Abstract
Yarrowia lipolytica produces brown extracellular pigments that correlate with tyrosine catabolism. During tyrosine depletion, the yeast accumulated homogentisic acid, p-hydroxyphenylethanol, and p-hydroxyphenylacetic acid in the medium. Homogentisic acid accumulated under all aeration conditions tested, but its concentration decreased as aeration decreased. With moderate aeration, equimolar concentrations of alcohol and p-hydroxyphenylacetic acid (1:1) were detected, but with lower aeration the alcohol concentration was twice that of the acid (2:1). p-Hydroxyphenylethanol and p-hydroxyphenylacetic acid may result from the spontaneous disproportionation of the corresponding aldehyde, p-hydroxyphenylacetaldehyde. The catabolic pathway of tyrosine in Y. lipolytica involves the formation of p-hydroxyphenylacetaldehyde, which is oxidized to p-hydroxyphenylacetic acid and then further oxidized to homogentisic acid. Brown pigments are produced when homogentisic acid accumulates in the medium. This acid can spontaneously oxidize and polymerize, leading to the formation of pyomelanins. Mn2+ accelerated and intensified the oxidative polymerization of homogentisic acid, and lactic acid enhanced the stimulating role of Mn2+. Alkaline conditions also accelerated pigment formation. The proposed tyrosine catabolism pathway appears to be unique for yeast, and this is the first report of a yeast producing pigments involving homogentisic acid.
Brown discoloration is a common defect in cheese. Yarrowia lipolytica is thought to be responsible for this discoloration in traditional Portuguese ewes' cheeses (7), Camembert (11), and Gorgonzola-type cheeses (27). The spoilage activity of this species seems to be related to its ability to produce brown pigments from tyrosine (5), but little is known of the mechanism involved.
Brown pigments produced from tyrosine are known as melanins. This is a general term that includes a wide variety of complex polyphenolic heteropolymers. Microorganisms may form melanin via l-tyrosine catabolism (8, 13, 32, 33) or a tyrosinase (EC 1.14.18.1)-mediated pathway (17, 21, 26, 29, 31, 37). In bacteria, tyrosine is degraded via pathways that involve either homoprotocatechuic (3,4-dihydroxyphenylacetic) acid (34) or homogentisic (2,5-dihydroxyphenylacetic) acid (HGA) (4, 30) as intermediates. Both of these intermediates can be melanin precursors, and their accumulation usually results from an enzymatic disruption of these pathways. Brown pigments are formed from the oxidation and polymerization of these intermediates (8, 13, 32, 33, 35, 36).
Melanin production in Y. lipolytica is reported to result from l-tyrosine degradation (6). It is a two-stage process in which the pigment precursor is first accumulated outside the cells and then autooxidizes and polymerizes. The chemical core of the resulting pigment has a structure typical of an intermediate of tyrosine catabolism, and this structure or compound seems to be the main monomer in the polymer (6). We hypothesize that HGA is the precursor or intermediate involved, since a mutant strain of Y. lipolytica unable to use tyrosine as the sole carbon source is known to accumulate this acid in a tyrosine-containing medium (2).
Our objectives in this study were (i) to identify the pigment precursor by studying the aromatic catabolites of tyrosine degradation by Y. lipolytica, (ii) to determine if aeration altered precursor accumulation, and (iii) to determine if the culture conditions altered the chemical autooxidation and polymerization of the precursor.
MATERIALS AND METHODS
Y. lipolytica strain, inoculum, and media.
Y. lipolytica ISA 1668, isolated from cheese (7) and considered to be a strong pigment producer (5), was used throughout the study. The medium used for inoculum preparation contained (per liter): 5 g of bacteriological peptone, 5 g of yeast extract, and 2 g of glucose. In all assays a loopful of young cells was inoculated into 50 ml of broth in 250-ml Erlenmeyer flasks and incubated overnight on a rotary shaker (150 rpm) at 25°C. Tyrosine catabolism and pigment production were assessed in a tyrosine medium previously developed for Y. lipolytica pigment production (6). This medium was supplemented with 50 mM lactic acid and 1 mM Mn2+ (MnSO4O · 5H2O) to maximize pigment production (6), except when specified otherwise in the results. Medium pH was adjusted to 5.0 or 5.5 with 10 M NaOH. All tyrosine media were inoculated with the inoculum suspension (cells/ml) for an initial optical density at 640 nm (OD640) of 0.02 to 0.04. [2-13C]l-tyrosine (Cambridge Isotope Laboratories) was used for the identification of tyrosine metabolites.
Incubation conditions.
Tyrosine catabolism and pigment production were followed under three different incubation conditions: (i) 400 ml of a tyrosine medium supplemented with 20 mM lactic acid in 2-liter Erlenmeyer flasks with a cotton plug and incubated in an orbital shaker (150 rpm) (high aeration); (ii) 250 ml of a tyrosine medium supplemented with 50 mM lactic acid in 500-ml Erlenmeyer flasks with a cotton plug and incubated in a water bath, with magnetic stirring (moderate aeration); (iii) 300 ml of a tyrosine medium supplemented with 50 mM lactic acid in 500-ml sidearm flasks with a rubber stopper and incubated in a water bath, with magnetic stirring (low aeration). Aeration levels were evaluated by measuring the rate of oxygen dissolution in media without cells. First, oxygen was removed from each medium by flushing with nitrogen gas. Each medium was then incubated under the appropriate condition, and the rates of oxygen dissolution were determined with a Clark type electrode (Diamond General Chemical Microsensor): high aeration, 52.3 μmol of O2/min; moderate aeration, 22.1 μmol of O2/min; low aeration, 10.9 μmol of O2/min.
Quantitative determinations.
After the appropriate periods of culturing, a sample of the culture medium was taken and growth was evaluated by measuring the OD640. After filtration (membrane pore size, 0.22 μm), pigment production was evaluated by measuring the OD400 of the filtrate (6) in a Spectronic 21 (Bausch & Lomb, Rochester, N.Y.) spectrophotometer. The samples were stored at −5°C (each sample contained 1 ml of the filtered sample and 0.1 ml of 1 M HCl) until high-performance liquid chromatography (HPLC) analysis.
Identification of aromatic tyrosine metabolites.
Tyrosine medium was used under controlled pH conditions (pH 4.8) in order to slow the increase in pH that occurs when Y. lipolytica is grown on this type of medium (5, 6). This procedure is needed to stabilize aromatic compounds that would otherwise oxidize at high pH. Cells were grown in 500-ml sidearm flasks containing 250 ml of tyrosine medium and incubated in a water bath, with magnetic stirring, at 25°C. pH was maintained at 4.8 with 1 M HCl by using a TTT 80 Titrator (Radiometer, Copenhagen, Denmark) connected to an electromagnetic valve (Radiometer) and to an electrode directly submerged in the culture medium. After 48 h of incubation, the cell suspension was filtered (membrane pore size, 0.22 μm) and the pH value of the filtrate was adjusted to 3.0 with 1 M HCl. The filtrate was reduced to 75 ml in a rotary evaporator. The residue was extracted three times with 75-ml portions of ethyl acetate; the organic layers were combined, dried over sodium sulfate, and evaporated to dryness by rotary evaporation. This procedure leaves the unreacted tyrosine in an aqueous solution. The residue (500 mg) was dissolved in a minimal amount of water, filtered, and analyzed by HPLC.
The HPLC method was assessed in a Merck (Darmstadt, Germany) HPLC system fitted with an RP18 reverse-phase column (4.4 by 250 mm; H50DS-250A; Hichrom, Reading, United Kingdom). The mobile phase consisted of a gradient of methanol–0.1% sulfuric acid in water, from 10 to 100% methanol in 40 min. Peaks eluting from the column were detected with a Merck-Hitachi L4750A diode array detector set between 220 and 400 nm. These HPLC conditions separated tyrosine from other aromatic tyrosine metabolites.
The isolation of the metabolites was conducted on the same system by the use of a semipreparative RP18 column (10 by 250 mm; Merck Lichrospher 100 RP-18). Three aromatic peaks were collected, concentrated, and extracted three times with 10-ml portions of ethyl acetate. The three ethyl acetate extracts were combined, dried over sodium sulfate, and evaporated to dryness by rotary evaporation to give 7 mg of the first component (retention time, 9.15 min), 22 mg of the second component (retention time, 18.03 min), and 15 mg of the third component (retention time, 21.89 min). All compounds were analyzed by nuclear magnetic resonance (NMR) spectroscopy. NMR spectra were determined with a Bruker ARX-400 MHz instrument (Germany). The same method was applied to the medium containing [2-13C]l-tyrosine.
Autooxidation of tyrosine metabolites.
All intermediates of tyrosine metabolism that accumulated in tyrosine medium during Y. lipolytica growth were screened for their potential to produce pigments through autooxidation and polymerization. For this purpose, tyrosine was replaced in the tyrosine medium with 1.5 mM concentrations of each intermediate (purchased from Sigma-Aldrich, St. Louis, Mo.) previously detected in the medium. These media where subjected to the same conditions as those used for cell incubation, i.e., orbital shaking (150 rpm) and 25°C, but without cells. The same media were used to assess the effect of pH by adjusting the initial value to 5.5, 6.5, or 7.4. Three different media were used to assess the effect of Mn2+ and EDTA on the autooxidation and polymerization of the intermediates (all at pH 5.5 and containing a 1.5 mM concentration of each intermediate): one was the same as that used for the pH studies, another contained 4 g of KH2PO4/liter, and the other contained 4 g of KH2PO4/liter and 50 mM lactic acid. Each of these media was tested with and without 1 mM Mn2+ and/or 10 mM EDTA.
All solutions were filter sterilized (membrane pore size, 0.22 μm) and tested in volumes of 100 ml in 250-ml Erlenmeyer flasks. Flasks were kept on a rotary shaker (150 rpm) at 25°C for several days, and autooxidation and polymerization were evaluated by measuring color development (at OD400).
All experiments were conducted in duplicate. Since similar trends in changes of the examined parameters were observed in all replicates (standard error of the mean was generally less than 10%), results of only one run are presented in the figures.
RESULTS
Identification of aromatic metabolites.
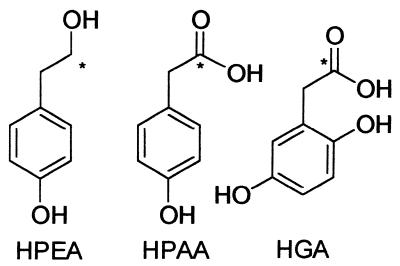
After 48 h of incubation, the tyrosine medium was still uncolored (OD400 of 0.01 ± 0.001), and after removing the cells the following compounds were identified in the filtrate by NMR spectroscopy (Fig. 1): p-hydroxyphenylethanol (p-HPEA; retention time, 18.0 min); p-hydroxyphenylacetic acid (p-HPAA; retention time, 21.9 min); HGA (retention time, 9.2 min). The same compounds were identified when [2-13C]l-tyrosine was used in the tyrosine medium. All the compounds had incorporated 13C into the marked position (Fig. 1), confirming that they are all derived from tyrosine.
FIG. 1.
Aromatic metabolites isolated from tyrosine medium, after growth of Y. lipolytica cells (pH 4.8), and identified by NMR spectroscopy. p-HPEA: 13C-NMR (100 MHz in aceton-d6) δ 39.4, 64.2, 115.8, 130.7, 130.9, and 156.5; 1H-NMR (400 MHz in aceton-d6) δ 2.72 (2H, t, J = 3 Hz), 3.70 (2H, m), 3.76 (1H, m), 6.74 (2H, d, J = 8 Hz), 7.05 (2H, d, J = 8 Hz), and 8.12 (1H, s); p-HPAA: 13C-NMR (100 MHz in aceton-d6) δ 40.8, 116.3, 126.9, 131.5, 157.5, and 173.6; 1H-NMR (400 MHz in aceton-d6) δ 3.50 (2H, s), 6.73 (2H, d, J = 8 Hz), 7.05 (2H, d, J = 8 Hz), 8.12 (1H, s), and 10.1 (1H, bl); HGA: 13C-NMR (100 MHz in aceton-d6) δ 36.0, 115.2, 116.7, 118.5, 123.2, 149.0, 151.1, and 173.4; 1H-NMR (400 MHz in aceton-d6) δ 3.57 (2H, s), 6.58 (1H, dd, Jo = 9 Hz, Jm = 3 Hz), 6.69 (1H, d, J = 9 Hz), 6.70 (1H, t, J = 3 Hz), 7.71 (1H, s), and 8.80 (1H, bl). Asterisks represent 13C where [2-13C]l-tyrosine was used in the tyrosine medium.
Tyrosine catabolism and pigment production. (i) Effect of aeration.
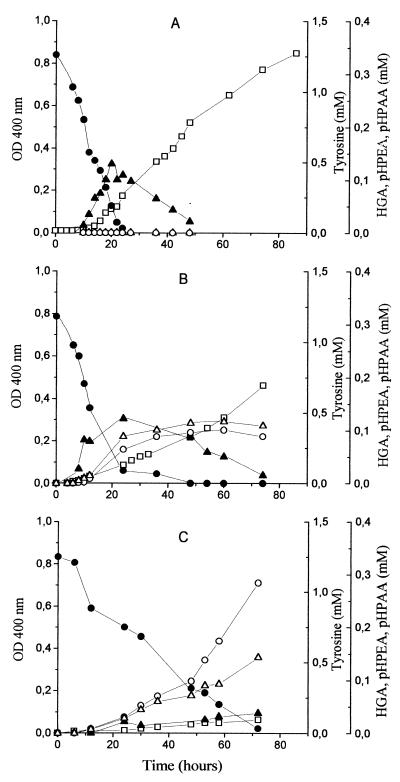
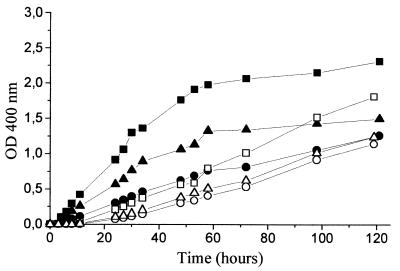
To assess the effect of aeration on tyrosine catabolism and pigment production by Y. lipolytica, three different incubation conditions were tested, corresponding to different aeration levels (high, moderate, and low). It was observed that both growth and pH increase were slower under lower aeration conditions. Tyrosine was completely consumed under all conditions (Fig. 2) but disappeared more slowly in the lowest aeration (Fig. 2C). This result is consistent with the differences in growth, indicating that tyrosine is catabolized during growth. Under high and moderate aeration (Fig. 2A and B), color development began during tyrosine consumption and HGA accumulation and further intensified during and after HGA depletion. Under the lowest aeration condition (Fig. 2C) almost no HGA or color was detected, even though tyrosine was completely consumed. p-HPEA and p-HPAA accumulated only under conditions of limited aeration. An equimolar relation was observed for these compounds in the moderate aeration condition (Fig. 2B), but in conditions of low aeration this relation began to change after about 48 h of incubation until it reached 2:1 (p-HPEA–p-HPAA) at the end of the assay (Fig. 2C). At this stage, the p-HPAA concentration was similar under both conditions. In all cases, tyrosine metabolites ceased to accumulate after tyrosine depletion.
FIG. 2.
Brown color (as measured by the OD400) (□), tyrosine (●), HGA (▴), p-HPEA (○), and p-HPAA (▵) evolution in tyrosine medium during Y. lipolytica growth. The tyrosine medium was supplemented with 1 mM Mn2+ and 50 mM lactic acid (20 mM for panel A) and incubated under high (A), moderate (B), and low (C) aeration conditions.
(ii) Effect of Mn2+.
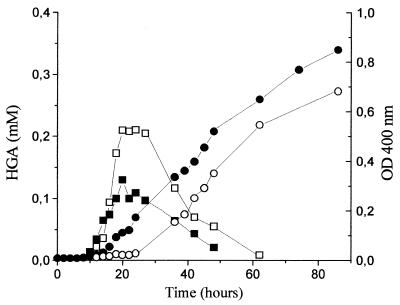
Pigment production by Y. lipolytica is strongly stimulated by Mn2+ (5, 7), but the mechanism involved is unknown. Growth, pH, and tyrosine consumption were not influenced by the removal of Mn2+. HGA also began to accumulate during tyrosine consumption, but its maximum concentration, reached after tyrosine depletion (about 24 h of incubation), was 0.21 mM, 60% higher than that observed in the presence of Mn2+ (Fig. 3). However, a delay of about 10 h was observed for pigment production in the absence of Mn2+. In both media the concentration of HGA decreased as color increased.
FIG. 3.
Effect of Mn2+ on the accumulation of HGA (▪, □) and on brown color formation, as measured by the OD400 (●, ○), during Y. lipolytica growth on tyrosine medium under high aeration conditions. Open symbols represent medium without Mn2+, and closed symbols represent medium with Mn2+.
(iii) Effect of pH.
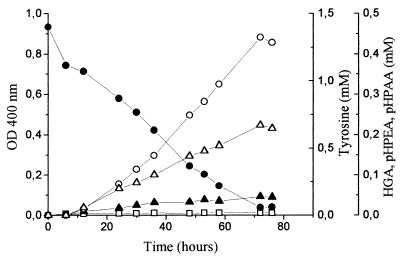
To assess whether the increasing of medium pH affects tyrosine catabolism, the lowest aeration was also studied under a controlled pH of 4.8. Figure 4 shows that tyrosine depletion and HGA accumulation were identical to those observed under noncontrolled pH conditions (Fig. 2C) in which the pH of the medium increased throughout incubation to a final value of about 7.0 after 72 h of incubation. However, the final concentrations of p-HPEA and p-HPAA were about 50% higher than those observed without pH control, and no color was produced.
FIG. 4.
Brown color (as measured by the OD400) (□), tyrosine (●), HGA (▴), p-HPEA (○), and p-HPAA (▵) evolution in tyrosine medium during Y. lipolytica growth under a constant pH of 4.8. The tyrosine medium was supplemented with 1 mM Mn2+ and 50 mM lactic acid and incubated under low aeration conditions.
Autooxidation of tyrosine metabolites.
Of the tyrosine metabolism intermediates that accumulated in the medium (HGA, p-HPEA, and p-HPAA), only HGA could autooxidize to brown pigments. The chemical conversion of HGA into brown pigments occurred spontaneously under the conditions used to incubate culture media.
HGA autooxidation.
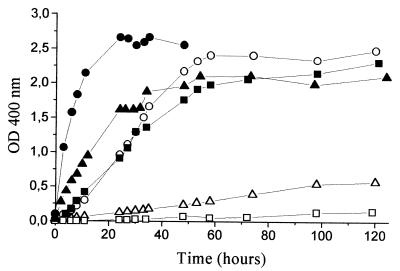
In the presence of Mn2+ the complete tyrosine medium was the most favorable environment for the autooxidation and polymerization of HGA (Fig. 5). The intensity of the brown color under these conditions was much higher than that in the tyrosine medium with cells (Fig. 2A). These results support the hypothesis that HGA is the precursor of the brown pigments, as its concentration in the presence of cells was much lower than the concentration used in these experiments (1.5 mM). In the absence of Mn2+, pigment formation due to HGA autooxidation was so slow that it resulted in almost an absence of color during the first 120 h of incubation. However, color continued to intensify at a constant rate throughout the time of the study. The combination of Mn2+ with lactic acid strongly accelerated and intensified HGA autooxidation (Fig. 5), but lactic acid did not promote the process in the absence of Mn2+. The addition of an excess of a chelating agent (10 mM EDTA) delayed the process but did not seem to affect the final intensity of color (Fig. 5). Higher pH values induced a higher rate of HGA autooxidation, both in the presence and absence of Mn2+ (Fig. 6). Slower rates of color formation and lower color intensities were observed in the absence of Mn2+ for pHs 5.5 and 6.5. Small differences were found between pH 7.4 without Mn2+ and pHs 5.5 and 6.5 with Mn2+. Initial pH values were stable throughout the experiments, except for both media at pH 7.4, where a slow decrease was observed after about 50 h of incubation, reaching 7.1 to 7.2 at the end of the assay. During this period, the pH 7.4 medium with Mn2+ also decreased in intensity of brown color, which is probably a result of some structural changes due to the high level of polymerization. This part of the curve is not presented in Fig. 6 because it varied widely among replicates. HGA autooxidation had a constant pattern of color development in all assays, characterized by an initial reddish color that further intensified and turned brown.
FIG. 5.
Brown color evolution by HGA autooxidation in chemically defined solutions (noninoculated), all containing 1.5 mM HGA and 1 mM Mn2+ (pH 5.5) and either the same composition of tyrosine medium but without tyrosine (▪, □), a solution of 4 g of KH2PO4/liter (●, ○), or a solution of 4 g of KH2PO4/liter plus 50 mM lactic acid (▴, ▵). Open symbols represent media with 10 mM EDTA, and closed symbols represent media without EDTA.
FIG. 6.
Brown color evolution by HGA autooxidation at the following pHs: 5.5 (▪, □), 6.5 (▴, ▵), and 7.4 (●, ○). A noninoculated solution with the same chemical composition of tyrosine medium but with 1.5 mM HGA instead of tyrosine was used with (closed symbols) and without (open symbols) 1 mM Mn2+.
HGA autooxidizes outside the cells, so it is not possible to determine the total amount accumulated in the medium. Instead, this concentration was inferred from the results obtained in the absence of Mn2+ (high aeration conditions), since under these conditions HGA did not seem to oxidize during its accumulation. Therefore, the maximum HGA concentration detected in the medium, 0.21 mM (Fig. 3), was used. Pigment formation was evaluated at two different pH values, 6.0 and 7.5. Higher pHs accelerated pigment formation, and the maximum color intensity detected was 0.4 (OD400). Higher color intensity was observed in the presence of cells (Fig. 2A), suggesting that either the total amount of HGA released from the cells was higher than 0.21 mM or other substances produced by the cells also are involved in pigment formation.
DISCUSSION
Our results identify HGA as the probable intermediate of tyrosine catabolism involved in pigment production by Y. lipolytica and suggest that pigment formation results from its accumulation and autooxidation. Brown pigments that result from tyrosine through HGA autooxidation are known as pyomelanins (24, 36). Since the chemical structure of melanins is still unknown and pyomelanin formation is presently proved by detecting the formation of HGA from tyrosine (8, 18, 36), it seems likely that the pigments of Y. lipolytica are pyomelanins. Furthermore, we have previously demonstrated that these pigments are polymers in which the main monomer is derived from tyrosine and has a general chemical structure compatible with the structure of HGA (6). To the best of our knowledge, the involvement of HGA in the production of brown pigments has not been reported for fungi. These organisms are known to produce a great variety of black-to-brown pigments, but the substrates involved usually are not tyrosine (1). Although Agaricus bisporus and Neurospora crassa both produce melanin from tyrosine, the pathway involved is tyrosinase mediated (31). HGA melanins have been reported in several bacteria (8, 13, 18, 33, 36).
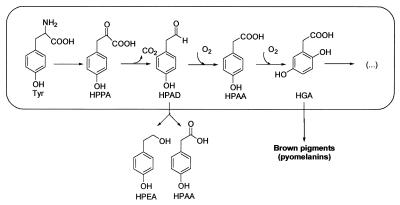
HGA is an intermediate in tyrosine catabolism in Y. lipolytica ISA 1668 and also has been found in a mutant strain of this species that cannot use tyrosine as the sole carbon source (2). There are two known pathways for the catabolism of tyrosine to HGA. The first step in both is the deamination of tyrosine, through a transamination reaction, to form p-hydroxyphenylpyruvic acid (p-HPPA). One of the pathways involves the enzyme p-hydroxyphenylpyruvate dioxygenase (EC 1.13.11.27), which catalyzes the oxidation of p-HPPA to HGA (15). This is the common pathway in mammals (22), and it also occurs in some bacteria (9, 23). In the alternate pathway, p-HPPA is metabolized to p-HPAA through p-hydroxyphenylacetaldehyde (p-HPAD), resulting in the formation of homoprotocatechuic acid (34) or HGA (3, 4, 14, 30, 34). Our results suggest that this alternate pathway occurs in Y. lipolytica (Fig. 7). We assume that p-HPPA is decarboxylated to p-HPAD, since p-HPAA and p-HPEA accumulate in the medium under oxygen-limiting conditions. Aldehydes can spontaneously decompose to the corresponding alcohol and carboxylic acid (25), and such a mechanism could be responsible for the p-HPEA and p-HPAA we observed in the medium. The next step of the pathway leads to the formation of HGA, but according to the literature (4, 30, 34) the aldehyde must be first oxidized to p-HPAA. HGA appears to be the last aromatic intermediate in the pathway. The proposed catabolic pathway has not been reported for a yeast. In general, yeasts are thought to use tyrosine as a nitrogen source only via a tyrosine ammonia-lyase (EC 4.3.1.5), but relatively few yeasts can use the resulting p-hydroxycinnamic acid as a carbon source (20). A similar pathway also has been described for Aspergillus nidulans, although HGA appears to be formed directly from p-HPPA by this fungus (12).
FIG. 7.
Proposed pathway for tyrosine degradation in Y. lipolytica. The box encloses the reactions that take place within the cell. Vertical arrows indicate the compounds released from the cell and their evolution in the extracellular medium (outside the box). Tyr, tyrosine; HPPA, p-hydroxyphenylpyruvic acid; HPAD, p-hydroxyphenylacetaldehyde; HPAA, p-hydroxyphenylacetic acid; HPEA, p-hydroxyphenylethanol.
Oxygen stimulates the oxidative degradation of tyrosine in Y. lipolytica by promoting the accumulation of HGA. This effect is probably related to an imbalance between the formation and degradation rates of HGA inside the cell. This effect also could explain the low levels of HGA observed when low aeration conditions were used. Oxygen also seems to affect the spontaneous breakdown of p-HPAD into p-HPAA and p-HPEA. The increase of the ratio of p-HPEA to p-HPAA (reduced-to-oxidized forms) from 1:1 to 2:1 observed in moderate and low aeration conditions, respectively, is consistent with the redox nature of the reaction.
Although our results do not rule out the involvement of other substances in pigment formation, the accumulation of HGA seems to be a key event in the process. Therefore, the chemical environment into which HGA is released is also important for pigment formation, since it may influence HGA's oxidative polymerization. High pH values are known to accelerate the process (19), and this was also confirmed in this study. HGA oxidation was also promoted by Mn2+, and this is probably a result of its ability to induce the activation of molecular oxygen, increasing the reactivity of melanin free radicals (16) and thus accelerating the polymerization process. The stimulating effect of lactic acid in the presence of Mn2+ is consistent with the known catalytic activity of Mn2+-hydroxy acid complexes on the oxidation and polymerization of substituted phenols (10). Other compounds present in tyrosine medium or produced by Y. lipolytica also may promote HGA oxidation.
The involvement of Y. lipolytica in the browning defects of cheese seems to result from its ability to produce brown pigments from tyrosine (6, 27). The technological conditions under which browning develops are not yet established, and it remains unclear why this phenomenon has a sporadic occurrence even in cheeses where this species is usually present. In this study we demonstrated that pigment formation depends on various conditions, suggesting that it may be triggered by specific abnormal conditions during cheese production and/or ripening. For example, an excessive level of Mn2+ in the milk or brine, or an imbalance in proteolysis that releases higher levels of free amino acids, including tyrosine, are conditions that might trigger the process. For Gorgonzola-style cheeses it has already been shown that defective cheeses do in fact have higher levels of tyrosine than cheeses which are not defective (28). In cheeses where this yeast is not a common contaminant, cheese producers should avoid its presence or limit its activity in case of contamination by controlling the Mn2+ levels and assuring adequate ripening conditions for proteolysis.
ACKNOWLEDGMENTS
We thank Alexandra Veiga for assistance in performing the oxygen analysis.
Financial support for A. Carreira was provided by grant PRAXIS XXI/BD 9150/96 from the Portuguese Ministry of Science and Technology.
REFERENCES
- 1.Bell A A, Wheeler M H. Biosynthesis and function of fungal melanins. Annu Rev Phytopathol. 1986;24:411–451. [Google Scholar]
- 2.Bigelis, E. R., and K. A. Black. September 1990. Biotransformation of l-tyrosine and l-phenylalanine to 2,5-dihydroxyphenylacetic acid. U. S. patent 4,956,279.
- 3.Blakley E R. Microbial conversion of p-hydroxyphenylacetic acid to homogentisic acid. Can J Microbiol. 1972;18:1247–1255. doi: 10.1139/m72-193. [DOI] [PubMed] [Google Scholar]
- 4.Boer L, Harder W, Dijkhuizen L. Phenylalanine and tyrosine metabolism in the facultative methylotroph Nocardia sp. 239. Arch Microbiol. 1988;149:459–465. [Google Scholar]
- 5.Carreira A, Loureiro V. A differential medium to detect Yarrowia lipolytica within 24 hours. J Food Mycol. 1998;1:3–12. [Google Scholar]
- 6.Carreira A, Ferreira L M, Loureiro V. Production of brown tyrosine pigments by the yeast Yarrowia lipolytica. J Appl Microbiol. 2001;90:372–379. doi: 10.1046/j.1365-2672.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 7.Carreira A, Paloma A, Loureiro V. Pigment producing yeasts involved in a brown surface discoloration of ewes' cheese. Int J Food Microbiol. 1998;41:223–230. doi: 10.1016/s0168-1605(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 8.Coon S L, Kotob S, Jarvis B B, Wang S, Fuqua W C, Weiner R M. Homogentisic acid is the product of MelA, which mediates melanogenesis in the marine bacterium Shewanella colwelliana D. Appl Environ Microbiol. 1994;60:3006–3010. doi: 10.1128/aem.60.8.3006-3010.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denoya C D, Skinner D D, Morgenstern M R. A Streptomyces avermitilis gene encoding a 4-hydroxyphenylpyruvic acid dioxygenase-like protein that directs the production of homogentisic acid and an ochronotic pigment in Escherichia coli. J Bacteriol. 1994;176:5312–5319. doi: 10.1128/jb.176.17.5312-5319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewar M J S, Nakaya T. Oxidative coupling of phenols. J Am Chem Soc. 1968;90:7134–7135. [Google Scholar]
- 11.Eliskases-Lechner F, Ginzinger W. Colour defects in dairy products. Lebensmittelindustrie Milchwirtschaft. 1999;120:102–106. [Google Scholar]
- 12.Fernández-Cañón J M, Peñalva M A. Fungal metabolic human type I hereditary tyrosinaemia. Proc Natl Acad Sci USA. 1995;92:9132–9136. doi: 10.1073/pnas.92.20.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin P H, Sopher C R. Brown pigmentation of Xanthomonas campestris pv. phaseoli associated with homogentisic acid. Can J Microbiol. 1993;40:28–34. [Google Scholar]
- 14.Hareland W A, Crawford R L, Peter C J, Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydrolase from Pseudomonas acidovorans. J Bacteriol. 1975;121:272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayaishi O, Nozaki M, Abbott M T. Oxygenases: dioxygenases. In: Boyer P D, editor. The enzymes, vol. 12. Oxidation-reduction, part B. Electron transfer (II) oxygenases oxidases (I). New York, N.Y: Academic Press, Inc.; 1975. pp. 119–191. [Google Scholar]
- 16.Hintz P, Kalayanaraman B. Metal ion-induced activation of molecular oxygen in pigmented polymers. Biochim Biophys Acta. 1986;883:41–45. doi: 10.1016/0304-4165(86)90132-7. [DOI] [PubMed] [Google Scholar]
- 17.Kelley S K, Coyne V E, Sledjeski D D, Fuqua W C, Weiner R M. Identification of a tyrosinase from a periphytic marine bacteria. FEMS Microbiol Lett. 1990;67:275–280. [Google Scholar]
- 18.Kotob S I, Coon S L, Quintero E J, Weiner R M. Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a Hyphomonas strain, and Shewanella colwelliana. Appl Environ Microbiol. 1995;61:1620–1622. doi: 10.1128/aem.61.4.1620-1622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Du B N. Alkaptonuria. In: Stanbury J B, Wyngaarden J B, Fredrickson D S, editors. The metabolic basis of the inherited disease. New York, N.Y: McGraw-Hill Book Company; 1972. pp. 268–282. [Google Scholar]
- 20.Large P J. Degradation of organic nitrogen compounds by yeasts. Yeast. 1986;2:1–34. [Google Scholar]
- 21.Lerch K, Ettlinger L. Purification and characterization of a tyrosinase from Streptomyces glaucescens. Eur J Biochem. 1972;31:427–437. doi: 10.1111/j.1432-1033.1972.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 22.Lindstedt S, Odelhög B. 4-Hydroxyphenylpyruvate dioxygenase from human liver. Methods Enzymol. 1987;142:139–142. doi: 10.1016/s0076-6879(87)42021-1. [DOI] [PubMed] [Google Scholar]
- 23.Lindstedt S, Odelhög B, Rundgren M. Purification and some properties of 4-hydroxyphenylpyruvate dioxygenase from Pseudomonas sp. P. J. 874. Biochemistry. 1977;16:3369–3377. doi: 10.1021/bi00634a013. [DOI] [PubMed] [Google Scholar]
- 24.Mann S. Ueber melaninbildende stämme von Pseudomonas aeruginosa. Arch Mikrobiol. 1969;65:359–379. [PubMed] [Google Scholar]
- 25.March J. Advanced organic chemistry. 4th ed. New York, N.Y: John Wiley and Sons, Inc.; 1992. pp. 1233–1235. [Google Scholar]
- 26.Mercado-Blanco J, Garcia F, Fernandez-Lopez M, Olivares J. Melanin production by Rhizobium meliloti GR4 is linked to nonsymbiotic plasmid pRme-Gr4b: cloning, sequencing and expression of the tyrosinase gene mepA. J Bacteriol. 1993;175:5403–5410. doi: 10.1128/jb.175.17.5403-5410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichol A W, Harden T J. Enzymic browning in mould ripened cheeses. Aust J Dairy Technol. 1993;48:71–73. [Google Scholar]
- 28.Nichol A W, Harden T J, Tuckett H. Browning defects in mould ripened cheese. Food Aust. 1996;48:136–138. [Google Scholar]
- 29.Pomerantz S H, Murthy V V. Purification and properties of tyrosinases from Vibrio tyrosinaticus. Arch Biochem Biophys. 1974;160:73–82. doi: 10.1016/s0003-9861(74)80010-x. [DOI] [PubMed] [Google Scholar]
- 30.Pometto A L, Crawford D L. l-Phenylalanine and l-tyrosine catabolism by selected Streptomyces species. Appl Environ Microbiol. 1985;49:727–729. doi: 10.1128/aem.49.3.727-729.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robb D A, Gutteridge S. Polypeptide composition of two fungal tyrosinases. Phytochemistry. 1981;20:1481–1485. [Google Scholar]
- 32.Ruzafa C, Sanchez-Amat A, Solano F. Characterization of the melanogenic system in Vibrio cholerae ATCC 14035. Pigment Cell Res. 1995;8:147–152. doi: 10.1111/j.1600-0749.1995.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Amat A, Ruzafa C, Solano F. Comparative tyrosine degradation in Vibrio cholerae strains. The strain ATCC 14035 as a prokaryotic melanogenic model of homogentisate-releasing cell. Comp Biochem Physiol. 1998;119:557–562. doi: 10.1016/s0305-0491(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 34.Sparnins V L, Chapman P J. Catabolism of l-tyrosine by the homoprotocatechuate pathway in gram-positive bacteria. J Bacteriol. 1976;127:362–366. doi: 10.1128/jb.127.1.362-366.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trias J, Viñas M, Guinea J, Loren J G. Brown pigmentation in Serratia marcescens cultures associated with tyrosine metabolism. Can J Microbiol. 1989;35:1037–1042. doi: 10.1139/m89-172. [DOI] [PubMed] [Google Scholar]
- 36.Yabuuchi E, Ohyama A. Characterization of “pyomelanin”-producing strains of Pseudomonas aeruginosa. Int J Syst Bacteriol. 1972;22:53–64. [Google Scholar]
- 37.Yoshimoto T, Yamamoto K, Tsuru D. Extracellular tyrosinase from Streptomyces sp. KY-453: purification and some enzymatic properties. J Biochem. 1985;97:1747–1754. doi: 10.1093/oxfordjournals.jbchem.a135233. [DOI] [PubMed] [Google Scholar]