Abstract
Objective
The aim of this study was to investigate whether the modified Atkins diet (MAD), a variant of the ketogenic diet, has an impact on bone‐ and calcium (Ca) metabolism.
Methods
Two groups of adult patients with pharmacoresistant epilepsy were investigated. One, the diet group (n = 53), was treated with MAD for 12 weeks, whereas the other, the reference group (n = 28), stayed on their habitual diet in the same period. All measurements were performed before and after the 12 weeks in both groups. We assessed bone health by measuring parathyroid hormone (PTH), Ca, 25‐OH vitamin D (25‐OH vit D), 1,25‐OH vitamin D (1,25‐OH vit D), phosphate, alkaline phosphatase (ALP), and the bone turnover markers procollagen type 1 N‐terminal propeptide (P1NP) and C‐terminal telopeptide collagen type 1 (CTX‐1). In addition, we examined the changes of sex hormones (estradiol, testosterone, luteinizing hormone, follicle‐stimulating hormone), sex hormone‐binding globulin, and leptin.
Results
After 12 weeks of MAD, we found a significant reduction in PTH, Ca, CTX‐1, P1NP, 1,25‐OH vit D, and leptin. There was a significant increase in 25‐OH vit D. These changes were most pronounced among patients <37 years old, and in those patients with the highest body mass index (≥25.8 kg/m²), whereas sex and type of antiseizure medication had no impact on the results. For the reference group, the changes were nonsignificant for all the analyses. In addition, the changes in sex hormones were nonsignificant.
Significance
Twelve weeks of MAD treatment leads to significant changes in bone and Ca metabolism, with a possible negative effect on bone health as a result. A reduced level of leptin may be a triggering mechanism. The changes could be important for patients on MAD, and especially relevant for those patients who receive treatment with MAD at an early age before peak bone mass is reached.
Keywords: antiseizure medications, bone health, ketogenic diet, leptin, sex hormones
Key Points.
MAD significantly affects calcium and bone metabolism
The effect was most pronounced in young patients and among those with BMI ≥ 25.8 kg/m²
A diet‐induced reduction of leptin levels is a possible explanation for the findings
The changes were independent of sex and type of antiseizure medications
1. INTRODUCTION
Over the past decades, ketogenic diets have been used in children and adults to treat pharmacoresistant epilepsy as an alternative or a supplement to antiseizure medications (ASMs). 1 , 2 This high‐fat, low‐carbohydrate diet induces a switch of brain metabolism from glucose to ketones as the main energy source, thereby mimicking the biochemical changes observed during fasting. 3 Much concern has been expressed regarding possible long‐term adverse effects of the diet, and a possible negative effect on bone health has been discussed. 4 Bone tissue is a highly specialized tissue that acts as a storage for minerals among other functions like being an active metabolic and endocrine organ.
Patients with epilepsy have a high risk of fractures. The fracture risk is 2–6 times higher in people with epilepsy compared with the general population, and may be caused by seizures themselves, seizure‐related falls, and adverse effects of ASMs, including decreased bone mineral density. 6 , 7 , 8 , 9
Reduced bone mineral density may be an adverse effect of ASMs. 8 Especially the enzyme‐inducing ASMs (EIASMs), which have impact on the hepatic cytochrome P450 enzyme system, have been associated with a reduction in bone mineral density. 10 , 11 , 12
According to some previous studies, usage of ketogenic diets in children has been associated with a decrease in bone mass and disordered mineral metabolism. 13 , 14 , 15 On the other hand, one study showed that the bone mass remained stable during 24 months of treatment with modified Atkins diet (MAD; a less strict variant of ketogenic diet) in 23 children. 16
Data from studies of ketogenic dietary treatment in adults are scarce. In a study by Bertoli et al., three adult patients used ketogenic diet from 5 to 6 years without any consequence for their bone health. 17 Cervenka et al. demonstrated osteoporosis in one adult patient after 14 years on a ketogenic diet without supplementation of vitamin D and calcium (Ca). 18 This topic has also been examined in animal models, which indicate that ketogenic diet may result in reduced bone mineral density, length growth, and mechanical properties. So far, the mechanisms behind these changes are unknown. 19 , 20
Another interesting finding is the link between the hormone leptin and parathyroid hormone (PTH) and its effect on Ca balance. Leptin seems to increase PTH. 21 , 22 Leptin is mainly secreted by adipose tissues, but has also been found in hypothalamus, neurons, and parathyroid glands. Because leptin levels are decreased with reduced body weight, one may speculate that leptin is one of the key hormones regulating bone metabolism in patients treated with ketogenic diet. In line with this, one study has confirmed a significant decrease of leptin in nine patients with epilepsy treated with MAD. 23 However, another study showed no changes of leptin in 10 children using ketogenic diet. 17 Body weight was included only in the last study, showing no significant changes.
Recently, bone turnover markers (BTMs) have been established as surrogate markers for bone metabolism. In particular, procollagen type 1 N‐terminal propeptide (P1NP) and C‐terminal telopeptide collagen type 1 (CTX‐1) are valid markers for bone formation and bone resorption, respectively. 24 , 25 The BTMs will typically increase with increased bone turnover and decrease if the turnover is decreased.
The aim of the present study was to evaluate the effect of 12 weeks of MAD in adult patients with pharmacoresistant epilepsy on bone metabolism measured by BTMs and alkaline phosphatase (ALP), parameters of Ca metabolism (PTH, Ca, 25‐OH vitamin D [25‐OH vit D], 1,25‐OH vitamin D [1,25‐OH vit D], and phosphate [Ph]). In addition, we measured sex hormones (estradiol, testosterone, luteinizing hormone [LH], follicle‐stimulating hormone [FSH]), sex hormone‐binding globulin (SHBG), and leptin.
2. MATERIALS AND METHODS
2.1. Patients
Fifty‐three adults with pharmacoresistant focal or generalized epilepsy were included from a tertiary referral epilepsy center to study the effect and tolerability of 12 weeks of treatment with MAD. The patients were merged from two studies by Kverneland et al., 26 , 27 and the results in the current article were based on data collected from these studies.
Screening and inclusion were performed by two senior neurologists and a clinical nutritionist. The inclusion criteria were (1) age > 16 years; (2) having tried at least three ASMs, including current treatment; (3) having a body mass index (BMI) > 18.5 kg/m2; and (4) having at least three countable seizures per month. The seizures had to be countable either by the patient or by relatives/caregivers. Exclusion criteria were pregnancy, use of a ketogenic diet the previous year, change of ASMs during the past 6 months, psychogenic nonepileptic seizures, status epilepticus in the previous 6 months, surgery or implantation of vagus nerve stimulator the last 12 months, or having psychosocial or physical comorbidities that contraindicated use of the diet.
The medication was kept unchanged, and oral contraceptives or other hormone therapies or nutrients that could affect the analyses were not allowed during the study period. All patients received vitamin D (5–7.5 µg “Multi,” Nycoplus, Takeda) and Ca supplements (800 mg pure Ca carbonate, Takeda) daily while consuming MAD.
The diet allowed intake of a maximum 16 g of carbohydrates daily (e.g., 5% carbohydrates, 70% fat, and 25% protein). 26 , 27 Adherence to the diet was checked by measuring ketosis in urine and blood. At home, the patients recorded urine ketosis twice daily (morning and evening) during the 12 weeks of dietary treatment. During the hospital stays after 4 and 12 weeks on the diet, the extent of blood ketosis was measured after an overnight fast by the concentration of ß‐hydroxybutyrate based on a finger‐prick blood sample in the morning using Precision Xtra Blood Ketone Test Strips (Abbott). Urine ketosis was also measured twice daily using urine dipsticks (Ketostix, Bayer Healthcare). The data were all reported in the work by Kverneland et al., and indicated satisfactory adherence to the dietary treatment. 26 , 27 , 28
The patients (and/or their caregivers) were motivated to adhere to the diet, and they were trained to be confident in preparing meals.
To observe possible changes over time, a reference group was gathered as follows. Nineteen of the 53 patients were recruited 3 months before starting on the MAD. In addition, nine patients ate their habitual diet for 3 months without starting on the MAD afterward. The reference group of 28 patients altogether consumed their habitual diet in line with a typical Norwegian diet with average energy distribution of 43%–44% carbohydrates, 34% fat, and 18% protein. 29 Blood samples were collected at start and after 3 months from the patients in the reference group.
The study was approved by the Regional Committee for Medical and Health Research Ethics South East Norway (number 2010/2326). Written informed consent was obtained from all patients.
2.2. Laboratory tests and other analyses
Venous serum was collected between 8 and 10 a.m. after an overnight fast for both the diet group and the reference group at baseline and after 12 weeks (i.e., 12 weeks on the MAD and 12 weeks on a habitual diet, respectively). These serum samples were stored at −80°C for 6–76 months. All samples were analyzed together in one batch. Ca metabolism was assessed by measuring PTH, free Ca (Ca2+), Ph, 25‐OH vit D, and 1,25‐OH vit D. Bone metabolism was measured by P1NP, CTX‐1, and ALP. PTH (intra‐assay coefficient of variation [CV] 9% at 3.5 pmol·L–1, detection limit = .60 pmol·L–1) was analyzed using noncompetitive immunoluminometric assays (Siemens Healthineers), Ca2+ (CV = 2% at 1.14 mmol·L–1, detection limit = .10 mmol·L–1) with potentiometric electrode (Cobas b221, Roche Diagnostics), and Ph (CV = 9%, limit of detection = .1 mmol·L–1) with a photometric method (Cobas c701, Roche Diagnostics). 25‐OH vit D (CV = 14% at 50 nmol·L–1, detection limit = 12 nmol·L–1) was analyzed using liquid chromatography–mass spectrometry (LCMS; Hormone laboratory, Oslo University Hospital, Oslo, Norway). 1,25‐OH vit D (CV = 10% at 184 pmol·L–1, detection limit = 12 pmol·L–1) was analyzed with chemiluminescent immunoassay (Liaison XL, DiaSorin). P1NP (CV = 5% at 45 μg/L, detection limit = 9 μg/L) and CTX‐1 (CV = 8% at .32 μg/L, detection limit = .07 μg/L) were analyzed with electrochemiluminescent immunoassay (Cobas e601, Roche Diagnostics). Total ALP (CV = 12% at 9.5 μg/L, detection limit = 1.5 μg/L) was analyzed using immunometric chemiluminescent immunoassay (Liaison XL, DiaSorin). LH, FSH, and SHBG were analyzed by noncompetitive immunoluminometric assays (Siemens Healthineers), estradiol by competitive chemiluminescence (DiaSorin), and testosterone by LCMS (Hormone Laboratory, Oslo University Hospital). Leptin was analyzed by enzyme‐linked immunosorbent assay (Mediagnost; CV = 12% at 972 nmol·L–1, detection limit = 63 nmol·L–1).
Electrolytes (Ca, magnesium [Mg], and sodium [Na]), Ph, ALP, aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), and creatinine were analyzed at the Department of Clinical Chemistry, Oslo University Hospital. All other analyses were performed at the Hormone Laboratory, Oslo University Hospital, and they were all accredited according to ISO 17025.
Vitamin D was analyzed as the biologically inert 25‐OH vit D and the active metabolite 1,25‐OH vit D. Vitamin D status is usually assessed by 25‐OH vit D.
Body weight was measured in the morning after an overnight fast. To examine differences in bone parameters according to body mass, the patients were divided into two groups by median BMI at baseline. Possible age‐related effects were studied by separating the patients into two groups according to the median age at baseline. We also analyzed for possible sex differences. The ASMs were categorized and analyzed according to type of ASM (i.e., EIASMs or non‐enzyme‐inducing ASMs [NEIASMs]; see Table 1). Patients who used one or more EIASMs were included in the EIASM group.
TABLE 1.
ASMs used by the participants
| Enzyme‐inducing ASMs, n = 12 | Non‐enzyme‐inducing ASMs, n = 41 |
| Phenytoin, n = 4 | Zonisamide, n = 4 |
| Phenobarbital, n = 2 | Levetiracetam, n = 14 |
| Carbamazepine, n = 7 | Valproate, n = 15 |
| Topiramate, n = 8 a | |
| Oxcarbazepine, n = 16 a | |
| Clobazam, n = 8 | |
| Lamotrigine, n = 16 | |
| Lacosamide, n = 6 | |
| Pregabalin, n = 2 | |
| Clonazepam, n = 2 |
n indicates number of patients using different ASMs.
Abbreviation: ASM, antiseizure medication.
Partial enzyme‐inducing ASMs.
2.3. Statistics
Normally distributed data were analyzed using parametric methods, and not normally distributed data were analyzed using nonparametric methods. Normality checks included Shapiro–Wilks test for significant deviation from the normal distribution as well as visual inspection of histograms and Q‐Q plots.
All bone analyses were analyzed and compared to baseline after 12 weeks on the diet using a Wilcoxon signed‐rank test because of not normally distributed data. Subgroup analyses were performed for sex, age, BMI, and type of ASMs. For the patients in the reference group using a habitual diet for 12 weeks, Wilcoxon signed‐rank test was used to test differences at start and after 12 weeks.
Significance was assumed for p < .05. All analyses were performed using SPSS Statistics v25 and v26.
3. RESULTS
3.1. Patient characteristics
The characteristics of both the diet group and reference group at baseline are described in Table 2. The groups differed in size, but were similar according to age, BMI, weight, the number of years with epilepsy and the mean number of ASMs used. Due to the wide variety in seizure frequency at baseline, median seizure frequency at baseline was also calculated.
TABLE 2.
Patient characteristics for 53 patients on MAD and 28 patients on a habitual diet (reference group)
| Characteristic | MAD, n = 53 | Reference group, n = 28 |
|---|---|---|
| Women/men, n | 33/20 | 13/15 |
| Age, years, mean (range) | 37.4 (16–65) | 36.0 (22–65) |
| Body weight, kg, mean (range) | 78.4 (51.6–138.8) | 78.8 (51.6–138.8) |
| BMI, kg/m², mean (range) | 26.8 (18.7–41.7) | 25.5 (18.7–35.1) a |
| Years with epilepsy, mean (range) | 25.0 (5–58) | 25.1 (3–58) |
| Seizure frequency per week, mean (range) | 15.7 (.08–268) | 22.8 (0–196) |
| Seizure frequency per week, median (IQR) | 3.5 (9.1) | 5.0 (14.0) |
| Mean ASMs used, n, current (range)/earlier (range) | 2.1 (1–3)/8 (3–18) | 2.0 (1–3)/8.6 (2–18) |
| Focal/generalized, n | 47/6 | 28/0 |
Abbreviations: ASM, antiseizure medication; BMI, body mass index; IQR, interquartile range; MAD, modified Atkins diet.
n = 18. Height was available for 18 patients in the control group.
3.2. Bone health parameters
Before start, all patients had bone marker values within normal range. The changes of these markers (PTH, CTX‐1, P1NP, Ph, Ca, ALP, and vitamin D) from start to after 12 weeks on the dietary treatment are shown in Table 3. There was a significant change in all values except for ALP. PTH, CTX‐1, P1NP, Ca, and 1,25‐OH vit D decreased significantly, and 25‐OH vit D and Ph increased significantly. There were no major sex differences. All values were within reference limits for the analyzing laboratory. For the patients in the reference group who received a habitual diet for 12 weeks, the changes were not significant (see Table 3).
TABLE 3.
Median (interquartile range) values of bone markers at baseline and after 12 weeks on modified Atkins diet or habitual diet (reference group)
| Baseline | 12 weeks | % change | p | |
|---|---|---|---|---|
| PTH, pmol·L–1 [ref: 1.2–7.1] | ||||
| All, n = 53 | 4.94 (3.34) | 4.12 (2.65) | −16.6 | <.01 |
| Min–max | 1.67–16.33 | .20–11.32 | ||
| Women, n = 33 | 5.06 (4.25) | 4.71 (2.12) | −7.0 | .06 |
| Men, n = 20 | 4.04 (2.85) | 3.37 (1.46) | −16.6 | <.01 |
| Reference group, n = 28 | 3.30 (2.87) | 3.50 (2.60) | 6.0 | .46 |
| Ca, mmol·L–1 [ref: 1.15–1.33] | ||||
| All, n = 53 | 1.25 (.06) | 1.22 (.05) | −2.4 | <.01 |
| Min–max | 1.17–1.36 | 1.14–1.32 | ||
| Women, n = 33 | 1.23 (.07) | 1.22 (.05) | −.82 | <.01 |
| Men, n = 20 | 1.27 (.06) | 1.24 (.06) | −2.4 | .16 |
| Reference group, n = 28 | 1.23 (.05) | 1.25 (.05) | 1.6 | .08 |
| 25‐OH vit D, nmol·L–1 [ref: 37–131] | ||||
| All, n = 46 a | 60 (25) | 73 (27) | 21.6 | <.01 |
| Min–max | 28–140 | 39–163 | ||
| Women, n = 30 | 62 (26) | 66 (25) | 4.8 | .10 |
| Men, n = 16 | 57.50 (32) | 81 (33) | 40.6 | .01 |
| Reference group, n = 24 | 58 (32.50) | 64 (27.75) | 10.3 | .06 |
| 1,25‐OH vit D, pmol·L–1 [ref: 39–193] | ||||
| All, n = 52 | 97 (52) | 90.50 (55) | −6.7 | .03 |
| Min–max | 22–162 | 31–162 | ||
| Women, n = 33 | 92 (51) | 93 (50) | 1.1 | .11 |
| Men, n = 19 | 103 (62) | 84 (69) | −18.4 | .13 |
| Reference group, n = 25 | 115.80 (57.70) | 101 (45.50) | −12.8 | .67 |
| Ph, mmol·L–1 [ref: .8−1.7] | ||||
| All, n = 52 a | 1.10 (.20) | 1.20 (.27) | 9.0 | .05 |
| Min–max | .80–1.70 | .90–1.90 | ||
| Women, n = 32 | 1.10 (.20) | 1.20 (.25) | 9.0 | .08 |
| Men, n = 20 | 1.10 (.27) | 1.15 (.27) | 4.5 | .45 |
| Reference group, n = 28 | 1.10 (.20) | 1.15 (.10) | 4.5 | .38 |
| ALP, U/L [ref: 35–105] | ||||
| All, n = 52 a | 66.50 (41) | 64 (34) | −4.0 | .08 |
| Min–max | 27–142 | 34–127 | ||
| Women, n = 32 | 58 (36) | 56.50 (35) | −2.6 | .04 |
| Men, n = 20 | 73 (40) | 67 (33) | −8.2 | .85 |
| Reference group, n = 28 | 75.50 (37) | 74 (37.75) | −2.0 | .73 |
| CTX−1, µg/L [ref: ≤.57] | ||||
| All, n = 53 | .45 (.33) | .40 (.36) | −11.0 | .03 |
| Min–max | .17–1.34 | .16–1.36 | ||
| Women, n = 33 | .36 (.32) | .29 (.27) | −19.5 | .11 |
| Men, n = 20 | .61 (.48) | .54 (.27) | −11.5 | .10 |
| Reference group, n = 28 | .54 (.28) | .49 (.30) | −9.3 | .47 |
| P1NP, µg/L [women, ref: ≥25 years : 11–94 µg/L; men, ref: ≥25 years: 20–91] | ||||
| All, n = 53 | 53 (27.97) | 43.19 (33.06) | −19.0 | <.001 |
| Min–max | 24.14–176.5 | 20.55–122 | ||
| Women, n = 33 | 47.52 (17.71) | 39.67 (25.52) | −16.6 | .03 |
| Men, n = 20 | 66.86 (41.32) | 55.80 (39.96) | −16.5 | <.001 |
| Reference group, n = 28 | 64.50 (23.75) | 63.12 (18.98) | −2.14 | .08 |
All reference values are according to adult patients.
Abbreviations: 1,25‐OH vit D, 1,25‐OH vitamin D; 25‐OH vit D, 25‐OH vitamin D; ALP, alkaline phosphatase; Ca, calcium; CTX‐1, C‐terminal telopeptide collagen type 1; max, maximum; Min, minimum; P1NP, procollagen type 1 N‐terminal propeptide; Ph, phosphate; PTH, parathyroid hormone; ref, reference value.
n according to available serum.
3.3. Sex hormones
In those patients using MAD, we analyzed LH, FSH, estradiol, and total testosterone in both women and men. All values were within normal reference values at baseline and after 12 weeks. There were no significant changes during the study period except for FSH in men (n = 19, p = .02). Removing the women >50 years of age from the analyses because of menopause (n = 6) did not change the results. SHBG was analyzed, and free androgen index (FAI = total testosterone [nmol·L–1] × 10 / SHBG [nmol·L–1]) was calculated as a surrogate marker for free testosterone. FAI showed significant reduction for men (n = 19, p = .02) and for women (n = 25, p < .01). SHBG increased significantly for both women (n = 25, p < .01) and men (n = 19, p = .02).
3.4. Age and BMI
We divided the patients on MAD into two groups according to a median age of 37 years (i.e., 26 patients younger than 37 years and 27 patients older than 37 years; maximum = 65 years). Both age groups were analyzed according to bone health parameters to identify possible age differences. In the youngest age group, there was a more substantial change, with a significant reduction in PTH, Ca, and P1NP. 25‐OH vit D was significantly increased only for the youngest patients (see Table 4).
TABLE 4.
Median (interquartile range) values of bone markers at baseline and after 12 weeks on modified Atkins diet in 53 patients divided according to median age 37 years, and 51 patients divided according to median BMI 25.82 kg/m²
| Age, n = 53 | BMI, n = 51 | |||
|---|---|---|---|---|
| <37 years, n = 26 | ≥37 years, n = 27 | <25.8 kg/m², n = 25 | ≥25.8 kg/m², n = 26 | |
| PTH, pmol·L–1 | ||||
| Baseline | 4.04 (2.99) | 5.04 (3.75) | 3.96 (2.86) | 5.11 (3.22) |
| 12 weeks | 3.43 (2.50) | 4.55 (2.79) | 4.12 (2.65) | 4.24 (2.07) |
| p | <.01 | .05 | .96 | <.01 |
| Ca, nmol·L–1 | ||||
| Baseline | 1.25 (.06) | 1.23 (.06) | 1.25 (.07) | 1.36 (.06) |
| 12 weeks | 1.26 (.07) | 1.22 (.05) | 1.22 (.04) | 1.22 (.05) |
| p | .01 | .09 | .04 | .01 |
| 25‐OH vit D, nmol·L–1 | ||||
| Baseline | 55.50 (26) | 65 (30) | 71 (40) | 59 (25) |
| 12 weeks | 77 (34) | 67.50 (26) | 80 (28) | 65 (30) |
| p | <.01 | .24 | .06 | .02 |
|
1,25‐OH vit D, nmol·L–1 |
n = 25 | n = 27 | ||
| Baseline | 103 (61.50) | 95.00 (41) | 88.00 (62) | 100 (46) |
| 12 weeks | 84 (63) | 93.00 (38) | 83.00 (49.50) | 93 (51) |
| p | <.23 | .05 | <.01 | .55 |
| Ph, mmol·L–1 | ||||
| Baseline | 1.10 (.20) | 1.10 (.20) | 1.10 (.20) | 1.10 (.25) |
| 12 weeks | 1.20 (.10) | 1.20 (.30) | 1.20 (.25) | 1.20 (.20) |
| p | .24 | .12 | .16 | .17 |
| ALP, U/L | ||||
| Baseline | 57 (45) | 67 (32) | 65 (39) | 70 (43) |
| 12 weeks | 64 (42) | 64 (27) | 59 (36) | 64 (41) |
| p | .42 | .12 | .44 | .09 |
| CTX−1, µg/L | ||||
| Baseline | .56 (.40) | .37 (.28) | .51 (.36) | .40 (.30) |
| 12 weeks | .49 (.26) | .32 (.27) | .47 (.41) | .33 (.21) |
| p | .09 | .12 | .32 | .02 |
| P1NP, µg/L | ||||
| Baseline | 52.97 (40.35) | 53 (24.80) | 54 (34.56) | 47.76 (24.85) |
| 12 weeks | 41.86 (37.67) | 44 (34.29) | 45.43 (39.11) | 41.21 (26.83) |
| p | <.001 | .07 | <.01 | .03 |
Abbreviations: 1,25‐OH vit D, 1,25‐OH vitamin D; 25‐OH vit D, 25‐OH vitamin D; ALP, alkaline phosphatase; BMI, body mass index; Ca, calcium; CTX‐1, C‐terminal telopeptide collagen type 1; P1NP, procollagen type 1 N‐terminal propeptide; Ph, phosphate; PTH, parathyroid hormone.
In those using MAD, BMI at baseline was mean (range) 26.8 (18.7–41.7) kg/m², and 25.2 (17.6–37.8) kg/m² after 12 weeks on the diet, resulting in a significant weight loss (p < .001). Twenty‐three patients had a normal BMI (18.5–24.9). For unknown reasons, two patients were not weighed at 12 weeks.
The patients using MAD were divided into two groups according to median BMI. At start, 25 patients had a BMI < 25.8 kg/m² and 26 patients had a BMI ≥ 25.8 kg/m². For the group with the highest BMI, there was a significant change in PTH, Ca, 25‐OH vit D, CTX‐1, and P1NP. For those with the lowest BMI, Ca and P1NP were significantly reduced (see Table 4).
3.5. Leptin
We found a significant decrease in leptin for all the patients on MAD. At baseline, median leptin was 963.50 (interquartile range [IQR] = 1949.25) pmol·L–1, which reduced to median 617.00 (IQR = 875.75) pmol·L–1 after 3 months (n = 52, p < .001). The reference values for leptin are for women with BMI 18–25 kg/m², 80–2500 pmol·L–1, and for men with BMI 18–25 kg/m², <950 pmol·L–1. Leptin will increase with increased BMI. As expected, women (n = 33) had higher values at both baseline (median = 1513.00 [IQR = 1901.00] pmol·L–1) and after 12 weeks on MAD (median = 847.00 [IQR = 1214.50] pmol·L–1). For men (n = 19), median leptin was 582.00 (IQR = 817.00) pmol·L–1 at baseline, and decreased to median = 225.00 (IQR = 445.00) pmol·L–1 during the study period. The changes for women and men were significant (p < .001) in both groups. For BMI, there was a significant reduction in leptin in those with low BMI (<25.8 kg/m², n = 26, p < .001) and those with high BMI (>25.8 kg/m², n = 26, p < .01). According to age, both patients younger than (n = 25) and older than (n = 27) the median age of 37 years had a significant decrease in leptin levels (p = .003 and p < .001, respectively). For the patients in the reference group, we observed no significant change from baseline (median = 738.00 [IQR = 1738.00] pmol·L–1) to 12 weeks (median = 815.00 [IQR = 2103.00] pmol·L–1; n = 25, p = .26).
3.6. Use of EIASMs and NEIASMs
At baseline, there were no significant differences for the bone markers between the EIASM and NEIASM groups. No patients used monotherapy. We also analyzed the NEIASM group without topiramate and oxcarbazepine, both being partial enzyme inducers, and classified this group the “NEIASM*” group. We found a significant change for Ca and 25‐OH vit D in the NEIASM group that was not observed in the NEIASM* group. Furthermore, there was a significant change in PTH and P1NP in both the EIASM group and the NEIASM group that was not significant in the NEIASM* group (see Table 5).
TABLE 5.
Changes in bone parameter levels from baseline to 12 weeks on modified Atkins diet according to type of antiseizure drug used: Median (interquartile range) values at baseline and after 12 weeks for EIASMs and NEIASMs
| Baseline | 12 weeks | p | % change | |
|---|---|---|---|---|
| PTH, pmol·L–1 [ref: 1.2 –7.7] | ||||
| EIASMs, n = 12 | 5.03 (5.76) | 4.25 (2.85) | .05 | −15.5 |
| NEIASMs, n = 41 | 4.60 (2.96) | 4.12 (2.72) | <.01 | −10.4 |
| NEIASMs, n = 19 a | 5.13 (2.14) | 4.71 (2.59) | .10 | −8.2 |
| Ca, mmol·L–1 [ref: 1.15–1.33] | ||||
| EIASMs, n = 12 | 1.21 (.09) | 1.24 (.06) | .59 | 2.5 |
| NEIASMs, n = 41 | 1.25 (.05) | 1.22 (.05) | .01 | −2.4 |
| NEIASMs, n = 15 a | 1.25 (.05) | 1.23 (.08) | .30 | −1.6 |
| 25‐OH vit D, nmol·L–1 [ref: 37–131] | ||||
| EIASMs, n = 11 | 55.00 (39.00) | 76.00 (24.00) | .19 | 38.2 |
| NEIASMs, n = 35 | 60.00 (23.00) | 72.00 (28.00) | .01 | 20.0 |
| NEIASMs, n = 15 a | 60.00 (32.00) | 65.00 (29.00) | .25 | 8.3 |
| 1,25‐OH vit D, pmol·L–1 [ref: 39–193] | ||||
| EIASMs, n = 12 | 87.00 (33.25) | 85.50 (55.75) | .66 | −1.8 |
| NEIASMs, n = 40 | 102.00 (59.00) | 93.50 (54.25) | .02 | −8.4 |
| NEIASMs, n = 18 a | 100.00 (62.50) | 89.50 (62.00) | .05 | −10.5 |
| Ph, mmol·L–1 [ref: .8 −1.7] | ||||
| EIASMs, n = 12 | 1.15 (.25) | 1.20 (.22) | .31 | 4.3 |
| NEIASMs, n = 40 | 1.10 (.20) | 1.20 (.27) | .11 | 9.0 |
| NEIASMs, n = 18 a | 1.10 (.30) | 1.10 (.22) | .20 | 0 |
| ALP, U/L [ref: 35–105] | ||||
| EIASMs, n = 12 | 76.50 (38.00) | 67.00 (35.00) | .18 | −12.4 |
| NEIASMs, n = 40 | 61.50 (38.00) | 59.50 (36.00) | .19 | −3.3 |
| NEIASMs, n = 18 a | 52.00 (28.00) | 42.00 (23.00) | .74 | −19.3 |
| CTX−1, µg/L [ref: ≤.57] | ||||
| EIASMs, n = 12 | .50 (.45) | .40 (.56) | .11 | −20.0 |
| NEIASMs, n = 41 | .45 (.33) | .40 (.35) | .09 | −11.2 |
| NEIASMs, n = 19 a | .36 (.25) | .35 (.28) | .52 | −2.8 |
| P1NP, µg/L [women, ref: ≥25 years : 11–94; men, ref: ≥25 years : 20–91] | ||||
| EIASMs, n = 12 | 64.01 (36.12) | 44.46 (25.47) | .01 | −30.6 |
| NEIASMs, n = 41 | 52.27 (30.74) | 41.16 (38.41) | .01 | −21.3 |
| NEIASMs, n = 19 a | 46.45 (19.76) | 40.07 (34.02) | .24 | −13.8 |
EIASMs: n = 12, n = 11 for 25‐OH vit D; NEIASMs: n = 41, n = 35 for 25‐OH vit D, n = 40 for Ph, and n = 40 for ALP.
Abbreviations: 1,25‐OH vit D, 1,25‐OH vitamin D; 25‐OH vit D, 25‐OH vitamin D; ALP, alkaline phosphatase; Ca, calcium; CTX‐1, C‐terminal telopeptide collagen type 1; EIASM, enzyme‐inducing antiseizure medication; NEIASM, non‐enzyme‐inducing antiseizure medication; P1NP, procollagen type 1 N‐terminal propeptide; Ph, phosphate; PTH, parathyroid hormone; ref, reference value.
NEIASMs without topiramate and oxcarbazepine (partial enzyme inducers).
3.7. Electrolytes, liver, and kidney function
Mean Mg and Na levels were within reference values at baseline and after 12 weeks on MAD. Mg was .85 (95% confidence interval [CI] = .83–.87) mmol·L–1 at baseline and significantly reduced to .83 (95% CI = .81–.85) mmol·L–1 after 12 weeks on MAD (p = .03). Mean Na was 138.3 (95% CI = 137.2–139.3) mmol·L–1 at baseline and reduced to 137.9 (95% CI = 136.8−138.9) mmol·L–1 after 12 weeks of dietary treatment. The reduction was not significant (p = .28).
Liver and kidney function were measured by ASAT, ALAT, and creatinine, which were all within reference values at baseline and after 12 weeks. Mean ASAT level at baseline was 19.3 (95% CI = 17.5–21.2) U/L compared to 20.1 (95% CI = 17.9–22.3) U/L after 12 weeks on MAD (p = .48). Mean ALAT levels were 25.3 (95% CI = 21.4–29.2) U/L and 25.6 (95% CI = 22.1–29.1) U/L, respectively (p = .84). For creatinine, mean levels were 72.9 (95% CI = 68.7–77.0) mmol·L–1 at baseline and 70.2 (95% CI = 65.6–74.8) mmol·L–1 after 12 weeks on MAD (p = .07).
For the patients in the reference group, the electrolytes, ASAT, ALAT, and creatinine were within reference values and the changes were not significant except for creatinine, which was significantly reduced, although within reference values (p = .05).
4. DISCUSSION
The main findings in this study of adult patients with pharmacoresistant epilepsy exposed to MAD for 12 weeks were a significant reduction of the blood levels of Ca, PTH, P1NP, CTX‐1, and 1,25‐OH vit D, whereas 25‐OH vit D and Ph increased significantly. The significant changes were all in contrast to the reference group that received a habitual diet for 12 weeks, and where no significant changes were observed. Despite the participants receiving Ca supplementation, we found a reduction of Ca levels without an expected increase of PTH values. Taken together, our findings indicate reduced bone metabolism for patients using MAD for 12 weeks. This may lead to reduced bone density and a possible increased risk of bone fractures over time.
As expected, we found an increase in 25‐OH vit D levels after 12 weeks on the MAD, as the participants received vitamin D supplementation. Thus, the decline in Ca levels cannot be explained by an inadequate level of vitamin D. However, the active metabolite of vitamin D, 1,25‐OH vit D, was significantly reduced during the study. This could imply an impaired conversion from 25‐OH vit D to 1,25‐OH vit D in the kidneys, possibly due to an unknown factor affecting one of the enzymes involved in this process and/or as a consequence of the reduced PTH observed. 30
Subgroup analysis with regard to age revealed that the reduction of Ca, PTH, and P1NP was most pronounced among the youngest patients (<37 years). Peak bone mineral density is obtained between 20 and 30 years of age, 5 , 31 and declines gradually the following years. Consequently, bone health early in life is essential for those who may experience bone loss later. The higher the peak bone mass achieved in young adult age, the more an individual is protected against osteoporosis and fractures later in life. 32 For patients treated with MAD at an early age, as children, adolescents, or young adults, this is a matter of particular concern and should be addressed in the follow‐up of such patients.
Leptin is mainly secreted in white adipose tissue, but also in smaller amounts in the hypothalamus and the parathyroid glands. 21 , 22 Leptin regulates energy balance, and the quantity mirrors the amount of body fat until a certain limit due to leptin tolerance in obesity. Women have higher leptin levels than men, even after correction for differences in body fat composition. A decrease in leptin levels in adult patients using the ketogenic diet has been reported. 23 , 33 In our study, leptin decreased significantly in both BMI groups and both women and men. For the patients on a habitual diet, we did not show any changes in leptin.
Potential links between leptin and PTH secretion have been discussed. 21 , 22 Animal studies have shown that leptin increases bone mineral density and bone mineral content. Low bone mineral density is seen in patients with anorexia nervosa and hypoleptinemia. 34 A possible explanation of our findings could be that the decrease in leptin levels seen in patients on MAD affects the parathyroid glands, leading to a reduction in PTH. The reduction in PTH consequently results in a lower conversion from 25‐OH vit D to 1,25‐OH vit D, which eventually results in a decrease in Ca reabsorption from the kidneys and reduced Ca absorption from the gut. We observed reduced Ca in our study. The reduced PTH also results in reduced bone turnover. 35 We suggest that reduced Ca metabolism, driven by leptin and PTH reduction, results in reduced bone turnover observed as a reduction of BTMs (Figure 1).
FIGURE 1.
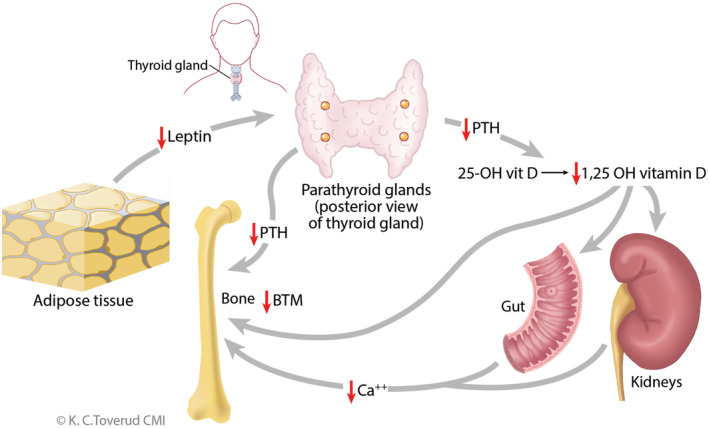
The possible mechanism on how modified Atkins diet influences calcium and bone metabolism by lowering leptin levels. Ca, calcium; 25‐OH vit D, 25‐OH vitamin D; BTM, bone turnover markers; PTH, parathyroid hormone
Impaired bone health due to usage of ASMs is reported in all ages and in both women and men. The degree of influence depends on type of drug, dosages, and duration of therapy. 30 , 31 To our surprise, we found no major differences between those using EIASMs and those using NEIASMs regarding changes in bone metabolism markers. The partial enzyme inducers topiramate and oxcarbazepine were then removed from the NEIASM group, and the remaining ASMs were organized in an NEIASM* group. We found no significant changes in the NEIASM* group in the study period. Multiple studies have revealed an increased risk of fractures in patients exposed especially to EIASMs, possibly due to a combination of mechanisms, including drug‐induced changes of liver enzymes, vitamin D, Ca, and hormones. 6 , 7 , 8 , 9 , 10 , 11 , 12 A possible explanation for not finding a significant change of bone markers in those using EIASMs among our patients could be that the number of patients was too low.
Low estrogen levels in women and low testosterone levels in men may lead to bone loss. 36 In our study, the sex hormones were within reference values according to age without significant changes during the study period. The calculation of FAI showed a significant decrease in both women and men during the 12 weeks on the MAD, although within reference values. The significant increase in SHBG, due to reduced weight and concomitant reduction of insulin resistance, may explain the reduction in FAI.
According to a study by Diemar et al., moderate and severe hyponatremia were associated with osteoporosis. 37 Hyponatremia was not found in our study. As expected, we found a significant reduction in Mg values during the study period due to a lower level of PTH. During the study period, we found no significant changes in analyses of liver and kidney function.
MAD is designed to resemble a state of fasting. At present, little is known about bone health during or after fasting in humans. However, one small animal study demonstrated that fasting reduced bone quality in rats. 38
Several factors are known to influence bone health, for example, nonmodifiable factors like genetics, ethnicity, increasing age, and sex, and modifiable factors like weight‐bearing exercise, adequate intake of Ca and vitamin D, immobilization, smoking, alcohol consumption, and sunlight exposure. Our study did not include genetic analyses, degree of physical activity, use of tobacco or alcohol, and extent of sunlight exposure. Ideally, the number of participants should have been higher. The patients were well controlled and monitored regularly at the same center by a specialized multidisciplinary team.
To our knowledge, this is the first study to assess the effects on bone health parameters in adult patients with pharmacoresistant epilepsy using MAD. Assessing bone health markers can be useful to recognize important changes of bone metabolism at an early stage, and thereby prevent osteopenia or osteoporosis and hopefully bone fractures. A newly published international guideline recommends bone density measurements with dual‐energy X‐ray absorptiometry (DXA) every fifth year in patients treated with ketogenic diets. 39 However, DXA may be of limited value, because the prevention of fractures should start a long time before pathological bone density is diagnosed.
Our findings may suggest that bone metabolism can be altered when using MAD. This is particularly important for the youngest patients, where bone metabolism is still more dynamic than among the older patients. We also suggest that the possible mechanism behind the altered bone metabolism is the effect of reduced leptin influence on Ca metabolism for patients treated with MAD. As prevention of osteoporosis begins at a young age, clinicians should be aware of factors having a negative impact on bone health, like smoking, excess alcohol, and low physical activity, when treating patients with epilepsy with MAD.
CONFLICTS OF INTEREST
E.M. received a payment from Nutricia for a lecture held in December 2019. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
We are grateful for the economic support from the Norwegian Epilepsy Association's Research Fund and the Norwegian Epilepsy Federation.
Molteberg E, Taubøll E, Kverneland M, Iversen PO, Selmer KK, Nakken KO, et al. Substantial early changes in bone and calcium metabolism among adult pharmacoresistant epilepsy patients on a modified Atkins diet. Epilepsia. 2022;63:880–891. 10.1111/epi.17169
REFERENCES
- 1. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–6. doi: 10.1016/S1474_4422(08)70092-9 [DOI] [PubMed] [Google Scholar]
- 2. Klein P, Tyrlikova I, Mathews GC. Dietary treatment in adults with refractory epilepsy: a review. Neurology. 2014;83(21):1978–85. doi: 10.1212/WNL.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 3. Höhn S, Dozières‐Puyravel B, Auvin S. History of dietary treatment from Wilder's hypothesis to the first open studies in the 1920s. Epilepsy Behav. 2019;101(Pt A):106588. doi: 10.1016/j.yebeh.2019.106588 [DOI] [PubMed] [Google Scholar]
- 4. Martin‐McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug‐resistant epilepsy. Cochrane Database Syst Rev 2020;6(6):CD001903. doi: 10.1002/14651858.CD001903.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stagi S, Cavalli L, Iurato C, Seminara S, Brandi ML, de Martino M. Bone metabolism in children and adolescents: main characteristics of the determinants of peak bone mass. Clin Cases Miner Bone Metab. 2013;10(3):172–9. [PMC free article] [PubMed] [Google Scholar]
- 6. Mattson RH, Gidal BE. Fractures, epilepsy, and antiepileptic drugs. Epilepsy Behav. 2004;5(Suppl 2):S36–40. doi: 10.1016/j.yebeh.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 7. Sheth RD, Gidal BE, Hermann BP. Pathological fractures in epilepsy. Epilepsy Behav. 2006;9(4):601–5. doi: 10.1016/j.yebeh.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 8. Nakken KO, Taubøll E. Bone loss associated with use of antiepileptic drugs. Expert Opin Drug Saf. 2010;9(4):561–71. doi: 10.1517/14740331003636475 [DOI] [PubMed] [Google Scholar]
- 9. Svalheim S, Røste LS, Nakken KO, Taubøll E. Bone health in adults with epilepsy. Acta Neurol Scand Suppl. 2011;191:89–95. doi: 10.1111/j.1600-0404.2011.01551.x [DOI] [PubMed] [Google Scholar]
- 10. Pack A. Bone health in people with epilepsy: is it impaired and what are the risk factors? Seizure. 2008;17(2):181–6. doi: 10.1016/j.seizure.2007.11.020 [DOI] [PubMed] [Google Scholar]
- 11. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(Suppl 2):S24–9. doi: 10.1016/j.yebeh.2003.11.029 [DOI] [PubMed] [Google Scholar]
- 12. Miziak B, Chrościńska‐Krawczyk M, Czuczwar SJ. An update on the problem of osteoporosis in people with epilepsy taking antiepileptic drugs. Expert Opin Drug Saf. 2019;18(8):679–89. doi: 10.1080/14740338.2019.1625887 [DOI] [PubMed] [Google Scholar]
- 13. Hahn TJ, Halstead LR, DeVivo DC. Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int. 1979;28(1):17–22. doi: 10.1007/BF02441213 [DOI] [PubMed] [Google Scholar]
- 14. Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88(6):1678–84. doi: 10.3945/ajcn.2008.26099 [DOI] [PubMed] [Google Scholar]
- 15. Simm PJ, Bicknell‐Royle J, Lawrie J, Nation J, Draffin K, Stewart KG, et al. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 2017;136:62–6. doi: 10.1016/j.eplepsyres.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 16. Svedlund A, Hallböök T, Magnusson P, Dahlgren J, Swolin‐Eide D. Prospective study of growth and bone mass in Swedish children treated with the modified Atkins diet. Eur J Paediatr Neurol. 2019;23(4):629–38. doi: 10.1016/j.ejpn.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 17. Bertoli S, Trentani C, Ferraris C, De Giorgis V, Veggiotti P, Tagliabue A. Long‐term effects of a ketogenic diet on body composition and bone mineralization in GLUT‐1 deficiency syndrome: a case series. Nutrition. 2014;30(6):726–8. doi: 10.1016/j.nut.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 18. Cervenka MC, Henry‐Barron BJ, Kossoff EH. Is there a role for diet monotherapy in adult epilepsy? Epilepsy Behav Case Rep. 2016;7:6–9. doi: 10.1016/j.ebcr.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, et al. Short‐term exposure to low‐carbohydrate, high‐fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res. 2010;25(2):275–84. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Huang Z, Wang X, Fu Z, Liu J, Huang Z, et al. Ketogenic diet compromises both cancellous and cortical bone mass in mice. Calcif Tissue Int. 2017;101(4):412–21. [DOI] [PubMed] [Google Scholar]
- 21. Polyzos SA, Duntas L, Bollerslev J. The intriguing connections of leptin to hyperparathyroidism. Endocrine. 2017;57(3):376–87. [DOI] [PubMed] [Google Scholar]
- 22. Hoang D, Broer N, Sosa JA, Abitbol N, Yao X, Li F, et al. Leptin is produced by parathyroid glands and stimulates parathyroid hormone secretion. Ann Surg. 2017;266(6):1075–83. doi: 10.1097/SLA.0000000000002004 [DOI] [PubMed] [Google Scholar]
- 23. Lambrechts DA, Brandt‐Wouters E, Verschuure P, Vles HS, Majoie MJ. A prospective study on changes in blood levels of cholecystokinin‐8 and leptin in patients with refractory epilepsy treated with the ketogenic diet. Epilepsy Res. 2016;127:87–92. doi: 10.1016/j.eplepsyres.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 24. Jorde R, Stunes AK, Kubiak J, Joakimsen R, Grimnes G, Thorsby PM, et al. Effects of vitamin D supplementation on bone turnover markers and other bone‐related substances in subjects with vitamin D deficiency. Bone. 2019;124:7–13. [DOI] [PubMed] [Google Scholar]
- 25. Svanevik M, Risstad H, Hofsø D, Blom‐Høgestøl IK, Kristinsson JA, Sandbu R, et al. Bone turnover markers after standard and distal roux‐en‐Y gastric bypass: results from a randomized controlled trial. Obes Surg. 2019;29(9):2886–95. doi: 10.1007/s11695-019-03909-1 [DOI] [PubMed] [Google Scholar]
- 26. Kverneland M, Molteberg E, Iversen PO, Veierød MB, Taubøll E, Selmer KK, et al. Effect of modified Atkins diet in adults with drug‐resistant focal epilepsy: a randomized clinical trial. Epilepsia. 2018;59(8):1567–76. doi: 10.1111/epi.14457 [DOI] [PubMed] [Google Scholar]
- 27. Kverneland M, Selmer KK, Nakken KO, Iversen PO, Taubøll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197–201. doi: 10.1016/j.yebeh.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 28. Kverneland M, Taubøll E, Molteberg E, Veierød MB, Selmer KK, Nakken KO, et al. Pharmacokinetic interaction between modified Atkins diet and antiepileptic drugs in adults with drug‐resistant epilepsy. Epilepsia. 2019;60(11):2235–44. doi: 10.1111/epi.16364 [DOI] [PubMed] [Google Scholar]
- 29. National Directorate of Health. Norkost 3 . A national dietary survey in Norway among adults age 18‐70, 2010‐11.IS‐2000. 2012. https://www.helsedirektoratet.no/ [Google Scholar]
- 30. Hamed SA. Markers of bone turnover in patients with epilepsy and their relationship to management of bone diseases induced by antiepileptic drugs. Expert Rev Clin Pharmacol. 2016;9(2):267–86. doi: 10.1586/17512433.2016.1123617 [DOI] [PubMed] [Google Scholar]
- 31. Heaney RP, Abrams S, Dawson‐Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020 [DOI] [PubMed] [Google Scholar]
- 32. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–386. doi: 10.1007/s00198-015-3440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djøseland O, et al. Reduction in serum leptin and IGF‐1 but preserved T‐lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18(2):209–14. [PubMed] [Google Scholar]
- 34. Bilezikian JPJ. Hypoparathyroidism. Clin Endocrinol Metab. 2020;105(6):1722–36. doi: 10.1210/clinem/dgaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015;64(1):105–13. doi: 10.1016/j.metabol.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pack AM, Walczak TS. Bone health in women with epilepsy: clinical features and potential mechanisms. Int Rev Neurobiol. 2008;83:305–28. doi: 10.1016/S0074-7742(08)00018-4 [DOI] [PubMed] [Google Scholar]
- 37. Diemar SS, Sejling AS, Eiken P, Suetta C, Jørgensen NR, Andersen NB. Hyponatremia and metabolic bone disease in patients with epilepsy: a cross‐sectional study. Bone. 2019;123:67–75. doi: 10.1016/j.bone.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 38. Hisatomi Y, Kugino K. Changes in bone density and bone quality caused by single fasting for 96 hours in rats. PeerJ. 2019;9(6):e6161. doi: 10.7717/peerj.6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mackenzie CC, Wood S, Bagary M, Balabanov A, Bercovici E, Brown M‐G, et al. International recommendations for the management of adults treated with ketogenic diet therapies. Neurol Clin Pract Oct 2021;11(5):385–397. doi: 10.1212/CPJ.0000000000001007 [DOI] [PMC free article] [PubMed] [Google Scholar]


