Abstract
Objective
Rural and remote patients with rheumatoid arthritis (RA) are at risk for inequities in health outcomes based on differences in physical environments and health care access potential compared to urban populations. The aim of this systematic review was to synthesize epidemiology, clinical outcomes, and health service use reported for global populations with RA residing in rural and remote locations.
Methods
Medline, Embase, HealthStar, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Library were searched from inception to June 2019 using librarian‐developed search terms for RA and rural and remote populations. Peer‐reviewed published manuscripts were included if they reported on epidemiologic, clinical, or health service use outcomes.
Results
Fifty‐four articles were included for data synthesis, representing studies from all continents. In 11 studies in which there was an appropriate urban population comparator, rural and remote populations were not at increased risk for RA; 1 study reported increased prevalence, and 5 studies reported decreased prevalence in rural and remote populations. Clinical characteristics of rural and remote populations in studies with an appropriate urban comparator showed no significant differences in disease activity measures or disability, but 1 study reported worse physical function and health‐related quality of life in rural and remote populations. Studies reporting on health service use provided evidence that rural and remote residence adversely impacts diagnostic time, ongoing follow‐up, access to RA‐care–related practitioners and services, and variation in medication access and use, with prominent heterogeneity noted between countries.
Conclusion
RA epidemiology and clinical outcomes are not necessarily different between rural/remote and urban populations within countries. Rural and remote patients face greater barriers to care, which increases the risk for inequities in outcomes.
INTRODUCTION
As rheumatology care providers, we are unified in our goals to deliver high‐quality care and implement effective evidence‐based approaches to secure optimal outcomes for patients who have evolving and established rheumatic diseases. Rural and remote population groups are acknowledged to be at risk for health inequities as a result of avoidable variations in social, political, cultural, and economic structures (1), and not only due to inequalities in disease distribution and outcomes alone. The PROGRESS‐Plus framework assists in conceptualizing which population groups are at risk for inequities (2, 3) and includes consideration of place of residence. Geography introduces risk for inequities in health outcomes for individuals with rheumatic disease residing in rural and remote locales based on differences in physical environments, social and economic capital, and health care access compared to urban populations. Identifying these inequities and implementing solutions and service enhancements to mitigate geographic influences on rheumatoid arthritis (RA) outcomes is a priority. Some factors to consider when determining appropriate solutions include the significant distance between provider location and affected population distribution (4, 5, 6, 7), the lack of a comprehensive understanding of existing outcomes for rural populations (8), and the acceptance of care models that bridge gaps in care using technology (9) and advanced skill practitioners (10). These solutions and enhancements need to be informed by a complete understanding of existing needs and gaps in RA care for rural and remote populations.
Significance & Innovations.
Epidemiologic and clinical outcomes in rheumatoid arthritis are similar between rural and remote and urban communities.
Rural and remote communities face barriers to health service access impacting length of diagnostic time, ongoing follow‐up, and allied health support.
Variations in medication access are of uncertain consequence but may result in outcome inequities.
The aim of this systematic review was to synthesize epidemiology (incidence, prevalence, mortality), clinical outcomes (indices of disease activity and severity, patient‐reported outcomes), and health service use (primary care visits, specialty care visits, hospitalizations, surgeries, medication access) reported for global populations with RA residing in rural and remote locations. This evidence will illuminate knowledge gaps, identify risk and resource factors to consider for care delivery, and inform clinical practice and policymaking.
MATERIALS AND METHODS
Search strategy and selection criteria
We conducted a systematic review with a broad search strategy applied to identify data for 3 separate outcomes (epidemiology, clinical outcomes, and health service use). Two search term filters were developed with the assistance of a systematic review methodologist. The population filter was developed to identify studies of inflammatory arthritis conditions. The second filter was developed to identify rural and remote studies specifically addressing health services, inequities, accessibility, and geographic health inequalities. Determination of rural and remote populations would have been made by the study's authors, reflecting their local situation and classification for these terms. Subject headings relating to rural health, rural health services and centers, and rural populations were modified or exempted as appropriate per database. The search filters were combined with the Boolean operator ‘AND’ and with an English language restriction, which is the primary language of all study team members (the search terms are available in Supplementary Table 1 on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24513). We performed the search using medical databases including Medline (1946 to June 2019), Embase (1980 to June 2019), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1996 to June 2019), HealthStar (1966 to June 2019), and the Cochrane Library (1996 to June 2019). References were extracted to a reference manager (EndNote X9.3.2, Clarivate Analytics), and reference duplicates were removed manually. We included all types of published studies (excluding conference abstracts) that reported on any of the epidemiologic, clinical, and health service utilization outcomes of interest. Epidemiologic outcomes included incidence and prevalence estimates. Clinical outcome measures could reflect disease activity measures, patient‐reported outcomes, and clinical phenotyping. Health service utilization outcomes included, but were not restricted to, diagnostic delay, provider and service utilization, and medication utilization. We included studies with stand‐alone rural and remote data and data from studies with an appropriate urban population comparator. For the present work, we reported on RA studies exclusively due to length constraints.
Data analysis
Three reviewers independently screened title and abstracts (SA screened the full set; EP and RH each screened one‐half of the set). A third reviewer resolved disagreements (EP or RH, as appropriate). EP and RH independently performed the full‐text review with consultation of a third reviewer (CB) upon disagreement. The authors independently abstracted data from studies deemed eligible in the full‐text review. EP and KC abstracted data from one‐half of the set, RH and SA from the other half of the set. CB reviewed and confirmed the entire data extraction. Each pair of authors came to a consensus on extractable data for each of the 3 outcome measures: clinical, epidemiologic, and health service utilization.
All included studies were appraised for quality using the Study Quality Assessment Tools developed by the National Heart, Lung, and Blood Institute (11). Extraction tables for case–control, cross‐sectional designs and observational cohorts were used as appropriate. Quality assessment was performed by EP and VU.
RESULTS
Study characteristics
The search generated 1,160 articles after removal of duplicates, and 138 articles were selected for full‐text review (Figure 1). From these, 54 articles were included for data synthesis (12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65) and are described in Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24513, with some studies reporting ≥1 outcome of interest. In total, 35 studies described an epidemiology outcome (n = 32 prevalence and n = 4 incidence), 15 described RA clinical characteristics of rural or remote populations, and 15 studies reported on health service utilization. In most cohort studies, patients met classification criteria appropriate to the study period (i.e., Rome criteria, American College of Rheumatology [ACR] 1987 criteria, or the ACR/European Alliance of Associations for Rheumatology 2010 criteria for RA [66, 67]) or had a clinical diagnosis conferred; in administrative data sources, case classification was by diagnosis codes. Definition of rurality was determined by location of residence, with some studies specifying the number of inhabitants in the region of study, geography (e.g., distance to a major city) or using descriptors such as postal codes or urbanization classifications. Studies from all continents were identified. Quality assessment found that of the 54 included studies, 21 were reported as good‐quality, 28 fair‐quality, and 3 poor‐quality studies. Two studies could not be assessed for quality (23, 33).
Figure 1.
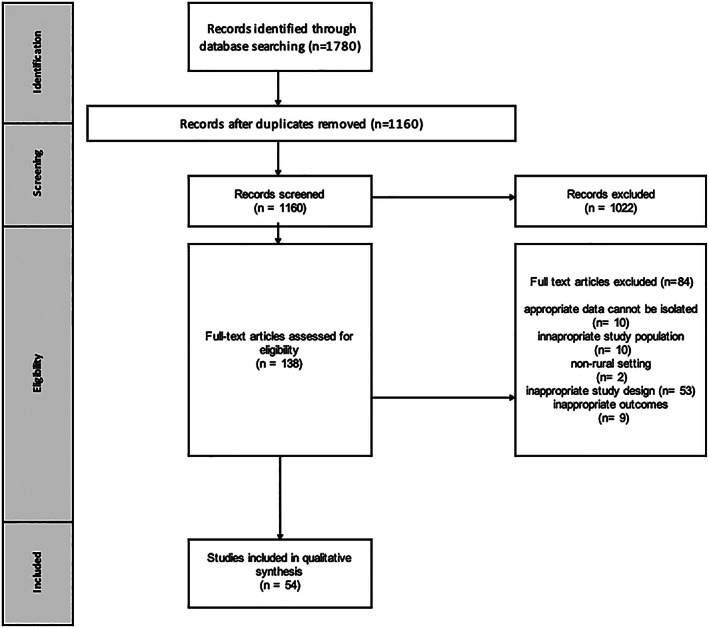
Study selection flow chart.
Epidemiology outcomes (incidence rates)
Four studies reported crude incidence rates. In a German population, incidence was reported as a proportion, with 0% of male patients and 6% of female patients developing RA over a 5‐year observation period (21). In 2 rural Bangladesh populations, the rate was 0.48 per 100 person‐years (95% confidence interval [95% CI] 0.27–0.85) (30) and 0.12 per 100 person‐years (95% CI 0.1–0.7) (65). In a Taiwanese population, the incidence rate was estimated at 0.02 per 100 person‐years (95% CI 0.02–0.3), which was not significantly different from that of the comparator urban population (relative risk 1.04 [95% CI 0.87–1.24], P = 0.67) (35).
Epidemiology outcomes (prevalence rates)
A total of 32 studies reported crude or adjusted prevalence rates with estimates ranging from 0 to 1.13% (12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 31, 32, 33, 34, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46) (Figure 2; detailed table available as Supplementary Table 3, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24513/abstract). In studies in which there was an appropriate urban population comparator, rural or remote populations were not at increased risk for RA (n = 11 studies) (14, 16, 17, 18, 19, 20, 24, 25, 29, 37, 41), whereas 1 study reported increased prevalence (13), and 5 studies reported decreased prevalence (12, 22, 28, 34, 43) in rural populations. Two studies from Taiwan contrasted RA prevalence in rural and urban populations. The study by Yang et al did not identify significant differences in the risk of RA by location of residence (35), whereas in a case–control study design by Chiang et al, there were lower odds of RA in rural residents (odds ratio [OR] 0.76 [95% CI 0.61–0.95], P = 0.021) (36).
Figure 2.
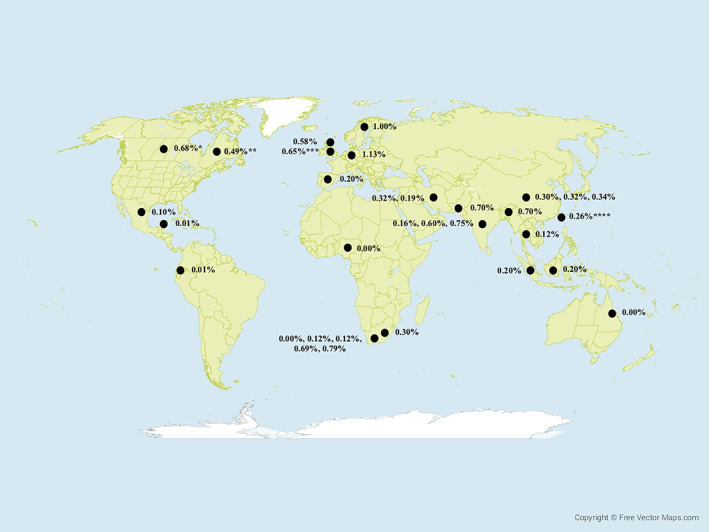
Prevalence estimates of rheumatoid arthritis in global rural and remote populations (crude data only). * = average of estimates in male and female groups of <45 and >45 years of age. ** = average of estimates in male and female groups of age‐adjusted estimates; *** = average of estimates in male and female groups; **** = age‐ and sex‐adjusted estimate. Map by FreeVectorMaps.com (https://freevectormaps.com/world‐maps/WRLD‐EPS‐02‐0009?ref=atr).
Patient‐reported outcomes and function
The impact of RA on rural and remote populations through function, assistive needs, and quality of life was described in 10 studies. In rural Chile, patients had a 2‐fold increased odds of moderate‐to‐severe disability relative to urban residents, although this was not statistically significant (OR 2.12 [95% CI 0.71–6.34]) (48). In the rural municipality of Chankom, Mexico, and in Southwest China, 59% of RA patients were reported to have disability using the Health Assessment Questionnaire (HAQ) (15, 51). Two studies describe functional impairments in a rural Finnish population; nearly all patients were classified as having class II or class III function according to the ACR 1987 criteria for RA (66) and using the HAQ functional disability index, two‐thirds had mild or moderate disability, and ~10% were severely disabled (49). The Keitel function test, which is a range of motion test reflecting the degree of functional limitation of joints and specific assessment of hand function, was completed in a Finnish population, with 10% of patient scores indicating severe impairment, primarily driven by hand dysfunction (50). In a study from Zimbabwe, rural and urban patients had similar HAQ scores, duration of morning stiffness, and proportion with joint deformities (54), whereas in Senegal, the proportion of rural patients with specific hand deformities was similar to those from urban residence (53). Darmawan and colleagues provided a description of grip strength, functional grade, and duration of morning stiffness in a small sample of RA patients from urban and rural Indonesia, with no significant differences between groups (37). Gong's study from China reported results of the Short Form 36 health survey physical component summary (PCS) score and mental component summary (MCS) score and total scores as an indication of health‐related quality of life (HRQoL), with rural patients having significantly lower PCS and HRQoL scores, but with no difference in MCS compared to urban patients (52). In a Canadian study of patients initiating their first biologic medication, HAQ scores, fatigue, and patient global scores were reported for rural and urban population comparisons using 2 different determinations for rural status (by postal code and by Statistics Canada population center), with no significant differences between populations (47).
Disease activity
Relatively few studies have reported disease activity in rural and remote populations. Darmawan provided erythrocyte sedimentation rate results for 8 rural Indonesian patients, which were similar to those of 3 urban patients (37). The proportion of patients with ≥3 joints affected by active disease was similar between rural and urban Zimbabwean patients (54). Disease Activity Score in 28 joints (DAS28) scores and swollen joint counts were similar between rural and urban patients in Senegal (53), and tender and swollen joint counts, DAS28 scores, Clinical Disease Activity Index scores, and physician global evaluation scores did not differ between rural and urban Canadian patients (47).
Extraarticular manifestations and comorbidities
The frequency of rheumatoid nodules, vasculitis, lung fibrosis, and secondary Sjögren's syndrome did not differ between rural and urban RA patients in Zimbabwe (54). Rural patients in Senegal more frequently had extraarticular manifestations (70% versus 49% urban) when considering sicca syndrome, nodules, fever, lymph nodes, interstitial lung disease, and hematologic or neurologic complications of RA (53). In a Han Chinese population, note was made that none of the patients had rheumatoid nodules (32). Rural Canadian patients did not have a higher mean number of comorbidities compared to urban patients (47).
Radiographic changes
Several studies reported the frequency of erosive changes that were present on radiographs (26, 27), with some providing grades of change (32, 45) or a radiographic erosion mean score (49) without a comparison population. In studies with an urban comparison group, all Indonesian patients, whether rural or urban, had erosive changes (37), and there was not a significant difference in the proportion of rural and urban patients with erosions in the Canadian study (47) or Zimbabwe study (54).
Serology
Several studies reported the frequency of rheumatoid factor in rural populations without a comparison population (26, 27, 32, 43, 45). Rheumatoid factor positivity was not more frequent in rural populations of Canada (47), Senegal (which also included anti–citrullinated protein antibody testing) (53), Zimbabwe (which also included antinuclear antibody, Ro, and La antibody testing) (54), or Indonesia (37).
Diagnostic delay
Rural patients from Saudi Arabia had a significantly longer time to diagnosis than urban patients, at a mean ± SD of 37.0 ± 17.1 months compared to a mean ± SD of 27.8 ± 14.9 months (P < 0.01) (64). The study from Senegal reported no difference in time to diagnosis (45 versus 50 months, rural versus urban; P = 0.21) (53). In a Canadian study, rural residence was associated with lower odds of being seen by a rheumatologist within 3 months of suspected diagnosis in the crude model; however, this did not remain significant in the adjusted model for patient demographic characteristics, clinical factors, primary care physician characteristics, provider continuity, and geographic characteristics (OR 0.92 [95% CI 0.83–1.01]); however, remote distance (>100 km to a rheumatologist) was significant in both crude and adjusted models (adjusted OR [ORadj] 0.51 [95% CI 0.41–0.64]) (55). In a study of rural Austrian patients, the median time to diagnosis was 7–12 months, with 57% having to consult 2–3 physicians before a diagnosis was made and 73% of patients diagnosed by rheumatologists (61).
Health service access
In a Scottish study, rural and urban patients had a similar number of musculoskeletal interventions (either joint aspirations or joint surgeries) over follow‐up, although the length of follow‐up in each group was not specified in the publication (18). In an American Medicare cohort, rural residents had higher odds of undergoing hand or wrist arthroplasty or arthrodesis (OR 1.31 [95% CI 1.04–1.66]) and tendon reconstructive procedures (OR 1.92 [95% CI 1.19–3.09]) (59). Hakala described the frequency of hospital contact due to RA (57% in preceding 2 years), general practitioner contact due to RA (83% in preceding 2 years), and frequency of joint replacement surgery (20%) or orthopedic operations (66%) in a rural Finnish population (49). An Austrian study described the path to diagnosis for rural patients, with the majority (80%) having accessed their general practitioner for their initial RA symptoms, with 13% directly seeking care from a rheumatologist, and with 57% of patients having to consult 2–3 physicians before their diagnosis was made (61). The use of general practitioner and rheumatology outpatient services in Estonia has been described, with rural patients seeking RA care from their general practitioner significantly more frequently (ORadj 2.84 [95% CI 1.63–4.95]) and from a rheumatologist significantly less frequently (ORadj 0.55 [95% CI 0.33–0.90]) than patients in the capital region (60). In Ontario, Canada, crude estimates suggested that rural patients were less likely to receive rheumatology care at 6 and 12 months, although the adjusted models were not statistically significant; however, remote distance (>100 km to a rheumatologist) was significant (6‐month ORadj 0.28 [95% CI 0.24–0.33]; 12‐month ORadj 0.33 [95% CI 0.26–0.43]) (55). A study from Saskatchewan, Canada, employed structured interviews to explore rural patients' experiences of availability of primary care, specialty care, and allied health professionals and services, including perceptions of accessibility, affordability and accommodation. Primary care availability was seen as a limitation for 28% of participants, with 27% of participants reporting traveling >4 hours to their rheumatologist. Allied health services were difficult to access, and of the 53% who accessed physical therapy, one‐half waited >1 month for the consultation. This was even more pronounced for occupational therapy, which 26% accessed, but 71% of patients waited >1 month, and 72% traveled outside the community for occupational therapy services, including 33% having to travel >2 hours. Distance to laboratory services impacted the completion of regular monitoring, and access to radiologic services and pharmacies was also difficult. Environmental barriers such as weather events and road quality impacted maintaining appointments for 67% of participants, and transportation availability and financial impacts were also reported (57).
Medication access
A Finnish study reported that 66% of rural patients were treated with disease‐modifying antirheumatic drug (DMARD) therapy, with the time from symptom onset to first DMARD being 2.4 years, and with 34% exposed to glucocorticoids, 18% treated with nonsteroidal antiinflammatory drugs alone, and 6% not receiving treatment (49). In Austria, 44% of rural patients took daily glucocorticoids, 61% were taking methotrexate, and 38% were receiving biologics (61). Urban and rural patients in Scotland had similar frequency of DMARD therapy (70% and 77%, respectively) and prednisone exposure (31%) (18). In 2 studies, compliance with treatment for RA was measured. In a Canadian cohort, rural residence and distance >25 km between patient residence and clinical site were not associated with the time to discontinuation of antirheumatic medications (hazard ratio [HR] 1.13 [95% CI 0.88–1.45] and HR 1.13 [95% CI 0.93–1.38], respectively), nor were these associated with time to discontinuation of conventional synthetic and biologic DMARDs specifically (HR 1.16 [95% CI 0.78–1.72] and 1.24 [95% CI 0.91–1.70]) (56). In an Egyptian cohort, rural residence was associated with lower adherence to medications and appointments (OR 12.4 [95% CI 1.2–128.7]) (63). Access to biologic therapy has been investigated in several studies. In Romania, rural patients had significantly lower access to biologics within their territory (74.1%) compared to urban patients (83.1%) (62). In an American study, rural residence increased the probability of initiation of biologic therapy once individual and contextual factors were considered (HR 1.96 [95% CI 1.28–2.99]) (58). In Taiwan, although there were no significant differences in exposure to methotrexate, non‐methotrexate DMARDs, antiinflammatory drugs, or non–tumor necrosis factor inhibitor (TNFi) biologics between most and least urbanized populations, those in less urbanized areas were less frequently treated with TNFi (1.3% versus 2.3%; P = 0.04) (36). In a Canadian study, rural patients were more frequently taking oral steroids (27.3% versus 20.0%), but living in a rural area had no significant impact on the first type of biologic DMARD used (TNFi or non‐TNFi) nor route of administration compared to urban residents. However, if the clinical site the patients attended to receive care was in a rural area, the patients were significantly more likely to be started on a TNFi biologic than a non‐TNFi one (ORadj 3.79 [95% CI 1.06–13.5]) and more likely to be started on subcutaneous therapy rather than intravenous therapy (ORadj 0.06 [95% CI 0.01–0.41]), with adjustment for sex, age, smoking, disease duration, function, concurrent use of antiinflammatory drugs, academic affiliated site, time period, and number of comorbidities (47).
DISCUSSION
This systematic review aimed to be comprehensive so as to provide a sufficient understanding of disease distribution, burden, and health service gaps existing for rural and remote populations with RA informative for health system leadership, especially where no jurisdictional‐level data may be available. Overall, the prevalence of RA does not appear to be increased in rural populations, nor was the severity of RA in these populations. However, the data on clinical outcomes are limited, highlighting a great need for more robust and contemporary data, and in particular, reporting variability within countries in the context of population‐level regional risk and prognostic factors and available health services and their quality. Although the contemporary Canadian study (47) may be reassuring in that clinical outcomes are not substantially different between rural, remote, and urban patients in a universal health care access setting, this study does represent patients enrolled in a particular registry, and results may not be generalizable to all clinical settings in Canada, and specifically other countries facing lower income levels. The identified data in fact support significant heterogeneity between countries, making interpretation of descriptive data of rural and remote results difficult when no urban comparator data were presented. Data from developing countries suggest that disability is highly prevalent in rural populations, but likely the determination of this does not attend to cultural differences in activities between populations and may reflect situations exacerbated by difficulties with access to supportive allied health services and assistive devices.
Our review does confirm that it is more difficult to secure appropriate health service access, including physician services for the studied rural and remote populations. Access issues create diagnostic delays and interruptions in the continuity of follow‐up and reassessment care, and longitudinal studies are necessary to determine the true impact of these disruptions. Variation in medication access for rural and remote populations between countries was also confirmed through our review, predominantly reflecting reduced availability. The impact of variability in selection of route of administration, with a preference for subcutaneous routes for rural and remote patients, may be negligible in observed disease outcomes but is worth querying through existing cohort studies.
We challenge health system leadership to implement structures and policies to support better outcomes in rural and remote populations. Access to health services is a recognized determinant of health, which presents the opportunity for actionable strategies and approaches to resolve inequities in care delivery and ensure that all populations can access timely and appropriate health care. Innovations in health care delivery including telehealth (9) and deployment of advanced skilled allied health professionals (10) are realistic solutions if sufficiently supported through infrastructure and remuneration. Rheumatology specialists are encouraged to build relationships and alliances with primary care providers in remote locations who would be critical components of these particular models. In‐person distributed care, such as through outreach clinic models (68) or through enhanced recruitment of rheumatology trainees from rural and remote locations, may be another effective strategy to provide in‐person services in rural and remote locales. Health information infrastructure is a key component to resource, serving to assist with health service model planning and ensuring high‐quality care delivery.
We recognize limitations of this review. The search strategy required exclusive use of search terms specifying ‘rural’ in subject headings, as the databases used have not yet included search terms for ‘remote’ within subject headings. Determination of rural or remote residence is not homogeneous globally and reflects country‐specific realities for population distribution. Although we included ‘rural’ terms in our supplementary searches, we did not specifically identify studies in which particular populations are overrepresented in rural and remote locations. For instance, data pertaining to rural indigenous populations would have been excluded from identification because indigenous search terms were not explicitly included. In considering the nature of a review, publication bias poses a risk. Government data sources, dissertations, or gray literature sources for unpublished research results were not specifically explored to supplement the medical database–driven search. Variation in prevalence estimates may reflect differences in case ascertainment methods, and outcomes related to clinical aspects of RA may have been limited by the fact that measurement tools have not been developed or adapted for various languages and cultures. The vast year difference of the included studies may not reflect contemporary influences and paradigms, while the small sample size in large countries may be misrepresentative of its true population, thereby limiting the modern‐day generalizability of our study findings. Covariates, such as sociodemographic factors or comorbidities that influence risk for RA trajectory or disease status, were not considered when synthesizing results. This situation may overrepresent causal pathways and conclusions drawn from the analysis.
In conclusion, RA epidemiology and clinical outcomes are not necessarily different between rural/remote and urban populations; however, rural and remote patients consistently face greater barriers to care, which increases the risk for inequities in outcomes.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Barnabe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Pianarosa, Hazlewood, Barnabe.
Acquisition of data
Pianarosa, Chomistek, Hsiao, Anwar, Umaefulam, Barnabe.
Analysis and interpretation of data
Pianarosa, Hazlewood, Barnabe.
Supporting information
Supplementary Table 1. MEDLINE Search
Supplementary Table 2. Included Studies*
Supplementary Table 3. Prevalence Estimates for Global Rural and Remote Populations
ACKNOWLEDGMENT
We thank Jordi Pardo for assistance in developing the search strategy.
Supported by the CIHR (Institute of Musculoskeletal Health and Arthritis Undergraduate Summer studentship to Ms. Pianarosa), the Alberta Children's Hospital (graduate award to Ms. Chomistek), the University of Calgary (Eyes High Postdoctoral scholarship to Dr. Umaefulam), a CIHR Canada Research Chair (Tier 2) in Rheumatoid Arthritis and Autoimmune Diseases, and the CIHR Foundation Scheme (grant FDN‐143284 to Dr. Barnabe).
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Arcaya MC, Arcaya AL, Subramanian SV. Inequalities in health: definitions, concepts, and theories. Glob Health Action 2015;8:27106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petkovic J, Epstein J, Buchbinder R, Welch V, Rader T, Lyddiatt A, et al. Toward ensuring health equity: readability and cultural equivalence of OMERACT patient‐reported outcome measures. J Rheumatol 2015;42:2448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petkovic J, Barton JL, Flurey C, Goel N, Bartels CM, Barnabe C, et al. Health equity considerations for developing and reporting patient‐reported outcomes in clinical trials: a report from the OMERACT Equity Special Interest group. J Rheumatol 2017;44:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barber CE, Jewett L, Badley EM, Lacaille D, Cividino A, Ahluwalia V, et al. Stand up and be counted: measuring and mapping the rheumatology workforce in Canada. J Rheumatol 2017;44:248–57. [DOI] [PubMed] [Google Scholar]
- 5. De Albuquerque CP. Inequality in the distribution of rheumatologists in Brazil: correlation with local of medical residency, Gross Domestic Product and Human Development Index. Rev Bras Reumatol 2014;54:166–71. [PubMed] [Google Scholar]
- 6. Pineda C, Sandoval H, Fraga‐Mouret A. Mexican rheumatology: where do we stand? Rheumatol Int 2019;39:585–93. [DOI] [PubMed] [Google Scholar]
- 7. Reveille JD, Muñoz R, Soriano E, Albanese M, Espada G, Lozada CJ, et al. Review of current workforce for rheumatology in the countries of the Americas 2012–2015. J Clin Rheumatol 2016;22:405–10. [DOI] [PubMed] [Google Scholar]
- 8. Hollick RJ, Macfarlane GJ. Association of rural setting with poorer disease outcomes for patients with rheumatic diseases: results from a systematic review of the literature. Arthritis Care Res (Hoboken) 2021;73:666–70. [DOI] [PubMed] [Google Scholar]
- 9. McDougall JA, Ferucci ED, Glover J, Fraenkel L. Telerheumatology: a systematic review. Arthritis Care Res (Hoboken) 2017;69:1546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Passalent LA, Kennedy C, Warmington K, Soever LJ, Lundon K, Shupak R, et al. System integration and clinical utilization of the Advanced Clinician Practitioner in Arthritis Care (ACPAC) Program–Trained Extended Role Practitioners in Ontario: a two‐year, system‐level evaluation. Healthc Policy 2013;8:56–70. [PMC free article] [PubMed] [Google Scholar]
- 11. National Heart Lung and Blood Institute . Study quality assessment tools. URL: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 12. Bernatsky S, Dekis A, Hudson M, Pineau CA, Boire G, Fortin PR, et al. Rheumatoid arthritis prevalence in Quebec. BMC Res Notes 2014;7:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor‐Gjevre R, Nair B, Jin S, Quail J. Geographic variation in incidence and prevalence rates for rheumatoid arthritis in Saskatchewan, Canada 2001–2014. Can J Public Health 2018;109:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez‐Amado J, Pelaez‐Ballestas I, Sanin LH, Esquivel‐Valerio JA, Burgos‐Vargas R, Perez‐Barbosa L, et al. Epidemiology of rheumatic diseases: a community‐based study in urban and rural populations in the state of Nuevo Leon, Mexico. J Rheumatol Suppl 2011;86:9–14. [DOI] [PubMed] [Google Scholar]
- 15. Loyola‐Sanchez A, Richardson J, Pelaez‐Ballestas I, Alvarez‐Nemegyei J, Lavis JN, Wilson MG, et al. The impact of arthritis on the physical function of a rural Maya‐Yucateco community and factors associated with its prevalence: a cross sectional, community‐based study. Clin Rheumatol 2016;35 Suppl 1:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guevara‐Pacheco S, Feican‐Alvarado A, Sanin LH, Vintimilla‐Ugalde J, Vintimilla‐Moscoso F, Delgado‐Pauta J, et al. Prevalence of musculoskeletal disorders and rheumatic diseases in Cuenca, Ecuador: a WHO‐ILAR COPCORD study. Rheumatol Int 2016;36:1195–204. [DOI] [PubMed] [Google Scholar]
- 17. Miall WE, Ball J, Kellgren JH. Prevalence of rheumatoid arthritis in urban and rural populations in South Wales. Ann Rheum Dis 1958;17:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Basu N, Steven M. A comparison of rural and urban rheumatoid arthritis populations. Scott Med J 2009;54:7–9. [DOI] [PubMed] [Google Scholar]
- 19. Neovius M, Simard JF, Askling J, ARTIS study group. Nationwide prevalence of rheumatoid arthritis and penetration of disease‐modifying drugs in Sweden. Ann Rheum Dis 2011;70:624–9. [DOI] [PubMed] [Google Scholar]
- 20. Carmona L, Villaverde V, Hernandez‐Garcia C, Ballina J, Gabriel R, Laffon A, et al. The prevalence of rheumatoid arthritis in the general population of Spain. Rheumatology (Oxford) 2002;41:88–95. [DOI] [PubMed] [Google Scholar]
- 21. Behrend T, Lawrence JS, Behrend H, Fischer K. Prevalence of rheumatoid arthritis in rural Germany. Int J Epidemiol 1972;1:153–6. [DOI] [PubMed] [Google Scholar]
- 22. Iltchev P, Sliwczynski A, Czeleko T, Sierocka A, Tlustochowicz M, Tlustochowicz W, et al. Epidemiology of rheumatoid arthritis (RA) in rural and urban areas of Poland: 2008–2012. Ann Agric Environ Med 2016;23:350–6. [DOI] [PubMed] [Google Scholar]
- 23. Forghanizadeh J, Abhari R, Shakibi MR, Samadi F, Piroozian M, Tavakoli S. Prevalence of rheumatic diseases in Fasham. RJMS 1995;2:182–91. [Google Scholar]
- 24. Davatchi F, Tehrani Banihashemi A, Gholami J, Faezi ST, Forouzanfar MH, Salesi M, et al. The prevalence of musculoskeletal complaints in a rural area in Iran: a WHO‐ILAR COPCORD study (stage 1, rural study) in Iran. Clin Rheumatol 2009;28:1267–74. [DOI] [PubMed] [Google Scholar]
- 25. Farooqi A, Gibson T. Prevalence of the major rheumatic disorders in the adult population of north Pakistan. Br J Rheumatol 1998;37:491–5. [DOI] [PubMed] [Google Scholar]
- 26. Malaviya AN, Kapoor SK, Singh RR, Kumar A, Pande I. Prevalence of rheumatoid arthritis in the adult Indian population. Rheumatol Int 1993;13:131–4. [DOI] [PubMed] [Google Scholar]
- 27. Chopra A, Patil J, Billempelly V, Relwani J, Tandle HS, WHO International League of Associations from Rheumatology Community Oriented Program From Control of Rheumatic Diseases. Prevalence of rheumatic diseases in a rural population in western India: a WHO‐ILAR COPCORD Study. J Assoc Physicians India 2001;49:240–6. [PubMed] [Google Scholar]
- 28. Kumar P, Alok R, Das SK, Srivastava R, Agarwal GG. Distribution of rheumatological diseases in rural and urban areas: an adapted COPCORD stage I phase III survey of Lucknow District in North India. Int J Rheum Dis 2018;21:1894–9. [DOI] [PubMed] [Google Scholar]
- 29. Haq SA, Darmawan J, Islam MN, Uddin MZ, Das BB, Rahman F, et al. Prevalence of rheumatic diseases and associated outcomes in rural and urban communities in Bangladesh: a COPCORD study. J Rheumatol 2005;32:348–53. [PubMed] [Google Scholar]
- 30. Ahmed M, Haq SA, Islam MN, Banik SK, Alam MN. Burden of rheumatic diseases in a rural community of Bangladesh. J Med 2014;15:125–30. [Google Scholar]
- 31. Beasley RP, Bennett PH, Lin CC. Low prevalence of rheumatoid arthritis in Chinese. Prevalence survey in a rural community. J Rheumatol Suppl 1983;10:11–5. [PubMed] [Google Scholar]
- 32. Wigley RD, Zhang NZ, Zeng QY, Shi CS, Hu DW, Couchman K, et al. Rheumatic diseases in China: ILAR‐China study comparing the prevalence of rheumatic symptoms in northern and southern rural populations. J Rheumatol 1994;21:1484–90. [PubMed] [Google Scholar]
- 33. Chaiamnuay P, Darmawan J, Muirden KD, Assawatanabodee P. Epidemiology of rheumatic disease in rural Thailand: a WHO‐ILAR COPCORD study. Community Oriented Programme for the Control of Rheumatic Disease. J Rheumatol 1998;25:1382–7. [PubMed] [Google Scholar]
- 34. Chou CT, Pei L, Chang DM, Lee CF, Schumacher HR, Liang MH. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol 1994;21:302–6. [PubMed] [Google Scholar]
- 35. Yang DH, Huang JY, Chiou JY, Wei JC. Analysis of socioeconomic status in the patients with rheumatoid arthritis. Int J Environ Res Public Health 2018;15:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chiang YC, Yen YH, Chang WC, Cheng KJ, Chang WP, Chen HY. The association between urbanization and rheumatoid arthritis in Taiwan. Int J Clin Pharm Ther 2016;54:1–10. [DOI] [PubMed] [Google Scholar]
- 37. Darmawan J, Muirden KD, Valkenburg HA, Wigley RD. The epidemiology of rheumatoid arthritis in Indonesia. Br J Rheumatol 1993;32:537–40. [DOI] [PubMed] [Google Scholar]
- 38. Wigley R, Manahan L, Muirden KD, Caragay R, Pinfold B, Couchman KG, et al. Rheumatic disease in a Philippine village. II: a WHO‐ILAR‐APLAR COPCORD study, phases II and III. Rheumatol Int 1991;11:157–61. [DOI] [PubMed] [Google Scholar]
- 39. Silman AJ, Ollier W, Holligan S, Birrell F, Adebajo A, Asuzu MC, et al. Absence of rheumatoid arthritis in a rural Nigerian population. J Rheumatol 1993;20:618–22. [PubMed] [Google Scholar]
- 40. Beighton P, Solomon L, Valkenburg HA. Rheumatoid arthritis in a rural South African Negro population. Ann Rheum Dis 1975;34:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solomon L, Beighton P, Valkenburg HA, Robin G, Soskolne CL. Rheumatic disorders in the South African Negro. Part I. Rheumatoid arthritis and ankylosing spondylitis. S Afr Med J 1975;49:1292–6. [PubMed] [Google Scholar]
- 42. Meyers OL, Daynes G, Beighton P. Rheumatoid arthritis in a tribal Xhosa population in the Transkei, Southern Africa. Ann Rheum Dis 1977;36:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meyers OL, Jessop S, Klemp P. The epidemiology of rheumatic disease in a rural and an urban population over the age of 65 years. S Afr Med J 1982;62:403–5. [PubMed] [Google Scholar]
- 44. Brighton SW, de la Harpe AL, van Staden DJ, Badenhorst JH, Myers OL. The prevalence of rheumatoid arthritis in a rural African population. J Rheumatol 1988;15:405–8. [PubMed] [Google Scholar]
- 45. Moolenburgh JD, Valkenburg HA, Fourie PB. A population study on rheumatoid arthritis in Lesotho, Southern Africa. Ann Rheum Dis 1986;45:691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Minaur N, Sawyers S, Parker J, Darmawan J. Rheumatic disease in an Australian Aboriginal community in North Queensland, Australia. A WHO‐ILAR COPCORD survey. J Rheumatol 2004;31:965–72. [PubMed] [Google Scholar]
- 47. Movahedi M, Joshi R, Rampakakis E, Thorne C, Cesta A, Sampalis JS, et al. Impact of residential area on the management of rheumatoid arthritis patients initiating their first biologic DMARD: results from the Ontario Best Practices Research Initiative (OBRI). Medicine (Baltimore) 2019;98:e15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alarcon AM, Munoz S, Kaufman JS, Martinez C, Riedemann P, Kaliski S. Contribution of ethnic group and socioeconomic status to degree of disability in rheumatoid arthritis in Chilean patients. Rheumatol Int 2015;35:685–9. [DOI] [PubMed] [Google Scholar]
- 49. Hakala M, Nieminen P, Koivisto O. More evidence from a community based series of better outcome in rheumatoid arthritis: data on the effect of multidisciplinary care on the retention of functional ability. J Rheumatol 1994;21:1432–7. [PubMed] [Google Scholar]
- 50. Hakala M, Nieminen P. Functional status assessment of physical impairment in a community based population with rheumatoid arthritis: severely incapacitated patients are rare. J Rheumatol 1996;23:617–23. [PubMed] [Google Scholar]
- 51. Zhao S, Chen Y, Chen H. Sociodemographic factors associated with functional disability in outpatients with rheumatoid arthritis in Southwest China. Clin Rheumatol 2015;34:845–51. [DOI] [PubMed] [Google Scholar]
- 52. Gong G, Mao J. Health‐related quality of life among Chinese patients with rheumatoid arthritis: the predictive roles of fatigue, functional disability, self‐efficacy, and social support. Nurs Res 2016;65:55–67. [DOI] [PubMed] [Google Scholar]
- 53. Lekpa FK, Ndongo S, Tiendrebeogo J, Ndao AC, Daher A, Pouye A, et al. Rheumatoid arthritis in Senegal: a comparison between patients coming from rural and urban areas, in an urban tertiary health care center in Senegal. Clin Rheumatol 2012;31:1617–20. [DOI] [PubMed] [Google Scholar]
- 54. Chikanza IC, Stein M, Lutalo S, Gibson T. The clinical, serologic and radiologic features of rheumatoid arthritis in ethnic black Zimbabwean and British Caucasian patients. J Rheumatol 1994;21:2011–5. [PubMed] [Google Scholar]
- 55. Widdifield J, Paterson JM, Bernatsky S, Tu K, Thorne JC, Ivers N, et al. Access to rheumatologists among patients with newly diagnosed rheumatoid arthritis in a Canadian universal public healthcare system. BMJ Open 2014;4:e003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahluwalia V, Rampakakis E, Movahedi M, Cesta A, Li X, Sampalis JS, et al. Predictors of patient decision to discontinue anti‐rheumatic medication in patients with rheumatoid arthritis: results from the Ontario best practices research initiative. Clin Rheumatol 2017;36:2421–30. [DOI] [PubMed] [Google Scholar]
- 57. Nair BV, Schuler R, Stewart S, Taylor‐Gjevre RM. Self‐reported barriers to healthcare access for rheumatoid arthritis patients in rural and Northern Saskatchewan: a mixed methods study. Musculoskeletal Care 2016;14:243–51. [DOI] [PubMed] [Google Scholar]
- 58. Yelin E, Tonner C, Kim SC, Katz JN, Ayanian JZ, Brookhart MA, et al. Sociodemographic, disease, health system, and contextual factors affecting the initiation of biologic agents in rheumatoid arthritis: a longitudinal study. Arthritis Care Res (Hoboken) 2014;66:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhong L, Chung KC, Baser O, Fox DA, Yuce H, Waljee JF. Variation in rheumatoid hand and wrist surgery among Medicare beneficiaries: a population‐based cohort study. J Rheumatol 2015;42:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Polluste K, Kallikorm R, Meiesaar K, Lember M. Use of general practice and rheumatology outpatient services in rheumatoid arthritis. Fam Pract 2012;29:433–40. [DOI] [PubMed] [Google Scholar]
- 61. Puchner R, Brezinschek HP, Herold M, Nothnagl T, Studnicka‐Benke A, Fritz J, et al. Quality of care of rural rheumatoid arthritis patients in Austria. Wien Klin Wochenschr 2014;126:360–7. [DOI] [PubMed] [Google Scholar]
- 62. Codreanu C, Popescu CC, Mogosan C. Area of residence and socioeconomic factors reduce access to biologics for rheumatoid arthritis patients in Romania. Biomed Res Int 2018;2018:7458361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ragab OM, Zayed HS, Abdelaleem EA, Girgis AE. Effect of early treatment with disease‐modifying anti‐rheumatic drugs and treatment adherence on disease outcome in rheumatoid arthritis patients. Egypt Rheumatologist 2017;39:69–74. [Google Scholar]
- 64. Hussain W, Noorwali A, Janoudi N, Baamer M, Kebbi L, Mansafi H, et al. From symptoms to diagnosis: an observational study of the journey of rheumatoid arthritis patients in Saudi Arabia. Oman Med J 2016;31:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haq SA, Darmawan J, Islam MN, Ahmed M, Banik SK, Rahman AK, et al. Incidence of musculoskeletal pain and rheumatic disorders in a Bangladeshi rural community: a WHO‐APLAR‐COPCORD study. Int J Rheum Dis 2008;11:216–23. [Google Scholar]
- 66. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 67. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 68. Nagaraj S, Kargard M, Hemmelgarn B, Fritzler M, White T, Barnabe C. Effectiveness of an outreach model of care for rheumatology specialty clinics to an on‐reserve first nations community. Int J Indig Health 2018;13:156–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. MEDLINE Search
Supplementary Table 2. Included Studies*
Supplementary Table 3. Prevalence Estimates for Global Rural and Remote Populations


