Abstract
The causal association between exposure to power‐frequency magnetic fields (MFs) and childhood leukemia has been under discussion. Although evidence from experimental studies is required for a conclusion to be reached, only a few studies have focused on the effects of MF exposure on the human hematopoietic system directly related to leukemogenesis. Here, we established an in vitro protocol to simulate the differentiation of human mesodermal cells to hematopoietic stem progenitor cells (HSPCs) using human‐induced pluripotent stem cells. Furthermore, we introduced MF in the protocol to study the effects of exposure. After a continuous exposure to 0–300 mT of 50‐Hz MFs during the differentiation process, the efficiency of differentiation of mesodermal cells into HSPCs was analyzed in a single‐blinded manner. The percentage of emerged HSPCs from mesodermal cells in groups exposed to 50‐Hz MFs indicated a lack of significant changes compared with those in the sham‐exposed group. These results suggest that exposure to 50‐Hz MFs up to 300 mT does not affect the differentiation of human mesodermal cells to HSPCs, which may be involved in the initial process of leukemogenesis. © 2022 The Authors. Bioelectromagnetics published by Wiley Periodicals LLC on behalf of Bioelectromagnetics Society.
Keywords: power‐frequency magnetic field, hematopoietic system, human induced pluripotent stem cell, differentiation
INTRODUCTION
Since first shown in 1979 [Wertheimer and Leeper, 1979], the association between exposure to power‐frequency magnetic fields (MFs) and the development of childhood leukemia has been discussed, while the findings of epidemiological studies have been influential [Ahlbom et al., 2000; Kheifets et al., 2010; Swanson et al., 2019]. Based on these findings, the International Agency for Research on Cancer (IARC) classified that extremely low‐frequency MFs (ELF‐MFs; up to 3 kHz) are possibly carcinogenic to humans (Group 2B) [IARC, 2002]. However, because of the low incidence of childhood leukemia and of various epidemiological confounding factors and biases, no conclusions have been drawn about the causal association between the two. Therefore, as the International Commission on Non‐Ionizing Radiation Protection (ICNIRP) mentioned in their recent statement [ICNIRP, 2020], results from experimental studies in vivo and in vitro under meticulously controlled conditions are needed to draw a conclusion.
Acute B‐lymphoblastic leukemia is the most common leukemia in childhood. It is known that in most cases, childhood leukemia develops as a result of the primary event, that is, chromosomal translocations [Iacobucci and Mullighan, 2017] that arise in utero [Greaves et al., 2003], and the additional secondary events that occur after birth [Sun et al., 2017]. Although some mechanisms have been clarified, a plausible mechanism underlying the association between exposure to MFs and leukemogenesis has not been proposed. The results of a pilot study on transgenic mice expressing the human leukemia‐specific fusion gene ETV6‐RUNX1, exposed to an MF of 50 Hz, were reported recently [Campos‐Sanchez et al., 2019], and subsequent reports are expected. However, based on the fact that acute B‐lymphoblastic leukemia does not develop spontaneously in commonly used rodent models, these results must be carefully evaluated in terms of the similarity of leukemia in mice and humans and the adaptability of these results to assess the corresponding health risks for humans. On the contrary, several in vitro studies have been performed to validate the carcinogenic initiation and promotional effects of power‐frequency MFs based on existing mechanisms; however, data supporting the correlation between MFs and childhood leukemia provided by epidemiological studies are limited [SCENIHR, 2015]. It is known that in various aspects, from gene expression pattern to sensitivity, cells exhibit differences in inter‐biological and cellular species and their stage of differentiation [Doseth et al., 2011; Ichii et al., 2014; Capinha et al., 2021; Luo et al., 2021; Magee and Signer, 2021], and it is considered that evaluation focused on the human hematopoietic system is important in in vitro risk assessment of childhood leukemia. Although human hematopoietic stem cells and lineage‐committed progenitors are specifically the most suitable subjects, the difficulty in the acquisition and handling of these cells prevents their application in the risk assessment of MFs.
Human‐induced pluripotent stem (hiPS) cells, which are generated by reprogramming somatic cells using a particular set of transcription factors, have the ability to differentiate into multiple cell lineages [Takahashi et al., 2007]. Cells prepared from healthy donor‐derived hiPS cells present similar characteristics and responsiveness to those of healthy human cells unlike immortalized cell lines. Therefore, several types of cells derived from hiPS cells have already been utilized in regenerative medicine, drug discovery, and toxicological evaluation [Mandai et al., 2017; Gintant et al., 2019; Kokubu et al., 2019]. hiPS cell technology is a powerful tool in health risk assessment. In a previous study, we used cardiomyocytes differentiated from hiPS cells to observe the effects of power‐frequency MFs on the human heart [Takahashi et al., 2017]. In this study, we establish a protocol to differentiate hiPS cells into mesodermal cells and HSPCs, which have the normal ability of terminally differentiating into hematopoietic cells. Furthermore, we evaluated the effects of 50‐Hz MFs on the differentiation of human mesodermal cells to HSPCs.
MATERIALS AND METHODS
Cell Culture
The human iPS cell line (7F3955‐pMXs#1) [Suzuki et al., 2013] was provided by the Stem Cell Bank of the University of Tokyo. Undifferentiated human iPS cells were maintained on a mitotically inactivated DR4 MEF (Applied StemCell, Milpitas, CA) feeder layer in Primate ES Cell Medium (ReproCELL, Yokohama, Japan) supplemented with 4 ng/ml basic FGF (Wako, Tokyo, Japan). The culture medium was changed daily, and the cells were passaged every 5 days. All cultures were grown in a humidified incubator containing 5% CO2 in air at 37°C.
Embryoid Body Formation and Differentiation to Hematopoietic Cells
After the removal of feeder cells by treatment with CTK dissociation solution (ReproCELL), the human iPS cell colonies were dispersed with Accutase solution (Millipore, Temecula, CA) containing 10 μM Y‐27632 (Wako) into a single cell suspension. To form an embryoid body (EB), the suspended human iPS cells (3 × 104 cells/well) were added in to an ultra‐low attachment 96‐well plate (spindle‐shaped bottom; Sumilon, Tokyo, Japan) containing Stemline II serum‐free medium (Sigma‐Aldrich, Saint Louis, MO) supplemented with 50 ng/ml BMP‐4 (R&D Systems, Minneapolis, MN) and 10 μM Y‐27632, and centrifuged at 100g. Half of the medium was changed on days 1 and 2, with the same final concentration of BMP‐4 plus basic FGF (20 ng/ml). On Day 4, the collected EBs were differentiated into HSPCs on a gelatin‐coated dish or plate in IMDM supplemented with 0.1 mM β‐mercaptoethanol, 0.1% polyvinyl alcohol (Sigma‐Aldrich), 50 ng/ml SCF (ACROBiosystems, Newark, DE), 25 ng/ml IGF‐1 (R&D systems), 20 ng/ml Flt3L, VEGF165, interleukin 6 (IL‐6) (ACROBiosystems), IL‐3 (Proteintech, Rosemont, IL), TPO (PeproTech, Cranbury, NJ), and basic FGF. Half the medium was changed every 2 or 3 days until day 12.
To test lineage‐specific differentiation of the emerged cells, the medium was changed completely to MEMα with 10% fetal bovine serum (Hyclone, Marlborough, MA) containing different cytokine cocktails on day 12. For the myeloid lineage, 50 ng/ml SCF, 20 ng/ml IL‐3, G‐CSF (Proteintech), and GM‐CSF (ACROBiosystems) were used. Half of the medium was changed every 3 days until day 34. For the lymphoid lineage, 50 ng/ml Flt3L, 10 ng/ml SCF, IL‐7 (ACROBiosystems), and IL‐3 were used. The medium without IL‐3 was used on and after the second medium change.
For the exposure test to benzoquinone, half of the medium was changed with a medium supplemented with 1,4‐benzoquinone (TCI, Tokyo, Japan) at a final concentration of 5 µM on day 11. Twwenty‐four hours later, flow cytometric analysis was performed.
MF Exposure
To expose cells to MFs, we used a previously reported system [Takahashi et al., 2017] with different settings. The system was comprised of a pair of saddle‐shaped coils and a window frame‐shaped iron core; it can generate a vertical sinusoidal MF at 50 Hz (Fig. 3A). The magnetic flux density in the exposure space was measured at an interval of 25 mm vertically and horizontally using a Gaussmeter (Model 9550; F.W. Bell, Orlando, FL), and the position of 50 mm × 50 mm from the center, where the uniformity of the magnetic flux density is more than 95%, was determined as the sample position. A water‐jacketed acrylic chamber, which can maintain the internal temperature at 37 °C ± 0.5 °C by circulating warm water inside all six sides of the chamber, was installed in the middle of the coils. While exposing the cells to MFs, 5% CO2 gas was supplied inside the chamber continuously.
Fig. 3.
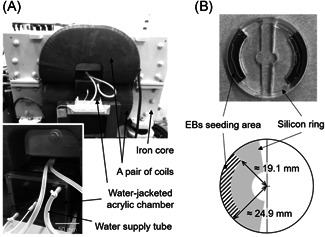
MF exposure system. (A) A water‐jacketed acrylic chamber was set in the middle of a pair of saddle‐shaped coils. (B) Detachable silicon rings were used to restrict the adhesive place for EBs to the periphery of the culture dish. EB = embryoid body; MF = magnetic fields
EBs derived from human iPS cells were seeded in the gelatin‐coated separated area of 35 mm culture dish with a silicon ring that is far from the center (Fig. 3B), and started inducing the differentiation into HSPCs. After EBs were attached on the dish 1 day later, the silicon ring was removed and the space was filled with the differentiation medium. The dishes were then positioned in the chamber immediately and exposed to 0, 100, 200, and 300 mT (rms) of 50‐Hz MFs. The cells were exposed to the MF continuously for 7 days except for a few minutes for medium change. The magnetic flux density in the sample position was confirmed using Narda ELT‐400 with 3 cm2 probe (Pfullingen, Germany). At the same time as exposure to MFs, a sham‐exposure test was performed. EB‐attached dishes were prepared in the same way and positioned inside another chamber with the same specifications and exposed to the same background level of MF as the exposed group. The technicians who exposed the cells to MFs and those analyzing the cells were different, and single‐blind tests were performed.
In our experiments, samples were exposed to vertically uniform MFs; therefore, the induced electric field in the culture medium was calculated using the following equation: E = πfBr; where E is the induced electric field strength, π is the circular constant, f is the frequency of MF, B is the magnetic flux density, and r is the radial distance from the center of the culture dish.
Gene Expression Analysis
The total RNA was extracted from 20 EBs using NucleoSpin RNA XS (Takara Bio, Shiga, Japan), and complementary DNA was synthesized with SuperScript IV reverse transcriptase (Thermo Fisher Scientific, Waltham, MA) and oligo(dT)20 primer. Polymerase chain reaction (PCR) was performed with the following primer set: 5′‐CTGGGTACTCCCAATGGGG‐3′ and 5′‐GGTTGGAGAATTGTTCCGATGA‐3′ for human Brachyury gene (PrimerBank ID: 19743811c2).
Flow Cytometric Analysis
After differentiation to hematopoietic cells and MF exposure tests, floating cells and adhesion cells were collected with dispase (50 U/ml) or collagenase/dispase (1 mg/ml). Following treatment with human FcR blocking reagent (BD Biosciences, San Jose, CA), the cells were stained with mouse monoclonal antibodies (mAbs) against human cell surface markers. PE‐conjugated anti‐CD38, PE/Cyanine5‐conjugated anti‐CD24, APC‐conjugated anti‐CD34 and anti‐CD11b, Alexa Fluor 700‐conjugated anti‐CD45, and APC/Fire 750‐conjugated anti‐CD10 mAbs were purchased from BioLegend (San Diego, CA). Brilliant Violet 711‐conjugated anti‐CD33 mAbs were purchased from BD Biosciences. Nonspecific signals and dead cells were excluded by appropriate fluorochrome‐conjugated isotype and 7‐AAD (BioLegend) staining, respectively. Flow cytometric analysis was conducted with a FACSAria III flow cytometer (BD Biosciences). Kaluza software (Beckman Coulter, Indianapolis, IN) was used for the analysis of flow cytometry data.
Statistical Analysis
Data are expressed as mean ± standard derivation (SD). Significant differences among the data groups were determined using the two‐sample Student's t test. Results were considered statistically significant at P < 0.05.
RESULTS
Differentiation of Human iPS Cells to Mesodermal Cells and HSPCs
We induced the differentiation of human iPS cells into mesodermal cells and HSPCs step‐by‐step using a protocol for EB formation (Fig. 1A and B). The emergence of mesodermal cells in EBs was confirmed by detecting the mesodermal gene brachyury [Papaioannou, 2014] (Fig. 2A). Next, we shifted the culture form of EBs from float to adhesion and induced the differentiation to HSPCs with various cytokines. The flow cytometric analysis showed that the cells after 8 days of treatment included a population of CD34+CD38− cells, which is a human HSPC‐enriched population (Fig. 2B) and revealed that mesodermal cells differentiated to HSPCs. We also detected the emergence of CD45dullCD34+CD38− cells (a highly enriched population of human hematopoietic stem cells) (Fig. 2B). To verify that HSPCs were induced in this protocol, we continued the cultivation of the differentiated cells by changing the medium to the one that contained a cytokine cocktail for lineage‐specific differentiation. In the additional differentiation with cytokines for lymphoid, the differentiation to B‐cell lineage was indicated via the detection of CD33−CD10+ cells (Fig. 2C, left panel). The differentiation process to CD10+CD24+ pro‐B cell was confirmed. On the other hand, CD10−CD33+CD11b+ myeloid cells were produced with cytokines for myeloid (Fig. 2C, right panel). These results suggested the successful development of a protocol to imitate the differentiation of mesodermal cells into human HSPCs in vitro using human iPS cells.
Fig. 1.
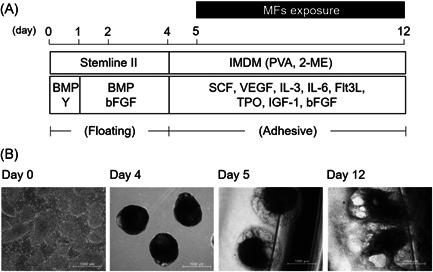
Differentiation of hiPS cells into HSPCs, and MF exposure during differentiation from mesodermal cells to HSPCs. (A) Schematic of the protocol for hematopoietic differentiation and MF exposure. (B) Morphology of the cells at each period. After EBs (day 4) were formed from hiPS colonies (day 0), the EBs were attached onto gelatin‐coated culture dishes (day 5). Exposure of MFs was performed for 7 days until day 12. Scale bars represent 1000 μm. EB = embryoid body; hiPS = human‐induced pluripotent stem; HSPC = hematopoietic stem progenitor cells; MF = magnetic field.
Fig. 2.
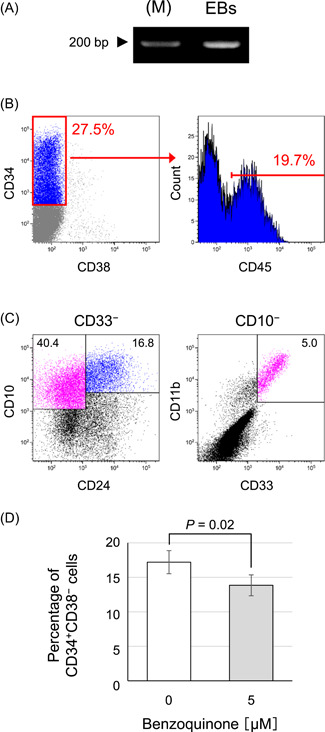
Characterization of the differentiated cells from hiPS cells. (A) Mesoderm‐specific gene, Brachyury, expression in EBs was detected by reverse‐transcription PCR (M; marker). (B) The cells that induced differentiation into HSPCs were analyzed using flow cytometry with antibody cocktail for detection of CD45dullCD34+CD38− cells (highly enriched population of human hematopoietic stem cells). (C) The HSPCs formed in this protocol were evaluated for their ability to terminally differentiate using a cytokine cocktail for lineage‐specific differentiation. Differentiation to lymphoid cell was analyzed by detecting CD33−CD10+(CD24+) cells (Left panel) and the myeloid cell was by detecting CD33+CD11b+CD10− cells (Right panel). (D) The percentage of CD34+CD38− cells in the total live cells was analyzed following benzoquinone exposure test. Data represent the mean ± standard deviation (SD; n = 4). EB = embryoid body; hiPS = human‐induced pluripotent stem; HSPC = hematopoietic stem progenitor cells; MF = magnetic field.
Next, to confirm the responsiveness of this established protocol to already‐known hematotoxic agent, exposure test to benzoquinone was performed. The percentage of emerged CD34+CD38− cells significantly decreased after exposure to 5 µM benzoquinone, the same level at which it showed hematotoxicity in a previous study [Mathialagan et al., 2020], in the last 24 h of the differentiation protocol (Fig. 2D); thus, hematotoxicity is detectable by this established protocol.
Evaluation of the Effects of Exposure to 50 Hz MFs on the Differentiation of Mesodermal Cells to HSPCs
To evaluate the effects of exposure of a power‐frequency MF, we exposed hiPS cell‐derived mesodermal cells to a 50‐Hz MF during the differentiation protocol to HSPCs for 7 days (Fig. 1A). For the exposure to MF, EBs were attached at the periphery of the culture dish using a detachable silicon ring and gelatin coating (Fig. 3B). As a result, we could expose the cells to MF under the condition of a stronger induced electric field. First, we validated the uniformity of the experimental conditions other than the MF between the exposed and sham‐exposed groups using the 0 mT MF exposure test. All exposure tests in this study were performed as single‐blind procedures. The percentage of CD34+CD38− cells did not change significantly between the groups (Fig. 4A). Next, we performed exposure tests of 50‐Hz MFs at 100, 200, and 300 mT. A sham‐exposed group was prepared for each test. In all conditions, the percentage of CD34+CD38− cells in the total live cells collected from the MF‐exposed group did not significantly change compared with that in the cells collected from the sham‐exposed group (Fig. 4A). The percentage of CD45dullCD34+CD38− cells, which was 2.3% ± 0.1% in 300 mT in the MF‐exposed group and 2.1% ± 0.4% in the sham‐exposed group, also did not change (P = 0.62, Fig. 4B). These results indicated that exposure to 50‐Hz MFs up to 300 mT did not affect the efficiency of differentiation from human mesodermal cells into HSPCs.
Fig. 4.
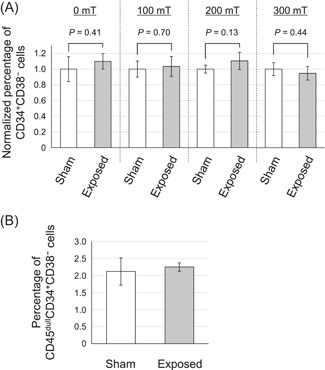
Differentiation of human mesodermal cells to HSPCs under 50‐Hz MF exposure. (A) The percentage of CD34+CD38− cells in the total live cells that emerged under the exposure of 50‐Hz MFs at 0–300 mT was evaluated. Sham‐exposure tests were performed in parallel with each MF‐exposure test, and the percentage of each HSPC was normalized with the value of its corresponding sham‐exposure group. (B) The percentage of CD45dullCD34+CD38− cells in the total live cells was analyzed following 300 mT MF exposure test. Data represent the mean ± standard deviation (SD; n ≥ 3). HSPC = hematopoietic stem progenitor cells; MF = magnetic field
DISCUSSION
Childhood leukemia is the most frequently observed malignancy in children aged 0–14 years, and the most common type is acute B‐lymphoblastic leukemia caused by B‐progenitors by acquiring dysregulated differentiation and abnormal proliferation abilities. Leukemia initiation occurs with chromosomal translocation, which is a hallmark of leukemia, and causes a shift from normal to pre‐leukemic state. Findings of studies on pairs of monozygotic monochorionic twins have strongly indicated that the first event arose through in utero development [Greaves et al., 2003], but the cellular origin has not been defined completely. Some studies have shown the presence of bone marrow mesenchymal stem cells harboring chromosomal translocations in childhood leukemia patients [Menendez et al., 2009; Shalapour et al., 2010], suggesting leukemia initiation in the differentiation stage before hematopoietic commitment. In this study, we simulated the differentiation of human mesodermal cells to HSPCs in vitro by utilizing hiPS cell technology. Furthermore, we exposed the differentiation process to MF to evaluate the effects of 50‐Hz MF exposure on them. Since previous studies with Ames test, γH2AX focal assay, and micronucleus assay have reported that ELF‐MFs do not interact with DNA directly or indirectly or are associated with chromosomal translocations, unlike ionizing radiation [Jin et al., 2012; Morandi et al., 1996; Sun et al., 2018], we focused on the effects of MF exposure on the differentiation process to HSPCs. To draw a conclusion about the causal association of power‐frequency MFs and development of childhood leukemia, we need to accumulate data from experimental studies in vivo and in vitro. Since a plausible mechanism regarding the association of MF exposure with leukemogenesis has not yet been proposed, comprehensive evaluations that focus on the hematopoietic system are considered to be effective. There are several studies on the effects of ELF‐MF exposure on commercialized human CD34+ HSPCs derived from cord blood; however, evaluation of the effects on the differentiation into human HSPCs is only possible by using pluripotent stem cells. To the best of our knowledge, this is the first study to report data on this issue.
Epidemiological studies have suggested that exposure to ≥0.4 μT residential MFs increases the risk of childhood leukemia [Ahlbom et al., 2000; Kheifets et al., 2010; Swanson et al., 2019]. In this study, we evaluated the effects of up to 300 mT (rms) of 50‐Hz MFs, which is considerably higher than the levels mentioned above, on the efficiency of differentiation from human mesodermal cells into HSPCs. It was considered to be of sufficient strength to detect the effects of MF, if any. For induced electric fields, we set strict conditions to determine some unknown effects of MFs on hematopoietic differentiation. In this study, the positioning of target cells at the periphery of the culture dish enabled us to expose them to stronger induced electric fields. Based on theoretical estimation, it was found that the cells were exposed to induced electric fields in the range of 0.90–1.17 V/m at 300 mT of MFs, which is 45‐ to 58.5‐fold higher than the basic restriction of the ICNIRP guideline for 50‐Hz MFs [ICNIRP, 2010]. The findings of this study would be helpful to draw an inference regarding the health risk of 50‐Hz MFs.
In conclusion, we successfully established an in vitro protocol to simulate the differentiation of human mesodermal cells to HSPCs using the hiPS cell technology, and we acquired some valuable insights to reflect the causal association between exposure to power‐frequency MFs and the development of childhood leukemia. We found that exposure to 50‐Hz MFs up to 300 mT may not affect the differentiation of human mesodermal cells into HSPCs. In the future, we need to accumulate further data regarding this association, with a focus on the human hematopoietic system and novel mechanisms. A hiPS cell line harboring the leukemia‐specific fusion gene ETV6‐RUNX1 [Takahashi and Yamazaki, 2019] would be useful for focusing on the participation of MFs in the development of leukemia from a pre‐leukemic state. Moreover, determining whether MFs contribute to some mechanisms of leukemogenesis without genetic changes, namely epigenetic alterations such as DNA methylation [Dunwell et al., 2009], histone modification [Li et al., 2010; Swaroop et al., 2019], and alterations of noncoding RNAs [Rodriguez et al., 2021], is also crucial.
ACKNOWLEDGMENTS
The authors wish to thank Ms. Chinami Ishiyama (CERES) for her assistance with the gene expression analysis. We also thank Editage for English language editing.
Conflicts of interest: None.
REFERENCES
- Ahlbom A, Day N, Feychting M, Roman E, Skinner J, Dockerty J, Linet M, McBride M, Michaelis J, Olsen JH, Tynes T, Verkasalo PK. 2000. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer 83:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos‐Sanchez E, Vicente‐Duenas C, Rodriguez‐Hernandez G, Capstick M, Kuster N, Dasenbrock C, Sanchez‐Garcia I, Cobaleda C. 2019. Novel ETV6‐RUNX1 mouse model to study the role of ELF‐MF in childhood B‐acute lymphoblastic leukemia: A pilot study. Bioelectromagnetics 40:343–353. [DOI] [PubMed] [Google Scholar]
- Capinha L, Jennings P, Commandeur JNM. 2021. Bioactivation of trichloroethylene to three regioisomeric glutathione conjugates by liver fractions and recombinant human glutathione transferases: Species differences and implications for human risk assessment. Toxicol Lett 341:94–106. [DOI] [PubMed] [Google Scholar]
- Doseth B, Visnes T, Wallenius A, Ericsson I, Sarno A, Pettersen HS, Flatberg A, Catterall T, Slupphaug G, Krokan HE, Kavli B. 2011. Uracil‐DNA glycosylase in base excision repair and adaptive immunity: Species differences between man and mouse. J Biol Chem 286:16669–16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwell TL, Hesson LB, Pavlova T, Zabarovska V, Kashuba V, Catchpoole D, Chiaramonte R, Brini AT, Griffiths M, Maher ER, Zabarovsky E, Latif F. 2009. Epigenetic analysis of childhood acute lymphoblastic leukemia. Epigenetics 4:185–193. [DOI] [PubMed] [Google Scholar]
- Gintant G, Burridge P, Gepstein L, Harding S, Herron T, Hong C, Jalife J, Wu JC. 2019. Use of human induced pluripotent stem cell‐derived cardiomyocytes in preclinical cancer drug cardiotoxicity testing: A scientific statement from the american heart association. Circ Res 125:e75–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves MF, Maia AT, Wiemels JL, Ford AM. 2003. Leukemia in twins: Lessons in natural history. Blood 102:2321–2333. [DOI] [PubMed] [Google Scholar]
- Iacobucci I, Mullighan CG. 2017. Genetic basis of acute lymphoblastic leukemia. J Clin Oncol 35:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC . 2002. Non‐ionizing radiation, part 1: Static and extremely low‐frequency (ELF) electric and magnetic fields. IARC Monogr Eval Carcinog Risks Hum 80:1–395. [PMC free article] [PubMed] [Google Scholar]
- Ichii M, Oritani K, Kanakura Y. 2014. Early B lymphocyte development: Similarities and differences in human and mouse. World J Stem Cells 6:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICNIRP . 2010. Guidelines for limiting exposure to time‐varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys 99:818–836. [DOI] [PubMed] [Google Scholar]
- ICNIRP . 2020. Gaps in knowledge relevant to the "guidelines for limiting exposure to time‐varying electric and magnetic fields (1 Hz‐100 kHz)". Health Phys 118:533–542. [DOI] [PubMed] [Google Scholar]
- Jin YB, Kang GY, Lee JS, Choi JI, Lee JW, Hong SC, Myung SH, Lee YS. 2012. Effects on micronuclei formation of 60‐Hz electromagnetic field exposure with ionizing radiation, hydrogen peroxide, or c‐Myc overexpression. Int J Radiat Biol 88:374–380. [DOI] [PubMed] [Google Scholar]
- Kheifets L, Ahlbom A, Crespi CM, Draper G, Hagihara J, Lowenthal RM, Mezei G, Oksuzyan S, Schuz J, Swanson J, Tittarelli A, Vinceti M, Wunsch, Filho V. 2010. Pooled analysis of recent studies on magnetic fields and childhood leukaemia. Br J Cancer 103:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu Y, Nagino T, Sasa K, Oikawa T, Miyake K, Kume A, Fukuda M, Fuse H, Tozawa R, Sakurai H. 2019. Phenotypic drug screening for dysferlinopathy using patient‐derived induced pluripotent stem cells. Stem Cells Transl Med 8:1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu J, Zhou R, Huang S, Huang S, Chen XM. 2010. Gene silencing of MIR22 in acute lymphoblastic leukaemia involves histone modifications independent of promoter DNA methylation. Br J Haematol 148:69–79. [DOI] [PubMed] [Google Scholar]
- Luo S, Lin H, Zhu L, Chen HT, Yang S, Li J, Liu M, Zheng L, Wu C. 2021. Optimized intracellular staining reveals heterogeneous cytokine production ability of murine and human hematopoietic stem and progenitor cells. Front Immunol 12:654094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JA, Signer RAJ. 2021. Developmental stage‐specific changes in protein synthesis differentially sensitize hematopoietic stem cells and erythroid progenitors to impaired ribosome biogenesis. Stem Cell Rep 16:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata KI, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M. 2017. Autologous induced stem‐cell‐derived retinal cells for macular degeneration. N Engl J Med 376:1038–1046. [DOI] [PubMed] [Google Scholar]
- Mathialagan RD, Abd Hamid Z, Ng QM, Rajab NF, Shuib S, Binti Abdul Razak SR. 2020. Bone marrow oxidative stress and acquired lineage‐specific genotoxicity in hematopoietic stem/progenitor cells exposed to 1,4‐benzoquinone. Int J Environ Res Public Health 17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez P, Catalina P, Rodriguez R, Melen GJ, Bueno C, Arriero M, Garcia‐Sanchez F, Lassaletta A, Garcia‐Sanz R, Garcia‐Castro J. 2009. Bone marrow mesenchymal stem cells from infants with MLL‐AF4+ acute leukemia harbor and express the MLL‐AF4 fusion gene. J Exp Med 206:3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi MA, Pak CM, Caren RP, Caren LD. 1996. Lack of an EMF‐induced genotoxic effect in the Ames assay. Life Sci 59:263–271. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE. 2014. The T‐box gene family: Emerging roles in development, stem cells and cancer. Development 141:3819–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PD, Paculova H, Kogut S, Heath J, Schjerven H, Frietze S. 2021. Non‐coding RNA signatures of B‐cell acute lymphoblastic leukemia. Int J Mol Sci 22:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCENIHR. 2015. Opinion on potential health effects of exposure to electromagnetic fields. Bioelectromagnetics 36:480–484. [DOI] [PubMed] [Google Scholar]
- Shalapour S, Eckert C, Seeger K, Pfau M, Prada J, Henze G, Blankenstein T, Kammertoens T. 2010. Leukemia‐associated genetic aberrations in mesenchymal stem cells of children with acute lymphoblastic leukemia. J Mol Med (Berl) 88:249–265. [DOI] [PubMed] [Google Scholar]
- Sun C, Chang L, Zhu X. 2017. Pathogenesis of ETV6/RUNX1‐positive childhood acute lymphoblastic leukemia and mechanisms underlying its relapse. Oncotarget 8:35445–35459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Wei X, Yimaer A, Xu Z, Chen G. 2018. Ataxia telangiectasia mutated deficiency does not result in genetic susceptibility to 50 Hz magnetic fields exposure in mouse embryonic fibroblasts. Bioelectromagnetics 39:476–484. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, Otsu M, Nakauchi H. 2013. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther 21:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Kheifets L, Vergara X. 2019. Changes over time in the reported risk for childhood leukaemia and magnetic fields. J Radiol Prot 39:470–488. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Oyer JA, Will CM, Huang X, Yu W, Troche C, Bulic M, Durham BH, Wen QJ, Crispino JD, MacKerell AD, Jr. , Bennett RL, Kelleher NL, Licht JD. 2019. An activating mutation of the NSD2 histone methyltransferase drives oncogenic reprogramming in acute lymphocytic leukemia. Oncogene 38:671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Saito A, Jimbo Y, Nakasono S. 2017. Evaluation of the effects of power‐frequency magnetic fields on the electrical activity of cardiomyocytes differentiated from human induced pluripotent stem cells. J Toxicol Sci 42:223–231. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamazaki S. 2019. Generation of a human induced pluripotent stem cell line, IMSUTi002‐A‐1, harboring the leukemia‐specific fusion gene ETV6‐RUNX1. Stem Cell Res 40:101546. [DOI] [PubMed] [Google Scholar]
- Wertheimer N, Leeper E. 1979. Electrical wiring configurations and childhood cancer. Am J Epidemiol 109:273–284. [DOI] [PubMed] [Google Scholar]


