Abstract
Background
Highly variable insulin sensitivity, susceptibility to hypoglycemia and inability to effectively communicate hypoglycemic symptoms pose significant challenges for young children with type 1 diabetes (T1D). Herein, outcomes during clinical MiniMed™ 670G system use were evaluated in children aged 2–6 years with T1D.
Methods
Participants (N = 46, aged 4.6 ± 1.4 years) at seven investigational centers used the MiniMed™ 670G system in Manual Mode during a two‐week run‐in period followed by Auto Mode during a three‐month study phase. Safety events, mean A1C, sensor glucose (SG), and percentage of time spent in (TIR, 70–180 mg/dl), below (TBR, <70 mg/dl) and above (TAR, >180 mg/dl) range were assessed for the run‐in and study phase and compared using a paired t‐test or Wilcoxon signed‐rank test.
Results
From run‐in to end of study (median 87.1% time in auto mode), mean A1C and SG changed from 8.0 ± 0.9% to 7.5 ± 0.6% (p < 0.001) and from 173 ± 24 to 161 ± 16 mg/dl (p < 0.001), respectively. Overall TIR increased from 55.7 ± 13.4% to 63.8 ± 9.4% (p < 0.001), while TBR and TAR decreased from 3.3 ± 2.5% to 3.2 ± 1.6% (p = 0.996) and 41.0 ± 14.7% to 33.0 ± 9.9% (p < 0.001), respectively. Overnight TBR remained unchanged and TAR was further improved 12:00 am–6:00 am. Throughout the study phase, there were no episodes of severe hypoglycemia or diabetic ketoacidosis (DKA) and no serious adverse device‐related events.
Conclusions
At‐home MiniMed™ 670G Auto Mode use by young children safely improved glycemic outcomes compared to two‐week open‐loop Manual Mode use. The improvements are similar to those observed in older children, adolescents and adults with T1D using the same system for the same duration of time.
Keywords: A1C, automated insulin delivery, hybrid closed loop, pediatric, time‐in‐range, type 1 diabetes
1. INTRODUCTION
The incidence and prevalence of type 1 diabetes (T1D) in children ≤6 years of age have increased, 1 , 2 and despite continued advances in T1D care, glycemic control remains a challenge in young children. 3 Known issues for this group include their low insulin requirements, high insulin sensitivity, limited on‐body real‐estate for diabetes management device placement, and inability to recognize and communicate symptoms of hypoglycemia. 4 , 5 , 6 While continuous subcutaneous insulin infusion pumps, continuous glucose monitoring (CGM), and sensor augmented pump technologies have been shown to improve glycemic outcomes for young children, 7 , 8 , 9 only a minority meet the recommended targets set by national and international diabetes organizations. 3 , 10
Feasibility and pivotal trials of automated insulin delivery systems have demonstrated their safety and efficacy across a variety of designs and patient populations. 11 , 12 , 13 , 14 , 15 , 16 , 17 The Medtronic MiniMed™ 670G system is available in several countries and pivotal trials have demonstrated increased time in range (71–180 mg/dl) from 68.8 ± 11.9% to 73.8 ± 8.4% (p < 0.001) in adults (22–75 years), 15 60.4 ± 10.9% to 67.2 ± 8.2% (p < 0.001) in adolescents (14–21 years), 15 and 56.2 ± 11.4% to 65.0 ± 7.7% (p < 0.001) in children (7–13 years). 16 Recently, real‐world use of the system by children aged 7–18 years showed improvements in A1C and TIR, as early as 1 month after use with outcomes maintained out to 12 months. 18 In the present study, results of the pivotal MiniMed™ 670G system trial in children aged 2–6 years are reported.
2. METHODS
This single‐arm trial conducted at seven sites in the United States involved a staged enrollment of, at least, 20 children (ages 2 to <7 years) with T1D for ≥3 months, an A1C <10%, prior insulin pump therapy for ≥3 months +/− CGM experience before screening, and a daily requirement of ≥8 units of insulin. The staged enrollment specifically required a data safety monitoring committee determination of study participation safety (based on the data review of 10 participants aged 5–6 years) prior to the enrollment of participants <5 years of age. Detailed study inclusion criteria are published elsewhere. 16
The study device included the MiniMed™ 670G insulin pump and Guardian™ CGM system (i.e., Guardian™ Sensor 3 glucose sensor with Guardian™ Link 3 transmitter). The parent(s)/guardian(s) of eligible participants were trained on system use and diabetes management principles (e.g., treatment of hyperglycemia and hypoglycemia), before study devices were worn or study start. Written informed consent was obtained from participant parent(s)/guardian(s) in accordance with the Code of Federal Regulations (CFR) Title 21, Part 50. The study was registered with ClinicalTrials.gov (ID: NCT02660827).
During the 2‐week run‐in period, open‐loop Manual Mode was used without low glucose management and the active insulin time, carbohydrate‐to‐insulin ratios, glucose targets, basal rates and sensitivity factors were set by investigators. During the 3‐month study phase, Manual Mode was used for 6 days before Auto Mode was enabled. Analyses were exploratory and p‐values were determined without multiplicity adjustment, as previously reported elsewhere. 16 The primary glycemic endpoint was change in A1C from baseline run‐in to the end of study. Secondary descriptive endpoints included comparisons of the following run‐in and study phase metrics: mean percentage of time at sensor glucose (SG) ranges (i.e., <50, <54, <70, 70–180, >180, >250, and >300 mg/dl), SG, SD of SG, coefficient of variation (CV) of SG, total daily insulin dose (TDD), total basal and bolus insulin, basal and bolus percentage, number of user‐initiated insulin boluses delivered, and body weight. Metrics were assessed for the overall (24‐h day) and overnight periods (i.e., 9:00 pm–12:00 am, 12:00 am–3:00 am, and 3:00 am–6:00 am), where applicable.
Safety endpoints included the number of serious adverse events, severe hypoglycemic and diabetic ketoacidosis events, serious adverse device effects (SADEs) and unanticipated adverse device effects (UADEs). Data values were analyzed using a paired t‐test or Wilcoxon signed‐rank test where indicated, and analyses were performed using SAS™ 9.4 (SAS Institute, Cary, NC).
3. RESULTS
Of the 52 children enrolled, there were five screen failures, one withdrawal during run‐in, and a total of 46 (N = 20 female) who entered the study phase. During the study phase, there was one withdrawal. At baseline, study participants were 4.6 ± 1.4 years of age with 2.9 ± 1.4 years T1D duration and an A1C of 8.0 ± 0.9% (min‐max, 6.0%–9.9%). Their weight, BMI (z‐score) and TDD were 20.6 ± 4.0 kg, 16.7 ± 1.7 kg/m2 (0.6 ± 1.0) and 0.75 ± 0.13 units/kg/day, respectively.
The median percentage of study phase CGM use and time spent in Auto Mode was 92.9% and 87.1%, respectively, and Auto Mode exits averaged 0.9/day. From run‐in to end of study, A1C and 24‐h day SG, TIR and time in hyperglycemia were improved (p < 0.001, for all), while time spent in hypoglycemia remained unchanged (Table 1). The SD of SG was reduced from 65.3 to 63.3 mg/dl (p = 0.024) and CV of SG was increased (p = 0.002), in part, due to lowered SG. Study phase‐enabled Auto Mode further increased TIR, lowered SG and reduced the time spent in hyperglycemia during the 12:00 am–6:00 am aspect of the overnight period. These early morning improvements were evident in the 24‐h SG profile (Figure 1), where run‐in versus study phase SG quartile ranges varied most, with lowering of the median study phase SG and an improved time in range from midnight to 6:00 am.
TABLE 1.
System use, glycemic outcomes, weight, and insulin delivery during the run‐in and study phase
| Overall 24‐h day (N = 46) | Overnight | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9:00 pm–12:00 am (N = 46) | 12:00 am–3:00 am (N = 46) | 3:00 am–6:00 am (N = 46) | ||||||||||
| Run‐in | Study | P | Run‐in | Study | P | Run‐in | Study | P | Run‐in | Study | P | |
| Auto Mode, % | — | 87.1% | — | — | 85.2% | — | — | 86.7% | — | — | 83.8% | — |
| A1C, % | 8.0 ± 0.9 | 7.5 ± 0.6 (N = 44) | <0.001 | — | — | — | — | — | — | — | — | — |
| CGM use, % | 87.9 ± 14.9 | 91.0 ± 5.6 | — | — | — | — | — | — | — | — | — | — |
| Percentage of time spent at sensor glucose (SG) ranges | ||||||||||||
| <50 mg/dl | 0.5 ± 0.5 | 0.5 ± 0.4 | 0.447 a | 0.4 ± 0.8 | 0.5 ± 0.7 | 0.107 a | 0.3 ± 0.5 | 0.3 ± 0.4 | 0.564 | 0.5 ± 1.3 | 0.5 ± 0.6 | 0.204 a |
| <54 mg/dl | 0.7 ± 0.8 | 0.7 ± 0.6 | 0.679 a | 0.7 ± 1.2 | 0.7 ± 1.0 | 0.786 | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.684 | 0.7 ± 1.4 | 0.7 ± 0.8 | 0.109 a |
| <70 mg/dl | 3.3 ± 2.5 | 3.2 ± 1.6 | 0.996 a | 2.3 ± 3.5 | 2.4 ± 2.6 | 0.297 a | 2.2 ± 2.6 | 1.7 ± 1.4 | 0.163 | 3.3 ± 3.2 | 3.6 ± 2.5 | 0.627 |
| 70–180 mg/dl | 55.7 ± 13.4 | 63.8 ± 9.4 | <0.001 | 48.6 ± 17.3 | 51.6 ± 12.0 | 0.174 | 45.6 ± 22.1 | 61.3 ± 14.0 | <0.001 | 60.6 ± 17.4 | 80.1 ± 10.3 | <0.001 |
| >180 mg/dl | 41.0 ± 14.7 | 33.0 ± 9.9 | <0.001 | 49.1 ± 19.0 | 46.0 ± 13.0 | 0.183 | 52.2 ± 23.0 | 37.0 ± 14.1 | <0.001 | 36.0 ± 17.9 | 16.3 ± 10.1 | <0.001 |
| >250 mg/dl | 14.6 ± 9.4 | 10.7 ± 5.9 | <0.001 a | 19.4 ± 14.7 | 15.9 ± 9.2 | 0.263 a | 20.3 ± 18.4 | 11.4 ± 8.3 | <0.001 a | 9.2 ± 9.8 | 4.2 ± 4.1 | <0.001 a |
| >300 mg/dl | 5.2 ± 4.9 | 3.7 ± 2.9 | 0.011 a | 7.5 ± 10.2 | 5.6 ± 4.9 | 0.951 a | 7.4 ± 10.4 | 3.7 ± 3.6 | 0.015 a | 2.0 ± 3.4 | 1.1 ± 1.4 | 0.021 a |
| SG, mg/dl | 173 ± 24 | 161 ± 16 | <0.001 | 186 ± 34 | 181 ± 22 | 0.166 | 189 ± 40 | 169 ± 21 | <0.001 | 163 ± 26 | 136 ± 16 | <0.001 |
| SD of SG, mg/dl | 65.3 ± 11.7 | 63.3 ± 9.9 | 0.024 | 64.2 ± 13.9 | 63.9 ± 10.6 | 0.848 | 59.5 ± 12.5 | 58.7 ± 11.8 | 0.591 | 54.5 ± 13.7 | 47.6 ± 12.6 | <0.001 |
| CV of SG, % | 37.7 ± 4.1 | 39.1 ± 3.3 | 0.002 | 34.6 ± 5.7 | 35.4 ± 4.9 | 0.202 | 31.9 ± 5.9 | 34.6 ± 4.2 | 0.005 | 33.3 ± 6.2 | 34.6 ± 6.1 | 0.124 |
| Weight, kg | 20.6 ± 4.0 | 21.3 ± 4.0 | <0.001 | — | — | — | — | — | — | — | — | — |
| TDD, units/kg/day | 0.75 ± 0.13 | 0.76 ± 0.15 | 0.759 a | 0.07 ± 0.02 | 0.08 ± 0.02 | <0.001 | 0.04 ± 0.02 | 0.05 ± 0.02 | <0.001 a | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.174 |
| Total basal, units/kg/day | 0.30 ± 0.09 | 0.31 ± 0.08 | 0.445 | 0.04 ± 0.01 | 0.05 ± 0.01 | <0.001 | 0.03 ± 0.01 | 0.04 ± 0.01 | <0.001 a | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.075 |
| Total bolus, units/kg/day | 0.45 ± 0.11 | 0.45 ± 0.11 | 0.951 | 0.03 ± 0.02 (N = 44) | 0.03 ± 0.02 | 0.684 | 0.01 ± 0.01 (N = 44) | 0.01 ± 0.01 | 0.066 a | 0.01 ± 0.01 (N = 44) | 0.01 ± 0.00 | 0.002 a |
| Basal percentage, % | 41.6 ± 10.1 | 39.8 ± 10.7 | 0.587 a | 73.1 ± 13.8 | 74.5 ± 10.8 | 0.418 | 81.0 ± 12.0 | 88.5 ± 5.3 | <0.001 a | 88.5 ± 8.4 | 93.4 ± 4.0 | <0.001 a |
| Bolus percentage, % | 58.4 ± 10.1 | 60.2 ± 10.7 | 0.587 a | 26.9 ± 13.8 | 25.5 ± 10.8 | 0.418 | 19.0 ± 12.0 | 11.5 ± 5.3 | <0.001 a | 11.5 ± 8.4 | 6.6 ± 4.0 | <0.001 a |
| Insulin‐to‐carb ratio b | 19.6 ± 5.6 | 18.5 ± 5.1 | <0.001 a | 22.7 ± 8.6 (N = 29) | 22.1 ± 8.0 | 0.002 a | 23.5 ± 6.1 (N = 11) | 28.0 ± 12.8 (N = 28) | 1.000 a | 21.5 ± 7.8 (N = 15) | 21.3 ± 7.9 (N = 31) | 0.156 a |
| Number of boluses | 9.0 ± 3.1 | 8.6 ± 2.0 | 0.236 a | 0.8 ± 0.5 | 0.8 ± 0.4 | 0.834 | 0.5 ± 0.3 | 0.3 ± 0.2 | 0.008 a | 0.3 ± 0.3 | 0.2 ± 0.1 | <0.001 a |
Note: The baseline run‐in period was 2 weeks and the study phase was 3 months. Values are shown as mean ± SD, excluding time in Auto Mode which is shown as median.
Abbreviations: CV, coefficient of variation; TDD, total daily insulin dose.
Wilcoxon signed rank test.
Based on carbohydrate announced.
FIGURE 1.
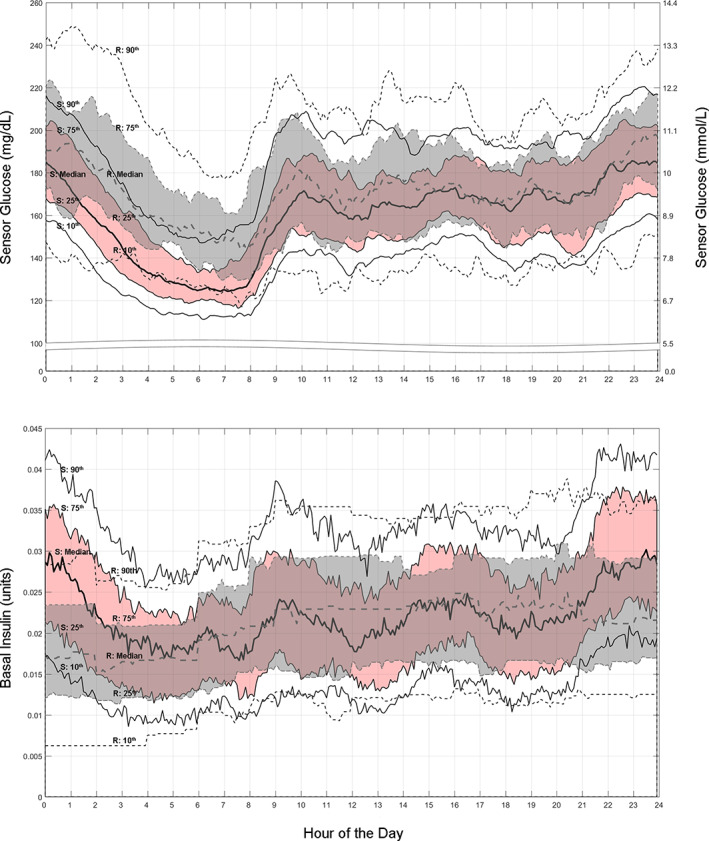
The median and 10th through 90th percentile ranges for sensor glucose (top) and basal insulin delivered (bottom) are shown for the 24‐h day of the baseline run‐in (gray band and dashed lines) and the Auto Mode‐enabled study phase (pink band and solid lines), for the intention to treat group (N = 46). Visually, and most notably during the overnight period (i.e., 9:00 pm–6:00 am), study phase automated basal insulin delivery is increased relative to preset basal insulin delivery during run‐in open loop. The more variable and increased insulin delivery with Auto Mode appear to partly underlie all percentile ranges of SG remaining within target range between approximately 5:00 am–8:00 am (i.e., waking hours). “R” is run‐in and “S” is study phase
The 24‐h day TDD and number of boluses administered did not change during Auto Mode use (Table 1). However, the daily boluses reduced from 0.5 ± 0.3/day to 0.3 ± 0.2/day (p = 0.008) and from 0.3 ± 0.3/day to 0.2 ± 0.1/day (p < 0.001), for the 12:00 am–3:00 am and 3:00 am–6:00 am periods, respectively. While 24‐h day basal insulin delivery during study phase also remained unchanged (Table 1), it increased during the 9:00 pm‐3:00 am overnight period and appeared more comparable to that of run‐in by 5:00 am (Figure 1).
3.1. Safety
There was no serious adverse event during run‐in or study phase. There was no SADE, UADE, or episode of severe hypoglycemia or DKA, during Auto Mode use. There were 10 episodes of device‐related severe hyperglycemia (blood glucose >300 mg/dl with ketones >0.6 mmol/L or symptoms of nausea, vomiting or abdominal pain) during run‐in (0.824/100 user‐days) and 39 during study phase (0.841/100 user‐days).
4. DISCUSSION
This study demonstrated that 3‐month at‐home MiniMed™ 670G system use was safe with no DKA or severe hypoglycemia, in a challenging and vulnerable population of very young children with T1D. These findings build upon the safety profile demonstrated in the previous pivotal trials conducted in adults, adolescents, and children. 15 , 16 Median CGM system use was high (92.9%, 22.3 h/day) and similar to that observed in the 7–13 years cohort (90.9%, 21.8 h/day), 16 while time spent in Auto Mode was higher (87.1% vs. 80.6%). Auto Mode use improved glycemia when compared to run‐in Manual Mode use, as demonstrated by TIR that increased from 55.7 ± 13.4% to 63.8 ± 9.4% and an associated decrease in A1C from 8.0 ± 0.9% to 7.5 ± 0.6%, without change in hypoglycemia. Interestingly, a small real‐world analysis of long‐term (6.3 ± 2.9 months) MiniMed™ 670G system Auto Mode use by children (N = 16, aged 2–6 years) also showed A1C improvement from 7.9 ± 1.1% to 7.4 ± 0.8% (p = 0.01) and TIR improvement from 42.8 ± 15.3% to 56.2 ± 12.8% (p < 0.001), although TBR increased from 1.3 ± 1.3% to 2.4 ± 2.1% (p = 0.04). 19
Auto Mode use revealed an increased and more variable insulin delivery during the overnight period, which corresponded with reductions in SG from midnight to 6:00 am. This difference in the extent of insulin delivery, contrasted by less glucose variability (GV), was most evident when viewed alongside Manual Mode use during run‐in (Figure 1). A similar situation in overnight insulin delivery variability and commensurate SG reduction was observed in the 7–13 years cohort using the system, where insulin delivery ranged from “suspended” for 1.3 h up to the maximum allowed for an average of 3.6 h. 20 This algorithm‐driven insulin delivery that addressed GV, an increasingly important clinical marker associated with complications risk, 21 appeared to lower diabetes burden by reducing user‐initiated boluses. It may, more importantly, help reduce complications risk in youth who must manage diabetes for years to come.
Pilot safety and effectiveness studies in young children have been performed on the t:slim X2™ with Control‐IQ (Tandem, Diabetes Care, San Diego, CA)17 and Omnipod™ Horizon (Insulet Corporation, Acton, MA) systems. 22 The former studied 12 children (4.7 ± 1.0 years with baseline A1C 7.3 ± 0.8%) during 48 h of supervised hotel use followed by 72 h of at‐home use and reported an improvement in TIR from 61.7% to 76.5% (hotel) and 68.0% (at home). Hypoglycemia increased from 3.7% during run‐in to 5.0% during the hotel and then reduced to 1.5% at home. The Omnipod™ Horizon study looked at 14 children (4.2 ± 0.9 years with baseline A1C 7.4 ± 1.0%) during 48–72 h of supervised hotel use and reported TIR improvement from 55.2% to 72.6%, and hypoglycemia that reduced from 5.1% to 2.9%.
The current study limitations include its open‐label and non‐randomized design and shorter‐duration run‐in. Direct comparisons between the present trial and the brief automated insulin delivery pilot studies are difficult due to different study durations and levels of supervision, and the better baseline glycemic control of participants in the pilot studies. However, noteworthy trends within the challenging young study populations include improvement in daily hypoglycemic exposure to <4% and clinically significant increases in TIR. Further analyses are warranted when more automated insulin delivery systems become available.
5. CONCLUSION
MiniMed™ 670G system Auto Mode use for 3 months by children 2–6 years of age versus open‐loop therapy for 2 weeks was safe and helped to improve glycemic control, similar to use observed in older cohorts with T1D.
CONFLICT OF INTEREST
The MiniMed™ 670G system is intended for the management of type 1 diabetes in persons 7 years of age and older.
AUTHOR CONTRIBUTIONS
The principal investigator authors (Gregory P. Forlenza, Laya Ekhlaspour, Linda A. DiMeglio, Larry A. Fox, Henry Rodriguez, Dorothy I. Shulman, Kevin B. Kaiserman, David R. Liljenquist, and Bruce A. Buckingham) received research support and compensation from Medtronic to manage the study conducted at their investigational centers, contributed to the analysis and interpretation of results, reviewed and approved the final manuscript, and agreed to be accountable for the manuscript and its content. John Shin and Scott W. Lee, employees of Medtronic, developed the study design, managed data analyses, and/or reviewed and edited the manuscript. Gregory P. Forlenza and Bruce A. Buckingham the guarantors of the work, had full access to all study data and take responsibility for the integrity of all data and the accuracy of the data analysis.
ETHICS STATEMENT
Study approval was obtained from either a central or local institutional review board.
ACKNOWLEDGMENTS
The authors thank the study participants and their families, and investigational center staff, for their time and dedication throughout the study. The authors acknowledge the contributions made by Medtronic employees involved in data analyses (Xiaoxiao Chen, PhD; Margaret Liu, BSc; Chenxiao Ling, PhD; and Vivian Chen, BSc), assistance with study management and monitoring (Thomas Troub and Cathy Rogert, RN), and manuscript development (Toni L. Cordero, PhD). This study (NCT02660827) was funded by Medtronic (Northridge, CA).
Forlenza GP, Ekhlaspour L, DiMeglio LA, et al. Glycemic outcomes of children 2–6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial. Pediatr Diabetes. 2022;23(3):324‐329. doi: 10.1111/pedi.13312
Funding information Medtronic, Grant/Award Number: NCT02660827
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available within the present article and supplemental material, if applicable.
REFERENCES
- 1. Writing Group for the Search for Diabetes in Youth Study Group , Dabelea D, Bell RA, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):12716‐12724. [DOI] [PubMed] [Google Scholar]
- 2. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for diabetes in youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336‐3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016‐2018. Diabetes Technol Ther. 2019;21(2):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dovc K, Boughton C, Tauschmann M, et al. Young children have higher variability of insulin requirements: observations during hybrid closed‐loop insulin delivery. Diabetes Care. 2019;42(7):1344‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep. 2014;14(9):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diabetes Research in Children Network Study Group , Tsalikian E, Tamborlane W, et al. Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well‐controlled type 1 diabetes. Diabetes Care. 2009;32(11):1954‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szypowska A, Schwandt A, Svensson J, et al. Insulin pump therapy in children with type 1 diabetes: analysis of data from the SWEET registry. Pediatr Diabetes. 2016;17(23):38‐45. [DOI] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor‐augmented insulin‐pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311‐320. [DOI] [PubMed] [Google Scholar]
- 9. Slover RH, Welsh JB, Criego A, et al. Effectiveness of sensor‐augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 2012;13(1):6‐11. [DOI] [PubMed] [Google Scholar]
- 10. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(27):105‐114. [DOI] [PubMed] [Google Scholar]
- 11. Tauschmann M, Allen JM, Nagl K, et al. Home use of day‐and‐night hybrid closed‐loop insulin delivery in very young children: a multicenter, 3‐week, randomized trial. Diabetes Care. 2019;42(4):594‐600. [DOI] [PubMed] [Google Scholar]
- 12. Brown SA, Kovatchev BP, Raghinaru D, et al. Six‐month randomized, multicenter trial of closed‐loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buckingham BA, Forlenza GP, Pinsker JE, et al. Safety and feasibility of the OmniPod hybrid closed‐loop system in adult, adolescent, and pediatric patients with type 1 diabetes using a personalized model predictive control algorithm. Diabetes Technol Ther. 2018;20(4):257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed‐loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230‐2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in‐home use of a hybrid closed‐loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forlenza GP, Pinhas‐Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7‐13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekhlaspour L, Schoelwer MJ, Forlenza GP, et al. Safety and performance of the Tandem t:slim X2 with Control‐IQ automated insulin delivery system in toddlers and preschoolers. Diabetes Technol Ther. 2021;23(5):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrovski G, Al Khalaf F, Campbell J, et al. One‐year experience of hybrid closed‐loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: drivers to successful outcomes. Acta Diabetol. 2021;58(2):207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salehi P, Roberts AJ, Kim GJ. Efficacy and safety of real‐life usage of MiniMed 670G AutoMode in children with type 1 diabetes less than 7 years old. Diabetes Technol Ther. 2019;21(8):448‐451. [DOI] [PubMed] [Google Scholar]
- 20. Roy A, Grosman B, Parikh N, et al. Overnight insulin delivery and glucose in children using the MiniMed™ 670G system. Diabetes. 2018;67(Supplement 1):A54. [Google Scholar]
- 21. Wilmot EG, Choudhary P, Leelarathna L, Baxter M. Glycaemic variability: the under‐recognized therapeutic target in type 1 diabetes care. Diabetes Obes Metab. 2019;21(12):2599‐2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buckingham B, Forlenza G, Jennifer S, et al. Safety and performance of the Omnipod hybrid closed‐loop system in young children aged 2‐6years with type 1 diabetes. Diabetes. 2019;68(Supplement 1). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available within the present article and supplemental material, if applicable.


