Abstract
One of the limitations of the use of corn in the food chain is its contamination with mycotoxins. Reduction in their levels can be achieved by processing the grain, which in the case of corn can be achieved by wet or dry milling. The aim of this study was to compare the distribution of aflatoxins B1 and B2, and fumonisin B1 in corn fractions obtained by dry and wet milling, aiming to identify conditions to mitigate the risk of exposure to these contaminants. Naturally, contaminated corn kernels were subjected to laboratory milling. The wet-milling conditions containing 1% lactic acid in the steeping solution and 18 h of steeping were the most efficient for mycotoxin reduction in the endosperm fraction, reducing aflatoxins B1 and B2 contamination to levels below the limit of quantification. Dry-milling reduced the concentration of these mycotoxins in the endosperm (98–99%). Fumonisin B1 contamination increased in the germ and pericarp fraction by more than three times in both dry and wet milling. Dry-milling reduced fumonisin B1 contamination in the endosperm to levels below the limit of quantitation. Wet and dry milling processes can be an efficient control method to reduce aflatoxins and fumonisin in the corn endosperm fraction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05373-9.
Keywords: Milling fractions, Aflatoxins, Fumonisin, Wet milling
Introduction
Corn (Zea mays L. ssp. mays) is part of the diet in Latin America, Asia, and Africa. Besides, corn consumption has increased in developed countries as it is used as an ingredient for breakfast cereals, snacks, dietetic products, and in particular for gluten-free foods whose consumption is increasing. Given the role of ground corn products as a staple food, their quality characterization becomes extremely important. From a nutritional point of view, corn and, its products are good sources of starch, proteins, lipids and, different bioactive compounds (Blandino et al. 2017).
The corn production chain includes the dry and wet milling processes that separate the grain into three main components: the germ (outer fraction), pericarp (outer fraction) and, endosperm (inner fraction). These fractions are used mainly for oil extraction, feed production and, human consumption, respectively. Wet milling differs fundamentally from dry milling as it contains a steeping step in which physical and chemical changes occur in the basic constituents, leading to the production of pure starch for industrial and food uses. Additionally, protein, fiber and, germ are obtained as by-products of the wet milling process (Malumba et al. 2015).
However, corn quality and safety may be reduced by the presence of mycotoxins, as kernels may be affected by fungi, that are capable of producing various metabolites in the field and during storage (Grenier and Oswald 2011). The association of corn with different fungi may lead to the co-occurrence of mycotoxins in this crop. In Nigeria, 65% of corn grains showed co-occurrence of aflatoxins and fumonisins (Adetunji et al. 2014). Similarly, this co-occurrence was observed in Korea (Park et al. 2018), EUA (Curry et al. 2019), and Brazil (Franco et al. 2019). This is a matter of concern since both fumonisin and aflatoxin are known human carcinogens and their co-occurrence may lead to a mechanism of action of complementary toxicity (Bryden 2007).
During the industrial milling process, mycotoxins are not destroyed and are redistributed between fractions (Castells et al. 2008; Savi et al. 2016). In general, fumonisins and aflatoxins tend to be concentrated in the germ and pericarp and to a smaller proportion in the endosperm and its derivatives (Bordini et al. 2019; Generotti et al. 2015; Park et al. 2018). However, these mycotoxin distributions are also dependent on the grinding process used, mycotoxin class and grain contamination level. Therefore, the milling process can be studied through multivariate techniques such as experimental design, where mathematical models are used to achieve the best reduction of these contaminants in the different fractions.
Considering the risk to human and animal health associated with the occurrence of mycotoxins, maximum limits are legislated by several countries. In Brazil and in the United States the maximum tolerable limits for the sum of aflatoxins B1, B2, G1 and G2 are 20 μg/kg and 10 μg/kg in European Union for corn or cornmeal. The limits for fumonisins (B1 and B2) for corn destined for further processing is 5000 μg/kg in Brazil, 4000 μg/kg in United States and European Union. For cornmeal, corn cream, flakes, hominy the limit is 1500 μg/kg in Brazil, 2000 μg/kg in the United States and 1000 μg/kg in European Union. For cornstarch and others corn products the limit is 1000 μg/kg in Brazil and for breakfast and snacks the limit is 800 μg/kg in European Union (Anvisa 2017; EC 2006, 2007, USFDA 2001).
Although the distribution of aflatoxins and fumonisins in corn milling processes has been evaluated in other studies (Oulkar et al. 2018; Kumar 2020), the simultaneous determination of these mycotoxins for the redistribution of these contaminants have been poorly performed. The aim of this study was to investigate and compare the simultaneous distribution of mycotoxins produced by field (fumonisin B1- FB1) and storage fungi (aflatoxins B2 (AFLA B2) and B1 (AFLA B1)) in fractions obtained by two milling processes performed under laboratory conditions and using experimental design as a tool to find the best conditions for mycotoxin reduction in the wet-milling process.
Material and methods
Standards, reagents and samples
Aflatoxins standards (B2 and B1) and fumonisin B1 were supplied by Sigma-Aldrich (Saint Louis, MO, USA) with purity > 98%. Aflatoxin stock solutions were prepared by dissolving the standards with toluene/acetonitrile (98:2, v/v); while fumonisin was dissolved in methanol:acetonitrile (50:50, v/v). After preparation, the mycotoxin standards were dried under nitrogen and stored at − 18 °C, to ensure their stability. The working solutions were prepared from stock solutions, and before use aflatoxins were quantified in a spectrophotometer, according to the AOAC (2000).
The solvents used as mobile phase in the chromatographic system (acetonitrile and methanol) with purity > 99.9% were supplied by JT Baker (Goiânia, GO, Brazil). Ultrapure H2O (> 18.2 MΩ/cm resistivity) was purified using a Milli-Q® SP Reagent Plus water system (Millipore Corp., Bedford, USA). The 0.1 M phosphate buffer pH 3.15 used as mobile phase in the chromatographic system for fumonisin B1 analysis was prepared to dilute 13.8 g of sodium phosphate monobasic in 1 L of ultrapure water, and adjusting the pH to 3.15 with 2 M hydrochloride acid.
The OPA-MCE derivatization reagent used during quantification of fumonisin B1 was prepared daily according to Kong et al. (2012) by mixing 100 mg ortho-phthaldialdehyde (OPA), 20 mL methanol, 500 µL 2-mercaptoethanol (MCE), and 950 mL of sodium tetraborate solution 0.05 M. This mixture was then brought to 1L by addition of ultrapure water. The OPA-MCE reagent was stored in a brown glass bottle and, when not in use, kept at 4 °C for up to 2 d. The mobile phase and derivatization reagent were always filtered through a 0.45 µm cellulose filter and then degassed in an ultrasonic bath before use.
Corn (Zea mays L.) samples were obtained in 2016 from a local farm in Matelândia (Paraná, Brazil). Ears of corn (100 ears) with the husks intact were stored after harvesting in a ventilated storage place for 10–12 months. Kernels were shelled and stored frozen in plastic containers until use.
Wet milling conditions
The wet milling until steeping was performed according to Malumba et al. (2015). The corn kernels were cleaned to remove broken grain, husk, and other impurities. The clean grains proceeded to the steeping step, which was evaluated by a CCRD 22 experimental design where the steeping time and lactic acid concentration were the variables of interest (Table 1). Steeping was carried out at a constant temperature of 50 ± 2 °C. Corn kernels (15 g) were added in the proportion (1:2.4 w/v) to the steeping solution containing lactic acid and sodium metabisulfite (0.6%), to release 0.2% SO2. After the steeping step, the corn grain was separated into 3 fractions: germ and pericarp (external fractions) and endosperm (internal fraction) (Supplementary Fig. S1).
Table 1.
CCRD matrix (coded and actual values) with the response of aflatoxins B1 and B2 and fumonisin B1 concentration
| Assay | X1 | X2 | AFLAB2 (ng/g) | AFLAB1 (ng/g) | FB1 (µg/g) |
|---|---|---|---|---|---|
| 1 | − 1 (6) | − 1 (0.6) | 0.40 | 0.01 | 1.53 |
| 2 | + 1 (18) | − 1 (0.6) | 0.42 | 0.35 | 0.68 |
| 3 | − 1 (6) | + 1 (1.0) | 0.38 | < LOD | 0.43 |
| 4 | + 1 (18) | + 1 (1.0) | 0.01 | < LOD | 0.82 |
| 5 | − 1.41 (3.54) | 0 (0.8) | 0.01 | 0.01 | 0.50 |
| 6 | + 1.41 (26.46) | 0 (0.8) | 0.19 | < LOD | 1.75 |
| 7 | 0 (12) | − 1.41 (0.52) | 0.08 | < LOD | 0.53 |
| 8 | 0 (12) | + 1.41 (1.28) | 0.01 | 0.01 | 0.21 |
| 9 | 0 (12) | 0 (0.8) | 3.78 | 10.28 | 0.71 |
| 10 | 0 (12) | 0 (0.8) | 3.98 | 12.87 | 0.88 |
| 11 | 0 (12) | 0 (0.8) | 3.80 | 10.01 | 0.79 |
X1: Steeping time (h); X2: Lactic acid concentration (%). LOD AFLA B1 0.04 ng/g
Endosperm samples were ground to 0.50 mm and homogenized for mycotoxin determination. The dependent variable was mycotoxin concentration in the endosperm since this fraction is destined for the production of breakfast cereals or ground for cornmeal production.
Mycotoxin distribution in wet and dry milling
Laboratory milling of naturally contaminated corn with aflatoxins and fumonisin was performed by the dry milling technique according to Somavat et al. (2016), with the following steps: cleaning the corn grains; tempering (moisture adjustment to 23.5% with the addition of water and orbital agitation at 200 rpm for 20 min); the first grind; drying at 49 °C for 2 h; separation of fractions; endosperm milling and sieving to obtain the cornmeal (Supplementary Fig. S2).
The wet milling was performed according to the conditions defined in item 2.2, where the corn kernels (30 g) were added to the steeping solution in the proportion (1:2.4 w/v), containing 1% lactic acid and 0.6% sodium metabisulphite for 0.2% SO2 release. Steeping was performed for 18 h. After that, the steeping water was separated from the kernels, which were then separated into 3 fractions: germ, pericarp, and endosperm.
Aflatoxins and fumonisin B1 determination
Aflatoxins B2 and B1 and fumonisin B1 were extracted from the milling fractions according to Massarolo et al. (2018). In short, 1 g of sample was macerated with 0.5 g of adsorbent C18, transferred to Falcon tube, and added 10 mL of acetonitrile:methanol (50:50, v/v). The mixture was vortexed for 3 min and centrifuged at 3220 × g, for 10 min. Two aliquots of the supernatant (one for aflatoxins and another for fumonisin B1) were removed and dried at 60 °C. For the determination of aflatoxins, the extract was resuspended in a mixture of ultrapure water and acetonitrile (90:10, v/v) and quantification in HPLC-FL with post-column derivatization was performed. The chromatographic conditions for identification, and quantification of aflatoxins B2 and B1 were performed as Massarolo et al. (2018).
Fumonisin B1 was first identified and quantified in a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) with post-column derivatization (unpublished data). After quantification, confirmation was performed using an Alliance Sampler Liquid Chromatograph equipped with an auto-sampler and Sequential Mass Detector Electrospray ionization mode following the chromatographic conditions of Scaglioni et al. (2018).
Distribution factor and distribution in the grain
Due to the variability associated with testing corn for mycotoxins, the estimation of mycotoxin concentration in the whole corn was carried out using mass balance according to previous studies (Belluco et al. 2017). The distribution factor was adopted to express the redistribution of mycotoxin content in each fraction (endosperm, germ, pericarp) compared to their respective concentrations estimated in whole kernels. The distribution factor is defined as the ratio between the concentration of mycotoxins in each fraction and the concentration of mycotoxins in whole corn (Bordini et al. 2017).
The distribution in the grain, which is defined as the multiplication of the mycotoxin content in the processed fraction and the percentage of the fraction in the whole grain, divided by the sum of the mycotoxin in all fractions (calculated using the percentage of the fraction).
Statistical analysis
Data normality was verified by the Kolmogorov–Smirnov test. To evaluate the influence and interaction of the independent variables of interest (steeping time and lactic acid concentration) with the response (mycotoxin concentration in the endosperm), the analysis of the effects on to the dependent variable measured (mycotoxin concentration in the endosperm), a statistical analysis was performed. Once the significance of the independent variables was verified, then an analysis of variance was performed to determine the mathematical model associated with the process, the F value, and the significance of the regression equation obtained. Response surfaces were generated to the process variables that led to the lowest levels of mycotoxins in the endosperm. Analyzes were performed using Statistica 6.0 software.
Results and discussion
Co-occurrence of mycotoxins in corn
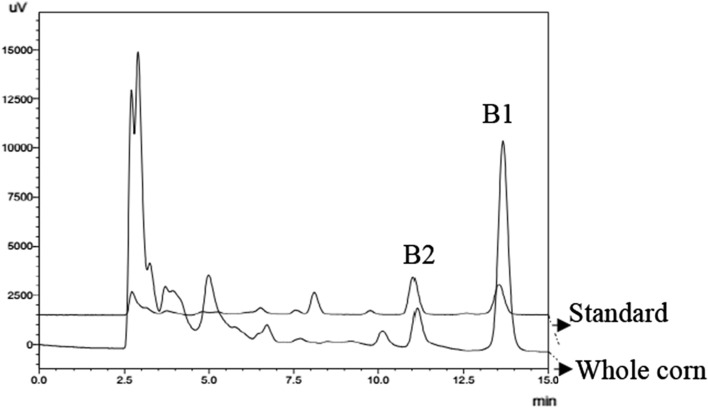
The corn used for this research was naturally contaminated with fumonisin B1 (Fig. 1) and aflatoxins B1 and B2 (Fig. 2), as can be observed in the chromatograms by the retention times of the compounds in the sample and the respective standard. As corn did not have the natural presence of aflatoxins G1 and G2, they were not evaluated in this work.
Fig. 1.
Chromatogram of standard fumonisin B1 1 µg/mL (a) and fumonisin B1 in the whole corn 0.81 µg/g (b)
Fig. 2.
Chromatogram of standard aflatoxins B1 (0.35 ng/mL) and B2 (0.10 ng/mL) and in the whole corn B1 (7.08 ng/g) and B2 (3.48 ng/g)
The use of naturally contaminated sample present advantages over the use of spiked samples because naturally contaminated corn is more applicable and representative for an industrial process. In addition, a naturally contaminated sample illustrates the actual behavior of the contaminant in corn, including possible interactions with corn macromolecules.
Wet milling conditions
The results of the reductions of aflatoxins B1 and B2, and fumonisin B1 in endosperm obtained by the wet milling evaluated by CCRD for the 2 variables under study (steeping time and lactic acid concentration) are shown in Table 1.
An estimate of the main effects was obtained by assessing the differences in process performance caused by a change from a lower level (−1.41) to a higher level (+ 1.41). The p-value was used to verify the significance of the factors under study (Supplementary Table S1). FB1 did not present an empirical model.
Steeping time and lactic acid concentration quadratic were the most significant variables in the reduction of aflatoxins B2 and B1, where aflatoxin levels decreased by 3.7% for B2 and 10.3% for B1 with increasing steeping time and amount of lactic acid. For fumonisin B1 none of the variables evaluated were significant at the 5% level. Therefore, process conditions that resulted in the greatest reduction in aflatoxins were a steeping time of 18 h and acid lactic at 1.0%(w/v).
Since the values obtained for the correlation between the dependent and independent variables were 0.99 for AFLA B2 and 0.95 for AFLA B1, mathematical models (Eqs. 1 and 2) were obtained from the coefficients presented in Table 2.
| 1 |
| 2 |
where Y = aflatoxin concentration, X1 = steeping time and X2 = lactic acid concentration.
Table 2.
Distribution of fumonisin B1 in wet and dry milling
| Fraction and % in the grain | Wet milling | Dry milling | ||||
|---|---|---|---|---|---|---|
| FB1 (µg/gfraction) | D.F (%) | D.G (%) | FB1 (µg/gfraction) | D.F (%) | D.G (%) | |
| Germ (11%) | 6.10 (6.4) | 369 | 40.6 | 2.53 (21.1) | 331 | 47.4 |
| Pericarp (7%) | 4.40 (12.2) | 266 | 18.7 | 4.41 (29.4) | 751 | 52.6 |
| Endosperm (82%) | 0.82 (15.2) | 41 | 40.7 | < LOQ | – | – |
Mean (CV) CV = coefficient of variation, n = 3. LOD = 0.2 µg/g, LOQ = 0.4 µg/g. D.F = distribution factor, D.G = distribution in the grain. Percentage as w/w
The calculated F-value (406.94 for AFLAB2 and 74.05 for AFLAB1) was higher than the tabulated F (4.46) and the determination coefficient obtained was close to 1 for AFLAB2 (R2 = 0.99) and AFLAB1 (R2 = 0.95). Thus, it is possible to state that the model is predictive and significant (Supplementary Table S2). From the equations obtained a contour curve was generated to illustrate the effect of the processing variables on the concentration of aflatoxin B1 and B2 (Fig. 3).
Fig. 3.
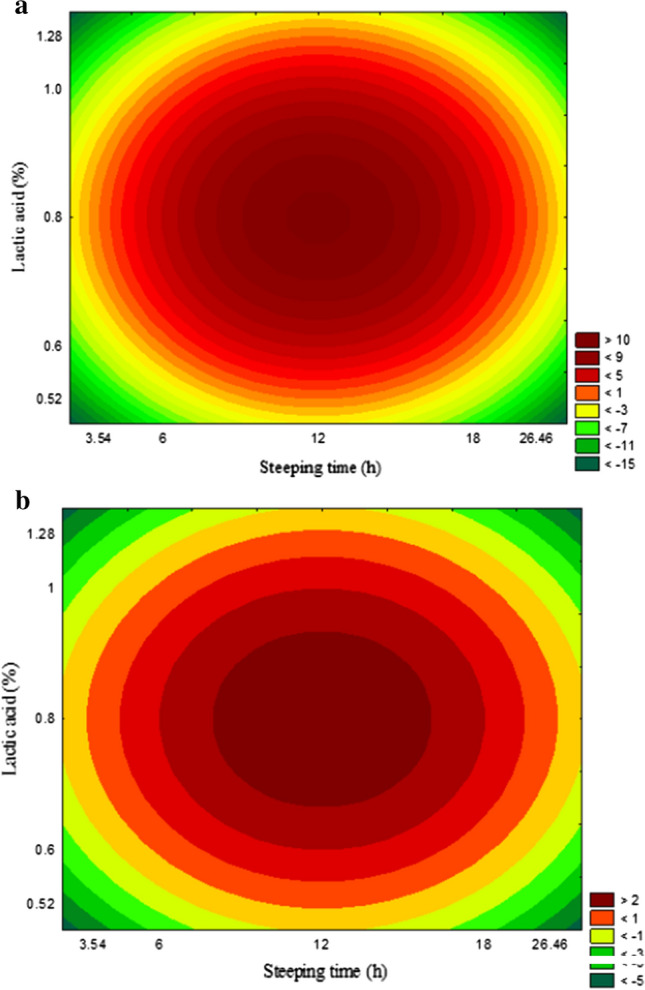
Contour diagram of aflatoxin B1 (A) and aflatoxin B2 (B) concentration as function of lactic acid concentration and steeping time
The lowest level of aflatoxin in the endosperm was obtained with a steeping time of 18 h and a lactic acid concentration of 1%. These conditions led to a reduction of aflatoxin concentration in the endosperm by 99.8% for B2 and 100% for B1. Thus, these steeping conditions were applied to evaluate the distribution of mycotoxins during corn wet milling.
Mycotoxin distribution in dry and wet milling
Fumonisin distribution
The fumonisin B1 content was determined in each fraction of the milled corn. In the wet milling, the greatest fumonisin content was observed in the germ fraction, followed by the pericarp and endosperm fractions. After the dry milling, the content of FB1 in the endosperm was below the limit of quantification, and the greatest FB1 content was detected in the pericarp followed by the germ fraction (Table 2).
The results are shown as distribution factor, defined as the ratio between the mycotoxin content in the processed fraction and the mycotoxin content in whole corn, and as distribution in the grain, which is defined as the multiplication of the mycotoxin content in the processed fraction and the percentage of the fraction in the whole grain, divided by the sum of the mycotoxin in all fractions (calculated using the percentage of the fraction).
After the wet milling, 40% of the fumonisin B1 was quantified in the germ and endosperm and only 18% in the pericarp; however, after the dry milling 52% was quantified in the pericarp and 47% in the germ.
The distribution factor for germ and pericarp ranged from 266 to 751%, which means that contamination in these fractions was increased in more than three-fold. The higher FB1 concentrations in germ and pericarp might be due to the location of the fungus in the tip cap and germ areas just beneath the pericarp (Katta et al. 1997). The germ is externally located in the kernel and rich in lipids, which favors the attack of molds and the subsequent mycotoxin productions (Brera et al. 2006).
The results of distribution factor in the germ (331%) in this study is similar to the results reported for industrial dry milling that was evaluated by Bordini et al. (2017). In the industrial evaluation the fate of fumonisins (B1 and B2) were evaluated in a dry milling process for two lots of non-transgenic corn. The fractions evaluated included germ, pericarp, endosperm, cornmeal and grits. Samples were collected from one of the major Brazilian milling industries in 2014 (n = 120) and 2015 (n = 120). The authors verified that fumonisins were concentrated in the germ and pericarp at a rate of 322% and 188% (lot 1) and 311% and 263% (lot 2), respectively.
Contamination trends for the different corn fractions in this study were similar to those found in other studies (Bordini et al. 2017; Castells et al. 2008; Generotti et al. 2015), in which an increase of fumonisin concentration in pericarp and germ fractions and a decrease in endosperm, a product intended for human consumption, was reported. Although fumonisin levels were higher in the corn germ, the alkaline treatment usually performed in the oil refining industry led to a degradation of these mycotoxins and negligible amounts of fumonisins were detected in refined oil and margarine for human consumption (Escobar et al. 2013).
The content of FB1 in the endosperm during the wet milling was higher than in the dry milling, probably due the fact that mycotoxins may have been carried by the steeping water into the endosperm. In the dry milling the surface parts of the grain is removed with minimum breakage of the endosperm by a physical process, and lower levels of mycotoxin is observed in the endosperm. The pericarp layer has the potential to act as a physical barrier preventing the mycelia from penetrating further in the kernel structure and transferring of fumonisins to the inner part of the kernel (Castells et al. 2008).
Aflatoxin distribution
The wet milling decreased the aflatoxins concentration in all fractions (Table 3), both aflatoxin B1 and B2 could not be measured in the endosperm.
Table 3.
Distribution of Aflatoxins B1 and B2 in wet and dry milling
| Fraction and % in the grain | Wet milling | |||||
|---|---|---|---|---|---|---|
| B1 (ng/gfraction) | D.F (%) | D.G (%) | B2 (ng/gfraction) | D.F (%) | D.G(%) | |
| Germ (11%) | 0.15 (8.4) | 620 | 68.2 | 0.07 (15.1) | 556 | 58.7 |
| Pericarp (7%) | 0.11 (12.2) | 455 | 31.8 | 0.07 (10.6) | 556 | 41.3 |
| Endosperm (82%) | < LOD | – | – | < LOQ | – | – |
| Fraction and % in the grain | Dry milling | |||||
| B1 (ng/gfraction) | D.F (%) | D.G (%) | B2 (ng/gfraction) | D.F (%) | D.G (%) | |
| Germ (11%) | 0.09 (21.0) | 61 | 6.7 | 0.21(20.9) | 228 | 9.9 |
| Pericarp (7%) | 1.15 (11.9) | 778 | 54.5 | 0.52 (20.7) | 563 | 16.1 |
| Endosperm (82%) | 0.07 (19.7) | 47 | 38.8 | 0.04 (14.5) | 43 | 74.0 |
Mean (CV) CV = coefficient of variation. LOD = B2 0.01 ng/g, B1 0.04 ng/g, LOQ = B2 0.02 ng/g, B1 0.07 ng/g. D.F = distribution factor, D.G = distribution in the grain. Percentage as w/w
After the wet milling 68% and 58% of the aflatoxin B1 and B2, respectively, were quantified in the germ and 31% and 41% of these aflatoxins in the pericarp. The relatively low contamination levels of aflatoxin in corn fractions possible can be explained by the low contamination in the whole corn and by the aflatoxin migration from kernels into steeping solution. Research conducted by Park et al. (2018) showed that aflatoxins were transferred from corn to steep water during the wet milling process.
A different aflatoxin distribution was observed in dry milling, and after this process aflatoxin B1 and B2 were detected in all fractions of the kernel. Regarding aflatoxin B1, 54.5% was quantified in the pericarp, 38% in the endosperm and only 6.7% in the germ; however, 74% of aflatoxin B2 was found in the endosperm, 16% in the pericarp and only 9.9% in the germ.
Higher aflatoxin levels were observed in pericarp and germ, and it could be the result of mold growth progress in the kernels. Lillehoj et al. (1976), showed that growth of Aspergillus flavus occurs from the external part of grain into the endosperm. The aflatoxin B1 and B2 levels were also higher in the pericarp than the germ in an industrial dry milling process using both conventional and organic corn (Brera et al. 2006). Contrary to that, Pietri et al. (2009) verified higher aflatoxin levels in germ than pericarp, and the conflicting results among these studies could be caused by the heterogeneity in the aflatoxin-contamination of corn kernels.
Dry and wet milling processing resulted in a concentration of both fumonisins and aflatoxins in pericarp and germen, which is widely used in the production of animal feeds. At the same time, these cereal milling fractions (pericarp and germ) represent a novel category of promising ingredients for human nutrition and health, due to other interesting functional properties (Schaffer-Lequart et al. 2017). Our study showed that the milling process is useful in reducing the levels of mycotoxins in the endosperm fraction and the outer layers of the cereal grain are more likely to be exposed to mycotoxins contaminants. This information is important to the food industry because the new trend in the food industry is the utilization of whole grain meals or flours because they contain higher amounts of minerals, vitamins, phytochemicals, and other nutraceuticals that favor human health (Serna-Saldivar and Carrillo 2019).
The observed variation in the levels of aflatoxins and fumonisin in the corn fractions can be associated with the yield of the milling process and the distribution of mycotoxins in the various parts of the grain as a consequence of the fungal attack.
Conclusion
The wet-milling process with 1% (w/v) lactic acid concentration and 18 h of steeping under laboratory conditions resulted in the lowest redistribution of aflatoxins in the endosperm fraction, however, for fumonisin B1 dry-milling is the most indicated. Considering that the mycotoxin levels in unprocessed corn do not reflect the contamination in their fractions obtained after milling, it is essential to evaluate the effect of milling on mycotoxin redistribution.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Abbreviations
- AFLAB2
Aflatoxin B2
- AFLAB1
Aflatoxin B1
- FB1
Fumonisin B1
- CCRD
Central composite rotatable design
- LOD
Limit of detection
- LOQ
Limit of quantification
Author contributions
K.C.M. conceived the work. K.C.M., E.B.F and L.K designed and planned the major experiments. K.C.M, P.R and C.F.J.F performed laboratory analysis..C.M. wrote the main manuscript text. K.C.M, L.K, and E.B.F contributed intellectually and reviewed the manuscript.
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kelly Cristina Massarolo, Email: kelly_massa@hotmail.com.
Priscila Rodrigues, Email: priscilasrdg@gmail.com.
Cláudia Fetter Jorge Ferreira, Email: fetterclaudia@gmail.com.
Larine Kupski, Email: larinekupski@yahoo.com.br.
Eliana Badiale-Furlong, Email: dqmebf@furg.br.
References
- Adetunji M, et al. Fungal and bacterial metabolites of stored maize (Zea mays, L.) from five agro-ecological zones of Nigeria. Mycotoxin Res. 2014;30:89–102. doi: 10.1007/s12550-014-0194-2. [DOI] [PubMed] [Google Scholar]
- ANVISA (2017) Resolução Da Diretoria Colegiada - RDC Nº 138, DE 8 DE FEVEREIRO DE 2017: Altera a Resolução da Diretoria Colegiada - RDC nº 7, de 18 de fevereiro de 2011, que dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos, para alterar os LMT da micotoxina deoxinivalenol (DON) em trigo e produtos de trigo prontos para oferta ao consumidor e os prazos para sua aplicação.
- AOAC (2000) Official methods of analysis international, 17. ed., CD- ROM
- Belluco B, de Camargo AC, da Gloria EM, dos Santos Dias CT, Button DC, Calori-Domingues MA. Deoxynivalenol in wheat milling fractions: a critical evaluation regarding ongoing and new legislation limits. J Cereal Sci. 2017;77:284–290. doi: 10.1016/j.jcs.2017.08.008. [DOI] [Google Scholar]
- Blandino M, Alfieri M, Giordano D, Vanara F, Redaelli R. Distribution of bioactive compounds in maize fractions obtained in two different types of large scale milling processes. J Cereal Sci. 2017;77:251–258. doi: 10.1016/j.jcs.2017.08.006. [DOI] [Google Scholar]
- Bordini JG, et al. Impact of industrial dry-milling on fumonisin redistribution in non-transgenic corn in Brazil. Food Chem. 2017;220:438–443. doi: 10.1016/j.foodchem.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Bordini JG, Ono MA, Garcia GT, Vizoni É, Amador IR, Hirozawa MT, Ono EYS. Transgenic versus conventional corn: fate of fumonisins during industrial dry milling. Mycotoxin Res. 2019;35:169–176. doi: 10.1007/s12550-019-00343-1. [DOI] [PubMed] [Google Scholar]
- Brera C, Catano C, de Santis B, Debegnach F, de Giacomo M, Pannunzi E, Miraglia M. Effect of industrial processing on the distribution of aflatoxins and zearalenone in corn-milling fractions. J Agric Food Chem. 2006;54:5014–5019. doi: 10.1021/jf060370s. [DOI] [PubMed] [Google Scholar]
- Bryden WL. Mycotoxins in the food chain: human health implications. Asia Pac J Clin Nutr. 2007;16:95–101. [PubMed] [Google Scholar]
- Castells M, Marín S, Sanchis V, Ramos AJ. Distribution of fumonisins and aflatoxins in corn fractions during industrial cornflake processing. Int J Food Microbiol. 2008;123:81–87. doi: 10.1016/j.ijfoodmicro.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Curry S, Hendel EG, Gott P, Murugesan G, Hofstetter-Schähs U. 170 Trends in mycotoxin contamination in the united states corn. J Anim Sci. 2019;97:93–94. doi: 10.1093/jas/skz122.169. [DOI] [Google Scholar]
- EC (2006) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Communitites
- EC (2007) Commission Regulation (EC) No 1126/2007 of of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in corn and corn products. Official Journal of the European Communitites,
- Escobar J, Lorán S, Giménez I, Ferruz E, Herrera M, Herrera A, Ariño A. Occurrence and exposure assessment of Fusarium mycotoxins in maize germ, refined corn oil and margarine. Food Chem Toxicol. 2013;62:514–520. doi: 10.1016/j.fct.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Franco LT, Petta T, Rottinghaus GE, Bordin K, Gomes GA, Oliveira CA. Co-occurrence of mycotoxins in maize food and maize-based feed from small-scale farms in Brazil: a pilot study. Mycotoxin Res. 2019;35:65–73. doi: 10.1007/s12550-018-0331-4. [DOI] [PubMed] [Google Scholar]
- Generotti S, Cirlini M, Dall'Asta C, Suman M. Influence of the industrial process from caryopsis to cornmeal semolina on levels of fumonisins and their masked forms. Food Control. 2015;48:170–174. doi: 10.1016/j.foodcont.2014.06.003. [DOI] [Google Scholar]
- Grenier B, Oswald I. Mycotoxin co-contamination of food and feed: meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4:285–313. doi: 10.3920/WMJ2011.1281. [DOI] [Google Scholar]
- Katta S, Cagampang A, Jackson L, Bullerman L. Distribution of Fusarium molds and fumonisins in dry-milled corn fractions. Cereal Chem. 1997;74:858–863. doi: 10.1094/CCHEM.1997.74.6.858. [DOI] [Google Scholar]
- Kong W, Xie T, Li J, Wei J, Qiu F, Qi A, Yang M. Analysis of fumonisins B 1 and B 2 in spices and aromatic and medicinal herbs by HPLC-FLD with on-line post-column derivatization and positive confirmation by LC-MS/MS. Analyst. 2012;137:3166–3174. doi: 10.1039/c2an35164a. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dhanshetty M, Banerjee K. Development and validation of a method for direct analysis of aflatoxins in animal feeds by ultra-high-performance liquid chromatography with fluorescence detection. J AOAC Int. 2020;103(4):940–945. doi: 10.1093/jaoacint/qsz037. [DOI] [PubMed] [Google Scholar]
- Lillehoj EB, Kwolek WF, Peterson RE, Shotwell OL, Hesseltine CW (1976) Aflatoxin contamination, fluorescence and insect damage in corn infected with Aspergillus flavus before harvest Cereal Chem 53:505–512
- Malumba P, Boudry C, Roiseux O, Bindelle J, Beckers Y, Béra F. Chemical characterisation and in vitro assessment of the nutritive value of co-products yield from the corn wet-milling process. Food Chem. 2015;166:143–149. doi: 10.1016/j.foodchem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Massarolo KC, Ferreira CFJ, Kupski L, Badiale-Furlong E. Optimization of matrix solid-phase dispersion method for extraction of aflatoxins from cornmeal food anal. Methods. 2018;11:3342–3351. [Google Scholar]
- Oulkar D, Goon A, Dhanshetty M, Khan Z, Satav S, Banerjee K. High-sensitivity direct analysis of aflatoxins in peanuts and cereal matrices by ultra-performance liquid chromatography with fluorescence detection involving a large volume flow cell. J Environ Sci Health Part B. 2018;53(4):255–260. doi: 10.1080/03601234.2017.1410416. [DOI] [PubMed] [Google Scholar]
- Park J, Kim DH, Moon JY, An JA, Kim YW, Chung SH, Lee C. Distribution analysis of twelve mycotoxins in corn and corn-derived products by LC-MS/MS to evaluate the carry-over ratio during wet-milling. Toxins. 2018;10:1–15. doi: 10.3390/toxins10080319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri A, Zanetti M, Bertuzzi T. Distribution of aflatoxins and fumonisins in dry-milled corn fractions. Food Addit Contam A. 2009;26:372–380. doi: 10.1080/02652030802441513. [DOI] [PubMed] [Google Scholar]
- Savi GD, Piacentini KC, Marchi D, Scussel VM. Fumonisins B1 and B2 in the corn-milling process and corn-based products, and evaluation of estimated daily intake. Food Addit Contam A. 2016;33:339–345. doi: 10.1080/19440049.2015.1124459. [DOI] [PubMed] [Google Scholar]
- Scaglioni PT, Blandino M, Scarpino V, Giordano D, Testa G, Badiale-Furlong E. Application of fungicides and microalgal phenolic extracts for the direct control of fumonisin contamination in corn. J Agric Food Chem. 2018;66:4835–4841. doi: 10.1021/acs.jafc.8b00540. [DOI] [PubMed] [Google Scholar]
- Schaffer-Lequart C, Lehmann U, Ross AB, Roger O, Eldridge AL, Ananta E, Wavreille AS. Whole grain in manufactured foods: current use, challenges and the way forward. Crit Rev Food Sci Nutr. 2017;57:1562–1568. doi: 10.1080/10408398.2013.781012. [DOI] [PubMed] [Google Scholar]
- Serna-Saldivar SO, Carrillo EP (2019) Food uses of whole corn and dry-milled fractions In Corn AACC international Press 435–467
- Somavat P, Li Q, De Mejia EG, Liu W, Singh V. Coproduct yield comparisons of purple, blue and yellow dent corn for various milling processes. Ind Crop Prod. 2016;87:266–272. doi: 10.1016/j.indcrop.2016.04.062. [DOI] [Google Scholar]
- USFDA - US FOOD & DRUG (2001) Guidance for industry: fumonisin levels in human foods and animal feeds, FDA-2013-S-0610. Accessed 20 Aug 2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.