Abstract
Objective
Overweight and obesity have been identified as risk factors for severe COVID‐19; however, prospective cohort studies investigating the association between overweight early in life and severity of COVID‐19 are lacking.
Methods
This study included 1,551,670 Swedish men, born between 1950 and 1987, with BMI registered at age 18 years. They were followed until January 9, 2021. COVID‐19 cases and comorbidities were identified through the National Patient, Intensive Care, and Cause of Death registries. Outcomes included the following: 1) hospitalization; 2) intensive care unit admission; and 3) death.
Results
The study found 4,315 cases (mean age = 56.4 years [SD 8.8]) of patients hospitalized because of COVID‐19, of which 729 were admitted to an intensive care unit, and altogether there were 224 deaths. The risk for hospital admission increased with higher values of BMI at age 18 years, despite adjustment for comorbidities, from an odds ratio (OR) of 1.19 (95% CI: 1.08‐1.31) at BMI = 22.5 to 25 to an OR of 1.68 (95% CI: 1.39‐2.02) at BMI ≥ 30, compared with BMI = 18.5 to 20. ORs for intensive care unit admission were 1.44 (95% CI: 1.13‐1.84) at BMI = 22.5 to 25 and 2.61 (95% CI: 1.73‐3.93) at BMI ≥ 30.
Conclusions
Higher BMI in early adulthood was associated with severe COVID‐19 many years later, with a risk increase starting already at BMI ≥ 22.5. This underlines the necessity of preventive actions against overweight in youth to offer protection against coming viral pandemics.
Study Importance.
What is already known?
-
►
Overweight and obesity have been identified as risk factors for severe COVID‐19; however, prospective cohort studies investigating the association between overweight early in life and severity of COVID‐19 are lacking.
What does this study add?
-
►
Higher BMI in early adulthood was associated with severe COVID‐19 many years later, with a risk increase starting already at BMI ≥ 22.5 kg/m2.
-
►
The risk for need of intensive care was more than twice as high with BMI ≥ 30 at a young age compared with BMI < 20.
How might these results change the direction of research or the focus of clinical practice?
-
►
Our results underline the necessity of preventive actions against overweight in youth to offer protection against coming viral pandemics.
INTRODUCTION
The COVID‐19 pandemic, caused by SARS‐CoV‐2, has caused millions of deaths around the world (1). Most infected people develop mild symptoms, whereas some require hospitalization or intensive care (2), and case fatality is high among the elderly (3). Studies of patients with COVID‐19 have reported that common characteristics among the severely ill include high age, male sex, hypertension, diabetes, cardiovascular disease, and other comorbidities, as well as high BMI (2, 4, 5, 6, 7). In a recent prospective cohort study of 6.9 million people in England, there was a linear association with intensive care unit admission owing to COVID‐19 across the whole BMI range, with a hazard ratio of 1.10 (95% CI: 1.09‐1.10) per unit increase in BMI (8).
The role of obesity has been recognized in other viral infections. During the H1N1 influenza pandemic in 2009, obesity was considered as a risk factor for severe disease and death (9). Moreover, obesity was found to increase the duration of viral shedding, such that patients with obesity and H1N1 influenza shed virus 42% longer than leaner peers (10). Longer viral shedding, hospital stay, and duration of oxygen treatment also were found in patients with COVID‐19 and obesity (11). Possible mechanisms by which overweight and obesity could affect risk, severity, and transmission of viral infections include an altered immune cell function, impaired host defense, mechanical ventilation challenges, and increased oxygen requirements (12, 13, 14, 15, 16). Moreover, the low‐grade chronic inflammation seen in people with obesity is associated with development of atherosclerosis, type 2 diabetes, and hypertension, all of which have a negative impact on the outcome in patients with COVID‐19 (17, 18). Taken together, elevated BMI appears to be a risk factor for severe COVID‐19, a matter of major concern because the number of individuals with overweight and obesity has increased dramatically worldwide during the past 30 years (19). A few prospective cohort studies have investigated the association between recent BMI status and severity of COVID‐19 (8, 20, 21); however, larger studies on BMI early in life are lacking. High adolescent BMI is associated with increased infectious disease mortality (22), and a long duration of overweight and obesity was found to predict cardiovascular disease risk (23). Even so, the relationship between early life BMI, intermediate cardiovascular risk factors, and risk of severe COVID‐19 in a life‐course perspective has not yet been established, to our knowledge. To address this, we followed more than 1.5 million Swedish men born after 1950 who were alive in the beginning of the COVID‐19 pandemic. Because more than 70% of patients with COVID‐19 in the intensive care units in Sweden are men (24), we were able to include a major part of the severely ill. The level of BMI in early adulthood and intermediate cardiovascular risk factors were investigated in relation to outcome in COVID‐19 later in life.
METHODS
Study population
The cohort comprised all young individuals who enlisted for military service between 1968 and 2005 in Sweden (n = 1,949,891), derived from the Swedish Military Service Conscription Register. The enlistment was mandatory for 18‐year‐old men during the study period, except for those in prison or with severe medical conditions incompatible with military service (altogether 2%‐3%/y). We excluded women (n = 10,631) and men with missing information regarding test center (n = 118), missing or invalid BMI (n = 206,951), and/or age <16 or >25 years (n = 3,791). For the purpose of the present study, only men alive on January 1, 2020, who had remained Swedish citizens were included. The final study sample encompassed 1,551,670 young men (Supporting Information Figure S1). During the assessment at conscription, standardized physical examinations included measurements of weight, height, and blood pressure. For a subgroup analysis, data from the Health Profile Institute AB, a private company offering occupational health services, were linked to the Military Service Conscription Register, which enabled a repeated BMI measurement in middle age for 151,693 conscripts.
Outcomes
The publicly funded Swedish national health system provides specialist and hospital care at a low cost to all citizens. Data from visits or discharges are recorded in the National Patient Register, including diagnostic codes according to the International Classification of Diseases (ICD). Patients with COVID‐19 who required hospital care were identified through the National Patient Register and the Swedish Intensive Care Registry from January 1, 2020, to January 9, 2021. Deaths due to COVID‐19 during the same period were found in the Cause of Death Register. The diagnostic codes used were U07.1 (COVID‐19, virus identified) and U07.2 (COVID‐19, virus not identified) in any diagnostic position. For all cases with COVID‐19 as a secondary diagnosis, the main diagnoses were assessed. Three clinically experienced physicians collectively decided which main diagnoses, with COVID‐19 as a secondary diagnosis, were to be included (Supporting Information Table S1). This decision was made to increase the likelihood that COVID‐19 was the main cause of admission. All cases were considered as severe COVID‐19 because they required hospital care. Patients with COVID‐19 were categorized into three groups for subsequent analyses: 1) hospitalizations; 2) intensive care unit admissions; and 3) deaths due to COVID‐19. All cases that were included as deaths had COVID‐19 as the main or secondary diagnosis in the National Patient Register. We also performed a subgroup analysis of patients with COVID‐19 with high‐flow nasal oxygen therapy, in which the ICD‐10 procedure code DG028 (heated humidified high‐flow nasal therapy) was used. Preexisting comorbidities before January 1, 2020, were identified in the National Patient Register (Supporting Information Table S2), except for diabetes mellitus type 1 and 2. Until 2016, these diagnoses were collected from the National Diabetes Register and, after this, from the National Patient Register.
Statistical analyses
Statistical calculations were performed with R version 4.0.3 software (http://www.R‐project.org). The follow‐up period started at the date of conscription (baseline), and patients were followed until registered hospitalization, intensive care unit admission, death due to COVID‐19, or until end of follow‐up (January 9, 2021). BMI, calculated as weight in kilograms divided by height in meters squared, was divided into six categories (BMI = 15‐18.5, 18.5‐20, 20‐22.5, 22.5‐25, 25‐30, and ≥30), with BMI = 18.5 to 20 as the reference. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals regarding the influence of BMI on severe COVID‐19. Test center and age in 2020 were considered as confounders and were adjusted for in model 1. In model 2, further adjustments were made for preexisting cardiovascular comorbidities registered before January 1, 2020. The models were not adjusted for year of conscription because of the strong collinearity with age in 2020. Owing to the low number of events, Firth’s biased reduction method was used to model COVID‐19 deaths (25, 26). Statistical significance was set at 0.05. For the subgroup of conscripts with repeated measurements of BMI, we performed a correlation and linear regression analysis between BMI in early adulthood and BMI in midlife.
RESULTS
Study population
The final study sample after exclusions contained 1,551,670 men born between 1950 and 1987, with a mean BMI at conscription of 21.9 (3.0) (Table 1). During 2020, a total of 4,315 of these men contracted severe COVID‐19 requiring hospital care, of which, 729 needed intensive care (Supporting Information Figure S1). Supporting Information Figure S2 shows the proportions of BMI groups among hospitalized patients with COVID‐19, divided by week of the pandemic. A total of 849 patients received high‐flow nasal oxygen therapy (445 of them [52%] were admitted to an intensive care unit). The number of reported deaths due to COVID‐19 reached 224, of which, 48 occurred outside hospital. Before the start of 2020, a total of 238,464 (15%) of the total study population had been diagnosed with one or more comorbidities. The proportion of men with comorbidities increased with increasing adolescent BMI (14% at BMI = 20‐22.5, increasing to 33% at BMI ≥ 30). Hypertension (n = 113,072), diabetes mellitus type 2 (n = 79,603), and cancer (n = 43,187) were the most frequent comorbidities (Table 1).
TABLE 1.
Baseline characteristics, age in 2020, comorbidities until 2020, and outcomes by BMI category in 1,551,670 young Swedish men born between 1950 and 1987
| All, n = 1,551,670 (100%) | BMI 15‐18.5, n = 125,003 (8%) | BMI 18.5‐20, n = 279,757 (18%) | BMI 20‐22.5, n = 626,698 (40%) | BMI 22.5‐25, n = 331,562 (21%) | BMI 25‐30, n = 153,812 (10%) | BMI ≥ 30, n = 34,838 (2%) | |
|---|---|---|---|---|---|---|---|
| Age (y), mean (SD) | 18.3 (0.6) | 18.3 (0.6) | 18.3 (0.6) | 18.3 (0.6) | 18.3 (0.7) | 18.3 (0.7) | 18.3 (0.7) |
| Height (m), mean (SD) | 179.3 (6.6) | 179.5 (6.8) | 179.4 (6.6) | 179.3 (6.5) | 179.1 (6.5) | 179.0 (6.6) | 179.3 (6.8) |
| Weight (kg), mean (SD) | 70.5 (11.0) | 57.1 (4.9) | 62.3 (4.8) | 68.3 (5.4) | 75.6 (6.0) | 85.8 (7.7) | 106.4 (12.7) |
| Systolic blood pressure (mm Hg), mean (SD) | 128.5 (11.1) | 125.5 (11.0) | 126.7 (10.9) | 128.2 (10.9) | 129.9 (10.9) | 131.6 (11.0) | 134.2 (11.3) |
| Diastolic blood pressure (mm Hg), mean (SD) | 67.5 (9.9) | 67.3 (9.7) | 67.2 (9.7) | 67.3 (9.8) | 67.6 (9.9) | 68.5 (10.1) | 70.6 (10.7) |
| Age in 2020 (y), mean (SD) | 52.1 (10.0) | 54.4 (10.2) | 53.8 (9.8) | 52.5 (9.8) | 50.9 (9.8) | 49.2 (9.8) | 46.5 (9.4) |
| Comorbidities a , n (%) | |||||||
| Any comorbidity | 238,464 (15.4) | 18,964 (15.2) | 39,565 (14.1) | 85,406 (13.6) | 50,527 (15.2) | 32,483 (21.1) | 11,519 (33.1) |
| Coronary heart disease | 42,661 (2.8) | 3666 (2.9) | 7913 (2.8) | 16,343 (2.6) | 9096 (2.7) | 4633 (3.0) | 1010 (2.9) |
| Heart failure | 14,233 (0.9) | 1156 (0.9) | 2375 (0.9) | 4972 (0.8) | 3046 (0.9) | 1996 (1.3) | 688 (2.0) |
| Hypertension | 113,072 (7.3) | 8697 (6.7) | 18,467 (6.6) | 40,620 (6.5) | 24,727 (7.5) | 15,797 (10.3) | 4764 (13.7) |
| Diabetes mellitus type 1 b | 9,493 (0.6) | 782 (0.6) | 1,664 (0.6) | 3,723 (0.6) | 2,068 (0.6) | 1,038 (0.7) | 218 (0.6) |
| Diabetes mellitus type 2 b | 79,603 (5.1) | 5,474 (4.4) | 11,005 (3.9) | 21,926 (3.5) | 15,907 (4.8) | 13,320 (8.7) | 4,784 (13.7) |
| Cancer | 43,187 (2.8) | 4,085 (3.3) | 8,670 (3.1) | 17,805 (2.8) | 8,392 (2.5) | 3,549 (2.3) | 686 (2.0) |
| Chronic obstructive pulmonary disease | 16,776 (1.1) | 1,943 (1.6) | 3,394 (1.2) | 6,078 (1.0) | 3,189 (1.0) | 1,725 (1.1) | 447 (1.3) |
| Obesity diagnosis | 21,053 (1.4) | 227 (0.2) | 809 (0.3) | 3,321 (0.5) | 4,346 (1.3) | 6,682 (4.3) | 5,668 (16.3) |
| Outcomes, n (%) | |||||||
| COVID‐19 admitted to hospital a | 4315 (0.3) | 349 (0.3) | 712 (0.3) | 1615 (0.3) | 932 (0.3) | 565 (0.4) | 142 (0.4) |
| COVID‐19 with high‐flow nasal oxygen therapy in hospital a | 849 (0.05) | 71 (0.06) | 137 (0.05) | 294 (0.05) | 198 (0.06) | 118 (0.08) | 31 (0.09) |
| COVID‐19 admitted to an intensive care unit c | 729 (0.05) | 67 (0.05) | 108 (0.04) | 249 (0.04) | 164 (0.05) | 110 (0.07) | 31 (0.09) |
| Death due to COVID‐19 d | 224 (0.01) | 30 (0.02) | 42 (0.02) | 66 (0.01) | 40 (0.01) | 40 (0.03) | 6 (0.02) |
Diagnoses received from the National Patient Register.
Diagnoses received from the National Diabetes Register until 2016 and from the National Patient Register from 2016.
Diagnoses received from the Intensive Care Register.
Diagnoses received from the Cause of Death Register.
When comparing the groups with and without COVID‐19 in 2020, hospitalized men with COVID‐19 had a significantly higher mean BMI at age 18 years compared with men without severe disease (22.3 [3.4] vs. 21.9 [3.0], p < 0.001; Table 2). Moreover, men hospitalized with COVID‐19 had a higher mean age than men who were not hospitalized with COVID‐19 (56.4 [8.8] years vs. 52.1 [10.0] years, p < 0.001). Preexisting comorbidities were more common in the hospitalized group, such that one in five had hypertension and 16% had diabetes mellitus type 2 before developing COVID‐19 (Table 2).
TABLE 2.
Characteristics of individuals with and without COVID‐19 requiring hospitalization in 2020 (n = 1,551,670)
| No COVID‐19 diagnosis, n = 1,547,355 (99.7%) | COVID‐19 diagnosis a , n = 4,315 (0.3%) | p value | |
|---|---|---|---|
| Age at conscription (y), mean (SD) | 18.3 (0.6) | 18.4 (0.9) | <0.001 |
| Height at conscription (m), mean (SD) | 179.3 (6.6) | 179.4 (6.9) | 0.295 |
| Weight at conscription (kg), mean (SD) | 70.5 (11.0) | 71.7 (12.2) | <0.001 |
| BMI at conscription (kg/m2), mean (SD) | 21.9 (3.0) | 22.3 (3.4) | <0.001 |
| Systolic blood pressure at conscription (mm Hg), mean (SD) | 128.5 (11.1) | 127.9 (10.8) | <0.001 |
| Diastolic blood pressure at conscription (mm Hg), mean (SD) | 67.5 (9.9) | 68.8 (9.6) | <0.001 |
| Age in 2020 (y), mean (SD) | 52.1 (10.0) | 56.4 (8.8) | <0.001 |
| Comorbidities a , n (%) | |||
| Any comorbidity | 23,6891 (15.3) | 1,573 (36.5) | <0.001 |
| Coronary heart disease | 42,397 (2.7) | 264 (6.1) | <0.001 |
| Heart failure | 14,066 (0.9) | 167 (3.9) | <0.001 |
| Hypertension | 112,190 (7.3) | 882 (20.4) | <0.001 |
| Diabetes mellitus type 1 b | 9,452 (0.6) | 41 (1.0) | <0.001 |
| Diabetes mellitus type 2 b | 78,913 (5.1) | 690 (16.0) | <0.001 |
| Cancer | 42,935 (2.8) | 252 (5.8) | <0.001 |
| Chronic obstructive pulmonary disease | 16,599 (1.1) | 177 (4.1) | <0.001 |
| Obesity diagnosis | 20,866 (1.4) | 187 (4.3) | <0.001 |
Diagnoses received from the National Patient Register.
Diagnoses received from the National Diabetes Register until 2016 and from the National Patient Register from 2016.
Higher BMI in early adulthood was associated with severe COVID‐19
When studying the relationship between BMI and severe COVID‐19, we found that higher BMI at a young age was associated with a significantly increased risk for hospitalization due to COVID‐19. Compared with the reference group of BMI = 18.5 to 20, the risk for in‐hospital admission increased gradually with higher values of BMI, from an adjusted OR of 1.19 (95% CI: 1.08‐1.31) at BMI = 22.5 to 25 to an OR of 1.68 (95% CI: 1.39‐2.02) at BMI ≥ 30. The risk elevation was even higher for intensive care unit admission, with adjusted ORs of 1.44 (95% CI: 1.13‐1.84) at BMI = 22.5 to 25 and 2.61 (95% CI: 1.73‐3.93) at BMI ≥ 30 (Figure 1A,B). A significantly increased risk for death due to COVID‐19 was seen for BMI = 25 to 30 (OR = 2.10 [95% CI: 1.34‐3.27]) compared with BMI = 18.5 to 20 (Supporting Information Table S3). We also performed two sensitivity analyses. First, we included individuals without any preexisting comorbidity; second, we included individuals younger than age 55 years. A similar risk increase as that found in the main analysis was found with elevated BMI for hospitalization due to COVID‐19 (Supporting Information Table S3).
FIGURE 1.
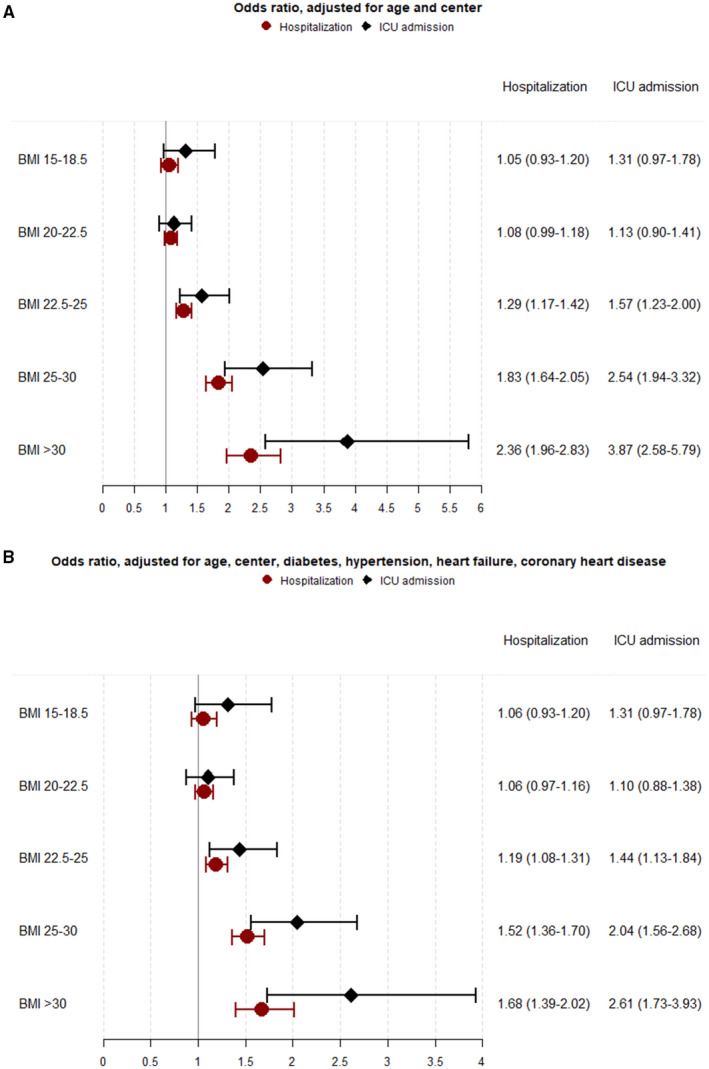
Odds ratios and 95% CIs for COVID‐19 hospitalization and intensive care unit admission divided by BMI groups. (A) Adjusted for age in 2020 and test center. (B) Further adjusted for preexisting comorbidities (before January 1, 2020), including diabetes mellitus type 1 and 2, hypertension, heart failure, and coronary heart disease
In further analyses, we found that age in 2020 was associated with an increased risk of developing COVID‐19 requiring intensive care (OR = 1.06 [95% CI: 1.05‐1.07]; Supporting Information Table S3). Moreover, preexisting hypertension and diabetes mellitus type 2 doubled the risk of COVID‐19 leading to intensive care unit admission compared with men without these comorbidities (OR = 1.93 [95% CI: 1.58‐2.35] and OR = 2.15 [95% CI: 1.75‐2.64], respectively). However, we found no association with COVID‐19 requiring intensive care for diabetes mellitus type 1, coronary heart disease, or heart failure (Supporting Information Table S3).
Comparing data of the first and second wave
To identify a potential difference between the first and second wave of the COVID‐19 pandemic in 2020 in Sweden, the year was divided in two parts, with the second wave starting on September 1. We identified 2,564 cases (59%) during the first wave and 1,751 cases (41%) during the second wave. BMI at a young age did not differ significantly between the waves (22.3 [3.4] vs. 22.0 [3.1]), nor did the occurrence of preexisting comorbidities (Supporting Information Table S4). However, the number of patients with COVID‐19 admitted to an intensive care unit was significantly higher during the first wave (564 cases [22%] vs. 165 cases [9%], p < 0.001), as were the number of patients needing high‐flow nasal oxygen therapy (559 cases [22%] vs. 290 cases [17%], p < 0.001) and the number of reported deaths due to COVID‐19 (162 cases [6%] vs. 14 cases [0.8%], p < 0.001).
Longitudinal measurements of BMI
Information on BMI in middle age for 151,603 conscripts (mean age = 45.2 [10.1] years), derived from the database at the Health Profile Institute AB, showed that mean BMI has increased from 21.8 (2.9) at age 18 years to 26.1 (5.6) in middle age (Supporting Information Table S5). Supporting Information Figure S3 shows the correlation between BMI at conscription and BMI at measurement in middle age. A total of 377 individuals (0.2%) in this subcohort developed severe COVID‐19 leading to hospital admission, of which, 58 were admitted to an intensive care unit. Mean BMI in middle age was significantly higher among men with COVID‐19 who required hospital care compared with men without hospitalization (27.9 [6.1] vs. 26.1 [5.6], p < 0.0001). The mean time from BMI measurement to development of severe COVID‐19 was 8.8 (5.3) years. Comorbidities preexisted in 28% of cases with severe COVID‐19 compared with 13% among men who did not develop COVID‐19 requiring hospitalization.
DISCUSSION
In this large prospective cohort study, we investigated the association between BMI in early adulthood and the risk of developing severe COVID‐19 during the pandemic in 2020. Including more than 1.5 million Swedish men in our analyses, we found that overweight and obesity early in life were associated with a higher risk of COVID‐19 requiring hospital care when they were middle‐aged and older. There was a gradual increase in risk with higher values of BMI, starting already at BMI of 22.5 to 25, compared with the reference group with BMI 18.5 to 20. Moreover, men with obesity in youth (BMI ≥ 30) more than doubled their risk of being admitted to an intensive care unit, and also an increased risk for death due to COVID‐19 in 2020 was seen for adolescent men with overweight. The associations persisted after adjusting for preexisting cardiovascular comorbidities. These findings support a strong and independent relationship between body weight in young men and severe COVID‐19 up to several decades later, probably mediated by a strong tracking of adolescent weight until middle age.
Obesity has previously been recognized as a risk factor for worse prognosis in respiratory infectious diseases (9, 27). This has received further attention during the current COVID‐19 pandemic, as a growing body of evidence has indicated that patients with obesity are more likely to suffer from severe symptoms and experience hospitalization (5, 8, 28). Despite a few large prospective cohort studies analyzing recent overweight and obesity as risk factors for severe COVID‐19 on a population level (8, 20, 21), our study is the first, to our knowledge, to establish an association between BMI in early adulthood and severe COVID‐19 later in life. Higher BMI in adolescence previously was recognized as a risk factor for infectious disease mortality among Israeli conscripts (22). Likewise, we included an unselected Swedish male population who enlisted for mandatory military service during the last decades, of which the majority has now reached an age at which they are at elevated risk for severe COVID‐19. Using adolescent BMI is reasonable considering that gaining excess weight at a young age frequently results in a lifetime of overweight or obesity (29).
We found a strong association between higher BMI in early adulthood and hospitalization for COVID‐19. The inclusion of all hospitalized patients with COVID‐19 during 2020 yielded a large number of cases, which enabled us to also discover risk elevations for narrower BMI intervals, even within the normal‐weight range. Notably, when compared with BMI of 18.5 to 20, a risk elevation was seen already at BMI 22.5 to 25 at a young age, which constitutes the upper part of the reference interval for normal weight (BMI = 18.5‐25) (30). This is supported by a recent prospective, community‐based cohort study from England that showed a linear increase in the risk of severe COVID‐19 leading to hospitalization or death at BMI > 23 and a linear increase in the risk of being admitted to an intensive care unit across the whole BMI range (8). In analogy with these findings, we recently showed that adolescent BMI at 22.5 to 25 also increases the risk for future heart failure and for venous thromboembolism compared with BMI 18.5 to 20 (31, 32). Therefore, having had a high‐normal adolescent BMI appears to be unfavorable for cardiovascular health, as well as for coping with viral infections such as COVID‐19. This may be explained by the fact that young individuals in the upper normal BMI range are more likely to develop overweight later in life (28), which exposes them to a chronic inflammatory state, impaired immune function, and higher long‐term cardiorespiratory demands (12, 13, 14, 16, 33). Regardless of the underlying cause, our findings indicate that a higher BMI during a lifetime has a negative impact not only on the cardiovascular system, but also on host defense against respiratory viruses such as SARS‐CoV‐2. The current and previous findings from our group suggest that high BMI in early adulthood is an important target for preventing obesity‐related health consequences, similar to high BMI later in life.
To investigate the independent role of overweight and obesity for COVID‐19, we adjusted for several common cardiovascular comorbidities that were reported to affect COVID‐19 outcome (17). Also, after adjusting for coronary heart disease, heart failure, hypertension, and diabetes mellitus, the association persisted between higher BMI and worse COVID‐19 prognosis. Therefore, in support of the findings by Gao et al. (8), overweight appears to be an independent risk factor for COVID‐19 leading to hospital and intensive care unit admission.
Strengths of the present study include the large sample size and the prospective‐based design. The longtime and almost complete follow‐up, with data from highly validated national registers, provides a strong ground for conclusions. During the pandemic, it has gradually become clear that several comorbidities predispose patients to a more severe form of the disease. Therefore, significant comorbidities among patients with COVID‐19 may have been registered in patient records to a higher degree than that of the remaining part of our cohort who did not develop severe COVID‐19, which introduces a potential selection bias. In order to avoid this bias, we only allowed comorbidities registered before 2020 for all study participants. Furthermore, information bias with misclassifications of the COVID‐19 diagnosis may also have occurred, as we used only register‐based diagnostic codes at discharge and not laboratory‐verified infection. However, a diagnosis of COVID‐19 in hospitalized patients is routinely confirmed with reverse transcriptase‐polymerase chain reaction (RT‐PCR) from nasopharyngeal swab aspirates on admission or later, when indicated. Unfortunately, we had no access to laboratory data verifying this, which is a limitation. Nevertheless, national hospital registers in Sweden have been found to have a high quality in former validation studies (34), and there is no reason to believe that this is not also true for patients with COVID‐19 in the hospital. Vaccination against COVID‐19 in Sweden was not yet possible during the study period; therefore, bias due to attitudes toward vaccination did not occur.
Further limitations of the present study are lack of generalizability regarding sex and ethnicity. It was not compulsory for women to conscript for military service during the study period, and the minor fraction of enlisting women at that time was not representative for the general Swedish female population. Therefore, they were excluded in this study. Immigrant status is a recognized risk factor for poorer prognosis in COVID‐19 (35). Because men who immigrated to Sweden in adulthood are not conscripted, they were not included in this cohort. Therefore, our results may not be representative for other populations or for women. Moreover, information on lifetime smoking status and lifestyle habits was not available. However, using the same study cohort, we have previously found that good cardiorespiratory fitness in youth is predictive of severe COVID‐19 later in life, independent of BMI (36). Furthermore, we used BMI as the exposure, which is a simple and internationally well‐known method to classify adiposity status, although it has no ability to account for alterations in regional fat distribution. Unfortunately, other anthropometric variables such as waist circumference and waist‐hip ratio were not available in our data set. Additionally, we were not able to differentiate between obesity classes because of the limited number of individuals with obesity in youth and later COVID‐19. Because extreme obesity has become more common among children and adolescents after the end of the inclusion period in 2005, our study may underestimate the current adolescent obesity levels (37).
CONCLUSION
In the present study, we found that higher BMI in early adulthood among Swedish men was a risk factor for hospitalization due to COVID‐19 decades later, during the first year of the pandemic. An increase in risk was seen already at BMI of 22.5 to 25, and it continued to rise with higher values of BMI, such that obesity entailed more than twice the risk for being admitted to an intensive care unit compared with BMI < 20. This underlines the necessity of preventive actions against overweight and obesity at a young age to offer protection against coming viral pandemics.O
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
JR, MÅ, AR, and MA were responsible for the conception and design of the study, as well as the acquisition and analysis of data. MA performed the statistical analyses. All authors took part in drafting the manuscript and approved the final version.
ETHICS STATEMENT
The study conforms to the principles outlined in the Declaration of Helsinki. The Ethics Committee of the University of Gothenburg and Confidentiality Clearance at Statistics Sweden approved the study (EPN Reference numbers: EPN Dnr 462‐14 and Dnr 567‐15; T174‐15/462‐14; T653‐17/462‐14; T196‐17/567‐15; T 2019‐05875/462‐14; T 2020‐01325/462‐14; T 2020‐02420/462‐14; T 2020‐03667/462‐14). Informed consent from the study participants was not required.
Supporting information
Supplementary Material
Robertson J, Adiels M, Lissner L, et al. BMI in early adulthood is associated with severe COVID‐19 later in life: A prospective cohort study of 1.5 million Swedish men. Obesity (Silver Spring). 2022;30:779–787. doi: 10.1002/oby.23378
Funding information
This work was supported by grants from the following: grants for the Swedish state under the agreement concerning research and education of doctors (grant number ALFGBG‐942707, ALFGBG‐813511); the Swedish Research Council for Health, Working Life and Welfare (2021‐00304); and the Aina Wallström and Mary‐Ann Sjöblom Foundation for Medical Research at the University of Gothenburg.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) pandemic. Accessed February 9, 2021. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019
- 2. Zunyou W, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 3. Yanez ND, Weiss NS, Romand J‐A, Treggiari M. COVID‐19 mortality risk for older men and women. BMC Public Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahlström B, Frithiof R, Hultström M, Larsson I‐M, Strandberg G, Lipcsey M. The Swedish COVID‐19 intensive care cohort: risk factors of ICU admission and ICU mortality. Acta Anaesthesiol Scand. 2021;65:525‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. J Intern Med. 2020;288:469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rawshani A, Kjölhede EA, Rawshani A, et al. Severe COVID‐19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg Health Eur. 2021;4:100105. doi: 10.1016/j.lanepe.2021.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao M, Piernas C, Astbury NM, et al. Associations between body‐mass index and COVID‐19 severity in 6,9 million people in England: a prospective, community‐based, cohort study. Lancet Diabetes Endocrinol. 2021;9:350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun Y, Wang Q, Yang G, Lin C, Zhang Y, Yang P. Weight and prognosis for influenza A(H1N1)pdm09 infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta‐analysis. Infect Dis (Lond). 2016;48:813‐822. [DOI] [PubMed] [Google Scholar]
- 10. Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moriconi D, Masi S, Rebelos E, et al. Obesity prolongs the hospital stay in patients affected by COVID‐19, and may impact on SARS‐COV‐2 shedding. Obes Res Clin Pract. 2020;14:205‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paich HA, Sheridan PA, Handy J, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring). 2013;21:2377‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nieman DC, Henson DA, Nehlsen‐cannarella SL, et al. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294‐299. [DOI] [PubMed] [Google Scholar]
- 14. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860‐867. [DOI] [PubMed] [Google Scholar]
- 15. Selim BJ, Ramar K, Surani S. Obesity in the intensive care unit: risks and complications. Hosp Pract. 2016;44:146‐156. [DOI] [PubMed] [Google Scholar]
- 16. Watson RA, Pride NB, Thomas EL, et al. Reduction of total lung capacity in obese men: comparison of total intrathoracic and gas volumes. J Appl Physiol. 2010;108:1605‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li BO, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol. 2020;109:531‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aghili SMM, Ebrahimpur M, Arjmand B, et al. Obesity in COVID‐19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta‐analysis. Int J Obes (Lond). 2021;45:998‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Afshin A, Forouzanfar MH, Reitsma MB, et al; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yates T, Razieh C, Zaccardi F, et al. Obesity, walking pace and risk of severe COVID‐19 and mortality: analysis of UK Biobank. Int J Obes (Lond). 2021;45:1155‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Twig G, Geva N, Levine H, et al. Body mass index and infectious disease mortality in midlife in a cohort of 2.3 million adolescents. Int J Obes (Lond). 2018;42:801‐807. [DOI] [PubMed] [Google Scholar]
- 23. Buscot M‐J, Thomson RJ, Juonala M, et al. Distinct child‐to‐adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018;39:2263‐2270. [DOI] [PubMed] [Google Scholar]
- 24. Swedish Intensive Care Registry . COVID‐19 in Swedish intensive care. Accessed September 16, 2021. https://www.icuregswe.org/en/data‐‐results/covid‐19‐in‐swedish‐intensive‐care/
- 25. Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:7‐38. [Google Scholar]
- 26. Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21:2409‐2419. [DOI] [PubMed] [Google Scholar]
- 27. Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis. 2011;53:413‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietri L, Giorgi R, Bégu A, et al. Excess body weight is an independent risk factor for severe forms of COVID‐19. Metabolism. 2021;117:154703. doi: 10.1016/j.metabol.2021.154703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta‐analysis. Obes Rev. 2016;17:95‐107. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . Obesity and overweight. Updated June 9, 2021. Accessed November 2, 2020. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight
- 31. Rosengren A, Åberg M, Robertson J, et al. Body weight in adolescence and long‐term risk of early heart failure in adulthood among men in Sweden. Eur Heart J. 2017;38:1926‐1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glise Sandblad K, Jern S, Åberg M, et al. Body mass index in adolescent men and risk of venous thromboembolism. J Intern Med. 2020;287:734‐745. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt M, Bøtker HE, Pedersen L, Sørensen HT. Young adulthood obesity and risk of acute coronary syndromes, stable angina pectoris, and congestive heart failure: a 36‐year cohort study. Ann Epidemiol. 2014;24:356‐361.e1. [DOI] [PubMed] [Google Scholar]
- 34. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national impatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drefahl S, Wallace M, Mussino E, et al. A population‐based cohort study of socio‐demographic risk factors for COVID‐19 deaths in Sweden. Nat Commun. 2020;11:5097. doi: 10.1038/s41467-020-18926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. af Geijerstam A, Mehlig K, Börjesson M, et al. Fitness, strength and severity of COVID‐19: a prospective register study of 1 559 187 Swedish conscripts. BMJ Open. 2021;11:e051316. doi: 10.1136/bmjopen-2021-051316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bendor CD, Bardugo A, Pinhas‐Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol. 2020;19:79. doi: 10.1186/s12933-020-01052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


