CONFLICT OF INTEREST
A.M. has received research grants, consulting fees, and/or speaker’s fees from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis, Pfizer, Sun Pharmaceutical Industries Taiho Pharmaceutical, Torii Pharmaceutical, Ushio, and UCB Pharma. K.I., K.T., Y.S., Y.K., A.N., and H.N. have no conflict of interest to declare.
Dear Editor,
Psoriasis is associated with a high risk of diabetes mellitus. 1 We previously reported that the Psoriasis Area and Severity Index (PASI) correlates with hemoglobin A1c (HbA1c) levels in psoriasis patients treated with biologics. 2 Treatment with interleukin‐17 decreases HbA1c levels. 2 Cyclic adenosine monophosphate (cAMP) is an important second messenger in the immune and metabolic systems. 3 The phosphodiesterase inhibitor (PDEI) roflumilast, which acts by increasing cAMP, is reported to improve insulin homeostasis and obesity. 4 Several studies have demonstrated that another PDEI, apremilast, improves obesity, but does not improve blood glucose levels. 5 Here, we investigated the effect of apremilast on insulin resistance in Japanese patients with psoriasis.
This study was approved by the Institutional Review Board of Nagoya City University (approval no. 60‐18‐0030). The study was a single‐center, retrospective, observational study performed from April 2017 to December 2017. Eighty‐six patients with skin psoriasis who were prescribed apremilast during the study period were enrolled. The laboratory data (glucose, HbA1c, insulin, and eicosapentaenoic acid/arachidonic acid [EPA/AA] ratio) at weeks 0 (baseline), 16, and 32 of apremilast treatment were investigated and analyzed using GraphPad Prism 8. The patients were asked to fast prior to having their blood drawn.
Patients enrolled in the study were mostly men (75.6%) with a median age of 62 years. Among the 86 patients, 11 were being treated for diabetes, and two of those were on insulin therapy. The median baseline glucose level was 102 mg/dL, HbA1c 5.7%, insulin 11.6 μU/mL (normal, 2–10 μU/mL; insulin resistance, ≥15 μU/mL), Homeostatic Model Assessment for Insulin Resistance (HOMA‐IR) 3.45 (normal, ≥1.6; insulin resistance, >2.5), and EPA/AA ratio 0.23 (normal, 0.05–0.61). The distribution of the body surface area covered by psoriatic plaques (BSA) is shown in Figure 1a, with 57.2% of the patients having a BSA of more than 20%. Consistent with our previous findings, 2 HbA1c levels correlated with the PASI, though this seemed to depend on the BSA. In the present study, PASI did not correlate with HbA1c among the total population, but significantly correlated with HbA1c in the group of patients with a BSA of 40% or more (Figure 1b). Changes in the serum insulin levels after apremilast treatment are shown in Figure 1c). In a group of clearly insulin‐resistant patients with insulin levels of 15 μU/mL or more, apremilast treatment significantly reduced the fasting serum insulin levels, and HOMA‐IR was also significantly reduced. In the group with low insulin levels (<15 μU/mL), apremilast treatment significantly increased the fasting serum insulin levels. BSA did not correlate with insulin levels of 15 or more and less than 15 μU/mL.
FIGURE 1.
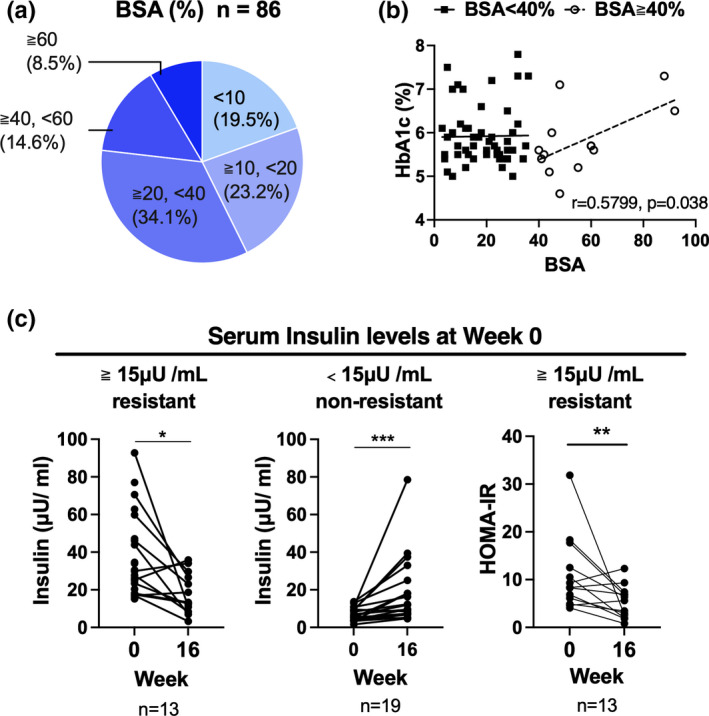
Serum insulin levels reduced after apremilast treatment. (a) Body surface area covered by psoriatic plaques (BSA) distribution (n = 86). (b) Scatter plot of BSA and hemoglobin A1c (HbA1c) levels. In the BSA ≥ 40% group, Pearson’s r was 0.5799 (95% confidence interval, 0.04243–0.8570; n = 13). (c) Changes in the serum insulin levels or Homeostatic Model Assessment for Insulin Resistance (HOMA‐IR) after apremilast treatment (week 16) in patients whose serum insulin levels were <15 (n = 13) and ≥15 μU/mL (n = 13) at week 0. The results were analyzed using the Wilcoxon matched‐pairs signed rank test. *p < 0.05. Scatter plot of BSA and insulin levels at week 0. Pearson’s r was 0.2186 and −0.0044 in patients whose serum insulin levels were <15 (n = 35) and ≥15 μU/mL (n = 42) at week 0, respectively
Previous reports indicated that PDE4 inhibitors affect glucose metabolism, and the present report extends these findings to show that treatment with a PDE4 inhibitor reduces fasting serum insulin levels in psoriasis patients with insulin resistance. Furthermore, fasting serum insulin levels and HOMA‐IR, an indicator of insulin resistance, were higher in psoriasis patients than the reference values. In conclusion, insulin resistance in patients with psoriasis may be improved by treatment with the PDE4 inhibitor apremilast.
REFERENCES
- 1. Lee MS, Lin RY, Lai MS. Increased risk of diabetes mellitus in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population‐based cohort study. J Am Acad Dermatol. 2014;70:691–8. [DOI] [PubMed] [Google Scholar]
- 2. Ikumi K, Odanaka M, Shime H, Imai M, Osaga S, Taguchi O, et al. Hyperglycemia is associated with psoriatic inflammation in both humans and mice. J Invest Dermatol. 2019;139:1329–38.e7. [DOI] [PubMed] [Google Scholar]
- 3. Wu C, Rajagopalan S. Phosphodiesterase‐4 inhibition as a therapeutic strategy for metabolic disorders. Obes Rev. 2016;17:429–41. [DOI] [PubMed] [Google Scholar]
- 4. Wouters EF, Bredenbröker D, Teichmann P, Brose M, Rabe KF, Fabbri LM, et al. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment‐naive, newly diagnosed type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:E1720–5. [DOI] [PubMed] [Google Scholar]
- 5. Dal Bello G, Gisondi P, Idolazzi L, Girolomoni G. Psoriatic arthritis and diabetes mellitus: a narrative review. Rheumatol Ther. 2020;7:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


