Abstract
Besides the well‐recognized risk factors, novel conditions increasing cardiovascular morbidity and mortality are emerging. Undesirable emotions and behavior such as anxiety and depression, appear to participate in worsening cardiovascular pathologies. On the other hand, deteriorating conditions of the heart and vasculature result in disturbed mental and emotional health. The pathophysiological background of this bidirectional interplay could reside in an inappropriate activation of vegetative neurohormonal and other humoral systems in both cardiovascular and psychological disturbances. This results in circulus vitiosus potentiating mental and circulatory disorders. Thus, it appears to be of utmost importance to examine the alteration of emotions, cognition, and behavior in cardiovascular patients. In terms of this consideration, recognizing the potential of principal cardiovascular drugs to interact with the mental state in patients with heart or vasculature disturbances is unavoidable, to optimize their therapeutic benefit. In general, beta‐blockers, central sympatholytics, ACE inhibitors, ARBs, aldosterone receptor blockers, sacubitril/valsartan, and fibrates are considered to exert anxiolytic effect in animal experiments and clinical settings. Statins and some beta‐blockers appear to have an equivocal impact on mood and anxiety and ivabradine expressed neutral psychological impact. It seems reasonable to suppose that the knowledge of a patient's mood, cognition, and behavior, along with applying careful consideration of the choice of the particular cardiovascular drug and respecting its potential psychological benefit or harm might improve the individualized approach to the treatment of cardiovascular disorders.
Keywords: aldosterone antagonists, angiotensin II type 1 receptor blockers, angiotensin‐converting enzyme inhibitors, anxiety, beta‐blockers, ivabradine, sacubitril/valsartan, statins
Abbreviations
- 5‐HIAA
5‐hydroxyindoleacetic acid
- 5‐HT1A receptor
serotonin 1 A receptor
- ACE
angiotensin‐converting enzyme
- ACTH
adrenocorticotropic hormone
- ADHD
attention deficit hyperactivity disorder
- Ang
angiotensin
- ANP
atrial natriuretic peptide
- ARBs
angiotensin II receptor blockers
- ARDS
acute respiratory distress syndrome
- ARNI
angiotensin receptor‐neprilysin inhibitor
- ASCOT
Anglo‐Scandinavian Cardiac Outcomes Trial
- AT1A receptor
angiotensin II type 1A receptor
- AT1 receptor
angiotensin II type 1 receptor
- AT2 receptor
angiotensin II type 2 receptor
- BAI
Beck Anxiety Inventory
- BDNF
brain‐derived neurotrophic factor
- beta‐blockers
beta‐adrenergic receptor blockers
- BNP
brain natriuretic peptide
- BSTvl
ventrolateral bed nucleus of the stria terminalis
- CAD
coronary artery disease
- CHARM
Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity
- CNS
central nervous system
- CONSENSUS
Cooperative North Scandinavian Enalapril Survival Study
- CoQ‐10
coenzyme Q‐10
- COVID‐19
Coronavirus disease 2019
- CRH
corticotropin‐releasing hormone
- CSF
cerebrospinal fluid
- CVD
cardiovascular disease
- DOCA
deoxycorticosterone acetate
- EPM
elevated plus maze
- EUROPA
European trial on Reduction Of cardiac events with Perindopril among patients with stable coronary Artery disease
- GABA
gamma‐aminobutyric acid
- GAD
generalized anxiety disorder
- GPCRs
G‐protein‐coupled receptors
- HAM‐A
Hamilton Anxiety Rating Scale
- HF
heart failure
- HMG‐CoA
3‐hydroxy‐3‐methyl‐glutaryl ‐coenzyme ACoA
- HOPE
Heart Outcomes Prevention Evaluation
- HPA axis
hypothalamic‐pituitary‐adrenal axis
- HSCL
Hopkins Symptom Checklist
- HSD2
hydroxysteroid dehydrogenase‐2
- ICD
implantable cardioverter defibrillator
- IL
interleukin
- i.p.
intraperitoneal
- LDB
light‐dark box
- LDL
low‐density lipoprotein
- LIFE study
Losartan Intervention For Endpoint reduction in hypertension study
- l‐NAME
NG‐nitro‐l‐arginine methyl ester
- LPS
lipopolysaccharide
- LV
left ventricle
- MAPK
p38mitogen‐activated protein kinase
- MCP‐1
monocyte chemoattractant protein‐1
- MI
myocardial infarction
- MPTP
1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NEP
neutral‐endopeptidase
- NF‐κB
nuclear factor kappa B
- NK‐1
neurokinin type 1
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NYHA
New York Heart Association
- OFT
open field test
- PARADIGM HF
Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure
- PEACE
Prevention of Events with ACE inhibition
- PPARα
peroxisome proliferator activated receptor alpha
- PTSD
posttraumatic stress disorder
- PVN
paraventricular nucleus
- QOL
quality of life
- RALES
Randomized Aldactone Evaluation Study
- RAS
renin‐angiotensin system
- RHR
renal hypertensive rats
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SAVE
Survival and Ventricular Enlargement trial
- SD rat
Sprague–Dawley rat
- SERT
serotonin reuptake transporter
- SF‐36
36‐Item Short Form questionnaire
- SHIFT
Systolic Heart failure treatment with the If inhibitor ivabradine Trial
- SHRs
spontaneously hypertensive rats
- SNS
sympathetic nervous system
- SOLVD
Studies of Left Ventricular Dysfunction
- SP
substance P
- STAI
State‐Trait Anxiety Inventory
- TBI
traumatic brain injury
- TNF‐α
tumor necrosis factor alpha
- TRACE
Trandolapril Cardiac Evaluation study
- V‐HeFT II
Veterans Administration Cooperative Vasodilator–Heart Failure Trial II
- WAG/Rij rats
Wistar‐Albino‐Glaxo from Rijswijk rats
1. INTRODUCTION
The most frequent cardiovascular disorders, hypertension and coronary artery disease, and the most common mental disturbances, such as anxiety and depressive disorders, dramatically increase morbidity and health care financial burden. Depressive disorders were the third leading cause of disability, after back pain and headache in 2017. 1 The link between cardiovascular and mental disorders has been discussed for decades. Obviously, psychosocial stressors such as anxiety disorder, type A behavior, hostility, stress, or conflict situations stimulate autonomic nervous system and neurohumoral cascade in varying degrees. Activation of neural and humoral mechanisms supports the development of hypertension, endothelial dysfunction, and atherosclerotic vascular changes. A meta‐analysis focused on anxiety as a risk factor of cardiovascular diseases (CVDs) revealed that anxiety patients have increased risk of coronary artery disease, stroke, heart failure, and cardiovascular death. 2 Vice versa, the neurohumoral activation is accompanied by the formation of anxiety disorders such as generalized anxiety disorder (GAD), panic disorder, posttraumatic stress disorder (PTSD), obsessive‐compulsive disorder, as well as conditions arising during the therapy of accompanying mental conditions such as depression (Figure 1). 3 , 4 , 5
Figure 1.

The potential link between anxiety and cardiovascular pathologies. Anxiety disorder activates stress, neurohumoral cascade, serotonergic and gamma‐aminobutyric acid (GABA) pathways and increases free radical burden. 6 , 7 The activation of neural and humoral mechanisms supports the development of endothelial dysfunction, hypertension, diabetes mellitus, and target organ damage. Conversely, the cardiovascular pathologies associated with neurohumoral imbalance result in the formation of various anxiety disorders. 8 , 9 , 10 [Color figure can be viewed at wileyonlinelibrary.com]
There seems to be a bidirectional relationship between negative emotions and mental disturbances in the form of distress, anxiety, or depression and increased risk of CVDs. CVDs coincide with anxiety development, 8 , 9 , 10 whilst patients with anxiety are more prone to CVD 11 , 12 , 13 , 14 (Figure 1). Cardiac patients and patients with anxiety were observed to manifest similar symptoms such as nervousness, palpitations, apprehension, fatigue, breathlessness, headache, sweating, dizziness, or insomnia. 15 Both anxiety and hypertension or coronary artery disease are common occurrences in primary medical practice, and a diagnosis of anxiety can even predict future adverse cardiovascular events. Thus, a knowledge of mutual interference and causal relationship between anxiety and CVD could be of value for the early detection and treatment of these pathologies. 16
Several review articles have examined the effects of only selected groups of cardiovascular drugs on emotions, cognition, and behavior. In contrast, this review provides data on the relationship between anxiety and the most commonly used groups of cardiovascular drugs in current cardiovascular practice. Two other specifics of this review may be of value. First, each presented drug group is introduced by a brief overview of its cardiovascular indications based on the evidence‐based approach. Second, the interactions of cardiovascular drugs with symptoms of anxiety tend to be presented from the experimental level to clinical implications. The presentation of the mutual interactions of pathophysiological, psychological, and cardiovascular alterations provides a comprehensive view of this multidisciplinary medicinal problem.
2. METHODS
The electronic database PubMed/MEDLINE was used to search for the following terms: CVDs, anxiety, RAS (renin‐angiotensin system) in brain and cardiovascular drugs beta‐blockers, central sympatholytic drugs, angiotensin‐converting enzyme inhibitors, angiotensin II type 1 receptor blockers, angiotensin (1–7), aldosterone antagonists, angiotensin II type 1 receptor inhibitors, neprilysin inhibitors, statins, fibrates, ivabradine, calcium channel blockers, diuretics, vasodilators, antihypertensives and antiarrhythmics in association with anxiety. The experimental studies, clinical studies, clinical guidelines, reviews, and meta‐analyses in the full text and in English were included. Articles without a direct correlation between particular drugs and anxiety were excluded. Finally, 323 records from 1951 to 2021 were used.
3. ANXIETY
Stress is considered one of the principal factors in the development of anxiety and depression. 17 , 18 These mental disturbances are the result of an inappropriate adaptation to stress, with a causal role of the hypothalamic‐pituitary‐adrenal (HPA) axis and the sympathoadrenal medullary system releasing corticosteroids and catecholamines, respectively. However, in contrast to acute and chronic stress, anxiety disorders are considered diagnosable mental illnesses. 6 , 19
3.1. Pathophysiological background
It is widely accepted that an interplay of genetic, ontogenetic, and environmental factors plays a role in the pathogenesis of anxiety, while several systems seem to participate in its development, such as gamma‐aminobutyric acid (GABA) system, sympathetic nervous system (SNS), HPA axis, oxidative metabolism, nitric oxide and serotonergic system. 6 , 7
3.1.1. Sympathetic nervous system
The excessive sympathetic response to stressors may be an important link between anxiety and development of cardiovascular events. The somatic symptoms of anxiety such as tachycardia, palpitations, hyperventilation, headache, diarrhea, and tremulousness are associated with autonomic nervous system hyperactivity. 20 The chronically enhanced sympathetic drive results in increased systemic vascular resistance and contractility contributing to increased arterial blood pressure. Moreover, increased levels of catecholamines induce myocardial damage including coronary spasms, coronary ischemia, and arrhythmias. 21 Taken together, the overactivity of sympathetic outflow in anxiety could increase the risk of CVD.
3.1.2. Hypothalamic‐pituitary‐adrenal axis
The paraventricular nucleus (PVN) of the hypothalamus is a central point of the HPA stress response that contributes to anxiety. 22 In anxiety disorders, the corticotropin‐releasing hormone (CRH) is released from the hypothalamus and stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH) into the circulation and finally, corticosterone from the adrenal gland. 6 Glucocorticoids contribute to cardiac dysfunction by prolonging the action duration, increasing the sensitivity of catecholamines to the myocardium, and promoting contractility, arrhythmias, and apoptosis leading to increased arterial blood pressure, tachycardia, and myocardial damage. 21 Cortisol also increases the level of angiotensinogen, the pressor responsiveness to angiotensin II (Ang II), thus contributing to hypertension development. Cortisol excess also leads to metabolic changes such as obesity, increased levels of fasting plasma glucose, or insulin resistance. 23 Since anxiety activates the HPA axis and promotes the corticosterone release from adrenal glands, it eventually results in hypertension development and metabolic disorders. 24 , 25
3.1.3. Nitric oxide pathway
Nitric oxide (NO) is a gaseous free radical synthesized by nitric oxide synthase (NOS) in the presence of oxygen. 26 It has been demonstrated that neurons expressing neuronal NOS (nNOS) are located in brain areas involved in anxiety. 27 It appears that activation of nNOS may play an ambivalent, sex‐dependent role in anxiety development. 28 Moreover, stress‐induced increase of NO production in PVN activates the release of CRH and ACTH and thus, the HPA axis. 29
3.1.4. Serotonergic system
The serotonergic system is implicated in the regulation of emotion and anxiety. The stimulation of serotonin 1A (5‐HT1A) receptors produced anxiolytic effects in both humans and animals. 30 Moreover, serotonin activates the HPA axis and contributes to anxiety. 31
3.1.5. Gamma‐aminobutyric acid system
Anxiety is related to GABA‐ergic modulation in various areas of the brain. In general, the GABA receptor antagonists induce anxiogenic effects, while GABA receptor agonists reduce anxiety and stress responses. 32 It has been found that systemic, intracerebral, or intracerebroventricular injections of GABA‐antagonists induce hypertension and tachycardia probably due to an increased sympathetic outflow to the cardiovascular system 33 which exerts deleterious effects on the cardiovascular system.
3.1.6. Oxidative stress
The HPA axis activation and the release of corticosterone and Ang II along with the SNS activation induce oxidative stress in specific brain regions controlling anxiety and depression. The damage via free radicals found in experimental animals with anxiety‐like behavior may result in neuroinflammation and neurodegeneration. 34
3.2. Methodological approaches to quantification of the anxiety level
For the purpose of the current review, the methods related to anxiety determination in experimental (rodents) and clinical (humans) settings are to be elucidated.
3.2.1. Anxiety indices in rodents
Anxiety symptoms in humans and rodents are difficult to compare, although both groups share some common or similar behavioral responses such as freezing, hypoactivity, increased attention, or tachycardia. 35 In animals, the term “anxiety‐like behavior” is used to describe the manifestation of experiencing anxiety rather than a statement indicating that an animal is anxious. Therefore, a variety of anxiety behavioral assays have been developed. For the needs of this review, only some of them are presented; however, extensive reviews are available. 36 , 37 Naturally, rodents prefer dark and closed spaces, which decrease the risk of potential threat. The excessive avoidance of light, open space, and novel objects is considered as a sign of anxiety‐like behavior in rodents. In the light‐dark box (LDB) the latency to enter and the shorter time spent in the light part indicates anxiety‐like behavior. In the elevated plus maze (EPM) the latency to enter, the shorter time spent in, and the decreased number of entries in the open arms are used as anxiety indices. Regarding the open field test (OFT), the anxiety indices include the latency to enter and the shorter time spent in the center of the arena. 37
3.2.2. Anxiety measures in clinical conditions
In clinical practice, various measures indicating anxiety symptoms and their severity in patients have been developed. The State‐Trait Anxiety Inventory (STAI) measures the presence and severity of anxiety symptoms via self‐reporting. 38 The Beck Anxiety Inventory (BAI) is used as an indicator of anxiety focused on somatic symptoms. 38 The Hamilton Anxiety Rating Scale (HAM‐A) reflects the severity of perceived anxiety symptoms. 39 The Hopkins Symptom Checklist (HSCL) is a self‐report symptom inventory including anxiety. 40
4. RENIN‐ANGIOTENSIN SYSTEM IN THE BRAIN AND ITS INTERACTION WITH CENTRAL NERVOUS SYSTEM
The RAS regulates not only the function of the cardiovascular system but also plays an important role in the regulation of the central nervous system (CNS). Circulating Ang II does not penetrate the blood–brain barrier. 41 It has been proposed that Ang II is synthesized by the local RAS in the brain. 42 However, van Thiel and colleagues showed that there was no local Ang I generation in the brain, therefore, the brain Ang II might represent Ang II originating from the blood that accumulates in the brain through damaged blood–brain barrier, rather than locally synthesized Ang II. 43 Regardless of its origin, the brain Ang II participates in the regulation of blood pressure and body fluid volume (sodium appetite, vasopressin, ACTH, and aldosterone release). Moreover, brain Ang II interacts as a neurotransmitter with catecholamines, serotonin or prostaglandins, 44 regulates the cerebral blood flow, blood–brain barrier, brain development, stress response 45 and is involved in sensory perception and emotional behavior (Figure 2). 42
Figure 2.
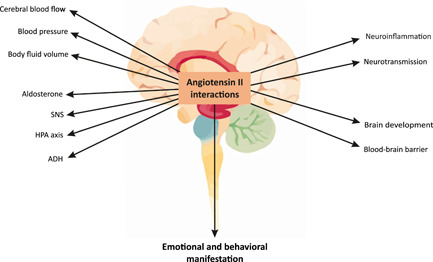
The role of brain angiotensin II. Angiotensin II in the brain modifies stress hormonal pathways, hemodynamic status in systemic and brain circulation, and structural and functional characteristics in the central nervous system. 44 , 45 All of these issues, individually or in concert, modulate the emotional and behavioral manifestation. 42 ADH, antidiuretic hormone; HPA axis, hypothalamic‐pituitary‐adrenal axis; SNS, sympathetic nervous system [Color figure can be viewed at wileyonlinelibrary.com]
Besides its regulatory role, brain Ang II may induce cerebrotoxicity via the enhancement of nicotinamide adenine dinucleotide phosphate (NADPH)‐oxidase activity, leading to the intracellular generation of reactive oxygen species activating redox‐sensitive signaling molecules, such as MAPKs (p38mitogen‐activated protein kinases), NH2‐terminal kinases, and extracellular signal‐regulated kinases 1 and 2. 19 In addition, Ang II‐induced enhancement of cellular and mitochondrial oxidative stress activates transcription factor nuclear factor kappa B (NF‐κB), promoting the production of inflammatory cytokines such as interleukin (IL)‐1β, IL‐6, and tumor necrosis factor alpha (TNF‐α) along with chemokines such as monocyte chemoattractant protein‐1 (MCP‐1). The result is the activation of an inflammatory response and apoptosis (Figure 2). 46
Psychological disorders are based on functional and structural disorders of neurons. Cerebral circulatory disorders due to cerebrovascular vasoconstriction and vessel remodeling, loss of vascular elasticity, impairment of autoregulatory mechanisms, or disorders of the blood–brain barrier are frequent causes of neuronal damage and dysfunction. 47 , 48 Another damaging factor is inflammation of the brain parenchyma due to the accumulation of inflammatory cytokines and hormones, activation of the microglia, as well as stress with consequent increased peripheral and cerebral sympathetic activity. 49 Structural and functional neuronal disorders lead to dual neuropsychiatric disorders:
-
1.
Affective, psychotic, or stress‐induced disorders being represented by somatic stress disorders, anxiety, PTSD, and depression, or autism and schizophrenia.
-
2.
Circulatory, traumatic, and neurodegenerative diseases in the form of vascular stroke, cognitive disability, Alzheimer's disease, Parkinson's disease, and others. 50
Because of the specific interaction of genetic equipment and the environment, there is an allostatic burden with consequent neuronal dysfunction and neuropsychiatric disorders. RAS appears to play a significant role in the pathogenesis and treatment of these disorders. 45
4.1. The brain angiotensin II in association with stress and anxiety
Overactivation of the brain pressor axis of the RAS has been implicated in the etiology of stress‐associated anxiety disorders. 19 Stressors activate renin release, increasing the production of β‐adrenergic receptors with enhanced peripheral RAS activity and increased production and release of Ang II in both the circulation and the brain. 51 The stimulation of angiotensin II type 1A (AT1A) receptors in the PVN of the hypothalamus leads to the release of CRH with the activation of HPA axis, resulting in stress‐associated anxiety. 51 However, it has been shown that low doses of Ang II injected bilaterally into the hippocampal CA1 area of male Sprague–Dawley (SD) rats 52 or intracerebroventricularly in male Wistar rats 53 , 54 attenuated anxiety, while higher acute intracerebroventricular doses in male Wistar rats 55 or chronic subcutaneous administration in C57BL6 mice 56 induced anxiety‐like behavior. Thus, the neuropsychiatric effect of Ang II is obviously dose‐dependent.
5. CARDIOVASCULAR DRUGS AND ANXIETY
A number of experimental and clinical evidence indicates that cardiovascular drugs interfering with the autonomic nervous system or the renin‐angiotensin‐aldosterone system along with several other groups of cardiovascular drugs such as statins or fibrates modulate the level of anxiety and related behavior in both experimental animals and humans.
5.1. Sympathetic nervous system and anxiety
5.1.1. Beta‐adrenergic receptor blockers
Beta‐adrenergic receptor blockers (beta‐blockers), which compete at the receptor level with catecholamines and attenuate the impact of the SNS, are used in a number of cardiovascular pathologies. Beta‐blockers (along with diuretics) were the first drugs supplying the evidence that reduction of hypertension reduces cardiovascular events and mortality. 57 , 58 In the secondary prevention of coronary artery disease (CAD), beta‐blockers reduce morbidity and mortality in patients with a recent myocardial infarction (MI) 59 or after percutaneous revascularization. 60 In the primary prevention within stable CAD, beta‐blockers remain the principal treatment for symptomatic relief. 61 Some beta‐blockers (carvedilol, metoprolol, bisoprolol, and nebivolol) are a cornerstone treatment in heart failure patients with systolic dysfunction. 61 , 62 , 63 , 64 Beta‐blockers are indicated also in various dysrhythmias. 65 , 66
5.1.1.1. Clinical studies
The idea of using beta‐blockers in the treatment of mental disorders began in the 1960s, when propranolol, a β1,2‐antagonist, seemed to be beneficial in managing the physical symptoms of anxiety, especially cardiovascular complaints 67 with tachycardia, palpitations, chest pain, physiological tremor, and a compulsion to over breathe under stress. 68 The anxiolytic effect of beta‐blockers has been studied in panic disorders, 69 , 70 specific phobias, 71 , 72 social phobias, 73 or PTSDs. 74 , 75 , 76 , 77 , 78 The use of propranolol after experiencing 79 or recalling 80 traumatic events reduced the symptoms of anxiety and PTSD. Metoprolol lowered the anxiety score in chronic heart failure patients 81 and atenolol reduced PTSD and anxiety symptoms in patients with mental health problems. 82 There is even some evidence of an anxiolytic effect of beta‐blockers in healthy individuals 83 (Table 1).
Table 1.
The effects of cardiovascular drugs on anxiety
| Drug | Model | Test | Effect | Refs. |
|---|---|---|---|---|
| Beta‐blockers | ||||
| Experimental studies | ||||
| Propranolol, nadolol, bisoprolol | Sprague–Dawley rats, social defeat | LDT, EPM | Anxiolytic (propranolol and nadolol, not bisoprolol) | Zaidi et al. 84 |
| Propranolol | ArcCreERT2 × eYFP mice, 129S6/SvEv mice, 4‐shock contextual fear conditioning followed by immediate or delayed context re‐exposures | Contextual Fear Conditioning, Cued Fear Conditioning, Context Fear Discrimination, EPM, OFT | Reduced fear, traumatic memory | Leal Santos et al. 78 |
| Propranolol | Male C57BL/6 mice, repeated social defeat | LDT | Anxiolytic | Wohleb et al. 85 |
| Clinical studies | ||||
| Propranolol | Patients with anxiety | Interview on a Five‐Point Scale | Anxiolytic | Granville‐Grossman and Turner 67 ; Kelly 68 |
| Propranolol | Patients with panic disorders | Panic and Anxiety Attack Scale, Marks‐Sheehan Phobia Scale, HAM‐A, Symptom Checklist Scale | Anxiolytic | Ravaris et al. 69 |
| Propranolol | Patients with specific phobias | Autonomic Perception Questionnaire, Self‐Reported Anxiety | Anxiolytic | Fagerström et al. 71 ; Liu et al. 72 |
| Propranolol | Patients with social phobias | Measures of specific fears, generalized social anxiety, self‐image, and global tension and anxiety | Anxiolytic | Falloon et al. 73 |
| Propranolol | Patients with PTSD | Script‐Driven Mental Imagery of Traumatic Event, Revised Children's Manifest Anxiety Scale | Anxiolytic | AlOkda et al. 74 ; Brunet et al. 75 ; Giustino et al. 76 ; Rosenberg et al. 77 |
| Propranolol | Healthy humans | Differential Fear‐Conditioning Procedure | Anxiolytic | Kindt et al. 83 |
| Propranolol | Subclinical population | Patient Health Questionnaire, Personal Report of Public Speaking Anxiety, Structured Clinical Interview for DSM‐5 social anxiety disorder | Public speaking anxiety decreased in questionnaire measures | Elsey et al. 79 |
| Propranolol | Children with PTSD symptoms | Child PTSD symptom scale, Children's Depression Inventory | PTSD symptoms decreased | Thierrée et al. 80 |
| Celiprolol | Patients with mitral valve prolapse syndrome | HADS | Anxiolytic | Bachmann et al. 70 |
| Metoprolol | Patients with chronic heart failure | HADS | Anxiolytic, increase in depression score | Wu et al. 81 |
| Atenolol | Patients with PTSD symptoms | Researcher's questionnaire | PTSD symptoms decreased | Armstrong and Kapolowicz 82 |
| Alpha antagonists | ||||
| Experimental studies | ||||
| Prazosin | Alcohol‐naïve male P rats, restraint stress | Social Approach/Avoidance Test, EPM | Suppression of stress‐induced anxiety during subsequent alcohol deprivation | Rasmussen et al. 87 |
| Prazosin | Sprague–Dawley rats, predator scent stress | EPM | Anxiolytic in traumatized rats, anxiogenic in controls | Ketenci et al. 88 |
| Prazosin, clonidine, yohimbine | Wistar rats, predator scent stress | OFT, EPM | Anxiolytic (prazosin and clonidine, not yohimbine) | Aykac et al. 89 |
| Clinical studies | ||||
| Prazosin | Active‐duty soldiers with PTSD | DSM‐IV criteria for PTSD | Reduced PTSD symptoms | Hendrickson et al. 90 |
| Prazosin | Inpatient children and adolescents with PTSD nightmares | Chart review and ICD code | Nightmare resolution | Hudson et al. 91 |
| Prazosin | Oncological patient | Self‐report for nightmares | Nightmare resolution | Santivasi et al. 92 |
| Prazosin | Patients with alcohol use disorder | PROMIS Anxiety, Depression and Anger T scores, STAI, BDI | Anxiolytic | Wilcox et al. 93 |
| Prazosin | Patients with alcohol use disorder | 10‐point visual analog scale, psychophysiological correlation of anxiety | Anxiolytic in alcohol que‐induced anxiety | Milivojevic et al. 94 |
| Central sympatholytic drugs | ||||
| Experimental studies | ||||
| Methyldopa | Wistar rats, Koletsky SHR | EPM | Anxiolytic in hypertensive rats | Golda and Petr 95 |
| Clonidine | Sprague–Dawley rats | Fear conditioning, Fear‐potentiated startle test, Sensitization by foot shocks, Light‐enhanced startle | Anxiolytic | Schweimer et al. 96 |
| Guanfacine | C57BL/6J mice | LDT, TST, FST, Locomotor activity | Anxiolytic | Mineur et al. 97 |
| Clinical studies | ||||
| Clonidine | Patients with anxiety | HAM‐A, Global Rating of Neurotic Symptoms, Global Rating of Somatic Symptoms, Global Rating of Persistent Anxiety, Global Rating of Anxiety Attacks, STAI, Somatic Symptoms Scale, Affects Balance Scale | Anxiolytic | Hoehn‐Saric et al. 98 |
| Guanfacine | Critically ill postoperative patient | Richmond Agitation Scale Score | Anxiolytic | Srour et al. 99 |
| Guanfacine | Children and adolescents with ADHD and PTSD | UCLA PTSD Reaction Index, GAD scale of Screen for Childhood Anxiety and Related Disorders, Columbia Impairment Scale, ADHD Rating Scale‐IV, clinician‐completed Clinical Global Impressions Severity Scale | Anxiolytic | Connor et al. 100 |
| Guanfacine | Children and adolescents with GAD, separation anxiety disorder, and/or social anxiety disorder | Dimensional anxiety scales: Pediatric Anxiety Rating Scale and Screen for Child Anxiety Related Emotional Disorders; Clinical Global Impression‐Improvement (CGI‐I) scale | Improvement in CGI‐I | Strawn et al. 101 |
| Clonidine, guanfacine | Patients with PTSD | NA | Anxiolytic, attenuated agitation, and hyperarousal | Belkin and Schwartz 102 |
| Angiotensin‐converting enzyme inhibitors | ||||
| Experimental studies | ||||
| Captopril | Doxorubicin‐treated Wistar rats | OF, EPM, LDB | Anxiolytic | Aziriova et al. 103 |
| Lisinopril | SHR | OF | Anxiolytic | Repova et al. 104 |
| Enalapril, losartan | RHR | OF | Anxiolytic, reduced hyperactivity | Srinivasan et al. 105 |
| Electroacupuncture, candesartan, perindopril | SHR with chronic cerebral hypoperfusion | OFT, NOR, MWM | Anxiolytic, improved memory | Feng et al. 106 |
| Egg white‐derived peptides TNGIIR and RVPSL | SHR | EPM | Anxiolytic | Yu et al. 107 |
| Clinical studies | ||||
| Captopril | Patients with CVD | NA | Elevated mood | Zubenko and Nixon 108 |
| Enalapril, captopril | Hypertensive patients | BDI, HSCL | Reversed depression and anxiety | Braszko et al. 109 |
| Angiotensin II type 1 receptor blockers | ||||
| Experimental studies | ||||
| Losartan | Bilaterally olfactory bulbectomized rats | EPM | Anxiolytic | Tashev and Ivanova 110 |
| Losartan | Female Long Evans rats, ovariectomy | EPM, OFT, NOR | Anxiolytic, improved memory | Campos et al. 111 |
| Losartan | Male BALB/c mice, LPS inflammation | MWM, NOR, passive avoidance, FST, EPM, marble burying task | Anxiolytic, improved learning and memory | Salmani et al. 112 |
| Candesartan | Wistar rats | EPM | Anxiolytic | Saavedra et al. 113 |
| Candesartan | SHR, LPS inflammation | MWM | Reduced memory impairment | Goel et al. 114 |
| Candesartan | Wistar Hannover rats, SHR, LPS inflammation | In vitro studies | Reduced brain inflammation | Benicky et al. 115 |
| Candesartan | Wistar Hannover rats, LPS inflammation, restraint stress | In vitro studies | Prevented LPS and restraint stress impact on CNS | Sánchez‐Lemus et al. 116 |
| Candesartan | Sprague–Dawley rats, transient focal cerebral ischemia | In vitro studies | Protection from brain ischemia | Singh et al. 117 |
| Candesartan | Sprague–Dawley rats | EPM, FST, novelty‐suppressed feeding test | Anxiolytic, antidepressant | Gong et al. 118 |
| Electroacupuncture, candesartan, perindopril | SHR, chronic cerebral hypoperfusion | OFT, NOR, MWM | Anxiolytic, improved memory | Feng et al. 106 |
| Irbesartan | Swiss albino mice, unpredictable chronic mild stress | Modified FST, TST, OFT | Anxiolytic, antidepressant | Ayyub et al. 119 |
| Telmisartan | C57BL/6N mice, C57BL/6J DIO mice, high fat diet | OFT, EPM | Anxiolytic | Huber et al. 120 |
| Clinical studies | ||||
| Valsartan | Anxiety‐naïve patient | Subjective anxiety symptoms of generalized type | Anxiolytic | Shad 121 |
| ARBs | Highly traumatized civilian medical population | PTSD Symptom Scale, Clinician‐Administered PTSD Scale | Decreased PTSD symptoms | Khoury et al. 122 |
| ARBs | Hypertensive patients | WMS‐R Logical Memory II subtest, Rey Auditory Verbal Learning Test, Wechsler Adult Intelligence Scale, Trail Making Tests A and B, Animal Fluency, Vegetable Fluency, Boston Naming Test | Improved memory | Ho et al. 123 |
| Angiotensin‐(1–7) | ||||
| Experimental studies | ||||
| i.c.v. Ang‐(1–7) | (mRen2)27 hypertensive rats | EPM | Anxiolytic | Almeida‐Santos et al. 124 |
| i.c.v. Ang‐(1–7) | Wistar rats | EPM | Anxiolytic | Bild and Ciobica 125 |
| i.v./i.c. Ang‐(1–7) | Wistar rats exposed to air‐jet stress | Blocked tachycardia and pressor response | Martins Lima et al. 126 ; Oscar et al. 127 | |
| NA | ACE2 knock‐in mice | EPM | Anxiolytic | Wang et al. 128 |
| NA | Transgenic rats TGR(A1–7)3292 overexpressing Ang‐(1–7) | EPM | Anxiolytic | Kangussu et al. 129 ; Moura Santos et al. 130 |
| NA | Transgenic rats TGR(A1–7)3292 overexpressing Ang‐(1–7) exposed to air‐jet stress | Reduced HR, reduced basal activity in renal sympathetic outflow | Moura Santos et al. 130 | |
| Aldosterone antagonists | ||||
| Experimental studies | ||||
| Spironolactone | Streptozotocin‐induced diabetic rats | Burying behavior test | Anxiolytic | López‐Rubalcava et al. 131 |
| Spironolactone | Sprague–Dawley rats, social defeat stress and mild traumatic brain injury | EPM | Anxiolytic | Fox et al. 132 |
| Eplerenone | Wistar rats | OF, EPM | Anxiolytic | Hlavacova and Jezova 133 |
| Clinical studies | ||||
| Spironolactone | Patients with primary hyperaldosteronism | SF‐36 questionnaire | Improved quality of life | Ahmed et al. 134 |
| ARNI | ||||
| Clinical studies | ||||
| Sacubitril/valsartan | HFrEF patients | Association between NYHA functional class and endorphin peptides | Improvement of patients' symptoms | Revuelta‐López et al. 135 |
| Sacubitril/valsartan | HFrEF patients | BDI‐II, BAI | Relief of depression and anxiety symptoms | Dereli et al. 136 |
| Statins | ||||
| Experimental studies | ||||
| Simvastatin | C57BL/6J mice | MWM, NOR, OFT, rotarod test, EPM | No effect on anxiety, impaired recognition, and spatial memory | Guo et al. 137 |
| Simvastatin | Sprague–Dawley rats | FST, EPM | Anxiolytic, antidepressant | Kilic et al. 138 |
| Atorvastatin | MPTP‐lesioned C57BL/6 mouse model of Parkinson's disease | TST, EPM | Anxiolytic, antidepressant | Yan et al. 139 |
| Atorvastatin, simvastatin | Wistar albino rats, methionine‐enriched diet with restricted vitamins B intake | OFT, EPM | Anxiolytic | Mijailovic et al. 140 |
| Atorvastatin, simvastatin, pravastatin | Wistar Albino Glaxo/Rijswijk rats, model of absence‐type epilepsy, epileptogenesis and low‐grade depression | FST, OF | Anxiolytic, antidepressant | Citraro et al. 141 |
| Rosuvastatin | Female Balb/c mice, chronic Toxoplasma gondii infection | OFT, NOR | Anxiolytic, improved memory | Evangelista et al. 142 |
| Simvastatin, rosuvastatin | Wistar rats | OF, EPM, MWM | Increased anxiety, impaired learning, and memory | Okudan and Belviranli 143 |
| Clinical studies | ||||
| Simvastatin | Patients with GAD | HAM‐A | No support for efficacy in GAD | Mirzaei et al. 144 |
| Statins | Humans | Adverse drug reaction reporting, nonadherence | Anxiety, depression, aggression, suicidal tendency | Tatley a Savage 145 ; Cham et al. 146 ; Golomb et al. 147 ; Duits a Bos 148 ; Korhonen et al. 149 |
| Statins | Swedish population aged 15 years or older | Neuropsychiatric outcomes: self‐injurious behavior or suicide attempt, death from suicide, depressive disorders, anxiety disorders, seizures | Reduced risk of depression, no effect on anxiety disorder | Molero et al. 150 |
| Fibrates | ||||
| Experimental studies | ||||
| Fenofibrate | NMRI mice, pentylenetetrazole‐induced kindling seizure | EPM | Anxiolytic | Sarahian et al. 151 |
| Fenofibrate | Wistar rats, propionic acid‐induced autism spectrum disorder | EPM | Anxiolytic | Mirza and Sharma 152 |
| Fenofibrate | Wistar rats, valproic acid‐induced autism spectrum disorder | EPM | Anxiolytic | Mirza and Sharma 153 |
| Endocannabinoid congener N‐palmitoylethanolamide | Swiss‐Webster mice, social isolation, contextual fear conditioning | EPM, OF, FST, TST | Anxiolytic, antidepressant | Locci and Pinna 154 |
| Ivabradine | ||||
| Experimental studies | ||||
| Ivabradine | Wistar rats | Phenotyper, OF, EPM, LDB, NOR | No disturbing effects on anxiety, locomotion, or learning | Aziriova et al. 155 ; Krajcirovicova et al. 156 |
| Ivabradine | Wistar rats, l‐NAME‐induced hypertension | Phenotyper | No disturbing effects on anxiety, locomotion, or learning | Aziriova et al. 155 |
| Clinical studies | ||||
| Ivabradine | CHF patients | SF‐36 questionnaire, European quality of life‐5 dimensions | Improved quality of life | Riccioni et al. 157 ; Zugck et al. 158 |
| Ivabradine | Patients with chronic stable angina | SF‐36 questionnaire | Improved quality of life | Riccioni et al. 159 |
| Calcium channel blockers | ||||
| Experimental studies | ||||
| Nifedipine, verapamil | Mice | Conditioned suppression of the motility test, the black and white box test | Anxiolytic (nifedipine in low dose), anxiogenic (nifedipine, verapamil in high dose) | Fulga and Stroescu 160 |
| Amlodipine | ICR mice, social defeat stress | EPM, TST | Anxiolytic, antidepressant | Joseph et al. 161 |
| Clinical studies | ||||
| Nifedipine | Phobic patients | Baseline anxiety ratings | No anxiolytic effect | Klein et al. 162 |
| Diuretics | ||||
| Experimental studies | ||||
| Furosemide, bumetanide | Long‐Evans rats | Contextual fear conditioning, fear‐potentiated startle, EPM, OFT | Anxiolytic effect on conditioned anxiety, no anxiolytic effect on unconditioned anxiety | Krystal et al. 163 |
| Vasodilators | ||||
| Experimental studies | ||||
| Nitroglycerin | Wistar rats, nitroglycerin‐induced migraine | Modified EPM, LDB | Anxiogenic | Farajdokht et al. 164 |
| Nitroglycerin | Wistar rats, nitroglycerin‐induced migraine | EPM, OFT, NOR | Anxiogenic, decreased locomotion, impaired spatial learning, and memory | Taheri et al. 165 |
| ICD | ||||
| Clinical studies | ||||
| ICD | Adults with an ICD | Depressive and anxiety disorders | Magyar‐Russell et al. 166 | |
Abbreviations: ACE2, angiotensin‐converting enzyme 2; Ang‐(1–7), angiotensin‐(1–7); ARNI, angiotensin receptor‐neprilysin inhibitor; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CGI‐I, Clinical Global Impression‐Improvement; CHF, chronic heart failure; CVD, cardiovascular disease; EPM, elevated plus maze; FST, forced swim test; GAD, generalized anxiety disorder; HADS, Hospital Anxiety and Depression Scale; HAM‐A, Hamilton Anxiety Rating Scale; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; HSCL, Hopkins Symptom Checklist; i.c.v., intracerebroventricular; ICD, implantable cardioverter‐defibrillator; LDB, light‐dark box test; LDT, light/dark test; l‐NAME, l‐NG‐Nitro arginine methyl ester; LPS, lipopolysaccharides; MWM, Morris water maze; NA, not applicable; NOR, novel object recognition test; NYHA, New York Heart Association; OF, open field test; PTSD, posttraumatic stress disorder; RHR, renal hypertensive rats; SF‐36 questionnaire, 36‐Item Short Form Survey; SHR, spontaneously hypertensive rats; STAI, State‐Trait Anxiety Index; TG, transgenic. TST, tail suspension test; UCLA, University of California at Los Angeles.
5.1.1.2. Experimental studies
SD rats pretreated with propranolol or nadolol spent more time in the lit area of the LDB and in the open arms of the EPM after social defeat, suggesting anxiolytic‐like behavior. 84 The pretreatment of mice with propranolol before social defeat reduced anxiety‐like behavior with increased time to enter the dark zone and decreased total time spent in the dark zone in the LDB. 85 However, the results of these studies are not equivocal and vary from hopeful to negative. According to a meta‐analysis performed by Steenen et al., 86 there is a lack of evidence that could confirm or exclude the beneficial impact of beta‐blockers in the treatment of anxiety disorders 86 (Table 1).
5.1.2. Alpha antagonists
Prazosin, an alpha‐1 antagonist, reduces blood pressure by direct dilation of the peripheral arteries. 167
5.1.2.1. Experimental studies
Prazosin increased the time spent in the open arms of the EPM after alcohol deprivation in alcohol‐naïve P rats. 87 In a rat model of PTSD induced by the predator scent test, prazosin promoted anxiolytic‐like behavior measured as decreased anxiety indices in the EPM. 88 , 89 On the contrary, in non‐stressed rats, prazosin decreased open arm entry in the EPM, suggesting anxiety‐like behavior in non‐traumatized individuals 88 (Table 1).
5.1.2.2. Clinical studies
The beneficial effect of prazosin in the treatment of PTSD and related sleep disorders, including nightmares, has been acknowledged. 90 , 91 , 92 Prazosin reduced subjective anxiety to high‐threat stimulus in patients with alcohol use disorder 93 and reduced stress‐ and alcohol cue—provoked anxiety in abstinent patients accompanied with decreased cortisol after stress cue 94 (Table 1).
5.1.3. Central sympatholytic drugs
Methyldopa, clonidine, and guanfacine are alpha‐2 agonists that decrease sympathetic outflow on the level of the CNS. Their original indication was the treatment of hypertension, 168 , 169 , 170 however, currently these drugs are being tested and starting to be used in different indications.
5.1.3.1. Methyldopa
The experimental and clinical data regarding the effect of methyldopa on anxiety are sparse.
5.1.3.2. Experimental study
Methyldopa administration in normotensive rats reduced the number of entries to the center and time spent in the open arms of the EPM, suggesting anxiety‐like behavior, while in hypertensive rats, methyldopa had the opposite effect. 95 There is a possibility, that like prazosin, methyldopa has different effects on anxiety in health and disease 95 (Table 1).
5.1.3.3. Clonidine
Clonidine is now being used for severe pain relief 171 and attention deficit hyperactivity disorder (ADHD) treatment. 172
5.1.3.4. Experimental study
Clonidine injections in the bed nucleus of the stria terminalis decreased learned and unlearned (anxiety) fear in rats 96 (Table 1).
5.1.3.5. Clinical studies
In patients with GAD and panic disorder, clonidine decreased the frequency of anxiety attacks and mental symptoms. 98 In patients with PTSD, clonidine relieved symptoms of agitation and hyperarousal 102 (Table 1).
5.1.3.6. Guanfacine
Guanfacine is currently indicated for ADHD treatment. 173
5.1.3.7. Experimental study
Guanfacine increased the time spent in the light compartment of the LDB which is a sign of anxiolytic‐like behavior in mice. Its possible mechanism includes activation of alpha2‐adrenergic receptors that decrease neuronal activity in amygdala 97 (Table 1).
5.1.3.8. Clinical studies
In a patient after cardiac surgery, guanfacine therapy effectively attenuated agitation and anxiety that was uncontrollable by conventional therapies. 99 In pediatric patients suffering from PTSD, 100 GAD, separation anxiety disorder, and social anxiety disorder, guanfacine extended‐release, was well‐tolerated and lead to global improvements 101 (Table 1).
5.2. Modification of renin‐angiotensin‐aldosterone system and anxiety
Recently, attention has been focused on the role of brain RAS and on the potential therapeutic benefit of blocking this neurohumoral system. In animal models, inhibition of the angiotensin II type 1 (AT1) receptor in the brain by angiotensin II receptor blockers (ARBs) or inhibition of Ang II formation by angiotensin‐converting enzyme (ACE) inhibitors exhibits neuroprotective effects, reduces stress response acceleration and anxiety, alleviates chronic cerebrovascular inflammation and reduces acute inflammatory response. 174 The ultimate consequence is the protection of neurons from structural damage, which may be responsible for improving cognitive functions in the brain and alleviating anxiety. 110 , 114 The meta‐analysis by Brownstein et al. 175 showed that the subjects receiving ACE inhibitors or ARBs presented better scores in the positive well‐being, mental, and anxiety domains of the Quality of Life Questionnaire.
5.2.1. Angiotensin‐converting enzyme inhibitors
ACE inhibitors reduce the level of Ang II by the blockade of ACE converting Ang I to Ang II. Thus, reduction of preload, afterload, and growth‐promoting and proliferating effect of angiotensin II is attenuated, resulting in hypotensive and anti‐remodeling effects in the heart and vascular wall. 176 , 177 , 178 , 179 , 180 , 181 , 183 Indeed, ACE inhibitors not only reduce blood pressure, hospitalizations, cardiovascular events, and death in hypertensive patients when compared with diuretics and/or beta‐blockers. 184 , 185 In the 90s, ACE inhibitors become a principal treatment of systolic heart failure (HF) with (SAVE, Survival and Ventricular Enlargement trial 186 ; TRACE, Trandolapril Cardiac Evaluation study 187 ) or without (CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study 188 ; V‐HeFT II, Veterans Administration Cooperative Vasodilator–Heart Failure Trial II 189 ; SOLVD, Studies of Left Ventricular Dysfunction 190 , 191 ) previous MI, improving survival. About 10 years later, ACE inhibitors were introduced in high‐risk patients to reduce complications of atherosclerosis and mortality (HOPE, Heart Outcomes Prevention Evaluation; EUROPA, European trial on Reduction Of cardiac events with Perindopril among patients with stable coronary Artery disease; PEACE, Prevention of Events with ACE inhibition). 192 Besides cardiovascular protection, ACE inhibitors were shown to reduce anxiety‐related behavior in rodent models and anxiety in clinical conditions (Figure 3).
Figure 3.
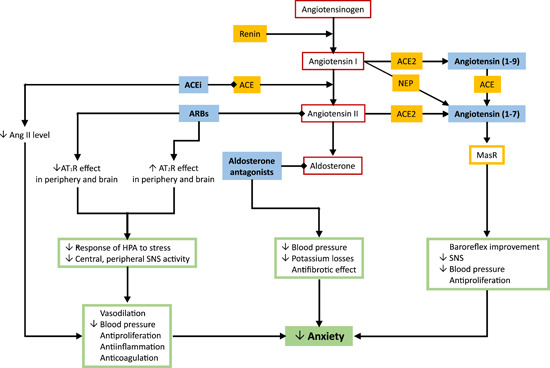
Modification of the renin‐angiotensin‐aldosterone system and its effect on anxiety.
The inhibition of the renin‐angiotensin system in the brain by angiotensin II type 1 receptor blockers (ARBs) or attenuating angiotensin II (Ang II) formation via angiotensin‐converting enzyme inhibitors (ACEi) exhibits neuroprotective effects and reduces the level of the stress response and anxiety. 103 , 104 , 109 , 192 The possible mechanisms underlying the anxiolytic effect of ARBs and ACEi include the upregulation of the Ang II type 2 receptor (AT2R) in the brain, and the enhancement of angiotensin (1–7) production acting on Mas receptors (MasR). 193 The stimulation of both AT2R by Ang II and MasR by angiotensin (1–7) is considered to protect the cardiovascular system via vasodilation and antiproliferative effects 194 , 195 while exerting anxiolytic effects. Similarly, aldosterone antagonists reduce hemodynamic burden, potassium losses, profibrotic effects, 196 , 197 and anxiety level. 132 , 133 ACE, angiotensin‐converting enzyme; AT1R, angiotensin II type 1 receptor; HPA, hypothalamic‐pituitary‐adrenal axis; NEP, neutral‐endopeptidase; SNS, sympathetic nervous system [Color figure can be viewed at wileyonlinelibrary.com]
5.2.1.1. Experimental studies
ACE inhibitor captopril exerted anxiolytic‐like effect in doxorubicin‐treated rats in a preventive experiment 103 and lisinopril reversed the alterations in terms of anxiety‐like behavior in spontaneously hypertensive rats (SHRs). 104 Analogically, egg white‐derived peptides TNGIIR and RVPSL that have ACE inhibitory activity, exerted an anxiolytic‐like effect in SHRs in the EPM. 107 Renal hypertensive rats (RHR) showed hyperactivity in OFT, and anxiety‐like behavior in the EPM. Treatment with enalapril and losartan significantly decreased the observed hyperactivity and anxiogenicity in RHR. 105 Perindopril increased the time spent in the central zone in the OFT, suggesting anxiolytic‐like behavior and improved scores in the novel object recognition test, thus representing improved memory and learning in SHR with chronic cerebral hypoperfusion 106 (Table 1).
5.2.1.2. Clinical studies
The mood‐modulating effect of captopril in humans has been described in 1984 by Zubenko and Nixon. They observed that captopril treatment due to another CVD elevated mood in patients with depressive symptoms. 108 In hypertensive patients, enalapril and captopril reversed depression and anxiety assessed by the Beck Depression Inventory and the Hopkins Symptom Checklist. 109 In another study of patients with anxiety or panic with stress‐induced hypertension, the anti‐anxiety effect of sublingual captopril was similar to diazepam 199 (Table 1).
5.2.2. Angiotensin II type 1 receptor blockers
ARBs reduce the effect of Ang II by blockade of AT1 receptors. Differently to ACE inhibitors, ARBs do not stimulate bradykinin production, thus partly avoiding side effects such as cough or angioedema. Similarly to ACE inhibitors, ARBs reduce hemodynamic burden and exert antiremodeling effect. 181 , 200 , 201 In the LIFE (Losartan Intervention For Endpoint reduction in hypertension) study with hypertensive patients, losartan exerted regression of left ventricular (LV) hypertrophy and reduction of cardiovascular events. 202 In heart failure CHARM (Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity) study, candesartan improved survival compared to placebo and co‐treatment with candesartan and ACE inhibitors dominated over ACE inhibitors alone. 203 Moreover, in several heart failure trials, ARBs were equally effective compared to ACE inhibitors with fewer side effects. 204
Analogically to ACE inhibitors, the pleiotropic nature of ARBs is projected into neuroprotective and mood modifying effects (Figure 3). Some preclinical and clinical studies with ARBs indicated the reduction of stress and anxiety in both rodents and humans. 193
5.2.2.1. Experimental studies
Losartan showed anxiolytic‐like behavior by increasing the number and time of open arms entries, the ratio of open/total entries and open/total time and decreasing the number and time of closed arm entries of the EPM in olfactory bulbectomized rats 110 and by the increased number of entries and percentage of time spent in the open arms of the EPM and increased time spent in the center of the OFT in ovariectomized Long Evans rats. 111 After i.p. lipopolysaccharide (LPS) injection in mice, losartan pretreatment improved memory impairment, increased the number of entries and time spent in open arms of the EPM, and decreased marble‐burying. 112 Irbesartan increased time spent in the center of the OFT after unpredictable mild stress in mice. 119 Candesartan reduced anxiety represented by increased time spent in and the number of entries to the open arm of the EPM in rats, 113 increased time spent in the central zone of the OFT in SHR with chronic cerebral hypoperfusion and 106 increased time spent in and entries to the open arm of the EPM in LPS‐induced neuroinflammation in SD rats. 118 Candesartan reduced memory impairment induced by LPS in SHRs 114 and in SHR with chronic cerebral hypoperfusion, 106 improved LPS‐induced brain inflammation in Wistar Hannover rats and SHRs 115 and also protected from ischemia in the SD rat's brain. 117 Telmisartan reduced anxiety‐like behavior in diet‐induced obesity in mice 120 (Table 1).
5.2.2.2. Clinical studies
ARBs have decreased PTSD symptoms in the highly traumatized civilian medical population 122 and may be associated with a decreased risk of mood disorders. 205 Hypertensive patients using ARBs were found to have better‐preserved memory. 123 The repeated onset of anxiety has been described in a patient after the discontinuation of valsartan therapy, while restarted valsartan treatment relieved anxiety symptoms completely 121 (Table 1).
All these actions of ARBs on mood and cognition are unrelated to cardiovascular effects. The possible mechanisms underlying the anxiolytic effect of ARBs include presumably the upregulation of angiotensin II type 2 (AT2) receptors in the brain, 194 while AT2 receptors exert antiproliferative, antioxidative, and anti‐inflammatory action. 195 Candesartan prevented alterations in cortical benzodiazepine 1 receptors that were under AT1 receptor control in stress. 116 Losartan reduced markers of brain inflammation and oxidative stress, 112 attenuated the response of the HPA axis to stress and prevented cortical alterations via corticotrophin‐releasing factor receptor 1 and benzodiazepine binding. 7 Valsartan decreased the activity of HPA axis and central and peripheral SNS activity in rats subjected to a forced swim stress 206 and restored hippocampal neurogenesis by upregulating the level of brain‐derived neurotrophic factor (BDNF) protein in the brain (Figure 3). 207
5.2.3. Angiotensin‐(1–7)
Angiotensin‐(1–7) is formed by hydrolysis of Ang II by ACE2, carboxypeptidases, and prolyl‐endopeptidases and by hydrolysis of Ang I by neutral‐endopeptidase (NEP), prolyl‐endopeptidase, and tymeth‐oligopeptidase. Mas receptors mediate the actions of Ang‐(1–7) in the CNS and peripheral tissues. 208 , 209 In the CNS, Ang‐(1–7) induces various cardiovascular, metabolic, and non‐cardiovascular effects. 196 , 210 Chronic administration of Ang‐(1–7) facilitated baroreflex bradycardia at the nucleus tractus solitarii in Wistar rats, 211 reduced cardiac sympathetic tone in fructose‐fed rats 212 and attenuated hypertension in deoxycorticosterone acetate (DOCA)–salt rats, 213 hypertensive transgenic (mRen2)27 rats 214 or Ang II‐induced hypertension in SD rats (Figure 3). 215
5.2.3.1. Experimental studies
Non‐cardiovascular effects of Ang‐(1–7) include attenuation of anxiety‐like behavior that has been demonstrated in several experimental studies. The activation of AT2 and Mas receptors in the medial amygdaloid nucleus indicates anxiolytic‐like behavior in mice. 216 Intracerebroventricular injection of Ang‐(1–7) in transgenic (mRen2)27 hypertensive rats increased the percentage of entries into the open arms of the EPM. 124 ACE2 knock‐in mice explored the open arms of the EPM significantly more than the wild type, 128 and intracerebroventricular administration of Ang‐(1–7) to Wistar rats increased percentage of time spent and frequency of entries in the open arms of the EPM. 125 Transgenic rats over‐expressing Ang‐(1–7) showed a significantly higher percentage in the number of open arms entries in the EPM. 129 They were also found to spend more time and enter the open arms of the EPM more often than the control SD rats 130 (Table 1). All these findings indicate the anxiolytic‐like effect of ACE2‐Ang‐(1–7) cascade.
Of note, Ang‐(1–7) has also the potential to modulate the cardiovascular response to emotional stress. In air‐jet stress, the transgenic rats over‐expressing Ang‐(1–7) attenuated elevated heart rate and expressed reduced basal activity in renal sympathetic outflow compared to SD rats. 130 In Wistar rats exposed to air‐jet stress, intravenous or intracerebral application of Ang‐(1–7) blocked tachycardia and the pressor response 126 , 127 (Table 1) and the bradycardic effect of Ang‐(1–7) was observed also after treatment with beta‐adrenergic agonist isoproterenol. 126 These results indicate that Ang‐(1–7) reduces the cardiovascular response to acute emotional stress and involves the Mas receptors. 126 , 127 , 130
5.2.4. Aldosterone antagonists
Aldosterone is produced by the zona glomerulosa of the adrenal cortex 217 and acts via mineralocorticoid receptors. 218 The principal role of aldosterone is the regulation of salt and water homeostasis by sodium and water absorption in the distal renal tubule. More recently, it was disclosed that aldosterone acts as a transcriptional factor of the cellular growth and proliferation in the heart, vasculature, and kidney, inducing excessive fibrosis and cardiovascular remodeling. 219 Aldosterone antagonists were originally considered to be potassium‐sparing diuretics, applied to prevent hypokalemia during the treatment with loop diuretics. 220 At present, aldosterone antagonists in combination therapy are considered to be the drug of choice in resistant hypertension (ASCOT, Anglo‐Scandinavian Cardiac Outcomes Trial). 221 , 222 , 223 Moreover, aldosterone antagonists spironolactone and eplerenone added to conventional treatment are established drugs in the reduction of morbidity and mortality in patients with severe (RALES, Randomized Aldactone Evaluation Study) 197 or with moderate heart failure. 198
In the brain, aldosterone acts on the hydroxysteroid dehydrogenase‐2 (HSD2) neurons that represent a major input to the ventrolateral bed nucleus of the stria terminalis (BSTvl), a key control point for generating negative affective state. Thus, aldosterone might influence behavioral arousal. 224 Indeed, the elevation of plasma aldosterone level resulted in increased anxiety‐like behavior in rats. 225
5.2.4.1. Experimental studies
The administration of spironolactone in streptozotocin‐induced diabetic rats showing increased anxiety‐like behavior in burying behavior test exerted an anxiolytic‐like effect. 131 Single subcutaneous injection of eplerenone, a selective aldosterone receptor antagonist, reduced ethological indices of anxiety‐like behavior related to exploration and risk assessment behavior in Wistar rats. 133 A single subcutaneous administration of either spironolactone or mifepristone (a glucocorticoid receptor antagonist) partially reduced anxiety‐like behavior in the EPM following social defeat stress and mild traumatic brain injury (TBI) in SD rats 132 (Table 1).
5.2.4.2. Clinical studies
In humans, primary hyperaldosteronism is linked to an elevated rate of GAD 226 and depressive symptoms. 227 , 228 Although spironolactone treatment in primary hyperaldosteronism improved the quality of life (QOL) measured by the 36‐Item Short Form (SF‐36) questionnaire, 134 unilateral adrenalectomy demonstrated faster and more profound QOL improvement (Table 1). 229
5.2.5. Simultaneous blockade of angiotensin II type 1 receptor and neprilysin (ARNI)
An additional approach to the inhibition of the RAS and SNS systems to attenuate vasoconstrictor, pro‐inflammatory and pro‐proliferative actions in CVDs could be the stimulation of the counterbalancing pathways such as the atrial (ANP) and brain natriuretic peptides (BNP). These peptides exert natriuretic, diuretic, and vasodilative effects while also inhibiting tissue growth and fibrosis. 230 , 231 Direct administration of these peptides requires a parenteral approach, which is technically demanding and not suitable for chronic heart diseases. 230 A simpler and more effective approach seems to be the slowing down of the splitting rate of these hormones by the inhibition of neprilysin (endopeptidase, vasopeptidase, neutral peptidase; NEP), the enzyme located in the cell membrane of various tissues. 233 Since neprilysin substrates include both, ANP/BNP and Ang II, its inhibition will not only increase the level of beneficial ANP/BNP but also adverse Ang II concentration, potentially counterbalancing the desirable vasodilative and antiproliferative effects of ANP/BNP. Therefore, sacubitril, an inhibitor of neprilysin, was combined with AT1 receptor blocker valsartan, thus mitigating the Ang II effects. 230 , 231 The PARADIGM HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) study with HF patients of NYHA (New York Heart Association) II/III severity showed that sacubitril/valsartan reduced the composite primary endpoint of cardiovascular death or HF hospitalization by 20% and even general mortality by 16% compared with the ACE inhibitor enalapril. 234 , 235 Sacubitril/valsartan has not only become one of the cornerstones in the treatment of HF with reduced ejection fraction 234 but may be considered for organ protection in a range of other cardiovascular pathologies.
Based on the rather complex pathophysiological background of sacubitril/valsartan actions, their potential impact on the mental state in terms of stress, anxiety, and depression modulation is difficult to predict. In addition to splitting natriuretic peptides, neprilysin degrades several other peptides exerting vasodilation and antiproliferation, such as bradykinin, substance P, and adrenomedullin 236 ; thus, the concentration of these substances supposedly increases during sacubitril/valsartan treatment. Indeed, in a study with 73 HF patients, sacubitril/valsartan resulted in the attenuation of soluble NEP activity along with an increase of ANP, substance P, and a glucagon‐like peptide 1. 237 Moreover, NEP seems to be the principle peptidase responsible for degrading enkephalins in the intercalated cells of the amygdala. 238 Furthermore, the limited cleavage of endorphin peptides by NEP inhibition was suggested to promote symptomatic improvement in HF. 135
Since ANP is produced both in the heart and in the CNS, its pleiotropic effects are assumed to contribute to neuropsychiatric diseases and stress‐related conditions such as anxiety, major depression, addictive behaviors, panic attacks, and PTSDs. 239 , 240 In 117 patients with diastolic heart failure and proven anxiety, the plasma concentration of pro‐ANP was negatively related to clinical anxiety, thus suggesting the potential anxiolytic effect of a circulating natriuretic peptide 241 ; this effect might be related to an ANP‐induced attenuation of ACTH and cortisol secretion. 242 Accordingly, the anxiety‐reducing effect of the exercise was correlated with increased plasmatic ANP concentrations. 243
Increasing the level of substance P, adrenomedullin, bradykinin, and enkephalins/endorphins could also modulate anxiety, although the data are sparse. Substance P (SP), a neuropeptide acting via a neurokinin type 1 (NK‐1) receptor, is elevated in stressed conditions, 244 while the amygdala was suggested as the primary region mediating the SP‐NK1 system on anxiety. 245 The NK‐1 receptor's pharmacological antagonism or genetic modulation resulted in an anxiolytic response. 244
Adrenomedullin, a vasoactive protein and tissue growth modulating factor, is not only a biomarker that predicts later cardiovascular pathologies, 246 but it seems to be related to psychological disturbances in terms of anxiety, stress, and depression. Five weeks of yoga training combined with psychoeducation led to lowered adrenomedullin levels, reduced anxiety, and sleep improvement. 247 Increased plasma adrenomedullin levels were associated with the development of anxiety disorders and LV hypertrophy in hypertensive patients. 248 On the other hand, mid‐regional proadrenomedullin concentrations were inversely associated with anxiety in patients with diastolic heart dysfunction, 241 and the lack of adrenomedullin in CNS in genetically modulated mice was linked with hyperactivity and overanxiousness compared with wild‐type animals. 249 It seems that adrenomedullin may have both beneficial and deleterious actions regarding anxiety depending on the particular model. Similarly, bradykinin via its B1 or B2 receptors is considered to exert either protective or deleterious effects on depression and anxiety, 250 , 251 , 252 while the impact of enkephalins/endorphins in anxiety modulation remains to be established.
5.2.5.1. Clinical study
Only one clinical study investigated the impact of sacubitril/valsartan on anxiety and depression. In 115 symptomatic patients with systolic HF, the switch from an ACE inhibitor or ARB to sacubitril/valsartan resulted in the significant improvement of heart function along with a reduction of both depression and anxiety symptoms (Table 1). 136
5.3. Lipid modifying agents
5.3.1. Statins
Statins competitively inhibit 3‐hydroxy‐3‐methyl‐glutaryl coenzyme A (HMG‐CoA) reductase, the key enzyme in cholesterol biosynthesis in the liver. 253 The primary effect resides in the reduction of low‐density lipoproteins (LDL). Statins were shown to reduce morbidity and mortality in a number of secondary prevention trials and even in primary prevention in patients with increased LDL but without organ affliction. 254 Additionally, statins express a number of pleiotropic effects including antioxidant, anti‐inflammatory, and antiapoptotic actions resulting in improvement of antiproliferative action, attenuation of endothelial dysfunction, and stabilization of atherosclerotic plaques. 179 , 255 , 256 , 257 , 258
Undesirably, the non‐adherence to statins due to a number of side effects limits their clinical benefit. Besides myopathy presumably determined by the reduced production of coenzyme Q‐10 (CoQ‐10; Figure 4), 259 a number of mood and behavior affections were observed during statin treatment.
Figure 4.
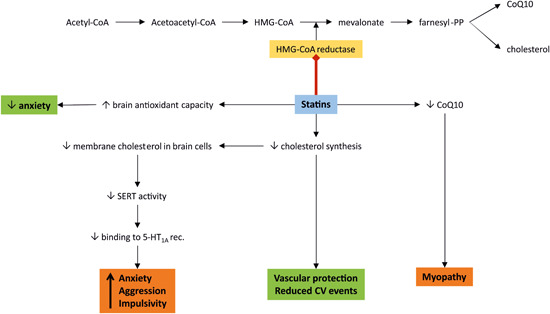
The role of statins on mood and behavior. Statins competitively inhibit 3‐hydroxy‐3‐methyl‐glutaryl coenzyme A (HMG‐CoA) reductase, the key enzyme in cholesterol biosynthesis in the liver. 252 Reducing the cholesterol level is considered protective for the vasculature, 254 while the simultaneous reduction of the synthesis of coenzyme Q‐10 (CoQ‐10) could result in undesirable myopathy. 258 However, a reduction of membrane cholesterol in the brain neurons decreased serotonin reuptake transporter (SERT) activity and attenuated the level of ligand binding to the serotonin1A (5‐HT1A) receptor 259 that resulted in anxiety, aggressive and impulsive behavior. 260 On the other hand, enhanced antioxidant capacity in the brain after statin use mitigates anxiety. 139 , 140 CoA, coenzyme A; CV, cardiovascular; Farnesyl‐PP, farnesyl pyrophosphate [Color figure can be viewed at wileyonlinelibrary.com]
5.3.1.1. Experimental study
A high dose of simvastatin and rosuvastatin in healthy Wistar rats decreased the time spent in the center zone in the OFT, as well as the number of entries in the open arms and time spent in the open arms of the EPM, suggesting anxiety‐like behavior 143 (Table 1).
5.3.1.2. Clinical studies
Pharmacovigilance databases reported anxiety, depression, aggression, suicidal tendency, cognitive, sleep, and other disorders, 145 and case studies reported irritability, aggression, 146 , 147 depressive symptoms, 146 , 148 nightmares, suicide attempts, 146 and experience of the somatic symptoms of anxiety 149 associated with antihyperlipidemic drugs, including statins (Table 1).
These adverse manifestations are supposedly linked to low cholesterol levels in plasma and brain. It has been observed that a low level of cholesterol is associated with violence, 262 suicidal attempts in patients with major depressive disorder, 263 aggression and hostility in suicide attempters, 264 while lower plasma levels of essential fatty acids are associated with self‐harm, impulsivity, and depression. 265
The brain contains a high proportion of cholesterol, representing 23% of free cholesterol present in the whole body. 266 Cholesterol is essential for determining the biophysical properties of membranes. In the mature brain, cholesterol is a part of the exocytosis apparatus in presynaptic terminals and of the biogenesis and transport of synaptic vesicles, which mediates axonal transport along microtubules, promotes cell adhesion between postsynaptic and presynaptic ends, and induces synaptogenesis. 267 Brain cholesterol is synthesized in situ with no evidence of the transfer of plasma lipoproteins through an intact blood–brain barrier. 268 Cholesterol may modulate the function of G‐protein‐coupled receptors (GPCRs) directly through a specific interaction with GPCRs with conformational change in the receptor, indirectly by altering the membrane physical properties or through a combination of both. 260 Upon statin treatment, a reduction of membrane cholesterol decreased the activity of serotonin reuptake transporter (SERT) and attenuated the level of ligand binding to 5HT1A receptor that belongs to the GPCRs family. 260 Indeed, there is an established relationship between depressed central serotonergic activity and aggressive and impulsive behavior (Figure 4). 261 , 269 In male cynomolgus monkeys, a low‐fat and low‐cholesterol diet decreased serotonergic activity within the hypothalamus 269 and led to aggressive behavior. 270 In human males, a correlation between low serum total cholesterol and low cerebrospinal fluid (CSF) levels of the main serotonin metabolite 5‐hydroxyindoleacetic acid (5‐HIAA) has been found, which predisposes the males to violent and risky behavior. 271 Low levels of serotonin and 5‐HIAA were found in post‐mortem examinations of brain‐stem tissues of suicide victims, 272 a low concentration of 5‐HIAA in the CSF were detected in attempted suicides who have killed their children 273 and in murderers and suicide attempters. 274
On the other hand, some experimental and clinical studies have not confirmed the link between statin use and suicide tendency, aggression, or anxiety.
5.3.1.3. Experimental studies
Simvastatin treatment in mice caused a deficiency in recognition and spatial memory but had no effect on motor ability or anxiety‐like behavior. 137 Simvastatin administration to healthy SD rats increased the time spent in the open arms of the EPM 138 ; atorvastatin increased the ratio time spent in the open arms of the EPM in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) mice 139 ; atorvastatin and simvastatin in rats fed with a methionine‐enriched diet improved exploratory and locomotor activity in the OFT and EPM 140 ; atorvastatin, simvastatin and pravastatin treatment in Wistar‐Albino‐Glaxo from Rijswijk (WAG/Rij) rats increased the time spent in and number of entries to the center of the OFT 141 ; and rosuvastatin increased the time spent in and locomotion in the central zone of the OFT in mice infected with the chronic ME‐49 strain of Toxoplasma gondii. 142 All of these findings point to anxiolytic‐like behavior after statin use in experimental animals. Possible mechanisms include decreased brain expression of NADPH oxidase 2, 139 lipid peroxidation and increased brain activity of the antioxidant enzymes, catalase, and superoxide dismutase after statin administration 140 (Table 1).
5.3.1.4. Clinical studies
Recent studies of Molero et al. 150 and Mirzaei et al. 144 have not found an association between statin treatment in patients and suicidality or anxiety disorders (Table 1).
5.3.2. Fibrates
Fenofibrate activates the peroxisome proliferator‐activated receptor alpha (PPARα) thus increasing lipolysis, activating lipoprotein lipase, and reducing apoprotein C‐III. It is used to treat primary hypercholesterolemia, mixed dyslipidemia, and severe hypertriglyceridemia. 275
5.3.2.1. Experimental studies
In the pentylenetetrazol‐induced kindling seizure model in mice, the fenofibrate treatment increased the time spent in the open arms and the percentage of open arm entries in the EPM. 151 Fenofibrate in autism spectrum disorder in rats increased the percentage of time spent in and number of entries to the open arm in the EPM. 152 , 153 It seems that this anxiolytic property of fenofibrate includes antioxidative, anti‐inflammatory, 153 and neurosteroidogenic effects through PPARα activation in the brain 154 (Table 1).
5.4. Other cardiovascular drugs and anxiety
5.4.1. Ivabradine
Increased heart rate is an independent risk factor of cardiovascular mortality. Ivabradine is a selective inhibitor of hyperpolarization‐activated channel in the sinoatrial node responsible for pacemaker generation through the If (funny) current. It reduces the spontaneous pacemaker activity, leading to a slowing of the heart rate without inducing negative inotropy as beta‐blockers do. 276 In the SHIFT (Systolic Heart failure treatment with the If inhibitor ivabradine Trial), ivabradine decreased the composite end‐point of mortality and hospitalizations for HF. 277 Moreover, a number of ivabradine pleiotropic effects have been described, including anti‐inflammatory, antiapoptotic, antiremodeling, oxidative stress‐reducing, and hypotensive actions, 278 , 279 , 280 , 281 , 282 , 283 that may be potentially beneficial in several off‐labeled indications. 284
The potential effects of ivabradine on mood, cognition, and behavior in experimental animals and humans remain elusive.
5.4.1.1. Experimental studies
In our laboratory, no disturbing effects of ivabradine were observed on anxiety, locomotion, or learning in healthy and NG‐nitro‐l‐arginine methyl ester (l‐NAME)‐induced hypertensive rats, while some of these parameters were even improved. 155 , 156 Moreover, the survival of rats with isoproterenol‐induced myocardial injury was significantly improved 285 (Table 1).
5.4.1.2. Clinical studies
Scarce clinical studies revealed that the administration of ivabradine in chronic heart failure patients 157 , 158 and patients with chronic stable angina pectoris 159 improved their quality of life (Table 1).
A recent study revealed that ivabradine may affect brain functions by its agonist activity in the GABAA channel in the brain, which is similar to diazepam. It was also demonstrated that ivabradine pretreatment attenuated pentylenetetrazol‐ and picrotoxin‐induced epileptic seizures in mice that were accompanied by a decrease of lipid peroxidation in the prefrontal cortex, hippocampus, and striatum, as well as by a reduction of cleaved‐caspase 3 expression, a marker of apoptosis, in various hippocampal regions. 286
5.4.2. Calcium channel blockers
Amlodipine, nifedipine, and verapamil are L‐type calcium channel blockers used to treat hypertension, 287 angina, 288 and to control supraventricular tachyarrhythmias. 289
5.4.2.1. Experimental studies
Amlodipine, 161 nifedipine, and verapamil 160 increased anxiety‐like behavior in mice (Table 1).
5.4.2.2. Clinical study
A single dose of nifedipine had no reducing effect on anxiety in phobic patients with generalized anxiety 162 (Table 1).
5.4.3. Diuretics
Furosemide is a loop diuretic indicated for the treatment of volume overload and edema associated with congestive heart failure, 290 liver failure with cirrhosis, 291 and renal failure, including nephrotic syndrome. 292
5.4.3.1. Experimental study
In conditioned models of anxiety in rats, furosemide decreased freezing in contextual fear‐conditioning, thus indicating anxiolytic‐like behavior 163 (Table 1).
5.4.4. Vasodilators
Nitroglycerin is a nitrate vasodilator used for symptomatic relief in myocardial ischemia, 293 and the treatment of MI, 294 hypertensive emergencies 295 and heart failure 296 predominantly through the venodilatation‐induced reduction of the hemodynamic burden.
5.4.4.1. Experimental studies
Chronic nitroglycerin administration in rats led to a decreased percentage of entries and time spent in the open arms of the EPM and reduced time to enter the dark part of the LDB with fewer transitions and decreased time spent in light part of the LDB, all indicating anxiety‐like behavior 164 , 165 (Table 1).
6. IMPLANTABLE CARDIOVERTER‐DEFIBRILLATOR AND ANXIETY
The implantable cardioverter‐defibrillator (ICD) represents an important tool for modifying cardiovascular mortality in patients with electrical instability and potentially fatal dysrhythmia. The ICD is used to intervene in life‐endangering ventricular dysrhythmias and prevent sudden cardiac death. 166 The ICD is an electronic device that continuously monitors heart rhythms and at the onset of an abnormal heart rhythm, it delivers energy in the form of pacing or shocks to restore sinus rhythm. 298 In the past, secondary prevention was administered to patients experiencing life‐threatening arrhythmia, but at present, primary prevention is mainly used for patients with serious left ventricular dysfunction who have not experienced potentially fatal dysrhythmia. 299
6.1. Clinical studies
Some ICD patients suffer from mental alterations in terms of depression and anxiety, which in turn, may influence adherence to the device. A number of conditions are thought to underlie mental disturbances, such as the severity of the cardiac disorder, concerns regarding cardiovascular prognosis, fear of ICD tolerance, and the unpredictable nature of ICD shocks. 299 , 300 Several reviews on the prevalence of depression/anxiety in ICD patients revealed inhomogeneous data due to small patient samples, different testing modes, or variable indications to ICD. 301 , 302 A systematic review of forty‐five studies including over 5000 patients concluded that based on the analyzed studies there is an approximately 20% prevalence rate for both anxiety and depression in patients with ICD, which is similar to other cardiovascular pathologies. 166 It has recently been revealed that patients with depression at the time of ICD implantation had a greater risk of mortality, while anxiety only showed a trend. 304 Moreover, in a large cohort of ICD recipients, the probability of anxiety and depression symptoms was associated with younger age, living alone, previous history of MI and heart failure, and female gender. 305 Suggestively, the patient's psychological characteristics are greater predictors of a poor quality of life than the actual shock experience. 306 A cognitive behavioral rehabilitation program for patients with ICD in terms of ICD shock and stress management seems to attenuate symptoms of depression and anxiety, 306 , 307 while specific factors should be addressed to improve outcomes (Table 1). 305
7. DISCUSSION
CVDs, anxiety, and depression are highly prevalent pathologies that deteriorate the quality of life and prognosis of patients. 309 Patients with current cardiovascular alterations such as hypertension‐induced target organ damage, MI, cerebral ischemia, heart failure, or kidney dysfunction frequently experience mental disorders, including feelings of worry, anxiety or depression. These states may result from or be deteriorated by the primary cardiovascular pathology. Vice‐versa, anxiety or depression may worsen the course of CVDs or trigger cardiac or vascular complications. Importantly, drugs used to treat cardiovascular disorders seem to affect the development and severity of anxiety or depression. 310
A considerable number of experimental and clinical studies have strived to determine the impact of different cardiovascular drug groups on anxiety or depression. This review has focused on presenting the impact of currently used, well‐established cardiovascular treatment for mental alterations regarding anxiety and related disorders and on delineating potential future clinical and research directions.
Beta‐blockers and drugs interfering with the renin‐angiotensin‐aldosterone system seem to be of considerable importance. Propranolol, the nonselective beta‐adrenergic receptor antagonist, exerted an anxiolytic effect in both experimental and clinical settings supposedly due to its sympatholytic action. 86 However, this drug is seldomly used in current clinical practice due to a number of novel beta‐blockers with higher receptor selectivity and fewer side effects. We assume that selective beta‐blockers could not only improve energy metabolism and reduce hemodynamic alterations and proarrhythmogenic potential induced by excessive sympathetic excitation 311 but they might be more effective in alleviating mental stress and anxiety disorders. Indeed, selective beta‐blockers decreased symptoms of anxiety in patients with chronic heart failure (metoprolol), 81 mitral valve prolapse syndrome (celiprolol) 70 and anxiety‐related mental health problems (atenolol). 82
Prazosin, the alpha‐1 antagonist, is currently used more often for indications other than hypertension. Thanks to its anxiolytic effect, it is used in the treatment of PTSD with related sleep disorders and nightmares 90 , 91 , 92 and in the treatment of alcoholic use disorder. 93 , 94 Central sympatholytic drugs such as clonidine and guanfacine represent another group of antihypertensives that have demonstrated a positive effect on anxiety symptoms. Clonidine 96 , 98 , 102 as well as guanfacine 97 , 99 , 100 , 101 exerted an anxiolytic effect in experimental and clinical settings and may be useful in the treatment of PTSD and GAD.
Both ACE inhibitors 103 , 104 , 105 , 106 , 108 , 109 , 198 and ARBs 106 , 110 , 111 , 112 , 113 , 114 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 193 , 194 , 195 , 205 , 206 , 207 indicate well‐established mental effects in reducing neuropsychological alterations, including stress and anxiety. The direct neurocellular protection on the level of the brain structure concerning their anti‐inflammatory and antiproliferative action along with improving hemodynamics of the CNS 112 , 194 , 195 , 207 could prove to be the underlying pathomechanism. The nonclassical ACE2/Ang‐(1–7)/Mas receptor pathway opposes the vasoconstriction, profibrotic and inflammatory action of the ACE/Ang II/AT1 receptor pathway protecting the cardiovascular system. 196 , 209 , 210 Its stimulation appears to be beneficial in terms of reducing undesirable stress response and anxiety 124 , 125 , 126 , 127 , 128 , 129 , 130 by exerting vasodilatation, antiproliferation, anti‐inflammation and oxidative stress reduction in the cardiovascular system 209 , 210 , 311 and potentially in the CNS. 196 Aldosterone, another important player of RAAS, seems to induce anxiety, 225 while aldosterone receptor blockers were shown to attenuate this undesirable emotional disorder. 131 , 132 , 133 , 134 The data presenting effects of ARNI on anxiety are sparse. The dual AT1/neprilysin blockade not only reduces the effect of Ang II but enhances the level of various peptides (ANP, bradykinin, substance P, enkephalins), which are considered to exert cardiovascular protection, 230 , 231 while some are believed to interfere with stress‐related conditions such as anxiety. 136 , 239 , 240 Well‐controlled prospective studies with angiotensin II/neprilysin inhibition focused on anxiety and depression are warranted.
Statin's various neuropsychiatric adverse effects in terms of aggression, depression, or impulsivity 145 , 146 , 147 , 148 , 149 appear to be related to low cholesterol and omega‐3 fatty acid levels 262 , 263 , 264 , 265 and correlate with decreased serotonergic activity in the brain. 260 , 261 , 269 , 271 Another potentially harmful condition could be statin‐induced CoQ‐10 depletion associated with anaerobic metabolism, mitochondrial bioenergetic impairment, 313 and increased oxidizability of LDL cholesterol, 314 which might induce ischemia and functional alterations in CNS. On the contrary, statin's pleiotropic effects including antiproliferation, antioxidation, and anti‐inflammation 256 could underlie some protective findings in terms of anxiety mitigation demonstrated in experimental settings 138 , 139 , 140 , 141 , 142 ; the same can be valid for fenofibrate's anxiolytic effects. 151 , 152 , 153
Although ivabradine's protection in HF patients is restricted, 277 it could be beneficial in several off‐labeled indications due to its various pleiotropic actions. 284 No disturbing effects of ivabradine on anxiety were observed in animal experiments, 155 , 156 while in patients with CVDs, ivabradine administration was even associated with improved quality of life. 157 , 158 , 159 The experimental and clinical studies regarding the effect of other cardiovascular drugs including antihypertensives, calcium channel blockers, diuretics, vasodilators, and antiarrhythmics regarding mood or behavior are sparse and not directly related to anxiety.
The coronavirus disease 2019 (COVID‐19) pandemic has influenced all areas of medical practice. Although acute respiratory distress syndrome (ARDS) is the most challenging and harmful condition resulting in the serious course of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, the affliction of the cardiovascular, 314 , 315 digestive, 317 and CNSs, among others, 318 are rather frequent complications. Moreover, stress and anxiety were disclosed in different groups of health care providers, such as dental practitioners, 319 other healthcare workers, 319 , 320 health profession students, 322 social workers 323 and in patients afflicted by COVID‐19. 324 The underlying reasons for mental disturbances involve fear of exposure to infection, proximity to death generated by the pandemic, extreme working demands, economic uncertainty, disruption of social life, insufficient knowledge of the disease and SARS‐CoV‐2‐induced physical disturbances in terms of endothelial inflammation, microvascular thrombosis, ischemia from pulmonary damage and multiple organ dysfunctions. 318 , 319 , 321 , 323 In addition to professional psychological support for those groups with increased risk of stress, anxiety, and depression development, the consideration of the potential mental effects of the current medical treatment of cardiovascular, respiratory, and other system dysfunctions could offer significant benefits for anxiety‐afflicted patients.
It is becoming obvious that health‐related quality of life depends in substantial measure on the mutual interactions between CVDs and mental disorders including anxiety. In the near future, anxiety resulting from cardiovascular pathology or developed independently from heart disorder could rank among the targeted risk factors deteriorating cardiovascular prognosis. Thus, knowledge of the interference of cardiovascular drugs with the mental state of the patient will improve the approach to the choice of optimal treatment, in particular, of cardiovascular affliction.
8. CONCLUSION
Negative emotions, mood and behavior alterations, and various cardiovascular pathologies are tightly bound, exerting a causal bidirectional relationship potentiating each other. The neurohumoral activation seems to underlie this cardiovascular‐psychological interference. It is of utmost importance to reveal signs and symptoms of altered mood, cognition, and behavior in terms of distress and anxiety in cardiovascular patients to prevent worsening of their conditions. Moreover, various principal cardiovascular drugs can interplay with anxiety and depression symptoms. In general, beta‐blockers, ACE inhibitors, ARBs, aldosterone receptor blockers, and sacubitril/valsartan are considered to exert an anxiolytic effect in animal experiments and clinical settings. Statins, fibrates, and central sympatholytic drugs exert a prevailingly protective impact on mood and anxiety, and ivabradine expressed a neutral mental and cognitive impact. Improving the level of knowledge of these therapeutics regarding their possible interference with mood, mind, and behavior could help manage both the cardiovascular and mental burden of the population with cardiovascular pathologies. It warrants future clinical trials focused not only on reducing cardiovascular morbidity and mortality but also on protecting patients' well‐being by preserving the normal state of mood and mental integrity.
ACKNOWLEDGMENTS
This study was supported by the VEGA grants for Scientific Research No 1/0035/19 and No 2/0112/19.
Biographies
Kristina Repova is an assistant professor at the Institute of Pathophysiology, Faculty of Medicine, Comenius University, Bratislava, Slovakia. She graduated from the same Faculty in 2009 and received her PhD in normal and pathological physiology from the Institute of Pathophysiology, Faculty of Medicine, Comenius University in 2014. Her area of research is cardiovascular diseases, specifically hypertension, heart failure, and the interactions of cardiovascular pathologies and cardiovascular drugs with emotions, cognition, and behavior. She is the author of 25 scientific papers and 2 textbooks (more than 300 citations, h‐index 11).
Silvia Aziriova is an assistant professor at the Institute of Pathophysiology, Faculty of Medicine, Comenius University, Bratislava, Slovakia. She graduated from the Faculty of Philosophy, Comenius University in 2010 in psychology and defended her PhD in normal and pathological physiology at the Institute of Pathophysiology, Faculty of Medicine, Comenius University in 2014. Her research is aimed on cardiovascular diseases and drugs in relation to human's mental state and animal's behavior. She published 16 papers and was cited more than 150 times with h‐index 8.
Kristina Krajcirovicova is a researcher at the Institute of Pathophysiology, Faculty of Medicine, Comenius University, Bratislava, Slovakia. Her research is focused on recent substances used in hypertension and heart failure treatment and their impact on animal's behavior. She is an author of 26 publications cited more than 420 times with h‐index 14.
Fedor Simko was born on April 13, 1956, in Bratislava, Slovak Republic. He is a professor of normal and pathological physiology, head of the Institute of Pathophysiology, Faculty of Medicine, Comenius University in Bratislava, Slovakia, and a cardiologist at the 3rd Clinic of Internal Medicine of the same faculty. He graduated from the Comenius University, Faculty of Medicine in 1981, defended his PhD thesis in 1987, became an associate professor in 1991 and professor in 2000. He received Alexander von Humboldt Fellowships from 1992 to 1994 and in 2000, and conducted research in Göttingen, Heidelberg, Berlin, Zurich, Vienna, and Prague. His scientific interests cover hypertension, heart failure, and the exploration of novel approaches in the protection of hypertensive and failing heart. Prof. Simko has received several awards for his research achievements and pedagogical work and in 2008 was awarded the prestigious “Scientist of the Year of the Slovak Republic” award for his extraordinary contributions to the research of hypertension. Prof. Simko has published 150 scientific papers, cited 1700×, with cumulative impact factor 270, h‐index 31, and served as a guest editor five times (J Hypertens 2009, J Hypertens 2010, Front Biosci 2013, Phys Res 2007, Int J Mol Sci 2020). He is a reviewer for 35 renowned journals including Lancet, Circulation, and Circulation Res, and is a co‐editor in Chief of the Bratislava Med Journal.
Repova K, Aziriova S, Krajcirovicova K, Simko F. Cardiovascular therapeutics: A new potential for anxiety treatment? Med Res Rev. 2022;42:1202‐1245. 10.1002/med.21875
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. The Lancet . GBD 2017: a fragile world. Lancet. 2018;392(10159):1683. [DOI] [PubMed] [Google Scholar]
- 2. Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BHM. Meta‐analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol. 2016;118(4):511‐519. [DOI] [PubMed] [Google Scholar]
- 3. Holwerda SW, Luehrs RE, Gremaud AL, et al. Relative burst amplitude of muscle sympathetic nerve activity is an indicator of altered sympathetic outflow in chronic anxiety. J Neurophysiol. 2018;120(1):11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reeves JW, Fisher AJ, Newman MG, Granger DA. Sympathetic and hypothalamic‐pituitary‐adrenal asymmetry in generalized anxiety disorder. Psychophysiology. 2016;53(6):951‐957. [DOI] [PubMed] [Google Scholar]
- 5. Won E, Kim YK. Stress, the Autonomic Nervous System, and the immune‐kynurenine pathway in the etiology of depression. Curr Neuropharmacol. 2016;14(7):665‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar A, Chanana P. Role of nitric oxide in stress‐induced anxiety: from pathophysiology to therapeutic target. Vitam Horm. 2017;103:147‐167. [DOI] [PubMed] [Google Scholar]
- 7. Pavel J, Benicky J, Murakami Y, Sanchez‐Lemus E, Saavedra JM. Peripherally administered angiotensin II AT1 receptor antagonists are anti‐stress compounds in vivo. Ann N Y Acad Sci. 2008;1148:360‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei TM, Wang L. Anxiety symptoms in patients with hypertension: a community‐based study. Int J Psychiatry Med. 2006;36(3):315‐322. [DOI] [PubMed] [Google Scholar]
- 9. Broomfield NM, Quinn TJ, Abdul‐Rahim AH, Walters MR, Evans JJ. Depression and anxiety symptoms post‐stroke/TIA: prevalence and associations in cross‐sectional data from a regional stroke registry. BMC Neurol. 2014;14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies SJC, Ghahramani P, Jackson PR, et al. Association of panic disorder and panic attacks with hypertension. Am J Med. 1999;107(4):310‐316. [DOI] [PubMed] [Google Scholar]
- 11. Boyle SH, Michalek JE, Suarez EC. Covariation of psychological attributes and incident coronary heart disease in U.S. Air Force veterans of the Vietnam war. Psychosom Med. 2006;68(6):844‐850. [DOI] [PubMed] [Google Scholar]
- 12. Jakobsen AH, Foldager L, Parker G, Munk‐Jørgensen P. Quantifying links between acute myocardial infarction and depression, anxiety and schizophrenia using case register databases. J Affect Disord. 2008;109(1–2):177‐181. [DOI] [PubMed] [Google Scholar]
- 13. Ostir GV, Goodwin JS. High anxiety is associated with an increased risk of death in an older tri‐ethnic population. J Clin Epidemiol. 2006;59(5):534‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen YH, Hu CJ, Lee HC, Lin HC. An increased risk of stroke among panic disorder patients: a 3‐year follow‐up study. Can J Psychiatry. 2010;55(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 15. Miles HHW, Barrabee EL, Finesinger JE. Evaluation of psychotherapy, with a follow‐up study of 62 cases of anxiety neurosis. Psychosom Med. 1951;13(2):83‐105. [DOI] [PubMed] [Google Scholar]
- 16. Pedersen SS, von Känel R, Tully PJ, Denollet J. Psychosocial perspectives in cardiovascular disease. Eur J Prev Cardiol. 2017;24(3_suppl):108‐115. [DOI] [PubMed] [Google Scholar]
- 17. Park H, Rhee J, Park K, Han JS, Malinow R, Chung C. Exposure to stressors facilitates long‐term synaptic potentiation in the Lateral Habenula. J Neurosci. 2017;37(25):6021‐6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ray A, Gulati K, Rai N. Stress, anxiety, and immunomodulation: a pharmacological analysis. Vitam Horm. 2017;103:1‐25. [DOI] [PubMed] [Google Scholar]
- 19. Bali A, Jaggi AS. Angiotensin as stress mediator: role of its receptor and interrelationships among other stress mediators and receptors. Pharmacol Res. 2013;76:49‐57. [DOI] [PubMed] [Google Scholar]
- 20. Hayes PE, Schulz SC. Beta‐blockers in anxiety disorders. J Affect Disord. 1987;13(2):119‐130. [DOI] [PubMed] [Google Scholar]
- 21. Adameova A, Abdellatif Y, Dhalla NS. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress‐induced heart disease. Can J Physiol Pharmacol. 2009;87(7):493‐514. [DOI] [PubMed] [Google Scholar]
- 22. Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315‐329. [DOI] [PubMed] [Google Scholar]
- 23. Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1(4):291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson HM. Anxiety and hypertension: is there a link? A literature review of the comorbidity relationship between anxiety and hypertension. Curr Hypertens Rep. 2019;21(9):66. [DOI] [PubMed] [Google Scholar]
- 25. Zhou Y, Dong Z, A R, et al. The prevalence of impaired glucose regulation in anxiety disorder patients and the relationship with hypothalamic‐pituitary‐adrenal axis and hypothalamic‐pituitary‐thyroid axis activity. J Evid Based Med. 2019;12(1):51‐55. [DOI] [PubMed] [Google Scholar]
- 26. Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411(2–3):217‐230. [DOI] [PubMed] [Google Scholar]
- 27. Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755‐784. [DOI] [PubMed] [Google Scholar]
- 28. Pitsikas N. The role of nitric oxide (NO) donors in anxiety. Lights and shadows. Nitric Oxide. 2018;77:6‐11. [DOI] [PubMed] [Google Scholar]
- 29. Karanth S, Lyson K, McCann SM. Role of nitric oxide in interleukin 2‐induced corticotropin‐releasing factor release from incubated hypothalami. Proc Natl Acad Sci USA. 1993;90(8):3383‐3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bauer EP. Serotonin in fear conditioning processes. Behav Brain Res. 2015;277:68‐77. [DOI] [PubMed] [Google Scholar]
- 31. Marcinkiewcz CA, Mazzone CM, D'agostino G, et al. Serotonin engages an anxiety and fear‐promoting circuit in the extended amygdala. Nature. 2016;537(7618):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62(1):42‐53. [DOI] [PubMed] [Google Scholar]
- 33. DeFeudis FV. gamma‐Aminobutyric acid and cardiovascular function. Experientia. 1983;39(8):845‐849. [DOI] [PubMed] [Google Scholar]
- 34. Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2(2):63‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1–3):199‐216. [DOI] [PubMed] [Google Scholar]
- 36. Bourin M. Animal models for screening anxiolytic‐like drugs: a perspective. Dialogues Clin Neurosci. 2015;17(3):295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lezak KR, Missig G, Carlezon WA. Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci. 2017;19(2):181‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Julian LJ. Measures of anxiety: State‐Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale‐Anxiety (HADS‐A). Arthritis Care Res. 2011;63(Suppl 11):S467‐S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson E. Hamilton Rating Scale for Anxiety (HAM‐A). Occup Med. 2015;65(7):601. [DOI] [PubMed] [Google Scholar]
- 40. Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self‐report symptom inventory. Behav Sci. 1974;19(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 41. Harding JW, Sullivan MJ, Hanesworth JM, Cushing LL, Wright JW. Inability of [125I]Sar1, Ile8‐angiotensin II to move between the blood and cerebrospinal fluid compartments. J Neurochem. 1988;50(2):554‐557. [DOI] [PubMed] [Google Scholar]
- 42. Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13(2):329‐380. [DOI] [PubMed] [Google Scholar]
- 43. van Thiel BS, Góes Martini A, Te Riet L, et al. Brain renin‐angiotensin system: does it exist? Hypertension. 2017;69(6):1136‐1144. [DOI] [PubMed] [Google Scholar]
- 44. Phillips MI. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413‐435. [DOI] [PubMed] [Google Scholar]
- 45. Saavedra JM, Sánchez‐Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kang YM, Ma Y, Zheng JP, et al. Brain nuclear factor‐kappa B activation contributes to neurohumoral excitation in angiotensin II‐induced hypertension. Cardiovasc Res. 2009;82(3):503‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin N Am. 2009;29(2):247‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15(4‐6):323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramirez K, Fornaguera‐Trías J, Sheridan JF. Stress‐induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Curr Top Behav Neurosci. 2017;31:155‐172. [DOI] [PubMed] [Google Scholar]
- 50. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Kloet AD, Wang L, Pitra S, et al. A unique “Angiotensin‐Sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J Neurosci. 2017;37(13):3478‐3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Belcheva I, Georgiev V, Chobanova M, Hadjiivanova C. Behavioral effects of angiotensin II microinjected into CA1 hippocampal area. Neuropeptides. 1997;31(1):60‐64. [DOI] [PubMed] [Google Scholar]
- 53. Braszko JJ, Wiśniewski K. Effect of angiotensin II and saralasin on motor activity and the passive avoidance behavior of rats. Peptides. 1988;9(3):475‐479. [DOI] [PubMed] [Google Scholar]
- 54. Hoły Z, Wiśniewski K. Examination of the influence of 3,5‐DHPG on behavioral activity of angiotensin II. Pol J Pharmacol. 2001;53(3):235‐243. [PubMed] [Google Scholar]
- 55. Braszko JJ, Kułakowska A, Winnicka MM. Effects of angiotensin II and its receptor antagonists on motor activity and anxiety in rats. J Physiol Pharmacol. 2003;54(2):271‐281. [PubMed] [Google Scholar]
- 56. Duchemin S, Belanger E, Wu R, Ferland G, Girouard H. Chronic perfusion of angiotensin II causes cognitive dysfunctions and anxiety in mice. Physiol Behav. 2013;109:63‐68. [DOI] [PubMed] [Google Scholar]
- 57. Cruickshank JM. The role of beta‐blockers in the treatment of hypertension. Adv Exp Med Biol. 2017;956:149‐166. [DOI] [PubMed] [Google Scholar]
- 58. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure‐lowering treatment on cardiovascular outcomes and mortality: 14 – effects of different classes of antihypertensive drugs in older and younger patients: overview and meta‐analysis. J Hypertens. 2018;36(8):1637‐1647. [DOI] [PubMed] [Google Scholar]
- 59. Andersson C, Shilane D, Go AS, et al. β‐blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64(3):247‐252. [DOI] [PubMed] [Google Scholar]
- 60. Peyracchia M, Errigo D, Raposeiras Rubin S, et al. Beta‐blocker therapy reduces mortality in patients with coronary artery disease treated with percutaneous revascularization: a meta‐analysis of adjusted results. J Cardiovasc Med. 2018;19(7):337‐343. [DOI] [PubMed] [Google Scholar]
- 61. Joseph P, Swedberg K, Leong DP, Yusuf S. The evolution of β‐blockers in coronary artery disease and heart failure (Part 1/5). J Am Coll Cardiol. 2019;74(5):672‐682. [DOI] [PubMed] [Google Scholar]
- 62. MERIT‐HF . Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in‐Congestive Heart Failure (MERIT‐HF). Lancet. 1999;353(9169):2001‐2007. [PubMed] [Google Scholar]
- 63. CIBIS‐II . The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353(9146):9‐13. [PubMed] [Google Scholar]
- 64. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349‐1355. [DOI] [PubMed] [Google Scholar]
- 65. Ackerman MJ, Priori SG, Dubin AM, et al. Beta‐blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: are all beta‐blockers equivalent? Heart Rhythm. 2017;14(1):e41‐e44. [DOI] [PubMed] [Google Scholar]
- 66. Sutter JD, Mendes M, Franco OH. Cardioprotective drugs. The ESC Textbook of Preventive Cardiology. Oxford University Press; 2017. [Google Scholar]
- 67. Granville‐Grossman KL, Turner P. The effect of propranolol on anxiety. Lancet. 1966;1(7441):788‐790. [DOI] [PubMed] [Google Scholar]
- 68. Kelly D. Clinical review of beta‐blockers in anxiety. Pharmakopsychiatr Neuropsychopharmakol. 1980;13(5):259‐266. [DOI] [PubMed] [Google Scholar]
- 69. Ravaris CL, Friedman MJ, Hauri PJ, McHugo GJ. A controlled study of alprazolam and propranolol in panic‐disordered and agoraphobic outpatients. J Clin Psychopharmacol. 1991;11(6):344‐350. [PubMed] [Google Scholar]
- 70. Bachmann S, Muller‐Werdan U, Huber M, Kasel M, Werdan K, Schmidt H. Positive impact of the β‐blocker celiprolol on panic, anxiety, and cardiovascular parameters in patients with mitral valve prolapse syndrome. J Clin Psychopharmacol. 2011;31(6):783‐785. [DOI] [PubMed] [Google Scholar]
- 71. Fagerström KO, Hugdahl K, Lundström N. Effect of beta‐receptor blockade on anxiety with reference to the three‐systems model of phobic behavior. Neuropsychobiology. 1985;13(4):187‐193. [DOI] [PubMed] [Google Scholar]
- 72. Liu HH, Milgrom P, Fiset L. Effect of a beta‐adrenergic blocking agent on dental anxiety. J Dent Res. 1991;70(9):1306‐1308. [DOI] [PubMed] [Google Scholar]
- 73. Falloon IR, Lloyd GG, Harpin RE. The treatment of social phobia. Real‐life rehearsal with nonprofessional therapists. J Nerv Ment Dis. 1981;169(3):180‐184. [DOI] [PubMed] [Google Scholar]
- 74. AlOkda AM, Nasr MM, Amin SN. Between an ugly truth and a perfect lie: wiping off fearful memories using beta‐adrenergic receptors antagonists. J Cell Physiol. 2019;234(5):5722‐5727. [DOI] [PubMed] [Google Scholar]
- 75. Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post‐retrieval propranolol on psychophysiologic responding during subsequent script‐driven traumatic imagery in post‐traumatic stress disorder. J Psychiatr Res. 2008;42(6):503‐506. [DOI] [PubMed] [Google Scholar]
- 76. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rosenberg L, Rosenberg M, Sharp S, et al. Does acute propranolol treatment prevent posttraumatic stress disorder, anxiety, and depression in children with burns? J Child Adolesc Psychopharmacol. 2018;28(2):117‐123. [DOI] [PubMed] [Google Scholar]
- 78. Leal Santos S, Stackmann M, Muñoz Zamora A, et al. Propranolol decreases fear expression by modulating fear memory traces. Biol Psychiatry. 2021;89:1150‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Elsey JWB, Filmer AI, Galvin HR, et al. Reconsolidation‐based treatment for fear of public speaking: a systematic pilot study using propranolol. Transl Psychiatry. 2020;10(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thierrée S, Richa S, Brunet A, et al. Trauma reactivation under propranolol among traumatized Syrian refugee children: preliminary evidence regarding efficacy. Eur J Psychotraumatol. 2020;11(1):1733248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu L, Zhang Q, Shu Q, Zhang R, Meng Y. Sex‐dependent changes in physical, mental, and quality of life outcomes in metoprolol‐treated Chinese chronic heart failure patients. Medicine. 2019;98(50):e18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Armstrong C, Kapolowicz MR. A preliminary investigation on the effects of atenolol for treating symptoms of anxiety. Mil Med. 2020;185(11–12):e1954‐e1960. [DOI] [PubMed] [Google Scholar]
- 83. Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256‐258. [DOI] [PubMed] [Google Scholar]
- 84. Zaidi S, Atrooz F, Valdez D, et al. Protective effect of propranolol and nadolol on social defeat‐induced behavioral impairments in rats. Neurosci Lett. 2020;725:134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wohleb ES, Hanke ML, Corona AW, et al. β‐Adrenergic receptor antagonism prevents anxiety‐like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277‐6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Steenen SA, van Wijk AJ, van der Heijden GJMG, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: systematic review and meta‐analysis. J Psychopharmacol. 2016;30(2):128‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rasmussen DD, Kincaid CL, Froehlich JC. Prazosin prevents increased anxiety behavior that occurs in response to stress during alcohol deprivations. Alcohol Alcohol. 2017;52(1):5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ketenci S, Acet NG, Sarıdoğan GE, Aydın B, Cabadak H, Gören MZ. The neurochemical effects of prazosin treatment on fear circuitry in a rat traumatic stress model. Clin Psychopharmacol Neurosci. 2020;18(2):219‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aykac A, Şehirli AÖ, Gören MZ. Evaluation of the effect of prazosin treatment on α‐2c adrenoceptor and apoptosis protein levels in the predator scent‐induced rat model of post‐traumatic stress disorder. J Mol Neurosci. 2020;70(7):1120‐1129. [DOI] [PubMed] [Google Scholar]
- 90. Hendrickson RC, Millard SP, Pagulayan KF, Peskind ER, Raskind MA. The relative effects of prazosin on individual PTSD symptoms: evidence for pathophysiologically‐related clustering. Chronic Stress. 2021;5:2470547020979780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hudson N, Burghart S, Reynoldson J, Grauer D. Evaluation of low dose prazosin for PTSD‐associated nightmares in children and adolescents. Ment Health Clin. 2021;11(2):45‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Santivasi W, Taylor E, Christensen A, Strand J. Prazosin for nightmares in serious illness. BMJ Support Palliat Care. 2021. [DOI] [PubMed] [Google Scholar]
- 93. Wilcox CE, Adinoff B, Clifford J, et al. Brain activation and subjective anxiety during an anticipatory anxiety task is related to clinical outcome during prazosin treatment for alcohol use disorder. Neuroimage Clin. 2020;26:102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Milivojevic V, Angarita GA, Hermes G, Sinha R, Fox HC. Effects of prazosin on provoked alcohol craving and autonomic and neuroendocrine response to stress in alcohol use disorder. Alcohol Clin Exp Res. 2020;44(7):1488‐1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Golda V, Petr R. Animal model of anxiety: the effect of methyldopa in the genetically hypertensive non‐obese rats of Koletsky type and in the rats of Wistar strain. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1990;33(5):529‐537. [PubMed] [Google Scholar]
- 96. Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. Eur J Pharmacol. 2005;507(1–3):117‐124. [DOI] [PubMed] [Google Scholar]
- 97. Mineur YS, Cahuzac EL, Mose TN, et al. Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety‐ and depression‐related behaviors in mice. Neuropsychopharmacology. 2018;43(10):2118‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hoehn‐Saric R, Merchant AF, Keyser ML, Smith VK. Effects of clonidine on anxiety disorders. Arch Gen Psychiatry. 1981;38(11):1278‐1282. [DOI] [PubMed] [Google Scholar]
- 99. Srour H, Pandya K, Flannery A, Hatton K. Enteral Guanfacine to treat severe anxiety and agitation complicating critical care after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2018;22(4):403‐406. [DOI] [PubMed] [Google Scholar]
- 100. Connor DF, Grasso DJ, Slivinsky MD, Pearson GS, Banga A. An open‐label study of guanfacine extended release for traumatic stress related symptoms in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23(4):244‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Strawn JR, Compton SN, Robertson B, Albano AM, Hamdani M, Rynn MA. Extended release Guanfacine in pediatric anxiety disorders: a pilot, randomized, placebo‐controlled trial. J Child Adolesc Psychopharmacol. 2017;27(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Belkin MR, Schwartz TL. Alpha‐2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Aziriova S, Repova Bednarova K, Krajcirovicova K, et al. Doxorubicin‐induced behavioral disturbances in rats: protective effect of melatonin and captopril. Pharmacol Biochem Behav. 2014;124:284‐289. [DOI] [PubMed] [Google Scholar]
- 104. Repova K, Aziriova S, Kovacova D, et al. Lisinopril reverses behavioural alterations in spontaneously hypertensive rats. Gen Physiol Biophys. 2019;38(3):265‐270. [DOI] [PubMed] [Google Scholar]
- 105. Srinivasan J, Suresh B, Ramanathan M. Differential anxiolytic effect of enalapril and losartan in normotensive and renal hypertensive rats. Physiol Behav. 2003;78(4–5):585‐591. [DOI] [PubMed] [Google Scholar]
- 106. Feng P, Wu Z, Liu H, et al. Electroacupuncture improved chronic cerebral hypoperfusion‐induced anxiety‐like behavior and memory impairments in spontaneously hypertensive rats by downregulating the ACE/Ang II/AT1R axis and upregulating the ACE2/Ang‐(1‐7)/MasR axis. Neural Plast. 2020;2020:9076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yu Z, Zhao W, Ding L, Yu Y, Liu J. Anxiolytic effects of ACE inhibitory peptides on the behavior of rats in an elevated plus‐maze. Food Funct. 2016;7(1):491‐497. [DOI] [PubMed] [Google Scholar]
- 108. Zubenko GS, Nixon RA. Mood‐elevating effect of captopril in depressed patients. Am J Psychiatry. 1984;141(1):110‐111. [DOI] [PubMed] [Google Scholar]
- 109. Braszko JJ, Karwowska‐Polecka W, Halicka D, Gard PR. Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol. 2003;14(4):323‐343. [DOI] [PubMed] [Google Scholar]
- 110. Tashev R, Ivanova M. Involvement of hippocampal angiotensin 1 receptors in anxiety‐like behaviour of olfactory bulbectomized rats. Pharmacol Rep. 2018;70(5):847‐852. [DOI] [PubMed] [Google Scholar]
- 111. Campos GV, de Souza AMA, Ji H, et al. The Angiotensin Type 1 receptor antagonist losartan prevents ovariectomy‐induced cognitive dysfunction and anxiety‐like behavior in long Evans rats. Cell Mol Neurobiol. 2020;40(3):407‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salmani H, Hosseini M, Baghcheghi Y, Moradi‐Marjaneh R, Mokhtari‐Zaer A. Losartan modulates brain inflammation and improves mood disorders and memory impairment induced by innate immune activation: the role of PPAR‐γ activation. Cytokine. 2020;125:154860. [DOI] [PubMed] [Google Scholar]
- 113. Saavedra JM, Armando I, Bregonzio C, et al. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress‐induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 2006;31(6):1123‐1134. [DOI] [PubMed] [Google Scholar]
- 114. Goel R, Bhat SA, Hanif K, Nath C, Shukla R. Angiotensin II receptor blockers attenuate lipopolysaccharide‐induced memory impairment by modulation of NF‐κB‐mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol. 2018;55(2):1725‐1739. [DOI] [PubMed] [Google Scholar]
- 115. Benicky J, Sánchez‐Lemus E, Honda M, et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36(4):857‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sánchez‐Lemus E, Honda M, Saavedra JM. Angiotensin II AT1 receptor blocker candesartan prevents the fast up‐regulation of cerebrocortical benzodiazepine‐1 receptors induced by acute inflammatory and restraint stress. Behav Brain Res. 2012;232(1):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Singh N, Sharma G, Singh N, Hanif K. A comparative study of neuroprotective effect of single and combined blockade of AT1 receptor and PARP‐1 in focal cerebral ischaemia in rat. Int J Stroke. 2014;9(5):560‐568. [DOI] [PubMed] [Google Scholar]
- 118. Gong X, Hu H, Qiao Y, et al. The involvement of renin‐angiotensin system in lipopolysaccharide‐induced behavioral changes, neuroinflammation, and disturbed insulin signaling. Front Pharmacol. 2019;10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ayyub M, Najmi AK, Akhtar M. Protective effect of Irbesartan an Angiotensin (AT1) receptor antagonist in unpredictable chronic mild stress induced depression in mice. Drug Res. 2017;67(1):59‐64. [DOI] [PubMed] [Google Scholar]
- 120. Huber G, Ogrodnik M, Wenzel J, et al. Telmisartan prevents high‐fat diet‐induced neurovascular impairments and reduces anxiety‐like behavior. J Cereb Blood Flow Metab. 2021;17:271678X211003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shad MU. Is there an association between anxiety symptoms and valsartan treatment? J Affect Disord. 2020;261:111‐112. [DOI] [PubMed] [Google Scholar]
- 122. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin‐angiotensin pathway in posttraumatic stress disorder: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ho JK, Nation DA. Alzheimer's disease neuroimaging initiative. Memory is preserved in older adults taking AT1 receptor blockers. Alzheimer's Res Ther. 2017;9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Almeida‐Santos AF, Kangussu LM, Moreira FA, Santos RAS, Aguiar DC, Campagnole‐Santos MJ. Anxiolytic‐ and antidepressant‐like effects of angiotensin‐(1‐7) in hypertensive transgenic (mRen2)27 rats. Clin Sci. 2016;130(14):1247‐1255. [DOI] [PubMed] [Google Scholar]
- 125. Bild W, Ciobica A. Angiotensin‐(1‐7) central administration induces anxiolytic‐like effects in elevated plus maze and decreased oxidative stress in the amygdala. J Affect Disord. 2013;145(2):165‐171. [DOI] [PubMed] [Google Scholar]
- 126. Martins Lima A, Xavier CH, Ferreira AJ, et al. Activation of angiotensin‐converting enzyme 2/angiotensin‐(1‐7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol. 2013;305(7):H1057‐H1067. [DOI] [PubMed] [Google Scholar]
- 127. Oscar CG, Müller‐Ribeiro FC, de Castro LG, et al. Angiotensin‐(1‐7) in the basolateral amygdala attenuates the cardiovascular response evoked by acute emotional stress. Brain Res. 2015;1594:183‐189. [DOI] [PubMed] [Google Scholar]
- 128. Wang L, de Kloet AD, Pati D, et al. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety‐like behavior in male mice by activating central Mas receptors. Neuropharmacology. 2016;105:114‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kangussu LM, Almeida‐Santos AF, Moreira FA, et al. Reduced anxiety‐like behavior in transgenic rats with chronically overproduction of angiotensin‐(1‐7): role of the Mas receptor. Behav Brain Res. 2017;331:193‐198. [DOI] [PubMed] [Google Scholar]
- 130. Moura Santos D, Ribeiro Marins F, Limborço‐Filho M, et al. Chronic overexpression of angiotensin‐(1‐7) in rats reduces cardiac reactivity to acute stress and dampens anxious behavior. Stress. 2017;20(2):189‐196. [DOI] [PubMed] [Google Scholar]
- 131. López‐Rubalcava C, Paez‐Martinez N, Oikawa J. Blockade of corticosteroid receptors induces anxiolytic‐like effects in streptozotocin‐induced diabetic mice, and synergizes with diazepam. Behav Pharmacol. 2013;24(4):320‐327. [DOI] [PubMed] [Google Scholar]
- 132. Fox LC, Davies DR, Scholl JL, Watt MJ, Forster GL. Differential effects of glucocorticoid and mineralocorticoid antagonism on anxiety behavior in mild traumatic brain injury. Behav Brain Res. 2016;312:362‐365. [DOI] [PubMed] [Google Scholar]
- 133. Hlavacova N, Jezova D. Effect of single treatment with the antihypertensive drug eplerenone on hormone levels and anxiety‐like behaviour in rats. Endocr Regul. 2008;42(4):147‐153. [PubMed] [Google Scholar]
- 134. Ahmed AH, Gordon RD, Sukor N, Pimenta E, Stowasser M. Quality of life in patients with bilateral primary aldosteronism before and during treatment with spironolactone and/or amiloride, including a comparison with our previously published results in those with unilateral disease treated surgically. J Clin Endocrinol Metab. 2011;96(9):2904‐2911. [DOI] [PubMed] [Google Scholar]
- 135. Revuelta‐López E, Núñez J, Gastelurrutia P, et al. Neprilysin inhibition, endorphin dynamics, and early symptomatic improvement in heart failure: a pilot study. ESC Heart Fail. 2020;7(2):559‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dereli S, Kılınçel O, Çerik İB, Kaya A. Impact of sacubitril/valsartan treatment on depression and anxiety in heart failure with reduced ejection fraction. Acta Cardiol. 2020;18:1‐9. [DOI] [PubMed] [Google Scholar]
- 137. Guo Y, Zou G, Qi K, et al. Simvastatin impairs hippocampal synaptic plasticity and cognitive function in mice. Mol Brain. 2021;14(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kilic FS, Ozatik Y, Kaygisiz B, Baydemir C, Erol K. Acute antidepressant and anxiolytic effects of simvastatin and its mechanisms in rats. Neurosciences. 2012;17(1):39‐43. [PubMed] [Google Scholar]
- 139. Yan J, Huang J, Liu A, et al. Atorvastatin improves motor function, anxiety and depression by NOX2‐mediated autophagy and oxidative stress in MPTP‐lesioned mice. Aging. 2020;13(1):831‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mijailovic N, Selakovic D, Joksimovic J, et al. The anxiolytic effects of atorvastatin and simvastatin on dietary‐induced increase in homocysteine levels in rats. Mol Cell Biochem. 2019;452(1–2):199‐217. [DOI] [PubMed] [Google Scholar]
- 141. Citraro R, Chimirri S, Aiello R, et al. Protective effects of some statins on epileptogenesis and depressive‐like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55(8):1284‐1291. [DOI] [PubMed] [Google Scholar]
- 142. Evangelista FF, Costa‐Ferreira W, Mantelo FM, et al. Rosuvastatin revert memory impairment and anxiogenic‐like effect in mice infected with the chronic ME‐49 strain of Toxoplasma gondii . PLOS One. 2021;16(4):e0250079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Okudan N, Belviranli M. High dose simvastatin and rosuvastatin impair cognitive abilities of healthy rats via decreasing hippocampal neurotrophins and irisin. Brain Res Bull. 2020;165:81‐89. [DOI] [PubMed] [Google Scholar]
- 144. Mirzaei E, Mirjalili M, Jahangard L, et al. Influence of simvastatin as augmentative therapy in the treatment of generalized anxiety disorder: a pilot randomized, placebo‐controlled study. Neuropsychobiology. 2021;80(3):242‐252. [DOI] [PubMed] [Google Scholar]
- 145. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid‐lowering agents. Drug Saf. 2007;30(3):195‐201. [DOI] [PubMed] [Google Scholar]
- 146. Cham S, Koslik HJ, Golomb BA. Mood, personality, and behavior changes during treatment with statins: a case series. Drug Saf Case Rep. 2016;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Golomb BA, Kane T, Dimsdale JE. Severe irritability associated with statin cholesterol‐lowering drugs. QJM. 2004;97(4):229‐235. [DOI] [PubMed] [Google Scholar]
- 148. Duits N, Bos FM. Depressive symptoms and cholesterol‐lowering drugs. Lancet. 1993;341(8837):114. [DOI] [PubMed] [Google Scholar]
- 149. Korhonen MJ, Pentti J, Hartikainen J, Kivimäki M, Vahtera J. Somatic symptoms of anxiety and nonadherence to statin therapy. Int J Cardiol. 2016;214:493‐499. [DOI] [PubMed] [Google Scholar]
- 150. Molero Y, Cipriani A, Larsson H, Lichtenstein P, D'Onofrio BM, Fazel S. Associations between statin use and suicidality, depression, anxiety, and seizures: a Swedish total‐population cohort study. Lancet Psychiatry. 2020;7(11):982‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sarahian N, Mohammadi MT, Darabi S, Faghihi N. Fenofibrate protects the neurovascular unit and ameliorates plasma corticosterone levels in pentylenetetrazole‐induced kindling seizure in mice. Brain Res. 2021;1758:147343. [DOI] [PubMed] [Google Scholar]
- 152. Mirza R, Sharma B. Selective modulator of peroxisome proliferator‐activated receptor‐α protects propionic acid induced autism‐like phenotypes in rats. Life Sci. 2018;214:106‐117. [DOI] [PubMed] [Google Scholar]
- 153. Mirza R, Sharma B. Benefits of Fenofibrate in prenatal valproic acid‐induced autism spectrum disorder related phenotype in rats. Brain Res Bull. 2019;147:36‐46. [DOI] [PubMed] [Google Scholar]
- 154. Locci A, Pinna G. Stimulation of peroxisome proliferator‐activated receptor‐α by N‐palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol Psychiatry. 2019;85(12):1036‐1045. [DOI] [PubMed] [Google Scholar]
- 155. Aziriova S, Repova K, Krajcirovicova K, et al. Effect of ivabradine, captopril and melatonin on the behaviour of rats in L‐nitro‐arginine methyl ester‐induced hypertension. J Physiol Pharmacol. 2016;67(6):895‐902. [PubMed] [Google Scholar]
- 156. Krajcirovicova K, Aziriova S, Baka T, et al. Ivabradine does not impair anxiety‐like behavior and memory in both healthy and L‐NAME‐induced hypertensive rats. Physiol Res. 2018;67(Supplum 4):S655‐S664. [DOI] [PubMed] [Google Scholar]
- 157. Riccioni G, Masciocco L, Benvenuto A, et al. Ivabradine improves quality of life in subjects with chronic heart failure compared to treatment with β‐blockers: results of a multicentric observational APULIA study. Pharmacology. 2013;92(5–6):276‐280. [DOI] [PubMed] [Google Scholar]
- 158. Zugck C, Martinka P, Stöckl G. Ivabradine treatment in a chronic heart failure patient cohort: symptom reduction and improvement in quality of life in clinical practice. Adv Ther. 2014;31(9):961‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Riccioni G, Prencipe G, Benvenuto A, et al. Ivabradine improves all aspects of quality of life assessed with the 36‐item short form health survey in subjects with chronic ischemic heart disease compared with beta‐blockers. Pharmacology. 2013;91(1–2):35‐38. [DOI] [PubMed] [Google Scholar]
- 160. Fulga IG, Stroescu V. Experimental research on the effect of calcium channel blockers nifedipine and verapamil on the anxiety in mice. Rom J Physiol. 1997;34(1–4):127‐136. [PubMed] [Google Scholar]
- 161. Joseph A, Thuy TTT, Thanh LT, Okada M. Antidepressive and anxiolytic effects of ostruthin, a TREK‐1 channel activator. PLOS One. 2018;13(8):e0201092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Klein E, Geraci M, Uhde TW. Inefficacy of single‐dose nifedipine in the treatment of phobic anxiety. Isr J Psychiatry Relat Sci. 1990;27(2):119‐123. [PubMed] [Google Scholar]
- 163. Krystal AD, Sutherland J, Hochman DW. Loop diuretics have anxiolytic effects in rat models of conditioned anxiety. PLOS One. 2012;7(4):e35417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Farajdokht F, Babri S, Karimi P, Alipour MR, Bughchechi R, Mohaddes G. Chronic ghrelin treatment reduced photophobia and anxiety‐like behaviors in nitroglycerin‐ induced migraine: role of pituitary adenylate cyclase‐activating polypeptide. Eur J Neurosci. 2017;45(6):763‐772. [DOI] [PubMed] [Google Scholar]
- 165. Taheri P, Mohammadi F, Nazeri M, et al. Nitric oxide role in anxiety‐like behavior, memory and cognitive impairments in animal model of chronic migraine. Heliyon. 2020;6(12):e05654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Magyar‐Russell G, Thombs BD, Cai JX, et al. The prevalence of anxiety and depression in adults with implantable cardioverter defibrillators: a systematic review. J Psychosom Res. 2011;71(4):223‐231. [DOI] [PubMed] [Google Scholar]
- 167. Jaillon P. Clinical pharmacokinetics of prazosin. Clin Pharmacokinet. 1980;5(4):365‐376. [DOI] [PubMed] [Google Scholar]
- 168. Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev. 2009;4:CD003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Garrett BN, Kaplan NM. Clonidine in the treatment of hypertension. J Cardiovasc Pharmacol. 1980;2(Suppl 1):S61‐S71. [DOI] [PubMed] [Google Scholar]
- 170. Sorkin EM, Heel RC. Guanfacine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31(4):301‐336. [DOI] [PubMed] [Google Scholar]
- 171. Neil MJ. Clonidine: clinical pharmacology and therapeutic use in pain management. Curr Clin Pharmacol. 2011;6(4):280‐287. [DOI] [PubMed] [Google Scholar]
- 172. Osland ST, Steeves TD, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2018;6:CD007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Childress A, Hoo‐Cardiel A, Lang P. Evaluation of the current data on guanfacine extended release for the treatment of ADHD in children and adolescents. Expert Opin Pharmacother. 2020;21(4):417‐426. [DOI] [PubMed] [Google Scholar]
- 174. Vian J, Pereira C, Chavarria V, et al. The renin‐angiotensin system: a possible new target for depression. BMC Med. 2017;15(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Brownstein DJ, Salagre E, Köhler C, et al. Blockade of the angiotensin system improves mental health domain of quality of life: a meta‐analysis of randomized clinical trials. Aust N Z J Psychiatry. 2018;52(1):24‐38. [DOI] [PubMed] [Google Scholar]
- 176. Regulski M, Regulska K, Stanisz BJ, et al. Chemistry and pharmacology of angiotensin‐converting enzyme inhibitors. Curr Pharm Des. 2015;21(13):1764‐1775. [DOI] [PubMed] [Google Scholar]
- 177. Repová‐Bednárová K, Aziriová S, Hrenák J, et al. Effect of captopril and melatonin on fibrotic rebuilding of the aorta in 24 hour light‐induced hypertension. Physiol Res. 2013;62(Suppl 1):S135‐S141. [DOI] [PubMed] [Google Scholar]
- 178. Simko F, Pechanova O, Repova K, et al. Lactacystin‐induced model of hypertension in rats: effects of melatonin and captopril. Int J Mol Sci. 2017;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Simko F, Pechanova O, Krajcirovicova K, et al. Effects of captopril, spironolactone, and simvastatin on the cardiovascular system of non‐diseased Wistar rats. Int J Cardiol. 2015;190:128‐130. [DOI] [PubMed] [Google Scholar]
- 180. Simko F, Pechanova O, Pelouch V, et al. Continuous light and L‐NAME‐induced left ventricular remodelling: different protection with melatonin and captopril. J Hypertens. 2010;28(Suppl 1):S13‐S18. [DOI] [PubMed] [Google Scholar]
- 181. Simko F, Simko J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiol Res. 2000;49(1):37‐46. [PubMed] [Google Scholar]
- 182. Simko F, Simko J. Heart failure and angiotensin converting enzyme inhibition: problems and perspectives. Physiol Res. 1999;48(1):1‐8. [PubMed] [Google Scholar]
- 183. Simko F, Pechanova O, Repova Bednarova K, et al. Hypertension and cardiovascular remodelling in rats exposed to continuous light: protection by ACE–inhibition and melatonin. Mediators of Inflamm. 2014;2014:703175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. CAPP Study Group . Effect of angiotensin‐converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPP) randomized trial. The Captopril Prevention Project (CAPP) Study Group. Curr Hypertens Rep. 1999;1(6):466‐467. [PubMed] [Google Scholar]
- 185. UKPDS Group . Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):713‐720. [PMC free article] [PubMed] [Google Scholar]
- 186. Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669‐677. [DOI] [PubMed] [Google Scholar]
- 187. Køber L, Torp‐Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin‐converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333(25):1670‐1676. [DOI] [PubMed] [Google Scholar]
- 188. CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429‐1435. [DOI] [PubMed] [Google Scholar]
- 189. Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine‐isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325(5):303‐310. [DOI] [PubMed] [Google Scholar]
- 190. SOLVD Investigators , Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685‐691. [DOI] [PubMed] [Google Scholar]
- 191. SOLVD Investigators , Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293‐302. [DOI] [PubMed] [Google Scholar]
- 192. Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin‐converting‐enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368(9535):581‐588. [DOI] [PubMed] [Google Scholar]
- 193. Saavedra JM. Beneficial effects of Angiotensin II receptor blockers in brain disorders. Pharmacol Res. 2017;125(Pt A):91‐103. [DOI] [PubMed] [Google Scholar]
- 194. Genaro K, Fabris D, Fachim HA, Prado WA. Angiotensin AT1 receptors modulate the anxiogenic effects of angiotensin (5‐8) injected into the rat ventrolateral periaqueductal gray. Peptides. 2017;96:8‐14. [DOI] [PubMed] [Google Scholar]
- 195. Guimond MO, Gallo‐Payet N. The Angiotensin II type 2 receptor in brain functions: an update. Int J Hypertens. 2012;2012:351758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin‐(1‐7)/MAS axis of the renin‐angiotensin system: focus on angiotensin‐(1‐7). Physiol Rev. 2018;98(1):505‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709‐717. [DOI] [PubMed] [Google Scholar]
- 198. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309‐1321. [DOI] [PubMed] [Google Scholar]
- 199. Grossman E, Nadler M, Sharabi Y, Thaler M, Shachar A, Shamiss A. Antianxiety treatment in patients with excessive hypertension. Am J Hypertens. 2005;18(9 Pt 1):1174‐1177. [DOI] [PubMed] [Google Scholar]
- 200. Omboni S, Volpe M. Angiotensin receptor blockers versus angiotensin converting enzyme inhibitors for the treatment of arterial hypertension and the role of olmesartan. Adv Ther. 2019;36(2):278‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Simko F, Simko J, Fabryova M. ACE‐inhibition and angiotensin II receptor blockers in chronic heart failure: pathophysiological consideration of the unresolved battle. Cardiovasc Drugs Ther. 2003;17(3):287‐290. [DOI] [PubMed] [Google Scholar]
- 202. Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995‐1003. [DOI] [PubMed] [Google Scholar]
- 203. Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet. 2003;362(9386):759‐766. [DOI] [PubMed] [Google Scholar]
- 204. Tokmakova M, Solomon SD. Inhibiting the renin‐angiotensin system in myocardial infarction and heart failure: lessons from SAVE, VALIANT and CHARM, and other clinical trials. Curr Opin Cardiol. 2006;21(4):268‐272. [DOI] [PubMed] [Google Scholar]
- 205. Boal AH, Smith DJ, McCallum L, et al. Monotherapy with major antihypertensive drug classes and risk of hospital admissions for mood disorders. Hypertension. 2016;68(5):1132‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Anil Kumar KV, Nagwar S, Thyloor R, Satyanarayana S. Anti‐stress and nootropic activity of drugs affecting the renin‐angiotensin system in rats based on indirect biochemical evidence. J Renin Angiotensin Aldosterone Syst. 2015;16(4):801‐812. [DOI] [PubMed] [Google Scholar]
- 207. Ping G, Qian W, Song G, Zhaochun S. Valsartan reverses depressive/anxiety‐like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol Biochem Behav. 2014;124:5‐12. [DOI] [PubMed] [Google Scholar]
- 208. Hrenak J, Paulis L, Simko F. Angiotensin A/Alamandine/MrgD Axis: another clue to understanding cardiovascular pathophysiology. Int J Mol Sci. 2016;17(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Simko F, Baka T. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers: potential allies in the COVID‐19 pandemic instead of a threat? Clin Sci. 2021;135(8):1009‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Steckelings UM, Sumners C. Correcting the imbalanced protective RAS in COVID‐19 with angiotensin AT2‐receptor agonists. Clin Sci. 2020;134(22):2987‐3006. [DOI] [PubMed] [Google Scholar]
- 211. Chaves GZ, Caligiorne SM, Santos RA, Khosla MC, Campagnole‐Santos MJ. Modulation of the baroreflex control of heart rate by angiotensin‐(1‐7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J Hypertens. 2000;18(12):1841‐1848. [DOI] [PubMed] [Google Scholar]
- 212. Guimaraes PS, Oliveira MF, Braga JF, et al. Increasing angiotensin‐(1‐7) levels in the brain attenuates metabolic syndrome‐related risks in fructose‐fed rats. Hypertension. 2014;63(5):1078‐1085. [DOI] [PubMed] [Google Scholar]
- 213. Guimaraes PS, Santiago NM, Xavier CH, et al. Chronic infusion of angiotensin‐(1‐7) into the lateral ventricle of the brain attenuates hypertension in DOCA‐salt rats. Am J Physiol Heart Circ Physiol. 2012;303(3):H393‐H400. [DOI] [PubMed] [Google Scholar]
- 214. Nautiyal M, Shaltout HA, de Lima DC, do Nascimento K, Chappell MC, Diz DI. Central angiotensin‐(1‐7) improves vagal function independent of blood pressure in hypertensive (mRen2)27 rats. Hypertension. 2012;60(5):1257‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin‐angiotensin system in protection against angiotensin II‐induced sensitization of hypertension. Am J Physiol Heart Circ Physiol. 2014;307(2):H191‐H198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Moreno‐Santos B, Marchi‐Coelho C, Costa‐Ferreira W, Crestani CC. Angiotensinergic receptors in the medial amygdaloid nucleus differently modulate behavioral responses in the elevated plus‐maze and forced swimming test in rats. Behav Brain Res. 2021;397:112947. [DOI] [PubMed] [Google Scholar]
- 217. Grundy HM, Simpson SA, Tait JF. Isolation of a highly active mineralocorticoid from beef adrenal extract. Nature. 1952;169(4306):795‐796. [DOI] [PubMed] [Google Scholar]
- 218. Arriza JL, Weinberger C, Cerelli G, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268‐275. [DOI] [PubMed] [Google Scholar]
- 219. Young MJ, Rickard AJ. Mechanisms of mineralocorticoid salt‐induced hypertension and cardiac fibrosis. Mol Cell Endocrinol. 2012;350(2):248‐255. [DOI] [PubMed] [Google Scholar]
- 220. Dettli L, Spring P. Therapy with combinations of diuretic agents: comparative studies. Ann N Y Acad Sci. 1966;139(2):471‐480. [DOI] [PubMed] [Google Scholar]
- 221. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53‐e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49(4):839‐845. [DOI] [PubMed] [Google Scholar]
- 223. Liu L, Xu B, Ju Y. Addition of spironolactone in patients with resistant hypertension: a meta‐analysis of randomized controlled trials. Clin Exp Hypertens. 2017;39(3):257‐263. [DOI] [PubMed] [Google Scholar]
- 224. Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol. 2009;297(3):F559‐F576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Hlavacova N, Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety‐like behavior. Horm Behav. 2008;54(1):90‐97. [DOI] [PubMed] [Google Scholar]
- 226. Sonino N, Fallo F, Fava GA. Psychological aspects of primary aldosteronism. Psychother Psychosom. 2006;75(5):327‐330. [DOI] [PubMed] [Google Scholar]
- 227. Khurshid KA, Weaver ME. Conn's syndrome presenting as depression. Am J Psychiatry. 2005;162(6):1226. [DOI] [PubMed] [Google Scholar]
- 228. Sonino N, Tomba E, Genesia ML, et al. Psychological assessment of primary aldosteronism: a controlled study. J Clin Endocrinol Metab. 2011;96(6):E878‐E883. [DOI] [PubMed] [Google Scholar]
- 229. Sukor N, Kogovsek C, Gordon RD, Robson D, Stowasser M. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2010;95(3):1360‐1364. [DOI] [PubMed] [Google Scholar]
- 230. McMurray JJV. Neprilysin inhibition to treat heart failure: a tale of science, serendipity, and second chances. Eur J Heart Fail. 2015;17(3):242‐247. [DOI] [PubMed] [Google Scholar]
- 231. Simko F, Dukat A. Inhibition of neprilysin – new horizon in heart failure therapy. Cardiol Lett. 2016;25(4):273‐276. [Google Scholar]
- 232. Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B‐type natriuretic peptide. J Am Coll Cardiol. 2012;60(22):2305‐2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Vanneste Y, Michel A, Dimaline R, Najdovski T, Deschodt‐Lanckman M. Hydrolysis of alpha‐human atrial natriuretic peptide in vitro by human kidney membranes and purified endopeptidase‐24.11. Evidence for a novel cleavage site. Biochem J. 1988;254(2):531‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54‐61. [DOI] [PubMed] [Google Scholar]
- 235. McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to determine impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail. 2013;15(9):1062‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. D'Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017;19(6):710‐717. [DOI] [PubMed] [Google Scholar]
- 237. Nougué H, Pezel T, Picard F, et al. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail. 2019;21(5):598‐605. [DOI] [PubMed] [Google Scholar]
- 238. Gregoriou GC, Patel SD, Winters BL, Bagley EE. Neprilysin controls the synaptic activity of neuropeptides in the intercalated cells of the Amygdala. Mol Pharmacol. 2020;98(4):454‐461. [DOI] [PubMed] [Google Scholar]
- 239. Meyer T, Herrmann‐Lingen C. Natriuretic peptides in anxiety and panic disorder. Vitam Horm. 2017;103:131‐145. [DOI] [PubMed] [Google Scholar]
- 240. Marazziti D, Barberi FM, Mucci F, Maglio A, Dell'Oste V, Dell'Osso L. The emerging role of atrial natriuretic peptide in psychiatry. Curr Med Chem. 2020;28:69‐79. [DOI] [PubMed] [Google Scholar]
- 241. Meyer T, Herrrmann‐Lingen C, Chavanon ML, et al. Higher plasma levels of MR‐pro‐atrial natriuretic peptide are linked to less anxiety: results from the observational DIAST‐CHF study. Clin Res Cardiol. 2015;104(7):574‐581. [DOI] [PubMed] [Google Scholar]
- 242. Wiedemann K, Jahn H, Kellner M. Effects of natriuretic peptides upon hypothalamo‐pituitary‐adrenocortical system activity and anxiety behaviour. Exp Clin Endocrinol Diabetes. 2000;108(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 243. Ströhle A, Feller C, Strasburger CJ, Heinz A, Dimeo F. Anxiety modulation by the heart? Aerobic exercise and atrial natriuretic peptide. Psychoneuroendocrinology. 2006;31(9):1127‐1130. [DOI] [PubMed] [Google Scholar]
- 244. Iftikhar K, Siddiq A, Baig SG, Zehra S. Substance P: a neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides. 2020;79:101993. [DOI] [PubMed] [Google Scholar]
- 245. Hoppe JM, Frick A, Åhs F, et al. Association between amygdala neurokinin‐1 receptor availability and anxiety‐related personality traits. Transl Psychiatry. 2018;8(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21(2):138‐167. [DOI] [PubMed] [Google Scholar]
- 247. Daukantaitė D, Tellhed U, Maddux RE, Svensson T, Melander O. Five‐week yin yoga‐based interventions decreased plasma adrenomedullin and increased psychological health in stressed adults: a randomized controlled trial. PLOS One. 2018;13(7):e0200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Kong DG, Gao H, Lu YQ, et al. Anxiety disorders are associated with increased plasma adrenomedullin level and left ventricular hypertrophy in patients with hypertension. Clin Exp Hypertens. 2014;36(1):27‐31. [DOI] [PubMed] [Google Scholar]
- 249. Fernández AP, Serrano J, Tessarollo L, Cuttitta F, Martínez A. Lack of adrenomedullin in the mouse brain results in behavioral changes, anxiety, and lower survival under stress conditions. Proc Natl Acad Sci USA. 2008;105(34):12581‐12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Rouhiainen A, Kulesskaya N, Mennesson M, et al. The bradykinin system in stress and anxiety in humans and mice. Sci Rep. 2019;9(1):19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Câmara AB, Brandão IA. Neural receptors associated with depression: a systematic review of the past 10 years. CNS Neurol Disord Drug Targets. 2020;19(6):417‐436. [DOI] [PubMed] [Google Scholar]
- 252. Luo H, Wu PF, Cao Y, et al. Angiotensin‐converting enzyme inhibitor rapidly ameliorates depressive‐type behaviors via bradykinin‐dependent activation of mammalian target of rapamycin complex 1. Biol Psychiatry. 2020;88(5):415‐425. [DOI] [PubMed] [Google Scholar]
- 253. Istvan ES. Structural mechanism for statin inhibition of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase. Am Heart J. 2002;144(6 Suppl):S27‐S32. [DOI] [PubMed] [Google Scholar]
- 254. Cholesterol Treatment Trialists' Collaboration . Efficacy and safety of statin therapy in older people: a meta‐analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120(1):229‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Simko F. Statins: a perspective for left ventricular hypertrophy treatment. Eur J Clin Invest. 2007;37(9):681‐691. [DOI] [PubMed] [Google Scholar]
- 257. Simko F, Pechanova O, Pelouch V, et al. Effect of melatonin, captopril, spironolactone and simvastatin on blood pressure and left ventricular remodelling in spontaneously hypertensive rats. J Hypertens Suppl. 2009;27(6):S5‐S10. [DOI] [PubMed] [Google Scholar]
- 258. Simko F, Matuskova J, Luptak I, et al. Effect of simvastatin on remodeling of the left ventricle and aorta in L‐NAME‐induced hypertension. Life Sci. 2004;74(10):1211‐1224. [DOI] [PubMed] [Google Scholar]
- 259. Apostolopoulou M, Corsini A, Roden M. The role of mitochondria in statin‐induced myopathy. Eur J Clin Invest. 2015;45(7):745‐754. [DOI] [PubMed] [Google Scholar]
- 260. Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin(1A) receptors. Biochemistry. 2010;49(26):5426‐5435. [DOI] [PubMed] [Google Scholar]
- 261. Wallner B, Machatschke IH. The evolution of violence in men: the function of central cholesterol and serotonin. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):391‐397. [DOI] [PubMed] [Google Scholar]
- 262. Golomb BA, Stattin H, Mednick S. Low cholesterol and violent crime. J Psychiatr Res. 2000;34(4):301‐309. [DOI] [PubMed] [Google Scholar]
- 263. Messaoud A, Mensi R, Mrad A, et al. Is low total cholesterol levels associated with suicide attempt in depressive patients? Ann Gen Psychiatry. 2017;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Suneson K, Asp M, Träskman‐Bendz L, Westrin Å, Ambrus L, Lindqvist D. Low total cholesterol and low‐density lipoprotein associated with aggression and hostility in recent suicide attempters. Psychiatry Res. 2019;273:430‐434. [DOI] [PubMed] [Google Scholar]
- 265. Garland MR, Hallahan B, McNamara M, et al. Lipids and essential fatty acids in patients presenting with self‐harm. Br J Psychiatry. 2007;190:112‐117. [DOI] [PubMed] [Google Scholar]
- 266. Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009;390(4):287‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Pfrieger FW, Ungerer N. Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res. 2011;50(4):357‐371. [DOI] [PubMed] [Google Scholar]
- 268. Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45(8):1375‐1397. [DOI] [PubMed] [Google Scholar]
- 269. Muldoon MF, Kaplan JR, Manuck SB, Mann JJ. Effects of a low‐fat diet on brain serotonergic responsivity in cynomolgus monkeys. Biol Psychiatry. 1992;31(7):739‐742. [DOI] [PubMed] [Google Scholar]
- 270. Kaplan JR, Manuck SB, Shively C. The effects of fat and cholesterol on social behavior in monkeys. Psychosom Med. 1991;53(6):634‐642. [DOI] [PubMed] [Google Scholar]
- 271. Markianos M, Koutsis G, Evangelopoulos ME, Sfagos C. Serum total cholesterol correlates positively to central serotonergic turnover in male but not in female subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):527‐531. [DOI] [PubMed] [Google Scholar]
- 272. Mann JJ, Arango V, Marzuk PM, Theccanat S, Reis DJ. Evidence for the 5‐HT hypothesis of suicide. A review of post‐mortem studies. Br J Psychiatry Suppl. 1989;8:7‐14. [PubMed] [Google Scholar]
- 273. Lidberg L, Asberg M, Sundqvist‐Stensman UB. 5‐Hydroxyindoleacetic acid levels in attempted suicides who have killed their children. Lancet. 1984;2(8408):928. [DOI] [PubMed] [Google Scholar]
- 274. Lidberg L, Tuck JR, Asberg M, Scalia‐Tomba GP, Bertilsson L. Homicide, suicide and CSF 5‐HIAA. Acta Psychiatr Scand. 1985;71(3):230‐236. [DOI] [PubMed] [Google Scholar]
- 275. Liu A, Patterson AD, Yang Z, et al. Fenofibrate metabolism in the cynomolgus monkey using ultraperformance liquid chromatography‐quadrupole time‐of‐flight mass spectrometry‐based metabolomics. Drug Metab Dispos. 2009;37(6):1157‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Silva FC, Paiva FA, Müller‐Ribeiro FC, et al. Chronic treatment with ivabradine does not affect cardiovascular autonomic control in rats. Front Physiol. 2016;7:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet. 2010;376(9744):875‐885. [DOI] [PubMed] [Google Scholar]
- 278. Baka T, Simko F. Ivabradine modulates the autonomic nervous system by affecting the “little brain” of the heart: a hypothesis. Med Hypotheses. 2019;129:109253. [DOI] [PubMed] [Google Scholar]
- 279. Baka T, Simko F. Ivabradine reversed nondipping heart rate in rats with l‐NAME‐induced hypertension. Clin Exp Pharmacol Physiol. 2019;46(6):607‐610. [DOI] [PubMed] [Google Scholar]
- 280. Heusch G, Kleinbongard P. Ivabradine: cardioprotection by and beyond heart rate reduction. Drugs. 2016;76(7):733‐740. [DOI] [PubMed] [Google Scholar]
- 281. Simko F, Baka T, Poglitsch M, et al. Effect of ivabradine on a hypertensive heart and the renin‐angiotensin‐aldosterone system in L‐NAME‐induced hypertension. Int J Mol Sci. 2018;19(10):3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Simko F, Baka T. Chronotherapy as a potential approach to hypertensive patients with elevated heart rate? Br J Clin Pharmacol. 2019;85(8):1861‐1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Simko F, Baka T. Ivabradine and blood pressure reduction: underlying pleiotropic mechanisms and clinical implications. Front Cardiovasc Med. 2021;8:607998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Oliphant CS, Owens RE, Bolorunduro OB, Jha SK. Ivabradine: a review of labeled and off‐label uses. Am J Cardiovasc Drugs. 2016;16(5):337‐347. [DOI] [PubMed] [Google Scholar]
- 285. Simko F, Baka T, Repova K, et al. Ivabradine improves survival and attenuates cardiac remodeling in isoproterenol‐induced myocardial injury. Fundam Clin Pharmacol. 2021;35(4):744‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Cavalcante TMB, De Melo JMA J, Lopes LB, et al. Ivabradine possesses anticonvulsant and neuroprotective action in mice. Biomed Pharmacother. 2019;109:2499‐2512. [DOI] [PubMed] [Google Scholar]
- 287. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269‐1324. [DOI] [PubMed] [Google Scholar]
- 288. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44‐e164. [DOI] [PubMed] [Google Scholar]
- 289. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199‐e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39(2):187‐192. [DOI] [PubMed] [Google Scholar]
- 292. Meena J, Bagga A. Current perspectives in management of edema in nephrotic syndrome. Indian J Pediatr. 2020;87(8):633‐640. [DOI] [PubMed] [Google Scholar]
- 293. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139‐e228. [DOI] [PubMed] [Google Scholar]
- 294. O'gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;61(4):e78‐e140. [DOI] [PubMed] [Google Scholar]
- 295. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 296. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776‐803. [DOI] [PubMed] [Google Scholar]
- 297. Defibrillators | NHLBI, NIH.
- 298. Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry's third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6(9):1397‐1401. [DOI] [PubMed] [Google Scholar]
- 299. Kamphuis HCM, De Leeuw JRJ, Derksen R, Hauer R, Winnubst JaM. A 12‐month quality of life assessment of cardiac arrest survivors treated with or without an implantable cardioverter defibrillator. Europace. 2002;4(4):417‐425. [DOI] [PubMed] [Google Scholar]
- 300. Sears SF, Burns JL, Handberg E, Sotile WM, Conti JB. Young at heart: understanding the unique psychosocial adjustment of young implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol. 2001;24(7):1113‐1117. [DOI] [PubMed] [Google Scholar]
- 301. Sears SF, Todaro JF, Lewis TS, Sotile W, Conti JB. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol. 1999;22(7):481‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Burke JL, Hallas CN, Clark‐Carter D, White D, Connelly D. The psychosocial impact of the implantable cardioverter defibrillator: a meta‐analytic review. Br J Health Psychol. 2003;8(Pt 2):165‐178. [DOI] [PubMed] [Google Scholar]
- 303. Andersen CM, Theuns DAMJ, Johansen JB, Pedersen SS. Anxiety, depression, ventricular arrhythmias and mortality in patients with an implantable cardioverter defibrillator: 7 years' follow‐up of the MIDAS cohort. Gen Hosp Psychiatry. 2020;66:154‐160. [DOI] [PubMed] [Google Scholar]
- 304. Thylén I, Dekker RL, Jaarsma T, Strömberg A, Moser DK. Characteristics associated with anxiety, depressive symptoms, and quality‐of‐life in a large cohort of implantable cardioverter defibrillator recipients. J Psychosom Res. 2014;77(2):122‐127. [DOI] [PubMed] [Google Scholar]
- 305. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933‐1940. [DOI] [PubMed] [Google Scholar]
- 306. Sears SF, Sowell LD, Kuhl EA, et al. The ICD shock and stress management program: a randomized trial of psychosocial treatment to optimize quality of life in ICD patients. Pacing Clin Electrophysiol. 2007;30(7):858‐864. [DOI] [PubMed] [Google Scholar]
- 307. Frizelle DJ, Lewin RJ, Kaye G, et al. Cognitive‐behavioural rehabilitation programme for patients with an implanted cardioverter defibrillator: a pilot study. Br J Health Psychol. 2004;9(Pt 3):381‐392. [DOI] [PubMed] [Google Scholar]
- 308. Senthilkumar A, Subitha L, Saravanan E, Giriyappa DK, Satheesh S, Menon V. Depressive symptoms and health‐related quality of life in patients with cardiovascular diseases attending a tertiary care hospital, Puducherry—a cross‐sectional study. J Neurosci Rural Pract. 2021;12(2):376‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Korosi B, Laszlo A, Tabak A, et al. The impact of currently recommended antihypertensive therapy on depression and other psychometric parameters: preliminary communication. Neuropsychopharmacol Hung. 2017;19(1):11‐22. [PubMed] [Google Scholar]
- 310. Gorre F, Vandekerckhove H. Beta‐blockers: focus on mechanism of action. Which beta‐blocker, when and why? Acta Cardiol. 2010;65(5):565‐570. [DOI] [PubMed] [Google Scholar]
- 311. Hrenak J, Simko F. Renin‐angiotensin system: an important player in the pathogenesis of acute respiratory distress syndrome. Int J Mol Sci. 2020;21(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. De Pinieux G, Chariot P, Ammi‐Saïd M, et al. Lipid‐lowering drugs and mitochondrial function: effects of HMG‐CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br J Clin Pharmacol. 1996;42(3):333‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Palomäki A, Malminiemi K, Metsä‐Ketelä T. Enhanced oxidizability of ubiquinol and alpha‐tocopherol during lovastatin treatment. FEBS Lett. 1997;410(2–3):254‐258. [DOI] [PubMed] [Google Scholar]
- 314. Simko F, Hrenak J, Dominguez‐Rodriguez A, Reiter RJ. Melatonin as a putative protection against myocardial injury in COVID‐19 infection. Expert Rev Clin Pharmacol. 2020;13(9):921‐924. [DOI] [PubMed] [Google Scholar]
- 315. Azevedo RB, Botelho BG, Hollanda J, et al. Covid‐19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35(1):4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Wong SH, Lui RN, Sung JJ. Covid‐19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744‐748. [DOI] [PubMed] [Google Scholar]
- 317. Alomari SO, Abou‐Mrad Z, Bydon A. COVID‐19 and the central nervous system. Clin Neurol Neurosurg. 2020;198:106116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Kamal AT, Sukhia RH, Ghandhi D, Sukhia HR. Stress and anxiety among dental practitioners during the COVID‐19 pandemic: a cross‐sectional survey. Dent Med Probl. 2021;58:139‐146. [DOI] [PubMed] [Google Scholar]
- 319. Conti C, Fontanesi L, Lanzara R, Rosa I, Doyle RL, Porcelli P. Burnout status of Italian healthcare workers during the first COVID‐19 pandemic peak period. Healthcare. 2021;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Gilleen J, Santaolalla A, Valdearenas L, Salice C, Fusté M. Impact of the COVID‐19 pandemic on the mental health and well‐being of UK healthcare workers. BJPsych Open. 2021;7(3):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Yu A, Wilkes M, Iosif AM, et al. Exploring the relationships between resilience and news monitoring with COVID distress in health profession students. Acad Psychiatry. 2021;45:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Martínez‐López JÁ, Lázaro‐Pérez C, Gómez‐Galán J. Death anxiety in social workers as a consequence of the COVID‐19 pandemic. Behav Sci. 2021;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID‐19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Simko F, Pechanova O, Repova Bednarova K, et al . Hypertension and cardiovascular remodelling in rats exposed to continuous light: protection by ACE‐inhibition and melatonin. Mediators of Inflamm. 2014;2014:703175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


