Abstract
Background and objective
Transcervical inflatable mediastinoscopic esophagectomy (TIME) is a novel method of minimally invasive esophagectomy (MIE) for esophageal cancer. However, whether TIME is effective and feasible as conventional MIE remains unclear. This study aimed to evaluate the efficacy of TIME by comparing it with thoracoscopic esophagectomy (TE).
Methods
Surgical outcomes and relapse‐free survival (RFS) rates of patients with local early‐ or intermediate‐stage thoracic esophageal squamous cell carcinoma that underwent TIME or TE from January 2017 to December 2019 were analyzed in this retrospective study. Propensity score matching was used to control the confounding factors.
Results
The mean operation time in TIME was shorter than that in TE (p < 0.05). Patients in the TIME group achieved postoperative ambulation earlier than those in the TE group (p < 0.05). The rate of pulmonary complications was lower in TIME than in TE (p < 0.05). The number of lymph nodes harvested during surgery and the RFS rates of two groups did not have significant differences.
Conclusion
TIME may be a feasible and safe method to treat local early‐ and intermediate‐stage thoracic esophageal squamous cell carcinoma effectively and it could be a supplementary surgical method of TE for patients with poor pulmonary function or cannot undergo TE.
Keywords: esophageal cancer, minimally invasive esophagectomy, thoracoscopic esophagectomy, transcervical inflatable mediastinoscopic esophagectomy
Abbreviations
- ASA
American Society of Anesthesiologists
- CT
computed tomography
- GE
gastroesophageal
- MIE
minimally invasive esophagectomy
- POD
postoperative day
- RAMIE
robot‐assisted minimally invasive esophagectomy
- RFS
relapse‐free survival
- RLN
recurrent laryngeal nerve
- TE
thoracoscopic esophagectomy
- TIME
transcervical inflatable mediastinoscopic esophagectomy
1. INTRODUCTION
Esophageal cancer, one of the most common malignant tumors, has high morbidity and a poor prognosis. 1 Surgical resection remains the primary treatment for this digestive system cancer. 2 Although radical esophagectomy with a three‐field approach is the standard and classic method for treating esophageal cancer, this method also has been considered to be one of the most invasive surgeries. 3 , 4 In 1992, the world's first thoracoscopic esophagectomy (TE) was successfully performed. 5 Then, minimally invasive surgery of esophageal cancer had a great progress. Hitherto, minimally invasive esophagectomy (MIE) mainly included TE, robot‐assisted MIE (RAMIE), and transcervical inflatable mediastinoscopic esophagectomy (TIME). Some studies have reported that TE is equally or more effective than open surgery via thoracotomy, with added advantages of a low respiratory complication rate, less postoperative pain, and better quality of life with comparable oncologic results. 6 , 7 , 8 Nowadays, TE has been the most popular minimally invasive method for treating esophageal cancer. It is also reported that RAMIE displayed similar effects and safety as TE and less pulmonary complications than conventional thoracotomy in the treatment of esophageal cancer. 9 , 10 As an option to MIE, mediastinoscopic esophagectomy was first described in 2001 and Professor Fujiwara first proposed TIME as a novel surgical method in 2015; however, it seems to be utilized mainly in Asia. 11 , 12 , 13 , 14 , 15 Whether TIME is effective and feasible as conventional MIE remains unclear and TIME is not yet recognized by many thoracic surgeons.
Since 2008, we have been vigorously performing MIE and more than 380 cases were managed with the procedure by 2019, and in 2016 we introduced TIME. Thus, we started the present study aimed to compare TIME with TE for the treatment of local early‐ and intermediate‐stage thoracic esophageal squamous cell carcinoma. Surgical and postoperative outcomes in patients who underwent TIME or TE from January 2017 in our center were retrospectively analyzed and propensity score matching was used to minimize potential bias.
2. MATERIALS AND METHODS
2.1. Patients
We retrospectively reviewed the records of 154 consecutive patients who underwent MIE at Shanghai Changzheng Hospital between January 2017 and December 2019. Before operation, each patient underwent enhanced computed tomography (CT), positron emission tomography/CT scan, esophagogastroduodenoscopy, cardiac doppler scan, cervical and supraclavicular lymph nodes ultrasound, and abdominal ultrasound for preoperative evaluation. All patients were confirmed with a malignant tumor of the esophagus by tumor biopsy, as assessed by esophagogastroduodenoscopy before the operation. Patients who underwent neoadjuvant chemotherapy, converted to open thoracotomy during operation, suspected lymph nodes around right recurrent laryngeal nerve (RLN) metastasis after preoperative evaluation, and diagnosed as pathological stage T4 or underwent palliative esophagectomy were excluded. All patients were categorized according to the eighth edition TNM classification of malignant tumors. 16 Preoperative risk assessment was performed according to the American Society of Anesthesiologists (ASA) risk classification. 17 Esophageal cancer of all patients was considered respectable and surgery was considered safe after preoperative evaluation. This study was approved by the institutional ethics committee.
2.2. Surgical procedure
For TE, the patient was placed in a left lateral to semiprone position after general anesthesia was administered in the supine position via a single‐lumen endotracheal tube. The artificial pneumothorax of the right thoracic cavity was facilitated by insufflating with carbon dioxide at 12–13 mmHg, which collapsed the right lung. Four access ports were inserted at the posterior axillary line in the fourth and seventh, and at the lower scapular line in the seventh and ninth intercostal spaces. The azygos vein was cut off and the thoracic esophagus was mobilized in the thoracic procedure. Concurrently, the thoracic paraesophageal lymph nodes and lymph nodes along RLN were exposed and dissected.
For TIME, as soon as general anesthesia was initiated with single‐lumen endotracheal intubation, the patient was placed in a supine position with the neck extended slightly. A 7 cm incision was made at the left cervical collar. Then, the cervical esophagus was mobilized and the cervical lymph nodes along the left RLN were dissected. A protective sleeve of 3.5 cm diameter (FF07; Hakko) and a specific protective cover (EZ Access; Hakko) that prevents gas leakage were placed in the cervical wound; subsequently, trocars were inserted into the wound through the cover. The procedure of mobilization of thoracic esophagus and lymph node dissection was started after mediastinal space was inflated with carbon dioxide (pressure of 12–14 mmHg). 12 , 14 Mediastinal retractors (Suzhou Sagemed Medical Technology Co., Ltd.) were used to fully expose the surgical field when mobilizing the esophagus and peripheral lymph nodes were dissected with the esophagus; lymph nodes around the subcarinal and inferior pulmonary vein could be double confirmed and resected during laparoscopic operation if not cleaned up completely during mediastinal procedure. 15
After the thoracic part, the abdominal procedure was initiated in the supine position, which was similar to two methods. The stomach was mobilized under laparoscopic surgery, as described previously. 18 The esophagus was cut off via the neck wound and the circumferential mobilized esophagus was exposed from the 5 cm wound in the upper medial abdomen. The gastric tube was pulled up to the cervical wound through the esophageal bed and the reconstruction of the digestive tract was completed via the neck wound. 19
All cases of esophagectomy were performed by seven surgeons, who were experienced in open esophagectomy and minimally invasive surgery of radical esophagectomy. All cases were supervised by three experienced surgeons.
2.3. Postoperative treatment
Patients were admitted to the intensive care unit after the operation and discharged when the vital signs of the patients were stable for >24 h. Antibiotics were administered 30 min before and 2 days after the operation for all patients. Patients with pneumonia were given antibiotics for an extended period. A bedside chest X‐ray was taken of the patient on the second day after the operation, to initially observe the patient's postoperative lung conditions, including the exclusion of pulmonary atelectasis. The standard for removing the chest tube was that the drainage volume in 24 h was <200 ml and the lungs expanded well. The removal of chest tube would be delayed if the amount of pleural drainage in 24 h was more than 200 ml or there was atelectasis. Typically, the chest tube removal of patients who underwent thoracoscopy was performed on postoperative day (POD) 2 or 3, and patients who underwent mediastinoscopy do not need to remove any chest tube, because there was no chest tube. Oral food intake was resumed around POD 7. Mortality was defined as the operation‐related or disease‐related death during hospitalization. All patients underwent a chest CT exam on POD 4 or when necessary. Patients suspected with anastomotic fistula were asked to drink a half cup of iodine water for an exact diagnosis. The recurrence of the disease was assessed by CT every 6 months and by electronic gastroscope annually postsurgery. The patients received enteral nutrition support via a jejunostomy tube on POD 3. If patients had no related complications after receiving oral feeding, the jejunostomy tube would be removed after 30 days. Patients diagnosed with pathological stage II–IV disease after the surgery underwent adjuvant chemotherapy (four courses of docetaxel and cisplatin).
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS version 23 (SPSS Statistics v23, IBM Co.). To improve the evidence level of the test and reduce the selection bias, a 1:1 nearest‐neighbor algorithm was applied using propensity score matching. The propensity scores were calculated using logistic regression. We used mediastinoscopy or thoracoscopy procedure as the dependent variable and five variables, such as age, sex, ASA score, tumor location, and pathological stage as the covariates. A t‐test, χ 2 test, or Fisher's exact test was used to compare the differences between two groups. The relapse‐free survival (RFS) rate was used to evaluate postoperative short‐term outcomes of the two groups. RFS was assessed using the Kaplan–Meier method and compared using the log‐rank test. p < 0.05 indicated statistical significance.
3. RESULTS
In total, 154 patients underwent MIE between January 2017 and December 2019. Of these, 129 patients diagnosed with squamous cell carcinoma in the thoracic esophagus, with pathological T1–3, N0–3, and M0 disease, were included in the analysis after excluding patients who underwent neoadjuvant chemotherapy (n = 9), converted to open thoracotomy during operation (n = 3), suspected lymph nodes around right RLN metastasis after preoperative evaluation (n = 4), diagnosed as pathological stage T4 (n = 5) or non‐squamous cell carcinoma (n = 3, one thyroid cancer esophageal metastasis; two small cell neuroendocrine carcinoma), and underwent palliative esophagectomy (n = 1). Of these 129 patients, 70 patients underwent thoracoscopy with laparoscopy (TE group) and 59 patients underwent transcervical inflatable mediastinoscopy combined with laparoscopy (TIME group). The confounding factors (age, sex, ASA score, tumor location, and pathological stage) in the two groups were adjusted by propensity score matching and 51 pairs of patients were identified after matching (Figure 1). In these 129 cases, the main part of all tumors was located in the thoracic esophagus and all tumors were squamous cell carcinomas. The distant of the highest upper thoracic esophageal cancer was 24 cm away from the incisor teeth. Meanwhile, the tumors in three cases extended to the gastroesophageal (GE) junction, of which the tumors in two cases just extended to the cardia and the tumor in one case crossed the GE junction 0.5 cm. All patients in this study received R0 resection.
Figure 1.
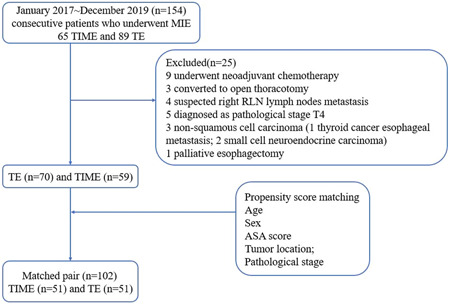
Flowchart of the propensity score matching. ASA, American Society of Anesthesiologists; RLN, recurrent laryngeal nerve; TE, thoracoscopic esophagectomy; TIME, transcervical inflatable mediastinoscopic esophagectomy
Tables 1 and 2 show the demographic and tumor‐related parameters of the two groups before and after propensity score matching. No significant differences were detected in age, sex, ASA score, tumor location, clinical T‐N status, pathological T‐N status, and pathological stage after matching.
Table 1.
Comparison of thoracoscope and mediastinoscope group (TE and TIME): demographic parameters
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| TE group (n = 70) | TIME group (n = 59) | p value | TE group (n = 51) | TIME group (n = 51) | p value | |
| Agea, years | 63.3 ± 7.6 | 66.2 ± 7.1 | 0.03 | 64.1 ± 7.0 | 65.5 ± 7.3 | 0.30 |
| Sexa, male (n, %) | 61 (87.1) | 49 (83.1) | 0.51 | 43 (84.3) | 41 (80.4) | 0.60 |
| BMI, kg/m2 | 22.8 ± 3.3 | 23.5 ± 4.1 | 0.46 | 22.3 ± 3.1 | 22.9 ± 4.4 | 0.57 |
| ASAa, score (n, %) | 0.06 | 0.47 | ||||
| Ⅰ | 49 (70.0) | 30 (50.8) | 34 (66.7) | 29 (56.9) | ||
| Ⅱ | 18 (25.7) | 22 (37.3) | 14 (27.5) | 16 (31.4) | ||
| Ⅲ | 3 (4.3) | 7 (11.9) | 3 (5.9) | 6 (11.8) | ||
| Smoking history (n, %) | 21 (30.0) | 19 (32.2) | 0.79 | 12 (23.5) | 16 (31.4) | 0.38 |
| Drinking history (n, %) | 18 (25.7) | 14 (23.7) | 0.80 | 9 (17.6) | 13 (25.5) | 0.34 |
| Hypertension (n, %) | 17 (24.3) | 18 (30.5) | 0.43 | 14 (27.5) | 13 (25.5) | 0.82 |
| Diabetes (n, %) | 5 (7.1) | 7 (11.9) | 0.36 | 5 (9.8) | 5 (9.8) | 1.00 |
Abbreviations: BMI, body mass index; TE, thoracoscopic esophagectomy; TIME, transcervical inflatable mediastinoscopic esophagectomy.
Covariates used for propensity score matching analysis.
Table 2.
Comparison of thoracoscope and mediastinoscope group (TE and TIME): tumor‐related parameters
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| TE group (n = 70) | TIME group (n = 59) | p value | TE group (n = 51) | TIME group (n = 51) | p value | |
| Tumor locationa(n, %) | 0.51 | 0.79 | ||||
| Upper thoracic | 6 (8.6) | 6 (10.2) | 5 (9.8) | 6 (11.8) | ||
| Middle thoracic | 37 (52.9) | 36 (61.0) | 30 (58.8) | 32 (62.7) | ||
| Lower thoracic | 27 (38.6) | 17 (28.8) | 16 (31.4) | 13 (25.5) | ||
| Clinical T‐status | 0.22 | 0.19 | ||||
| T1 | 16 (22.9) | 15 (25.4) | 13 (25.5) | 13 (25.5) | ||
| T2 | 25 (35.7) | 28 (47.5) | 15 (29.4) | 23 (45.1) | ||
| T3 | 29 (41.4) | 16 (27.1) | 23 (45.1) | 15 (29.4) | ||
| Clinical N‐status | 0.01 | 0.58 | ||||
| N0 | 40 (57.1) | 48 (81.4) | 36 (70.6) | 40 (78.4) | ||
| N1 | 22 (31.4) | 9 (15.3) | 11 (21.6) | 9 (17.6) | ||
| N2 | 8 (11.4) | 2 (3.4) | 4 (7.8) | 2 (3.9) | ||
| Pathological T‐status | 0.27 | 0.82 | ||||
| T1 | 19 (27.1) | 24 (40.7) | 17 (33.3) | 20 (39.2) | ||
| T1a/T1b | 7/12 | 9/15 | 7/10 | 8/12 | ||
| T2 | 24 (34.3) | 16 (27.1) | 16 (31.4) | 14 (27.5) | ||
| T3 | 27 (38.6) | 19 (32.2) | 18 (35.3) | 17 (33.3) | ||
| Pathological N‐status | 0.00 | 0.07 | ||||
| N0 | 31(44.3) | 46 (78.0) | 29 (56.9) | 38 (74.5) | ||
| N1 | 29(41.4) | 7 (11.9) | 17 (33.3) | 7 (13.7) | ||
| N2 | 10(14.3) | 6 (10.2) | 5 (9.8) | 6 (11.8) | ||
| Pathological stagea(n, %) | 0.00 | 0.11 | ||||
| Ⅰ | 12 (17.1) | 27 (45.8) | 12 (23.5) | 21 (41.2) | ||
| Ⅱ | 25 (35.7) | 22 (37.3) | 22 (43.1) | 20 (39.2) | ||
| Ⅲ | 33 (47.1) | 10 (16.9) | 17 (33.3) | 10 (19.6) | ||
| Adjuvant chemotherapy after operation (n, %) | 58 (82.9) | 32 (54.2) | 0.00 | 39 (76.5) | 30 (58.8) | 0.06 |
Abbreviations: TE, thoracoscopic esophagectomy; TIME, transcervical inflatable mediastinoscopic esophagectomy.
Covariates used for propensity score matching analysis.
Table 3 shows the surgical outcomes of the two groups before and after matching. The mean operative time in the TIME group was shorter than that in the TE group after matching (TE group vs. TIME group: 275.1 min vs. 240.8 min; p < 0.05). Similarly, the mechanical ventilation time in TIME group was also shorter than that in TE group after matching (TE group vs. TIME group: 291.1 min vs. 257.5 min; p < 0.05), although the mean number of lymph nodes harvested during surgery was not significantly different between the two groups after matching (p = 0.07) and the ratio of positive/total lymph nodes was also not significantly different between the two groups after matching (p = 0.10).
Table 3.
Outcomes of MIE in the thoracoscope versus mediastinoscope group (TE vs. TIME)
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| TE group (n = 70) | TIME group (n = 59) | p value | TE group (n = 51) | TIME group (n = 51) | p value | |
| Operation time, min | 277.3 ± 61.7 | 242.0 ± 65.8 | 0.00 | 275.1 ± 66.7 | 240.8 ± 69.2 | 0.01 |
| Mechanical ventilation time, min | 293.2 ± 60.8 | 258.6 ± 65.1 | 0.00 | 291.1 ± 65.7 | 257.5 ± 69.1 | 0.01 |
| Blood loss, ml | 243.6 ± 74.7 | 225.7 ± 50.3 | 0.12 | 244.7 ± 78.2 | 229.1 ± 47.5 | 0.23 |
| Time to postoperative ambulation, days | 1.9 ± 0.7 | 1.3 ± 0.5 | 0.00 | 1.9 ± 0.7 | 1.4 ± 0.5 | 0.00 |
| Hospitalization after surgery, days | 16.5 ± 10.8 | 16.0 ± 13.1 | 0.81 | 16.7 ± 10.5 | 16.9 ± 13.8 | 0.92 |
| Number of harvested lymph nodes | 23.1 ± 14.0 | 18.9 ± 6.0 | 0.03 | 22.7 ± 14.6 | 18.5 ± 6.2 | 0.07 |
| Ratio of positive/total lymph nodes (%) | 11.5 | 14.9 | 0.14 | 10.8 | 14.9 | 0.10 |
| Pulmonary complications | 16 (22.9) | 5 (8.5) | 0.03 | 14 (27.5) | 4 (7.8) | 0.01 |
| Hoarseness | 9 (12.9) | 15 (25.4) | 0.07 | 6 (11.8) | 12 (23.5) | 0.12 |
| Anastomotic fistula | 5 (7.1) | 8 (13.6) | 0.23 | 3 (5.9) | 8 (15.7) | 0.11 |
| Atrial fibrillation | 4 (5.7) | 2 (3.4) | 0.69 | 4 (7.8) | 1 (2.0) | 0.36 |
| Fat liquefaction of incision | 7 (10.0) | 5 (8.5) | 0.77 | 7 (13.7) | 3 (5.9) | 0.18 |
| Chylothorax (n, %) | 1 (1.4) | 1 (1.7) | 1.00 | 1 (2.0) | 1 (2.0) | 1.00 |
| Reoperation | 1 (1.4) | 1 (1.7) | 1.00 | 1 (2.0) | 1 (2.0) | 1.00 |
| Mortality | 1 (1.4) | 2 (3.4) | 0.59 | 0 | 2 (3.9) | 0.50 |
| Relapse‐free survival rates (%) | 84.3 | 84.7 | 0.91 | 82.4 | 86.3 | 0.63 |
Abbreviations: MIE, minimally invasive esophagectomy; TE, thoracoscopic esophagectomy; TIME, transcervical inflatable mediastinoscopic esophagectomy.
Moreover, the time of patients to postoperative ambulation in the mediastinoscope group was significantly lesser than that in the thoracoscope group after matching (1.9 days vs. 1.4 days; p < 0.05). The rate of postoperative pulmonary complication (pneumonia and atelectasis) was higher in the thoracoscope group than in the mediastinoscope group after matching (27.5% vs. 7.8%; p < 0.05). The rate of anastomosis fistula and hoarseness caused by RLN palsy did not show any significant difference in the two groups. Most of the cases with hoarseness in this study returned to normal within 1–4 months after operation. Only one patient in the TIME group had permanent hoarseness, while the symptom was relieved 6 months after the operation. The rate of other complications also was not significantly different between the two groups. Also, the rate of mortality and reoperation were similar in the two groups. The patient in TE group died of pneumonia and respiratory failure caused by postoperative anastomotic leakage. One patient in the mediastinoscope group died due to a sudden heart attack and the other died of postoperative pneumonia and respiratory failure. These three cases represented the entirety deaths within 30 days after operation in this study. The patient in the thoracoscope group underwent reoperation because of shock caused by thoracic hemorrhage and the patient in the mediastinoscope group underwent reoperation because of bleeding in the cervical incision.
Figures 2 and 3 show the RFS before and after matching. The RFS of the two groups were similar after matching (thoracoscope group vs. mediastinoscope group: 82.4% vs. 86.3%; p = 0.63).
Figure 2.
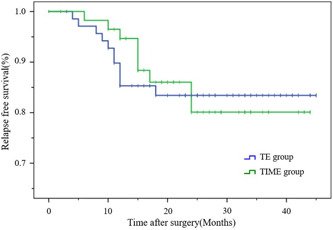
Kaplan–Meier relapse‐free survival (RFS) curves for the transcervical inflatable mediastinoscopic esophagectomy (TIME) and thoracoscopic esophagectomy (TE) before matching. The RFS rate of the two groups were similar before matching. p = 0.91
Figure 3.
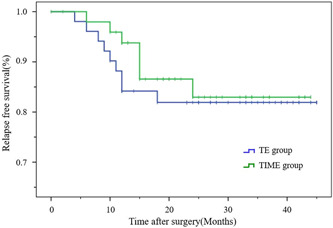
Kaplan–Meier relapse‐free survival (RFS) curves for the transcervical inflatable mediastinoscopic esophagectomy (TIME) and thoracoscopic esophagectomy (TE) after matching. The RFS rate of the two groups were also similar after matching. p = 0.63
4. DISCUSSION
Based on the concept of minimally invasive surgery, TIME was reported as a new MIE since 2015. 12 , 14 , 20 However, whether TIME is effective as conventional MIE is still controversial; thus, we designed this study to evaluate the safety, feasibility, and effectiveness of TIME by comparing it with TE. Unlike previous research, 21 , 22 propensity score match analysis was adopted to regulate the confounding factors for reducing the selection bias and the RFS rates was used to evaluate the early and midterm effects of TIME in this study.
In TIME, artificial pneumomediastinum maybe the most mainframe procedure that is quite distinct from conventional nontransthoracic esophagectomy. 23 , 24 , 25 Compared with conventional nontransthoracic procedure, the mediastinal space that inflated with carbon dioxide enhanced visualization of the mediastinum structures and suppressed venous flow and capillary oozing. 26 , 27 All of these can effectively reduce the operation duration and improve the safety and efficiency of the surgery. Undergoing TE, patients needed to change the position after the thoracic procedure, whereas in mediastinoscope group patients were performed in the supine position, which attributed to the shortened operation time and avoided single‐lung ventilation. Less operation time could reduce the lung injury caused by mechanical ventilation under anesthesia, whereas two‐lung ventilation could maintain oxygenation and uniformly distributed lung perfusion during the operation, which contribute to less postoperative pulmonary complications. 28 Furthermore, the other reason why the rate of pulmonary complications lower in TIME group maybe that the unique nontransthoracic method could avoid the injury that was caused by lung traction during the operation. 22 , 29 , 30
Meanwhile, compared to TE, TIME had three distinctive advantages. On one hand, it is a nontransthoracic procedure, which did not need chest drainage after the operation. On the other hand, as nontransthoracic operations for esophageal cancer, it possessed the advantage of less chest pain after surgery. Furthermore, the less postoperative pain made it easier for patients to achieve effective cough and expectoration after operation, which contributed to the low incidence of pulmonary complications. 30 Therefore, without postoperative pain caused by thoracic wounds, chest tube, and the fear of chest tube falling off, patients who underwent TIME may easier achieve effective cough and early ambulation. Early postoperative mobilization has been advocated for patients undergoing major surgery to improve functional capacity and to enhance recovery. 31 , 32 The hospitalization in the two groups was similar, which might be due to the oral feeding time of patients that was generally on the POD 7, and patients were discharged from the hospital if no related complications were observed after oral feeding. It was worth mentioning that one patient was transferred from thoracoscope procedure to mediastinoscope because of the severe thoracic adhesion after probed under thoracoscope. This might inspire us that mediastinoscope could be an alternative choice for patients with severe thoracic adhesion or poor pulmonary function that could not tolerate TE.
Although there are some advantages of TIME, the procedure still has some limitations. The most controversial one is dissection of lymph nodes along the right RLN. Recently, Koterazawa et al. 33 reported that prophylactic cervical lymph node dissection increased the postoperative complications, instead of improving the long‐term survival of patients with esophageal cancer, which may support that the mediastinoscope method could be applied to patients with early‐stage esophageal cancer or without lymph node metastasis around RLN. However, comprehensive and systematic lymph node dissection is the key for TIME to become an eligibly radical operation of esophageal cancer. Fortunately, several surgeons have already improved lymphadenectomy procedure, especially the dissection of lymph nodes along the right RLN, of this new MIE. 13 , 34 The other limitation might be the poor surgical field of the mediastinoscope procedure. Although the mediastinal space was full of carbon dioxide, the surgical field was still smaller in the mediastinum than that in the thorax when performing TE. A successful surgical manipulation in the narrow space required skill. Although Fujiwara et al. 13 reported that the transcervical approach of mediastinoscope had advantages in the dissection of the subaortic arch or tracheobronchial lymph nodes and lymph nodes along the left RLN, we still found that the operation was challenging, and the lack of experience in the first few cases might underlie the detection of fewer lymph nodes in the mediastinoscope group. In the current study, there was no significant difference in lymph node dissection between the two groups and the result was similar to the study of Liu et al.; 21 however, there was still some differences between the two studies. In our research, patients underwent neoadjuvant chemotherapy or suspected lymph nodes around the right RLN metastasis before operation were excluded from the study because of the limitations of TIME. In addition, patients only with local early‐ or intermediate‐stage of esophageal cancer were included in the study and propensity score match analysis was also adopted to obtain a more credible result of the RFS. However, whether before or after the propensity matching analysis, the percentage of patients with Ⅲ stage in the TE group was higher than that in the TIME group. Although it was not statistically significant, it was still a shortcoming of the study, which might be caused by the limitation of the number of cases in the study or by accidental lymph node metastasis after surgery in some cases that led to the escalation of final pathological stage. Therefore, although the final RFS of the two groups in this single‐center study were similar after matching, this result only implied that TIME might have the potential to treat local early‐ and intermediate‐stage esophageal squamous cell carcinoma effectively. A further study and the long‐term outcomes must be followed up.
5. CONCLUSION
TIME may be a feasible and safe method to treat local early‐ and intermediate‐stage thoracic esophageal squamous cell carcinoma effectively and it could be a supplementary surgical method of TE for patients with poor pulmonary function or cannot undergo TE.
SYNOPSIS
Transcervical inflatable mediastinoscopic esophagectomy (TIME) is a novel method of minimally invasive esophagectomy (MIE) for esophageal cancer. In this study, we compared TIME and conventional thoracoscopic esophagectomy (TE) for the treatment of local early‐ and intermediate‐stage thoracic esophageal squamous cell carcinoma in terms of surgical outcomes and relapse‐free survival (RFS) rates. Finally, we found that TIME may be a feasible and safe method to treat local early‐ and intermediate‐stage thoracic esophageal squamous cell carcinoma effectively, and it could also be a supplementary surgical method of TE for patients who cannot undergo TE.
ACKNOWLEDGEMENTS
We thank the patients and their caregivers for participating in this study. This study was performed by Department of Minimally Invasive Thoracic Surgery Center and Department of Thoracic Surgery of Changzheng Hospital.
Chen Z, Huang K, Wei R, et al. Transcervical inflatable mediastinoscopic esophagectomy versus thoracoscopic esophagectomy for local early‐ and intermediate‐stage esophageal squamous cell carcinoma: A propensity score‐matched analysis. J Surg Oncol. 2022;125:839‐846. 10.1002/jso.26798
Zihao Chen, Kenan Huang, and Rongqiang Wei contributed equally to this work.
Contributor Information
Xinyu Ding, Email: dingxy269@163.com.
Hua Tang, Email: tangh_mits@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;6:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 2. Esophagus. 2019;1:25‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;4:1170‐1176. [DOI] [PubMed] [Google Scholar]
- 4. Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Complications and costs after high‐risk surgery: where should we focus quality improvement initiatives? J Am Coll Surg. 2003;5:671‐678. [DOI] [PubMed] [Google Scholar]
- 5. Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;1:7‐11. [PubMed] [Google Scholar]
- 6. Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg. 2015;8:1986‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osugi H, Takemura M, Higashino M, Takada N, Lee S, Kinoshita H. A comparison of video‐assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg. 2003;1:108‐113. [DOI] [PubMed] [Google Scholar]
- 8. Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open‐label, randomised controlled trial. Lancet. 2012;9829:1887‐1892. [DOI] [PubMed] [Google Scholar]
- 9. Jin D, Yao L, Yu J, et al. Robotic‐assisted minimally invasive esophagectomy versus the conventional minimally invasive one: a meta‐analysis and systematic review. Int J Med Robot. 2019;3:e1988. [DOI] [PubMed] [Google Scholar]
- 10. Tsunoda S, Obama K, Hisamori S, et al. Lower incidence of postoperative pulmonary complications following robot‐assisted minimally invasive esophagectomy for esophageal cancer: propensity score‐matched comparison to conventional minimally invasive esophagectomy. Ann Surg Oncol. 2020;28:639‐647. [DOI] [PubMed] [Google Scholar]
- 11. Ikeda Y, Niimi M, Kan S, et al. Mediastinoscopic esophagectomy using carbon dioxide insufflation via the neck approach. Surgery. 2001;4:504‐506. [DOI] [PubMed] [Google Scholar]
- 12. Fujiwara H, Shiozaki A, Konishi H, et al. Single‐port mediastinoscopic lymphadenectomy along the left recurrent laryngeal nerve. Ann Thorac Surg. 2015;3:1115‐1117. [DOI] [PubMed] [Google Scholar]
- 13. Fujiwara H, Shiozaki A, Konishi H, et al. Perioperative outcomes of single‐port mediastinoscope‐assisted transhiatal esophagectomy for thoracic esophageal cancer. Dis Esophagus. 2017;10:1‐8. [DOI] [PubMed] [Google Scholar]
- 14. Fujiwara H, Shiozaki A, Konishi H, Otsuji E. Mediastinoscope and laparoscope‐assisted esophagectomy. J Vis Surg. 2016;2:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li GS. Prof. Qingdong Cao: single‐port inflatable mediastinoscopy combined with laparoscopy for the radical treatment of esophageal cancer. J Thorac Dis. 2016;9:E1108‐E1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donohoe CL, Phillips AW. Cancer of the esophagus and esophagogastric junction: an 8(th) edition staging primer. J Thorac Dis. 2017;3:E282‐E284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woodfield JC, Beshay NM, Pettigrew RA, Plank LD, van Rij AM. American Society of Anesthesiologists classification of physical status as a predictor of wound infection. ANZ J Surg. 2007;9:738‐741. [DOI] [PubMed] [Google Scholar]
- 18. Fujiwara H, Shiozaki A, Konishi H, et al. Hand‐assisted laparoscopic transhiatal esophagectomy with a systematic procedure for en bloc infracarinal lymph node dissection. Dis Esophagus. 2016;2:131‐138. [DOI] [PubMed] [Google Scholar]
- 19. McKeown KC. Total three‐stage oesophagectomy for cancer of the oesophagus. Brit J Surg. 1976;4:259‐262. [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Li X, Cheng H, et al. Single‐port inflatable mediastinoscopy combined with laparoscopic‐assisted small incision surgery for radical esophagectomy is an effective and safe treatment for esophageal cancer. J Gastrointest Surg. 2019;8:1533‐1540. [DOI] [PubMed] [Google Scholar]
- 21. Liu W, Guo X, Zhao H, et al. Mediastinoscopy‐assisted transhiatal esophagectomy versus thoraco‐laparoscopic esophagectomy for esophageal cancer: a single‐center initial experience. J Thorac Dis. 2020;9:4908‐4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin Y, Lu X, Xue L, Zhao X. Retrospective comparison of two minimally invasive esophagectomy in the treatment of esophageal cancer: pneumatic mediastinoscopy versus thoracoscopy. J Laparoendosc Adv S. 2019;29:5. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web‐based database. Ann Surg. 2014;2:259‐266. [DOI] [PubMed] [Google Scholar]
- 24. Tangoku A, Yoshino S, Abe T, et al. Mediastinoscope‐assisted transhiatal esophagectomy for esophageal cancer. Surg Endosc. 2004;3:383‐389. [DOI] [PubMed] [Google Scholar]
- 25. Bumm R, Hölscher AH, Feussner H, Tachibana M, Bartels H, Siewert JR. Endodissection of the thoracic esophagus. Technique and clinical results in transhiatal esophagectomy. Ann Surg. 1993;1:97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonavina L, Bona D, Binyom PR, Peracchia A. A laparoscopy‐assisted surgical approach to esophageal carcinoma. J Surg Res. 2004;1:52‐57. [DOI] [PubMed] [Google Scholar]
- 27. Ikeda Y, Takami H, Sasaki Y, Kan S, Niimi M. Endoscopic neck surgery by the axillary approach. J Am Coll Surg. 2000;3:336‐340. [DOI] [PubMed] [Google Scholar]
- 28. Miura S, Nakamura T, Miura Y, et al. Long‐term outcomes of thoracoscopic esophagectomy in the prone versus lateral position: a propensity score‐matched analysis. Ann Surg Oncol. 2019;11:3736‐3744. [DOI] [PubMed] [Google Scholar]
- 29. Wang QY, Tan LJ, Feng MX, et al. Video‐assisted mediastinoscopic resection compared with video‐assisted thoracoscopic surgery in patients with esophageal cancer. J Thorac Dis. 2014;6:663‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu B, Xue L, Qiu M, et al. Video‐assisted mediastinoscopic transhiatal esophagectomy combined with laparoscopy for esophageal cancer. J Cardiothorac Surg. 2010;1:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (ERAS(R)) society recommendations. Br J Surg. 2014;10:1209‐1229. [DOI] [PubMed] [Google Scholar]
- 32. Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum. 2017;8:761‐84. [DOI] [PubMed] [Google Scholar]
- 33. Koterazawa Y, Oshikiri T, Takiguchi G, et al. Prophylactic cervical lymph node dissection in thoracoscopic esophagectomy for esophageal cancer increases postoperative complications and does not improve survival. Ann Surg Oncol. 2019;9:2899‐904. [DOI] [PubMed] [Google Scholar]
- 34. Gan X, Wang X, Zhang B, et al. Lymphadenectomy along bilateral recurrent laryngeal nerve under single‐incision mediastinoscopy. Ann Thorac Surg. 2020;109:e449‐e452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


