Abstract
The hydro-alcoholic extract of raw and processed Macrotyloma uniflorum seeds, an underutilized food legume was analysed for its bioactive compounds, Type-II diabetes enzyme regulation and antiurolithiatic potential. The study aimed to establish and promote the introduction of these new grains and enlarge the market of novel functional foods. The seed extract had phenolic content of 35.6 and 30.4 mg GAE/g dm, for PAIYUR-2 and GPM-6 respectively. Chlorogenic, coumaric, vanillic and ellagic were the major and, sinapic and syringic were limiting phenolic acids. The raw seeds extract exhibited ferric ion reducing potential (1125 and 1236 mmol Fe II/mg extract dm), free radical inhibition (EC50, 3.58 and 3.78 g dm/g DPPH), hydroxyl ion inhibition (46.71 and 45.44%) and superoxide ion inhibition (36.93 and 33.37%) for PAIYUR-2 and GPM-6 respectively. Further, considerable α-amylase (49.34 & 45.89%) and α-glucosidase (62.72 & 60.33%) inhibition potentials were observed along with antiurolithiatic activity of 48.12 and 46.31% in PAIYUR-2 and GPM-6 respectively. Although, processing had significant (p ≤ 0.05) impact on grain quality but still the significant (p ≤ 0.05) functional properties were retained. This proves the grain utility as a functional food in maintaining human health.
Keywords: Horse Gram, Antioxidant Potential, α-Amylase Inhibition, α-Glucosidase Inhibition, Antiurolithiatic Activity
Introduction
Epidemiological studies have shown that plant-based sources such as legumes offer an excellent alternative source of natural antioxidant and associated with decreased incidence of cancer and cardiovascular diseases (Ravichandran, et al. 2012). Oxidative stress has been reported as a precursor of various diseases linked with metabolic or vascular disorders including diabetes and hypertension (Van der Zwan, et al. 2010; Sreerama, et al. 2012). Therefore, it is essential to regulate both blood glucose level and cellular redox status for managing these diabetic complications. This can be achieved by enriching our diet with legumes having bioactive compounds with antioxidant potential and α-amylase and α-glucosidase regulating potential (Vandivel, et al. 2012).
Horse gram is a legume belonging to family Fabaceae, is a potentially underutilized legume having excellent nutritional and remedial properties with superior climatic resilience to adapt against hostile environment conditions (Bhartiya, et al. 2015). It is largely cultivated in dry areas of Australia, Burma, India and Sri Lanka. It is used as a traditional food in India and established as a poor man’s pulse in India. The nutritional quality of this traditional underutilized legume is well known as a potential source of protein (17–25%), carbohydrate (51.9–60.9%), minerals (2–3%) and dietary fibre (5–7%) (Vashishth, et al. 2017). It contains isoflavone, diglycoside, 5-hydroxy-7,3’,4’-trimethoxy-8-methylisoflavones, 5-O-α-L-rhamnopyranonyl (1 → 2)-O-β-D-glucopyranoide (Mitra & Joshi, 1983; Sreerama, et al. 2012). Consumption of horse gram can provide protection against health problems, reduce oxidative damage of lipid and enhance the antioxidant status of human (Petchiammal & Hooper, 2014). The antioxidant potential of horse gram is associated with its radical scavenging potential which helps in preventing cancer and cardiovascular diseases (Ramesh, et al. 2011). The seed decoction or cooked seeds from horse gram on consumption helps in diluting renal stone (Siddhuraju & Manian, 2007). It can be used to control diabetes as it has been reported to significantly reduce the blood glucose level, reduce cholesterol, reduce body weight and reduce hyperlipidaemia in albino rats (Upadhyay, et al. 2015).
Even though the nutritional and functional properties of horse gram seeds were reported earlier, the information regarding the role of these bioactive components of horse gram as antioxidant and enzymatic activity regulators are limited. In current scenario research in this direction is of prime importance to combat free radical-mediated chronic disease by using the functional properties of legume. These exhibited by the presence of phytochemical components in legume. The bioactive components in legumes were reported to be susceptible to oxidation and degradation by light, heat and conditions present during processing (Ravichandran, et al. 2012). Therefore, the current investigation was conducted to evaluate the phenolic profile, antioxidant potential, Type-II diabetes-related enzyme regulatory properties and antiurolithiatic property of phenolic extract from raw and processed horse gram.
Material and methods
Two varieties of horse gram seeds namely GPM-6 was procured from University of Horticulture Sciences, Bhagalkot, Karnataka, India and PAYIUR-2 were procured from Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India. Samples were cleaned and sun-dried for a day and packed in polyethene pouches (75 µ) for further analysis.
Preparation of precooked and dehydrated samples of horse gram
Pressure cooking (PC)
Two kg raw horse gram was cleaned thoroughly and soaked overnight at grain to distilled water ratio of 1:10 at 35 ± 2 °C. Seeds were cooked (gelatinized edible grain) in the same soaking water for better retention of nutrients, at 15 psi for 17 min and dried at 80 °C in a fluidized bed drier (FBD) (Model PD 01 CMP, Maharashtra, India) to a moisture content below 10%.
Pressure cooking and flaking (PCF)
Horse gram seeds were steam cooked after soaking as mentioned earlier and conditioned to a moisture content of 30–35% using FBD at 80 °C. These conditioned grains were flaked in roller flaker (ER and Turner 460, Ipswich, England) while maintaining 0.2 mm clearance between the rollers. Further, the grains were dried to a moisture content below 10% using FBD at 80 °C.
Microwave cooking (MWC)
Overnight soaked grains of both varieties were cooked in the microwave (MG607APR, LG Electronics India Pvt Ltd, India) using water to grain ratio of 8:1 for 30 min. Further, the cooked grains were dried using FBD at 80 °C to a moisture content below 10%.
Micronization cooking (IRC)
Raw grains were conditioned to a moisture content of 50% by soaking for 4 h at 35 ± 2 °C and cooked in two successive cycles of 500 KW power in Infra-red processing unit (Thermen Heating Technologies Pvt Ltd., Bangalore, India) equipped with ceramic IR heater. Cooked grains were brought down to moisture content below 10% using FBD at 80 °C.
Extrusion cooking
The raw seeds were powdered and sieved (0.5 µm). The flour was equilibrated to a moisture content of 21% and passed through the extruder (Brabender GmbH & Co. KG) (815804E00-00, 2008; screw diameter-25 mm, length-800 mm) under the following condition; screw speed of 100 rpm, temperature 160 ± 2 °C, feeding rate 10 kg/h.
Solvent extraction
10 g of each raw and processed horse gram from both varieties were defatted in soxhlet apparatus using petroleum ether. The phenolic extract was extracted using the method of Siddharaju & Manian, (2007). The dried grains were extracted in 100 ml of the hydro-alcoholic mixture (70% methanol) for 24 h at 25 °C and filtered using Whatman No. 4 filter paper. The residue was re-extracted with 50 ml of 70% methanol. The extract was collected and evaporated using a rotary vacuum evaporator at 40 °C. The remaining of the moisture was removed by lyophilisation. The dried extract was used for further analysis.
Total phenol content
The total phenolic and tannin content of extract from raw and processed seeds was determined according to the method of Suriano, et al. (2018). The amount of total phenol was calculated as Gallic acid equivalent from the calibration curve (Suriano, et al. 2018).
Phenolic profile
The estimation of phenolic acid profile in raw and processed sample was performed by following the method of Ti, et al. (2015) with slight modification. Gallic, vanillic, syringic, caffeic, chlorogenic, ferulic, sinapic, coumaric and ellagic acids were estimated along with catechin, epicatechin, quercetin, kaempferol, myricetin, daidzein and genistein in the sample using HPLC (Agilent-1260 Infinity, 2014, Agilent Technology, India) equipped with an auto-injector, quaternary pump, PDA detector and RP-C18 column (250 mm × 4.5 mm). Briefly, the gradient elution was conducted with 0.1% formic acid (A) and methanol (B), programmed as follows: 0–55 min, 85:25, 57 min 20:80 and 60 min 85:15 at a constant flow rate of 0.8 ml/min and 20 µl of aliquot was injected for all the samples. The detection of compounds was carried out at 280 nm. Before injecting, samples were filtered through 0.22 µm membrane filter (Merk, India). The phenolic components for all the samples were identified and estimated based on retention time, area under the curve of authenticated standards (Sigma Aldrich, India). The percentage recovery of phenolic acid was 94–98%.
Superoxide anion (O2*) radical scavenging activity by NBT method
The superoxide anion (O2*) scavenging activity of phenolic extract from raw and processed grains was performed using photo-induced reaction with 20 W fluorescent lamp by following the procedure explained by Zhishen, et al. (1999). All solutions were prepared in phosphate buffer of 0.05 M and pH 7.8. The total volume of reactive mixture was 5.0 ml containing riboflavin (3 × 10–2 mol/L), methionine (1 × 10–2 mol/L) and nitro blue tetrazolium (NBT) (1 × 10–4 mol/L) respectively. Based on the absorbance value the superoxide scavenging activity was calculated and expressed on percent basis.
Ferric ion reducing/antioxidant potential
The ferric reducing/antioxidant power of phenolic extract from raw and processed grains was determined according to the method described by Pulido, et al. (2000). Aqueous methanol was taken as the reagent blank. The FRAP reagent contained 2.5 ml of 20 mmol/L TPTZ solution in 40 mmol/L HCl and 2.5 ml of 20 mmol/L FeCl3.6H2O, 25 ml of 0.3 mol/L acetate buffer pH 3.6. 900 µl of frap freshly prepared FRAP reagent was incubated at 37 ºC and mixed with 90 µl of dH2O and 30 µl of test sample, blank in place of the sample was also kept in water bath at 37 ºC for 30 min. Absorbance was taken immediately at 593 nm using a spectrophotometer. The calibration curve was made by using the methanolic solution of Fe(II) known concentration range from 100 to 2000 µmol/L (FeSO4.7H2O). The reducing power of the sample was determined as the concentration of antioxidant having a ferric TPTZ reducing activity equivalent to that of 1 mmol/L FeSO4 (Nithiyanantham, et al. 2012).
DPPH
The DPPH radical scavenging activity of extract from horse gram sample was estimated according to the method described by Nithiyanantham, et al. 2012. The decrease in absorbance was measured after each minute till 60 min at 515 nm wavelength.
ABTS* and Hydroxyl (OH־) radical-scavenging activity (HRSA)
ABTS* or the total antioxidant activity (TEAC) and HRSA of seed extract was measured by radical cation decolourization assay involving ABTS* radical cation given by Siddharaju & Bekar, (2007); Nithiyanantham, et al. (2012).
α-Amylase and α-glucosidase inhibition activity
α-Amylase and α-glucosidase inhibition activity assay for phenolic extract were performed by following the method of Vaidivel & Biesalski, (2012). α-Amylase solution of activity 4.5 units/ml/min and α-glucosidase solution of activity 1 unit/ml/min were used during the assay. The results are expressed on % basis.
Antiurolithiatic activity of horse gram extract
Preparation of calcium oxalate precipitate and semi permeable membrane was achieved by following the method described by Jain, et al. (2012). 10 mg of calcium oxalate was weighed and mixed with 1 ml (5 mg/ml) phenolic extract of horse gram and packed in semipermeable membrane produced from the egg. Standard (Cystone) and Control (10 mg of calcium oxalate) were also packed in the same manner. The packed material was allowed to suspend in a conical flask containing 100 ml of 0.1 M Tris buffer. All the flasks were incubated for 7 h at 37 ± 1 °C with occasional stirring. The content for each sample from the membrane was transferred to a separate test tube using 2 ml of 1 N HCl. 0.2 ml of suspension from each sample was transferred to another test tube containing 2 ml of O-cresopthaleincomplexone (O-CPC) indicator (Hodgkinson & William, 1972). The intensity of colour formed was measured at 570 nm using a spectrophotometer. The concentration of calcium was estimated using the standard curve of calcium oxalate (Satish, et al. 2010).
Statistical analysis
The experiments were performed in triplicates and represented as mean ± standard deviation. The results were analysed using analysis of variance (ANOVA) (SPSS, 2002). The Duncan multiple range test was used to separate the means and accepted at the level of p ≤ 0.05.
Result and discussion
Percent recovery
The yield percentage of extract recovered from raw and processed seeds along with their phytochemical content namely phenols and tannins content are depicted in Table 1. Maximum recovery for both the varieties was obtained from micronized grains followed by extrusion, MWC, PC, PCF and Raw seeds. This can be attributed to the solubility of phenolic and other compounds (Siddharaju, 2006). The percent yield obtained was greater than the previously reported yield percent of horse gram by Sreeramulu, et al. (2012).
Table 1.
Recovery percentage, phenols and tannin content of horse gram extract
| Sample | PAIYUR-2 | GPM-6 | ||||
|---|---|---|---|---|---|---|
| Recovery (g/100 g) | Phenols (mg GAE/g dm) | Tannins (mg/g dm) | Recovery (g/100 g) | Phenols (mg GAE/g dm) | Tannins (mg/g dm) | |
| Raw | 5.7 ± 0.41a | 35.6 ± 11.7bc | 09.1 ± 0.7b | 5.7 ± 0.21a | 30.4 ± 0.99c | 0.76 ± 0.09b |
| PC | 6.0 ± 0.36ab | 25.1 ± 8.7bc | 11.4 ± 2.2c | 5.9 ± 0.27a | 24.1 ± 0.91b | 1.12 ± 0.18c |
| PCF | 5.8 ± 0.41ab | 24.5 ± 7.7 b | 10.8 ± 3.6bc | 5.8 ± 0.44ab | 23.8 ± 0.71b | 0.91 ± 0.25c |
| IRC | 6.2 ± 0.49ab | 26.8 ± 4.4bc | 17.7 ± 1.3d | 6.2 ± 0.32b | 25.4 ± 0.92bc | 1.77 ± 0.22d |
| MWC | 6.0 ± 0.22a | 28.8 ± 11.9ab | 12.9 ± 2.9c | 6.0 ± 0.29ab | 27.7 ± 0.89b | 1.11 ± 0.29c |
| Extrusion | 6.1 ± 0.55ab | 21.8 ± 2.8a | 05.3 ± 0.2a | 6.1 ± 0.29b | 20.3 ± 0.84a | 0.48 ± 0.13a |
All data were means of triplicates (n = 3), values are mean of three independent determinations ± Standard deviation; Raw: Untreated horse gram 70% methanolic extract; PC: Pressure processed horse gram70% methanolic extract; PCF:Pressure processed and flaked horse gram70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
Total phenolic content and phenolic acid profile
The phenolic content reported as a major constituent of secondary metabolites in legume such as simple phenols (phenolic acid, phenyl-propanoids etc.) to highly polymerized compound (lignins, lignans, tannins etc.) that might prevent the development of chronic diseases such as oxidative changes in blood, heart attack and cancer (Suja, et al. 2005). The phenol content of aqueous methanolic extract from raw seeds of PAYIUR-2 and GPM-6 varieties was 35.6 and 30.4 mg GAE/g dm respectively and among processed grains, it varied from 21.8–28.8 mg GAE/g dm for PAYIUR-2 and 20.3–27.7 mg GAE/g dm for GPM-6 respectively. The tannin content of the grains was also significantly (p ≤ 0.05) affected during processing. The observed values of phenol content were lower than previously reported values of black variety of horse gram (3.63–12.63 g/100 g dm) (Siddhuraju, & Manian, 2007) but higher than brown variety (1.74–9.67 g/100 g dm) and other legumes such as white bean (3.08 g catechin equivalent 100 g) and Pea (3.48 g catechin equivalent/100 g) (Amarowicz & Raab, 1997). The change in extractable phenolic content during processing may be attributed to the change in solubility of phenols during thermal processing (Sreeramulu, et al. 2012).
Extract from raw and processed grains was also analysed for their phenolic acid compositional changes during processing. Vanillic, chlorogenic, p-coumaric, ellagic, catechin and caffeic acid were the predominant phenolic acids making up to 75.98 and 78.03% of total phenolic acids present in raw grains of both the varieties PAYIUR-2 and GPM-6 respectively. The sinapic acid (phenol), myricetin, daidzein and genistein (flavonoids) were the limiting acid making up to 4.59 and 4.42% of the total phenolic acid present (Table 2). Gallic acid concentration in both the varieties of grain increased during microwave processing and micronization, and decreased significantly (p ≤ 0.05) from 4.53 to 0.92% for PAYIUR-2 and 3.13 to 0.49% for GPM-6 variety during extrusion processing. Kaempferol content increased significantly (p ≤ 0.05) from 5.26 to 7.25% and 4.95 to 9.29% in PAIYUR-2 and GPM-6 varieties respectively during extrusion processing. The changes in concentration of phenolic acid can be attributed to their chemical structure due to which the phenolic acids and their conjugated forms can get converted to one another during processing (Sreerama, 2012). The extrusion processing affected the phenolic acid content significantly (p ≤ 0.05) in comparison to other processing techniques suggesting a higher degree of oxidation in extruded grains due to the presence of a lower concentration of phenolic acids (Vadivel, et al. 2012).
Table 2.
Phenolic acid profile of raw and processed horse gram extract (mg/100gdm)
| VARIETY | PAIYUR-2 | GPM-6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S.No | Phenolic Acids | Raw | AC | AC + FLK | MWC | IR | EXTRUSION | Raw | AC | AC + FLK | MWC | IR | EXTRUSION | |
| 1 | Gallic acid | 4.53d | 1.47c | 1.46c | 6.74e | 7.13f | 0.92a | 3.13d | 1.03b | 1.25b | 5.54d | 5.63d | 0.49a | |
| 2 | Catechin | 14.83 h | 6.19d | 3.80b | 10.25f | 9.05e | 1.69a | 17.33i | 6.37d | 5.43c | 9.29e | 13.24 g | 3.75b | |
| 3 | Vanillic acid | 32.54 h | 13.96e | 12.35d | 15.93f | 11.39d | 5.45b | 24.67 g | 5.96b | 4.90ab | 8.24c | 5.29b | 4.06a | |
| 4 | Syringic acid | 4.38f | 2.29c | 1.48b | 3.22d | Nd | nd | 4.15e | nd | nd | 0.99a | Nd | nd | |
| 5 | Caffeic acid | 14.48i | 8.33 g | 6.71f | 9.91 h | 8.26 g | 4.38b | 9.16gh | 7.56 cd | 6.36c | 8.08e | 9.94d | 2.89a | |
| 6 | Chlorogenic acid | 30.92j | 17.51f | 14.38e | 23.75 g | 27.01i | 4.46b | 26.72 h | 10.31d | 8.18c | 15.96e | 22.60 g | 2.42a | |
| 7 | Ferullic acid | 11.17i | 5.07f | 4.17e | 6.16 g | 6.97 h | 4.86c | 6.96 g | 4.59d | 3.46b | 4.90c | 5.47e | 1.91a | |
| 8 | Sinapic acid | 3.67d | nd | 1.37b | 2.56c | Nd | nd | 2.34c | nd | nd | 0.70a | Nd | nd | |
| 9 | p-coumaric acid | 16.88f | 8.24d | 8.39d | 12.43e | 6.56c | 4.08b | 9.84c | nd | nd | 3.48a | Nd | nd | |
| 10 | Epicatechin | nd | nd | nd | 3.26c | 0.73b | nd | nd | nd | nd | 4.18d | 0.62a | nd | |
| 11 | Ellagic acid | 16.69 h | 11.71c | 11.58c | 13.23f | 14.03d | 8.41a | 16.89 g | 12.80b | 11.67b | 13.38e | 14.13a | nd | |
| 12 | Quercitin | 6.96e | 6.32de | 6.82de | 9.22f | 10.07 g | 0.83a | 3.88b | 3.88b | 3.94b | 5.36c | 6.08 cd | 1.31a | |
| 13 | Kaempferol | 5.26c | 6.14d | 5.01bc | 6.31d | 6.42d | 7.25e | 4.95bc | 4.39b | 3.31a | 4.19b | 4.20b | 9.29f | |
| 14 | Myricetin | 1.90b | nd | 0.42a | Nd | 0.49a | nd | 2.92c | nd | nd | nd | Nd | nd | |
| 15 | Daidzein | 0.75a | nd | nd | Nd | Nd | nd | Nd | nd | nd | nd | Nd | nd | |
| 16 | Genistein | 1.32b | nd | nd | Nd | Nd | nd | 0.56a | nd | nd | nd | Nd | nd | |
Values are mean ± standard deviation of three measurements, mean value with different superscripts in rows are significantly different (p ≤ 0.05). The standard deviation in the observed sample was not more than 5 percent; Raw: Untreated horse gram 70% methanolic extract; PC: Pressure processed horse gram70% methanolic extract; PCF:Pressure processed and flaked horse gram70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
Ferric ion reducing antioxidant power (FRAP) assay
The FRAP assay estimates the antioxidant potential of substance/substances in the reaction medium as the reducing potential of these compounds towards Fe(III) complex. The antioxidant potential of raw and processed seed extract was estimated from their ability to reduce TPTZ Fe(III) complex to TPTZ-Fe(II) complex. FRAP value of raw and processed grains varied from 736 to 1718 mg dm/mmol Fe(II) for GPM-6 and 625–1518 mg dm/mmol Fe(II) for PAIYUR-2 (Fig. 1). The maximum FRAP value for aqueous methanolic extract was observed in extrusion processed grains (1718 and 1518 mg dm/mmol Fe(II)) and minimum (736 and 625 mg dm/mmol Fe(II)) was observed in raw grains of GPM-6 and PAIYUR-2 respectively. The trend of FRAP value was similar for both the varieties (Extrusion > IRC > PCF > PC > MWC > Raw). Hence, the highest antioxidant potential was obtained in raw grains. The variation in ferric-reducing potential of processed horse gram can be accorded to the change in reducing power of low and high molecular weight bioactive compounds of processed grain which undergoes changes during wet and dry heating (Sidduraju and Manian, 2007). The observed values for horse gram varieties were significantly higher than V. unguiculate grains (487–547 mg dm/mmol Fe(II)) and C. arietinum processed grains (771–758 mg dm/mmol Fe(II)) but lower than previously reported values for horse gram (Siddhuraju, 2007) and other underutilized grains Vigna vexillata (1967 mmol Fe(II)/mg extract) by Sowndhararajan, et al. 2011.
Fig. 1.
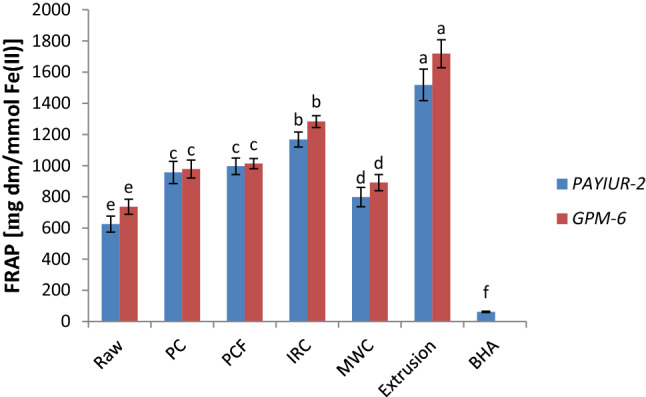
Ferric ion reducing/antioxidant power (FRAP) ofhorse gram aqueous methanolic extract (mg dm/mmol Fe(II). Raw: Untreated horse gram 70% methanolic extract; PC: Pressure Processed horse gram70% methanolic extract; PCF:Pressure processed and flaked horse gram70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
DPPH (Radical-scavenging activity on α-α diphenyl-β-picrylhydrazine) and ABTS* assay
The free radical scavenging activity of aqueous methanolic extract from raw and processed grains in terms of EC50 for both the varieties was determined by DPPH and results are shown in Table 3. The EC50 for raw grains was 3.58 and 3.78 g dm/g DPPH for PAIYUR-2 and GPM-6 varieties respectively. The maximum increase in EC50 was observed for extrusion processed (61.18 and 63.75%) and minimum was observed for microwave processed grains (6.98 and 5.29%) for PAIYUR-2 and GPM-6 respectively. The trend observed for both the varieties was Raw ≤ MWC ≤ IRC ≤ PC ≤ PCF ≤ Extrusion. The increase in EC50 concentration after processing was directly proportional to the reduction in phenolic content of respective grains. The results obtained were higher than the Phaseolus vulgaris var. Bayo victoria (Rocha-Guzman, et al. 2007) and comparable with previously reported result of underutilized legume Mucuna pruriens (Randhir, et al. 2009). The DPPH inhibition activity of the extract is the result of antioxidant potential of phenolic compounds as well as the intermediate compound formed in maillard reaction during processing (Yen & Duh, 1993).
Table 3.
DPPH radical and ABTS* radical scavenging activity of from horse gram extract
| Sample | PAIYUR-2 | GPM-6 | ||
|---|---|---|---|---|
| DPPH (EC50) (g dm/g DPPH) | TEAC (mmol/100 g dm) | DPPH (EC50) (g dm/gDPPH) | TEAC (mmol/100 g dm) | |
| Raw | 3.58 ± 0.34a | 65.31 ± 4.07d | 3.78 ± 0.42a | 62.80 ± 7.20d |
| PC | 4.12 ± 0.61ab | 58.87 ± 5.61b | 4.84 ± 0.46ab | 53.24 ± 2.20b |
| PCF | 4.22 ± 0.22c | 58.37 ± 3.33b | 4.87 ± 0.64c | 51.38 ± 1.81b |
| IRC | 4.01 ± 0.38bc | 59.92 ± 2.08c | 4.13 ± 0.33bc | 55.19 ± 3.01b |
| MWC | 3.83 ± 0.41ab | 63.68 ± 3.30 cd | 3.98 ± 0.67ab | 60.49 ± 4.30c |
| Extrusion | 5.77 ± 0.78d | 41.73 ± 1.43a | 6.19 ± 0.96d | 37.46 ± 1.80a |
All data were means of triplicates (n = 3), values are mean of three independent determinations ± Standard deviation; Raw: Untreated horse gram 70% methanolic extract; PC: Pressure processed horse gram70% methanolic extract; PCF:Pressure processed and flaked horse gram70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
In ABTS* radical cation scavenging method the activity of tested seed extract was expressed as trolox equivalent. Total antioxidant activity (TEAC) of raw and processed grain is shown in Table 3. The raw seeds extract from both the varieties have shown the maximum TEAC (62.80 and 65.31 mmol/100 g dm) for GPM-6 and PAIYUR-2 respectively in comparison with processed grains which ranged from 41.73–63.68 mmol/100 g dm for PAIYUR-2 and 37.46–60.49 mmol/100 g dm for GPM-6. The trend of TEAC for both PAIYUR-2 and GPM-6 was Raw > MWC > IRC > PC > PCF > Extrusion. The TEAC of PAIYUR-2 variety was higher than that of GPM-6 variety. The higher free radical quenching potential of MWC processed and micronized grains can be justified based on their combined phenols and tannin content as the radical scavenging potential of antioxidants depend on the molecular weight, number of aromatic compounds and nature of hydroxyl groups association than the specific functional groups (Hagerman, et al. 1998). Horse gram seed extract exhibited significant free radical scavenging capacities which can be used as a natural antioxidant (Petchiammal & Hopper, 2014).
Superoxide anion radical and hydroxyl radical scavenging activity
The estimation of superoxide radical scavenging activity of biological compounds becomes an important parameter to prove their antioxidant potential as these radicals play an important role in the oxidation of unsaturated fatty acids and other susceptible substances. The superoxide radical scavenging activity of grains is exhibited in Table 4. The investigation of seed extract of raw grains exhibited 36.93 and 33.37% superoxide radical scavenging activity for PAIYUR-2 and GPM-6 respectively. The superoxide radical scavenging activity for the processed grains ranged from 26.61–33.4 and 24.86–31.39% for PAIYUR-2 and GPM-6 respectively. The extrusion processing caused maximum reduction in radical scavenging activity (27.94 and 25.50%) and microwave treated grains showed the best radical scavenging activity (33.44 and 31.39%) among the processed grains for PAIYUR-2 and GPM-6 respectively. In both the varieties, PAIYUR-2 had the greater radical scavenging activity than GPM-6. The trend of radical scavenging activity for both PAIYUR-2 and GPM-6 was Raw > MWC > IRC > PC > PCF > Extrusion. The results obtained were higher than the previously reported data of black horse gram variety (23.43%) (Siddharaju, 2006), light brown (32%) and dark brown (32.8%) variety of cow pea (Siddharaju & Becker, 2007) and lower than Bauhinia vahil (Sowndhararajan, et al. 2009). The aqueous methanolic extract of horse gram has shown the substantial activity which proves its utility in controlled prevention of oxidative stress if incorporated in the diet.
Table 4.
Superoxide and Hydroxyl radical scavenging activity of horse gram extract (dm)
| Sample | PAIYUR-2 | GPM-6 | ||
|---|---|---|---|---|
| Hydroxyl (% Inhibition) | Superoxide (% inhibition) | Hydroxyl (% Inhibition) | Superoxide (% Inhibition) | |
| Raw | 46.71 ± 2.98c | 36.93 ± 1.19c | 45.44 ± 2.22c | 33.37 ± 1.91c |
| PC | 41.09 ± 2.53ab | 30.14 ± 2.61b | 39.21 ± 2.92ab | 27.08 ± 2.74ab |
| PCF | 40.59 ± 1.97ab | 28.25 ± 1.53ab | 38.22 ± 0.54ab | 26.19 ± 1.11ab |
| IRC | 45.09 ± 2.10bc | 31.83 ± 1.94b | 43.73 ± 2.35bc | 29.97 ± 2.28bc |
| MWC | 43.37 ± 2.47b | 33.44 ± 1.56bc | 41.84 ± 2.19b | 31.39 ± 3.11bc |
| Extrusion | 39.62 ± 1.81a | 26.61 ± 2.03a | 35.19 ± 1.76a | 24.86 ± 1.52a |
All data were means of triplicates (n = 3), values are mean of three independent determinations ± Standard deviation; Raw: Untreated horse gram 70% methanolic extract; PC: Pressure processed horse gram 70% methanolic extract; PCF: Pressure processed and flaked horse gram 70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
The potential of the phenolic extract of seeds in scavenging hydroxyl radical is shown in Table 4. The raw seeds of horse gram have shown maximum hydroxyl radical scavenging activity (46.71 and 45.44%) for PAIYUR-2 and GPM-6 varieties respectively. The hydroxyl scavenging activity for processed grain ranged from 39.62 to 45.09 and 35.19 to 43.73% for PAIYUR-2 and GPM-6 varieties respectively. The extrusion processing caused maximum reduction in scavenging activity (15.18 and 22.56%) and micronized grains showed the best scavenging activity (45.09 and 43.73%) among the processed grains for PAIYUR-2 and GPM-6 respectively. In both the varieties, PAIYUR-2 had a greater scavenging capability than GPM-6. The trend of hydroxyl radical scavenging activity for both PAIYUR-2 and GPM-6 was Raw > IRC > MWC > PC > PCF > Extrusion. The high scavenging ability against superoxide and hydroxyl ions for microwave processed and micronized grains can be attributed to the phenolic content and possible bioactive compounds formed during maillard reaction which may have occurred during grain processing. Similar results were also reported for Cicer arietinum L. and Pisum sativum L (Nithiyanantham, et al. 2012), raw seed extracts of M. uniflorum (Siddharaju & Manian, 2007) and D. lablab (Hagerman et al. 1998).
α- Amylase and α- glucosidase inhibition activity
α- Amylase and α- glucosidase inhibition activity plays a vital role in regulating the hyperglycaemia (Vadivel & Biesalski, 2011). The aqueous methanolic extract of raw seeds of both varieties PAIYUR-2 and GPM-6 has shown 49.34 and 45.89% α-amylase inhibition respectively (Table 5). The inhibition activity of the grains reduced after processing and ranged from 14.13–33.37% and 12.40–30.63% for PAIYUR-2 and GPM-6 respectively. Among processed grains, extrusion processing caused maximum reduction in inhibition activity (71.36 and 72.97%) whereas, the minimum reduction was observed for MWC treated grains (32.36 and 33.25%) for PAIYUR-2 and GPM-6 respectively. In both the varieties, PAIYUR-2 had a greater inhibition activity than GPM-6. The trend of α- amylase inhibition activity for both PAIYUR-2 and GPM-6 was Raw > MWC > PC > PCF > IRC > Extrusion. The higher reduction of α-amylase inhibition potential in extrusion processed and micronized grains can be attributed to the change in activity of inhibitors as both the processing techniques imply the dry heating at high temperature during processing in comparison with other used technologies (Sreerama, et al. 2012). The estimated % inhibition was higher than mung beans (65%) (Randhir, et al. 2009), foxtail millet (32%) (Kim, et al. 2011) but lower than P. pinnata (77.92%) (Vadivel & Biesalski, 2011) and Canavalia ensiformis (77.56%) (Vadivel, et al. 2012).
Table 5.
α- Amylase andα-Glucosidase inhibition activity of horse gram extract (dm)
| Sample | PAIYUR-2 | GPM-6 | ||
|---|---|---|---|---|
| α-Amylase (% Inhibition) | α-glucosidase (% Inhibition) | α-Amylase (% Inhibition) | α-glucosidase (% Inhibition) | |
| Raw | 49.34 ± 2.34e | 62.72 ± 4.21e | 45.89 ± 2.91e | 60.33 ± 4.77e |
| PC | 32.75 ± 1.81c | 43.31 ± 3.69c | 28.32 ± 2.01c | 41.44 ± 3.82c |
| PCF | 32.33 ± 1.57bc | 41.17 ± 2.21c | 27.21 ± 0.97c | 39.88 ± 1.82c |
| IRC | 28.91 ± 2.02b | 35.60 ± 3.02b | 23.21 ± 1.66b | 32.88 ± 2.31b |
| MWC | 33.37 ± 3.38d | 46.62 ± 3.84d | 30.63 ± 3.07d | 41.73 ± 3.68de |
| Extrusion | 14.13 ± 0.91a | 24.59 ± 1.77a | 12.40 ± 0.99a | 20.19 ± 1.52a |
All data were means of triplicates (n = 3), values are mean of three independent determinations ± Standard deviation; Raw: Untreated horse gram 70% methanolic extract; PC: Pressure processed horse gram70% methanolic extract; PCF: Pressure processed and flaked horse gram70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
The absorption of glucose in the intestine can be retarded by the use of α- glucosidase inhibitors. Similar to α-amylase inhibition activity, α- glucosidase inhibition activity also decreased during processing (24.59–46.62% for PAIYUR-2 and 20.19–41.73% for GPM-6) and found directly proportional to the phenolic content of the grains (Table 4). The % inhibition for raw horse gram extract of PAIYUR-2 and GPM-6 were 62.72 and 60.33% respectively (Table. 5). Among processed grains extrusion caused maximum reduction in inhibition activity (60.79 and 66.53%) whereas minimum reduction was observed for MWC treated grains (25.66 and 30.83%) for PAIYUR-2 and GPM-6 respectively. The trend of glucosidase inhibition activity for both PAIYUR-2 and GPM-6 varieties was Raw > MWC > PC > PCF > IRC > Extrusion. The lower enzymatic inhibition of micronized and extrusion processed grains can be attributed to the sensitivity of these active compounds towards high temperature and pressure involved in these processing methods (Sreerama, et al. 2012). The reported results were lower than the P. pinnata seeds (86.50%), Proso millet (77%) and foxtail millet (82.5%) (Vadivel & Biesalski, 2011; Kim, et al. 2011). The phenolics from legumes are considered safe due to their moderate α-amylase and higher α-glucosidase inhibition activity. The desired inhibition activity of phenolic from legumes makes them quite useful in delaying the absorption of dietary carbohydrates and reducing the glycaemic index of food, leading to the reduction in postprandial blood glucose level without creating any adverse effect.
In-vitro antiurolithiatic activity
Percent stone dissolving capacity of raw grains were 48.12 and 46.31% in PAIYUR-2 and GPM-6 varieties respectively and ranged from 15.66 to 42.34% for the processed grains (Fig. 2). The antiurolithiatic activity of seed extracts was significantly (p ≤ 0.05) affected during processing. Extrusion processing resulted in maximum reduction (63.07 and 66.18%) in antiurolithiatic potential whereas least effect was observed for grains processed under pressure (12.01 and 9.71%) in PAYIUR-2 and GPM-6 varieties respectively. The antiurolithiatic activity of PAYIUR-2 variety was in accordance with the previously reported results (Atodariya, et al. 2013) but significantly (p ≤ 0.05) lower for GPM-6. This can be attributed to the cultivar difference of grain (De-Mejia, et al. 2003). This may also be attributed to the change in functionality of phenols due to the change in the functional group during processing. Hence, suitable processing for an optimum time interval can actually prevent higher loss in the quality of grain and maintain its nutritional and functional quality.
Fig. 2.
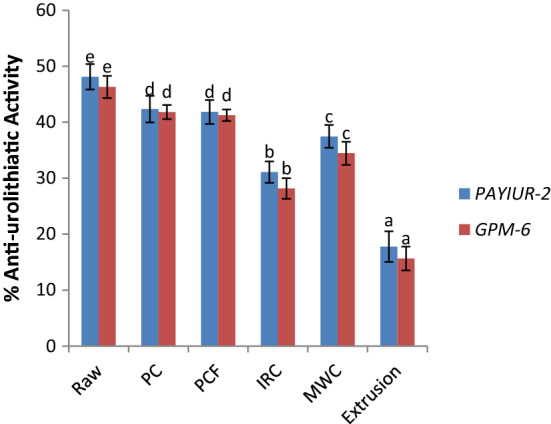
Anti-urolithiatic activity of horse gram extract from raw and processed samples (the concentration of extract used was 5 mg/ml). Raw: Untreated horse gram 70% methanolic extract; PC: Pressure processed horse gram 70% methanolic extract; PCF: Pressure processed and flaked horse gram 70% methanolic extract; IRC: Micronized horse gram 70% methanolic extract; MWC: Microwave processed horse gram 70% methanolic extract; Extrusion: Extrusion processed horse gram 70% methanolic extract
Conclusion
The present study shows that the aqueous methanolic extract of horse gram seeds possesses appreciable levels of phenolic content and other nutrients even after the processing of grain. The extract has shown prodigious antioxidant and functional properties such as enzymatic inhibition and renal stone dissolving properties. The extrusion processing has significantly (p ≤ 0.05) affected the antioxidant and functional properties of grain extract. Even though the processing of grain has shown the deleterious effects on the phenolic content and functional properties of grain but they still possess significant antioxidant potential and other functional properties. Hence, they can still be appreciably utilized as an important ingredient in daily diet. All the grains have shown a significant correlation between antioxidant potential and phenolic content of the grain while it did not exist for enzyme inhibition activity. Hence, the processing technologies can effectively be used in the processing of legume and these legumes can be envisaged as one of the main ingredients in the development of supplementary food with better nutritional and functional properties.
Acknowledgements
The authors are grateful to Director, Defence Food Research Laboratory, DRDO, Mysore, India for providing us with all facilities and financial assistance to carry out the research work.
Abbreviations
- GPM-6
Horse gram variety
- NBT
Nitro blue tetrazolium
- FRAP
Ferric reducing antioxidant power
- TPTZ
2,4,6-Tri(2-pyridyl)-1,3,5-triazine
- ABTS
2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid
- AOAC
Association of official agricultural chemist
- ANOVA
Analysis of variance
- SPSS
Statistical package for the social sciences
Author contributions
*Rest all the contributors to the article are given due authorship in article itself. 1) RV: Conducted research and wrote the article. 2) ADS: Research guide, research framework, Scientific Language Correction, 3) MPM: Instrumental analysis, Scientific Language Correction and proof reading. 4) MAK: Statistical Evaluation of Data, 5) CG: Proof Reading and Correction.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. All the funding was by the research institute of DRDO-DFRL.
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
After the consent of all the authors and as a corresponding author of this manuscript I declare that.
-
(i)
the work described has not been published before (except in the form of an abstract, a published lecture or academic thesis),
-
(ii)
it is not under consideration for publication elsewhere,
-
(iii)
its submission to JFST publication has been approved by all authors as well as the responsible authorities–tacitly or explicitly–at the institute where the work has been carried out,
-
(iv)
if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder, and.
-
(v)
JFST will not be held legally responsible should there be any claims for compensation or dispute on authorship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amarowicz R, Raab B. Antioxidative activity of leguminous seed extracts evaluated by chemi-luminescence methods. Z Naturforsch. 1997;52:709–712. doi: 10.1515/znc-1997-9-1022. [DOI] [Google Scholar]
- Atodariya U, Roshni B, Upadhyay S, Upadhyay U. Anti-Urolithiatic activity of Dolicus biflorus seeds. J Pharmacogn Phytochem. 2013;2:209–213. [Google Scholar]
- Bhartiya A, Aditya JP, Kant L. Nutritional and remedial potential of an underutilized food legume horse gram (Macrotyloma uniflorum): a review. J Anim Plant Sci. 2015;25:908–920. [Google Scholar]
- De-Mejia EG, Guzman-Maldonado GH, Acosta-Gallegos JA, Reynoso-Camacho R, Ramirez-Rodriguez A. Effect of cultivar and growing location on the trypsin inhibitors, tannins, and lectins of common beans (Phaseolus vulgaris L.) grown in the semi-arid high lands of Mexico. J Agric Food Chem. 2003;51:5962–5966. doi: 10.1021/jf030046m. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Hodgkinson A, William A. An improved colorimetric procedure for urine oxalate. Int J Clin Chem Dignos Lab Med. 1972;36:127–132. doi: 10.1016/0009-8981(72)90167-2. [DOI] [PubMed] [Google Scholar]
- Jain M, Bhandari A, Bhandari A, Patel P. Isolation, characterization and In vitro Antiurolithiatic activity of Cerpegin Alkaloid from Ceropegiabulbosa var. Lushii root. Int J Drug Dev Res. 2012;4:154–160. [Google Scholar]
- Kim JS, Hyun TK, Kim MJ. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chem. 2011;124:1647–1651. doi: 10.1016/j.foodchem.2010.08.020. [DOI] [Google Scholar]
- Mitra J, Das A, Joshi T. An isoflavone-diglycoside from the seeds of Dolichos biflorus. Phytochemistry. 1983;22:1063–1064. doi: 10.1016/0031-9422(83)85069-9. [DOI] [Google Scholar]
- Nithiyanantham S, Selvakumar S, Siddhuraju P. Total phenolic content and antioxidant activity of two different solvent extracts from raw and processed legumes, Cicer arietinum L. and Pisum sativum L. J Food Comp Anal. 2012;27:52–60. doi: 10.1016/j.jfca.2012.04.003. [DOI] [Google Scholar]
- Petchiammal C, Hopper W. Antioxidant activity of proteins from fifteen varieties of legume seeds commonly consumed in India. Int J Pharm Sci. 2014;6:476–479. [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/ antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Ramesh CK, Rehman ABT, Prabhakar BR, Vijay A, Rao ASJ. Antioxidant potential in sprout vs. seeds of Vigna radiate and Macrotyloma uniflorum. J Appl Pharm Sci. 2011;1:99–103. [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Improved health-relevant functionality in dark germinated Mucuna pruriens sprouts by elicitation with peptide and phytochemical elicitors. Bioresour Technol. 2009;100:4507–4514. doi: 10.1016/j.biortech.2009.01.078. [DOI] [PubMed] [Google Scholar]
- Ravichandran K, Ahmed AR, Knorr D, Smestanska I. The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Res Int. 2012;48:16–20. doi: 10.1016/j.foodres.2012.01.011. [DOI] [Google Scholar]
- Rocha-Guzman NE, Gonzalez-Laredo RF, Ibarra-Perez FJ, Nava-Berumen CA, Gallegos-Infante JA. Effect of pressure cooking on the antioxidant activity of extracts from three common bean (Phaseolus vulgaris L.) cultivars. Food Chem. 2007;100:31–35. doi: 10.1016/j.foodchem.2005.09.005. [DOI] [Google Scholar]
- Satish H, Raman D, Kashama D, Shivananda BG, Shridhar KA. In vitro anti-lithatic activity study of Tribuluster restris fruits and Boerhaaviadiffusa roots. Scholars Res Library Der Pharm Lett. 2010;2:12–20. [Google Scholar]
- Siddhuraju P. The antioxidant activity and free radical scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006;99:149–157. doi: 10.1016/j.foodchem.2005.07.029. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seeds extract. Food Chem. 2007;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Siddhuraju P, Manian S. The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chem. 2007;105:950–958. doi: 10.1016/j.foodchem.2007.04.040. [DOI] [Google Scholar]
- Sowndhararajan K, Siddhuraju P, Manian S. Antioxidant and free radical scavenging capacity of the underutilized legume, Vigna vexillata (L.) A. rich. J Food Comp Anal. 2011;24:160–165. doi: 10.1016/j.jfca.2010.09.016. [DOI] [Google Scholar]
- Sreerama YN, Vadakkoot B, Sashikala V, Pratape M. Phenolic compounds in cowpea and horse gram flours in comparison to chickpea flour: evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chem. 2012;133:156–162. doi: 10.1016/j.foodchem.2012.01.011. [DOI] [Google Scholar]
- Sreeramulu D, Reddy VK, Raghunath M. Antioxidant activity of commonly consumed cereals, millets, pulses and legumes in India. Indian J Biochem Bio. 2012;46:112–115. [PubMed] [Google Scholar]
- Suja KP, Jayalekshmy A, Arumughan C. In vitro studies on antioxidant activity of lignans isolated from sesame cake extract. J Sci Food Agric. 2005;85:1779–1783. doi: 10.1002/jsfa.2170. [DOI] [Google Scholar]
- Suriano S, Iannucci A, Codianni P, Fares C, Russo M, Pecchioni N, Marciello U, Savino M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties in coloured barley grown in southern Italy. Food Res Int. 2018;113:221–233. doi: 10.1016/j.foodres.2018.06.072. [DOI] [PubMed] [Google Scholar]
- Ti H, Zhang MZ, Wei Z, Chi J, Deng Y, Zhang Y. Effect of extrusion on phyto-chemical profile in milled fractions of black rice. Food Chem. 2015;178:186–194. doi: 10.1016/j.foodchem.2015.01.087. [DOI] [PubMed] [Google Scholar]
- Upadhyay SU, Jain VC, Upadhyay UM. Glossary of Dolicus biflorus- A legume with miraculous activities. Res J Pharm Pharmacody. 2015;7:103–116. doi: 10.5958/2321-5836.2015.00021.X. [DOI] [Google Scholar]
- Vadivel V, Biesalski HK. Contribution of phenolic compounds to the antioxidant potential and type II diabetes related enzyme inhibition properties of Pongamiapinnata L. Pierre Seeds Process Biochem. 2011;46:1973–1980. doi: 10.1016/j.procbio.2011.07.007. [DOI] [Google Scholar]
- Vadivel V, Cheong JN, Biesalski HK. Antioxidant and type II diabetes related enzyme inhibition properties of methanolic extract of an underutilized food legume, Canavalia ensiformis (L.) DC: Effect of traditional processing methods. LWT-Food Sci Technol. 2012;47:255–260. doi: 10.1016/j.lwt.2012.01.014. [DOI] [Google Scholar]
- Van der Zwan LP, Scheffer PG, Dekker JM, Stehouwer CDA, Heine RJ, Teerlink T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxide and blood pressure. Hypertension. 2010;55:1366–1372. doi: 10.1161/HYPERTENSIONAHA.109.147231. [DOI] [PubMed] [Google Scholar]
- Vashishth R, Semwal AD, Pal Murugan M, Sharma GK. Post-harvest processing, cooking and textural properties of horse gram (Mycrotyloma uniflorum) varieties. J Food Meas Charact. 2017;12:257–261. doi: 10.1007/s11694-017-9636-9. [DOI] [Google Scholar]
- Yen GC, Duh PD. Antioxidative properties of methanolic extracts from peanut hulls. J Amer Oil Chem Soc. 1993;70:383–386. doi: 10.1007/BF02552711. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoids contents on mulberry and their scavenging effect on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Zielinski H, Kozlowska H, Lewczuk B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov Food Sci Emerg. 2001;2:159–169. doi: 10.1016/S1466-8564(01)00040-6. [DOI] [Google Scholar]


