Abstract
Background
Leaves of Urtica simensis (U. simensis) have been used traditionally for wound healing in different communities in Ethiopia. In spite of this, there were no scientific data documented regarding the wound healing activity of this plant. There is a need to investigate herbal remedies for the treatment of wounds in order to overcome the limitations of conventional drugs.
Aim of the Study
Aim of the study was to evaluate the wound healing activity of extract and solvent fractions of the leaves of U. simensis in mice.
Methods
Leaves of U. simensis were washed, dried under shade and ground into coarse powder and then extracted by 80% methanol with three consecutive macerations. Part of the extract was fractionated with n-hexane, chloroform and water. In excision and burn wounds, healing progress was measured by wound contraction, epithelialization period and histopathology investigation whereas incision wound healing was assessed by skin breaking strength.
Results
In excision wound model, the 5% and 10% crude extract ointments showed significant (p < 0.001) wound contractions during day 8 to day 16 evaluations. Similarly, in burn wound model, both 5% and 10% crude extract ointments produced significant (p < 0.001) wound contractions starting from day 12 and 10, respectively. In both models, the periods of epithelialization were also significantly reduced and favorable histopathologic changes were produced by the crude extract ointments. The solvent fractions of the crude extract as well produced significant wound contractions as evaluated in excision wound model. The fractions also significantly reduced the period of epithelialization in this model. The aqueous fraction found to be more active than either chloroform or n-hexane fraction in wound healing.
Conclusion
Results of this study indicated that methanol extract and aqueous fractions of the leaves of U. simensis possess dose-dependent wound healing activity, thus supporting traditional claims.
Keywords: wound healing activity, wound contraction, Urtica simensis, mice
Background
Wound is defined as an opening or breaking in the integrity of the skin that causes disruption of anatomical and functional integrity of living tissues.1 Every time the integrity of the cutaneous barrier is compromised, a wound is created. This can range from a simple break in the epithelial integrity of the skin or it can be deeper, extending into subcutaneous tissue.2 About 8.2 million people had wounds with or without infections in United States.3 In Africa wounds account for about 30–42% of hospital attendance and 9% death every year.4 According to a study conducted in 2015 in Amhara Regional State Referral Hospitals, Ethiopia, 55.6% of emergency visits were due to injury.5 Hypertrophic scars are common after injuries and cause physical, social, emotional, and psychological burdens.6
Wounds can be classified based on the nature and the way of the injury causing the wounds as open wounds, closed wounds, puncture wounds and burn wounds.7 Based on the time required to heal, wounds are also classified whether acute or chronic. Acute wounds are those that heal through the routine processes of healing, whereas chronic wounds are often associated with underlying pathological conditions that contribute to prolonged and impaired healing.8
Normal wound healing process comprises four distinct but overlapping phases: hemostasis, inflammation, proliferation and maturation and remodeling. This begins directly after wounding and might last for years depending on the severity of the wound. Hemostasis occurs immediately following an injury through vasoconstriction and platelet activation and resulting in the deposition of a fibrin clot at the site of injury.9 Inflammation occurs within the first 24 hours after injury and can last for up to two weeks in normal wounds and significantly longer in chronic wounds. Neutrophils and macrophages are key cells during the inflammatory phase with a function of phagocytosis of bacteria and tissue debris. However, if remaining in the wound over time, neutrophils cause more harm than good. For example neutrophil proteases can degrade extracellular matrix components as well as the proteins important for repair, they produce free oxygen radicals resulting in oxidative stress, which further damage tissue and delay healing. Therefore, removal of neutrophils is prerequisite for wounds to progress into the proliferative phase.10 The activated macrophages also mediate the transition from the inflammatory phase to the proliferative phase by releasing of a wide variety of growth factors and cytokines.11,12 During proliferative phase the provisional wound matrix that was formed during hemostasis is replaced by granulation tissue. Granulation tissue is highly vascularized connective tissue composed of macrophages, proliferating fibroblasts, newly formed capillaries and residual inflammatory cells.10 The formation of granulation tissue into an open wound allows the epithelialization process to take place.13 Maturation and remodeling phase involves remodeling of collagen from type III to type I and cellular activity and the number of blood vessels in the wounded area becomes decreased. During this phase the new tissue slowly gains flexibility and tensile strength limited to 80% of the pre-injured strength.14
Many factors can interfere with one or more phases of healing, these causing improper or impaired wound healing.15
Wound management possesses challenges for the medical community. Only 1–3% of drugs listed in Western pharmacopeias are intended for cure and healing of wounds. In clinical practice, wound dressings and topical products are used to create and maintain a moist environment and provide adequate conditions for healing. However, they are often expensive or ineffective and may generate adverse reactions.16
Plant-derived extracts and/or isolates induce healing and tissue regeneration through multiple interrelated mechanisms. Many of these are affordable and cause minimal unwanted side effects.17 Traditional use of plants by health practitioners for wound management is limited by what is immediately at hand or locally acquired plant features.11 It is needed to investigate, identify and formulate plants or combinations derived from plants for the treatment of wounds.18
The Genus Urtica (Urticaceae family) commonly known as nettle family comprises more than 2000 species and are mostly found in the tropical and subtropical regions of the world.19 Urtica genus are medicinally well known throughout the world and locally used against various diseases. Among the species; U. simensis, Urtica dioica, Urtica incaica, Urtica deltoidea, Urtica gracilenta, Urtica sondenii, Urtica australis, Urtica ferox and Urtica urens are used for the treatment of rheumatism, arthritis, anemia, prostate diseases, wounds including burns and skin rashes in the area of folk medicine.20,21
An aqueous extract of leaves of Urtica dioica on in vitro studies can enhance cell proliferation, possess significant anti-inflammatory and multifaceted antioxidant properties. The aqueous extract of this plant also clearly demonstrated in the potential for wound healing applications in animal models.22 In another study methanol extract of leaves of Urtica dioica exhibited significant antioxidant and hepatoprotective activities comparable to standard antioxidant compound.23 There is also a scientific report on the healing effect of nettle extract on second degree burn wounds.24
U. simensis is one of the nettle species in the genus Urtica that is endemic in Ethiopia. It is a dark green, dioecious, erect, perennial plant which grows up to 1 m tall and widely known for its stinging hairs located on the stems and lower leaf surface.25U. simensis grows all around the year in the highlands of Ethiopia especially in the North and South Gondar, North and South Wello, North Shewa, Wag Hamra, highland of Sidama zone in Southern region and Arsi zone of Oromia region. This makes it easily harvested whenever there is a need. The leaves are used as a popular vegetable, especially in times of famine in some areas of Ethiopia.26
U. simensis has been used traditionally as a medicinal plant in Ethiopia. Different parts are used for the treatment of different illness and disorders. The leaf part of the plant is used for wound healing in various ways and preparations: grind the leaf and cream with butter on wound area,27,28 leaf is crushed and applied topically on wound area,29–31 pounding and squeezing the leaf and dropped to the injured area (eye),32 rub the cream of the leaf on the affected skin in case of warts,33 and leaves are crushed and pasted on the affected part in case of snakebite.31 Furthermore, the leaves of this plant have been used in Ethiopian traditional medicine for the treatment of gastritis,27,34–36 sexual transmitted disease such as gonorrhea,37,38 acute stomachache,37 common cold and heart failure.30
In addition to assessment and documentation of the uses of the leaves of U. simensis in traditional medicine, there are some experimental works done on this part. It has been verified by an experimental study that the leaves of U. simensis have antidiabetic activity.39 A study on chemical composition of U. simensis revealed that its leaves contain a significant level of dietary and health important biochemicals including carbohydrates, minerals and fats rich in essential fatty acids.40 In another investigation, it has been shown that the leaves of U. simensis contain appreciable amount of phytochemical constituents (phenolic compounds, flavonoids and tannins) and possess a potentially good antioxidant activity.26 Antibacterial activities of the leaves of U. simensis were also evaluated against gram positive bacterial species (S. pneumonia, S. aureus and S. pyogenes) and gram negative bacterial species (K. pneumonia, S. flexneri and P. aeruginosa) and the results showed that the plant has relatively higher antimicrobial activities against gram positive than gram negative bacteria.41
As indicated above, the leaf part of U. simensis has extensive traditional use for wound healing and experimentally verified antibacterial and antioxidant activities; both of which can be implicated for wound healing effects of different medicinal plants.42 However, no scientific study has been conducted to validate either the wound healing activity or safety of U. simensis. Hence, this study was designed to evaluate wound healing activity and acute toxicity of the crude extract and solvent fractions of the leaves of U. simensis in animal models.
Materials and Methods
Materials
Drugs and Chemicals
Wool fat, hard paraffin, white soft paraffin, cetostearyl alcohol, methanol absolute (Sisco Research Laboratories, New Mumbai, India) chloroform (Carlo Erba Reagents S.A.S), n-hexane (Pentoky Organy, India LTD), distilled water (Dialysis center, University of Gondar Hospital) nitrofurazone ointment 0.2% (Shanghai General Pharmaceutical Co. LTD, China), bees wax, sodium hydroxide, ketamine hydrochloride injection, diazepam injection, normal saline, formalin 10% buffered solution, hematoxylin, eosin, 6 M HCl, perchloric acid and 70% alcohol were obtained from respective suppliers. All the drugs, chemicals and reagents were with the required standard and analytical grade.
Supplies, Instruments and Apparatus
Sensitive digital weighing balance, lyophilizer, rotary evaporator, deep freezer, light, vacuum pump, desiccator, mini orbital shaker, electrical hair clipper series 3000, water bath, mortar and pestle, ointment slab, sharp sterilized scissors, surgical threads with curved needles, forceps, surgical scalpel blade, Erlenmeyer conical flask, beaker, adhesive plaster, Whatman filter paper (№ 1), gloves, cotton swab, permanent marker and transparent polythene graph paper were used.
Plant Material
The fresh leaves of U. simensis were collected in March 2020 from the side of Kiha River in Gondar town, Amhara regional state, Ethiopia. The plant material was identified and authenticated by a botanist in Department of Biology, College of Natural and Computational science, University of Gondar, and a specimen with a voucher number 001BAA/2020 was deposited there in the herbarium for future reference.
The collected leaves of the plant were washed with tap water and allowed to dry under shade. The dried material was crushed to coarse powder and weighed and stored in air tight containers until extraction.
Experimental Animals
Healthy white Swiss albino mice of either sex (25–35 g, and 6–8 weeks of age) were obtained from animal house. The animals were housed in cages under standard conditions (24 ± 2 °C, 40–70% relative humidity, and 12 hours light and dark cycles) and had free access to pellet diet and water. A week before starting the experiment, the animals were acclimatized to the laboratory condition. The animals were handled throughout the experiment according to international laboratory animal use and care guidelines.43,44 The research was performed as per the agreement of the ethical clearance document Ref. No SoP4/285/12.
Methods
Extraction
One kilogram of the coarse powder was macerated with eight liter of 80% (v/v) methanol in divided volume at room temperature in Erlenmeyer flasks and the flasks were covered with aluminum foil. It was then kept for 72 hours with occasional shaking using mini orbital shaker. After 72 hours the extract was filtered through Whatman filter paper (No. 1) using pressurized suction. The marc was re-macerated in fresh and same volume of 80% methanol twice and extracted and filtered in the same fashion in order to maximize the yield. Each filtrate was combined and concentrated using a rotary evaporator set at 40°C to remove the methanol part. The remaining aqueous mixture was placed in a deep freezer (−20°C) overnight to freeze and then it was dried by using lyophilizer. The dried extract was weighed and packed in air tight container and stored until used for the preparation of crude topical formulation and solvent fractionation for the intended experiment.45
Fractionation
Extract (90 gram) was suspended in distilled water in 1 to 8 ratio and then an equal amount to water of n-hexane was added. The mixture was left for phase separation (hexane at the top and water at the bottom). The n-hexane fraction was collected in a separate flask and this fractionation process was repeated in triplicate by adding equal amount of n-hexane solvent. Equal amount to water of chloroform was added to the aqueous layer and was left for phase separation (aqueous at the top and chloroform at the bottom) and the chloroform fraction was collected. The process repeated 3 times. The n-hexane and chloroform portions were evaporated using a rotary evaporator and the residues were placed in an oven (40°C) until dried. The aqueous fraction was frozen and dried in a lyophilizer. The % yields of each of the dried fractions were calculated.
Preliminary Phytochemical Screening
The crude extract was screened for the presence of secondary metabolites such as alkaloids, steroids, phenolic compounds, glycosides, flavonoids, saponins, tannins, terpenoids, and anthraquinones using standard screening procedures.46
Preparation of Topical Formulation
Two strengths, 5% and 10%, ointments for the crude extract as well as for each solvent fraction were determined after acute dermal toxicity study.
Simple Ointment Formulation
Simple ointment was first prepared from hard paraffin, cetostearyl alcohol, white soft paraffin, and wool fat for each wound model by the formula and proportion described in British Pharmacopoeia (Table 1).47
Table 1.
Formula for Preparation of Simple Ointment
| Ingredients | Master Formula (g) | Reduced Formula (g) |
|---|---|---|
| Hard paraffin | 50 | 5 |
| Cetostearyl alcohol | 50 | 5 |
| White soft paraffin | 850 | 85 |
| Wool fat | 50 | 5 |
| Total | 1000 | 100 |
All ingredients were weighed using an electronic weighing balance. Hard paraffin was placed first into an evaporating dish and melted over a water bath. The other ingredients were added in descending order of melting point until all were melted (the order being cetostearyl alcohol, then wool fat, and lastly white soft paraffin). The mixture was continuously stirred to ensure homogeneity. The prepared simple ointment was used as vehicle for the preparation of ointments of the crude extract and solvent fractions and as a dummy preparation for negative control.
The medicated ointment containing 5% and 10% strengths of the crude extract and each solvent fraction were prepared by using 95 g and 90 g of simple ointment and then adding 5 g and 10 g of the test substances, respectively. The mixture was levigated on ointment slab until the product got uniform consistency and smooth texture. The 100 gram of the entire non-medicated ointment base was taken for negative control group.48
Acute Dermal Toxicity
Dermal irritation evaluation was carried out according to OECD 402 guideline. A total of six female mice with normal skin texture (25–35 g and 6–8 weeks of age) were used and grouped in to two groups of each with three mice. The animals were housed individually in a cage and acclimatized to the laboratory condition for five days prior to the test. Then, around 10% of the body surface area fur was shaved from the dorsal area of the trunks of the test animals 24 hours prior the test procedure.
First, a testing dose, 2000 mg/kg (limit dose), of 10% crude extract ointment and simple ointment base were applied to single mice in each test and control group, respectively. Then covered by gauze and a non-occlusive bandage soon after and left for 24 hours. After 24 hours, the entire materials and residual test substance were removed with care and the test site of each animal was washed with distilled water. The animals were observed for development of any adverse skin reaction for 24 hours. Then, four additional mice, two from each group, were taken and dosed, covered and washed following the same procedure and similarly observed for 24 hours for any skin reaction. All the animals were observed for development of any adverse skin reactions, in terms of edema and erythema with close follow up for 24 hours after washing and then daily for 14 days.49
Grouping and Dosing of Experimental Animals
Animals were randomly assigned in to groups for evaluation of wound healing activity of the crude extract in excision, incision and burn wound models and solvent fractions in excision wound model.
In evaluation of the wound healing effect of the crude extract using excision and burn wound models, four groups containing six mice per group were used in each model and dosed as follows.
Group I: Simple ointment (negative control)
Groups II & III: 5% and 10% crude extract ointments, respectively
Group IV: 0.2% nitrofurazone ointment (positive control)
The wound healing activities of the solvent fractions were evaluated in circular excision wound model. Eight groups, each containing six mice, were used in the evaluation and received the treatments as follows.
Group I: Simple ointment (negative control)
Groups II & III: 5% and 10% aqueous fraction ointments, respectively
Groups IV & V: 5% and 10% chloroform fraction ointments, respectively
Groups VI & VII: 5% and 10% n-hexane fraction ointments, respectively
Group VIII: 0.2% nitrofurazone ointment (positive control)
In the evaluation of the wound healing activity of the crude plant extract in incision wound model, five groups, each containing six mice, were used. The groups were treated as follows.
Group I: Simple ointment (negative control)
Group II & III: 5% and 10% crude extract ointment, respectively
Group IV: 0.2% nitrofurazone ointment (positive control)
Group V: Left untreated (untreated negative control)
Wound Healing Activity
Excision Wound Model
Excision wound model was used as per the method described by previous studies.50,51 The mice were anesthetized by ketamine (80 mg/kg) plus diazepam (5 mg/kg) through intraperitoneal route. The skin of the dorsolateral flank area 1–1.5 cm away from the vertebral column on either side and 5 cm away from the ear was shaved with shaving machine after disinfected with 70% alcohol. The anticipated circular wound area 300 mm2 was marked with thin permanent marker and 2 mm depth excised wound was created along the markings carefully by using forceps and small sharp sterilized scissors. Hemostasis was achieved by blotting the wound with cotton swab soaked in normal saline. The entire wound was left open. The wounding day was considered as day 0. The treatments were applied once daily topically till the wound in the test group will completely healed. The wound healing progress was assessed by wound closure rate, period of epithelialization and histopathology investigations.
The wound closure rate, expressed as a reduction in percentage of the original wound size, was assessed taking measurements at 2nd, 4th, 6th, 8th, 10th, 12th and 16th days of post- wounding. The wound healing effects, as measured by percent wound contraction, of the extract and solvent fraction were calculated using the following formula.50,52
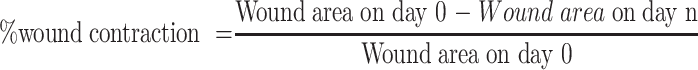 |
Where, n is number of days post-wounding.
Period of Epithelialization
The number of days required for falling scar without any residue raw wound were observed.53
Histopathology Investigation
At the end of experiment, the mice from each group were sacrificed by 320 mg/kg of ketamine plus 20 mg/kg of diazepam (four times the anesthetic dose) intraperitoneally. The cross-sectional full thickness skin specimens were collected. Samples were fixed in 10% buffered formalin, processed, and blocked with paraffin and then sectioned into 5 µm sections and stained with hematoxylin and eosin.53 The phases in wound healing processes (inflammation, proliferation, angiogenesis and remodeling) were analyzed by blinded (unaware) senior pathologist. Then results were compared with those of the control groups.
Incision Wound Model
In this model, the experimental animals were anesthetized in the same way as described for excision wound model. The dorsal fur of each mouse was shaved after rubbed by 70% alcohol. Three cm long and 2 mm depth of longitudinal paravertebral incision was made with a sterile blade through the shaved skin on either side at the distance of 1.5 cm from the dorsal midline. Wounds were closed with interrupted sutures 1 cm apart using surgical sutures (No. 000) and curved needle (no. 11). After stitching, wounds were left undressed.50
The wounding day was considered as day 0. The animals in Group I–IV were given the respective treatments topically as described in grouping dosing section above. The treatments were given starting 24 hours after wound creation until 9th day. The mice in Group V were left untreated and were used as untreated negative control.
The sutures were removed on 8th day, and the skin breaking strength of the healed wound were measured on the day 10 post wounding using continuous water flow technique.7 The tensile strengths of the test groups were compared with those of negative control, positive control and untreated negative control groups. The percent tensile strengths (% TS) of the groups were calculated using the following formulas.
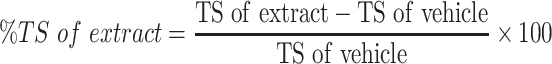 |
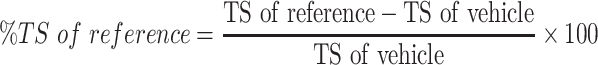 |
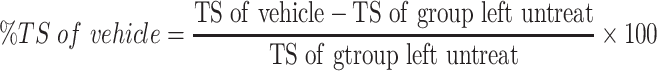 |
Burn Wound Model
Animals were anesthetized with ketamine (80 mg/kg) and diazepam (5 mg/kg) subcutaneously and the dorsal fur of each mouse was shaved after decontaminated with 70% alcohol. Partial thickness burn wound was created on four groups of mice through hot molten wax. The hot molten wax at 80°C was poured into a cylinder of 300 mm2 circular opening and placed on the shaven skin of each animal until it got solidified. Solidification of wax normally takes 10–12 minutes. Then the cylinder was removed and the demarked partial thickness circular burn wound was created on each animal.54
The animals were placed in individual cages. Treatments were applied over the wound area with respective groups, as described in the grouping and dosing section, every day starting from the wounding day until the day of scab falling of the test group. The progress of healing was examined every 2 days by measuring the wound contraction starting on 2 days of wounding. Epithelialization time and histopathology investigations were done after healing.55
Histopathology Analysis
Pathologic analysis was done based on the methods explained in excision wound model in the above.53
Statistical Analysis
The raw data were entered in to SPSS version 23 computer software and the group means were determined. The results were expressed as mean ± SEM (standard error of the mean). The differences between groups means were statistically analyzed using one-way analysis of variance (ANOVA) followed by Post Hoc Tukey’s tests. The differences were considered statistically significant at p < 0.05.
Results
Percentage Yield of the Crude Extract
From 1 kg coarse powder of the leaves of U. simensis, 157 g crude extract was obtained. Accordingly, the percentage yield of the crude extract was 15.7%. In fractionation of 90 g crude extract, 70.47, 6.12, and 3.24 g of aqueous, chloroform and n-hexane fractions, respectively, were obtained.
Phytochemical Screening Test
The qualitative phytochemical screening test on the crude extract of the plant revealed the presence of alkaloids, flavonoids, phenols, tannins, steroids, glycosides, anthraquinones, and terpenoids and no saponins.
Acute Dermal Toxicity Test
Topical application of a limit dose of 2000 mg/kg of 10% extract ointment formulation did not show any sign of skin reaction on close follow up for 24 hours after washing the applied formulation and daily observation for 14 days.
Wound Healing Activity of the Crude Extract
Excision Wound Model
Wound Contraction
Compared to the simple ointment, the crude extract topical preparations showed significant wound contraction at 5% (p < 0. 01) and 10% (p < 0.001) on day-6 measurement. In measurements taken from day-8 to day-16, progressive and of similar level of significance (p < 0.001) wound contractions were recorded in both 5% and 10% crude extract ointment treated groups, compared to the negative control. In comparison between treatment groups, the 10% crude extract ointment and the standard treatment produced significantly more wound contractions from day-6 to day-16 than 5% crude extract ointment. In all groups, the percent wound contractions were improving with post wounding days. On day 16, 90%, 97.81%, 100% and 99.85% of wound contractions were produced by SO, 5% CEO, 10% CEO, NFO 0.2%, respectively (Table 2).
Table 2.
Effect of Extract Ointments on Excision Wound Contraction
| Days | Group (Treatment) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 (SO) | Group 2 (5% CEO) | Group 3 (10% CEO) | Group 4 (NFO 0.2%) | |||||
| Wound Area (mm2) | % Wound Contraction# | Wound Area (mm2) | % Wound Contraction# | Wound Area (mm2) | % Wound Contraction# | Wound Area (mm2) | % Wound Contraction# | |
| 2 | 283.16±3.85 | 5.61 | 276.17±5.85 | 7.94 | 268±2.13a* | 10.67 | 273.5±2.96 | 8.83 |
| 4 | 246±2.48 | 18.00 | 240±2.41 | 20.00 | 229.50±4.09a* | 23.50 | 232.3±3.84a* | 22.57 |
| 6 | 193.17±3.83 | 35.61 | 176±3.27a** | 41.33 | 153.67±3.83a***b*** | 48.78 | 162.5±1.18a***b* | 45.83 |
| 8 | 146.17±3.70 | 51.28 | 93.17±2.39a*** | 68.94 | 76.83±2.62a***b** | 74.39 | 80.33±0.88a***b* | 73.22 |
| 10 | 101±3.61 | 66.33 | 63±2.53a*** | 79.00 | 48.17±2.87a***b** | 83.94 | 51.5±1.71a***b* | 82.83 |
| 12 | 78.67±3.11 | 73.78 | 34.67±1.56a*** | 88.44 | 15.25±1.82a***b*** | 94.92 | 16.08±2.05a***b*** | 94.64 |
| 14 | 50.33±3.20 | 83.22 | 17.33±2.38a*** | 94.22 | 1.10±0.37a***b** | 99.63 | 2.38±0.43a***b** | 99.21 |
| 16 | 30±2.13 | 90.00 | 6.58±0.99a*** | 97.81 | 0.00±0.00a***b** | 100.00 | 0.50±0.27a***b* | 99.83 |
Notes: Wound areas are expressed as mean ± SEM (n = 6). aCompared to SO, bcompared to 5% CEO, *p < 0.05, **p < 0.01, ***p < 0.00, #% wound contraction is from the initial wound area (300 mm2).
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Period of Epithelialization
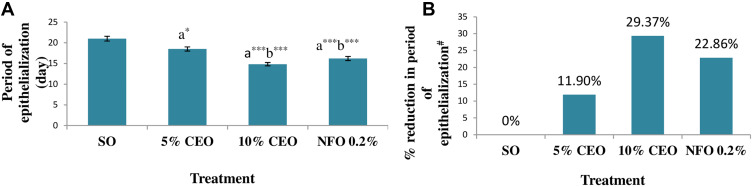
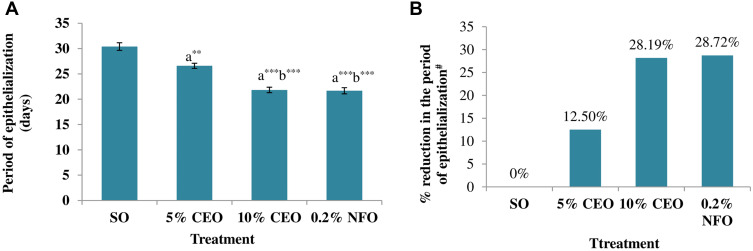
The time for complete epithelialization of the excision wound was short in groups treated by both the 5% and 10% CEO and nitrofurazone 0.2% ointment. The 10% CEO and nitrofurazone treatments significantly (p< 0.001) shorten period of epithelialization as compared to the 5% crude extract and the negative control. Relative to the negative control, the 5% extract also significantly (p< 0.05) reduced the epithelialization period. The percent reductions in periods of epithelialization, from that of the negative control, were 11.90%, 29.37% and 22.86% in groups treated by 5% and 10% the crude extract ointments and nitrofurazone, respectively (Figure 1).
Figure 1.
Effect of the crude extract ointments on the period of epithelialization of excision wound (A) and percentage reduction in the period of epithelialization (B).
Notes: The periods of epithelialization are expressed as mean ± SEM (n = 6). aCompared to SO, bCompared to 5% CEO, *p < 0.05, ***p < 0.001, #% reduction in period of epithelialization is from the SO treated group.
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Histopathology Analysis
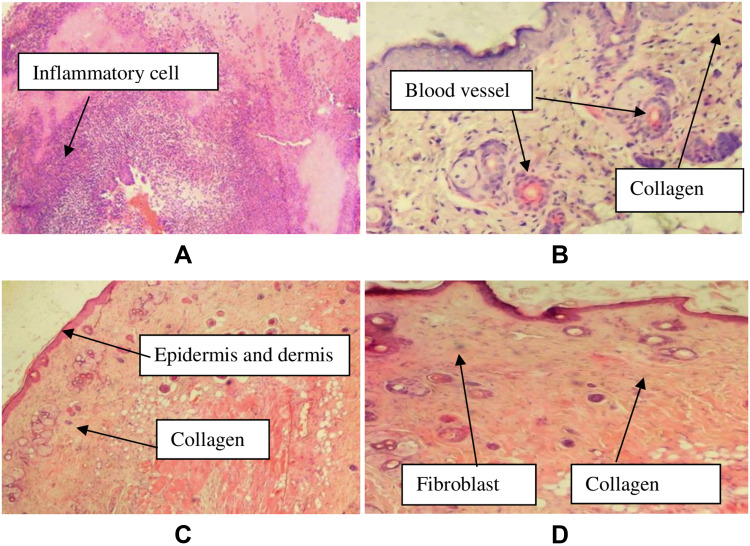
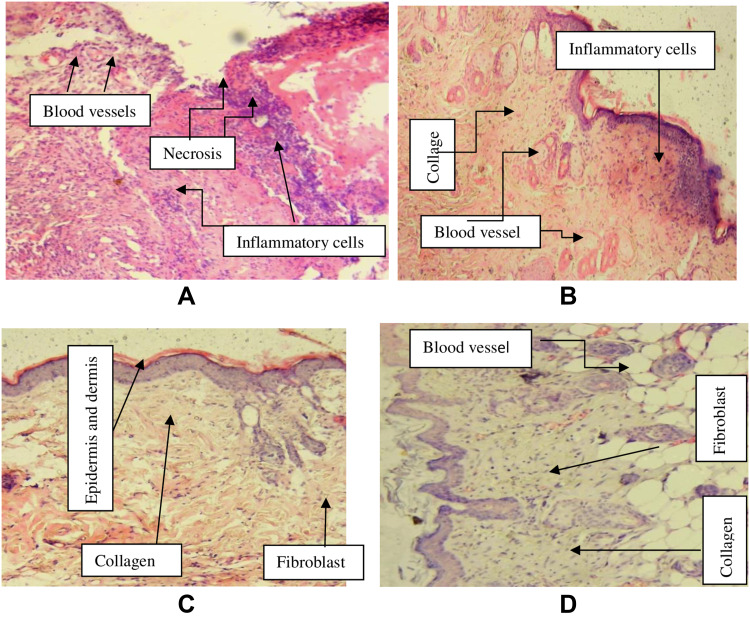
Histological specimens of the excised wound from crude extract treated groups were taken on day 14. The sample specimens were taken from one animal in each group. In histopathological analysis, the 10% CEO treated wounds showed improved healing as revealed by the presence of moderate concentration of fibroblasts and collagen depositions, neovascularization and absence of inflammatory cells. Analysis of tissue from a mouse in the negative control group showed sheet of inflammatory cells, low concentration of fibroblasts, collagen fiber and proliferating blood capillaries. The specimen from nitrofurazone treated animal showed moderate fibroblast proliferation, high collagen deposition, and low concentrations of polymorphonuclear and mononuclear cells (Table 3, Figure 2). Furthermore, Figure 3 shows the physical improvements of the wound texture of the mice received different treatments.
Table 3.
Histopathological Examination of the Effects of the Crude Extract of the Plant on Excision Wound
| Group | FP | CD | PMNC | MNC | NV |
|---|---|---|---|---|---|
| SO | + | + | +++ | +++ | + |
| 5% CEO | + | + | + | + | ++ |
| 10% CEO | ++ | ++ | – | – | + |
| NFO 0.2% | ++ | +++ | + | + | + |
Notes: Absent (-), low concentration (+), moderate concentration (++), and high concentration (+++) for epidermal and/or dermal remodeling.
Abbreviations: FP, fibroblast proliferation; CD, collagen depositions; MNC, mononuclear cells; PMNC, polymorphonuclear cells; NV, neovascularization; SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Figure 2.
Images of histological sections of excision wound tissue.
Notes: (A): histological section of wound tissue from negative control, (B): histological section of wound tissue of 5% extract treated mouse, (C): histological section of wound from 10% extract treated mouse, (D): histological section of wound from nitrofurazone 0.2% treated mouse.
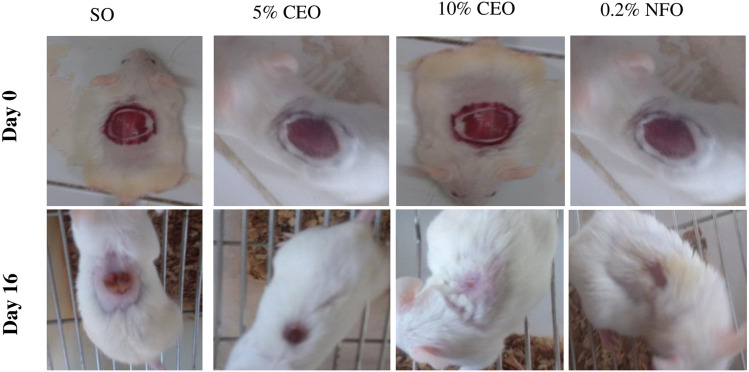
Figure 3.
Images showing improvement of excision wound tissue in the crude extract and nitrofurazone treated and control groups.
Notes: The images were randomly selected from such pair of images that were taken for each animal in the treatment and control groups.
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Burn Wound Model
Wound Contraction
In this model, the crude extract ointments showed significant (p<0.001) wound contraction effect at strengths of 5% and 10% starting from day 12 and 10, respectively, as compared to negative control. The standard treatment started to show significantly (p < 0.05) higher wound contraction on day 8 compared to the negative control. This treatment also produced more significant (p < 0.001) wound contraction on day 10 and onwards compared to the negative control. From day 10 to day 24 observations, both the 10% CEO and the standard drug were found to produce more significant (p < 0.001) wound contractions than the 5% CEO. The percent wound contraction produced by each treatment was progressive with post wounding days. Minimal percent wound contractions were recorded on day 2 of post wounding in all groups. Complete, 100%, wound contractions were achieved by 10% CEO and nitrofurazone treatment on day 24 of post wounding (Figure 4). On this day evaluation, the wound contraction in 5% CEO treat group was 94.13% whereas in simple ointment treated groups the value was 77.00% (Table 4).
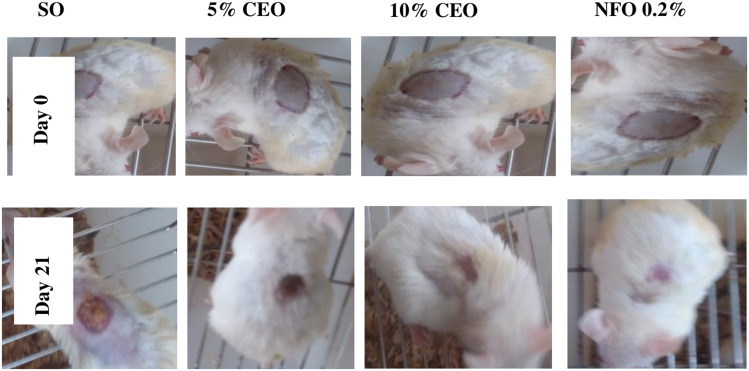
Figure 4.
Images showing healing progress of burn wound in the crude extract treated and control groups.
Note: The images were randomly selected from such pair of images that were taken for each animal in the treatment and control groups.
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Table 4.
Effect of the Crude Extract on Wound Contraction of Burn Wound
| Days | Group (Treatment) | |||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 (SO) | Group 2 (5% CEO) | Group 3 (10% CEO) | Group 4 (NFO 0.2%) | |||||
| Wound Area (mm2) | % Wound Contraction# | Wound Area (mm2) | % Wound Contraction# | Wound Area (mm2) | % Wound Contraction# | Wound Area (mm2) | % Wound Contraction# | |
| 2 | 291.50 ±1.26 | 2.83 | 291.33±1.05 | 2.89 | 291.00±1.03 | 3.00 | 290.17±1.14 | 3.28 |
| 4 | 283.17±1.08 | 5.61 | 282.50±0.76 | 5.83 | 282.00±0.97 | 6.00 | 281.83±1.35 | 6.06 |
| 6 | 274.5±1.34 | 8.50 | 273.33±0.88 | 8.89 | 273.00±0.73 | 9.00 | 272.33±1.36 | 9.22 |
| 8 | 264.67±1.58 | 11.78 | 264.33±0.71 | 11.89 | 260.67±1.05 | 13.11 | 258.50±1.45a*b* | 13.83 |
| 10 | 244.83±1.56 | 18.39 | 243.08±0.58 | 18.97 | 231.00±1.18a***b*** | 23.00 | 227.67±1.12a***b*** | 24.11 |
| 12 | 234.00±1.37 | 22.00 | 201.67±1.76a*** | 32.78 | 166.75±1.28a***b*** | 44.42 | 164.00±1.06a***b*** | 45.33 |
| 14 | 203.33±1.95 | 32.22 | 164.92±1.52a*** | 45.03 | 106.67±2.01a***b*** | 64.44 | 103.58±1.98a***b*** | 65.47 |
| 16 | 173.42±1.57 | 42.19 | 133.33±1.33a*** | 55.56 | 64.00±1.75a***b*** | 78.67 | 62.67±1.46a***b*** | 79.11 |
| 18 | 157.33±1.05 | 47.56 | 107.17±2.26a*** | 64.28 | 30.33±1.36a***b*** | 89.89 | 30.00±1.75a***b*** | 90.00 |
| 20 | 134.00±1.46 | 55.33 | 72.40±1.72a*** | 75.87 | 1.92±0.57a***b*** | 99.36 | 1.83±0.67a***b*** | 99.39 |
| 22 | 103.00±2.19 | 65.67 | 33.20±1.28a*** | 88.93 | 0.42±0.27a***b*** | 99.86 | 0.20±0.20a***b*** | 99.93 |
| 24 | 69.00±1.79 | 77.00 | 17.60±1.08a*** | 94.13 | 0.00±0.00a***b*** | 100.00 | 0.00±0.00a***b*** | 100.00 |
Notes: Wound areas are expressed as mean ± SEM (n = 6). aCompared to SO, bcompared to 5% CEO, *p < 0.05, ***p < 0.001, #% wound contraction is from the initial wound area (300 mm2).
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Period of Epithelialization
Similar to the results in excision wound model, the period for complete epithelialization was relatively short in the treatment groups. Accordingly, epithelialization period was significantly shorter in groups treated with 5% (p < 0.01), 10% CEO (p < 0.001), and NFO 0.2% (p < 0.001), compared to the negative control. The 10% CEO and NFO 0.2% treatments were able to more significantly shorten the period of epithelialization than the 5% CEO treatment. There was no significant difference in the effects of the 10% CEO and nitrofurazone treatments on the period of epithelialization. For groups treated with 5%, 10% CEO, and NFO 0.2%, the mean period required for epithelialization was 26.60, 21.83, and 21.67 days, respectively, whereas for the negative control group it was 30.40 days. The percent reduction in the period of epithelization by 5%, 10% CEO, and NFO 0.2% was 12.50%, 28.19%, and 28.72%, respectively (Figure 5).
Figure 5.
Effect of the crude extract on epithelialization period of burn wound (A) and percent reduction in the period of epithelialization (B).
Notes: The periods of epithelialization are expressed as mean ± SEM (n = 6). aCompared to SO, bCompared to 5% CEO, **p < 0.01, ***p < 0.001, #% reduction in period of epithelialization is relative to the SO treated group.
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Histopathology Analysis
Specimens of wound tissues were taken on day 21 for histopathology examination. Tissues from the 10% CEO and the standard drug treated animals showed moderate concentration of fibroblast and collagen depositions, low concentration of inflammatory cells, polymorphonuclear cells, and mononuclear cells in the examination. The specimen taken from 5% CEO treated animal also showed moderate collagen deposition and neovascularization and low fibroblast fibrillation. On the other hand, tissue taken from simple ointment (negative control) treated mouse showed the presence of necrotic tissue, moderate concentration of inflammatory cells and neovascularization and absence of fibroblast proliferation and collagen depositions (Table 5, Figure 6).
Table 5.
Histopathological Examination of the Effects of the Crude Extract of the Plant on Burn Wound
| Group | FP | CD | PMNC | MNC | NV |
|---|---|---|---|---|---|
| SO | – | – | ++ | ++ | ++ |
| 5% CEO | + | ++ | + | + | ++ |
| 10% CEO | ++ | ++ | – | + | + |
| NFO 0.2% | ++ | ++ | + | + | ++ |
Notes: Low concentration (+), moderate concentration (++), absent (-) for epidermal and/or dermal remodeling,
Abbreviations: FP, fibroblast proliferation; CD, collagen depositions; MNC, mononuclear cells; PMNC, polymorphonuclear cells; NV, neovascularization; SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Figure 6.
Images of histological sections of burn wound tissue in the crude extract treated and control groups.
Notes: (A): histological section of wound tissue from negative control mice, (B): histological section of wound tissue of 5% extract treated mice, (C): histological section of wound from 10% extract treated mice, (D): histological section of wound from 0.2% nitrofurazone treated mice.
Incision Wound Model
The tensile strength (wound breaking strength) of the incision wound was measure on day 10 of post wounding (Figure 7). The tensile strength was significantly (p < 0.001) higher in 10% crude extract and nitrofurazone treated groups as compared to the simple ointment treated and untreated groups. Both treatments also significantly (p < 0.01) increased the tensile strength in comparison with the 5% crude extract. The tensile strength was significantly increased in the 5% crude extract treated group compared to the negative control (p < 0.05) and untreated groups (p < 0.01). Relative to untreated group, the percent increases of the tensile strength were 30.59%, 59.467%, and 60.55% in 5%, 10% CEO, and NFO 0.2% treated groups, respectively (Table 6).
Figure 7.
Incision wound at day zero (A) and tensile strength measurement (B) during experiment.
Table 6.
Effect of the Extract Ointments on Tensile Strength in Incision Wound Model
| Group (Treatment) | Tensile Strength (g) | % Increase in Tensile Strength# |
|---|---|---|
| Group 1 (untreated) | 267. 25±8.57 | – |
| Group 2 (SO) | 282.67±9.35 | 5.77 |
| Group 3 (5% CEO) | 369.14±15.60a**b** | 30.59 |
| Group 4 (10% CEO) | 450.74±12.18a***b***c** | 59.46 |
| Group 5 (NFO 0.2%) | 453.82±25.79a***b***c** | 60.55 |
Notes: Tensile strengths are expressed as mean ± SEM (n = 6). aCompared to untreated group, bcompared to SO, ccompared to 5% CEO, **p < 0.01, ***p < 0.001, #% increase in tensile strength is relative to untreated group.
Abbreviations: SO, simple ointment; CEO, crude extract ointment; NFO, nitrofurazone ointment.
Wound Healing Activity of the Solvent Fractions
Excision Wound Model
Wound Contraction
All of the solvent fractions of the plant extract were found active in decreasing the wound area. Compared to the negative control, the 5% AFO showed significant wound contraction effect on day 6 (p < 0.05), and day 8–16 (p < 0.001) evaluations. The 10% AFO showed significant wound contraction starting from day 4 (p < 0.05) and was progressive on day 6 (p < 0.01) and day 8–16 (<0.001) measurements. The 10% AFO produced significantly (p < 0.001) more wound contraction than 5% AFO on day 8–12 evaluations and the effect of this preparation was comparable to the effects of the standard treatment. In measurements taken on day 8–16, the 5% CFO was found to produce significant (p < 0.05) reduction in the mean wound area, compared to the negative control. The 10% CFO showed significant wound contraction on day 8, 10, 12, 14 (p < 0.01), and 16 (p < 0.001) evaluations as compared to the simple ointment. The chloroform fraction was found to be less active than the aqueous fraction in wound healing. The 5% n-HFO caused significant (p < 0.05) wound contraction, compared to the negative control, in measurements taken on day 8–16. The 10% ointment of this fraction had significant wound contraction effects on day 8, 10, 12, 14 (p < 0.05), and 16 (p < 0.01) evaluations, compared to the negative control. Similar to chloroform fraction preparations, the n-hexane ointments were generally less effective than the AF ointments in wound contraction. The standard treatment, nitrofurazone 0.2% ointment, was significantly more effective than the 5% AFO and 5% and 10% CFO and h-HFO (Table 7).
Table 7.
The Effect of Solvent Fractions on Excision Wound Contraction
| Days | Wound Area (mm2) | |||||||
|---|---|---|---|---|---|---|---|---|
| SO | 5% AFO | 10% AFO | 5% CFO | 10% CFO | 5% n-HFO | 10% n-HFO | NFO 0.2% | |
| 2 | 274.67±0.99 | 272.00±1.37 | 270.67±0.88 | 273.83±1.19 | 273.50±1.12 | 274.17±1.22 | 273.67±0.88 | 272.33±1.02 |
| 4 | 235.83±1.01 | 234.00±1.39 | 230.67±0.88A* | 234.67±1.12 | 234.50±0.96 | 235.00±1.24 | 234.83±0.87 | 231.00±0.93A* |
| 6 | 169.33±0.88 | 164.67±1.20A* | 163.33±0.80A** | 168.67±0.88 | 168.5±1.26C* | 168.83±1.17 | 168.58±0.86C* | 163.67±1.05A** |
| 8 | 117.83±1.01 | 100.75±1.40A*** | 72.67±1.26A***B*** | 112.00±1.23A*B*** | 111.17±1.35A**B***C*** | 112.33±0.67A*B*** | 111.83±1.08A*B***C*** | 77.33±0.88A***B*** |
| 10 | 83.50±1.54 | 39.17±0.91A*** | 19.17±1.74A***B*** | 77.17±1.40A*B*** | 76.00±1.18A**B***C*** | 77.33±1.36A*B*** | 77.00±0.97A*B***C*** | 24.00±1.29A***B*** |
| 12 | 48.33±1.12 | 13.67±1.15A*** | 3.33±0.40A***B*** | 43.33±1.28A*B*** | 42.17±0.95A**B***C*** | 43.42±0.95A*B*** | 43.00±1.18A*B***C*** | 4.25±0.57A***B*** |
| 14 | 28.61±5.67 | 5.25±0.66A*** | 0.08±0.08A*** | 17.17±1.45A*B* | 16.50±1.34A**B*C*** | 17.33±1.20A*B** | 17.00±1.39A*B*C*** | 0.67±0.28A*** |
| 16 | 14.83±0.79 | 0.08±0.20A*** | 0.00±0.00A*** | 10.92±1.02A*B*** | 7.42±0.82A***B***C*** | 11.08±1.05A*B*** | 10.83±0.90A**B***C*** | 0.00±0.00A*** |
Notes: Results are expressed as mean ± SEM (n = 6). ACompared to SO, Bcompared to 5% AFO, Ccompared to 10% AFO, *p < 0.05, **p < 0.01 and ***p < 0.001.
Abbreviations: SO, simple ointment; AFO, aqueous fraction ointment; CFO, chloroform fraction ointment; n-HFO, n-hexane fraction ointment; NFO, nitrofurazone ointment.
As shown in Table 8 below, the percent wound contractions produced by the solvent fractions are progressive with post wounding days. Minimal wound contractions were recorded on day 2 of post wounding in all groups and increased with time. On day 16, 100% wound closure was recorded in 10% AFO and nitrofurazone treated groups. On this day, almost equal percent wound contraction (99.97%) was also observed in 5% AFO treated group.
Table 8.
Percent Wound Contractions Produced by Solvent Fractions of the Plant Extract
| Group (Treatment) | % Wound Contraction# | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | Day 16 | |
| Group 1 (SO) | 8.44 | 21.39 | 43.56 | 60.72 | 72.17 | 83.89 | 90.46 | 95.06 |
| Group 2 (5% AFO) | 9.33 | 22.00 | 45.11 | 66.42 | 86.94 | 95.44 | 98.25 | 99.97 |
| Group 3 (10% AFO) | 9.78 | 23.11 | 45.56 | 75.78 | 93.61 | 98.89 | 99.97 | 100.00 |
| Group 4 (5% CFO) | 8.72 | 21.78 | 43.78 | 62.67 | 74.28 | 85.56 | 94.28 | 96.36 |
| Group 5 (10% CFO) | 8.83 | 21.83 | 43.83 | 62.94 | 74.67 | 85.94 | 94.50 | 97.53 |
| Group 6 (5% n-HFO) | 8.61 | 21.67 | 43.72 | 62.56 | 74.22 | 85.53 | 94.22 | 96.31 |
| Group 7 (10% n-HFO) | 8.78 | 21.72 | 43.81 | 62.72 | 74.33 | 85.67 | 94.33 | 96.39 |
| Group 8 (NFO 0.2%) | 9.22 | 23.00 | 45.44 | 74.22 | 92.00 | 98.58 | 99.78 | 100.00 |
Note: #% wound contraction is from the initial wound area (300 mm2).
Abbreviations: SO, simple ointment; AFO, aqueous fraction ointment; CFO, chloroform fraction ointment; n-HFO, n-hexane fraction ointment; NFO, nitrofurazone ointment.
Period of Epithelialization
In case of solvent fractions, epithelialization period was significantly reduced (p< 0.001) in groups treated by 5% and 10% AFO as compared to in groups treated by the simple ointment. The effect of the AFO in decreasing the time required for complete epithelialization was comparable to that of the standard treatment. The period for complete epithelialization was also significantly shorten in groups treated by 5% (p < 0.05) and 10% (p < 0.001) CFO. Similarly, the period of epithelialization was significantly reduced by 5% (p < 0.05) and 10% (p < 0.01) n-HFO compared to the simple ointment. Compared to the aqueous fraction, both chloroform and n-hexane fractions were less effective in reducing the time for complete epithelialization. The highest percent reduction in the period of epithelialization, from the negative control group, was produced by 10% AFO (34.37%) followed by nitrofurazone (27.99%) (Table 9).
Table 9.
Effect of Solvent Fractions on the Period of Epithelialization in Excision Wound Model
| Group (Treatment) | Period of Epithelialization (Days) | % Reduction in the Period of Epithelialization# |
|---|---|---|
| Group 1 (SO) | 20.83±0.31 | - |
| Group 2 (5% AFO) | 15.83±0.31a*** | 24 |
| Group 3 (10% AFO) | 13.67±0.33a***b** | 34.37 |
| Group 4 (5% CFO) | 19±0.52a*b*** | 8.78 |
| Group 5 (10% CFO) | 18±0.65a***b**c*** | 13.59 |
| Group 6 (5% n-HFO) | 19±0.67a*b*** | 8.78 |
| Group 7 (10% n-HFO) | 18.67±0.42a**b***c*** | 10.45 |
| Group 8 (NFO 0.2%) | 15±0.52a*** | 27.99 |
Notes: Periods of epithelialization are expressed as mean ± SEM (n = 6). aCompared to SO, bcompared to 5% AFO, ccompared to 10% AFO, *p < 0.05, **p < 0.01, ***p < 0.001, #% reduction in period of epithelialization is from the SO treated group.
Abbreviations: SO, simple ointment; AFO, aqueous fraction ointment; CFO, chloroform fraction ointment; n-HFO, n-hexane fraction ointment; NFO, nitrofurazone ointment.
Discussion
The leaf part of U. simensis has been traditionally used for wound healing in different parts of Ethiopia and this was the initiative to conduct this study. The leaf juice of U. simensis is applied topically on affected parts as it is or after blending with butter.27 In this study, the crude extract of the plant and the solvent fractions ointment was prepared with the hydrophobic ointment base. This preparation agrees (at least in part) with the use of the plant material after blending with butter in the traditional settings. The wound healing activity of the plant extract was evaluated using three models: excision, incision and burn wound models while of the solvent fractions using excision wound model only. In excision and burn wound models the parameters measured were wound contractions from the initial, period of epithelialization and histopathologic changes and in incision wound model the tensile strength. The wound healing effect of each treatment was demonstrated by enhanced rate of wound contractions, rapid epithelialization, favorable histopathological changes and increase in tensile strength.
In excision wound model both 10% w/w and 5%w/w plant extract produce dose dependent increase in wound contraction rate and decrease in periods of epithelialization. This might be associated with the concentration of the bioactive constituents found in the extract ointment. In the current study 94.92% percent of wound contraction resulted by 10% extract on day 12 is comparable with other studies conducted on the plant in same genus (Urtica dioica) that shows (92.39%) of excision wound contraction on the 11th day of wounding.56
This enhanced wound contraction rate and decreased time required to complete epithelialization indicates better efficacy of the plant extract on wound healing. These could be associated with either the individual or synergistic effects of bioactive molecules present in the plant extract. During wound repair, stimulation of fibroblasts proliferation and anti-inflammatory activity is the mechanism by which herbal extracts might enhance the wound repair process.7
The findings in current study might be related to ability of the extract to enhance fibroblast proliferation, collagen synthesis and anti-inflammatory activity. In support of this, those preparations of the crude extract showed favorable histopathologic changes for wound healing as demonstrated by improved concentration of fibroblasts and collagen depositions, and the presence of low or no inflammatory cell depositions in histopathologic analysis of specimens from treatment groups. This is consistent with the previous study reports, which shows Urtica dioica possess wound healing activities in excision wounds primarily by accelerating cell proliferation, cell migration, and anti-inflammatory activities of the extract.22
The medicinal values of the plant extracts lie on their phytochemical constituents. The bioactive constituents may possess antioxidant and antimicrobial activities. According to previous study, 80% methanol extract of the leaves of U. simensis rich in polyphenolic content and had a good antioxidant activities.26 Oxidants are inhibitory factors to wound healing due to their damage on extracellular structure proteins, lipids, and DNA and stimulate signal transduction pathways to prolong the inflammatory phase of wound healing.57 This antioxidant activity of the leaves U. simensis extract might partly contribute to the resulted wound healing effect. This is consistent with another study on saponin extract of Urtica dioica that demonstrated antioxidant activity and significantly stimulate wound contraction (p<0.05) and allow for shortening the period of epithelialization.58
The other possible mechanism which may be attributed to the wound healing effect could be antibacterial activities of U. simensis extract reported in the previous study.41 Bacteria can delay wound healing via toxin secretion, these toxins tend to cause local necrosis and disrupt the delicate balance of critical inflammatory mediators which are necessary for healing progression.59 This is supported by another study that reported as topical administration of Urtica dioica extract containing gold nanoparticles nanocomposites ointment accelerated wound healing through reducing bacteria colonization and wound rate enhancing collagen biosynthesis and re-epithelialization on methicillin-resistant Staphylococcus aureus in infected wound model in mouse.60 The methanolic extracts of both leaves and stem bark of Holoptelea integrifolia (Urticaceae) also have an activity against the growth of different bacterial strains and demonstrated an accelerated wound-healing process.61
In case of solvent fractions, despite a general increase in percent wound contractions and short period of epithelialization upon application of the test substance on excision wound, the aqueous fraction showed better effect. Both 5% and 10% doses of aqueous fraction showed a significant (p< 0.001) rate of wound contraction effect and epithelialization as compared to negative control. This result is supported by a previous study stated that aqueous fraction of the same plant, U. simensis, showed the highest cardio protective and a dose-dependent free radical scavenging activity in comparison to hexane fractions.62 In addition, this result is comparable with a study on wound healing potential of the aqueous extract of Urtica dioica, suggests that the extract promotes cell migration along with cell proliferation in vitro and exhibited statistically significant (0.01) wound closure rate as compared to non-treated wounds in rats.22
In this study the result might be due either to the plant’s majority of bioactive constituents involved in cell proliferation as well as antioxidant activity are water soluble in nature or the delivering ability of oleaginous bases for water soluble active ingredients. On the other hand in this study there was no significant difference in wound contraction rate up to 14 days as well as period of epithelialization between the chloroform and hexane fraction treated groups. The reason might be no difference in kind or quantity of bioactive constituents of the chloroform and hexane fractions.
In the present study, the efficacy of the extract in wound healing was evaluated in partial thickness burn wound model. Partial thickness burn wounds can advance to full thickness after initial injury due to a number of mechanisms. Likely; microbial colonization, reduced dermal microcirculation, free radical generation and the release of a large number of cytokines and wound modulators. These cause protein denaturation and necrosis, makes the repair process more complicated.63
The results of this study indicated that the extract showed significant (p<0.001) wound contraction effect as compared to negative control and epithelialization time was significantly (p<0.001) reduced from the mean day of 30.40±0.75 (negative control) to 21.83±0.54 (10% extract ointment). The occurrence of enhanced epithelialization and wound contraction could be due to the ability of the extract to enhance fibroblast proliferation, collagen synthesis or antioxidant/anti-inflammatory activity as evidenced by histopathological improvements as demonstrated by moderate fibroblast proliferation and collagen deposition which were absent in simple ointment treated group (Figure 6). The wound healing activity of 10% extract ointment were closer to that of the standard drug in the course of treatment. These are indicative of the healing effect of the crude extract on burn wound as well and these findings are comparable with a previous study conducted on healing effect of nettle extract on second degree burn wounds that reported as the nettle extract showed a significance difference in angiogenesis and collagen deposition after 10 days of treatment as compared to negative control.24
The wound healing activity of the crude extract of the leaves of U. simensis was further demonstrated by measuring its effect on tensile (breaking) strength in incision wound model. The tensile strength was significantly increased in groups treated by the 5% (p < 0.01) and 10% (p < 0.001) crude extract ointments compared with that in the simple ointment treated and untreated groups. Tensile strength shows the resistance of the tissue being repaired to tension and can indicate the quality of the tissue.53 The increase in tensile strength by the extract might be associated with fibroblasts proliferation and migration to the wounded area and collagen deposition which gives strength and integrity to the wound matrix. This is consistent with another study conducted on the plant Holoptelea integrifolia (Urticaceae), both the leave and stem bark extracts have shown high breaking strength in incision wound with enhanced fibroblast proliferation, angiogenesis, keratinization and epithelization as compared to control group.61
The observed wound healing activity of the crude extract and solvent fractions may be primarily attributed to the phytochemicals identified in the qualitative phytochemical screening test. The crude extract of the leaves of U. simensis contain alkaloids, flavonoids, phenols, tannins, steroids, glycosides, anthraquinones, and terpenoids as confirmed in the screening test. This finding is consistent with other reports of poly phenolic content determination of the same plant.26
Phenolic compounds have been documented to possess anti-inflammatory, antioxidant and free radical scavenging effect.64 In the early inflammatory phase, neutrophils are abundant in the wound and they are essential for decontamination. However, if remaining in the wound over time cause excessive and prolonged inflammation results in delayed healing and increased scar formation. Therefore, removal of neutrophils is prerequisite for wounds to progress into the proliferative phase.10 In order to shorten the healing period as well as for minimal pain and scar, anti-inflammatory activity is required.65
Tannins could promote the process of wound healing and prevent complex consequences of infection due to their antibacterial, astringent and angiogenic properties and also can precipitate proteins in damaged tissues, resulting in rapid scab formation.55 Alkaloids facilitate the growth of colonies from fibroblast precursors and enhance wound healing in phases. Anthraquinones have been shown to stimulate tissue regeneration on excisional wound repair.66 Flavonoids and their derivatives improve vascularity and decrease lipid peroxidation. Terpenoids possess astringent and antimicrobial activities and promote the wound healing process.67 Glycosides possess antioxidant, antimicrobial, analgesic, anti-inflammatory, and immunomodulatory effects.68 These activities of the phytochemical constituents support the wound healing activity of the leaves of U. simensis.
These all are supported by experiments that show Urtica genus exhibit anti-inflammatory, immunomodulatory as well as antioxidant activities, all of which contribute towards the wound healing effectiveness of the plant.26
Conclusion
Generally, in the present study 80% methanol extract of the leaves of U. simensis and solvent fractions produced wound healing effect. Among the solvent fractions, the aqueous fraction elicited better wound healing activity. The result of this study provides scientific evidence supporting the medicinal use of the leaves of U. simensis for wound healing in the traditional medicine.
Acknowledgment
We would like to thank College of Medicine and Health Sciences, University of Gondar for its substantial contribution in facilitating laboratory rooms and instruments. We would like also to express our heartfelt gratitude to Dr. Wubayehu Kahaliw for his constructive suggestions and comments in the writing up of the manuscript. In addition, we are grateful to Mr. Abiyu Mola for identification and authentication of the plant material.
Data Sharing Statement
The data set not included in the article is available from both of the corresponding authors upon reasonable request.
Ethics Approval
The experimental animals were handled and cared in the experimental procedures in step with the local ethical and internationally accepted laboratory animal use, care, and welfare guidelines like Basel declaration, ICLAS Ethical Guideline, and EU directive on the protection of animals used for scientific purposes (46, 47). The study protocol was submitted and approved by the ethical review committee of Department of Pharmacology, College of Medicine and Health Sciences, University of Gondar with a Reference number of SoP4/285/12. The research was performed as per the agreement.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest regarding this work.
References
- 1.Alam G, Singh MP, Singh A. Wound healing potential of some medicinal plants. Int j Pharm Sci Rev Res. 2011;9(1):136–145. [Google Scholar]
- 2.Yadav S, Rawal G, Baxi M. Vacuum assisted closure technique: a short review. Pan Afr Med J. 2017;28:246. doi: 10.11604/pamj.2017.28.246.9606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care. 2019;8(2):39–48. doi: 10.1089/wound.2019.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Builders PF, Builders MI. Wound care: traditional African medicine approach. Worldwide Wound Healing. 2016;3:1–24. [Google Scholar]
- 5.Bashah DT, Dachew BA, Tiruneh BT. Prevalence of injury and associated factors among patients visiting the Emergency Departments of Amhara Regional State Referral Hospitals, Ethiopia: a cross-sectional study. BMC Emerg Med. 2015;15(1):1–7. doi: 10.1186/s12873-015-0044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT. Cutaneous scarring: basic science, current treatments, and future directions. Adv Wound Care. 2018;7(2):29–45. doi: 10.1089/wound.2016.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid-Based Compl Alt Med. 2011;2011:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejiram S, Kavalukas S, Shupp J, Barbul A. Wound healing. Wound Healing Biomaterials. 2016;45:3–39. [Google Scholar]
- 9.Wallace HA, Basehore BM, Zito PM. Wound healing phases. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 10.Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid-Based Compl Alt Med. 2019;2019:2684108. doi: 10.1155/2019/2684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 13.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi: 10.1089/wound.2013.0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. doi: 10.1159/000339613 [DOI] [PubMed] [Google Scholar]
- 15.Anderson K, Hamm RL. Factors that impair wound healing. J Am College Clin Wound Specialists. 2012;4(4):84–91. doi: 10.1016/j.jccw.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta D. Wound healing medicinal plants: an exhaustive literature; 2015.
- 17.Maver T, Kurečič M, Smrke DM, Kleinschek KS, Maver U. Plant-derived medicines with potential use in wound treatment. Herbal Med. 2018. doi: 10.5772/intechopen.72813 [DOI] [Google Scholar]
- 18.Farahpour MR. Medicinal plants in wound healing. Wound Healing-Current Perspect. 2019;45:33–47. [Google Scholar]
- 19.Wu Z-Y, Monro AK, Milne RI, et al. Molecular phylogeny of the nettle family (Urticaceae) inferred from multiple loci of three genomes and extensive generic sampling. Mol Phylogenet Evol. 2013;69(3):814–827. doi: 10.1016/j.ympev.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 20.Vogl S, Picker P, Mihaly-Bison J, et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J Ethnopharmacol. 2013;149(3):750–771. doi: 10.1016/j.jep.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain M. Medicinal plant genus Urtica- Traditional uses phytochemical and pharmacological review; 2019.
- 22.Kasouni AI, Chatzimitakos TG, Stalikas CD, Trangas T, Papoudou-Bai A, Troganis AN. The unexplored wound healing activity of urtica dioica l. extract: an in vitro and in vivo study. Molecules. 2021;26(20):6248. doi: 10.3390/molecules26206248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarma Kataki M, Murugamani V, Rajkumari A, Singh Mehra P, Awasthi D, Shankar Yadav R. Antioxidant, hepatoprotective, and anthelmintic activities of methanol extract of urtica dioica L. Leaves. Pharm Crops. 2012;3(1):38–46. [Google Scholar]
- 24.Akbari H, Fatemi MJ, Iranpour M, et al. The healing effect of nettle extract on second degree burn wounds. World J Plastic Surg. 2015;4(1):23. [PMC free article] [PubMed] [Google Scholar]
- 25.Eskedar G, Gulelat D, Getachew A. Nutritional profile of samma (Urtica simensis Steudel) leaves grown in Ethiopia. Int J Sci Innov Disc. 2013;3(1):153–160. [Google Scholar]
- 26.Seifu T, Mehari B, Atlabachew M, Chandravanshi B. Polyphenolic content and antioxidant activity of leaves of Urtica simensis grown in Ethiopia. Latin Am Appl Res. 2017;47(1):35–40. doi: 10.52292/j.laar.2017.295 [DOI] [Google Scholar]
- 27.Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4. doi: 10.1186/1746-4269-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitew H, Gebregergs H, Tuem KB, Yeshak MY. Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. Ethiopian J Health Develop. 2019;33(2):45. [Google Scholar]
- 29.Gebrezgabiher G, Kalayou S, Sahle S. An ethno-veterinary survey of medicinal plants in woredas of Tigray region, Northern Ethiopia. Int J Biodivers Conserv. 2013;5(2):89–97. [Google Scholar]
- 30.Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. [Google Scholar]
- 31.Birhan Y, Kitaw S, Alemayehu Y, Mengesha N. Ethnobotanical study of medicinal plants used to treat human diseases in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. SM J Med Plant Stud. 2017;1(1):1–9. doi: 10.36876/smjmps.1006 [DOI] [Google Scholar]
- 32.Meragiaw M, Asfaw Z, Argaw M. The status of ethnobotanical knowledge of medicinal plants and the impacts of resettlement in Delanta, northwestern Wello, northern Ethiopia. Evid-Based Compl Alt Med. 2016;2016:1–24. doi: 10.1155/2016/5060247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):55. doi: 10.1186/s13002-017-0182-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regassa R, Bekele T, Megersa M. Ethnobotanical study of traditional medicinal plants used to treat human ailments by Halaba people, southern Ethiopia. J Med Plants Stud. 2017;5(4):36–47. [Google Scholar]
- 35.Seble WY, Zemede A, Ensermu K. Ethnobotanical study of medicinal plants used by local people in Menz Gera Midir District, North Shewa Zone, Amhara Regional State, Ethiopia. J Med Plants Res. 2018;12(21):296–314. doi: 10.5897/JMPR2018.6616 [DOI] [Google Scholar]
- 36.Dalle MGG. Ethnobotanical Study of Medicinal Plants in Nagelle Arsi District. Ethiopia: West Arsi Zone of Oromia; 2019. [Google Scholar]
- 37.Alemayehu G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa Zone of Amhara Region, Ethiopia. J Med Plants Stud. 2015;3(6):01–11. [Google Scholar]
- 38.Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):25. doi: 10.1186/s13002-015-0014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsegaye W, Urga K, Asres K. Antidiabetic activity of samma (urtica simensis hochst. ex. a. rich.) in streptozotocin-induced diabetic mice. Ethiopian Pharm J. 2009;27(2):75–82. [Google Scholar]
- 40.Bayba K, Dubale A, Mehari B, Atlabachew M. Chemical composition of urtica simensis grown in different regions of Ethiopia. J Chem. 2020;2020:1–8. doi: 10.1155/2020/9546178 [DOI] [Google Scholar]
- 41.Kassa F, Nedi T, Feleke A, Eguale T, Alemayehu H, Shibeshi W. In vitro antimicrobial activity of 80% methanol extract and solvent fractions of urtica simensis hochst. ex. A. Rich. (Urticaceae) leaves against pathogenic bacteria and fungi. Ethiopian Pharm J. 2020;36(2):97–108. doi: 10.4314/epj.v36i2.3 [DOI] [Google Scholar]
- 42.Süntar I, Akkol EK, Nahar L, Sarker SD. Wound healing and antioxidant properties: do they coexist in plants? Free radic antioxid. 2012;2(2):1–7. doi: 10.5530/ax.2012.2.2.1 [DOI] [Google Scholar]
- 43.Council NR. Guide for the care and use of laboratory animals; 2010.
- 44.Mohr BJ, Fakoya FA, Hau J, Souilem O, Anestidou L. The governance of animal care and use for scientific purposes in Africa and the Middle East. ILAR j. 2016;57(3):333–346. doi: 10.1093/ilar/ilw035 [DOI] [PubMed] [Google Scholar]
- 45.Ayal G, Belay A, Kahaliw W. Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (Fabaceae) in mice. Wound Med. 2019;25(1):100151. doi: 10.1016/j.wndm.2019.100151 [DOI] [Google Scholar]
- 46.Senbeta A, Awas T, Gure A. The qualitative and quantitative phytochemical investigation of crinum species in Ethiopia. Int J Photochem Photobiol. 2019;3(1):1. doi: 10.11648/j.ijpp.20190301.11 [DOI] [Google Scholar]
- 47.Namunana S, Lutoti S, Nyamaizi G, et al. Formulation, development and validation of a wound healing herbal ointment from extracts of bidens pilosa and aloe barbadensis. J Pharm Pharmacol Res. 2018;2:32–38. doi: 10.26502/jppr.0008 [DOI] [Google Scholar]
- 48.Allen LV Jr, Ansel HC. Ansel’s pharmaceutical dosage forms and drug delivery systems; 2014.
- 49.OECD. Test No. 402: acute dermal toxicity; 2017.
- 50.Demilew W, Adinew GM, Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of acanthus polystachyus Delile (Acanthaceae). Evid-Based Compl Alt Med. 2018;2018:1–9. doi: 10.1155/2018/2047896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):341. doi: 10.1186/s12906-015-0878-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma GN, Dubey SK, Sati N, Sanadya J. Evaluation of wound healing activity of Aegle marmelos seeds. Pharmacologyonline. 2011;2:171–178. [Google Scholar]
- 53.Beshir K, Shibeshi W, Ejigu A, Engidawork E. In-vivo wound healing activity of 70% ethanol leaf extract of Beciumgrandiflorum Lam. (Lamiaceae) in mice. Ethiop Pharm J. 2016;32:117–130. [Google Scholar]
- 54.Kumar V, Khan A, Nagarajan K. Animal models for the evaluation of wound healing activity. Int Bull Drug Res. 2013;3:93–107. [Google Scholar]
- 55.Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9). doi: 10.5812/ircmj.19960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zouari Bouassida K, Bardaa S, Khimiri M, et al. Exploring the urtica dioica leaves hemostatic and wound-healing potential. Biomed Res Int. 2017;2017:1047523. doi: 10.1155/2017/1047523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.HdS P, Pereira A, Blasius MB, et al. In vitro evaluation of the antioxidant activity and wound healing properties of Jaboticaba (Plinia peruviana) fruit peel hydroalcoholic extract. Oxid Med Cell Longev. 2016;2016:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Razika L, Thanina AC, Nadjiba C-M, Narimen B, Mahdi DM, Karim A. Antioxidant and wound healing potential of saponins extracted from the leaves of Algerian Urtica dioica L. Pak J Pharm Sci. 2017;30:1023–1029. [PubMed] [Google Scholar]
- 59.Mendoza RA, Hsieh J, Galiano RD. The impact of biofilm formation on wound healing. Wound Healing-Current Perspect. 2019;10:e65. [Google Scholar]
- 60.Choodari Gharehpapagh A, Farahpour MR, Jafarirad S. The biological synthesis of gold/perlite nanocomposite using Urtica dioica extract and its chitosan-capped derivative for healing wounds infected with methicillin-resistant Staphylococcus aureus. Int J Biol Macromol. 2021;183:447–456. doi: 10.1016/j.ijbiomac.2021.04.150 [DOI] [PubMed] [Google Scholar]
- 61.Reddy BS, Reddy RK, Naidu V, et al. Evaluation of antimicrobial, antioxidant and wound-healing potentials of Holoptelea integrifolia. J Ethnopharmacol. 2008;115(2):249–256. doi: 10.1016/j.jep.2007.09.031 [DOI] [PubMed] [Google Scholar]
- 62.Tesfaye BA, Berhe AH, Wondafrash DZ, Berhe DF. Cardioprotective effect of crude extract and solvent fractions of Urtica simensis leaves on cyclophosphamide-induced myocardial injury in rats. J Exp Pharmacol. 2021;13:147. doi: 10.2147/JEP.S270038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singer AJ, Boyce ST. Burn wound healing and tissue engineering. J Burn Care Res. 2017;38(3):e605–e13. doi: 10.1097/BCR.0000000000000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Begashaw B, Mishra B, Tsegaw A, Shewamene Z. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Complement Altern Med. 2017;17(1):1–11. doi: 10.1186/s12906-017-1841-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma Y, Jeyabalan G, Singh R. Potential wound healing agents from medicinal plants: a review. Pharmacologia. 2013;4(5):349–358. doi: 10.5567/pharmacologia.2013.349.358 [DOI] [Google Scholar]
- 66.Tsala DE, Amadou D, Habtemariam S. Natural wound healing and bioactive natural products. Phytopharmacology. 2013;4(3):532–560. [Google Scholar]
- 67.Belachew TF, Asrade S, Geta M, Fentahun E. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce) JF Gmel in mice. Evid-Based Compl Alt Med. 2020;2020:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganesh S, Vennila JJ. Screening for antimicrobial activity in Acanthus ilicifolius. Arch Appl Sci Res. 2010;2(5):311. [Google Scholar]