Abstract
Purpose
To report the baseline prevalence of myopia among school children in Tamil Nadu, South India from a prospective cohort study.
Methods
Children between the ages of 5 and 16 years from 11 schools in two districts of Tamil Nadu underwent vision screening. All children underwent visual acuity assessment using a Pocket Vision Screener followed by non‐cycloplegic open‐field autorefraction (Grand Seiko WAM‐5500). Myopia was defined as a spherical equivalent (SE) refraction of ≤−0.75 D and high myopia was defined as SE ≤ −6.00 D. Distribution of refraction, biometry and factors associated with prevalence of myopia were the outcome measures.
Results
A total of 14,699 children completed vision screening, with 2% (357) of them having ocular abnormalities other than refractive errors or poor vision despite spectacle correction. The remaining 14,342 children (7557 boys; 52.69%) had a mean age of 10.2 (Standard Deviation [SD] 2.8) years. A total of 2502 had myopia in at least one eye, a prevalence of 17.5% (95% CI: 14.7–20.5%), and 74 (0.5%; 95% CI: 0.3–0.9%) had high myopia. Myopia prevalence increased with age (p < 0.001), but sex was not associated with myopia prevalence (p = 0.24). Mean axial length (AL; 23.08 (SD = 0.91) mm) and mean anterior chamber depth (ACD; 3.45 (SD = 0.27) mm) positively correlated with age (p < 0.001). The mean flat (K1; 43.37 (SD = 1.49) D) and steep (K2; 44.50 (SD = 1.58) D) corneal curvatures showed negative correlation with age (p = 0.02 and p < 0.001, respectively). In the multivariable logistic regression, older age and urban school location had higher odds for prevalence of myopia.
Conclusion
The baseline prevalence of myopia among 5‐ to 16‐year‐old children in South India is larger than that found in previous studies, indicating that myopia is becoming a major public health problem in this country.
Keywords: myopia, prevalence, refraction, school children, South India
Key Points.
To the best of our knowledge, for the first time, a large cohort of school children in India were screened with both refraction and ocular biometry measurements being analysed.
The prevalence of myopia was noted to be higher among children studying in urban school locations indicating a role for the environment in modifying the prevalence of myopia.
One out of four children had myopia at the age of 15, an increase from previous studies, implying that myopia is becoming a major public health problem in the country.
INTRODUCTION
Myopia among children is a growing public health concern worldwide with a very high prevalence found among children in Asian countries, especially East Asia. 1 High myopia is associated with various sight threatening conditions like myopic maculopathy, glaucoma and retinal detachment. 2 , 3 Even low degrees of myopia are associated with these complications. 3 Furthermore, predictions suggest that 50% of the global population will have myopia by 2050, with almost 10% having high myopia. 4
Despite the wealth of data regarding myopia prevalence in south east Asian countries such as Singapore, Japan, Hong Kong, Taiwan and China, few studies have reported the prevalence of myopia and its risk factors in Indian populations. In India, the recent studies that have reported the prevalence of myopia and its risk factors have been restricted to the northern regions. 5 , 6 Investigations in North India reported the prevalence of myopia to be 13%, 5 and 21% 6 among children 5–15 years of age, which is lower than the prevalence of myopia seen in other Asian ethnicities 7 but higher than that reported in earlier decades in India. An earlier study in North India reported a myopia prevalence of 7% among 5‐ to 15‐year‐old school children, 8 while studies from other regions reported a prevalence of myopia between 3% and 10% in a similar age group. 9 , 10 , 11 , 12
With a population of one billion, of which 30% are under 15 years of age, India has one of the largest child populations in the world. 13 Up‐to‐date studies examining the rising prevalence of myopia and its risk factors among Indian children across various regions are limited; previous studies were done more than a decade ago. 9 , 10 , 11 , 12 Therefore, it becomes essential to understand the current and changing trends in myopia prevalence and the factors associated with it.
The Sankara Nethralaya Tamil Nadu Essilor Myopia (STEM) study is a longitudinal investigation designed to assess the prevalence, incidence and risk factors associated with myopia among school children between the ages of 5 and 16 years in South India. This article presents the methodology, baseline demographic characteristics and prevalence estimates of myopia among school children in Tamil Nadu, South India.
METHODS
Study design
The STEM study is a prospective, school‐based, longitudinal epidemiological cohort study with an aim to examine and follow 15,000 school children from grades 1 to 10 (aged 5–16 years) in the districts of Chennai and Tiruvallur in Tamil Nadu, a state of South India. Chennai is the state capital and is completely urban, while Tiruvallur is an adjacent district with a mix of both rural and urban settings. According to the Chennai District Human Development Report 2017, 14 the percentage of children enrolled in primary school education is 100%. The percentage of 6 to 14 year old children enrolled in education in the Tiruvallur district is also high, at 99.92%. 15 Therefore, the selection of schools in these districts provided an ideal representation of the child population in the state. Out of the identified schools located close to the Sankara Nethralaya Hospital (a total of 114 schools within 50 km from the base hospital), 20 schools were chosen based on simple randomisation. A total of 11 schools, six from Chennai and five from Tiruvallur district, consented to participate and were included in the study. According to the 2011 Census of India, a region is classified as urban if the minimum population is 5000, 75% of the male working population is engaged in non‐agricultural activities and the region has a minimum population density of 400 people per square kilometre. 16 , 17 In Tamil Nadu, there are regions called town panchayats, which are classified as a transitional zone between urban and rural regions, where the population is more than 5000 and has an annual income of not less than one lakh (100,000) rupees (approximately US$ 1400). 18 Based on this definition, four schools in the Tiruvallur district that formed the suburbs of Chennai city were classified as suburban location schools and seven schools (one from Tiruvallur and six from Chennai city) were classified as urban location schools.
Recruitment modality
Prior to the commencement of the STEM study, investigators met formally with the principal, teachers and parents of the participating schools and explained the purpose and relevance of the study. A written informed consent form that provided details of the study protocol, both in English and the local vernacular Tamil, was distributed to all children through their class teachers. Only children whose parents provided signed consent for enrolling their child in the study underwent the detailed examinations.
Sample size
The sample size required to determine a precise estimate of myopia prevalence among children aged between 5 and 15 years in South India was calculated. We assumed the prevalence to be 13% based on the estimates from the North India Myopia (NIM) Study. 5 Assuming a myopia prevalence of 13% in the age group of 5–15 years, a design effect of 2, relative precision in calculating the prevalence to be 1%, and a response rate of 70%, the calculated sample size required was 12,414 with 95% confidence.
Process of vision screening
Data collection for the baseline assessment commenced in January 2019. A total of 11 schools were screened from January 2019 to December 2019. The entire process of the school screening programme is shown in Figure 1.
FIGURE 1.
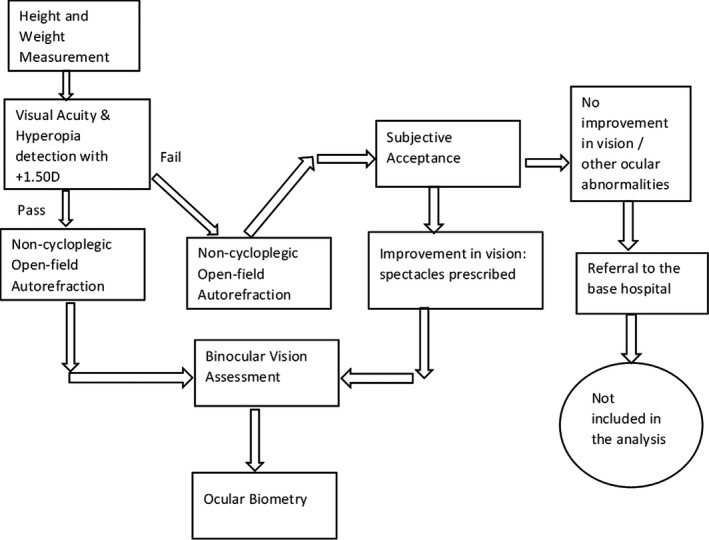
Stages and measurements of the ocular examination at the school premises in the Sankara Nethralaya Tamil Nadu Essilor Myopia (STEM) Study
Participants were provided with a unique study ID for the purpose of identifying them in the follow up period. All children underwent height and weight measurements, followed by visual acuity examination with a Pocket Vision Screener (PVS, Elite School of Optometry, eso.sankaranethralaya.org) 19 specifically designed for school vision screening programmes. The PVS has three rows of five letters sized 0.2 log MAR (6/9.5) at 3 m, and participants were considered to have passed the vision assessment if they read three out of the five letters on one line correctly. Visual acuity was assessed for each eye separately. If a participant wore spectacles, vision was assessed with the spectacles in place. If the PVS test was passed, a +1.50 D lens was introduced in front of each eye and the test was repeated to check for any latent hyperopia. Failure to read through the +1.50 D lens was considered a pass. If a participant passed the vision screening, they proceeded to binocular vision assessment and objective open‐field autorefraction measurements. Participants who failed the visual acuity testing underwent refractive correction and prescription of spectacles, at no cost to the participant. If visual acuity did not improve with spectacles or other ocular pathologies were detected, the participant was sent to the base hospital for further investigation and was excluded from the study. Participants who passed the visual acuity screening with the new spectacle prescription underwent binocular vision assessment and open‐field autorefraction measures.
Open‐field autorefraction
Non‐cycloplegic, open‐field autorefraction using the Grand Seiko WAM 5500 (Grand Seiko, grand‐seiko.com) was conducted on all participants following visual acuity assessment. Distance autorefraction measurements were made while the participant viewed a non‐accommodative Maltese cross target placed at 6 m. A series of five readings were taken and the average of the five measurements was documented as the distance refractive error. The use of this open field refractor has been validated. 20
Definition of myopia
Myopia was defined as a non‐cycloplegic refractive error with a spherical equivalent (SE) refraction of ≤−0.75 D (Dioptre) 20 in either eye. High myopia was defined as SE ≤ −6.00 D. 21
Binocular vision assessment
All participants were assessed for non‐strabismic binocular vision anomalies (NSBVA) using the near point of convergence (NPC) measurement through a red filter, heterophoria measurement with a muscle imbalance measure card and accommodative facility. These tests were identified to be the minimum test battery to screen for NSBVA in a community screening by Hussaindeen et al. 22 NPC was measured objectively using a pen torch and red filter in front of the right eye, with the end point determined by the deviation of one of the eyes indicating a loss of fusion. Horizontal heterophoria status was determined using the muscle imbalance measure card based on the modified Thorington method. 22 Participants were asked to fixate on the central white light in the muscle imbalance card with a Maddox rod held in front of the right eye, and the deviation noted based on the participant's response.
In addition, all participants with a refractive error (newly detected and habitual spectacle wearers) as well as a subset of emmetropic participants (all participants in grades 1, 4 and 6) also underwent near point of accommodation (NPA), accommodative response and accommodative facility assessment. The NPA was measured using the standard push up test, 16 whereby N8 size print was brought towards the participant until the first report of sustained blur. NPA was measured in each eye monocularly and binocularly, with measured distances converted to dioptres. Measures of accommodative response were also made with participants observing a near accommodative target (Maltese cross subtending 4° at 40 cm) through the open‐field autorefractor, with the full distance refractive correction in place. The average of five readings for each eye was taken as the accommodative response, with lag or lead of accommodation calculated by subtracting the accommodative response from the accommodative demand (+2.50 D). Accommodative facility was measured using ±2.00 D accommodative flippers monocularly in front of the right eye. 22 Three‐letter, N8 sized words at 40 cm were used as the target for participants up to grade 3, with five‐letter word grid cards used for the higher grades. The number of cycles per minute (one cycle being clearing the target through both the positive and negative lenses of the flippers) was recorded as the accommodative facility.
Ocular biometry
Ocular biometry measurements were made with a non‐contact ocular biometer (IOLMaster Version 5.4, Carl Zeiss‐Meditec, zeiss.com), and recorded as the average of five readings. All participants from the first two schools had biometry measurements, but due to logistics and time constraints, only a subset of participants in the subsequent nine schools had ocular biometry measurements taken. For these nine schools, participants with a refractive error requiring correction underwent ocular biometry measures, as well as emmetropic individuals from grades 1, 4 and 6. Axial length (AL), anterior chamber depth (ACD) and flat and steep corneal curvature (K1 and K2) measurements were recorded as the average of five readings. Overall, 7901 children (4166 male, 3735 female) had biometry measurements recorded.
Risk factors questionnaire
Baseline data for future assessment of risk factors for myopia development and progression were collected using a modified version of the Sydney myopia questionnaire. 23 Approval was obtained from the investigators of the Sydney Myopia Study, and the questionnaire was modified to suit local cultural and ethnicity considerations through circulation to a group of 10 experts in paediatric eye care research through Delphi technique sessions. The modified versions of the parent and student questionnaires were translated into the local vernacular language of Tamil with the help of a linguistic expert.
The parent questionnaire was distributed to the parents of participants in grades 1–10 via the children by the class teachers. This was filled in by the parents and returned by the students, along with the signed parental consent form, on the day of the baseline measurements at the school. The student questionnaire was administered to the students from grades 8 to 10 at school on the day of the measurements.
Follow‐up
The study was planned as a two‐year longitudinal study to assess risk factors for myopia incidence and progression with outcome measures at 12 and 24 months. Due to the emergence of SARS‐CoV‐2 (COVID‐19) in December 2019, 24 lockdowns were implemented globally as a pandemic was declared. In Tamil Nadu, this meant that the school screening visits were unable to continue. 25 Due to the pandemic, the plan for the STEM Study outcome measures was modified to make outcome measures at 24 and 36 months (instead of 12 and 24 months), local restrictions permitting. All examinations, including the questionnaires, will be repeated for each participant at each follow‐up visit as per the baseline measures that were made.
Statistical analysis
The data analysed in the present study included the baseline demographic data, baseline refractive error data (including myopia prevalence) and baseline ocular biometric data. The binocular vision status and questionnaire data would be analysed once follow‐up data have been collected, as part of the risk factor analysis for myopia incidence and progression in the longitudinal STEM Study. Data analysis was performed with Stata Version 16.0 (StataCorp, stata.com). The sphero‐cylindrical refraction was converted to power vector form of M, J0 and J45, 26 where M equals the mean SE refraction, for the purpose of analysis. Pearson correlation test was used to compare the SE refraction between the two eyes. There was a strong correlation between the right and left eye refractions (Pearson correlation of 0.90; p < 0.001). Therefore, only the right eye data were analysed. The prevalence of myopia was expressed as percentages with 95% confidence intervals (CI). The t‐test was used to compare two independent groups and analysis of variance (ANOVA) was used to compare means of more than two groups. Z test for proportions was used for comparison of proportions. Multivariable logistic regression analysis was carried out to identify factors associated with myopia prevalence among children. The confidence intervals and the odds ratio were adjusted for cluster sample design.
RESULTS
Of 16,436 children in the 11 schools, 14,699 (89.4%) underwent vision screening. The 1737 children who did not undergo assessment, 54.9% of whom were boys, were absent from school during the time period in which the study was conducted in each of the schools. The children who did not undergo the vision assessment were younger (mean age of 8.9 standard deviation (SD) 2.8 years) than the group of children who underwent vision assessment (mean age 10.2 (SD = 2.8) years).
Of 14,699 children who underwent screening, 357 (2.4%) were excluded from the study due to ocular abnormalities and were referred to the hospital for further management and investigation of poor vision despite full refractive correction.
Non‐cycloplegic refraction data were available for 14,342 children, of whom 52.7% (n = 7557) were boys. The mean age of the children was 10.2 (SD = 2.8) years (range 5–16 years). The overall mean SE refraction was −0.18 (SD = 1.11) D (range: −12.00 D to +9.00 D) in the right eye and −0.11 (SD = 1.13) D (range: −13.00 D to +6.00 D) in the left eye.
Prevalence of myopia and high myopia
A total of 2502 children had myopia (SE ≤ −0.75 D) in at least one eye. The prevalence of myopia in the entire sample (n = 14,342) was 17.5% (95% CI: 14.7–20.5%). Bilateral myopia was present amongst 12.5% of the sample (n = 1793; 95% CI: 9.8–15.8%), while 373 children had myopia only in the right eye and 336 had myopia only in the left eye. Only 0.5% (n = 74; 95% CI: 0.3–0.9%) of the entire sample had high myopia (≤−6.00 D), which represented a prevalence of 3% of the myopic individuals (95% CI: 2.7–4.2%).
Spherical equivalent refraction by age
One‐way ANOVA revealed a significant difference in the right eye SE refraction with age (F (9,14,332) = 68.05, p < 0.001). A post‐hoc Bonferroni test showed that SE was significantly more myopic from the age of 11 years (p < 0.001). There was a significant difference in refraction between the 5–10, 11–12 and 13–16 year age groups (post hoc Bonferroni, p < 0.001). The sample was therefore binned into the three age groups based on the one‐way ANOVA analysis, and the refraction distributions for each of the three groups are shown in Figure 2.
FIGURE 2.
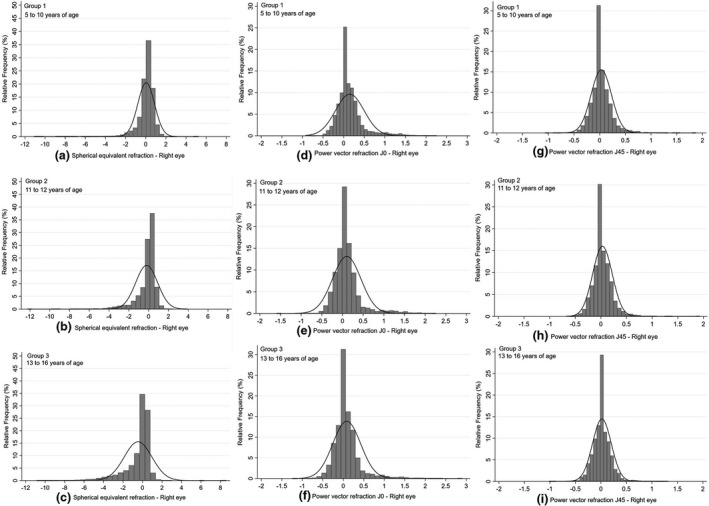
Distribution of spherical equivalent (SE) or M values (a, b, c), J0 (d, e, f) and J45 (g, h, i) in the right eye for the three age groups of children. Group 1, ages 5–10 years (a, d, g; n = 7697); Group 2, ages 11–12 years (b, e, h; n = 2926); and Group 3, ages 13–16 years (c, f, i; n = 3719). Refraction represented in dioptres
Refraction distribution and myopia prevalence in the three age groups
The mean refraction (M) in the 5‐ to 10‐year age group (group 1; n = 7697) was 0.04 (SD = 0.83) D, which decreased to −0.20 (SD = 1.11) D in the 11‐ to 12‐year age group (group 2; n = 2926) and −0.50 (SD = 1.43) D in the 13‐ to 16‐year age group (group 3; n = 3719). These differences were statistically significant (F (2,14,339) = 278.48, p < 0.001). Post‐hoc analysis showed significant differences between all the three age groups (p < 0.001). The distribution of SE is shown in Figure 2a–c.
Likewise, the prevalence of myopia increased with age, and there was a statistically significant difference in the prevalence of myopia among the three age groups. There was significantly less myopia in the 5‐ to 10‐year age group (10.2%; 95% CI: 9.6–10.9%) than either the 11‐ to 12‐year age group (p < 0.001; 16.9%, 95% CI: 15.6–18.3%) or the 13‐ to 16‐year age group (p < 0.001; 23.8%, 95% CI: 22.4–25.2%). Myopia prevalence in the 13‐ to 16‐year age group was also significantly higher than in the 11‐ to 12‐year age group (p < 0.001).
The distribution of J0 across the three age groups was significantly different (F (2,14,339) = 51.65, p < 0.001; Figure 2d–f). The mean J0 vector component decreased from 0.15 (SD = 0.35) D at the age of 5–10 years (group 1) to 0.10 (SD = 0.34) D at 11–12 years (group 2) and 0.08 (SD = 0.34) D at 13–16 years of age (group 3). Post‐hoc analysis showed a significant difference between the 5–10 and 11–12 year age groups (p < 0.001) as well as the 5–10 and 13–16 year age groups (p < 0.001). There was no statistically significant difference between the middle (11–12 years of age) and the older age (13–16 years of age) groups (p = 0.11).
The distribution of J45 among the three age groups is shown in Figure 2g–i. There was a significant difference in the J45 refraction among the three groups (F (2,14,339) = 4.83; p = 0.01). One‐way ANOVA showed that the differences were significant between the 5–10 and 13–16 year age groups (p = 0.01), but not between the other groups.
Refraction by sex
Overall, the mean spherical equivalent refraction was not significantly different between boys (−0.14 [SD = 1.07] D, n = 7557) and girls (−0.14 [SD = 1.12] D, n = 6785; p = 0.94). The prevalence of myopia among boys was slightly lower at 14.8% (95% CI: 14.0–15.6%) compared to the girls (15.5%; 95% CI: 14.6–16.3%), but was not significantly different (p = 0.24). There was a statistically significant difference in J0 (mean difference 0.02 (SE 0.01) D; p = 0.01) and J45 (mean difference 0.02 (SE 0.003) D; p < 0.001) between boys and girls.
Refraction and myopia prevalence based on school location
There was a statistically significant difference in the prevalence of myopia between children studying in urban (16.4%) and suburban (12.5%) school locations, with a higher prevalence observed in urban schools (p < 0.001; Table 1). The mean age in both groups were similar (10.14 [SD = 0.04] years in the suburban vs. 10.25 [SD = 0.03] years in the urban group). The ages ranged from 5 to 16 years in both school locations. There was no difference in distribution with sex between the school locations (z test for proportions; p = 0.38).
TABLE 1.
Refraction and prevalence of myopia based on the location of schools
| School location | N | SE (D) | J0 (D) | J45 (D) | Myopia prevalence (%) |
|---|---|---|---|---|---|
| Urban | 9611 |
−0.19 (SD = 1.16) 95% CI (−0.22 to −0.17) |
0.13 (SD = 0.36) 95% CI (0.12–0.14) |
0.03 (SD = 0.20) 95% CI (0.03–0.04) |
16.4 95% CI (15.6–17.1) |
| Suburban | 4731 |
−0.03 (SD = 0.93) 95% CI (−0.06 to −0.003) |
0.10 (SD = 0.32) 95% CI (0.09–0.11) |
0.02(SD = 0.19) 95% CI (0.01–0.02) |
12.5 95% CI (11.6–13.5) |
| P Value | <0.001* | <0.001* | <0.001* | <0.001** |
Abbreviations: D, dioptres; J0, astigmatic power vector component; J45, oblique astigmatic power vector component; SE, mean spherical equivalent refraction.
*Independent samples t‐test, **Z test for proportions ‐ difference between urban and suburban children.
On further analysis by age, the mean difference in SE between suburban and urban locations was 0.04 D (SD = 0.83 D) among the 5‐ to 10‐year‐old group, 0.24 D (SD = 1.05 D) in the 11‐ to 12‐year‐old group and 0.26 D (SD = 1.36 D) in the 13‐ to 16‐year‐old group. The differences in SE refraction among the three age groups between the locations were statistically significant (p < 0.001), whereas there were no significant differences for astigmatic components J0 (p = 0.15) and J45 (p = 0.07) in the age groups between the two locations.
Distribution of ocular biometry parameters between different ages and sexes
Overall, ocular biometry data were available for 7901 children. A total of 1941 of them had myopia (24.57%; SE ≤ −0.75 D). The mean AL was 23.08 (SD = 0.91) mm (range 20.08–28.66 mm); the mean ACD was 3.45 (SD = 0.27) mm (range 2.37–4.6 mm) and the mean K1 and K2 were 43.37 (SD = 1.49) D (range 38.31–49.94 D) and 44.50 (SD = 1.58) D (range 39.06–51.29 D), respectively (Figure 3).
FIGURE 3.
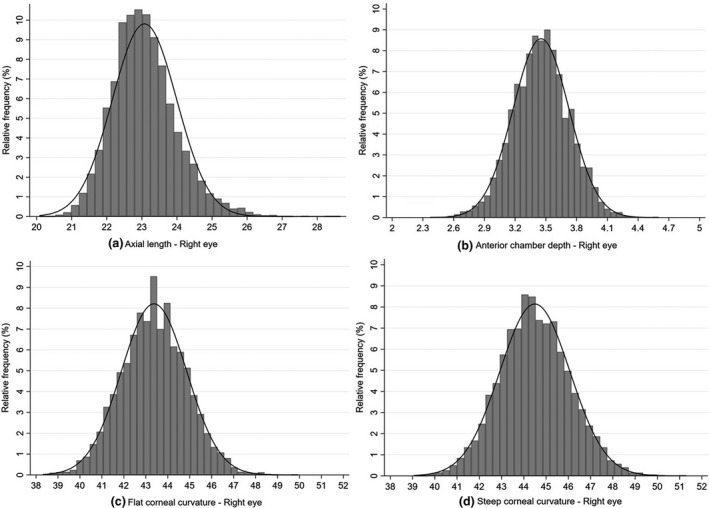
Distribution of ocular biometry parameters of the right eye in a sub‐group of the sample (n = 7901; consisting of all children in the first two schools, and all children with a refractive error requiring correction plus all emmetropic children from grades 1, 4 and 6 from the other nine schools) for (a) Axial length (in mm); (b) Anterior chamber depth (in mm); (c) flattest corneal meridian (K1) (in dioptres) and (d) steepest corneal meridian (K2) (in dioptres)
There was a significant difference in AL (F (2,7898) = 887.99; p < 0.001), ACD (F (2,7898) = 447.47; p < 0.001) and K2 (F (2,7898) = 15.44; p < 0.001) between the three age groups (Table 2). Post‐hoc analysis showed significant differences between all the three age groups for AL and ACD (p < 0.001). There was no significant difference noted in K2 between groups 2 and 3 (p = 0.20). There was no significant difference noted between the age groups for K1 (p = 0.08).
TABLE 2.
Mean ocular biometry parameters for the three age groups
| Age (years) | N |
AL (mm) Mean (SD) |
ACD (mm) Mean (SD) |
K1 (D) Mean (SD) |
K2 (D) Mean (SD) |
|---|---|---|---|---|---|
| 5–10 | 4526 | 22.77 (0.78) | 3.38 (0.25) | 43.41 (1.49) | 44.56 (1.58) |
| 11–12 | 1960 | 23.25 (0.82) | 3.51 (0.26) | 43.32 (1.47) | 44.35 (1.55) |
| 13–16 | 1415 | 23.80 (0.99) | 3.60 (0.27) | 43.35 (1.48) | 44.45 (1.58) |
Abbreviations: ACD, anterior chamber depth; AL, axial length; D, dioptres; K1, flat corneal curvature in dioptres; K2, steep corneal curvature in dioptres.
Ocular biometry parameters and sex
Boys had a longer AL (mean difference 0.49 (SD = 0.02) mm; 95% CI: 0.45–0.52 mm), deeper ACD (mean difference 0.07 (SD = 0.01) mm; 95% CI: 0.06–0.08 mm) and flatter corneal curvatures (flat K mean difference −0.74 (SD = 0.03) D; 95% CI: −0.81 to −0.68 D; steep K mean difference −0.74 (SD = 0.03) D; 95% CI: −0.81 to −0.68 D) than girls. There was a significant difference noted in all the biometry parameters between the sexes (p < 0.001; Table 3).
TABLE 3.
Mean ocular biometry parameters of the right eye between boys and girls
| Parameters | Boys | Girls | p Value* |
|---|---|---|---|
| AL (mm) | |||
| Mean (SD) | 23.31 (0.01) | 22.82 (0.01) | <0.001 |
| ACD (mm) | |||
| Mean (SD) | 3.49 (0.004) | 3.41 (0.004) | <0.001 |
| K1 (D) | |||
| Mean (SD) | 43.02 (0.02) | 43.77 (0.02) | <0.001 |
| K2 (D) | |||
| Mean (SD) | 44.14 (0.02) | 44.89 (0.03) | <0.001 |
Abbreviations: AL, axial length; ACD, anterior chamber depth; D, dioptres; K1, flat corneal curvature in dioptres; K2, steep corneal curvature in dioptres.
Independent samples t‐test.
Correlation of ocular biometry parameters with age and refraction
There was a significant increase in AL and ACD with age (p < 0.001), whereas K1 and K2 decreased with age (Table 4). There was a strong correlation between SE refraction and AL and a moderate correlation with ACD.
TABLE 4.
Pearson correlation between ocular biometry parameters, refraction and age in the study sample
| Parameters | AL (mm) | ACD (mm) | K1 (D) | K2 (D) |
|---|---|---|---|---|
| Age (in years) | 0.46 | 0.37 | −0.03 | −0.06 |
| p < 0.001 | p < 0.001 | p = 0.02 | p < 0.001 | |
| SE (D) | −0.61 | −0.33 | −0.05 | −0.16 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| J0 (D) | 0.06 | −0.11 | −0.16 | 0.25 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| J45 (D) | 0.05 | −0.07 | −0.13 | 0.07 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
Abbreviations: ACD, anterior chamber depth; AL, axial length; D, dioptres; J0, astigmatic power vector component; J45, oblique astigmatic power vector component; K1, flat corneal curvature; K2, steep corneal curvature; SE, mean spherical equivalent refraction.
Ocular biometry parameters between myopes and non‐myopes
There was a statistically significant difference noted in all of the biometry parameters between myopes and non‐myopes. Myopes had longer axial length, deeper anterior chamber and steeper corneal curvatures than non‐myopes (Table 5).
TABLE 5.
Mean ocular biometry parameters between myopes and non‐myopes
| Ocular biometry | Myopes | Non‐myopes | p Value* |
|---|---|---|---|
| AL (mm) | |||
| Mean (SD) | 23.87 (0.02) | 22.82 (0.01) | <0.001 |
| ACD (mm) | |||
| Mean (SD) | 3.60 (0.01) | 3.41 (0.003) | <0.001 |
| K1 (D) | |||
| Mean (SD) | 43.46 (0.03) | 43.35 (0.02) | 0.004 |
| K2 (D) | |||
| Mean (SD) | 44.95 (0.04) | 44.35 (0.02) | <0.001 |
Abbreviations: ACD, anterior chamber depth; AL, axial length; D, dioptres; K1, flat corneal curvature in dioptres; K2, steep corneal curvature in dioptres.
Independent samples t‐test.
Factors influencing the prevalence of myopia
In the multivariate model, the odds for myopia increased with age and urban school location. Adjusting for age, gender and clustering of the schools, the odds for urban school location was 2.75 (95% CI: 1.17–6.44; p = 0.02). Previous studies have shown that as axial length changes with both age and gender, it is both correlated and acts as a confounder with refractive error. 27 The results of the multivariate model including axial length show that older age (13–16 years), urban location and axial length all contribute significantly to the odds of developing myopia in our cohort (Table 6). Adjusting for axial length also results in sex becoming significant in the model (odds ratio 1.06 without adjusting for axial length; 95% CI: 0.97–1.15; p = 0.18).
TABLE 6.
Factors associated with myopia prevalence – univariate and multivariate logistic regression analysis
| Variables | Unadjusted odds ratio | 95% CI | p Value | Odds ratio c | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age a | ||||||
| 5–10 years (ref) | – | |||||
| 11–12 years | 1.63 | 1.37–1.97 | <0.01 | 0.87 | 0.68–1.11 | 0.27 |
| 13–16 years | 6.89 | 6.04–7.86 | <0.001 | 2.61 | 1.40–4.88 | 0.003 |
| Gender a | ||||||
| Male (ref) | ||||||
| Female | 1.05 | 0.95–1.16 | 0.36 | 2.37 | 2.05–2.73 | <0.001 |
| School location a | ||||||
| Suburban (ref) | ||||||
| Urban | 2.23 | 2.04–2.54 | <0.001 | 2.53 | 1.13–5.68 | 0.02 |
| Axial length (mm) b | 4.76 | 4.11–5.11 | <0.001 | 4.97 | 4.18–5.91 | <0.001 |
Categorical variables
Continuous variable.
Odds are adjusted for clustering and all other variables in the regression model.
DISCUSSION
This study found the prevalence of myopia to be 17.5% among 14,342 children (5‐ to 16‐ years‐old) living predominantly in urban and suburban localities of Tamil Nadu, Southern India. Previous large‐scale studies of children in South India conducted 20 years ago reported a markedly lower prevalence of myopia of around 4% in both rural and urban regions for children up to 15 years of age. 9 , 10 Consistent with our finding of increasing myopia prevalence in South India, a similar trend has been observed in North India with prevalence increasing from 7% in 2002 8 to 21% in 2019 among similar age groups of children. 6 Our findings are consistent with the predicted increase in total myopia burden in South Asia from 14.4% in 2000 to 28.6% by 2020, as calculated by Holden et al. 4 Given the peak in myopia prevalence in the population expected to occur after 20 years of age, an increase in myopia prevalence from 4% to 17% in 5‐ to 15‐year‐olds in South India aligns with the predicted model. 4
Another finding from our study is the increasing prevalence of myopia with age. With increasing age, there is an increase in academic activity and near work amongst children, which could explain the increased prevalence of myopia seen with older age, as education and intense near work are both identified as key risk factors for myopia. 28 , 29 In India, the academic demands in the school system remain relatively uniform up to grade 10 (until 15 years of age). In grade 10, the demand on children increases as this marks the beginning of significantly increased academic activity and expectations. At this age, children are required to decide on their preferred area of focus for their studies and usually change schools after grade 10 depending on their interest. The levels of academic work also begin to vary between schools depending upon the area of study after the age of 15 years. For these reasons, we included children up to grade 10 to ensure relatively consistent academic demands and curriculum between all schools. Based on our results, one in four children completing high school education (grade 10) in the urban regions could be myopic. Increased time spent outdoors is considered an effective option to prevent myopia onset, 30 and raising awareness among parents and school authorities on the importance of sunlight exposure and increased outdoor time are needed to address the rising prevalence of myopia among children.
A pooled myopia prevalence of 8% (95% CI: 7.4–8.1) among children in India younger than 15 years of age was reported in a recent systematic review, but a higher prevalence of myopia among children from urban regions (18.7%; 95% CI: 17.7–19.6) was reported compared with children from rural regions (4.8%; 95% CI: 4.5–5.1). 31 This study showed a similar pattern with children whose schools were located in an urban region having a higher prevalence of myopia (16.4%), compared with children whose schools were in suburban areas (12.5%). Although the mean age of the urban school children was slightly older than the suburban cohort (mean difference 0.11 years), the adjusted odds of myopia increased to 2.5 times when children studied in schools located in urban areas as compared with children from suburban areas. The odds of myopia prevalence in the urban versus suburban cohort reduced from 2.75 to 2.53 when adjusted for axial length, which is in keeping with a confounding effect of axial length on myopia prevalence observed between different ethnic groups, 32 and the variable correlation between axial length and refraction with age and gender. 27 , 33 This suggests that while some of the increased myopia prevalence observed in urban children can be accounted for by an increase in myopia with age, a location effect is also present (3.9% greater prevalence in urban cohort, and 0.26 D more myopic mean refraction in the 13 to 16 years age group). The statistical significance of the location effect on myopia could be attributed to the large sample size, and the clinical significance and the reasons for the observed 0.26 D difference between urban and suburban cohorts in the older age group needs to be explored further. Similar observations were noted in previous studies. 1 , 34 , 35 Although the reason for this difference is not clear, even minor environmental modifications or differences could exacerbate the prevalence of myopia. 35 Studies have also found other factors of urbanisation like increased population density 36 and crowded housing 35 , 37 to be associated with myopia, independent of near and outdoor activities.
The schools included in our study had a similar curriculum and academic demands and differed only in their geographic locations. Since there is a requirement of a child's home to be located within a 1 km radius of a school zone for primary school children and within 3 km for middle school children in the state, 38 the school location can be taken as a surrogate indicator of a child's residence, and the observed difference in myopia prevalence can thus be attributed to regional differences rather than differing academic demands.
The assessment of ocular biometry parameters and their association with myopia among Indian children is another important contribution from this study, which, to the best of our knowledge, is the first to assess ocular biometry on a large‐scale among children in India. We found a significant increase in mean axial length with age from 22.47 mm at the age of 5–6 years to 23.80 mm at the age of 13–16 years. This increasing trend of axial length with age is similar to other studies reporting ocular biometry distribution among children. 39 , 40 , 41 There was no significant difference noted in corneal curvature with age, and a decreasing trend was noted in the astigmatic component J0 with the mean towards with‐the‐rule astigmatism. There was no change in the oblique astigmatic component J45 with age. This is in agreement with previous studies that have assessed trends of astigmatism among children. 42 , 43 , 44
There were also differences in ocular biometry parameters between boys and girls. In the present study, boys had a significantly longer axial length and deeper anterior chamber when compared with girls. This observation is consistent with ocular biometry parameters assessed in other ethnicities. 39 , 40 , 41 In spite of differences in ocular biometry between sexes, we did not find any significant difference in the prevalence of myopia or SE refraction between boys and girls. This is in contrast to the NIM study, 5 where female sex was associated with an increased prevalence of myopia, whereas in the study by Singh et al., 6 boys had a significantly higher prevalence of myopia than girls. In the NIM study, girls spent less time outdoors and engaged in increased near work when compared with boys, and in the study by Singh et al., boys spent more time playing video games. Hence, these differences could be related to the environmental and behavioural differences between the sexes rather than a true biological cause. Longitudinal data from the STEM study will enable future investigation of these parameters in this cohort.
In the present investigation, high myopia (≤−6.00 D) was present in 3% (95% CI: 2.7–4.1%) of the myopic study population. There are very few recent studies that have reported the prevalence of high myopia among school children in India, with none in South India. The prevalence of high myopia (1.5%) in North India 5 was lower than the present findings. Nevertheless, an increase in prevalence of myopia with no preventive or myopia control strategies would likely result in an increase in the prevalence of high myopia. This again emphasises the need for implementing public health policies and control strategies in the community.
Moreover, the results of this cohort represent the baseline data of a longitudinal study. The results of longitudinal data can be influenced by changes in the lifestyle of children in the country brought by the nationwide lockdown due to the global COVID‐19 pandemic. 45 , 46 We are currently investigating the changes in the lifestyle habits of the children enrolled in our study before and after COVID‐19 lockdown. We plan to recommence the school‐based study once the pandemic subsides. This will help us to understand the change in refraction and ocular status due to COVID‐19.
There are a few limitations to our study. India is a country with large geographic variation and the lifestyle and habits of people also differ based on the region of residence. Since myopia is multifactorial, with both genetic and environmental components, our results may not be generalizable to other parts of the country. 47 , 48 Considering a sample of 1000 children per school and the annual follow up required as per the study design, we could include a maximum of 12 schools. Anticipating non‐participation by some invited schools due to practical reasons (a time period of three weeks to one month was required to complete one school) we randomly selected 20 schools, and the inclusion of only those schools that gave consent could have introduced some clustering bias into our results. Although the specific reasons for non‐participation was not collected, it is possible that the school management did not provide consent owing to the need to provide space for the screening in the school campus and the need to remove children from their classes during the school hours. 49 The within school participation rate was still higher than the anticipated rate (89% actual vs. 70% expected) and the reason for non‐participation of students within a school was mainly due to children being absent during the screening. We included schools from predominantly urban regions, and the prevalence reported in the study is therefore reflective of urban regions of South India. Another limitation is the use of non‐cycloplegic refraction for estimating the prevalence of myopia, which could cause overestimation of myopia prevalence. However, we previously found that using an open‐field autorefractor with a higher threshold of SE ≤ −0.75 dioptres to define myopia agreed well with the cycloplegic refraction prevalence estimates 20 ; hence the prevalence estimates reported here can be considered to be in line with prevalence estimates under cycloplegia. A further drawback was the logistical limitation of collecting biometric data on all participants. We prioritised biometry collection for those participants who displayed a myopic refraction at baseline to better understand how their baseline measurements correlated with refractive changes longitudinally. We also collected representative data from emmetropic participants in grades 1, 4 and 6. While we can make inferences about the biometric differences in our study, extrapolation to the whole population is a limitation of the study that must be approached cautiously due to the sub‐sampling employed in our protocol.
To conclude, our baseline study confirmed the changing trend of increasing myopia prevalence amongst 5‐ to 16‐year‐old children in South India, indicating that myopia is becoming a major public health problem in the country. Preventive measures and increasing awareness regarding myopia among the general public and stakeholders are needed to curb this rise. Future longitudinal data collected as part of the STEM Study will enable the investigation of factors associated with myopia onset and progression in children, and will help to improve the understanding of risk factors for the development of myopia.
CONFLICT OF INTEREST
Dr Yee Ling Wong is an employee of the funder (Essilor International, Singapore) and was involved in developing the methodology and reviewing the manuscript. The author reports no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
AUTHOR CONTRIBUTION
Aparna Gopalakrishnan: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Funding acquisition (supporting); Investigation (lead); Methodology (lead); Project administration (equal); Resources (equal); Validation (lead); Visualization (lead); Writing – original draft (lead); Writing – review & editing (lead). Jameel Rizwana Hussaindeen: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Supervision (equal); Validation (equal); Writing – original draft (lead); Writing – review & editing (lead). Viswanathan Sivaraman: Conceptualization (supporting); Funding acquisition (equal); Methodology (equal); Project administration (equal); Supervision (supporting); Writing – original draft (supporting); Writing – review & editing (supporting). Meenakshi Swaminathan: Conceptualization (equal); Methodology (equal); Resources (equal); Writing – original draft (supporting); Writing – review & editing (supporting). Yee Ling Wong: Funding acquisition (lead); Methodology (equal); Project administration (supporting); Writing – original draft (supporting); Writing – review & editing (supporting). James A Armitage: Conceptualization (equal); Methodology (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Alex Gentle: Conceptualization (equal); Methodology (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Simon Backhouse: Conceptualization (equal); Formal analysis (supporting); Methodology (equal); Supervision (lead); Writing – original draft (lead); Writing – review & editing (lead).
ACKNOWLEDGEMENTS
We acknowledge and thank Dr. Sudharsanam Manni Balasubramaniam, Global Health Director, SCOPE, Helsinki, Finland for his input with the statistical analysis.
Gopalakrishnan A, Hussaindeen JR, Sivaraman V, et al. Prevalence of myopia among urban and suburban school children in Tamil Nadu, South India: findings from the Sankara Nethralaya Tamil Nadu Essilor Myopia (STEM) Study. Ophthalmic Physiol Opt 2022;42:345–357. doi: 10.1111/opo.12943
Funding information
This work was supported by Essilor International, Singapore.
REFERENCES
- 1. Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta‐analysis: implications for aetiology and early prevention. Br J Ophthalmol 2016;100:882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saw SM, Gazzard G, Shih‐Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt 2005;25:381–91. [DOI] [PubMed] [Google Scholar]
- 3. Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012;31:622–60. [DOI] [PubMed] [Google Scholar]
- 4. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123:1036–42. [DOI] [PubMed] [Google Scholar]
- 5. Saxena R, Vashist P, Tandon R, et al. Prevalence of myopia and its risk factors in urban school children in Delhi: the North India myopia study (NIM study). PLoS One 2015;10:e0117349. 10.1371/journal.pone.0117349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh NK, James RM, Yadav A, et al. Prevalence of Myopia and associated risk factors in schoolchildren in North India. Optom Vis Sci 2019;96:200–5. [DOI] [PubMed] [Google Scholar]
- 7. Ding B‐Y, Shih Y‐F, Lin LLK, Hsiao CK, Wang IJ. Myopia among schoolchildren in East Asia and Singapore. Surv Ophthalmol 2017;62:677–97. [DOI] [PubMed] [Google Scholar]
- 8. Murthy G, Gupta SK, Ellwein LB, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci 2002;43:623–31. [PubMed] [Google Scholar]
- 9. Dandona R, Dandona L, Srinivas M, et al. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci 2002;43:615–22. [PubMed] [Google Scholar]
- 10. Dandona R, Dandona L, Naduvilath TJ, et al. Refractive errors in an urban population in Southern India: the Andhra Pradesh Eye Disease Study. Invest Ophthalmol Vis Sci 1999;40:2810–8. [PubMed] [Google Scholar]
- 11. Kalikivayi V, Naduvilath TJ, Bansal AK, Dandona L. Visual impairment in school children in Southern India. Indian J Ophthalmol 1997;45:129–34. [PubMed] [Google Scholar]
- 12. Padhye AS, Khandekar R, Dharmadhikari S, et al. Prevalence of uncorrected refractive error and other eye problems among urban and rural school children. Middle East Afr J Ophthalmol 2009;16:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Government of India Ministry of Statistics and Programme Implementation . Children in India 2018 – a statistical appraisal; 2018. Available at: http://www.mospi.gov.in/download‐reports?main_cat=NzI2&cat=All&sub_category=All Accessed April 8, 2020.
- 14. State Planning Commission Tamil Nadu . District human development report 2017 – Chennai district; 2017. Available at: http://www.spc.tn.gov.in/tnhdr2017.html Accessed April 23, 2020.
- 15. State Planning Commission Tamil Nadu . District human development report 2017 – Tiruvallur district; 2017. Available at: http://www.spc.tn.gov.in/tnhdr2017.html Accessed April 23, 2020.
- 16. Hussaindeen JR, George R, Swaminathan M, et al. Binocular vision anomalies and normative data (BAND) in Tamil Nadu–study design and methods. Vis Dev Rehabil 2015;1:260–70. [Google Scholar]
- 17. Census of India 2011 . Provisional Population Totals Urban Agglomerations and Cities. Available at: censusindia.gov.in Accessed April 23, 2020.
- 18. Government of Tamil Nadu . Directorate of Town Panchayats. Available at: www.tn.gov.in/dtp/introduction.htm Accessed February 23, 2021.
- 19. Raja M, Ramamurthy D, Srinivasan K, Varadharajan LS. Development of Pocket Vision Screener and its effectiveness at screening visual acuity deficits. Indian J Ophthalmol 2014;62:1152. 10.4103/0301-4738.149137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gopalakrishnan A, Hussaindeen JR, Sivaraman V, et al. The Sankara Nethralaya Tamil Nadu Essilor Myopia (STEM) study‐defining a threshold for non‐cycloplegic myopia prevalence in children. J Clin Med 2021;10:1215. 10.3390/jcm10061215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flitcroft DI, He M, Jonas JB, et al. IMI – defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 2019;60:M20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussaindeen JR, Rakshit A, Singh NK, et al. The minimum test battery to screen for binocular vision anomalies: report 3 of the BAND study. Clin Exp Optom 2018;101:281–7. [DOI] [PubMed] [Google Scholar]
- 23. Ojaimi E, Rose KA, Smith W, et al. Methods for a population‐based study of myopia and other eye conditions in school children: the Sydney Myopia study. Ophthalmic Epidemiol 2005;12:59–69. [DOI] [PubMed] [Google Scholar]
- 24. Coronaviridae Study Group of the International Committee on Taxonomy of V . The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol 2020;5:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vashist P, Senjam SS, Gupta V, et al. Community eye‐health and vision center guidelines during COVID‐19 pandemic in India. Indian J Ophthalmol 2020;68:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci 1997;74:367–75. [DOI] [PubMed] [Google Scholar]
- 27. Ip JM, Huynh SC, Kifley A, et al. Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol Vis Sci 2007;48:4846–53. [DOI] [PubMed] [Google Scholar]
- 28. Mountjoy E, Davies NM, Plotnikov D, et al. Education and myopia: assessing the direction of causality by Mendelian randomisation. Br Med J (Clin Res Ed) 2018;361:k2022. 10.1136/bmj.k2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children—a systematic review and meta‐analysis. PLoS One 2015;10:e0140419. 10.1371/journal.pone.0140419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta‐analysis and systematic review. Acta Ophthalmol 2017;95:551–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheeladevi S, Seelam B, Nukella PB, et al. Prevalence of refractive errors in children in India: a systematic review. Clin Exp Optom 2018;101:495–503. [DOI] [PubMed] [Google Scholar]
- 32. Rudnicka AR, Owen CG, Nightingale CM, Cook DG, Whincup PH. Ethnic differences in the prevalence of myopia and ocular biometry in 10‐ and 11‐year‐old children: the Child Heart and Health Study in England (CHASE). Invest Ophthalmol Vis Sci 2010;51:6270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ojaimi E, Rose KA, Morgan IG, et al. Distribution of ocular biometric parameters and refraction in a population‐based study of Australian children. Invest Ophthalmol Vis Sci 2005;46:2748–54. [DOI] [PubMed] [Google Scholar]
- 34. Dong L, Kang YK, Li Y, Wei WB, Jonas JB. Prevalence and time trends of myopia in children and adolescents in China: a systemic review and meta‐analysis. Retina 2019;40:399–411. [DOI] [PubMed] [Google Scholar]
- 35. Ip JM, Rose KA, Morgan IG, Burlutsky G, Mitchell P. Myopia and the urban environment: findings in a sample of 12‐year‐old Australian school children. Invest Ophthalmol Vis Sci 2008;49:3858–63. [DOI] [PubMed] [Google Scholar]
- 36. Zhang M, Li L, Chen L, et al. Population density and refractive error among Chinese children. Invest Ophthalmol Vis Sci 2010;51:4969–76. [DOI] [PubMed] [Google Scholar]
- 37. Wu X, Gao G, Jin J, et al. Housing type and myopia: the mediating role of parental myopia. BMC Ophthalmol 2016;16:151. 10.1186/s12886-016-0324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Department of School Education Government of Tamil Nadu . Implementation of the right of children to free and compulsory education act 2009. Available at: http://ssa.tnschools.gov.in/ssa‐tn/rte Accessed December 26, 2020.
- 39. Li S‐M, Li S‐Y, Kang M‐T, et al. Distribution of ocular biometry in 7‐and 14‐year‐old Chinese children. Optom Vis Sci 2015;92:566–72. [DOI] [PubMed] [Google Scholar]
- 40. Hashemi H, Jafarzadehpur E, Ghaderi S, et al. Ocular components during the ages of ocular development. Acta Ophthalmol 2015;93:e74–81. [DOI] [PubMed] [Google Scholar]
- 41. Lira RPC, Arieta CEL, Passos THM, et al. Distribution of ocular component measures and refraction in Brazilian school children. Ophthalmic Epidemiol 2017;24:29–35. [DOI] [PubMed] [Google Scholar]
- 42. Read SA, Collins MJ, Carney LG. A review of astigmatism and its possible genesis. Clin Exp Optom 2007;90:5–19. [DOI] [PubMed] [Google Scholar]
- 43. Gwiazda J, Grice K, Held R, McLellan J, Thorn F. Astigmatism and the development of myopia in children. Vision Res 2000;40:1019–26. [DOI] [PubMed] [Google Scholar]
- 44. O'Donoghue L, Breslin KM, Saunders KJ. The changing profile of astigmatism in childhood: the NICER study. Invest Ophthalmol Vis Sci 2015;56:2917–25. [DOI] [PubMed] [Google Scholar]
- 45. Wang J, Li Y, Musch DC, et al. Progression of myopia in school‐aged children after COVID‐19 home confinement. JAMA Ophthalmol 2021;139:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alvarez‐Peregrina C, Martinez‐Perez C, Villa‐Collar C, et al. Impact of COVID‐19 home confinement in children’s refractive errors. Int J Environ Res Public Health 2021;18:5347. 10.3390/ijerph18105347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agarwal D, Saxena R, Gupta V, et al. Prevalence of myopia in Indian school children: meta‐analysis of last four decades. PLoS One 2020;15:e0240750. 10.1371/journal.pone.0240750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Priscilla JJ, Verkicharla PK. Time trends on the prevalence of myopia in India – a prediction model for 2050. Ophthalmic Physiol Opt 2021;41:466–74. [DOI] [PubMed] [Google Scholar]
- 49. Logan NS, Shah P, Rudnicka AR, Gilmartin B, Owen CG. Childhood ethnic differences in ametropia and ocular biometry: the Aston Eye study. Ophthalmic Physiol Opt 2011;31:550–8. [DOI] [PubMed] [Google Scholar]


