Abstract
Objective
The objective of this study was to compare and evaluate the diagnostic value of serum carbohydrate antigen 125 (CA125) and/or human epididymis protein 4 (HE4) and a panel of novel multiple biomarkers in patients with ovarian tumors to identify more accurate and effective markers for screening ovarian cancer.
Methods
Candidate ovarian cancer biomarkers were selected based on a literature search. Dozens of candidate biomarkers were examined using 143 serum samples from patients with ovarian cancer and 157 healthy serum samples as non-cancer controls. To select the optimal marker panel for an ovarian cancer classification model, a set of biomarker panels was created with the number of possible combinations of eight biomarkers. Using the set of biomarkers as an input variable, the optimal biomarker panel was selected by examining the performance of the biomarker panel set using the Random Forest algorithm as a non-linear classification method and a 10-fold cross-validation technique.
Results
The final selected optimal combination of five biomarkers (CA125, HE4, cancer antigen 15-3, apolipoprotein [Apo] A1, and ApoA2) exhibited a sensitivity of 93.71% and specificity of 93.63% for ovarian cancer detection during validation.
Conclusion
Combining multiple biomarkers is a valid strategy for ovarian cancer diagnosis and can be used as a minimally invasive screening method for early ovarian cancer. A panel of five optimal biomarkers, including CA125 and HE4, was verified in this study. These can potentially be used as clinical biomarkers for early detection of ovarian cancer.
Keywords: Ovarian cancer, Screening, Biomarkers, Algorithm
Introduction
Ovarian cancer is one of the most common malignant tumors of the female reproductive system and the 5th most common cause of death in women. Unfortunately, nearly 80% of ovarian cancers are diagnosed at advanced stages (III/IV) because of their asymptomatic nature [1,2]. Thus, the early detection of ovarian cancer is critical for reducing mortality. However, early-stage diagnosis has been difficult to achieve because ovarian cancer exhibits a wide range of morphological, clinical, and genetic variations during tumor progression in addition to asymptomatic progression [3,4]. Thus, the biggest challenge in ovarian cancer diagnosis is the lack of effective screening methods.
Ovarian cancer diagnosis requires surgery and biopsy. Owing to the anatomical location of the ovary within the abdominal cavity, biopsy for diagnosis of ovarian cancer is impossible, except with surgical methods [5]. However, tissue biopsy has disadvantages in that the patient is severely affected. Tissue biopsy also increases the risk of infection, which may require a recovery period after hospitalization and examination.
In contrast, a non-invasive method using blood (blood collection) has the advantages of being simple and painless. It does not require a recovery period after hospitalization or examination. In addition, liquid biopsy for early ovarian cancer diagnosis avoids the adverse effects associated with tissue biopsy. Early diagnosis is possible even in patients who have not yet developed ovarian cancer. It is also advantageous in periodic monitoring of the progress of treatment in patients with ovarian cancer [6]. Therefore, screening high-risk patients with ovarian cancer using liquid biopsy and developing more effective early diagnostic methods can compensate for the problems associated with the existing tissue biopsy methods and considerably reduce medical expenses. Thus, an accurate, non-invasive diagnostic method for ovarian cancer is necessary to increase the early diagnosis rate. Unfortunately, the information provided by a single tumor marker is limited and it is almost impossible to detect cancer using a single tumor marker [7,8].
Several tumor biomarkers have been evaluated in laboratory examinations. Carbohydrate antigen 125 (CA125) is a traditional marker used for ovarian cancer screening. It was first described in the early 1980s. In cases of ovarian cancer, serum CA125 levels may be elevated. However, this marker has low sensitivity in the early stages of ovarian cancer [9]. Increased CA125 levels have also been reported in other physiological or pathological conditions such as menstruation, pregnancy, endometriosis, and inflammatory diseases of the peritoneum [10]. Other biomarkers, such as human epididymis protein 4 (HE4) [11], have been developed to improve the detection specificity of ovarian carcinomas. HE4 is overexpressed in ovarian cancer. A meta-analysis of 13 studies on HE4 showed that serum HE4 is a common indicator of ovarian cancer and the diagnostic accuracy of CA125 can be improved when combined with HE4 [12].
Ovarian cancer screening based on the simultaneous measurement of multiple markers known to be associated with ovarian cancer may be an alternative and effective method. Over the past few decades, considerable efforts have been made to identify novel cancer biomarkers for use in clinical practice. However, a striking discrepancy exists between the amount of effort directed toward biomarker discovery and the number of markers used in clinical practice. To use multiple markers for ovarian cancer diagnosis, it is necessary to develop and apply a unique algorithm to analyze the expression patterns of multiple ovarian cancer-related markers [13,14].
We previously reported diagnostic models for lung, gastric, and breast cancers using multiple serum biomarkers. These algorithms can accurately classify a test set and a training set [15–18]. The objective of this study was to compare and evaluate the diagnostic value of serum CA125 and/or HE4 and a novel panel of multiple biomarkers for patients with ovarian tumors to identify more accurate and effective markers for screening ovarian cancer.
Materials and methods
1. Cohorts and serum samples
The specimens used in this study were approved by the Institutional Review Board of the BIOINFRA Life Science Inc. (No. 1-700097-B-N-01). Serum samples from patients with ovarian cancer were obtained before initiating any therapeutic approach. Data were obtained from the Korea Regional Biobank of Keimyung University Hospital, Kangwon National University Hospital, and Inje University Pusan Paik Hospital. Serum samples from asymptomatic healthy donors were obtained from the Korea Regional Biobank of Seoul National University Hospital. Healthy controls with a known history of cancer, high-grade dysplasia, autoimmune disease, chronic kidney disease, pregnancy, or inflammatory conditions requiring medical management were excluded from the study. Samples from healthy controls and patients with ovarian cancer were collected after obtaining appropriate consent from the individuals, clearly labeled (sex of individual), and stored at −80°C or in liquid nitrogen without repeated re-thawing until analysis. In addition, staging information for ovarian cancer specimens was assigned according to the TNM clinical classification (cTNM), TNM patho-taxonomic classification (pTNM), or a standard equivalent. Human resources were used in this study. To identify the optimal multi-biomarker combination, sera from 143 patients with ovarian cancer and 157 healthy controls were used to create and validate a classification model in this study (Table 1). Ovarian cancer stage was based on the final pathological diagnosis after resection. Ovarian cancer patient and control samples were age-matched.
Table 1.
Characteristics of ovarian cancer patient and healthy control samples
| Characteristic | Healthy control | Epithelial ovarian cancer | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | CA125 | HE4 | N | CA125 | HE4 | |
| Age (yr) | 157 | 13.70±8.48 | 46.93±14.82 | 143 | 572.18±936.35 | 315.77±430.56 |
|
| ||||||
| <50 | 66 | 16.83±10.45 | 41.69±8.31 | 60 | 455.37±827.43 | 212.02±338.72 |
|
| ||||||
| <60 | 56 | 10.87±6.11 | 44.88±9.86 | 51 | 609.81±985.35 | 289.59±422.24 |
|
| ||||||
| <70 | 22 | 12.79±5.57 | 54.71±18.35 | 20 | 733.28±959.36 | 623.51±599.92 |
|
| ||||||
| ≥70 | 13 | 11.51±4.39 | 69.18±25.10 | 12 | 727.79±1,219.93 | 417.88±300.07 |
|
| ||||||
| Stage, total | - | - | - | 143 | 572.18±936.35 | 315.77±430.56 |
|
| ||||||
| I | - | - | - | 64 | 206.98±402.85 | 126.24±165.46 |
|
| ||||||
| II | - | - | - | 21 | 1,110.26±1,376.20 | 381.36±365.22 |
|
| ||||||
| III | - | - | - | 39 | 934.64±1,160.06 | 520.82±585.90 |
|
| ||||||
| VI | - | - | - | 16 | 437.37±464.63 | 521.91±519.76 |
|
| ||||||
| N/A | - | - | - | 3 | 603.57±996.33 | 135.01±117.46 |
Values are presented as mean±standard deviation or number.
CA125, carbohydrate antigen 125; HE4, human epididymis protein 4; N/A, not available.
2. Measurement of serum protein markers
The following eight biomarkers were measured and screened in this study: apolipoprotein A1 (ApoA1), transthyretin, CA125, carcinoembryonic antigen, cytokeratin fragment 21-1, cancer antigen 15-3, and HE4. They were measured on Cobas c501 or Cobas e601 (Hoffmann-La Roche AG., Basel, Switzerland) using electrochemiluminescent detection. ApoA2 was measured using a Toshiba 120FR by immunonephelometry, according to the manufacturer’s instructions. It may be noted that the analysis of multiple biomarkers in the proposed model is expected to cost approximately $32 and the cost of testing is expected to be approximately $56.
3. Statistical methods
In this study, before generating a classification model to distinguish ovarian cancer from healthy controls, the distinguishing abilities of eight selected candidate biomarkers were reviewed through basic statistical analyses, such as boxplot and Mann-Whitney U-test. A set of biomarker panels was created to identify the optimal marker panel for the ovarian cancer classification model. The number of possible biomarker combinations was eight. Using the set as an input variable, the optimal biomarker panel was selected by examining the performance of each biomarker panel set using Random Forest (RF) algorithm, a non-linear classification method, and a 10-fold cross-validation technique.
To evaluate and compare candidate biomarker sets, we used the area under the curve (AUC) and receiver operating characteristic (ROC) curve, which are performance metrics used in classification problems. The ROC curve is a graph that displays the performance for all thresholds simultaneously. The AUC refers to the lower area of the ROC curve. A value closer to 1 indicates an excellent model, whereas a value closer to 0 indicates a poor model. Detailed performance by stage was compared using sensitivity, indicating the probability of classifying actual ovarian cancer as ovarian cancer, and specificity, indicating the probability of classifying actual healthy controls as healthy. All analyses were performed using R statistical analysis tool (version 4.0.3; R Core Team, Auckland, New Zealand).
To determine whether the selected optimal biomarker panel shows excellent classification performance in other classification methods, the performance was verified using three other algorithms (general linear model [GLM], extreme gradient boosting [XGBoost], and generalized linear model random forest [GLMRF]). Logistic regression is a special case of a GLM. It is a representative method used in various classification and prediction models. This method has the advantage of being able to interpret prediction results using formulas. RF and XGBoost are widely used machine-learning algorithms as ensemble techniques. In general, these methods exhibit high performance in classification problems. However, they have the disadvantages of a high possibility of overfitting and difficulty interpreting the results. We also used the GLMRF model, which has the advantage of both linear (explanatory) and non-linear classification models.
Results
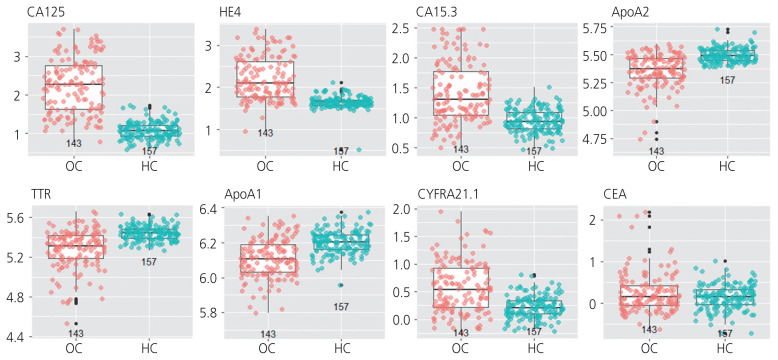
First, we selected eight candidate biomarkers for screening high-risk ovarian cancer based on literature searches [19–27]. The expression levels of these eight candidate biomarkers were predicted to be important for screening ovarian cancer in healthy controls and patients with ovarian cancer (Table 2). To analyze the performance of each biomarker, the expression levels of the eight candidate biomarkers were measured and analyzed in samples from 157 healthy control subjects and 143 patients with ovarian cancer. Seven out of the eight biomarkers were found to be effective in distinguishing between healthy controls and patients with ovarian cancer using the Mann-Whitney U-test. This was confirmed using box plots (Fig. 1).
Table 2.
Preclinical study report of eight candidate biomarkers
| Biomarker | Function | Ref. |
|---|---|---|
| CA125 | CA125 is a high molecular weight glycoprotein with increased expression in approximately 90% of patients with advanced epithelial ovarian cancer and is well known as one of the most important biomarkers used to monitor epithelial ovarian cancer. As a screening indicator, CA125 has been applied to screen out patients with ovarian cancer in the population and to differentiate ovarian cancer from benign diseases. | [19] |
| HE4 | HE4 might play a protective role in the progression of epithelial ovarian cancer by inhibiting cell proliferation and is expressed in malignant ovarian tissues at a significantly higher rate than that of benign tumors and normal ovarian tissue and is regarded as a serum tumor marker which has high sensitivity and specificity in screening tests. HE4 can be used in the detection and staging of treatment for ovarian cancer. | [20,21] |
| CA15.3 | CA15.3 is an aspecific tumor marker characteristic of cancer proliferation and the elevated levels of CA15.3 have been associated with ovarian malignancy, measurement of this biomarker has been proposed to be of some clinical value in combination with CA125 for diagnosis and monitoring of ovarian cancer. | [22,23] |
| ApoA2 | ApoA2 is the second most abundant apolipoprotein of high-density lipoproteins, which modulate cholesterol transport and metabolism and concentrations of ApoA2 is higher in malignant compared with either borderline or benign ovarian cyst fluids. | [24] |
| TTR | TTR is a normal serum protein synthesized primarily in the liver, the choroid plexus and the retina and is found to be downregulated in ovarian cancer. Lower level of serum TTR level has been observed in ovarian cancer. TTR has been used with other biomarkers to diagnose ovarian cancer. | [25] |
| ApoA1 | ApoA1 played an important role in the inhibition of tumor progression through different pathways and is decreased in the serum of ovarian cancer patients, has been evaluated as a screening and diagnostic biomarker of epithelial ovarian cancer. | [24] |
| CYFRA21.1 | The serum CYFRA21.1 level was significantly correlated with many prognostic factors, including recurrence and survival and acts as a good complementary diagnostic biomarker and may be superior to CA125 as a prognostic indicator in epithelial ovarian cancer. | [26] |
| CEA | CEA is one of the longest known tumor antigens and elevated serum levels is demonstrated in patients with ovarian adenocarcinomas, up to 87–88% in mucinous histotypes. The CEA in combination with CA125 can differentiate between malignant ovarian disease and malignant non-ovarian disease. | [27] |
Ref., reference; CA125, carbohydrate antigen 125; HE4, human epididymis protein 4; CA15.3, cancer antigen 15-3; ApoA2, apolipoprotein A2; TTR, transthyretin; ApoA1, apolipoprotein A1; CYFRA21.1, cytokeratin fragment 21-1; CEA, carcinoembryonic antigen.
Fig. 1.
Box plots and Mann-Whitney U-test of single biomarker. Seven out of the eight biomarker candidates were found to be effective in distinguishing healthy controls from patients with ovarian cancer in Mann-Whitney U-test and box plots. CA125, carbohydrate antigen 125; OC, ovarian cancer; HC, healthy control; HE4, human epididymis protein 4; CA15.3, cancer antigen 15-3; ApoA2, apolipoprotein A2; TTR, transthyretin; ApoA1, apolipoprotein A1; CYFRA21.1, cytokeratin fragment 21-1; CEA, carcinoembryonic antigen.
For model generation, the samples were randomized and 10-fold cross-validation and RF methods were used. At this time, the age information was reflected. Table 3 shows examples of representative sets of multiple biomarkers with improved performance compared with single biomarkers. The results revealed that several biomarkers showed high accuracy in ovarian cancer diagnosis. A combination of five biomarkers consisting of CA125, HE4, ApoA1, and ApoA2 was confirmed to be most optimal among the various combinations. Specifically, the optimal set of these five biomarkers had an AUC of 0.97, sensitivity of 93.71%, and specificity of 90.45%.
Table 3.
The area under the curve values and accuracy of various multiple biomarkers (at fixed specificity)
| Biomarker set | AUC | Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| CA125, HE4, CA15.3, CEA, ApoA1, ApoA2 | 0.965 | 90.45 | 93.71 | 89.93 | 94.04 |
| CA125, HE4, CYFRA21.1, CA15.3, ApoA1, ApoA2 | 0.965 | 90.45 | 93.01 | 89.86 | 93.42 |
| CA125, HE4, ApoA1 | 0.966 | 90.45 | 91.61 | 89.73 | 92.21 |
| CA125, HE4, CA15.3, CEA, ApoA1 | 0.966 | 90.45 | 93.01 | 89.86 | 93.42 |
| CA125, HE4, CEA, ApoA1, ApoA2 | 0.966 | 90.45 | 93.71 | 89.93 | 94.04 |
| CA125, HE4, CYFRA21.1, ApoA1, ApoA2 | 0.966 | 90.45 | 93.01 | 89.86 | 93.42 |
| CA125, HE4, CA15.3, TTR, ApoA1 | 0.967 | 90.45 | 91.61 | 89.73 | 92.21 |
| CA125, HE4, ApoA1, ApoA2 | 0.967 | 90.45 | 93.71 | 89.93 | 94.04 |
| CA125, HE4, CEA, ApoA1 | 0.967 | 90.45 | 91.61 | 89.73 | 92.21 |
| CA125, HE4, CA15.3, ApoA1, ApoA2 | 0.970 | 90.45 | 93.71 | 89.93 | 94.04 |
AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; CA125, carbohydrate antigen 125; HE4, human epididymis protein 4; CA15.3, cancer antigen 15-3; CEA, carcinoembryonic antigen; ApoA1, apolipoprotein A1; ApoA2, apolipoprotein A2; CYFRA21.1, cytokeratin fragment 21-1; TTR, transthyretin.
To compare the performance of CA125 and/or HE4, the single biomarkers commonly used for screening ovarian cancer, with the optimal combination of biomarkers identified in this study, the mean serum concentrations of these five biomarkers were compared between healthy controls and ovarian cancer samples. Ovarian cancer was determined based on reference values used in clinical practice (CA125 reference range for normal: ≤35 U/mL; HE4 reference range for normal: ≤60.50 pmol/L for <40 years old; ≤76.20 pmol/L for 40–49 years old; ≤74.30 pmol/L for 50–59 years old; ≤82.90 pmol/L for 60–69 years old; and ≤104.00 pmol/L for ≥70 years old). Although combining CA125 and HE4 showed improved sensitivity compared to CA125 or HE4 as single biomarkers, the combination exhibited lower performance than the optimal set of five biomarkers (Table 4). The optimal set of five multiple biomarkers was found to have a sensitivity of 93.71%, which was higher than that of the combination of CA125 and HE4 (85.31%).
Table 4.
Comparison of area under the curve values and accuracy of CA125 and/or HE4 vs. multiple biomarkers
| Biomarker set | Specificity (%) | Sensitivity (%) | |
|---|---|---|---|
| CA125 vs. multiple marker set | CA125, HE4, CA15.3, ApoA1, ApoA2 | 96.18 | 88.11 |
| CA125 | 96.18 | 80.42 | |
| HE4 vs. multiple marker set | CA125, HE4, CA15.3, ApoA1, ApoA2 | 97.45 | 86.71 |
| HE4 | 97.45 | 69.23 | |
| CA125+HE4 vs. multiple marker set | CA125, HE4, CA15.3, ApoA1, ApoA2 | 93.63 | 93.71 |
| CA125+HE4 | 93.63 | 85.31 |
CA125, carbohydrate antigen 125; HE4, human epididymis protein 4; CA15.3, cancer antigen 15-3; ApoA1, apolipoprotein A1; ApoA2, apolipoprotein A2.
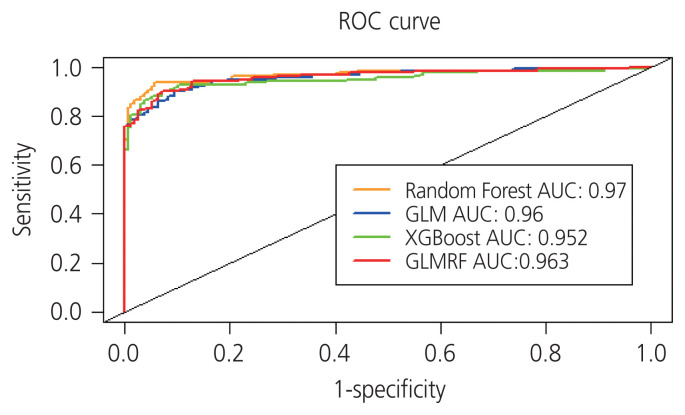
Three analytical methods, including GLM, XGBoost, and GLMRF, were used to validate the performance of the selected optimal combination of markers. ROC curves were generated to determine the sensitivity of the optimal set of five biomarkers. ROC curves for patients with ovarian cancer and healthy controls using the four classification methods are shown in Fig. 2. The multiple biomarker set was found to have a sensitivity of 88.11–93.71% and a specificity of approximately 90%.
Fig. 2.
Two receiver operating characteristic (ROC) curves of 10-fold cross-validation for four classification methods for optimal biomarker sets. All four methods showed similar Random Forest performances. The X-axis represents the 1-specificity and the Y-axis represents sensitivity. The set of multiple biomarkers exhibited the highest performance in early diagnosis of ovarian cancer. AUC, area under the curve; GLM, general linear model; XGBoost, extreme gradient boosting; GLMRF, generalized linear model random forest.
The results showed that the three analytical methods showed similar RF performance (Fig. 2). In particular, in the case of early ovarian cancer (stages 1 and 2), it showed a high level of sensitivity, ranging from 82.35–91.76%. Based on these results, the multiple biomarker set identified in this study is expected to play an important role in the early diagnosis of ovarian cancer.
Discussion
Ovarian cancer is the most common malignancy of the female reproductive system. It is the most lethal malignancy owing to its advanced stage at presentation. To reduce the mortality rate of ovarian cancer, it is important to develop more effective and accurate diagnostic methods.
Unfortunately, effective screening tools for early detection of ovarian cancer have not yet been established. Although most gynecologic oncologists use multimodal screening based on transvaginal ultrasonography and CA125 for early detection of ovarian cancer, such screening methods are expensive. In addition, these tests are not very sensitive or specific [28]. For this reason, several attempts have been made recently to develop methods to identify patients at high risk for ovarian cancer. Liquid biopsy has the advantage of being non-invasive for the early detection of cancer. It also enables multiple repetitions and easy disease monitoring.
Most ovarian tumor markers reported to date have poor sensitivity and specificity, thus limiting their use in differentiating between malignant and benign ovarian masses. CA125 is the most widely used tumor marker not only for the diagnosis of epithelial ovarian cancer, but also for monitoring treatment success. However, CA125 has some limitations in terms of sensitivity and specificity, as it is elevated only in 50% of women with early-stage disease. In addition, it is detected in other benign gynecological conditions, such as endometriosis and pregnancy, as well as in non-gynecological conditions, such as cirrhosis and congestive heart diseases [9,10].
Recently, several new markers have been proposed and investigated for early detection of ovarian cancer. Among these, HE4, a precursor of the human epididymis protein, has received special attention. HE4 is as specific and sensitive as CA125. Additionally, it has superior sensitivity in detecting stage I ovarian cancer. The serum level of HE4 is elevated in association with preoperative CA125, advanced stage, grade, and the presence of residual tumors. Moreover, HE4 levels correlates with the prognosis and overall survival of patients with ovarian cancer [11]. In addition, both HE4 and the risk of ovarian malignancy algorithm (ROMA) scores are superior to CA125 in the diagnosis of ovarian cancer in Korean women with pelvic masses [29].
The ROMA scores were developed to improve the inherent characteristics of a single biomarker. The ROMA model has attracted considerable attention in recent years. ROMA incorporates CA125, HE4, and menopausal status to assign women with an adnexal mass into high- or low-risk groups for finding an ovarian malignancy [30]. Although the clinical value of the ROMA index in the diagnosis of ovarian cancer has been widely reported in the literature, most of these studies used small sample sizes and the results are inconsistent. However, its reliability needs to be explored further. Increasing the sample size can improve the credibility of the results and resolve the inconsistency problem among results of these studies. Although these markers have reliable specificity, they are not very sensitive.
The results of the present study revealed the sensitivity of the optimal set of five multiple biomarkers to be 93.71%, which is higher than that of the combination of CA125 and HE4 (85.31%). A similar RF performance was observed even when three analytical methods were used (Fig. 2). In the case of early ovarian cancer (stages 1 and 2), the sensitivity was high at 82.35–91.76%. Based on these results, the multiple biomarker set identified in this study is expected to play an important role in the early diagnosis of ovarian cancer. Whether the multiple biomarker set identified in this study can differentiate between benign ovarian disease and ovarian cancer remains to be clarified. Future research using samples from patients with benign ovarian disease should be conducted to test its ability to differentiate between benign ovarian disease and ovarian cancer.
In conclusion, the set of multiple biomarkers identified in this study shows higher accuracy in the early diagnosis of ovarian cancer than the combination of CA125 and HE4, the two currently used biomarkers. This optimal set of biomarkers, including CA125 and HE4, was selected from eight biomarkers by measuring their levels in the serum samples. In terms of their clinical significance, these diagnostic indicators can improve the performance of existing ovarian cancer screening tests and thus improve the survival rates of patients by enabling effective and early detection of ovarian cancer. However, additional analyses (calculation of cancer discrimination rate through a model supplemented with benign disease and cancer data) are required to strengthen the clinical significance and effectiveness of the model established in this study.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The specimens used in this study were approved by the BIOINFRA Life Science Inc. Institutional Review Board (No. 1-700097-B-N-01).
Patient consent
Subjects whose serum samples were used in this study provided written informed consent for publication of their data.
Funding information
Funding was received from BIOINFRA Life Science Inc.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021;64:444–53. doi: 10.5468/ogs.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95 (Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 4.Brun JL, Fritel X, Levêque J. Clinical practice guidelines: presumed benign ovarian tumors--aims, methods, and organization. J Gynecol Obstet Biol Reprod (Paris) 2013;42:710–4. doi: 10.1016/j.jgyn.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Vaisbuch E, Dgani R, Ben-Arie A, Hagay Z. The role of laparoscopy in ovarian tumors of low malignant potential and early-stage ovarian cancer. Obstet Gynecol Surv. 2005;60:326–30. doi: 10.1097/01.ogx.0000161373.94922.33. [DOI] [PubMed] [Google Scholar]
- 6.Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018;19:2877. doi: 10.3390/ijms19102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates SE, Longo DL. Tumor markers: value and limitations in the management of cancer patients. Cancer Treat Rev. 1985;12:163–207. doi: 10.1016/0305-7372(85)90037-4. [DOI] [PubMed] [Google Scholar]
- 8.Holdenrieder S, Pagliaro L, Morgenstern D, Dayyani F. Clinically meaningful use of blood tumor markers in oncology. Biomed Res Int. 2016;2016:9795269. doi: 10.1155/2016/9795269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban N, McIntosh MW, Andersen M, Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. 2003;17:989–1005. doi: 10.1016/s0889-8588(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 10.Buamah P. Benign conditions associated with raised serum CA-125 concentration. J Surg Oncol. 2000;75:264–5. doi: 10.1002/1096-9098(200012)75:4<264::aid-jso7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–700. [PubMed] [Google Scholar]
- 12.Ahmed AA, Abdou AM. Diagnostic accuracy of CA125 and HE4 in ovarian carcinoma patients and the effect of confounders on their serum levels. Curr Probl Cancer. 2019;43:450–60. doi: 10.1016/j.currproblcancer.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Qing L, Xiaoling S, Yuqin Yang. Research progress of ROMA in early diagnosis of ovarian epithelial carcinoma. Adv Mod Biomed Sci. 2011;11:4999–5000. [Google Scholar]
- 14.Zhang Z. An in vitro diagnostic multivariate index assay (IVDMIA) for ovarian cancer: harvesting the power of multiple biomarkers. Rev Obstet Gynecol. 2012;5:35–41. [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HJ, Kim YT, Park PJ, Shin YS, Kang KN, Kim Y, et al. A novel detection method of non-small cell lung cancer using multiplexed bead-based serum biomarker profiling. J Thorac Cardiovasc Surg. 2012;143:421–7. doi: 10.1016/j.jtcvs.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Ahn HS, Shin YS, Park PJ, Kang KN, Kim Y, Lee HJ, et al. Serum biomarker panels for the diagnosis of gastric adenocarcinoma. Br J Cancer. 2012;106:733–9. doi: 10.1038/bjc.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BK, Lee JW, Park PJ, Shin YS, Lee WY, Lee KA, et al. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009;11:R22. doi: 10.1186/bcr2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon HI, Kwon OR, Kang KN, Shin YS, Shin HS, Yeon EH, et al. Diagnostic value of combining tumor and inflammatory markers in lung cancer. J Cancer Prev. 2016;21:187–93. doi: 10.15430/JCP.2016.21.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fawzy A, Mohamed MR, Ali MA, Abd El-Magied MH, Helal AM. Tissue CA125 and HE4 gene expression levels offer superior accuracy in discriminating benign from malignant pelvic masses. Asian Pac J Cancer Prev. 2016;17:323–33. doi: 10.7314/apjcp.2016.17.1.323. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang H, Gao J, Hu Z, Liu J, Liu D, Lin B. Co-expression of Lewis Y antigen with human epididymis protein 4 in ovarian epithelial carcinoma. PLoS One. 2013;8:e68994. doi: 10.1371/journal.pone.0068994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–9. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 22.Scambia G, Benedetti P, Baiocchi G, Perrone L, Greggi S, Di Roberto P, et al. CA 15-3 serum levels in ovarian cancer. Oncology. 1988;45:263–7. doi: 10.1159/000226575. [DOI] [PubMed] [Google Scholar]
- 23.Lotzniker M, Pavesi F, Scarabelli M, Vadacca G, Franchi M, Moratti R. Tumour associated antigens CA 15.3 and CA 125 in ovarian cancer. Int J Biol Markers. 1991;6:115–21. doi: 10.1177/172460089100600206. [DOI] [PubMed] [Google Scholar]
- 24.Podzielinski I, Saunders BA, Kimbler KD, Branscum AJ, Fung ET, DePriest PD, et al. Apolipoprotein concentrations are elevated in malignant ovarian cyst fluids suggesting that lipoprotein metabolism is dysregulated in epithelial ovarian cancer. Cancer Invest. 2013;31:258–72. doi: 10.3109/07357907.2013.789896. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Chen S, Li L, Liu X, Liu X, Dai S, et al. Evaluation of HE4 and TTR for diagnosis of ovarian cancer: comparison with CA-125. J Gynecol Obstet Hum Reprod. 2018;47:227–30. doi: 10.1016/j.jogoh.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Jin C, Yang M, Han X, Chu H, Zhang Y, Lu M, et al. Evaluation of the value of preoperative CYFRA21-1 in the diagnosis and prognosis of epithelial ovarian cancer in conjunction with CA125. J Ovarian Res. 2019;12:114–21. doi: 10.1186/s13048-019-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sørensen SS, Mosgaard BJ. Combination of cancer antigen 125 and carcinoembryonic antigen can improve ovarian cancer diagnosis. Dan Med Bull. 2011;58:A4331. [PubMed] [Google Scholar]
- 28.Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009;361:170–7. doi: 10.1056/NEJMcp0901926. [DOI] [PubMed] [Google Scholar]
- 29.Cho HY, Park SH, Park YH, Kim HB, Kang JB, Hong SH, et al. Comparison of HE4, CA125, and risk of ovarian malignancy algorithm in the prediction of ovarian cancer in Korean women. J Korean Med Sci. 2015;30:1777–83. doi: 10.3346/jkms.2015.30.12.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui R, Wang Y, Li Y, Li Y. Clinical value of ROMA index in diagnosis of ovarian cancer: meta-analysis. Cancer Manag Res. 2019;28:2545–51. doi: 10.2147/CMAR.S199400. [DOI] [PMC free article] [PubMed] [Google Scholar]