outcomes when switching to ocrelizumab. Mult Scler 2022;28:13524585211049986. 10.1177/13524585211049986 [DOI] [PubMed] [Google Scholar]
- 27. Holmøy T, Torkildsen Øivind, Zarnovicky S. Extensive multiple sclerosis reactivation after switching from fingolimod to rituximab. Case Rep Neurol Med 2018;2018:1–3. 10.1155/2018/5190794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt S, Schulten T. Severe rebound after cessation of fingolimod treated with ocrelizumab with coincidental transient aggravation: report of two cases. Ther Adv Neurol Disord 2019;12:1756286419846818. 10.1177/1756286419846818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2017;4:e320. 10.1212/NXI.0000000000000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 2018;75:320–7. 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1A as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–28. 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 32. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–39. 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 33. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010;362:416–26. 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.