Abstract
Background
Coformulated sodium phenylbutyrate/taurursodiol (PB/TURSO) was shown to prolong survival and slow functional decline in amyotrophic lateral sclerosis (ALS).
Objective
Determine whether PB/TURSO prolonged tracheostomy/ventilation-free survival and/or reduced first hospitalisation in participants with ALS in the CENTAUR trial.
Methods
Adults with El Escorial Definite ALS ≤18 months from symptom onset were randomised to PB/ TURSO or placebo for 6 months. Those completing randomised treatment could enrol in an open-label extension (OLE) phase and receive PB/TURSO for ≤30 months. Times to the following individual or combined key events were compared in the originally randomised treatment groups over a period spanning trial start through July 2020 (longest postrandomisation follow-up, 35 months): death, tracheostomy, permanent assisted ventilation (PAV) and first hospitalisation.
Results
Risk of any key event was 47% lower in those originally randomised to PB/TURSO (n=87) versus placebo (n=48, 71% of whom received delayed-start PB/TURSO in the OLE phase) (HR=0.53; 95% CI 0.35 to 0.81; p=0.003). Risks of death or tracheostomy/PAV (HR=0.51; 95% CI 0.32 to 0.84; p=0.007) and first hospitalisation (HR=0.56; 95% CI 0.34 to 0.95; p=0.03) were also decreased in those originally randomised to PB/TURSO.
Conclusions
Early PB/TURSO prolonged tracheostomy/PAV-free survival and delayed first hospitalisation in ALS.
Trial registration number
Keywords: ALS, RANDOMISED TRIALS, MOTOR NEURON DISEASE, NEUROMUSCULAR
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive motor neuron disorder typically culminating in death from respiratory failure.1 2 Non-invasive ventilation (NIV) or, when NIV is not possible, tracheostomy and invasive ventilation may prolong survival and maintain or improve quality of life in people with ALS.1 3 From a societal standpoint, interventions such as assisted ventilation and hospitalisation are significant drivers of aggregate costs attributable to ALS in the USA each year.4 5 By slowing disease progression, therapies have the potential to reduce the short-term clinical burden associated with ALS.6
A fixed-dose sodium phenylbutyrate/taurursodiol (PB/TURSO) coformulation was designed to reduce neuronal death by simultaneously mitigating endoplasmic reticulum and mitochondrial dysfunction. PB/TURSO safety and efficacy were evaluated in the CENTAUR trial consisting of a randomised, double-blind, placebo-controlled phase (NCT03127514) and open-label extension (OLE) phase (NCT03488524). PB/TURSO administration was associated with a significantly slower rate of decline in ALS Functional Rating Scale–Revised (ALSFRS-R) total score compared with placebo over the 6-month randomised phase (primary outcome).7 In an intent-to-treat (ITT) analysis encompassing all 137 randomised participants in CENTAUR followed for up to 3 years after randomisation, long-term survival duration was significantly increased among those originally randomised to PB/TURSO versus placebo.8 Here, we report the results of prespecified analyses evaluating the occurrence of key events in addition to death in CENTAUR, including tracheostomy, permanent assisted ventilation (PAV), and first hospitalisation.
Methods
Detailed methodology for the trial is reported elsewhere,7 8 (see online supplemental file 2 for the full trial protocol and amendments). Briefly, adults with Definite ALS (revised El Escorial criteria)9 who were ≤18 months from symptom onset with a slow vital capacity >60% of predicted value were randomised 2:1 to receive PB/TURSO (3 g PB/1 g TURSO) or placebo by mouth or feeding tube for 6 months.7 Those completing randomised treatment were eligible to enrol in an OLE phase and receive PB/TURSO for up to 30 months. Continuation of a stable dose of riluzole and/or edaravone was permitted throughout the trial. Investigators, evaluators and participants were blinded to originally randomised treatment assignments.
jnnp-2022-329024supp002.pdf (4.3MB, pdf)
Rates of the following key events were evaluated as a secondary efficacy outcome in CENTAUR: death (all-cause), tracheostomy (either for respiratory distress or airway clearance), PAV (defined as NIV>22 hours/day for >7 days), and hospitalisations specifically for ALS-related procedures (placement of a feeding tube, tracheostomy for management of secretions or respiratory support, or diaphragm pacing system) or due to a severe or serious adverse event, including those relating to progression or complications of ALS. The prespecified outcome was the composite of these key events.
Statistical analysis
The scope and methodology for all analyses are graphically summarised in online supplemental figure 1. The analyses encompassed occurrence of key events from the point of randomisation through a cut-off date of 20 July 2020 (longest postrandomisation follow-up, 35 months). The prespecified analysis population was the modified ITT (mITT) population, comprising all randomised participants who received at least one dose of originally assigned trial medication and had at least one postbaseline ALSFRS-R total score. All randomised participants within this population were included in the analyses, including those who discontinued from the trial, were lost to follow-up, or did not continue into the OLE phase.
jnnp-2022-329024supp001.pdf (1.2MB, pdf)
Vital status was assessed for all randomised participants via prospective monitoring during the randomised and OLE phases of the trial or, in participants who discontinued, were lost to follow-up, or did not continue into the OLE phase, by a participant locating service (OmniTrace), via search of public records (online supplemental figure 1). The vital status of all but one randomised participant was successfully confirmed. Occurrence of other key events was primarily captured prospectively during participant monitoring within the trial (online supplemental figure 1). Analyses compared the time to each of the following individual or combined events in the originally randomised treatment groups: death; death, tracheostomy or PAV; first hospitalisation; and any key event. HRs were estimated using a Cox proportional hazards model with covariates of age at randomisation, prebaseline ALSFRS-R slope and baseline ALSFRS-R total score, as previously described.7 8 Median times to event(s) and associated IQRs were estimated from Kaplan-Meier curves. Tests were declared significant if the two-tailed p value was ≤0.05.
Results
Of 177 screened individuals, 137 were randomised in the double-blind phase (PB/TURSO, n=89; placebo, n=48); of 98 participants eligible for OLE phase enrolment, 90 (92%) elected to enrol (56 and 34 originally randomised to PB/TURSO and placebo, respectively). Two participants in the PB/TURSO group who died shortly after randomisation did not undergo a postbaseline ALSFRS-R assessment and were excluded from the prespecified mITT population. Detailed baseline characteristics of the mITT population are published elsewhere.7 Average participant age was 58 years, with mean durations of 13.5 and 6.0 months since ALS symptom onset and diagnosis, respectively. The majority (77%) of participants, including 71% originally randomised to PB/TURSO and 88% originally randomised to placebo, were receiving riluzole and/or edaravone at or prior to trial entry; among participants originally randomised to PB/TURSO and placebo, 68% and 77%, respectively, were receiving riluzole and 25% and 50%, respectively, were receiving edaravone. PB/TURSO exposure data for the originally randomised groups are summarised in online supplemental table 1.
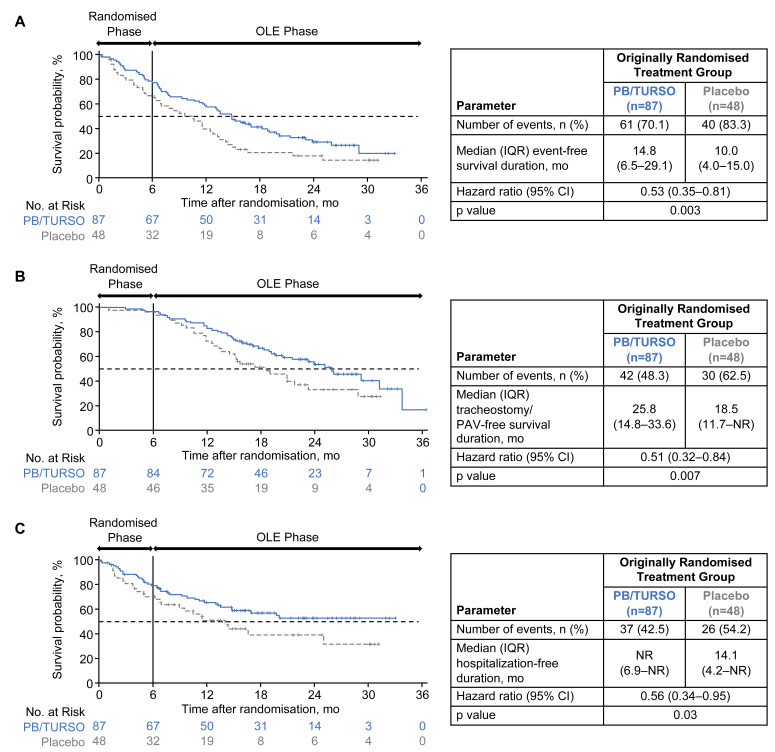
Over the period spanning randomisation to the analysis cut-off date (longest postrandomisation follow-up, 35 months), the risk of any key event was 47% lower in those originally randomised to PB/TURSO versus placebo (HR=0.53; 95% CI 0.35 to 0.81; p=0.003); median (IQR) event-free durations were 14.8 (6.5–29.1) and 10.0 (4.0–15.0) months, respectively (figure 1A). Risk of death or tracheostomy/PAV was 49% lower among those originally randomised to PB/TURSO versus placebo (HR=0.51; 95% CI 0.32 to 0.84; p=0.007), with median (IQR) tracheostomy/PAV-free survival durations of 25.8 (14.8–33.6) months and 18.5 months (11.7 months–not reached (NR)), respectively (figure 1B). Risk of first hospitalisation was 44% lower in the group originally randomised to PB/TURSO (HR=0.56; 95% CI 0.34 to 0.95; p=0.03); median (IQR) hospitalisation-free duration was NR (6.9 months–NR) in those originally randomised to PB/TURSO versus 14.1 months (4.2 months–NR) in those originally randomised to placebo (figure 1C). Similar to the previously published ITT survival analysis, which included the two participants excluded from the mITT population who died shortly after randomisation,8 results of the death-only analysis in the prespecified mITT population showed a significantly lower risk of death with early-start PB/TURSO (online supplemental figure 2). Results for the remaining key event analyses were likewise similar between the mITT and ITT populations (online supplemental table 2).
Figure 1.
Kaplan-Meier analyses of time to key events. Time to (A) any key event (ie, death, tracheostomy, PAV or first hospitalisation), (B) death or tracheostomy/PAV and (C) first hospitalisation and corresponding median event-free duration estimates are shown for each originally randomised group in the modified intent-to-treat population (ie, all randomised participants who received at least one dose of originally assigned trial drug and had at least one postbaseline Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised assessment; N=135). HRs and p values were estimated using a Cox proportional hazards model. The numbers at risk exclude participants who experienced the analysed event(s) or were censored before that time point. OLE, open-label extension; PAV, permanent assisted ventilation (defined as non-invasive ventilation >22 hours/day for >7 days); PB/TURSO, sodium phenylbutyrate/taurursodiol.
Discussion
In this long-term analysis of CENTAUR, the risk of key events including death, tracheostomy, PAV and first hospitalisation was significantly lower in those originally randomised to PB/TURSO compared with those originally randomised to placebo, most of whom went on to receive 6-month delayed-start PB/TURSO in the OLE phase. Median key event-free survival duration was 4.8 months longer in participants originally randomised to PB/TURSO versus placebo, and median tracheostomy/PAV-free survival duration was 7.3 months longer. As of the analysis cut-off, median time to first hospitalisation was not yet reached in the group originally randomised to PB/TURSO, compared with 14.1 months in the group originally randomised to placebo.
Among riluzole and edaravone, the two US Food and Drug Administration–approved therapies for ALS, only riluzole has shown a survival benefit in randomised clinical trials.10 11 However, the impact of riluzole on function in ALS is currently unclear.11 PB/TURSO has shown a dual benefit on survival8 and function7 in ALS. The findings of prolonged tracheostomy/PAV-free survival and reduced hospitalisation incidence in the analyses described herein support potential added benefits of PB/TURSO on reducing health burden in ALS.
Limitations
Because most participants who were originally randomised to placebo continued into the OLE phase and thus crossed over to PB/TURSO, the observed effect of early PB/TURSO may have been somewhat diluted in these analyses. In addition, while the use of a participant-locating firm allowed for definitive determination of vital status for all randomised participants but one, ascertainment of other key events was limited to the on-trial period, as these events are not captured in public records. As such, some postdropout tracheostomy, PAV, or hospitalisation events may not have been recorded in the subset of participants who discontinued from the trial, were lost to follow-up, or did not enrol in the OLE despite eligibility.
Conclusions
Early administration of PB/TURSO in the phase 2 CENTAUR trial prolonged tracheostomy/PAV-free survival and reduced hospitalisation risk in ALS, thereby potentially reducing drivers of individual health burden. Adding to the previously reported overall survival and functional benefits attributable to PB/TURSO, these findings support a modifying effect of PB/TURSO on disease progression in ALS. The phase 3 PHOENIX trial (NCT05021536), which began enrolment in late 2021, will further evaluate PB/TURSO safety and efficacy outcomes, including the incidence of the key events analysed in CENTAUR, over 48 weeks in a more heterogeneous, international population of individuals with ALS.
Acknowledgments
The authors would like to thank the people living with ALS who participated in the CENTAUR trial, as well as their families and caregivers; without them, this trial would not have been possible. We also thank the CENTAUR coordination centre and trial site staff. Lara Primak, MD, and Theresa Leichner, PhD, of PRECISIONscientia provided medical writing assistance with the development and revision of the manuscript under the direction of the authors, with financial support from Amylyx Pharmaceuticals, Inc. and in compliance with international Good Publication Practice guidelines.
Footnotes
Contributors: SP, SH, SPD, NK, JC, JK, PDY, and MEC contributed to conceptualisation and design of the trial and drafting of the manuscript, had full access to all trial data, and take responsibility for the integrity of the data and the accuracy of the data analysis. SH, SPD, and NK performed all statistical analyses. JC and JK obtained funding for the trial. All authors contributed to acquisition of data and critically reviewed interim and final versions of the manuscript.
Funding: The CENTAUR trial was funded by Amylyx Pharmaceuticals, Inc., ALS Finding a Cure®, and The ALS Association. The trial sponsor, Amylyx Pharmaceuticals, Inc. collaborated with the Northeast ALS Consortium network (www.neals.org) in the design and execution of the trial. Amylyx provided active drug and, during the randomised trial phase, placebo; participated in data analysis and manuscript development; and provided funding for writing support in the development of the manuscript. All other funders had no role in any aspect of the trial or in manuscript development.
Competing interests: SP reports grant support from Amylyx during the conduct of the study; grant support from Revalesio, Biohaven, UCB, Clene, Prilenia, and Seelos outside the submitted work; and consulting fees from Orion, Cytokinetics, and Medscape outside the submitted work. SH is owner, and SH, SPD, and NK are employees of Pentara Corporation, which was contracted by Amylyx for statistical analysis. JDB reports clinical trial support from Amylyx Pharmaceuticals, Inc, during the conduct of the study; grant support from Alexion, Biogen, Mitsubishi Tanabe Pharma America (MTPA), Anelixis Therapeutics, Brainstorm Cell Therapeutics, nQ Medical, the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, ALS One, Amylyx, MT Pharma Holdings of America, The ALS Association, ALS Finding a Cure®, and RAPA Therapeutics outside the submitted work; and personal fees from Biogen, Clene Nanomedicine, MTPA, MT Pharma Holdings of America, Sawai Pharmaceutical Co, Ltd, and Janssen outside the submitted work. MAE reports payment and personal fees from Amylyx for advisory board and speakers bureau participation outside the submitted work. CK reports consulting fees from Alnylam, Takeda, CSL Behring, Argenx, Akcea/Ionis, and Sanofi; honoraria from Alnylam; and advisory board participation fees from Alnylam and Orphazyme, all outside the submitted work. JBC reports grants from AZ Therapies Inc, MTB Pharmaceuticals, and Cytokinetics Inc, lecture honorarium from Northwest Area Health Education Center, and consulting fees from the Department of Defense Congressionally Directed Medical Research Programs grant review board, all outside the submitted work. JW reports research funding from Amylyx during the conduct of the study and presentation honoraria from Amylyx outside the submitted work. SAG reports institutional funding from Amylyx and The ALS Association during the conduct of the study; institutional funding from the National Institute of Environmental Health Sciences and The ALS Association outside the submitted work; consulting fees from Biogen and ITF Pharma outside the submitted work; lecture fees from Illinois State Neurological Society Lecture and Spectrum Health outside the submitted work; and personal fees for Data Safety Monitoring Board (DSMB) participation from Watermark outside the submitted work; and is an inventor on institutional patent US10660895B2. DH reports advisory board participation for Amylyx outside the submitted work. TDH-P reports clinical trial funding from Amylyx during the conduct of the study; institutional grant support from Cytokinetics, Orion, Bio 3, MTPA, the Sean M. Healey and AMG Center for ALS & the Neurological Clinical Research Institute, UCB Pharma, Alexion, and AB Sciences outside the submitted work; institutional contract work with Samus outside the submitted work; consulting fees from Samus, Alpha Insights, and Evidera outside the submitted work; payment for educational deliverables from Platform Q Health, WebMD, MJH Holdings, IQVIA, P Value, and Vindico Medical Education outside the submitted work; and advisory board fees from MTPA, Cytokinetics, AB Bio, Alexion, Biogen, and Orphazyme outside the submitted work; and is President of the ALS Hope Foundation. CEJ reports grant support from the Muscular Dystrophy Association, The ALS Association, and the National Institutes of Health and advisory board participation for Brainstorm Cell Therapeutics, MTPA, and Anelixis, all outside the submitted work, and is President Elect of the American Academy of Neurology. CQ reports advisory board fees from Amylyx outside the submitted work. JDR reports clinical trial funding from Amylyx during the conduct of the study; licensing agreement and nonfinancial support from Ionis Pharmaceuticals; nonfinancial support from Calico, Biogen, and IBM Watson; research grant support from the National Institute of Neurological Disorders and Stroke, National Institute on Aging, Department of Defense, the Chan Zuckerberg Initiative, Microsoft, The ALS Association, the Muscular Dystrophy Association, Target ALS, F Prime, ALS Finding A Cure, Answer ALS, Robert Packard Center for ALS Research, GlaxoSmithKline, Travelers Insurance, American Airlines, Caterpillar, and the National Football League; and personal consulting fees from Expansion Therapeutics and Team Gleason, all outside the submitted work. JK reports consulting fees from Calico, Denali, Biogen, MTPA, and Amylyx outside the submitted work. LJ reports advisory board participation for Cytokinetics outside the submitted work. SL reports advisory board fees from Amylyx outside the submitted work. TMM reports licensing agreements with Ionis Pharmaceuticals and C2N, consulting fees from Cytokinetics, Ionis Pharmaceuticals, and Disarm Therapeutics, lecture honorarium from Regeneron, advisory board participation for Biogen and UCB Pharma, and receipt of reagents from Ionis Pharmaceuticals, all outside the submitted work, and is co-chair of the Northeast ALS Consortium. SNS reports funding from Amylyx during the conduct of the study; grant support from Orion Pharma and Alexion outside the submitted work; lecture (grand rounds) honorarium from New York University outside the submitted work; and payment for expert testimony from Martin Clearwater & Bell LLP; Shaub, Ahmuty, Citrin & Spratt LLP; MCIC Vermont; and Aaronson Rappaport Feinstein and Deutsch, LLP outside the submitted work. THV reports institutional funding from Amylyx during the conduct of the study; institutional grant support from Alexion, Alector, Annexon Bio, Apellis, Biogen, Cytokinetics, Healey ALS Platform Trial, MT Pharma, and Sanofi outside the submitted work; and honoraria from Amylyx, Alexion, and Cytokinetics outside the submitted work. CNF reports institutional funding from Amylyx during the conduct of the study. AS reports institutional grant support from Amylyx during the conduct of the study and from the Rare Diseases Clinical Research Network, National Institutes of Health, Healey ALS Platform Trial, Cytokinetics, and Wyck Foundation outside the submitted work; honorarium from Weill Cornell Medicine – Qatar (residency recruitment seminar) outside the submitted work; uncompensated DSMB participation for the University of Kansas outside the submitted work; and compensated independent data monitoring committee participation for Alexion outside the submitted work. NAG reports grants from Alexion, Annelixis, Annexon, Brainstorm Cell Therapeutics, Cytokinetics, Fulcrum, the Sean M. Healey and AMG Center for ALS & the Neurological Clinical Research Institute, Kezar, Medicinova, Octapharma, Orion, and Orphazyme and advisory board participation for Alexion, Argenx, AstraZeneca, CSL Behring, MT Pharma, Sanofi Genzyme, Sarepta, and UCB, all outside the submitted work. GLP reports consulting fees from MT Pharma and honoraria from MT Pharma and Cytokinetics, all outside the submitted work. GK reports provision of trial supplies from Amylyx during the conduct of the study. JMS reports grant support from the National Institutes of Health, The ALS Association, Amylyx, Biogen, Biotie Therapies (now Acorda Therapeutics), Cytokinetics Incorporated, MTPA, Alexion, Medicinova, Ionis, Alector, and Orphazyme; royalties from UpToDate; consulting fees from Amylyx, Apic Biosciences, Neurosense, Cytokinetics, Denali, GSK, MTPA, Orphazyme, Pinteon, RRD, Swanbio, Helixsmith, Novartis, Sanofi, and EMD Serono; and honoraria from Amylyx (symposium) and Oakstone (online presentation); all outside the submitted work. JC and JK are co-CEOs of and own stock in Amylyx, the trial sponsor, and report grant support from ALS Finding a Cure and The ALS Association during the conduct of the study. RET is Founding Chair of Amylyx’s Scientific Advisory Board and holds equity in Amylyx. Dr Gilbert reports stock ownership in Amylyx. PDY reports full-time employment and stock option ownership with Amylyx outside the submitted work. MEC reports consulting fees from Faze, Regeneron, AB Sciences, Avexis, Orion, Lilly, Biohaven, Mt Pharma, Revalasio, Aclipse, Anelexis, Cytokinetics, Disarm, ALS Pharma, Immunity Pharma, Wave, Sunovian, Pontifex, Denali, Transposen, Quralis, Helixsmith, and RRD and is a board member for Praxis, all outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Deidentified participant data will be made available upon reasonable request. Requests for data sharing can be sent to info@amylyx.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involved human participants and was approved by Partners Human Research Committee (central IRB, no reference number or ID is associated). Participants gave informed consent to participate in the study before taking part.
References
- 1. Niedermeyer S, Murn M, Choi PJ. Respiratory failure in amyotrophic lateral sclerosis. Chest 2019;155:401–8. 10.1016/j.chest.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 2. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet 2017;390:2084–98. 10.1016/S0140-6736(17)31287-4 [DOI] [PubMed] [Google Scholar]
- 3. Ang K, Lim MY, Srinivasan S. Ethical and legal issues of tracheostomy ventilation in patients with amyotrophic lateral sclerosis. Proc Singapore Healthc 2019;28:193–202. 10.1177/2010105819828753 [DOI] [Google Scholar]
- 4. Gladman M, Zinman L. The economic impact of amyotrophic lateral sclerosis: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2015;15:439–50. 10.1586/14737167.2015.1039941 [DOI] [PubMed] [Google Scholar]
- 5. Larkindale J, Yang W, Hogan PF, et al. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve 2014;49:431–8. 10.1002/mus.23942 [DOI] [PubMed] [Google Scholar]
- 6. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol 2017;81:479–84. 10.1002/ana.24897 [DOI] [PubMed] [Google Scholar]
- 7. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N Engl J Med 2020;383:919–30. 10.1056/NEJMoa1916945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paganoni S, Hendrix S, Dickson SP, et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve 2021;63:31–9. 10.1002/mus.27091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9. 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 10. Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2012;2012:CD001447. [DOI] [PubMed] [Google Scholar]
- 11. Andrews JA, Jackson CE, Heiman-Patterson TD, et al. Real-World evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2020;21:509–18. 10.1080/21678421.2020.1771734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-329024supp002.pdf (4.3MB, pdf)
jnnp-2022-329024supp001.pdf (1.2MB, pdf)
Data Availability Statement
Deidentified participant data will be made available upon reasonable request. Requests for data sharing can be sent to info@amylyx.com.