Abstract
Idiopathic intracranial hypertension (IIH) is more common in women of reproductive age who have obesity, yet there is little information on its management specifically in pregnancy. Women with IIH should plan their pregnancy including discussing contraception before pregnancy, recognising that hormonal contraceptives are not contraindicated. Potentially teratogenic medications including acetazolamide and topiramate are not recommended during pregnancy or in those with immediate plans to conceive; prescribing acetazolamide in pregnancy must only follow discussion with the patient and their obstetrician. Ideally, patients should aim to achieve disease remission or control before pregnancy, through optimising their weight. Although weight gain is expected in pregnancy, excessive weight gain may exacerbate IIH and increase maternal and fetal complications; evidence-based recommendations for non-IIH pregnancies may help in guiding optimal gestational weight gain. The vast majority of women with IIH can have a normal vaginal delivery, with spinal or epidural anaesthesia if needed, provided the papilloedema is stable or the IIH is in remission.
Keywords: BENIGN INTRACRAN HYP, OBSTETRICS, METABOLIC DISEASE, NEUROOPHTHALMOLOGY
Introduction
Idiopathic intracranial hypertension (IIH) is characterised by raised intracranial pressure that frequently causes chronic headaches and visual loss. It predominately affects women of reproductive age with obesity.1 Its exact pathophysiological mechanisms are not yet fully established.2 IIH is associated with central adiposity3 and recent weight gain4 and IIH adipocytes appear transcriptionally and metabolically primed towards lipogenesis and adipose accumulation.5 Other features of metabolic dysregulation in IIH include a doubled risk of cardiovascular disease,6 androgen excess,7 and insulin resistance.5 The document ‘IIH: the international consensus guideline’ is a useful source of information for its management.8
Reproductive health in patients with IIH can be complex and there is currently limited guidance to support clinical care. We aim to provide practical guidance for managing women with IIH when approaching and during pregnancy. We discuss its medical and surgical management in relation to pregnancy and suggest limits on gestational weight gain.
Prepregnancy
Contraception
Hormonal contraceptives are not contraindicated in IIH8 and there is no preferred contraceptive method. Previously, case reports have linked oral contraceptives to secondary causes of raised intracranial pressure (pseudotumour cerebri); however, more recent literature has shown that they are not associated with increased incidence of raised intracranial pressure.9–11 We have noted that weight gain can be associated with starting oral contraceptives, and this may be something to explore sensitively with patients. Advice to withhold contraception may put women at risk of unplanned pregnancy. Where there is a clear temporal relationship between a patient starting the contraceptive and developing raised intracranial pressure, our pragmatic approach is to withdraw the contraceptive initially (with discussion about alternative methods) and to investigate for secondary causes, that is, coagulopathies.12 If the intracranial hypertension does not remit and no secondary cause is identified, then it is unlikely to be driven by the contraceptive, and so we would restart the medication with monitoring.8
Medical management
When a woman with IIH wishes to conceive, the aim is to achieve disease remission or stable disease with mild papilloedema. The International IIH Guideline details this management and follow-up dependent on papilloedema severity and visual function.8 We recommend using optical coherence tomography (OCT) imaging to monitor papilloedema and to track disease activity.13 The increased monitoring needed in pregnancy is discussed later.
Medication
Women of childbearing age need to have a careful risk–benefit discussion about medications that may be teratogenic. Several medications are used off label in IIH aiming to reduce intracranial pressure.
Acetazolamide, a carbonic anhydrase inhibitor, is the most commonly used medication in IIH. It can modestly improve visual function in IIH patients who have mild visual loss14, and improve quality of life.15 However, there is a high (48%) likelihood of discontinuation due to adverse effects when using a mean daily dose of 1.5 g.16 Animal studies have shown teratogenicity and so in the UK it is not recommend for those planning a pregnancy or during pregnancy.8 However, opinion internationally varies8 17 and a retrospective series of 50 pregnancies found no increase in adverse events.18 Women should be counselled that acetazolamide is potentially teratogenic (table 1) and its prescription in pregnancy must only follow discussion with the patient and their obstetrician.
Table 1.
Main adverse effects and teratogenic risks of acetazolamide and topiramate
| Medication | Main adverse effects | Teratogenicity |
| Acetazolamide | Diarrhoea, paraesthesia, fatigue, renal stones, altered taste, nausea, tinnitus, vomiting and depression8 | In animals at 200–1000 mg/kg79 80 but none identified in a study of 50 human pregnancies.18 |
| Topiramate | Nausea, dizziness, depression, cognitive slowing and weight loss.8
Can reduce efficacy of hormonal contraceptives at higher doses23 especially >200 mg/day.22 |
Major congenital malformations in women with epilepsy—RR 3.8 (95% CI 1.4 to 10.6).21 81
Increased risk of: |
RR, relative risk.
Topiramate is used off label in IIH supported by in vivo19 and open-label study evidence.20 It is also effective in migraine prevention19 and sometimes can drive weight loss through appetite suppression.8 Human pregnancy registry studies have highlighted its teratogenicity21 (table 1) and so topiramate should be avoided in women actively planning to conceive. It is also important to counsel patients that at higher doses (>200 mg/day) it can reduce the efficacy of the combined contraceptive pill.22 23
Folic acid supplementation ≥400 μg daily is recommended for all women wishing to become pregnant, starting at least 1–3 months before conception and continuing during the first trimester. In line with the International Federation of Gynaecology and Obstetrics guidelines we recommend considering a higher dose (5 mg) for women with IIH and obesity, as obesity in pregnancy is a risk factor for fetal neural tube defects.24
Weight management
Body weight is a potentially modifiable risk factor in IIH; thus, women ideally should achieve a healthy body weight before conception.25 The WHO defines obesity as a body mass index (BMI) ≥30 kg/m2; however, in ethnic minorities this is lower at 27.5 kg/m2 due to their associated higher risks.26–28 Increased weight is a stigmatised area of healthcare and clinicians should broach this topic sensitively and with the patient’s permission. Local weight management services can offer practical support for first-line interventions8 with diet, physical activity and behaviour change. The target for initial weight loss in women with obesity planning a pregnancy is typically 5%–10% of preconception weight;29 a normal BMI (18.5 to 24.9 kg/m2) target,24 29 although ideal, may not be realistic. Bariatric surgery can achieve lower and sustained reductions in intracranial pressure and put IIH into remission but is not appropriate for all.30 Following bariatric surgery, guidance suggests women wait at least 18 months before conceiving to enable optimisation of weight management and management of nutritional deficiencies.31 Dietary supplementation may be required, and we recommend consulting with a physician or dietician with expertise in the management of bariatric surgery patients for those planning conception or are newly pregnant.
During pregnancy
Medical management
Most pregnant women with IIH come under obstetric consultant care. Patients with existing stable IIH typically do very well during pregnancy; those newly diagnosed with IIH while pregnant can be more challenging as their disease may be more aggressive.
During pregnancy, we aim to review patients who are in ocular remission every trimester, for the reassurance of both the patient and healthcare professionals involved in their care. This allows information to be passed to the obstetric team regarding safe delivery methods. For those not in ocular remission, the frequency of visits depends on their papilloedema and field status and is based on clinical judgement and experience. In the vast majority we review as frequently as every 6–8 weeks; however, some visits maybe done to evaluate visual assessment alone, with remote virtual review by the consultant with OCT imaging and visual fields.
Medication
Patients ideally should stop potentially teratogenic drugs (eg, acetazolamide, topiramate) before pregnancy, in order to avoid their use during the critical stages of embryogenesis in the first trimester. Pregnancies detected after the first trimester, may not benefit from rapid withdrawal and therefore should be based on IIH disease control. We recommend having individual patient discussions where there is ongoing actively raised ICP and if a patient is unable or unwilling to delay pregnancy. We understand that there are multifactorial reasons behind this decision and as clinicians our role is to enable an informed choice (table 1). We cannot, however, recommend their routine use in pregnancy. Teratogenicity in humans has not been reported for acetazolamide unlike topiramate, however, this is based on a small case series of 50 patients and not by large data, therefore, this should be factored into a risk–benefit assessment. From our experience, where use of these medications would be indicated during pregnancy, our patients can be managed by alternative methods outlined later in this article.
Headache management during pregnancy is important. When there is a new or changed headache in pregnancy, it is essential to identify red flag symptoms or signs32 33 and to investigate accordingly. The headache phenotype in IIH is typically migraine-like34 35 and, therefore, its management during pregnancy is based on migraine management. Clinicians should weigh the risks and benefits of headache medication taken during pregnancy. Table 2 summarises appropriate treatments, based on published reviews and guidance.33 36 Unrestricted acute treatment of headache symptoms increases the risk of medication overuse headache in IIH during pregnancy; this should be discussed and managed appropriately.
Table 2.
Acute headache recommendations in pregnancy
| First line | Second line | Third line |
| Analgesic Paracetamol33—recommended to use the lowest effective dose for the shortest time, as it might alter fetal development86 |
Triptan if severe (sumatriptan has no documented teratogenicity87)33 36 88 | Opiates—for limited use due to risks including: |
| Antiemetic (short-term use) Cyclizine first line36 (alternatives metoclopramide or prochlorperazine)36 88 91 |
Ibuprofen—avoid in third trimester due to risk of premature closure of ductus arteriosus33 | |
Non-pharmacological treatments36 92:
|
Greater occipital nerve blocks36 94 |
Weight management
Weight management is a key issue in IIH. Weight loss is disease-modifying and can put IIH into remission30 37 whereas weight gain can exacerbate it.38 39 Pregnancy leads to weight gain and consequently risks exacerbating IIH. Excessive weight gain during pregnancy also increases the risk of complications for baby and mother.40–42 Providing advice to pregnant women with IIH on how to approach weight gain in pregnancy is essential.
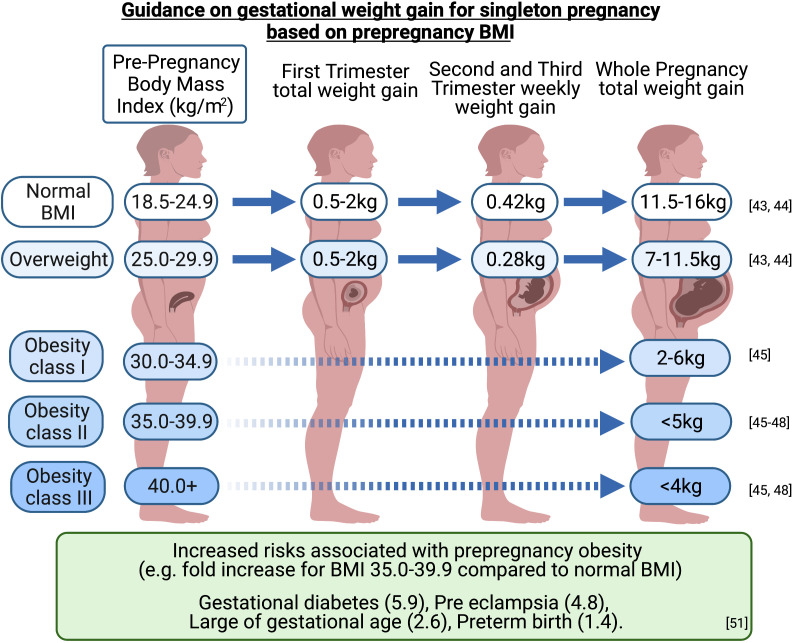
There are currently no evidence-based guidelines for limiting weight gain in pregnancy for women with IIH. The US Institute of Medicine (IoM) guidelines (2009) are used internationally to guide weight gain in pregnancy for women with a BMI ≤30 kg/m2.24 43 44 Those with a BMI ≥30 kg/m2 are recommended to gain less weight during pregnancy than patients with lower BMIs. The IoM suggests a gain of 5–9 kg during a singleton pregnancy in women with obesity, however, does not differentiate by obesity class.43 44 Subsequent studies suggest (figure 1) that those with a preconception BMI of:
Figure 1.
Weight gain in pregnancy guide. Pregnancy complications/risks are higher with higher BMIs. Figures for obesity class II (BMI 35.0–39.9 kg/m2) are provided as a guide. Infographic created with BioRender.com. BMI, body mass index.
30.0–34.9 kg/m2 should aim to gain 2–6 kg45.
35.0–39.9 kg/m2 should aim to gain less than 5 kg.45–48
>40 kg/m2 should aim to gain less than 4 kg.45 48
These targets must balance the risks of having large-for-gestational-age infants, small-for-gestational-age babies, preterm births and postpartum weight retention.44 49–51
The foundations of weight management during pregnancy are diet, physical activity and behavioural interventions. Weight loss programmes are not recommended during pregnancy, as the inadequate nutrition may harm the unborn child’s health.29 While some neurologists may not feel confident to provide general nutrition advice during pregnancy, all clinicians can dispel myths around ‘eating for two’. Women have no increased energy needs in the first and second trimesters, and only a slight increase in the third trimester—even then, it is only around 200 calories per day.29 52 Women may need advice about the benefits of physical activity for managing gestational weight gain, and its other health benefits during pregnancy. Pregnant women should be supported to reduce sedentary time and incorporate 150 min per week of moderately intense physical activity into their daily life,53 for example, walking or swimming. However, it is important to consider a woman’s baseline level of physical activity and build this up gradually according to capabilities.
We recommend an individualised approach so as not to obscure important biocultural factors, including socioeconomic status, ethnicity and comorbidities.54–56 We recommend referral to a dietitian (if resources permit) or an appropriately trained health professional to provide personalised advice on healthy eating and physical activity during pregnancy.29 This may be a referral initiated by the IIH clinic or liaising with maternity services or the general practitioner/family physician.
Many factors may influence management decisions, including IIH disease activity, risk of deterioration and prepregnancy BMI. A combined approach of diet, physical activity and behaviour change approaches has good evidence to reduce gestational weight gain.57 This is important as excessive gestational weight gain is also associated with increased postpartum weight retention. This can significantly increase the long-term weight gain trajectory of a woman of childbearing age,58 particularly if she has multiple pregnancies,59 and therefore, potentially increases the risk of IIH deterioration or recurrence.
Serial lumbar punctures
These are not usually recommended for management of IIH,8 but our experience suggests that in pregnancy they can sometimes provide short-term treatment.60 61 It is useful to note that in a commonly cited pregnancy publication, single lumbar punctures (LPs) were only needed in 25% (3/12).60 From our experience, although serial LPs or a lumbar drain may be required in severe papilloedema, these can often be avoided in those with mild papilloedema and stable visual function. Hence, we would not recommend routinely using serial LPs in pregnancy. If it is done, an LP in pregnancy could be performed with a Quincke (cutting) needle, which is more likely to lead to a cerebrospinal fluid (CSF) leak than an atraumatic needle. The patient should be counselled regarding exacerbation of headache.62 63 The volume drained is not as important as the type of needle as CSF will be replaced within a few hours.64–66 We would aim to normalise the CSF pressure (approx. 18 cmH2O) or by half if the opening pressure >40 cmH2O. We use OCT imaging to guide the impact of this intervention on papilloedema 24–48 hours later, however, this is based on clinical judgement.13 If the papilloedema is reduced or stabilised, we continue to monitor using OCT depending on papilloedema severity.
If the papilloedema increases and again poses a risk of rapidly declining vision, we would repeat the lumbar puncture. Our experience is that this type of bridging is most useful in the first trimester, since thereafter things often naturally start to improve. The timing of this intervention is judged primarily on the severity of the papilloedema, aided by OCT measures, including retinal nerve fibre layer (RNFL) thickness and optic nerve head volume; and on the impact of the visual function as measured by formal visual fields.67 Active IIH in the context of pregnancy should have specialist oversight, either a neuro-ophthalmologist or neurologist who regularly manages IIH. We would proceed to a more permanent surgical intervention only if the severity of the disease dictates it or if repeated lumbar punctures, or indeed a lumbar drain, could not hold the disease.
Surgical management
If a pregnant woman requires sight saving surgery, there are several options, as with women who are not pregnant. In reality, it is the local expertise and multidisciplinary team preferences that typically dictate which procedure is performed.
Ventriculoperitoneal shunting has been recommended by consensus as the optimal surgical procedure for CSF diversion as it has the most evidence of efficacy in IIH.8 68 However, there is an increased risk of ventriculoperitoneal shunt failure due to the increased intra-abdominal pressure in late pregnancy.69 For that reason, some surgeons prefer to use lumbar peritoneal shunts in pregnancy70 although they can be technically difficult with a gravid uterus.71 Of note, those with CSF shunts inserted before becoming pregnant can have a normal pregnancy and delivery.70 72
Optic nerve sheath fenestration is less commonly performed, as evidenced from data from the UK73 and in the USA74 due to limited local surgical expertise, the complication rate and scanty data on the longevity of the procedure.68 75 Furthermore, the procedure decompresses the optic nerves but does not necessarily help the headache. Given the possible problems with CSF shunting, optic nerve sheath fenestration may be preferable if there is local expertise.
Dural venous sinus stenting requires preprocedure and postprocedure anticoagulation, which would potentially further complicate pregnancy.75 It is rarely performed in the UK73 and its use remains controversial, with evidence only from case series and no randomised clinical trials.75 76 A randomised controlled trial comparing CSF shunting with venous sinus stenting in IIH is ongoing in the UK (IIH: Intervention). Therefore, in our opinion, we would not recommend this in pregnancy. Interestingly, one report noted that venous sinus stenting failed, with a recurrence rate of 10% following becoming pregnant.77
Delivery and peripartum
Patients with IIH do not need any specific mode of delivery and there is no clear evidence to support the reported trend towards caesarean section.78 The concern in theory is that a prolonged second stage of labour, with consequent Valsalva manoeuvre, could exacerbate optic nerve ischaemia and damage, but in reality the duration of contractions during the second stage of labour are relatively short. Our clinical impression (we have managed >50 cases) is that there no evidence of declining vision following labour. In practice, a normal vaginal delivery poses a negligible risk to women with IIH and in addition caesarean sections are associated with their own risks both intra and post operatively. The mode of delivery should therefore be decided by obstetric factors only.8 70 However, we would advise caution in those rarer cases with severe papilloedema who have rapidly declining vision at delivery; such patients should be managed in a specialist centre.
Lumbar punctures and spinal anaesthesia are safe in pregnancy. We do recommend caution, and where possible avoidance, if there is a lumbar peritoneal shunt in situ.
Conclusion
Pregnancy in IIH requires additional considerations. Ideally women should achieve disease remission or stabilisation before pregnancy. Drugs including acetazolamide and topiramate have teratogenic risks and we avoid these during pregnancy. An important aspect is the risk of weight gain during pregnancy, as this can exacerbate IIH. There are useful guidelines on weight gain targets in pregnancy for those with obesity; these should be actively discussed with the patient and supported. We recommend increased frequency of IIH outpatient reviews during pregnancy to identify any clinical deterioration early and provide reassurance.
Case vignettes
Scenario 1
A 28-year-old woman with mildly active IIH (normal visual fields). Her headache frequency has improved following topiramate monotherapy (100 mg nocte). Her BMI is 36 kg/m2 with only minimal weight reduction since presentation. She is not using any contraception, is in a relationship and now has plans for children.
Bespoke recommendations would include a sensitive discussion regarding weight management and family planning. We would recommend weaning the topiramate before trying to conceive and suggest folic acid supplementation. Should pregnancy occurs while on topiramate, we would stop topiramate or wean over a week if on higher doses, with close monitoring of the ocular status.
Scenario 2
A 26-year-old woman with IIH, 25 weeks pregnant has a 2 week history of deteriorating vision, with transient visual obscurations and increased pulsatile tinnitus. She undergoes a full assessment of visual function including visual fields and OCT imaging. Her visual acuity is 6/7.5 bilaterally, perimetric mean deviation −3 dB right eye and −2.75 dB left eye (in a well performed Humphrey visual field). The global peripapillary RNFL thickness is 220 µm (right eye) and 200 µm (left eye), in properly segmented scans. Given her symptoms, if left she will be at risk of potential visual loss. We recommended emergency lumbar puncture with a cutting needle. She was re-evaluated 2 days later and the papilloedema had reduced on OCT examination (RNFL 188 µm right eye and 172 µm left eye). One week later, there had been a further reduction, no further intervention was organised, and she remained under close observation until she had a normal delivery.
However, if her OCT had shown that the papilloedema was increasing beyond what was noted the previous week we would have considered a lumbar drain. If we determined that she had rapidly declining vision with increasing papilloedema on OCT imaging and that this approach was unsuccessful, we would escalate to an emergency CSF diversion procedure.
Key points.
Prepregnancy care should aim for remission or stability of idiopathic intracranial hypertension (IIH); hormonal contraception is not contraindicated.
Acetazolamide and topiramate have teratogenic risks and we advise avoiding them in women actively planning a pregnancy or those who become pregnant.
Women with IIH require frequent and active monitoring throughout pregnancy.
Pregnancy weight gain can exacerbate IIH; those with a prepregnancy body mass index (BMI) ≥30 kg/m2 should be guided to gain less weight in pregnancy than those with lower BMIs.
Further reading.
Mollan SP et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry 2018 89(10):1088–1100.
Santos S et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019 126(8):984–995.
Mollan SP, A.A. Tahrani and A.J. Sinclair. The Potentially Modifiable Risk Factor in Idiopathic Intracranial Hypertension: Body Weight. Neurology Clinical Practice 2021, doi:10.1212/CPJ.0000000000001063.
Footnotes
Twitter: @MarkHeadache, @AbdTahrani, @DrMollan, @DrIIHBirmingham
Contributors: All authors have contributed to the creation and review of this manuscript.
Funding: AJS is funded by a Sir Jules Thorn Award for Biomedical Science.
Competing interests: BRW—Invex therapeutics consultancy AAT—I have received support for research and meetings, honorarium for advisory work and equipment support from: ANSAR, Aptiva, AstraZeneca. Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, ImpetoMedical, Janssen, Merck Sharp & Dohme, Napp, Novo Nordisk, Philips Resporinics, Resmed, Sanofi Aventis. SPM—Invex therapeutics, advisory board (2020) and consulting fees (2021); Heidelberg engineering, speaker fees (2021); Neurodiem, advisor (2019, 2020, 2021); Novartis, speaker fees (2020); and Scope Ophthalmics, speaker fees (2021). AJS - Novartis and Allergan Advisory board. Speaker fees Novartis. Invex therapeutics, company director with salary and stock options (2019, 2020) No other authors contributing have a conflict of interest in the subject matter.
Provenance and peer review: Provenance and peer review. Commissioned. Externally peer reviewed by Angela O’Neal, Boston, USA.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Grech O, Mollan SP, Wakerley BR, et al. Emerging themes in idiopathic intracranial hypertension. J Neurol 2020;267:3776–84. 10.1007/s00415-020-10090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hornby C, Mollan SP, Botfield H, et al. Metabolic concepts in idiopathic intracranial hypertension and their potential for therapeutic intervention. J Neuroophthalmol 2018;38:522–30. 10.1097/WNO.0000000000000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hornby C, Botfield H, O'Reilly MW, et al. Evaluating the fat distribution in idiopathic intracranial hypertension using dual-energy X-ray absorptiometry scanning. Neuroophthalmology 2018;42:99–104. 10.1080/01658107.2017.1334218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews LE, Liu GT, Ko MW. Idiopathic intracranial hypertension and obesity. Horm Res Paediatr 2014;81:217–25. 10.1159/000357730 [DOI] [PubMed] [Google Scholar]
- 5. Westgate CS, Botfield HF, Alimajstorovic Z, et al. Systemic and adipocyte transcriptional and metabolic dysregulation in idiopathic intracranial hypertension. JCI Insight 2021;6. 10.1172/jci.insight.145346. [Epub ahead of print: 24 May 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adderley NJ, Subramanian A, Nirantharakumar K, et al. Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol 2019;76:1088–98. 10.1001/jamaneurol.2019.1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Reilly MW, Westgate CS, Hornby C, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight 2019;4. 10.1172/jci.insight.125348. [Epub ahead of print: 21 Mar 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry 2018;89:1088–100. 10.1136/jnnp-2017-317440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kilgore KP, Lee MS, Leavitt JA, et al. A population-based, case-control evaluation of the association between hormonal contraceptives and idiopathic intracranial hypertension. Am J Ophthalmol 2019;197:74–9. 10.1016/j.ajo.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sodhi M, Sheldon CA, Carleton B, et al. Risk of pseudotumor cerebri syndrome (PTCS) with hormonal contraceptive use. Int J Reprod Contracept Obstet Gynecol 2018;7:778. 10.18203/2320-1770.ijrcog20180854 [DOI] [Google Scholar]
- 11. Sundholm A, Burkill S, Waldenlind E, et al. A national Swedish case-control study investigating incidence and factors associated with idiopathic intracranial hypertension. Cephalalgia 2021;41:1427–36. 10.1177/03331024211024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee BWH, Lau FS, Francis IC. In pseudotumor cerebri, hormonal contraception is not associated, and the diagnosis remains idiopathic intracranial hypertension. Am J Ophthalmol 2019;203:116. 10.1016/j.ajo.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 13. Vijay V, Mollan SP, Mitchell JL, et al. Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol 2020;138:1264–71. 10.1001/jamaophthalmol.2020.4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee, Wall M, McDermott MP, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 2014;311:1641–51. 10.1001/jama.2014.3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruce BB, Digre KB, McDermott MP, et al. Quality of life at 6 months in the idiopathic intracranial hypertension treatment trial. Neurology 2016;87:1871–7. 10.1212/WNL.0000000000003280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ball AK, Howman A, Wheatley K, et al. A randomised controlled trial of treatment for idiopathic intracranial hypertension. J Neurol 2011;258:874–81. 10.1007/s00415-010-5861-4 [DOI] [PubMed] [Google Scholar]
- 17. Lee AG, Pless M, Falardeau J, et al. The use of acetazolamide in idiopathic intracranial hypertension during pregnancy. Am J Ophthalmol 2005;139:855–9. 10.1016/j.ajo.2004.12.091 [DOI] [PubMed] [Google Scholar]
- 18. Falardeau J, Lobb BM, Golden S, et al. The use of acetazolamide during pregnancy in intracranial hypertension patients. J Neuroophthalmol 2013;33:9–12. 10.1097/WNO.0b013e3182594001 [DOI] [PubMed] [Google Scholar]
- 19. Scotton WJ, Botfield HF, Westgate CS, et al. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia 2019;39:209–18. 10.1177/0333102418776455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Çelebisoy N, Gökçay F, Şirin H, et al. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand 2007;116:322–7. 10.1111/j.1600-0404.2007.00905.x [DOI] [PubMed] [Google Scholar]
- 21. Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev 2016;11:CD010224. 10.1002/14651858.CD010224.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bialer M, Doose DR, Murthy B, et al. Pharmacokinetic interactions of topiramate. Clin Pharmacokinet 2004;43:763–80. 10.2165/00003088-200443120-00001 [DOI] [PubMed] [Google Scholar]
- 23. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol 2010;3:183–92. 10.1586/ecp.10.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAuliffe FM, Killeen SL, Jacob CM, et al. Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO pregnancy and non-communicable diseases Committee: a FIGO (International Federation of gynecology and obstetrics) guideline. Int J Gynaecol Obstet 2020;151 Suppl 1:16–36. 10.1002/ijgo.13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mollan SP, Tahrani AA, Sinclair AJ. The potentially modifiable risk factor in idiopathic intracranial hypertension: body weight. Neurol Clin Pract 2021;11:e504–7. 10.1212/CPJ.0000000000001063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organisation . Body Mass Index - BMI. Available: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [Accessed 27 Jul 2020].
- 27. National Institute for Health and Care Excellence . Obesity: identification, assessment and management Clinical guideline [CG189], 2014. [PubMed] [Google Scholar]
- 28. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence . Weight management before during and after pregnancy, 2010. [Google Scholar]
- 30. Mollan SP, Mitchell JL, Ottridge RS, et al. Effectiveness of bariatric surgery vs community weight management intervention for the treatment of idiopathic intracranial hypertension: a randomized clinical trial. JAMA Neurol 2021;78:678–86. 10.1001/jamaneurol.2021.0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciangura C, Coupaye M, Deruelle P, et al. Clinical practice guidelines for childbearing female candidates for bariatric surgery, pregnancy, and post-partum management after bariatric surgery. Obes Surg 2019;29:3722–34. 10.1007/s11695-019-04093-y [DOI] [PubMed] [Google Scholar]
- 32. Mitsikostas DD, Ashina M, Craven A, et al. European headache Federation consensus on technical investigation for primary headache disorders. J Headache Pain 2015;17:5. 10.1186/s10194-016-0596-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Negro A, Delaruelle Z, Ivanova TA, et al. Headache and pregnancy: a systematic review. J Headache Pain 2017;18:106. 10.1186/s10194-017-0816-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wakerley BR, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: update on diagnosis and management. Clin Med 2020;20:384–8. 10.7861/clinmed.2020-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedman DI, Quiros PA, Subramanian PS, et al. Headache in idiopathic intracranial hypertension: findings from the idiopathic intracranial hypertension treatment trial. Headache 2017;57:1195–205. 10.1111/head.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jarvis S, Dassan P, Piercy CN. Managing migraine in pregnancy. BMJ 2018;360:k80. 10.1136/bmj.k80 [DOI] [PubMed] [Google Scholar]
- 37. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 2010;341:c2701. 10.1136/bmj.c2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol 2007;143:635–41. 10.1016/j.ajo.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 39. Ko MW, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology 2011;76:1564–7. 10.1212/WNL.0b013e3182190f51 [DOI] [PubMed] [Google Scholar]
- 40. Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 2017;317:2207–25. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hill B, Skouteris H, Boyle JA, et al. Health in preconception, pregnancy and postpartum global alliance: international network pregnancy priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J Clin Med 2020;9. 10.3390/jcm9030822. [Epub ahead of print: 18 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev 2012;13:347–67. 10.1111/j.1467-789X.2011.00965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rasmussen K, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academy Press, 2009. [PubMed] [Google Scholar]
- 44. American College of Obstetricians and Gynecologists . ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol 2013;121:210–2. 10.1097/01.aog.0000425668.87506.4c [DOI] [PubMed] [Google Scholar]
- 45. LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, Voerman E, Santos S, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019;321:1702–15. 10.1001/jama.2019.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roussel E, Touleimat S, Ollivier L, et al. Birthweight and pregnancy outcomes in obese class II women with low weight gain: a retrospective study. PLoS One 2019;14:e0215833. 10.1371/journal.pone.0215833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol 2007;110:752–8. 10.1097/01.AOG.0000278819.17190.87 [DOI] [PubMed] [Google Scholar]
- 48. Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of medicine recommendations. Obstet Gynecol 2011;117:1065–70. 10.1097/AOG.0b013e318214f1d1 [DOI] [PubMed] [Google Scholar]
- 49. Kapadia MZ, Park CK, Beyene J, et al. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and meta-analysis. Obes Rev 2015;16:189–206. 10.1111/obr.12238 [DOI] [PubMed] [Google Scholar]
- 50. Kapadia MZ, Park CK, Beyene J, et al. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and meta-analyses of maternal and infant outcomes. PLoS One 2015;10:e0132650. 10.1371/journal.pone.0132650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019;126:984–95. 10.1111/1471-0528.15661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Department of Health . Dietary reference values. Department of Health ed. London: HMSO, 1991. [Google Scholar]
- 53. UK Chief Medical Officers . Uk chief medical officers' physical activity guidelines, 2019. [Google Scholar]
- 54. Bowers K, Laughon SK, Kiely M, et al. Gestational diabetes, pre-pregnancy obesity and pregnancy weight gain in relation to excess fetal growth: variations by race/ethnicity. Diabetologia 2013;56:1263–71. 10.1007/s00125-013-2881-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldstein RF, Abell SK, Ranasinha S, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med 2018;16:153. 10.1186/s12916-018-1128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Melby MK, Yamada G, Schwartz DA, et al. One size does not fit all: examining ethnicity in gestational weight gain guidelines. Health Care Women Int 2019;40:365–85. 10.1080/07399332.2018.1531864 [DOI] [PubMed] [Google Scholar]
- 57. Farpour-Lambert NJ, Ells LJ, Martinez de Tejada B, et al. Obesity and weight gain in pregnancy and postpartum: an evidence review of lifestyle interventions to inform maternal and child health policies. Front Endocrinol 2018;9:546. 10.3389/fendo.2018.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nehring I, Schmoll S, Beyerlein A, et al. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 2011;94:1225–31. 10.3945/ajcn.111.015289 [DOI] [PubMed] [Google Scholar]
- 59. Cohen AK, Chaffee BW, Rehkopf DH, et al. Excessive gestational weight gain over multiple pregnancies and the prevalence of obesity at age 40. Int J Obes 2014;38:714–8. 10.1038/ijo.2013.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huna-Baron R, Kupersmith MJ. Idiopathic intracranial hypertension in pregnancy. J Neurol 2002;249:1078–81. 10.1007/s00415-002-0791-4 [DOI] [PubMed] [Google Scholar]
- 61. Alves S, Sousa N, Cardoso L, et al. Multidisciplinary management of idiopathic intracranial hypertension in pregnancy: case series and narrative review. Braz J Anesthesiol 2021. 10.1016/j.bjane.2021.02.030. [Epub ahead of print: 20 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scotton WJ, Mollan SP, Walters T, et al. Characterising the patient experience of diagnostic lumbar puncture in idiopathic intracranial hypertension: a cross-sectional online survey. BMJ Open 2018;8:e020445. 10.1136/bmjopen-2017-020445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yiangou A, Mitchell J, Markey KA, et al. Therapeutic lumbar puncture for headache in idiopathic intracranial hypertension: minimal gain, is it worth the pain? Cephalalgia 2019;39:245–53. 10.1177/0333102418782192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brown PD, Davies SL, Speake T, et al. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 2004;129:957–70. 10.1016/j.neuroscience.2004.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Monserrate AE, Ryman DC, Ma S, et al. Factors associated with the onset and persistence of post-lumbar puncture headache. JAMA Neurol 2015;72:325–32. 10.1001/jamaneurol.2014.3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wright EM. Transport processes in the formation of the cerebrospinal fluid. Rev Physiol Biochem Pharmacol 1978;83:3–34. [PubMed] [Google Scholar]
- 67. Fraser C, Lueck CJ. Optical coherence tomography: a window to the brain? Pract Neurol 2021;21:313–21. 10.1136/practneurol-2020-002824 [DOI] [Google Scholar]
- 68. Spitze A, Malik A, Al-Zubidi N, et al. Optic nerve sheath fenestration vs cerebrospinal diversion procedures: what is the preferred surgical procedure for the treatment of idiopathic intracranial hypertension failing maximum medical therapy? J Neuroophthalmol 2013;33:183–8. 10.1097/WNO.0b013e318292d06f [DOI] [PubMed] [Google Scholar]
- 69. Hwang S-C, Kim T-H, Kim B-T, et al. Acute shunt malfunction after cesarean section delivery: a case report. J Korean Med Sci 2010;25:647–50. 10.3346/jkms.2010.25.4.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tang RA, Dorotheo EU, Schiffman JS, et al. Medical and surgical management of idiopathic intracranial hypertension in pregnancy. Curr Neurol Neurosci Rep 2004;4:398–409. 10.1007/s11910-004-0087-4 [DOI] [PubMed] [Google Scholar]
- 71. Kesler A, Kupferminc M. Idiopathic intracranial hypertension and pregnancy. Clin Obstet Gynecol 2013;56:389–96. 10.1097/GRF.0b013e31828f2701 [DOI] [PubMed] [Google Scholar]
- 72. Al-Saadi TD, Glisic M, Al Sharqi A, et al. Safety of pregnancy in ventriculoperitoneal shunt dependent women: meta-analysis and systematic review of the literature. Neurol India 2020;68:548–54. 10.4103/0028-3886.288995 [DOI] [PubMed] [Google Scholar]
- 73. Mollan SP, Mytton J, Tsermoulas G, et al. Idiopathic intracranial hypertension: evaluation of admissions and emergency readmissions through the hospital episode statistic dataset between 2002-2020. Life 2021;11:417. 10.3390/life11050417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hamedani AG, Thibault DP, Revere KE, et al. Trends in the surgical treatment of pseudotumor cerebri syndrome in the United States. JAMA Netw Open 2020;3:e2029669. 10.1001/jamanetworkopen.2020.29669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gurney SP, Ramalingam S, Thomas A, et al. Exploring the current management idiopathic intracranial hypertension, and understanding the role of dural venous sinus stenting. Eye Brain 2020;12:1–13. 10.2147/EB.S193027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kamdar H, Gullo T, Strohm T. Idiopathic intracranial hypertension in a pregnant female improved with venous sinus stenting (4990). Neurology 2021;96. [Google Scholar]
- 77. Labeyrie M-A, Fantoni M, Vever U, et al. Intracranial venous sinus stenting for the treatment of lateral sinus stenoses: an analysis of 200 patients. Diagn Interv Imaging 2021;102:619–27. 10.1016/j.diii.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 78. Mollan SP, Aguiar M, Evison F, et al. The expanding burden of idiopathic intracranial hypertension. Eye 2019;33:478–85. 10.1038/s41433-018-0238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Holmes LB, Kawanishi H, Munoz A. Acetazolamide: maternal toxicity, pattern of malformations, and litter effect. Teratology 1988;37:335–42. 10.1002/tera.1420370407 [DOI] [PubMed] [Google Scholar]
- 80. Kojima N, Naya M, Makita T. Effects of maternal acetazolamide treatment on body weights and incisor development of the fetal rat. J Vet Med Sci 1999;61:143–7. 10.1292/jvms.61.143 [DOI] [PubMed] [Google Scholar]
- 81. Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78:1692–9. 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 82. Alsaad AMS, Chaudhry SA, Koren G. First trimester exposure to topiramate and the risk of oral clefts in the offspring: a systematic review and meta-analysis. Reprod Toxicol 2015;53:45–50. 10.1016/j.reprotox.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 83. Hunt S, Russell A, Smithson WH, et al. Topiramate in pregnancy: preliminary experience from the UK epilepsy and pregnancy register. Neurology 2008;71:272–6. 10.1212/01.wnl.0000318293.28278.33 [DOI] [PubMed] [Google Scholar]
- 84. Vajda FJE, O'Brien TJ, Graham J, et al. Associations between particular types of fetal malformation and antiepileptic drug exposure in utero. Acta Neurol Scand 2013;128:228–34. 10.1111/ane.12115 [DOI] [PubMed] [Google Scholar]
- 85. Castilla-Puentes R, Ford L, Manera L, et al. Topiramate monotherapy use in women with and without epilepsy: pregnancy and neonatal outcomes. Epilepsy Res 2014;108:717–24. 10.1016/j.eplepsyres.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 86. Bauer AZ, Swan SH, Kriebel D, et al. Paracetamol use during pregnancy - a call for precautionary action. Nat Rev Endocrinol 2021;17:757–66. 10.1038/s41574-021-00553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Spielmann K, Kayser A, Beck E, et al. Pregnancy outcome after anti-migraine triptan use: a prospective observational cohort study. Cephalalgia 2018;38:1081–92. 10.1177/0333102417724152 [DOI] [PubMed] [Google Scholar]
- 88. Saldanha IJ, Cao W, Bhuma MR, et al. Management of primary headaches during pregnancy, postpartum, and breastfeeding: a systematic review. Headache 2021;61:11–43. 10.1111/head.14041 [DOI] [PubMed] [Google Scholar]
- 89. Yazdy MM, Desai RJ, Brogly SB. Prescription opioids in pregnancy and birth outcomes: a review of the literature. J Pediatr Genet 2015;4:56–70. 10.1055/s-0035-1556740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bateman BT, Hernandez-Diaz S, Straub L, et al. Association of first trimester prescription opioid use with congenital malformations in the offspring: population based cohort study. BMJ 2021;372:n102. 10.1136/bmj.n102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Koren G. Safety considerations surrounding use of treatment options for nausea and vomiting in pregnancy. Expert Opin Drug Saf 2017;16:1227–34. 10.1080/14740338.2017.1361403 [DOI] [PubMed] [Google Scholar]
- 92. Macgregor EA. Headache in pregnancy. Continuum 2014;20:128–47. 10.1212/01.CON.0000443841.40933.9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kropp P, Meyer B, Meyer W, et al. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017;17:1059–68. 10.1080/14737175.2017.1377611 [DOI] [PubMed] [Google Scholar]
- 94. Tang Y, Kang J, Zhang Y, et al. Influence of greater occipital nerve block on pain severity in migraine patients: a systematic review and meta-analysis. Am J Emerg Med 2017;35:1750–4. 10.1016/j.ajem.2017.08.027 [DOI] [PubMed] [Google Scholar]