Abstract
Salts that meet the standard quality are enriched with micronutrients, such as potassium iodate, at least 30 ppm. The iodization can be carried out directly in crystallization ponds (in-situ iodization) in salt fields. This paper reports the effectiveness of in-situ iodization technology combined with the enrichment of Halophilic bacteria consortium and Haloferax spp. to produce bio-based NaCl salt. The brine was first crystallized under sunlight exposure for approximately five days with water temperatures of 32–39 °C and an average wind speed of 2.8–6.0 m/s in each pond with a dimension of 20 × 20 m. Following this, the performance of these bacteria was analyzed in terms of the resulting final concentration of KIO3 (ppm), NaCl concentration (v/v), and water content (v/v). Results showed that the treatment with in situ iodization and Haloferax spp. successfully produce better bio-based salt quality in terms of KIO3 level, NaCl purity, and water contents. Moreover, the method did not produce aqueous and solid wastes, unlike in the conventional salt industry. The optimum condition was found at 50 ppm of KIO3 with the addition of Haloferax spp. SEM analysis shows that the treatment using Haloferax spp. resulted in a larger rectangular and harder crystal salt than the controls.
Keywords: Haloferax spp., In-situ iodization, NaCl, KIO3, Salt crystallization
Introduction
Industrial food production and processing is closely related to the use of quality salt. The functional properties of good quality salt in food processing and food production go well beyond taste. Salt is widely used as a preservative, seasoning, coloring agent, texture modifier, and to regulate fermentation, yeast and mold. In addition, salt is also needed by most industries, including non-food industries. Indonesia has its national quality standards for salts, called Standar Nasional Indonesia (SNI), divided into two categories. The raw iodized salt for domestic (referring to SNI 3556: 2016) and the raw iodized salt for industry (referring to SNI 4435: 2017). For industrial-grade or standard, the salt for consumption must contain at least 30 ppm of KIO3 (Baroroh et al. 2018). To meet this standard, salt iodization can be carried out by inducing KIO3 homogeneously. Generally, the process needs three grams of KIO3 dissolved with water for every 100 kg of salt to obtain a solution of 2–4% of iodine or equal to 40 ppm KIO3 (Nilawati and Marihati 2015).
The addition of iodine for domestic salt consumption is necessary for human health. Iodine deficiency inhibits normal human development and leads to mental retardation (García Ascaso et al. 2019; McGee et al. 2017). Fortified salt with iodine can reduce micronutrient deficiency. Iodine is a trace element needed by the body to prevent mumps disease. Iodine also plays a significant role in preventing breast cancer (Malya et al. 2018; Medrano-Macías et al. 2016) and acts as an antioxidant (Liu et al. 2015; Diosady et al. 2002). However, the iodine content in a raw iodized salt is usually unstable. Iodine content can gradually decrease by 0.2–1.3 ppm up to four months during storage (Nilawati and Marihati 2015). To stabilize the iodine content, 50 mg of iodine and 1000 mg of iron per kilogram of NaCl salt are introduced. This enrichment retains more than 75% iodine at 40 °C and 100% RH (Marihati et al. 2014).
Several studies related to the addition of halophilic bacteria consortium to increase the purity of sodium chloride salt have been reported (Nilawati and Marihati 2015; Marihati et al. 2014). The reports showed that high-purity NaCl could be produced by inducing red Haloferax spp. during bio-based salt production. The enrichment of Haloferax spp. in bio-based salt production can be more effective if carried out during the iodization when the KIO3 solution is added to the salt. In addition, direct enrichment of Haloferax spp. produces less waste; hence it is environmentally friendly. However, the study about Haloferax spp. enrichment during the iodization of salt was limited (Oren 2010a).
Generally, iodized salt production consists of sequential processes, such as raw materials washing, draining, iodization, briquettes printing, cooking or drying, cooling, and packaging (Nilawati and Marihati 2015). During iodization, the brine raw materials have to meet a minimum concentration of 22–25 Bé°. Several washing steps can reach this minimum concentration. If the concentration already exceeds 25 Bé°, most washing water must be removed since it contains MgSO4, MgCl2, CaSO4, and a small amount of other salt impurities. The volume of discharged washing water is about 1.5 m3, equivalent to 500 kg of salt or 10 tonnes of washed salt (McGee et al. 2017; Nilawati et al. 2019). This washing water or aqueous waste is quite large and requires more effort to minimize. Salt liquid waste that is dumped around would pollute the environment because there is metal and chemical contamination. Alternatives to prevent pollution environment industry salt with do research iodization salt in situ so, in industry salt, there is no need to wash salt before processing.
The other research that the iodization process's laboratory analysis has been shown to directly reduce the iodine concentration for one to four months to 0.2–1.3 ppm during storage. Iodine enriched and iron in salt, obtaining the most stable combination, containing 50 mg iodine and 1000 mg of iron per kg of salt, retains more than 75% iodine added during addition at 40 °C, 100% RH. Iodine is essential for health. Various studies have been conducted, such as research in China, monitoring and evaluating iodine salt consumption and iodine deficiency in children and pregnant women in 31 provinces, and the prevalence of mumps disease in children 2.6% (Wang et al. 2020; Sun et al. 2017).
Polumbryk et al. (2019) reported the performance of Haloferax spp. enrichment in the iodization to simultaneously improve the purity of NaCl salt and obtain a proper concentration of KIO3. The proposed methods were performed using a combination of in situ flow injection technology and Haloferax spp. enrichment. This study was aimed to produce NaCl salt with homogeneous iodine content and high purity (minimum of 94.7%) that meet the SNI 4435-2010 standard. Furthermore, the proposed method will skip the sequence of washing and iodizing during salt production.
In contrast to the conventional salt production, the results of bio-based salt with this bacterial bio-process technology can be used directly for the food production and food processing industries because it already contains iodine and a NaCl value that is above 94%. This bio-based salt can be applied in the soy sauce, gravy, biscuit industry, as well as children's extruded food which is a micronutrient to prevent stunting. Finally, a sustainable industry with environmental insight, cost-efficiency, and less energy consumption during the salt production stages can be achieved.
Materials and methods
The research was divided into two stages. The first stage is the enrichment of the Haloferax spp. and the halophilic bacterial consortium on a lab scale. The second stage is performance analysis of the in-situ iodization and enrichment of Haloferax spp. to obtain high-purity NaCl. The last is conducted directly in farmers' ponds in Pati, Central Java, Indonesia.
Materials
For the bacteria consortium medium, the ingredients used were yeast extract 0.25 gr/l (Oxoid), trypton 0.5 gr/l (Oxoid), 20 gr/l MgSO4·7H2O (99% Merck Millipore), KCl 2 gr/l (99% Merck Millipore), trisodium citrate 3 gr/l (99%, Merck Millipore), NaCl 240 gr/l (99%, Merck Millipore), 0.036 gr/l of FeCl3·3H2O (99% Merck Millipore), brine of 22° Be, Haloferax spp. isolate and halophilic bacteria consortium inoculum. The material used as a culture medium was microbiological and analytical grade, respectively. Haloferax spp. isolates were obtained from BBTPPI (Balai Besar Teknologi Pencegahan Pencemaran Industri or Center for Industrial Pollution Prevention Technology) laboratory stock culture, and halophilic bacteria consortium inoculum was obtained from enriched brine from Sampang Madura solar saltern in East Java, Indonesia. The preparation of the bacteria medium was referred to in the previous reports (Tripathi et al. 2016; Schneegurt 2012; Nilawati et al. 2017).
Methods
Five liters of halophilic consortium inoculum and Haloferax spp. are prepared according to Malik (2019) and Nilawati et al. (2019). The iodization of flow injectors is carried out to achieve KIO3 levels of 30 ppm in salt. In this study, there are two variables to be observed. The first variable (the independent variable) is KIO3, consisting of eight different concentrations, namely 0, 40, 50, 60, 70, 80, 90, and 100 ppm. These concentrations were selected based on standard operating procedures in the salt industry that require a minimum of 80 ppm KIO3 to be spread to salt in the iodization. The second variable (the fixed variable) is the type of bacteria, consisting of control (without bacteria), halophilic consortium, and Haloferax spp. It is important to note that we did not differentiate the concentration value of each type of bacteria in this variable. Note that halophilic consortium and Haloferax spp. are bacteria that can improve the quality of salt.
Each experiment was conducted in triplicates. KIO3 solution and each bacteria (1% v/v) were added to the brine and ready to crystallize. The crystallization was carried out by heating the mixture underexposed to sunlight for approximately five days. Subsequently, salt was harvested and analyzed for KIO3 concentration, moisture content, and NaCl purity refers to the SNI 3556:2016. Saltwater that has formed salt crystals was sampled and analyzed for KIO3 concentration, NaCl purity, and water content. These analyses were done by performing an independent test of KIO3 concentration by titration method, water content by gravimetric method, and NaCl purity by titration method.
The Scanning Electron Microscope-Energy Dispersive X-Ray (SEM) was used to analyze the morphology of the salt and its component. Meanwhile, the mean differences of the KIO3 among treatments were determined by two-way analysis of variance and followed by Tukey’s HSD test along with multiple comparisons. The statistical analysis was performed using α = 0.05 as a significance level in Two-Way MANOVA (Priyanto 2013). MANOVA was chosen since the dependent variable was > 1, KIO3, water content, and NaCl.
Results and discussions
Effect of KIO3 addition on final KIO3 concentration in the bio-based salt product
Adding a KIO3 solution is carried out in the crystallization pool after the bacteria were enriched with micronutrients in the pool, as presented in Fig. 1. Statistical test results showed a link between the amount of KIO3 added in brine, the addition of bacteria, and the final KIO3 content in salt products. In the KIO3 concentration range given in brine solution, the remaining concentration of KIO3 in salt products for control, halophilic consortium, and Haloferax spp. was 62.40, 62.07, and 40.21 ppm, respectively (Table 1). Figure 2a shows the relationship between KIO3 concentration added in the brine solution against the final KIO3 concentration in the bio-based salt product. The MANOVA test also showed similar results, namely the addition of bacterial types, KIO3 concentrations, and interactions between bacterial types and KIO3 in salt solutions affecting KIO3 in salt products, with the administration of Sig. 0.00 < 0.05.
Fig. 1.

Workflow of KIO3 iodization. (1) activation of Haloferax spp. and halophilic consortium in the laboratory, (2) enrichment of Haloferax spp. and halophilic consortium in enrichment ponds (salt fields brine of 220 Bé°), (3) iodization of KIO3 in the crystallization pool on brine 250 Bé°, and (4) salt crystals were formed after drying under the sunlight on brine of 290 Bé°
Table 1.
Effect of the addition of halophilic bacteria and KIO3 to the final concentration of KIO3 in the salt product
| Bacteria type | N | Subset | |
|---|---|---|---|
| 1 | 2 | ||
| Turkey HSD | |||
| Haloferax spp. | 24 | 40.21 | – |
| Halophilic Consortium | 24 | – | 62.07 |
| Control | 24 | – | 62.40 |
| Sig | 1.00 | 0.76 | |
Means for groups in homogeneous subsets are displayed. Based on observed means. The error term is Mean Square (Error) = 2.663. Harmonic Mean Sample Size (N) = 24 and alpha value (α) = 0.05
Fig. 2.

Effect of KIO3 and bacteria added to the final salt products: a KIO3 concentration, b NaCl concentration, and c water content
The results also showed the difference between KIO3 in salt products versus the addition of KIO3 content in brine. In the 0 ppm of KIO3 addition, the final concentration of KIO3 in the salt product is 0.32 ppm. Meanwhile, for the addition of 40, 50, and 60 ppm, the final KIO3 concentration was found at 32.97, 51.86, and 54.23 ppm, respectively (Table 2). Note that there is an insignificant difference between 50 and 60 ppm of KIO3 addition compared to the 70–100 ppm. The result showed that this type of bacteria has a significant effect on the final concentration of KIO3.
Table 2.
The effect of KIO3 concentration on NaCl content in salt products
| [KIO3] (ppm) | N | Subset | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Turkey HSD | ||||||||
| 0 | 9 | 0.32 | ||||||
| 40 | 9 | 32.98 | ||||||
| 50 | 9 | 51.86 | ||||||
| 60 | 9 | 54.23 | ||||||
| 70 | 9 | 62.39 | ||||||
| 80 | 9 | 73.14 | ||||||
| 90 | 9 | 77.81 | ||||||
| 100 | 9 | 86.40 | ||||||
| Sig | 1.00 | 1.00 | 0.06 | 1.00 | 1.00 | 1.00 | ||
Means for groups in homogeneous subsets are displayed. Based on observed means. The error term is Mean Square (Error) = 2.663. Harmonic Mean Sample Size (N) = 9 and alpha value (α) = 0.05
The results indicate that halophilic consortium and control did not significantly differ from the addition of Haloferax spp., which gave KIO3 the lowest concentration. It is possibly because Haloferax spp. is one of the halophilic types that are very active in the ion exchange process. The possibility of iodide bonding with other cations is less so that the iodide in the crystallization pool tends to be volatile and becomes more easily lost during the crystallization. Meanwhile, the halophilic consortium may still be halotolerant that does not carry out ion uptake as high as Haloferax spp.
Halophilic bacteria are microorganisms whose habitat is in waters with high bio-based salt content (Shivanand and Mugeraya 2011). Most of the halophilic bacteria that live at high salinity levels have color pigments (Mani et al. 2012). The color pigments possessed by halophilic bacteria are generally carotenoids and bacterioruberins (Rodrigo-Baños et al. 2015). These color pigments are closely related to the need for sunlight to produce energy and protect intracellular halophilic bacteria from oxidation due to exposure to UV light (Waditee-Sirisattha et al. 2016). Red halophilic organisms significantly improve the quality of salt produced in salt farming (Oren 2010a; Ventosa et al. 2011). In general, most members of the halophilic family have the ability to accumulate cations, especially K+, in their bodies. Another possibility is the Haloferax spp. having more reddish pigment in color, where the bacteria in the photosynthesis process absorb more heat than the halophilic consortium that performs photosynthesis. However, the color is not as red as the Haloferax spp. (Empadinhas et al. 2008). Treatment with Haloferax spp. produces a higher temperature so that the possibility of iodine being released into the air becomes higher than other treatments.
Another reason for the decrease in KIO3 may be due to Fe2+ and Cu2+ cations present in saline, which decomposes KIO3 into I2 (Saksono 2010). The higher the concentration of reductor compounds in salt, the higher the amount of KIO3 decomposes into I2(g) (Saksono 2010). The Cu concentrations in this study ranged from 0.80 to 1.40 ppm. Cu will react with iodine as follows: 2Cu2+ + I ⇔ 2 CuI + I2. The decrease of iodine concentration was also influenced by wind speed and temperature, which are 2.8–6.0 m/s and 32–39 °C, respectively. These results show that optimum condition was achieved at 50 ppm of KIO3 in Haloferax spp. treatment.
Effect of adding two types of bacteria to the purity of the NaCl bio-based salt
The results showed that the overall average concentration of NaCl salt on control, halophilic consortium, and Haloferax spp. was 87.38, 90.64, and 94.06% (Table 3), respectively. NaCl for each type of halobacteria is shown in Fig. 2b. Several factors may cause the purity of NaCl not to reach above 90%. First is the brine source, which obtained from salt fields has been mixed with water after crystallization. This is a characteristic of the salt production scheme in Pati, Central Java, which always mixes the remaining water (above 30 Bé°) with the 10 Bé° water then crystallizes again. Meanwhile, the remaining water after crystallization, called bitern, contains many Mg and Ca cations, decreasing salt purity. Second, on the laboratory scale, rapid evaporation of water occurred during crystallization. This condition yielding higher viscosity and exceeds “the salt point” above 30 Bé°. The salt point is a critical point for harvest salt to get the highest quality. Meanwhile, with the halophilic consortium and Haloferax spp., the NaCl level is higher. The statistical tests showed that the NaCl levels differed significantly between the halophilic consortium treatments, Haloferax spp. and control treatment (Table 3). Treatment with Haloferax spp. results in the highest concentration of NaCl salt. This type of bacteria can accumulate large amounts of Na+. In addition, if there are K+ present, the Na+ that has been accumulated will be exchanged for K+ through the ion pump on the bacterium cell wall.
Tabel 3.
The effect of bacteria type on NaCl content in salt products
| Bacteria type | N | Subset | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Tukey HSD | ||||
| Control | 24 | 87.38 | ||
| Halophilic Consortium | 24 | 90.64 | ||
| Haloferax spp. | 24 | 94.06 | ||
| Sig | 1.00 | 1.00 | 1.00 | |
Means for groups in homogeneous subsets are displayed. Based on observed means. The error term is Mean Square (Error) = 2.663. Harmonic Mean Sample Size (N) = 24 and alpha value (α) = 0.05
The ability of Haloferax spp. in accumulating ions will significantly assist the crystallization process because when there is an exchange process between K+ that enter the bacteria and Na+ that released, there will be a saturation shift so that the Na+ and Cl− around the bacterial cells will be higher and thus initiates more the formation of salt crystals. NaCl v/v levels for treatment of zero and 90 ppm are not significantly different. The control treatment was found no significant difference with the addition of 40, 50, 60, 70, 80, 90, 100 ppm KIO3 (Table 4). However, in treating different types of bacteria, the obtained NaCl v/v levels were different. The control is significantly different from halophilic treatment but not with Haloferax spp. treatment. The highest value of NaCl v/v yield in Haloferax spp. treatment corresponds to the previous report (Nilawati et al. 2017).
Table 4.
The effect of KIO3 concentration on NaCl content in salt products
| [KIO3] (ppm) | N | Subset | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Turkey HSD | ||||
| 0 | 9 | 90.31 | 90.31 | |
| 40 | 9 | 90.71 | 90.71 | |
| 50 | 9 | 91.14 | 91.14 | |
| 60 | 9 | 91.08 | 91.08 | |
| 70 | 9 | 90.67 | 90.67 | |
| 80 | 9 | 91.38 | ||
| 90 | 9 | 89.63 | ||
| 100 | 9 | 90.65 | 90.65 | |
| Sig | 0.41 | 0.17 | 0.32 | |
Means for groups in homogeneous subsets are displayed. Based on observed means. The error term is Mean Square (Error) = 2.663. Harmonic Mean Sample Size (N) = 9 and alpha value (α) = 0.05
NaCl concentrations in control treatment ranged from 85.12 to 90.37% v/v to an average of 87.54% v/v. For the addition of halophilic consortium, NaCl levels were achieved at 88.81 to 91.85% v/v with an average value of 90.15% v/v. Meanwhile, treatment with Haloferax spp. achieved NaCl concentration between 92.54 to 94.87% v/v or an average value of 94.0% v/v (Table 4). For negative control (NaCl salt from farmers in Pati, Central Java is 88.22% v/v). The optimal condition was found in concentrations of 80 ppm.
Water content in the bio-based salt product
The results showed that the higher the increase in KIO3 brine consumption, the higher the moisture content. The average value of water content for control, halophilic consortium, and Haloferax spp. were found at 7.71, 7.08, and 7.54% v/v, respectively (Table 5). The average water content is 7.44% v/v. The test results of a subset of homogeneity showed that the halophilic consortium's performance is different from control and Haloferax spp. (Table 6). The lowest water content (v/v) value is obtained in the treatment of halophilic consortium addition. During the water evaporation process (crystallization), treatment with the halophilic consortium evaporates faster due to red pigment, absorbing higher heat. Within five days of harvest, the water content was lower than the other treatments.
Table 5.
The effect of KIO3 concentration on the water content in salt products
| [KIO3] (ppm) | N | Subset | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Tukey HSD | ||||
| 0 | 9 | 6.33 | ||
| 40 | 9 | 7.22 | ||
| 50 | 9 | 7.44 | 7.44 | |
| 60 | 9 | 7.67 | 7.67 | |
| 70 | 9 | 7.33 | 7.33 | |
| 80 | 9 | 8.00 | ||
| 90 | 9 | 7.67 | 7.67 | |
| 100 | 9 | 7.89 | 7.89 | |
| Sig | 1.00 | 0.09 | 0.09 | |
Means for groups in homogeneous subsets are displayed. Based on observed means. The error term is Mean Square (Error) = 2.663. Harmonic Mean Sample Size (N) = 9 and alpha value (α) = 0.05
Table 6.
The effect of halobacteria addition on the water content in salt products
| Bacteria type | N | Subset | |
|---|---|---|---|
| 1 | 2 | ||
| Tukey HSD | |||
| Halophilic Consortium | 24 | 7.08 | |
| Haloferax spp. | 24 | 7.54 | |
| Control | 24 | 7.71 | |
| Sig | 1.00 | 0.47 | |
Means for groups in homogeneous subsets are displayed. Based on observed means. The error term is Mean Square (Error) = 0.24. Harmonic Mean Sample Size (N) = 24 and alpha value (α) = 0.05
SEM analysis
SEM analysis was performed on five different samples. Cortes and Ochoa (Lopez-Cortes and Ochoa 1998) suggest that the bacterial cell of the Haloferax spp. is a Halite/NaCl crystals' formation template. Organic compounds produced by Haloferax spp. also influence the shape and formation of bio-based salt. The secretion of organic substances from Haloferax spp. such as glycine, cocamidopropyl, and polysaccharides affects the resulting morphology of salt (Oren 2010b). There is a resemblance to the cube shape or rectangular tendency of salt crystals. Each treatment has different characteristics of crystal form, treatment with Haloferax spp. has a regular cubic crystal shape, and the resulting salt crystals are relatively harder in texture and slightly reddish-white (Fig. 3a, b). One of the physical characteristics of salt crystallized with halophilic bacteria and Haloferax spp. is that the color of salt when harvested will be slightly reddish. This is due to the presence of halophilic consortium or Haloferax spp. trapped in the salt crystals. In general, the form of bio-based salt with the halophilic consortium and Haloferax spp. is more likely to be cubic and hard (Fig. 3c, d), whereas the control salts (without bacteria treatment) were irregular and somewhat brittle (Fig. 3e). However, the salt morphology appears to be a crystal-small shape for salt fields (originated from Pati in Central Java, Figure 3e) without bacteria treatment. Meanwhile, using the proposed method in the field using halophilic bacteria, the morphology of salt appears in a different form, such as large-curved cubes, small cubes, and pyramids-shaped (Malik 2019).
Fig. 3.
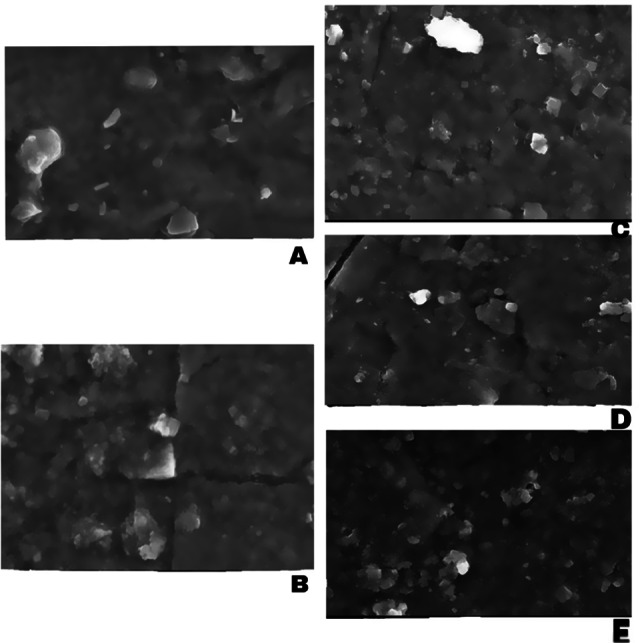
SEM micrographs of resulting salts from different treatments. a control with halophilic, b control without the addition of KIO3, c Haloferax spp. with the addition of 50 ppm KIO3, d halophilic consortium with the addition of 60 ppm KIO3, and e conventional salt from the farmer in Pati, Central Java
Conclusion
The results showed that the treatment with a combination of in situ iodization and Haloferax spp. resulted in good quality of bio-based salt. The treatment by adding bacteria significantly affects KIO3 concentration, NaCl concentration or purity, and water content in the resulting salt. The highest value of NaCl purity was obtained in the treatment with Haloferax spp. The optimum conditions for NaCl salt production were obtained at the addition of 50–60 ppm of KIO3 and Haloferax spp.
Abbreviations
- SEM
Scanning electron microscopy
- SNI
Standar Nasional Indonesia
- RH
Relative humidity
- BBTPPI
Balai Besar Teknologi Pencegahan Pencemaran Industri (Center for Industrial Pollution Prevention Technology)
- MANOVA
Multivariate analysis of variance
- Bé°
The Baumé scale or hydrometer scale used to indicate the density of various liquids
Author contributions
Nilawati (Corresponding aurthor): conducted all the experiments and wrote the manuscript. Rame: conducted experiment on in situ iodization and wrote the manuscript partially. RM: conducted experiment on bacteria preparation and wrote the manuscript partially. RY (Principal Investigator): providing language help and revised the manuscript.
Funding
The research was financialy supported by the Industrial Research and Development Agency, Ministry of Industry of the Republic of Indonesia.
Declarations
Conflict of interest
The authors declare no competing financial and conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baroroh I, Suwasono B, Hardianto D. The fortication of iodine in salt consumption using manual gun spray. MATEC Web Conf. 2018;177:1–8. doi: 10.1051/matecconf/201817701004. [DOI] [Google Scholar]
- Diosady LL, Alberti JO, Ramcharan K, Venkatesh Mannar MG. Iodine stability in salt double-fortified with iron and iodine. Food Nutr Bull. 2002;23(2):196–207. doi: 10.1177/156482650202300209. [DOI] [PubMed] [Google Scholar]
- Empadinhas N, da Costa MC. Osmoadaptation mechanisms in prokaryotespdf. Int Microbiol. 2008;11:151–161. [PubMed] [Google Scholar]
- García Ascaso MT, Pérez PR, Alcol EC, López AL. Nutritional status of iodine in children: when appropriateness relies on milk consumption and not adequate coverage of iodized salt in households. Clin Nutr ESPEN. 2019;30:52–58. doi: 10.1016/j.clnesp.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Liu D, Lin X, Yu F, Zhang M, Chen H, Bao W, Wang X. Effects of 3,5-diiodotyrosine and potassium iodide on thyroid function and oxidative stress in iodine-excess wistar rats. Biol Trace Elem Res. 2015;168(2):447–452. doi: 10.1007/s12011-015-0371-y. [DOI] [PubMed] [Google Scholar]
- Lopez-Cortes A, Ochoa JL. The biological significance of Halobacteria on nucleation and sodium chloride crystal growth. J Chem Inf Model. 1998;53(9):903–923. [Google Scholar]
- Malik RA et al. (2019). Aplikasi Bakteri Halofilik Berwarna Merah Terimmobilisasi Dalam Meningkatkan Kualitas Garam Dalam Proses Produksi Garam Berbasis Air Laut, pp 224–231
- Malya FU, Kadioglu H, Hasbahceci M, Dolay K, Guzel M, Ersoy YE. The correlation between breast cancer and urinary iodine excretion levels. J Int Med Res. 2018;46(2):687–692. doi: 10.1177/0300060517717535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani K, Salgaonkar BB, Braganca JM. Culturable halophilic archaea at the initial and crystallization stages of salt production in a natural solar saltern of Goa, India. Aquatic Biosyst Saline Syst. 2012;8(1):1. doi: 10.1186/2046-9063-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marihati M, Nani H, Muryati M, Nilawati N, Eddy S, Dan DWH. Penggunaan Bakteri Halofilik Sebagai Biokatalisator Untuk Meningkatkan Kualitas dan Produktifitas Garam NaCl di Meja Kristalisasi. Jurnal Riset Industri. 2014;8(3):191–196. [Google Scholar]
- McGee EJT, Sangakkara AR, Diosady LL. Double fortification of salt with folic acid and iodine. J Food Eng. 2017;198:72–80. doi: 10.1016/j.jfoodeng.2016.11.019. [DOI] [Google Scholar]
- Medrano-Macías J, Leija-Martínez P, González-Morales S, Juárez-Maldonado A, Benavides-Mendoza A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front Plant Sci. 2016;7:1–20. doi: 10.3389/fpls.2016.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilawati N, Marihati M. Pemurnian Dan Yodisasi In Situ Pengolahan Limbah Padat Blotong Menjadi Garam Konsumsi Di Industri Garam Beryodium. Biopropal Industri. 2015;1:43–51. [Google Scholar]
- Nilawati N, Marihati M, Malik RA. Kemampuan Isolat Bakteri Haloferax spp dalam meningkatkan Kemurnian Garam NaCl pada Proses Kristalisasi. Jurnal Riset Teknologi Pencegahan Pencemaran Industri. 2017;8:92–103. doi: 10.21771/jrtppi.2017. [DOI] [Google Scholar]
- Nilawati N, et al. Application of iodized in farm and haloferax bacteria technology for salt production in order to make zero waste salt consumption industry. E3S Web Conf. 2019;3:8–11. [Google Scholar]
- Oren A. Thoughts on the “missing link” between saltworks biology and solar salt quality. Global NEST J. 2010;12(4):417–425. [Google Scholar]
- Oren A. Industrial and environmental applications of halophilic microorganisms. Environ Technol. 2010;31(8–9):825–834. doi: 10.1080/09593330903370026. [DOI] [PubMed] [Google Scholar]
- Polumbryka M, Kravchenkob V, Pasichnyia V, Omelchenko C. The effect of intake of sausages fortified with β-CD-I2 complex on iodine status and thyroid function: a preliminary study. J Trace Elem Med Biol. 2019;51:159–163. doi: 10.1016/j.jtemb.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Priyanto D (2013) Analisis Korelasi, Regresi dan Multivariate dengan SPSS. Penerbit Gaya Media
- Rodrigo-Baños M, et al. Carotenoids from Haloarchaea and their potential in biotechnology. Mar Drugs. 2015;13(9):5508–5532. doi: 10.3390/md13095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksono N. Studi Pengaruh Proses Pencucian Garam Terhadap Komposisi Dan Stabilitas Yodium Garam Konsumsi. MAKARA of Technol Ser. 2010 doi: 10.7454/mst.v6i1.34. [DOI] [Google Scholar]
- Schneegurt MA (2012) Chapter 2 : media and conditions for the growth of halophilic and media and conditions for the growth of halophilic and halotolerant bacteria and archaea. 10.1007/978-94-007-5539-0
- Shivanand P, Mugeraya G. Halophilic bacteria and their compatible solutes -osmoregulation and potential applications. Curr Sci. 2011;100(10):1516–1521. [Google Scholar]
- Sun D, Codling K, Chang S, Zhang S, Shen H, Su X, Chen Z, Scherpbier RW, Yan J. Eliminating iodine deficiency in China: achievements, challenges and global implications. Nutrients. 2017;9(4):1–21. doi: 10.3390/nu9040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R, Singh P, Singh A, Chagtoo M, Khan S, Tiwari S, Agarwal G, Meeran SM, Godbole MM. Zoledronate and molecular iodine cause synergistic cell death in triple negative breast cancer through endoplasmic reticulum stress. Nutr Cancer. 2016;68(4):679–688. doi: 10.1080/01635581.2016.1158293. [DOI] [PubMed] [Google Scholar]
- Ventosa A, Oren A, Ma Y. Halophiles and hypersaline environments, springer science. London: Springer International Publishing; 2011. [Google Scholar]
- Waditee-Sirisattha R, Kageyama H, Takabe T. Halophilic microorganism resources and their applications in industrial and environmental biotechnology. AIMS Microbiol. 2016;2(1):42–54. doi: 10.3934/microbiol.2016.1.42. [DOI] [Google Scholar]
- Wang Y, Cui Y, Chen C, Duan Y, Wu Y, Li W, Zhang DD, Li F, Hou C. Stopping the supply of iodized salt alone is not enough to make iodine nutrition suitable for children in higher water iodine areas: a cross-sectional study in northern China. Ecotoxicol Environ Saf. 2020;188(September):109930. doi: 10.1016/j.ecoenv.2019.109930. [DOI] [PubMed] [Google Scholar]


