Abstract
We included 97 patients with unilateral rhegmatogenous retinal detachment (RRD) with posterior vitreous detachment who underwent vitrectomy, and examined pigmentary lesion (PL) characteristics around the sites of original tears using pre- and postoperative ultra-widefield scanning light ophthalmoscopy, green light fundus autofluorescence (FAF) imaging, and intraoperative digital video. If PL did not involve RRD, we used OCT to preoperatively assess any pathologic changes to the lesion. A total of 116 retinal tears (mean count, 1.2 ± 0.5; range, 1–4 per eye) were observed in the detached retina. Overall, 102 (88%), 63 (54%), 14 (12%), and 25 (22%) tears were accompanied by lattice degeneration (LD) or PL, both LD and PL, only LD, and only PL, respectively. In green FAF images, LD showed normal to mild-hyper fluorescence, whereas all PL showed hypofluorescence. On OCT, PL were located at the RPE level, while choroid abnormalities were unclear. In the retinal areas of 22 eyes, which were not affected by RRD, we observed PL without retinal tears; some were accompanied by vitreous traction and tractional retinal detachment. Pre-, intra-, and post-operative assessments of original flap tears suggested that PL might be a risk factor for RRD, developing alongside or separately from LD.
Subject terms: Diseases, Medical research, Risk factors, Signs and symptoms
Introduction
Rhegmatogenous retinal detachment (RRD) is a representative vitreoretinal disease caused by a hole or flap tear in the retina1–5. It is accompanied by a flap (horseshoe-like) tear and is associated with the progression of posterior vitreous detachment (PVD)6,7. In general, RRD with a flap tear rarely resolves on its own and requires surgeries in almost all cases8. The visual outcomes of RRD have improved since adjuvant agents (e.g., triamcinolone acetonide9,10 and perfluorocarbon liquids11,12) and a wide-viewing system13,14 ecame available. However, visual prognosis is poor in cases of RRD involving the macula, and those with re-detachment, long-term RRD, or RRD progressing to proliferative vitreoretinopathy.
Lattice degeneration (LD) is a retinal lesion associated with RRD2,6,15–19 that is implicated in the onset of 20% to 65% of RRD cases3,4,20–24. Ophthalmoscopically, a typical case of LD presents with a white-striped appearance and slightly elevated margin16,17. Pathological findings revealed that the retina is thin in LD and loses its layered-structure, and that the inner limiting membrane is missing and replaced by retinal glial cells16. Concentrated vitreous adheres to the margin of LD, and proliferated glial cells extend into the vitreous. In eyes with these pathological characteristics, the retina can be pulled as PVD progresses, leading to the development of retinal flap tear and RRD16,17,20,25,26.
Alongside the wide-viewing systems in vitrectomy, ultra-widefield (UWF) imaging is commonly used in clinical practice27–30. These modalities have made it easier than it was before to evaluate and treat peripheral retinal lesions. In our practice, we have noticed high incidence rates of pigmentary lesions in RRD eyes with flap tears, which may differ from those observed in typical LD, as pigmentary lesions (PLs) are often observed behind the retinal tears and may be located at the RPE or choroidal levels. Our preliminary observations suggest that this PL may coexist with typical LD in some cases. PLs not accompanied by LD may suggest a condition other than LD.
In the present study, we refer to this pigmented change observed in eyes with RRD and a flap tear as a pigmentary lesion (PL). This study aimed to elucidate the clinical features of PL and their associations with retinal flap tears and RRD in pre-, intra-, and post-operative imaging findings.
Methods
This observational study was approved by the Institutional Review Board of the Kyoto University Graduate School of Medicine (Kyoto, Japan) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained at the initial visit from each subject prior to commencing the study. The study was performed in accordance with all the relevant guidelines and regulations.
This study included patients with unilateral RRD with PVD and retinal flap tears who visited the Department of Ophthalmology, Kyoto University Hospital, between January 2019 and February 2021. We excluded patients with RRD associated with atrophic hole, atopic dermatitis, trauma, macular hole, proliferative vitreoretinopathy, hereditary vitreoretinal degeneration (e.g., Wagner syndrome); patients with recurrent RRD; and patients with media opacities that made it difficult to observe the retinal features preoperatively. A total of 97 patients met the eligibility criteria.
UWF imaging of pre- and post-operative fundus color scanning light ophthalmoscopy (SLO) and green light fundus autofluorescence (FAF) was performed using an Optos 200T× imaging system or Optos Silverstone (Optos PLC, Dunfermline, UK). In addition, we reviewed intraoperative digital video recordings of vitrectomy using a non-contact 128 diopter front lens (Resight, Carl Zeiss Meditec AG) to evaluate the vitreous, retinal flap tears, and degeneration around the tears.
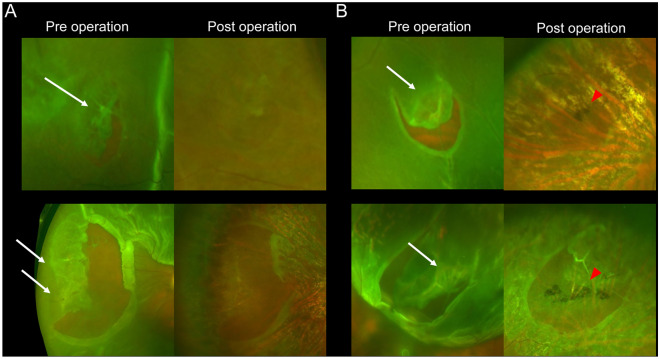
We used the pre- and postoperative UWF SLO images and intraoperative digital video images to examine the structural features of the original flap tears and degenerative lesions. Based on previous reports, we defined LD as a circumferential and well-defined lesion in the retina located from the equator to its periphery, accompanied by the whitening of retinal vessels and thinning of the retina (Fig. 1).
Figure 1.
Lattice degeneration (LD) associated with rhegmatogenous retinal detachment (RRD) examined by pre- and postoperative ultra-widefield (UWF) color scanning light ophthalmoscopy (SLO). (A) LD without a pigmentary lesion (PL) in two representative cases. (B) LD with PL in two representative cases. The preoperative UWF images show the white vessels and retinal thinning within the LD lesions. White arrows indicate LD, and red arrowheads indicate PL.
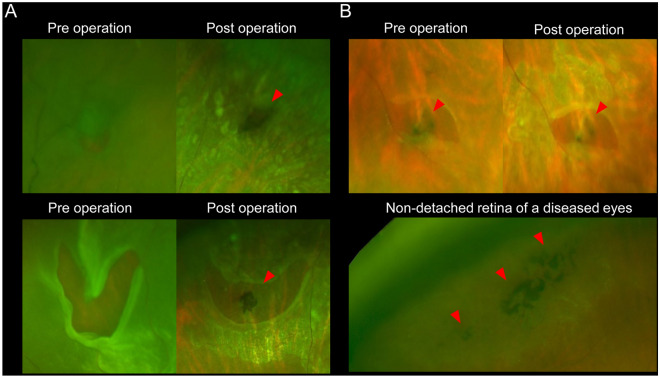
We additionally defined PLs as a pigmented change in the deep retina or behind the retinal tears, reaching from the equator to the further periphery, that were distinct from typical LD (Fig. 2). LD or PL were classified independently by two retinal specialists (MI, KK). In cases of disagreement, the senior retinal specialist (YMu) made the final decision.
Figure 2.
Pigmentary lesion (PL) of eyes with rhegmatogenous retinal detachment (RRD) examined by pre- and postoperative ultra-wide field (UWF) scanning light ophthalmoscopy (SLO). (A) Pigmentary lesion (PL) without lattice degeneration (LD) in representative two RRD-eyes. The preoperative UWF-SLO images do not clearly show the PL. However, the postoperative UWF-SLO images show the PL in and behind the retinal tears, at the retinal pigment epithelial level. In the eyes, the retinal tears do not accompany the LD lesion. (B) PL without LD in other RRD-eyes. The pre- and postoperative SLO images show the PL. Red arrowheads indicate PL.
Preoperative examination of PLs (to which the RRD did not extend) in eyes with RRD was conducted using the Silverstone OCT (Optos PLC, Dunfermline, UK), Xephilio OCT-S1 (Canon Medical Systems, Japan), and Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) devices. These OCT devices and protocol used for examination (extended field imaging in Spectralis31) can capture wide-field images of the retina and depict equatorial retinal lesions.
Statistical analysis
Statistical analysis was performed using PASW Statistics version 18.0 (SPSS, Chicago, IL). Values are presented as mean ± standard deviation. For statistical analysis, visual acuity (VA) values measured with a Landolt chart were converted to a logarithm of the minimum angle of resolution (logMAR). The Dunnett test was used to compare the respective VAs of eyes without both LD and PL, eyes with LD and without PL, eyes without LD and with PL, and eyes with both LD and PL.
Results
This study included RRD patients with PVD and retinal flap tears. Table 1 shows the clinical characteristics of the included patients. The total sample size was 97 patients (women: 27, and men: 70), and the mean age was 56.8 ± 8.5 (range: 35–77) years. We performed pars plana vitrectomy (PPV; 87 eyes received 25 gauge PPV, and 10 eyes received 27 gauge PPV) for all eyes with RRD. Sulfur hexafluoride gas was used as the tamponade agent in 93 eyes and silicon oil was used in 4 eyes. All retinal tears were blocked intraoperatively using laser photocoagulation. The retinal reattachment rate was 100% (97eyes/97 eyes).
Table 1.
Characteristics of patients with rhegmatogenous retinal detachment.
| Total number of patients (women/men) | 97 (27/70) |
| Mean age (years, range) | 56.8 ± 8.5 (35–77) |
| Duration from onset of symptom (days) | 11.0 ± 19.5 |
| Visual acuity, logMAR | 0.34 ± 0.62 |
| Snellen visual acuity (range) | 20/13–20/200 |
| Retinal detachment area, n (%) | |
| < 1 quadrant | 37 (38.1) |
| ≥ 1 to < 2 quadrants | 40 (41.2) |
| ≥ 2 to < 3 quadrants | 15 (15.5) |
| ≥ 3 to ≤ 4 quadrants | 5 (5.2) |
| Eyes with macula-off, n (%) | 39 (40.2) |
| Number of retinal tears in the detached retina, n (range) | 1.2 ± 0.49 (1–4) |
| Number of retinal tears outside of the detached retina, n (range) | 0.12 ± 0.39 (0–2) |
| Mean axial length, (mm) ± S.D | 26.3 ± 1.8 (22.8–30.8) |
| Phakia/pseudophakia, n | 81/16 |
The data are shown as the mean ± standard deviation unless otherwise indicated.
We classified participants into the following 4 groups based the presence of PL and LD, and compared the preoperative and postoperative VA among the groups (Table 2). Eyes without both LD and PL were designated as the control group. The respective pre-, and post-operative VAs of other three groups were compared to those of the control group. The preoperative VA did not differ significantly among the groups, except the postoperative VA of the group with LD and without PL, which was significantly worse than that of the control group (P = 0.044).
Table 2.
Preoperative and postoperative visual acuity based the presence or absence of lattice degeneration and pigmentary lesion.
| LD − , PL − | LD + , PL − | LD − , PL + | LD + , PL + | P * | P ** | P *** | |
|---|---|---|---|---|---|---|---|
| Preoperative VA (logMAR) | 0.06 ± 0.41 | 0.41 ± 0.42 | 0.30 ± 0.70 | 0.45 ± 0.64 | 0.35 | 0.52 | 0.18 |
| Postoperative VA (logMAR) | − 0.06 ± 0.11 | 0.13 ± 0.26 | − 0.04 ± 0.14 | 0.05 ± 0.20 | 0.044 | 1.0 | 0.19 |
The VA values are presented as the mean ± standard deviation.
VA visual acuity, logMAR logarithm of the minimum angle of resolution, LD lattice degeneration .PL pigmentary lesion.
The Dunnett test was used to compare the respective VAs of eyes without both LD and PL, eyes with LD and without PL*, eyes without LD and with PL**, and eyes with both LD PL***.
There were 116 (mean count, 1.2 ± 0.5; range, 1–4 per eye) retinal tears in the detached retina of the included eyes. One-hundred and two (88%) of the retinal tears were accompanied by LD, PL, or another type of degeneration. A total of 63 (54%), 14 (12%), and 25 (22%) retinal tears were accompanied by both LD and PL, LD only, and PL only, respectively (Supplementary Table S1).
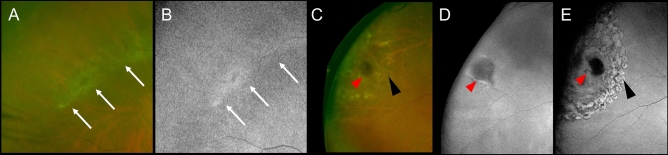
In green FAF images, LD lesions showed normal to mild-hyper fluorescence. In contrast, all PLs showed hypofluorescence (Fig. 3).
Figure 3.
Green fundus autofluorescent (FAF) images of lattice degeneration (LD) and pigmentary lesion (PL). (A) and (B) LD in a non-detached retina of an eye with rhegmatogenous retinal detachment (RRD). The ultrawide field (UWF) scanning light ophthalmoscopy (SLO) image of LD (A). The green FAF shows normal to mild-hyper fluorescence in the LD lesion (B). White arrows indicate LD. (C)–(E) The PL seen in an original flap tear of another RRD-eye. The postoperative UWF SLO image shows the PL and retinal photocoagulation (PC) scars (C). The pre- and post-operative green FAF images of the PL in the original retinal flap tear, respectively (D, E). The PL shows hypofluorescence, which is distinguished from the appearances of the laser scars around the retinal tear. Red arrowheads indicate PL. Black arrowheads indicate PC scars.
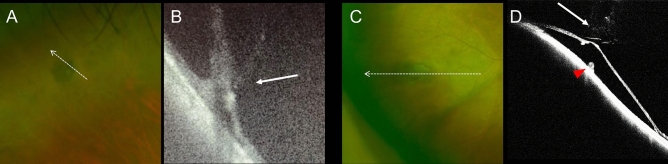
On OCT examination, PLs were located at the RPE level, which was elevated. PL-associated abnormalities were unclear on the OCT images that segmented the choroid. In the retinal areas of 22 eyes that were not affected by RRD, we observed PLs without obvious retinal tears. Some of the PLs were accompanied by vitreous traction and tractional retinal detachment (Fig. 4).
Figure 4.
Optical coherence tomography (OCT) images of pigmentary lesion (PL) at retinal areas that are not affected by rhegmatogenous retinal detachment (RRD). (A) The ultrawide field (UWF) scanning light ophthalmoscopy (SLO) image of PL. This retinal area is not affected by the RRD. (B) The OCT image of PL along the dotted arrow. Vitreous traction to the PL is seen. (C) The UWF SLO image of PL of other RRD-eyes. This retinal area is not affected by the RRD. (D) The OCT image of the PL along the dotted arrow. In addition to the vitreous traction (arrow), tractional retinal detachment and elevation of the retinal pigment epithelial level (red arrowhead) are seen.
Discussion
This study aimed to examine the characteristics of PL observed in eyes with RRD and a flap tear. The pre- and post-operative UWF imaging and intraoperative direct observation of eyes with RRD suggested that PLs were frequently present at the site of the original flap tear, which tended to occur at the level of the RPE and behind the retinal tear.
Retinal detachment is a condition in which the sensory retina detaches from the RPE. Depending on the underlying causes, retinal detachment can be divided into serous (exudative) and tractional retinal detachments, and RRD. RRD is associated with the formation of retinal holes or tears, and it has an incidence of approximately 1 per 10,000 persons per year. Retinal holes generally occur on the atrophic peripheral retina of myopic eyes and are weakly associated with PVD. In contrast, retinal flap tears occur with the development and extension of PVD. The PVD itself is an age-dependent physiological phenomenon. However, in pathologic areas with abnormal adhesions to the retina, the retina is pulled anteriorly during the progression of PVD, resulting in the formation of retinal tear, and RRD6,7.
The LD of the retina is considered a typical lesion associated with RRD. The rate of LD-related RRD has been previously reported in the range of 46.8–65.7% in Japan4,21, which was higher than the estimates reported for Europe and the United States. This discrepancy may be accounted for by the fact that myopia is more prevalent in Asia, including Japan, than elsewhere. In the present study, LD was found in 77 (66.4%) of 116 original retinal tears. The results of this study were consistent with those of previous studies in the Japanese population4,23. The LD is ophthalmoscopically characterized by circumferential degeneration of the retina with clear borders accompanied by vascular whitening and thinning of the retina16,17. The pathological features of LD are mainly seen within the retina or vitreoretinal interface, and include the absence of the inner limiting membrane, abnormality of retinal vessels16, disruption of the layered-structure16, replacement of retinal tissue by glia cells16, liquefaction of the nearby vitreous25, and strong vitreous adhesion at the degeneration edge25. Pathological changes to the RPE in LD remain unclear, which was not consistent with the presence of RPE findings in PL of the current study.
In this study, we examined the characteristics of PLs in eyes with flap tear-associated RRD based on pre-, intra-, or postoperative observations. However, we did not include RRD eyes without retinal flap tear, since most cases of RRD without flap tear are considered to be the result of retinal atrophic holes, which are commonly seen in the eyes of young patients with high myopia. Since these eyes are generally not treated with intraocular surgery (vitrectomy) but with extraocular buckling surgery, intraoperative observation is not applicable for these eyes. Hence, we excluded eyes with RRD caused by retinal hole from this study, which did not allow us to compare the frequency of PL between RRD eyes with and without flap tears. We endeavor to examine these aspects in a future study.
The postoperative VA was significantly worse in the group with LD and without PL compared to the group without both LD and PL. However, the relationship between the presence of LD or PL and postoperative VA is unclear. In general, we believe that VA in eyes with RRD is greatly influenced by whether or not the retinal detachment involves the macula.
In the present study, PL was found in more than 75% of all original tears and was co-localized with LD in 54% of all flap tears. This high rate of co-localization suggests that PL may be associated with LD. However, LD and PL were found alone in 12% and 22% of the patients, respectively, suggesting that they may be separate pathologies. The depth of PL may have reached the level of the RPE and/or choroid because the lesion was intraoperatively observed through the retinal tears and on the RPE surface. However, our OCT examination did not clearly show whether the lesion involved the choroid because the pigment of the lesions obscured the boundary between the RPE and choroid. On green FAF images, the LDs appeared as normal to slightly hyperfluorescent structures, whereas PLs appeared as hypofluorescent structures in all cases, suggesting that the two entities show different properties on FAF imaging. Overall, it is likely that the low fluorescence of PL was not due to the RPE atrophy, but rather due to the melanin pigment blocking the fluorescent substance. In summary, in this study, PL was a pigmented change located at the site of the original retinal tear and present at the RPE level, and with some features distinct from those of LD.
In this study, we obtained preoperative OCT images of PLs observed in retinal areas that were not affected by RRD. These images revealed that PL was occasionally accompanied by vitreous traction and tractional retinal detachment (a tear was not evident) (Fig. 4). We speculate that PL may have caused the vitreous adhesion and tractional retinal detachment, which in turn caused retinal flap tears and RRD. However, PL may be a secondary change that occurs after retinal detachment. Although laser photocoagulation performed for the retinal tears can cause pigmentary change around the PL, we could differentiate between the PL and the laser photocoagulation scars because we evaluated the PL preoperatively and intraoperatively. Additionally, we could also differentiate the PL from the laser photocoagulation scars postoperatively (Fig. 3). In cases where retinal detachment resolves spontaneously, we occasionally observe pigmentary changes around the edge of the detachment area16. However, serous and tractional retinal detachments are unlikely to accompany pigmentary changes; thus, it is unlikely that the observed PL was secondary to RRD. Given the high frequency of PL at the original tear sites and occasional co-occurrence of PL and tractional RD along with vitreous traction, PL might be a risk factor for RRD.
This study has some limitations. First, it was a retrospective observational study. Consequently, it remains unclear what percentage of the general population is affected by PL, and how many patients with PL progress to RRD. Second, although we used color SLO and intraoperative video findings to distinguish LD and PLs based on previous findings, the classification remains subjective. In addition, because we observed the RPE lesion through the retinal flap tears, we could not comprehensively evaluate the RPE, including the retinal areas that were not detached. Finally, because the RRD patients were often seen outside of office hours, OCT imaging of the retinal flap tears was not always performed. However, despite these limitations, the present study suggests that PL may present at RPE levels behind the original tears in eyes with RRD. These findings suggest that PL might be a risk factor for RRD, developing alongside or separately from LD. Prospective studies are required to validate these findings.
Supplementary Information
Acknowledgements
This work was supported in part by a grant-in-aid for scientific research (no. 20K09771) from the Japan Society for the Promotion of Science (Tokyo, Japan) and Canon Inc. (Tokyo, Japan). These organizations had no role in the design or conduct of this research. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Conception and design of the study, MI, YM; data analysis and interpretation, MI, YM, KK, MI, SK, NN; writing of the article, MI and YM; critical revision of the article, TK, KI, Manabu M, Miyake M, TM, SO, and AT; All authors have approved the final version of the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16508-5.
References
- 1.Wilkes SR, Beard CM, Kurland LT, Robertson DM, O'Fallon WM. The incidence of retinal detachment in Rochester, Minnesota, 1970–1978. Am. J. Ophthalmol. 1982;94:670–673. doi: 10.1016/0002-9394(82)90013-7. [DOI] [PubMed] [Google Scholar]
- 2.Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch. Ophthalmol. 1982;100:289–292. doi: 10.1001/archopht.1982.01030030291012. [DOI] [PubMed] [Google Scholar]
- 3.Törnquist R, Stenkula S, Törnquist P. Retinal detachment. A study of a population-based patient material in Sweden 1971–1981. I. Epidemiology. Acta Ophthalmol. 1987;65:213–222. doi: 10.1111/j.1755-3768.1987.tb07003.x. [DOI] [PubMed] [Google Scholar]
- 4.Ideta H, et al. Epidemiologic characteristics of rhegmatogenous retinal detachment in Kumamoto, Japan. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995;233:772–776. doi: 10.1007/BF00184088. [DOI] [PubMed] [Google Scholar]
- 5.Rowe JA, et al. Retinal detachment in Olmsted County, Minnesota, 1976 through 1995. Ophthalmology. 1999;106:154–159. doi: 10.1016/S0161-6420(99)90018-0. [DOI] [PubMed] [Google Scholar]
- 6.Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16:411–421. doi: 10.1038/sj.eye.6700197. [DOI] [PubMed] [Google Scholar]
- 7.Sebag J. Anomalous posterior vitreous detachment: A unifying concept in vitreo-retinal disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004;242:690–698. doi: 10.1007/s00417-004-0980-1. [DOI] [PubMed] [Google Scholar]
- 8.Gartry DS, Chignell MAH, Franks WA, Wong D. Pars plana vitrectomy for the treatment of rhegmatogenous retinal detachment uncomplicated by advanced proliferative vitreoretinopathy. Br. J. Ophthalmol. 1993;77:199–203. doi: 10.1136/bjo.77.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyman GA, Cheema R, Conway MD, Fang T. Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. Retina. 2000;20:554–555. doi: 10.1097/00006982-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Yamakiri K, et al. Reduced incidence of intraoperative complications in a multicenter controlled clinical trial of triamcinolone in vitrectomy. Ophthalmology. 2007;114:289–297. doi: 10.1016/j.ophtha.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Chang S, Zimmerman NJ, Iwamoto T, Ortiz R, Faris D. Experimental vitreous replacement with perfluorotributylamine. Am. J. Ophthalmol. 1987;103:29–37. doi: 10.1016/S0002-9394(14)74165-0. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Ozmert E, Zimmerman NJ. Intraoperative perfluorocarbon liquids in the management of proliferative vitreoretinopathy. Am. J. Ophthalmol. 1988;106:668–674. doi: 10.1016/0002-9394(88)90698-8. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M. Wide-angle viewing system. Dev. Ophthalmol. 2014;54:87–91. doi: 10.1159/000360453. [DOI] [PubMed] [Google Scholar]
- 14.Lai CT, et al. Outcome of primary rhegmatogenous retinal detachment using microincision vitrectomy and sutureless wide-angle viewing systems. BMC Ophthalmol. 2019;19:1–8. doi: 10.1186/s12886-019-1238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schepens CL. Subclinical retinal detachments. AMA Arch. Ophthalmol. 1952;47:593–606. doi: 10.1001/archopht.1952.01700030607005. [DOI] [PubMed] [Google Scholar]
- 16.Straatsma BR, Zeegen PD, Foos RY, Feman SS, Shabo AL. Lattice degeneration of the retina. Am. J. Ophthalmol. 1974;77:619–649. doi: 10.1016/0002-9394(74)90525-X. [DOI] [PubMed] [Google Scholar]
- 17.Byer NE. Lattice degeneration of the retina. Surv. Ophthalmol. 1979;23:213–248. doi: 10.1016/0039-6257(79)90048-1. [DOI] [PubMed] [Google Scholar]
- 18.Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96:1396–1402. doi: 10.1016/S0161-6420(89)32713-8. [DOI] [PubMed] [Google Scholar]
- 19.Lewis H. Peripheral retinal degenerations and the risk of retinal detachment. Am. J. Ophthalmol. 2003;136:155–160. doi: 10.1016/S0002-9394(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 20.Dumas J, Schepens CL. Chorioretinal lesions predisposing to retinal breaks. Am. J. Ophthalmol. 1966;61:620–621. doi: 10.1016/0002-9394(66)91198-6. [DOI] [PubMed] [Google Scholar]
- 21.Murakami-Nagasako F, Ohba N. Phakic retinal detachment associated with atrophic hole of lattice degeneration of the retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 1983;220:175–178. doi: 10.1007/BF02186664. [DOI] [PubMed] [Google Scholar]
- 22.Laatikainen L, Tolppanen EM. Characteristics of rhegmatogenous retinal detachment. Acta Ophthalmol. 1985;63:146–154. doi: 10.1111/j.1755-3768.1985.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br. J. Ophthalmol. 2010;94:678–684. doi: 10.1136/bjo.2009.157727. [DOI] [PubMed] [Google Scholar]
- 24.Mitry D, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118:1429–1434. doi: 10.1016/j.ophtha.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Foos RY, Simons KB. Vitreous in lattice degeneration of retina. Ophthalmology. 1984;91:452–457. doi: 10.1016/S0161-6420(84)34266-X. [DOI] [PubMed] [Google Scholar]
- 26.Manjunath V, Taha M, Fujimoto JG, Duker JS. Posterior lattice degeneration characterized by spectral domain optical coherence tomography. Retina. 2011;31:492–496. doi: 10.1097/IAE.0b013e3181ed8dc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witmer MT, et al. Ultra-wide-field autofluorescence imaging in non-traumatic rhegmatogenous retinal detachment. Eye. 2012;26:1209–1216. doi: 10.1038/eye.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witmer MT, Kiss S. Wide-field imaging of the retina. Surv. Ophthalmol. 2013;58:143–154. doi: 10.1016/j.survophthal.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Navaratnam J, Salvanos P, Vavvas DG, Bragadóttir R. Ultra-widefield autofluorescence imaging findings in retinoschisis, rhegmatogenous retinal detachment and combined retinoschisis retinal detachment. Acta Ophthalmol. 2021;99:195–200. doi: 10.1111/aos.14521. [DOI] [PubMed] [Google Scholar]
- 30.Choudhry N, Golding J, Manry MW, Rao RC. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology. 2016;123:1368–1374. doi: 10.1016/j.ophtha.2016.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uji A, Yoshimura N. Application of extended field imaging to optical coherence tomography. Ophthalmology. 2015;122:1272–1274. doi: 10.1016/j.ophtha.2014.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.