Abstract
Despite public concern on the role of free‐roaming cats as reservoirs of zoonotic agents, little is known about the influence of urban and peri‐urban landscapes on the exposure risk. We evaluated the seroprevalence of three zoonotic agents (Chlamydia felis, Coxiella burnetii and Toxoplasma gondii) in domestic cats (Felis catus). Two hundred and ninety‐one free‐roaming cats were trapped in Murcia municipality (Southeast Spain), and their sera were tested for specific antibodies against T. gondii using a modified agglutination test (MAT), and for C. felis, C. burnetii and feline immunodeficiency virus (FIV) antibodies with ELISA technique. Pathogen seroprevalence at 95% CI was calculated for each sex and age category (up to and over 12 months) and compared with a chi‐squared test. The role of human population density and urban landscape characteristics on the risk of pathogen exposure in the cat population was explored using generalized linear models. Seropositivity against a single pathogen was found in 60% of the cats, while 19% was seropositive for two or three pathogens. Seroprevalence of C. felis was 8% (CI95%: 5–11), 37% (CI95%: 31–42) for C. burnetii and 42% (CI95%: 36–47) for T. gondii. In addition to these three pathogens, FIV seropositivity was low (1%, CI95%: −0.1 to 2) and adult cats were more likely to be seropositive to C. burnetii than young individuals (OR: 2.3, CI95%: 1.2–4.2). No sex or age class differences in seroprevalence were observed for the rest of the pathogens. Seropositivity was correlated with water surface areas for C. felis, and not with crop areas. Coxiella burnetii seropositivity was correlated with the percentage of urban areas (continuous with only buildings and discontinuous, that include buildings, parks, and pedestrian and urban green areas), human population size and peri‐urban areas with shrubs, and not correlated with other agricultural landscapes (orchards and crop areas). However, the seroprevalence of T. gondii was only associated with agricultural landscapes such as orchards. The detection of hotspot areas of high pathogen exposure risk is the basis for municipal services to implement surveillance and risk factor control campaigns in specific‐risk areas, including (a) efficient health management of urban cat colonies by geographical location, population census and health status monitoring of the components of each cat colony, (b) improvement of hygiene and sanitary conditions at the feeding points of the cat colony and (c) free‐roaming cat trapping for health monitoring and, in the long term, to know the evolution of the health status of their populations.
Keywords: Chlamydia felis; Coxiella burnetii; free‐roaming cat; geo‐epidemiology; landscape, urbanization, rural area; Toxoplasma gondii
IMPACTS.
Landscape features affect Chlamydia felis, Coxiella burnetii and Toxoplasma gondii distribution.
Cats in rural environments are exposed to C. felis, C. burnetii and T. gondii.
Cats are more exposed to C. burnetii in urban than in peri‐urban/rural environments.
Free‐roaming cats are a sentinel to detect zoonotic pathogens in anthropized areas.
1. INTRODUCTION
The domestic cat (Felis catus) is the most abundant carnivore in urban and peri‐urban areas with high human density (Sims et al., 2008). Feral cats include not only the offspring of domestic cats adapted to living on their own in rural and urban areas, but also un‐owned individuals that have left or lost their domestic home, surviving in the urban environment as stray cats (Ogan & Jurek, 1997). Both types are referred to as free‐roaming cats because of their behaviour. Given that more than 75% of emerging human infectious diseases are of zoonotic origin (Jones et al., 2008), information on pathogens that may be transmitted to sympatric wildlife or humans is needed. In this context, free‐roaming cats are a major public health concern due to their large populations in contact with humans and their capacity to harbour pathogens that produce disease in both humans and animals (Taetzsch et al., 2018). The range of pathogens transmitted depends primarily on the host communities, with the presence and frequency of free‐roaming pets having a significant effect on risk of disease transmission to humans (Polley, 2005). The prevalence of viruses, bacteria and parasites is usually higher in free‐roaming cats because outdoor access (Little, 2011) and roaming behaviour (Patronek, 1998) increase the risk of infection. Indeed, free‐roaming cats have unrestricted entry to public areas such as parks and playgrounds where different potential hosts (e.g. rodents, birds, lizards and snakes) are present (Montoya et al., 2018). Moreover, in many cities in Europe and other continents, free‐roaming cat colonies are tolerated, deliberately fed and kept by citizens, resulting in extremely high densities of cats in certain urban and peri‐urban areas (Crawford et al., 2019).
Among the zoonotic agents associated with cats that can infect a wide variety of host species are Toxoplasma gondii, whose only definitive hosts are felids (Elmore et al., 2010), and Coxiella burnetii, the etiological agent of Q fever, of which an upsurge in urban cases in both humans and animals, has been reported (Meredith et al., 2014; Shapiro et al., 2015). In Spain, T. gondii is widespread in domestic and wild animals, with seroprevalence ranging between 37% and 85% (Gauss et al., 2003; Millán et al., 2009; Miró et al., 2004), and C. burnetii has been diagnosed in animals and people in urban areas with varying seroprevalence (Espejo et al., 2014; van der Hoek et al., 2010; Ruiz‐Seco et al., 2011). In addition to these pathogens, cats can also be infected with Chlamydia felis, an obligate intracellular bacterium with a predilection for conjunctival epithelial cells, the most common cause of feline chlamydiosis. Moreover, there is controversy about its zoonotic potential, having been described as an etiological agent of different diseases in humans (Browning, 2004; Halánová et al., 2011). In fact, pet cats seem to play an important role as sources of C. felis infection in humans (Trávnicek et al., 2002). Although many surveys on C. felis have been carried out worldwide (Sykes, 2005), limited information is available for Spain on the presence of this pathogen in cats (Millán & Rodríguez, 2009; Ravicini et al., 2016). On the contrary feline immunodeficiency virus (FIV) is a pathogen specific to cats that can create concomitant immunosuppressive infections. This virus induces progressive lymphadenopathy and immunosuppression that usually ends in a fatal disease after several years of latency (Hofmann‐Lehmann et al., 1997). In fact, FIV has been associated with an increased risk of co‐infection with a wide range of parasites, from viruses as feline leukaemia virus (Bandecchi et al., 2006) to protozoa as T. gondii (Danner et al., 2007; Serrano & Millán, 2014).
Recent studies indicate that human‐induced landscape changes are an important factor causing modifications in the transmission of zoonoses (Bradley & Altizer, 2007; Mackenstedt et al., 2015). This is of particular importance considering that, in urban and peri‐urban environments, a complex community of wild and domestic animals, acting as reservoirs of zoonotic pathogens, is present (Despommier et al., 2006). Spatial distribution of diseases depends not only on land cover (e.g. vegetation cover, surface moisture, topography, soil types) but also on land use (e.g. landscape uses influenced by the political, social and economic context), since these environmental features determine the probability of contact between human and animal hosts (Lambin et al., 2010). In spite of the large number of methods to investigate spatiotemporal patterns of infectious diseases (Blasdell et al., 2016; Chowell & Rothemberg, 2018; Lewis et al., 2017; Ortega et al., 2020), these have rarely been applied to study pathogen spread in free‐roaming cats from urban and peri‐urban areas.
In this work, we explore the role of urban landscape features and human population density on exposure risk to three zoonotic pathogens (C. felis, C. burnetii and T. gondii) in cats from Murcia municipality (Southeast Spain). We assume that there are high densities of wild and synanthropic hosts species increasing the risk of tick infestations and amplifying the sources of C. burnetii (Barbu et al., 2013; Meredith et al., 2014; Pfäffle et al., 2013; Rizzoli et al., 2014), T. gondii (Aronson et al., 2017; Barros et al., 2018; Hill & Dubey, 2002) and C. felis (Halánová et al., 2019). The high environmental stability of C. burnetii, which allows it to survive in soil for long periods (Eldin et al., 2017), the route of infection via inhalation (Angelakis & Raoult, 2010) and the possibility of wind currents spreading from manure accumulates (King et al., 2011; Tissot‐Dupont et al., 2004) influence the prevalence of infection in agricultural landscapes with small ruminant farms (De Lange et al., 2014; Eldin et al., 2017; van der Hoek et al., 2010). In the same sense, humidity linked to green areas (agricultural and urban parks) favours the survival of infective T. gondii oocysts (Afonso et al., 2008; Shapiro et al., 2019; Yilmaz & Hopkins, 1972). Chlamydia felis spreads through direct contact, and transmission between cats is due to contact with infectious eye secretions (Halánová et al., 2011). Moreover, free‐roaming cats usually show higher C. felis seroprevalence than cats kept strictly indoors (Helps et al., 2005), probably linked to malnutrition and a possibly weakened immune system, and increased exposure in overpopulated urban cat colonies (Halánová et al., 2019).
Our hypothesis is that the landscape influences the exposure risk to cat pathogens, especially in those urban and peri‐urban areas where land cover and land use favour the persistence of infective forms in the environment. Also, areas of high landscape biodiversity would influence free‐roaming cats' interaction (direct/indirect contact or predation) with infected and spreader hosts, either conspecifics or other animal species. Finally, urban landscapes are likely to be areas with higher cat pathogens seroprevalences due to the presence of urban colonies of this felid. The objectives of this study were (a) to determine the seroprevalence of zoonotic pathogens (C. burnetii, T. gondii and C. felis) in free‐roaming cats from Murcia municipality (SE Spain) and (b) to evaluate the host and environmental risk factors associated with exposure to these zoonotic pathogens.
2. MATERIAL AND METHODS
2.1. Study area
The study area covers the Municipality of Murcia, located in the Southeast of Spain (37º59′10″N, 1º7′49″W, Figure 1), which is a complex geomorphologic territory. Broadly, the study area is made up of two large tertiary basins occupied by areas of high agricultural activity, separated by the Alpine massif of the Sierra de Carrascoy, which is part of the Penibetic System of the Baetic Mountain Ranges (Estrella et al., 2018) and divides the municipality into Southern/Western (predominantly unirrigated landscape) and Northern (predominantly irrigated and urbanized landscape) regions. The city of Murcia, settled in the basin to the north of the Sierra de Carrascoy, is drained by the Segura River, an area that has been traditionally cultivated giving rise to the ‘Huerta de Murcia’, one of the six orchard landscapes that exist in Europe (Martí‐Ciriquián & Moreno‐Vicente, 2014). In the southern basin, the traditional predominant use was unirrigated agriculture, but in recent years, this has changed to intensive irrigated agriculture, especially in an extensive area known as Campo de Cartagena. The study area has a hot semi‐arid climate, with mild winters and very hot summers, sometimes with absolute maximum temperatures above 45ºC. The average annual rainfall is less than 300 mm, showing irregular but intense rains, which can cause large floods (Espín Sánchez et al., 2018). From a social point of view, the municipality of Murcia has a population of 459,403 inhabitants (INE, 2020), unevenly distributed due to the fact that the city of Murcia and its surroundings comprise more than 90% of the study area, with a human population density of 525 inhabitants/km2. Administratively, the study area is divided into 55 districts (called ‘pedanías’, Figure 2) formed from small population centres scattered throughout the territory. The land covers and uses are very heterogeneous, showing an unequal distribution throughout the study area. The municipality covers 88,473 hectares, of which 10% is dedicated to anthropic uses (half of which is urban area), with agricultural activity accounting for the largest part at 65%, and natural surfaces cover the remaining 25% (Corine Land Cover (CLC), 2018).
FIGURE 1.

Geographic location of the municipality of Murcia. Demarcation and description of the landscape of the municipal districts
FIGURE 2.

Number of cats sampled per district in the municipality of Murcia
2.2. Cat sampling
In the period 2005–2007, 291 free‐roaming cats were trapped in the study area (Figure 2), as a part of an annual programme run by the Department of Public Health of Murcia. Animals were trapped during October, November and December in different urban and peri‐urban areas and euthanized for sanitary reasons (according to European Union protocols regarding animal welfare and bioethics) by official Veterinary Services at Centro Municipal de Control de Zoonosis de la ciudad de Murcia (CMCZ). Cats were grouped by sex, age and provenance (district where they were trapped). Sex was determined by visual inspection during the necropsy, and the age of the animals assigned by observing tooth replacement (The Humane Society of United States, 2006). Two classes were considered for age: young (up to 12 months old) and adult (more than 12 months old). The sample population included 128 males, 162 females, 69 young and 221 adult cats. The age and sex of one of the animals could not be determined.
2.3. Serological analysis of samples
Immediately after death, a blood sample was taken from each cat, which was then allowed to coagulate at laboratory temperature for 24 hr to obtain the serum samples. The serum obtained was kept in aliquots at −20ºC until analysis. Sera were tested for C. felis‐ and C. burnetii‐specific antibodies using a commercially available ELISA kit according to the manufacturer protocols (CHEKIT© Chlamydia, Bonmelli Diagnost 2356789 ics. IDEXX Laboratories B.V. Netherlands; CHEKIT© Q‐fever C. burnetii Antibody Test Kit, IDEXX Switzerland AG, Bern, Switzerland). The test showed a sensitivity of 90.9% and specificity of 85.9% to detect Chlamydia LPS antigen, and a sensitivity of 99% and specificity of 88.57% to detect an inactivated mixture of C. burnetii phase I and II anFIVtigens (Nine Mile strain). Both tests were modified in house with a monoclonal goat anti‐cat IgM antibody HRP conjugate (Bethyl A20‐100P©, Bionova Científica S.L. Madrid, Spain), according to the recommendations of Candela et al. (2017). The samples were also tested for FIV antibody detection using INGEZIM FIV‐R.16.FIV.K.1© (Ingenasa, Inmunología y Genética Aplicada, S.A., Madrid, Spain), assuming that the presence of virus‐specific antibodies in serum is indicative of FIV infection (Fish & Altman, 1989).
A modified agglutination test (MAT) was used to detect T. gondii antibodies using a commercial test kit; the antigen (Antigène ToxoAD, Catalogue No. 7‐542‐2, Lot No. 640558‐A) and control serum (Toxotrol A, Catalogue No. 7‐543‐1, Lot No. 613896‐A) were obtained from bioMerièux (Marcy l’Etoile, 69260‐Charbonnieres‐les‐Bains, France).
2.4. Statistical analysis
Pathogen seroprevalence (i.e. number of seropositive animals over the total sampled) was calculated for each sex and age class and compared with a chi‐squared test (χ 2 test). Differences were considered significant at p‐values <.05. Odds ratio (OR) was used as a measure of the strength of association between variables. Confidence intervals (CI) at 95% were also calculated using Epi InfoTM 7.0 version (Dean et al., 2000).
In regard to the spatial analysis, the landscape information covered land use and land cover characteristics potentially linked to zoonosis risk at the district level (Barros et al., 2018; McFarlane et al., 2013) and cat ranging behaviour (Hanmer et al., 2017; Metsers et al., 2010). Land cover is related to landscape attributes such as vegetation cover, surface moisture, topography and soil types, among others. Land uses are related to anthropogenic landscape uses influenced by the political, social and economic context (Lambin et al., 2010). All landscape variables used were representative of the study area, selected to respond to the hypotheses proposed on the risk of potential contact of free‐roaming cats with the pathogens selected and, consequently, to achieve the objectives of our study. Some similar or spatially under‐represented landscape categories were grouped into a new spatial variable. We have used nine landscape categories, which group specific landscape variables and characteristics and are detailed in Table 1. The proportion of land cover in each district was calculated intersecting a shapefile containing the urban districts in SIOSE (Sistema de Información sobre Ocupación del Suelo de España, downloaded from Instituto Geográfico Nacional, https://www.ign.es/web/ign/portal). We checked for collinearity between landscape characteristics using variance inflation factors (VIF) in the ‘HH’ package Version 3.1‐43 (Heiberger, 2018). Highly correlated variables were removed based on their multicollinearity (VIF > 5) (Kim, 2019) (Table 1). Information on human population size was used as an additional covariate. Details on the covariates are provided in Table 1. Some districts were excluded because of the low sample size (e.g. less than one cat sampled).
TABLE 1.
Overall listing of variables considered in the study (* variable with VIF>5 and removed because of multicollinearity)
| Variable | Description | Source |
|---|---|---|
| Human population size | Number of resident human population at district level |
Instituto Nacional de Estadística (INE) |
| % of crops area | The category includes herbaceous crops different from rice |
SIOSE (Sistema de Información sobre Ocupación del Suelo de España) downloaded from Instituto Geográfico Nacional (IGN) |
| % of unproductive area | The category includes rocky outcrops, nude soil, ground without construction | |
| % of orchards and other farming area | The category includes citrus fruit plants, non‐citrus fruit plants, olive grove, vineyard and other woody plants. | |
| % of pasture area* | The category includes pasture | |
| % of urban area | The category includes buildings (as continuous urban areas), and buildings mixed with parks, pedestrian and urban green areas (as discontinuous urban areas) | |
| % of water surface area | The category includes water courses and artificial water surfaces | |
| % of wood area | The category includes coniferous trees and leafy trees | |
| % of shrubs and small bush areas | The category includes scrubs and small brushes |
Quantitative analysis of risk factors was performed using generalized linear models (GLM) with binomial family and logit link. These were fit using the function glm (R Core Team, 2018). Pathogen occurrence (0 = absence and 1 = presence) was considered as a response variable and landscape characteristics at the district level as the explanatory variables (Table 1).
Model selection was done using a backward approach based on the Akaike information criterion (Akaike, 1973) (Table S2). The models with the lowest AIC were used. Goodness of fit was evaluated using diagnostic plots of the residuals. Overdispersion, however, was assessed using the ‘testOverdispersion’ function implemented in the DHARMa package (Harting, 2019). All statistical analyses were conducted in the R software 3.5.2 version (R Core Team, 2018). The final model for each pathogen was used to build a risk map using QGIS 3.6.0 version (QGIS Development Team, 2017).
3. RESULTS
The overall seroprevalence was 8% (CI95%: 5–11) for C. felis, 37% (CI95%: 31–42) for C. burnetii and 42% (CI95%: 36–47) for T. gondii. There were only three FIV positive cats (P: 1%, CI 95%: −0.1 to 2); they were all adults, specifically one female and two males. Seroprevalence for all the different groups is presented in Table 2. Statistically significant differences were observed between age and the seroprevalence of C. burnetii, with the adult cats at higher risk (OR: 2.3, CI 95%: 1.2–4.2, χ 2 = 6.4, p‐value = .01). All the information related to odds ratio and chi‐squared values performed with sex and age variables is detailed in Table S1.
TABLE 2.
Seroprevalences (%) and CI95% of Chlamydia felis, Coxiella burnetii, Toxoplasma gondii and feline immunodeficiency virus (FIV) according to host sex and age
| Agent | Sex | Age | ||
|---|---|---|---|---|
| Male | Female | Young | Adult | |
| C. felis | 9 (4–10) | 9 (4–12) | 3 (0–6) | 10 (6–14) |
| C. burnetii | 34 (26–42) | 40 (30–46) | 23 (5–33) | 40 (34–47) |
| T. gondii | 40 (30–46) | 45 (37–52) | 32 (12–42) | 45 (39–51) |
| FIV | 1.5 (0–3) | 0.6 (0–1) | 0 | 1.3 (0–2) |
Seropositivity against more than two pathogens was found in 19% of the cats studied (56/291). Specifically, 16% (47/291) were positive to two pathogens (47/291) and 3% (9/291) to three. Among the cats that tested positive to three pathogens, two were positive to FIV.
The statistically significant factors selected in the final GLMs were (a) for C. felis, the percentage of water surface areas (OR: 1.74e−08, CI 95%: 1.3e−18–0.065) with a p‐value equal to the cut‐off alpha; (b) for C. burnetii, the percentage of shrub area (OR:4.15e−09, CI 95%:3.7e‐16–0.01, p‐value: .01), the percentage of urban area (OR: 5.06, CI 95%:1.3–20, p‐value: .02) and the human population size (OR:0.99, CI 95% 0.993–0.99, p‐value: .02); and (c) for T. gondii, the percentage of orchards (OR: 0.13, CI 95%: 0.02–0.7, p‐value: .02) in peri‐urban areas.
Final models for each pathogen species are shown in Table 3. Neither residual patterns nor overdispersion was observed in our GLM. All models with their AIC values and weights are available in the Table S2.
TABLE 3.
GLM results for Chlamydia felis, Coxiella burnetii and Toxoplasma gondii
| Predictors | OR (CI95%) | p‐value |
|---|---|---|
| Reduced GLM of C. felis | ||
| Percentage of crop area | 0.04 (0.0003–1) | .14 |
| Percentage of water surface area | 1.74e−08 (1.3e−18–0.065) | .05 |
| AIC:69 | ||
| quasi R 2: .23 | ||
| Reduced GLM of C. burnetii | ||
| % of crop area | 0.12 (0.006–1) | .15 |
| % of orchard and other farming areas | 6.86 (0.89–56) | .067 |
| % of shrub and small bush areas | 4.15e−09 (3.7e−16–0.01) | .01 |
| % of urban area | 5.06 (1.3–20) | .02 |
| Human population size | 0.99 (0.9999873–0.99999885) | .02 |
| AIC:93 | ||
| quasi R 2: .45 | ||
| Reduced GLM of T. gondii | ||
| % of orchard and other farming areas | 0.13 (0.02–0.7) | .02 |
| AIC: 87 | ||
| quasi R 2: .16 | ||
A map of infection risk for each pathogen at the district scale is shown in Figures 3, 4, 5. The probability of C. felis occurrence is low in all districts ranging from 0.09 to 0.18 (mean: 0.11; Figure 3), whereas for C. burnetii, it ranged from 0 to 0.70 (mean: 0.38) with some districts at higher risk (probability: 0.68–1, Figure 4). Contact risk for C. burnetii in urban landscapes was higher than in peri‐urban areas (OR: 6.86). On the contrary, cats from peri‐urban areas are more prone to contact with T. gondii (probability from 0.34 to 0.67) than their counterparts living in more urbanized areas (0.21–0.22, Figure 5).
FIGURE 3.
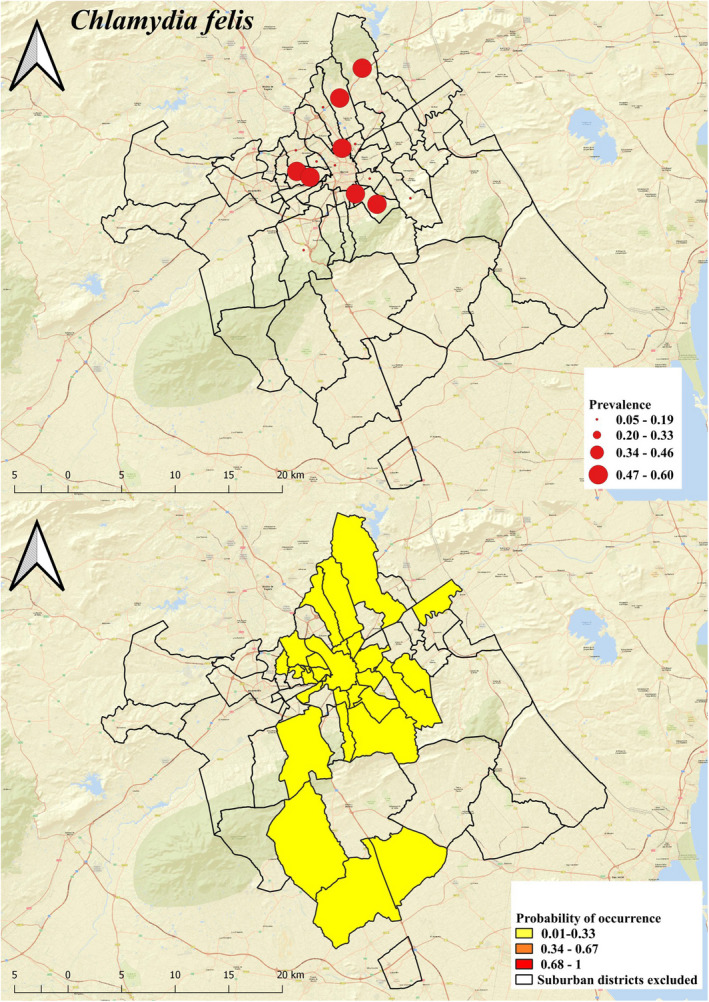
Probability of occurrence and intervals of Chlamydia felis prevalence in the municipality of Murcia
FIGURE 4.

Probability of occurrence and intervals of Coxiella burnetii prevalence in the municipality of Murcia
FIGURE 5.
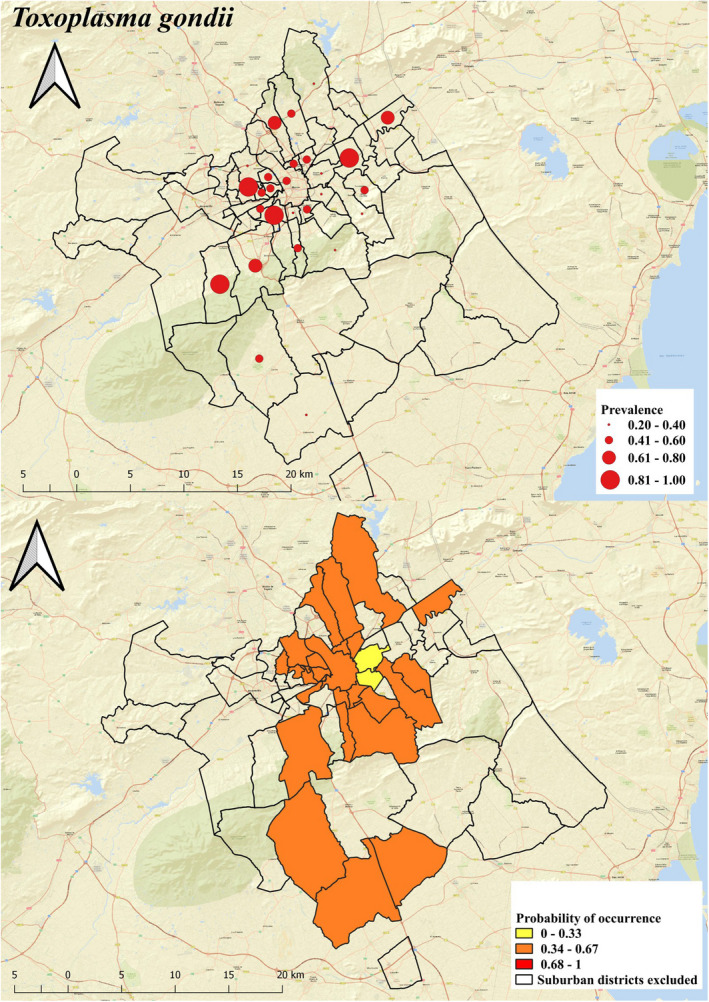
Probability of occurrence and intervals of Toxoplasma gondii prevalence in the municipality of Murcia
4. DISCUSSION
This work aimed to explore the exposure of free‐roaming cats to three selected zoonotic pathogens by understanding the landscape variables that influence pathogen contact rates with cats. This approach was based on the use of complementary research tools to determine risk factors associated with the environmental characteristics of the study area. This provides more precise knowledge of the risk factors and, consequently, allows for more effective and localized implementation of control plans for these zoonoses in urban and peri‐urban environments. Here, we provide a comprehensive assessment of the effect of landscape on the exposure of cats to C. felis, C. burnetii and T. gondii in a highly urbanized landscape. One of the limitations of our study has been that the sampled cats could not be accurately geo‐referenced. Another limitation has been the number of samples per district, which in some cases was less than 5. Finally, although serological studies based on the detection of antibodies should be interpreted with caution, since they do not unequivocally indicate that the animal is a carrier of the pathogens investigated, they are useful to know, indirectly, the spatial distribution of pathogens, since contact with them generates a serological response in the host.
Several surveys have been carried out on domestic ruminants, highlighting the relevant role of C. burnetii as a zoonotic agent (Astobiza et al., 2012; García‐Pérez et al., 2009; Ruiz‐Fons et al., 2010), although the risk of transmission from cats to humans has not been previously investigated in the Iberian Peninsula. Candela et al. (2017) reported C. burnetii infection in free‐living European wildcats (Felis sylvestris sylvestris) in Central Spain, suggesting that this wild feline should be considered as part of the epidemiological cycle, just like domestic and feral cats. Other authors have reported high seroprevalence of C. burnetii in cats; specifically, Meredith et al. (2014) found a prevalence of 61.5% in rural cats in the United Kingdom, and Komiya et al. (2003) detected a prevalence of 41.6% in stray cats in Japan. Our study revealed a high probability of C. burnetii exposure (seroprevalence close to 40%) in adult free‐roaming cats from urban and peri‐urban areas. Together with the above‐mentioned studies, our findings provide a valuable overview of high seroprevalence of C. burnetii in cats and, consequently, the potential zoonotic risk to humans should be of wide interest, considering the public health threat posed by this pathogen (Espejo et al., 2014; Murcia et al., 2002; Ruiz‐Seco et al., 2011).
The GLM analysis highlighted the link between urban and peri‐urban landscapes and the risk of pathogen infection at the district level. We detected a significant relationship between C. burnetii exposure, not only with more urbanized and densely populated areas, but also with the more populated traditional orchard areas surrounding the metropolitan area of Murcia. C. burnetti has a very wide host range that may serve to amplify hosts, favouring transmission to humans in densely populated urban areas (Comer et al., 2001; Meredith et al., 2014). These hosts include domestic animals (cats, dogs and cattle), commensal rodents (Rattus norvegicus and Mus musculus), wild birds and lagomorphs. Important landscape alterations have occurred in the metropolitan area of Murcia over the centuries, resulting in alterations to ecotones and the creation of new and multiple human–domestic–wild interfaces, which have shaped changes in density and distribution of wildlife species (Mata Olmo & Fernández Muñoz, 2004). Under this perspective, free‐roaming cats have the opportunity to consume wild prey, which can be reservoirs of C. burnetii (Meredith et al., 2014). Other factors that may increase contact between conspecifics, and thus, the risk of disease transmission in urban areas is the smaller (Dards, 1978) and overlapping home ranges of free‐roaming cats, due to the greater abundance of trophic resources, either because of prey abundance (Fitzgerald & Karl, 1979; Lieber, 1980) or the supplementary food provided to cat colonies by local residents (Natoli et al., 2006).
Although the transmission of C. burnetii by ticks is still debated, the rise of tick populations in urban and peri‐urban areas is of particular importance considering that this arthropod is able to amplify sources of C. burnetii infection by vertical transmission to their progeny (i.e. transtadially and transovarially) (Walker & Fishbein, 1991). In urban and peri‐urban habitats in Europe, rodents, hedgehogs, shrews, birds, lizards and pets (dogs and cats), as well as red foxes (Vulpes vulpes), roe deer (Capreolus capreolus) and wild boar (Sus scrofa) in peri‐urban areas, play a major role as hosts and reservoirs of tick‐borne pathogens (Pfäffle et al., 2013). Some authors have also claimed that ticks may play an important role in the maintenance of C. burnetii infection in rodents and lagomorphs (Marrie et al., 1993), and thus, a very complex network of transmission could be present in an urban area. In fact, increasing urbanization may alter the biology and population density of ticks and hosts and, accordingly, could lead to an increased risk of vector‐borne disease emergence in peri‐urban and urban areas (Barbu et al., 2013; Rizzoli et al., 2014).
It is interesting to note that the percentage of shrub and small bush areas in peri‐urban zones in the selected model shows a protective effect, which contradicts our initial hypothesis that hedgerows and shrubs, which harbour greater biodiversity and therefore more ticks, may favour exposure to C. burnetii. Interpreting this result is difficult, but it is known that in semi‐arid landscapes (such as Murcia), shrubs and small bushes are important determinants of biodiversity (Chu et al., 2015; Giladi et al., 2007) and ecosystem enrichment (Eldridge et al., 2011). Thus, these landscapes favour the presence of predators (medium‐sized birds of prey, lizards, snakes, etc.) that reduce rodent populations in urban and peri‐urban areas (Aronson et al., 2017; Lepczyk et al., 2017). No correlation was detected between seroprevalence and unirrigated agricultural landscapes, frequent in the south of Murcia, and with a greater presence of sheep and goat farms.
Cats play a central role in the epidemiology of T. gondii (Robert‐Gangneux & Dardé, 2012) because felids are the only definitive hosts that can excrete oocysts in the environment. Although cats shed oocysts for a short period after primoinfection (Dubey, 2010), some individuals may have new episodes of oocyst shedding during their lifetime (Zulpo et al., 2018). In this study, the seroprevalence of T. gondii was the highest among the pathogens studied (42%). A comparison to other studies in the Iberian Peninsula shows a similar prevalence, within the range observed in the central (52%, Gauss et al., 2003) and north‐eastern part of the Iberian Peninsula (37%, Miró et al., 2004). In these same studies, a statistically significant higher prevalence in adults was found. A similar difference was found in our study, although not statistically significant. However, these studies are difficult to compare due to different epidemiological situations of the cat populations. The prevalence of T. gondii may differ between specific areas of a country and even within a single city (Barros et al., 2018; Dubey, 2010). This is because the prevalence depends on factors such as the presence of intermediate hosts (mainly birds and small mammals), the availability of infected prey (Hill & Dubey, 2002; although some studies did not detect a relationship between prevalence in rodents and infected cats—see De Feo et al., 2002) and oocyst accumulation in the soil (Shapiro et al., 2019). In urban areas, the survival of oocysts can be influenced by the increase in green spaces (Aronson et al., 2017; Yilmaz & Hopkins, 1972) and by the patterns of defecation of cats. Cat colonies often defecate repeatedly in specific areas called latrines, and areas favourable for cat defecation (such as urban parks and gardens) have been shown to increase the presence of oocysts in the soil (Afonso et al., 2008; Simon et al., 2019).
In our study, T. gondii seroprevalence does not appear to be influenced by urbanization and human population density, nor by the increased presence of intermediate hosts associated with peri‐urban environments (Aronson et al., 2017; Barros et al., 2018; Hill & Dubey, 2002). The risk of T. gondii is medium to high (0.34–0.67) in almost the entire municipality of Murcia, and based on results from the GLM analysis, the presence of orchard and farming areas was a decreasing factor to infection in peri‐urban environments. Studies indicate that rural cats are more likely to be infected than urban cats (Oi et al., 2015), because in rural areas, which have a higher percentage of permanent crop areas, cats may have larger home ranges and thus less focused deposition places. The seroprevalence found in the peri‐urban territory studied does not correlate with non‐irrigated cropland landscapes, and decreases in our case, with the existence of trees and permanent crops in irrigated areas. However, further studies should be carried out to elucidate these issues.
The presence of trees and permanent crops in irrigated peri‐urban areas, and shrubs and bushes in dryland areas, are landscape features that reduce the exposure of free‐roaming cats to pathogens such as T. gondii and C. burnetii. Conversely, urbanization and population density increase the contact with C. burnetii. Therefore, seroprevalence in cats in a given area may serve as a sentinel not only for the prevalence of T. gondii infection in the definitive host (Dubey et al., 1995), but also for anthropogenic disturbance for the maintenance of T. gondii infections (Barros et al., 2018), bacteria and oocyst environmental contamination and, consequently, for the health risk to the human population in this area.
To our knowledge, very few studies have been carried out on C. felis in the Iberian Peninsula. Specifically, Millán and Rodriguez (2009) reported a seroprevalence of 27% in wild cats in central Spain, and Ravicini et al. (2016) found that 2.6% of stray cats were positive to C. felis with PCR in Catalonia. Several studies have reported C. felis prevalence in feline populations in other countries; in Slovakia, an overall positivity of 17.1% was found by PCR in cats living in different environments (Halánová et al., 2011); in central China, a seroprevalence of 5.9% was found in domestic cats (Wu et al., 2013), and a 20% seroprevalence was found in Iran (Maazi et al., 2016). In our study, C. felis seroprevalence was lower (8%) in comparison with most of these studies. Differences in C. felis exposure could be due to different environmental and climatic factors. Specifically, Murcia is characterized by the typical Mediterranean semi‐arid subtropical climate, with hot summers and mild winters, which could reduce the extra‐host survival of the bacterium (Gruffydd‐Jones et al., 2009), a pattern that was well depicted by the risk map, with a low probability of C. felis occurrence in the whole municipality. The GLM identified the percentage of water surface as a protective factor. C. felis seems to be highly unstable outside the host and remains infectious for a short time in the environment (Halánová et al., 2019), although other Chlamydia‐like organisms are heat‐resistant and able to survive in the environment and in artificial water networks (Coulon et al., 2012). Considering the dry landscape of Murcia and the surrounding neighbourhood, most water areas are human‐made water supplies to agriculture. The presence of these areas implies a reduction in biological and ecological diversity. Thus, they might be interpreted as a proxy of low animal density as well as a low richness of potential host for C. felis.
In our study, the prevalence rate for FIV was low and in accordance with those reported in other areas of Spain (Arjona et al., 2000; Ravicini et al., 2016). Of the three cats positive for FIV, two were also seropositive for other pathogens. About 20% of free‐roaming cats tested were found to have two pathogens, and 15% had three pathogens, suggesting that some areas have higher exposure to various pathogens than others, and that there are risk factors that favour these rates of pathogen contact in cats (Candela et al., 2009).
To ensure public health in urban and peri‐urban landscapes, preventive measures need to focus on cat colony management, environmental enrichment, conservation of dryland bush landscapes and health education for sheep and goat farmers. To reduce pathogen exposure in free‐roaming cats and cat colonies in the Municipality of Murcia, we recommended (a) enhancing sanitary surveillance measures in cat colonies (serological testing, complying with the principles of hygiene, deworming, therapy and vaccination of animals) because contact with free‐roaming cats or cats living in colonies could be a C. burnetii risk factor for keepers and veterinarians (Komiya et al., 2003); (b) free‐roaming cat population surveillance, taking into account that these animals in urban landscapes are more likely to be infected with C. burnetii than indoor pet cats and are considered an important route to transmission to humans (Ma et al., 2020); (c) implementing population control measures in cat colonies, and controlling cat feed support and over‐densification through planned captures of cats for sterilization (Natoli et al., 2006), because high population density may be associated with the spread of C. felis and with disease severity (Gonsales et al., 2016; Halánová et al., 2019); (d) eliminating free‐roaming cat feeding points that do not provide adequate sanitary and hygiene conditions to prevent C. felis human disease (Wu et al., 2013); and (e) measuring soil contamination by T. gondii oocyst estimation in green areas near cat colonies (Afonso et al., 2008).
Trees and permanent crops in irrigated peri‐urban areas, and shrubs and bushes in dryland areas, are landscape features that reduce the exposure of feral cats to pathogens such as T. gondii and C. burnetii. Conversely, urbanization and population density increase the presence of C. burnetii. To reduce the risk of human exposure to these pathogens by increasing biodiversity, the environmental restoration of vegetation loss in urban areas and its conservation in peri‐urban environments is likely necessary (Aronson et al., 2017; Barros et al., 2018; Lepczyk et al., 2017). On the contrary, cats suspected of having been exposed to C. burnetii at a positive farm might shed infective forms during parturition, becoming a potential and mobile vector for spreading the infection to other conspecifics, nearby farms and homes in the area (Cyr et al., 2021). In addition, health education of small ruminant farmers should be provided, especially regarding sheep vaccination, indoor lambing, appropriate disposal of placentas and litter to prevent the spread of the pathogen to other domestic and wild species that might consume these lambing remains. Wind spreading of manure infected with C. burnetii in unirrigated agricultural landscapes is a factor that can only be monitored and not prevented. Finally, tick prevention measures are recommended (Egberink et al., 2013).
Operational strategies to increase the effectiveness of zoonotic pathogen detection and apply appropriate prevention and control measures should be focussed on those hotspots where pathogen spread or risk factors have been detected (Ortega et al., 2020). The detection of seroprevalence in cats in a given area may serve as a proxy not only for the prevalence of infection in the host (Dubey et al., 1995), but also for anthropogenic disturbance for the maintenance of infections (Barros et al., 2018), bacteria and oocyst environmental contamination, and consequently for the health risk to humans in this area. Interdisciplinary collaboration of policy makers and public health, veterinary and human health professionals is required to improve our understanding on the dynamics of zoonoses in urban areas.
5. CONCLUSION
Disease dynamics in urban and peri‐urban areas are complex, as they involve the presence of varied hosts in a human–domestic–wild interface. Therefore, a One Health approach, bringing together professionals from human medicine, veterinary medicine, biology, geography and landscaping, is necessary to address the challenges posed by the spread of zoonotic pathogens in areas with high human population density. Our study indicates that (a) free‐roaming cats are sentinels for detecting zoonotic pathogens in anthropized areas, (b) peri‐urban agricultural areas influence the contact of free‐roaming cats with C. felis, C. burnetii and T. gondii, and (c) urbanization is a risk factor for C. burnetii infection in free‐roaming cats. These results should be the basis of further studies to deepen knowledge on the epidemiology of these pathogens. The findings of our study highlight the potential public health threat posed by cats in urban areas, if proper preventive and control measures, as well as proper hygienic practices, are not applied.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Mónica G. Candela worked on the conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, writing of the original draft, and reviewing and editing of the manuscript. Angela Fanelli worked on the formal analysis, validation and writing of the original draft. João Carvalho worked on the formal analysis, validation, and reviewing and editing of the manuscript. Emmanuel Serrano worked on the conceptualization, formal analysis, validation, writing of the original draft, and review and editing of the manuscript. Guillermo Domenech worked on data curation and investigation. Francisco Alonso worked on visualization, methodology, data curation and investigation. Carlos Martínez‐Carrasco worked on the conceptualization, data curation, methodology, supervision, validation, and reviewing and editing of the manuscript.
ANIMAL ETHICS
The authors declare no animal ethics conflict. Animal samples were from animals euthanized according to European Union protocols regarding animal welfare and bioethics and provided by the Centro Municipal de Control de Zoonosis de la ciudad de Murcia (CMCZ).
Supporting information
Table S1
Table S2
ACKNOWLEDGEMENTS
Preliminary results were presented as an oral presentation at the 37èmes Rencontres du GEEFSM. Etroubles, Vallee d'Aoste, Italia, 13‐16 Jun 2019. We thank the Department of Public Health of Murcia and the official Veterinary Services at Centro Municipal de Control de Zoonosis de la ciudad de Murcia (CMCZ). This research was partially funded by the City Council of Murcia through the OTRI research contract awarded to the Department of Animal Health of the University of Murcia, ‘Epidemiological study of parasitic and infectious agents affecting stray cats in the municipality of Murcia. Zoonotic implications of the cat and its role as an environmental biomarker'. The authors would like to thank the official Veterinary Services and trappers at Centro Municipal de Control de Zoonosis from the Municipality of Murcia (CMCZ) for providing us with euthanized animals according to European Union protocols for animal welfare and bioethics. We are also very grateful to Dr. Pedro Pérez Cutillas for his supervision, review and improvement of the text describing the study area. Emmanuel Serrano is supported by the Spanish Ministerio de Ciencia, Innovación y Universidades (MICINN) through a Ramon y Cajal agreement (RYC‐2016‐21120). João Carvalho was supported by a research contract (CEECIND/01428/2018) from the Fundação para a Ciência e a Tecnologia (FCT). Thanks for financial support are also due to CESAM (UID/AMB/50017/2019), to FCT/MCTES through national funds and co‐funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. Finally, we would also like to acknowledge the inspiring comments of two anonymous reviewers who helped to improve the understanding of the work presented.
Candela, M. G. , Fanelli, A. , Carvalho, J. , Serrano, E. , Domenech, G. , Alonso, F. , & Martínez‐Carrasco, C. (2022). Urban landscape and infection risk in free‐roaming cats. Zoonoses and Public Health, 69, 295–311. 10.1111/zph.12919
Mónica G. Candela and Angela Fanelli contributed equally to this article
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Afonso, E. , Lemoine, M. , Poulle, M.‐L. , Ravat, M.‐C. , Romand, S. , Thulliez, P. , Villena, I. , Aubert, D. , Rabilloud, M. , Riche, B. , & Gilot‐Fromont, E. (2008). Spatial distribution of soil contamination by Toxoplasma gondii in relation to cat defecation behaviour in an urban area. International Journal of Parasitology, 38, 1017–1023. 10.1016/j.ijpara.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Akaike, H. (1973). Information theory and an extension of the maximum likelihood principle. In Petrov B. N., & Csaki F. (Eds.), Proceedings of the 2nd international symposium on information theory (pp. 267–281). Akadémiai Kiadó. [Google Scholar]
- Angelakis, E. , & Raoult, D. (2010). Q fever. Veterinary Microbiology, 140(3‐4), 297–309. 10.1016/j.vetmic.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Arjona, A. , Escolar, E. , Soto, I. , Barquero, N. , Martin, D. , & Gomez‐Lucia, E. (2000). Seroepidemiological survey of infection by feline leukemia virus and immunodeficiency virus in Madrid and correlation with some clinical aspects. Journal of Clinical Microbiology, 38, 3448–3449. 10.1128/JCM.38.9.3448-3449.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson, M. F. J. , Lepczyk, C. A. , Evans, K. L. , Goddard, M. A. , Lerman, S. B. , MacIvor, J. S. , Nilon, C. H. , & Vargo, T. (2017). Biodiversity in the city: Key challenges for urban green space management. Frontiers in Ecology and the Environment, 15, 189–196. 10.1002/fee.1480 [DOI] [Google Scholar]
- Astobiza, I. , Ruiz‐Fons, F. , Piñero, A. , Barandika, J. F. , Hurtado, A. , & García‐Pérez, A. L. (2012). Estimation of Coxiella burnetii prevalence in dairy cattle in intensive systems by serological and molecular analyses of bulk‐tank milk samples. Journal of Dairy Sciences, 95, 1632–1638. 10.3168/jds.2011-4721 [DOI] [PubMed] [Google Scholar]
- Bandecchi, P. M. , Dell'Omodarme, M. , Magi, M. , Palamidessi, A. , & Prati, M. C. (2006). Feline leukaemia virus (FeLV) and feline immunodeficiency virus infections in cats in the Pisa district of Tuscany and attempts to control FeLV infection in a colony of domestic cats by vaccination. Veterinary Record, 158, 555–557. 10.1136/vr.158.16.555 [DOI] [PubMed] [Google Scholar]
- Barbu, C. , Hong, A. , Manne, J. , Small, D. , Quintanilla Calderón, J. , Sethuraman, K. , … Levy, M. Z. (2013). The effects of city streets on an urban disease vector. PLoS Computational Biology, 9, e1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, M. , Cabezón, O. , Dubey, J. P. , Almería, S. , Ribas, M. P. , Escobar, L. E. , Ramos, B. , & Medina‐Vogel, G. (2018). Toxoplasma gondii infection in wild mustelids and cats across an urban‐rural gradient. PLoS One, 13(6), e0199085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdell, K. , Morand, S. , Henttonen, H. , Tran, A. , & Buchy, P. (2016). Hantavirus seropositivity in rodents in relation to habitat heterogeneity in human‐shaped landscapes of Southeast Asia. Spatial and Spatio‐Temporal Epidemiology, 17, 27–35. 10.1016/j.sste.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Bradley, C. A. , & Altizer, S. (2007). Urbanization and the ecology of wildlife diseases. Trends in Ecology & Evolution, 22(2), 95–102. 10.1016/j.tree.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning, G. (2004). Is Chlamydophila felis a significant zoonotic pathogen? Australian Veterinary Journal, 82, 695–696. [DOI] [PubMed] [Google Scholar]
- Candela, M. , Caballol, A. , & Atance, P. (2017). Wide exposure to Coxiella burnetii in ruminant and feline species living in a natural environment: Zoonoses in a human‐livestock‐wildlife interface. Epidemiology and Infection, 145, 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela, M. , Serrano, E. , Martínez‐Carrasco, C. , Martín‐Atance, P. , Cubero, M. , Alonso, F. , & León, L. (2009). Coinfection is an important factor in epidemiological studies: The first serosurvey of the aoudad (Ammotragus lervia). European Journal of Clinical Microbiology and Infectious Diseases, 28(5), 481–489. 10.1007/s10096-008-0654-8 [DOI] [PubMed] [Google Scholar]
- Chowell, G. , & Rothenberg, R. (2018). Spatial infectious disease epidemiology: On the cusp. BMC Medicine, 16, 192. 10.1186/s12916-018-1184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, J. , Yang, H. , Lu, Q. , & Zhang, X. (2015). Endemic shrubs in temperate arid and semiarid regions of northern China and their potentials for rangeland restoration. Annals of Botany Plants, 7, plv063. 10.1093/aobpla/plv063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer, J. , Paddock, C. , & Childs, J. (2001). Urban zoonoses caused by Bartonella, Coxiella, Ehrlichia, and Rickettsia species. Vector Borne and Zoonotic Diseases, 1(2), 91–118. 10.1089/153036601316977714 [DOI] [PubMed] [Google Scholar]
- Corine Land Cover (CLC), Copernicus Land Monitoring Service (2018) European Environment Agency (EEA)", f.ex. in 2018: © European Union, Copernicus Land Monitoring Service 2018. European Environment Agency (EEA). [Google Scholar]
- Coulon, C. , Eterpi, M. , Greub, G. , Collignon, A. , McDonnell, G. , & Thomas, V. (2012). Amoebal host range, host‐free survival and disinfection susceptibility of environmental Chlamydiae as compared to Chlamydia trachomatis . FEMS Immunology and Medical Microbiology, 64(3), 364–373. 10.1111/j.1574-695X.2011.00919.x [DOI] [PubMed] [Google Scholar]
- Crawford, H. M. , Calver, M. C. , & Fleming, P. A. (2019). A case of letting the cat out of the bag‐why trap‐neuter‐return is not an ethical solution for stray cat (Felis catus) management. Animals, 16, 171. 10.3390/ani9040171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr, J. , Turcotte, M. È. , Desrosiers, A. , Bélanger, D. , Harel, J. , Tremblay, D. , Leboeuf, A. , Gagnon, C. A. , Côté, J. C. , & Arsenault, J. (2021). Prevalence of Coxiella burnetii seropositivity and shedding in farm, pet and feral cats and associated risk factors in farm cats in Quebec, Canada. Epidemiology and Infection, 149, e57. 10.1017/S0950268821000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner, R. M. , Goltz, D. M. , Hess, S. G. , & Banko, P. C. (2007). Evidence of feline immunodeficiency virus, feline leukemia virus, and Toxoplasma gondii in feral cats on Mauna Kea, Hawaii. Journal of Wildlife Diseases, 43, 315–318. 10.7589/0090-3558-43.2.315 [DOI] [PubMed] [Google Scholar]
- Dards, J. (1978). Home ranges of feral cats in Portsmouth Dockyard. Carnivore Genetics Newsletter, 3, 242–255. [Google Scholar]
- De Feo, M. , Dubey, J. , Mather, T. , & Rhodes, R. (2002). Epidemiologic investigation of seroprevalence of antibodies to Toxoplasma gondii in cats and rodents. American Journal of Veterinary Research, 63, 1714–1717. [DOI] [PubMed] [Google Scholar]
- De Lange, M. M. , Schimmer, B. , Vellema, P. , Hautvast, J. L. , Schneeberger, P. M. , & Van Duijnhoven, Y. T. (2014). Coxiella burnetii seroprevalence and risk factors in sheep farmers and farm residents in The Netherlands. Epidemiology and Infection, 142(6), 1231–1244. 10.1017/S0950268813001726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, A. , Sullivan, K. M. , & Zubieta, J. (2000). Epi Info 2000: a database, and statistics program for public health professionals for use on Windows 95, 98, and NT computers. Centers for Disease Control and Prevention (U.S.); Epidemiology Program Office; Global Programme on AIDS (World Health Organization). https://stacks.cdc.gov/view/cdc/23207 [Google Scholar]
- Despommier, D. , Ellis, B. , & Wilcox, B. (2006). The role of ecotones in emerging infectious diseases. EcoHealth, 3, 281–289. 10.1007/s10393-006-0063-3 [DOI] [Google Scholar]
- Dubey, J. (2010). Toxoplasmosis in animals and humans (2nd ed., p. 313). CRC Press. [Google Scholar]
- Dubey, J. , Lappin, M. , & Thulliez, P. (1995). Long‐term antibody responses of cats fed Toxoplasma gondii tissue cysts. Journal of Parasitology, 81, 887–893. 10.2307/3284035 [DOI] [PubMed] [Google Scholar]
- Egberink, H. , Addie, D. , Belák, S. , Boucraut‐Baralon, C. , Frymus, T. , Gruffydd‐Jones, T. , Hartmann, K. , Hosie, M. J. , Lloret, A. , Lutz, H. , Marsilio, F. , Möstl, K. , Pennisi, M. G. , Radford, A. D. , Thiry, E. , Truyen, U. , & Horzinek, M. C. (2013). Coxiellosis/Q fever in cats: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery, 15(7), 573–575. 10.1177/1098612X13489216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin, C. , Mélenotte, C. , Mediannikov, O. , Ghigo, E. , Million, M. , Edouard, S. , Mege, J. L. , Maurin, M. , & Raoult, D. (2017). From Q Fever to Coxiella burnetii infection: A paradigm change. Clinical Microbiology Reviews, 30(1), 115–190. 10.1128/CMR.00045-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge, D. J. , Bowker, M. A. , Maestre, F. T. , Roger, E. , Reynolds, J. F. , & Whitford, W. G. (2011). Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecology Letters, 14(7), 709–722. 10.1111/j.1461-0248.2011.01630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore, S. , Jones, J. , Conrad, P. , Patton, S. , Lindsay, D. , & Dubey, J. (2010). Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends in Parasitology, 26, 190–196. 10.1016/j.pt.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Espejo, E. , Gil‐Díaz, A. , Oteo, J. , Castillo‐Rueda, R. , García‐Alvarez, L. , Santana‐Báez, S. , & Bella, F. (2014). Clinical presentation of acute Q fever in Spain: Seasonal and geographical differences. International Journal of Infectious Diseases, 26, 162–164. 10.1016/j.ijid.2014.06.016 [DOI] [PubMed] [Google Scholar]
- Espín‐Sánchez, D. , García, C. , & Porcel, G. (2018). Temperature inversions due to warm air advections at low levels: Significant thermal contrast in the Vega Media of the Segura River (Southeast Spain). In Daniels J. A. (Ed.), Advances in environmental research publisher. 64 (pp. 139–178). New York, NY: Nova Science Publishers. [Google Scholar]
- Estrella, T. , García, C. , Navarro‐Hervás, F. , Pérez‐Cutillas, P. , & Mariana, F. (2018). Evidence of holocene tectonic activity affecting alluvial fill evolution in the Vega Media of the Segura river, southeastern Spain. Geografia Fisica e Dinamica Quaternaria, 50, 99–117. 10.4461/gfdq [DOI] [Google Scholar]
- Fisch, H. , & Altman, N. (1989). Feline immunodeficiency virus infection in a population of pet cats from southeastern Florida. Journal of Veterinary Diagnostic Investigation, 1, 339–342. 10.1177/104063878900100411 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, A. , & Karl, B. (1979). Foods of feral house cats (Felis catus L.) in forest of the Orongorongo valley, Wellington. New Zealand Journal of Zoology, 6, 107–126. [Google Scholar]
- García‐Pérez, A. , Astobiza, I. , Barandika, J. , Atxaerandio, R. , Hurtado, A. , & Juste, R. (2009). Investigation of Coxiella burnetii occurrence in dairy sheep flocks by bulk‐tank milk analysis and antibody level determination. Journal of Dairy Sciences, 92, 1581–1584. [DOI] [PubMed] [Google Scholar]
- Gauss, C. , Almería, S. , Ortuño, A. , García, F. , & Dubey, J. (2003). Seroprevalence of Toxoplasma gondii antibodies in domestic cats from Barcelona. Journal of Parasitology, 89, 1067–1068. [DOI] [PubMed] [Google Scholar]
- Giladi, I. , Segoli, M. , & Ungar, E. (2007). The effect of shrubs on the seed rain of annuals in a semiarid landscape. Israel Journal of Plant Sciences, 55, 83–92. 10.1560/IJPS.55.1.83 [DOI] [Google Scholar]
- Gonsales, F. F. , Brandão, P. E. , Melville, P. A. , Zuniga, E. , & Benites, N. R. (2016). Chlamydia felis: Lack of association between clinical signs and the presence of the cryptic plasmid. Microbial Pathogenesis, 97, 14–18. 10.1016/j.micpath.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Gruffydd‐Jones, T. , Addie, D. , Belák, S. , Boucraut‐Baralon, C. , Egberink, H. , Frymus, T. , Hartmann, K. , Hosie, M. J. , Lloret, A. , Lutz, H. , Marsilio, F. , Pennisi, M. G. , Radford, A. D. , Thiry, E. , Truyen, U. , & Horzinek, M. C. (2009). Chlamydophila felis infection ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery, 11(7), 605–609. 10.1016/j.jfms.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halánová, M. , Petrová, L. , Halán, M. , Trbolová, A. , Babinská, I. , & Weissová, T. (2019). Impact of way of life and environment on the prevalence of Chlamydia felis in cats as potential sources of infection for humans. Annals of Agricultural and Environmental Medicine, 26(2), 222–226. 10.26444/aaem/100655 [DOI] [PubMed] [Google Scholar]
- Halánová, M. , Sulinová, Z. , Čisláková, L. , Trbolová, A. , Páleník, Ľ. , Weissová, T. , Halán, M. , Kalinová, Z. , & Holičková, M. (2011). Chlamydophila felis in cats ‐ Are the stray cats dangerous source of infection? Zoonoses and Public Health, 58, 519–522. 10.1111/j.1863-2378.2011.01397.x [DOI] [PubMed] [Google Scholar]
- Hanmer, H. J. , Thomas, R. L. , & Fellowes, M. D. E. (2017). Urbanisation influences range size of the domestic cat (Felis catus): Consequences for conservation. Journal of Urban Ecology, 3(1), 1–11. 10.1093/jue/jux014 [DOI] [Google Scholar]
- Harting, F. (2019). DHARMa: Residual diagnostics for hierarchical (Multi‐Level / Mixed). R Packag version 024. [Google Scholar]
- Heiberger, R. M. (2018). HH: Statistical analysis and data display: Heiberger and Holland. R package version 3.1‐35. https://cran.r‐project.org/package=HH [Google Scholar]
- Helps, C. R. , Lait, P. , Damhuis, A. , Björnehammar, U. , Bolta, D. , Brovida, C. , Chabanne, L. , Egberink, H. , Ferrand, G. , Fontbonne, A. , Pennisi, G. , Gruffydd‐Jones, T. , Gunn‐Moore, D. , Hartmann, K. , Lutz, H. , Malandain, E , Möstl, K. , Stengel, C. , Harbour, A. , & Graat, E. A. (2005). Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: Experience from 218 European catteries. The Veterinary Record, 156(21), 669–673. 10.1136/vr.156.21.669 [DOI] [PubMed] [Google Scholar]
- Hill, D. , & Dubey, J. P. (2002). Toxoplasma gondii: Transmission, diagnosis and prevention. Clinical Microbiology and Infection, 8(10), 634–640. 10.1046/j.1469-0691.2002.00485.x [DOI] [PubMed] [Google Scholar]
- Hofmann‐Lehmann, R. , Holznagel, E. , Ossent, P. , & Lutz, H. (1997). Parameters of disease progression in long‐term experimental feline retrovirus (feline immunodeficiency virus and feline leukemia virus) infections: Hematology, clinical chemistry, and lymphocyte subsets. Clinical and Diagnostic Laboratory Immunology, 4, 33–34. 10.1128/cdli.4.1.33-42.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- INE (2020). https://www.ine.es/index.htm
- Jones, K. E. , Patel, N. G. , Levy, M. A. , Storeygard, A. , Balk, D. , Gittleman, J. L. , & Daszak, P. (2008). Global trends in emerging infectious diseases. Nature, 451, 990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H. (2019). Multicollinearity and misleading statistical results. Korean Journal of Anesthesiology, 72(6), 558–569. 10.4097/kja.19087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, L. A. , Goirand, L. , Tissot‐Dupont, H. , Giunta, B. , Giraud, C. , Colardelle, C. , Duquesne, V. , Rousset, E. , Aubert, M. , Thiéry, R. , Calatayud, L. , Daurat, G. , Hocqueloux, L. , Cicchelero, V. , Golliot, F. , & de Valk, H. (2011). Outbreak of Q fever, Florac, Southern France, Spring 2007. Vector Borne and Zoonotic Diseases, 11(4), 341–347. 10.1089/vbz.2010.0050 [DOI] [PubMed] [Google Scholar]
- Komiya, T. , Sadamasu, K. , Kang, M.‐I. , Tsuboshima, S. , Fukushi, H. , & Hirai, K. (2003). Seroprevalence of Coxiella burnetii infections among cats in different living environments. Journal of Veterinary Medicine and Science, 65, 1047–1048. 10.1292/jvms.65.1047 [DOI] [PubMed] [Google Scholar]
- Lambin, E. , Tran, A. , Vanwambeke, S. , Linard, C. , & Soti, V. (2010). Pathogenic landscapes: Interactions between land, people, disease vectors, and their animal hosts. International Journal of Health Geographics, 9, 54. 10.1186/1476-072X-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepczyk, C. A. , Aronson, M. F. J. , Evans, K. L. , Goddard, M. A. , Lerman, S. B. , & MacIvor, J. S. (2017). Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience, 67(9), 799–807. 10.1093/biosci/bix079 [DOI] [Google Scholar]
- Lewis, J. S. , Logan, K. A. , Alldredge, M. W. , Carver, S. , Bevins, S. N. , Lappin, M. , Van de Woude, S. , & Crooks, K. R. (2017). The effects of demographic, social, and environmental characteristics on pathogen prevalence in wild felids across a gradient of urbanization. PLoS One, 12, e0187035. 10.1371/journal.pone.0187035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberg, O. (1980). Spacing patterns in a population of rural free roaming domestic cats. Oikos, 35, 336–349. 10.2307/3544649 [DOI] [Google Scholar]
- Little, S. (2011). A review of feline leukemia virus and feline immunodeficiency virus seroprevalence in cats in Canada. Veterinary Immunology and Immunopathology, 143, 243–245. 10.1016/j.vetimm.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Ma, G. C. , Norris, J. M. , Mathews, K. O. , Chandra, S. , Šlapeta, J. , Bosward, K. L. , & Ward, M. P. (2020). New insights on the epidemiology of Coxiella burnetii in pet dogs and cats from New South Wales. Australia. Acta Tropica, 205, 105416. 10.1016/j.actatropica.2020.105416 [DOI] [PubMed] [Google Scholar]
- Maazi, N. , Jamshidi, S. , Kayhani, P. , & Momtaz, H. (2016). Occurrence of Chlamydophila felis, feline herpesvirus 1 and calcivirus in domestic cats of Iran. Iran Journal of Microbiology, 8(5), 312–315. [PMC free article] [PubMed] [Google Scholar]
- Mackenstedt, U. , Jenkins, D. , & Romig, T. (2015). The role of wildlife in the transmission of parasitic zoonoses in peri‐urban and urban areas. International Journal for Parasitology: Parasites and Wildlife, 4, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie, T. , Embil, J. , & Yates, L. (1993). Seroepidemiology of Coxiella burnetii among wildlife in Nova Scotia. American Journal of Tropical Medicine and Hygiene, 49, 613–615. 10.4269/ajtmh.1993.49.613 [DOI] [PubMed] [Google Scholar]
- Martí‐Ciriquián, P. , & Moreno‐Vicente, E. (2014). Land‐use transformations in the city of Murcia and its surroundings (1977‐2010). Estudios Geográficos, 276, 261–309. ISSN: 0014‐1496, eISSN: 1988‐8546. 10.3989/estgeogr.201407 [DOI] [Google Scholar]
- Mata Olmo, R. , & Fernández Muñoz, S. (2004). La Huerta de Murcia: Landscape guidelines for a peri‐urban territory. Landscape Research, 29, 385–397. 10.1080/0142639042000289028 [DOI] [Google Scholar]
- McFarlane, R. A. , Sleigh, A. C. , & McMichael, A. J. (2013). Land‐use change and emerging infectious disease on an island continent. International Journal of Environmental Research and Public Health, 10, 2699–2719. 10.3390/ijerph10072699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith, A. , Cleaveland, S. , Denwood, M. , Brown, J. , & Shaw, D. (2014). Coxiella burnetii (Q‐Fever) seroprevalence in prey and predators in the United Kingdom: Evaluation of infection in wild rodents, foxes and domestic cats using a modified ELISA. Transboundary Emerging Diseases, 62, 639–649. [DOI] [PubMed] [Google Scholar]
- Metsers, E. , Seddon, P. , & van Heezik, Y. (2010). Cat‐exclusion zones in rural and urban‐fringe landscapes: How large would they have to be? CSIRO Wildlife Research, 37, 47–56. 10.1071/WR09070 [DOI] [Google Scholar]
- Millán, J. , Cabezón, O. , Pabón, M. , Dubey, J. P. , & Almería, S. (2009). Seroprevalence of Toxoplasma gondii and Neospora caninum in feral cats (Felis silvestris catus) in Majorca, Balearic Islands, Spain. Veterinary Parasitology, 165, 323–326. 10.1016/j.vetpar.2009.07.014 [DOI] [PubMed] [Google Scholar]
- Millán, J. , & Rodríguez, A. (2009). A serological survey of common feline pathogens in free‐living European wildcats (Felis silvestris) in central Spain. European Journal of Wildlife Research, 55, 285–291. 10.1007/s10344-008-0246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró, G. , Montoya, A. , Jiménez, S. , Frisuelos, C. , Mateo, M. , & Fuentes, I. (2004). Prevalence of antibodies to Toxoplasma gondii and intestinal parasites in stray, farm and household cats in Spain. Veterinary Parasitology, 126, 249–255. 10.1016/j.vetpar.2004.08.015 [DOI] [PubMed] [Google Scholar]
- Montoya, A. , García, M. , Gálvez, R. , Checa, R. , Marino, V. , Sarquis, J. , Barrera, J. P. , Rupérez, C. , Caballero, L. , Chicharro, C. , Cruz, I. , & Miró, G. (2018). Implications of zoonotic and vector‐borne parasites to free‐roaming cats in central Spain. Veterinary Parasitology, 251, 125–130. 10.1016/j.vetpar.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Murcia, J. , Reus, S. , Climent, V. , Manso, M. , López, Í. , & Tello, A. (2002). Acute myocardial failure in a young man: Q‐fever myocarditis. Revista Española de Cardiología, 55, 875–877. [DOI] [PubMed] [Google Scholar]
- Natoli, E. , Maragliano, L. , Cariola, G. , Faini, A. , Bonanni, R. , Cafazzo, S. , & Fantini, C. (2006). Management of feral domestic cats in the urban environment of Rome (Italy). Preventive Veterinary Medicine, 77, 180–185. 10.1016/j.prevetmed.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Ogan, C. V. , & Jurek, R. M. (1997). Biology and ecology of feral, free‐roaming and stray cats. In Harris J. E., & Ogan C. V. (Eds.), Mesocarnivores of Northern California: Biology, management and survey techniques, workshop manual (pp. 87–92). Humbolt State University. [Google Scholar]
- Oi, M. , Yoshikawa, S. , Maruyama, S. , & Nogami, S. (2015). Comparison of Toxoplasma gondii seroprevalence in shelter cats and dogs during 1999–2001 and 2009–2011 in Tokyo, Japan. PLoS One, 10, e0135956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, N. , Fanelli, A. , Serrano, A. , Martínez‐Carrasco, C. , Escribano, F. , Tizzani, P. , & Candela, M. G. (2020). Salmonella seroprevalence in wild boar from Southeast Spain depends on host population density. Research in Veterinary Science, 132, 400–403. 10.1016/j.rvsc.2020.07.026 [DOI] [PubMed] [Google Scholar]
- Patronek, G. (1998). Free‐roaming and feral cats–their impact on wildlife and human beings. Journal of the American Veterinary Medical Association, 212, 218–226. [PubMed] [Google Scholar]
- Pfäffle, M. , Littwin, N. , Muders, S. V. , & Petney, T. N. (2013). The ecology of tick‐borne diseases. International Journal of Parasitology, 43, 1059–1077. 10.1016/j.ijpara.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Polley, L. (2005). Navigating parasite webs and parasite flow: Emerging and re‐emerging parasitic zoonoses of wildlife origin. International Journal for Parasitology, 35(11–12), 1279–1294. 10.1016/j.ijpara.2005.07.003 [DOI] [PubMed] [Google Scholar]
- QGIS Development Team (2017). QGIS geographic information system. Open Source Geospatial Foundation. [Google Scholar]
- R Core Team (2018). A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Ravicini, S. , Pastor, J. , Hawley, J. , Brewer, M. , Castro‐López, J. , Beall, M. , & Lappin, M. R. (2016). Prevalence of selected infectious disease agents in stray cats in Catalonia, Spain. Journal of Feline Medicine and Surgery Open Reports, 16(2), 205511691663410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli, A. , Silaghi, C. , Obiegala, A. , Rudolf, I. , Hubálek, Z. , Földvári, G. , Plantard, O. , Vayssier‐Taussat, M. , Bonnet, S. , Špitalská, E. , & Kazimírová, M. (2014). Ixodes ricinus and its transmitted pathogens in urban and peri‐urban areas in Europe: New hazards and relevance for public health. Frontiers in Public Health, 2, 251. 10.3389/fpubh.2014.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert‐Gangneux, F. , & Dardé, M. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews, 25, 264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Fons, F. , Astobiza, I. , Barandika, J. , Hurtado, A. , Atxaerandio, R. , Juste, R. , & García‐Pérez, A. L. (2010). Seroepidemiological study of Q fever in domestic ruminants in semi‐extensive grazing systems. BMC Veterinary Research, 6, 1–6. 10.1186/1746-6148-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Seco, M. , López‐Rodríguez, M. , Estébanez‐Muñoz, M. , Pagán, B. , Gómez‐Cerezo, J. , & Barbado‐Hernández, F. (2011). Fiebre Q: 54 nuevos casos de un hospital terciario de Madrid. Revista Clínica Española, 211, 240–244. 10.1016/j.rce.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Serrano, E. , & Millán, J. (2014). What is the price of neglecting parasite groups when assessing the cost of co‐infection? Epidemiology and Infection, 142, 1533–1540. 10.1017/S0950268813002100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, A. , Bosward, K. , Heller, J. , & Norris, J. (2015). Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Veterinary Microbiology, 177, 154–161. 10.1016/j.vetmic.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Shapiro, K. , Bahia‐Oliveira, L. , Dixon, B. , Dumètre, A. , de Wit, L. A. , Van Wormer, E. , & Villena, I. (2019). Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food and Waterborne Parasitology, 15, e00049. 10.1016/j.fawpar.2019.e00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, J. A. , Chancel, E. , Hubert, P. , Aubert, D. , Villena, I. , Gilot‐Fromont, E. , & Poulle, M. L. (2019). Pattern of latrine use by domestic cats on dairy farms and the implications for Toxoplasma gondii transmission. Veterinary Parasitology, 273, 112–121. 10.1016/j.vetpar.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Sims, V. , Evans, K. , Newson, S. , Tratalos, J. , & Gaston, K. J. (2008). Avian assemblage structure and domestic cat densities in urban environments. Diversity and Distribution, 14, 387–399. 10.1111/j.1472-4642.2007.00444.x [DOI] [Google Scholar]
- Sykes, J. (2005). Feline chlamydiosis. Clinical Techniques in Small Animal Practice, 20, 129–134. 10.1053/j.ctsap.2004.12.018 [DOI] [PubMed] [Google Scholar]
- Taetzsch, S. , Bertke, A. , & Gruszynski, K. (2018). Zoonotic disease transmission associated with feral cats in a metropolitan area: A geospatial analysis. Zoonoses and Public Health, 65, 412–419. 10.1111/zph.12449 [DOI] [PubMed] [Google Scholar]
- The Humane Society of United States (2006). How to determine a cat's or dog's age. The Humane Society of United States. http://www.hsus.org [Google Scholar]
- Tissot‐Dupont, H. , Amadei, M. A. , Nezri, M. , & Raoult, D. (2004). Wind in November, Q fever in December. Emerging Infectious Diseases, 10(7), 1264–1269. 10.3201/eid1007.030724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trávnicek, M. , Mardzinová, S. , Cisláková, L. , Valocký, I. , & Weissová, T. (2002). Chlamydial infection of cats and human health. Folia Microbiologica, 47(4), 441–444. 10.1007/BF02818705 [DOI] [PubMed] [Google Scholar]
- van der Hoek, W. , Dijkstra, F. , Schimmer, B. , Schneeberger, P. M. , Vellema, P. , Wijkmans, C. , van Schegget, R. , Hackert, V. , & Duynhoven, Y. (2010). Q fever in the Netherlands: An update on the epidemiology and control measures. Euro Surveillance, 15(12), 19520. 10.2807/ese.15.12.19520-en [DOI] [PubMed] [Google Scholar]
- Walker, D. , & Fishbein, D. (1991). Epidemiology of rickettsial diseases. European Journal of Epidemiology, 7, 237–245. 10.1007/BF00145672 [DOI] [PubMed] [Google Scholar]
- Wu, S. M. , Huang, S. Y. , Xu, M. J. , Zhou, D. H. , Song, H. Q. , & Zhu, X. Q. (2013). Chlamydia felis exposure in companion dogs and cats in Lanzhou, China: A public health concern. BMC Veterinary Research, 9, 104. 10.1186/1746-6148-9-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz, S. , & Hopkins, S. (1972). Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. Journal of Parasitology, 58, 938–939. [PubMed] [Google Scholar]
- Zulpo, D. L. , Sammi, A. S. , dos Santos, J. R. , Sasse, J. P. , Martins, T. A. , Minutti, A. F. , Cardim, S. T. , de Barros, L. D. , Navarro, I. T. , & Garcia, J. L. (2018). Toxoplasma gondii: A study of oocyst re‐shedding in domestic cats. Veterinary Parasitology, 249, 17–20. 10.1016/j.vetpar.2017.10.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


