Abstract
The aim of the study was evaluating the effect of probiotic bacteria on chemical values, texture profile and sensory attributes of Mozzarella cheese which produced from cow and buffalo milk during the storage. The acidity, dry matter content, amount of protein and ripening index of sample increased throughout the storage (P < 0.05). Storage time influenced acidity, dry matter content, amount of protein and ripening index of samples (P < 0.001). Lightness and redness decreased while yellowness increased (P < 0.05). Storage time influenced lightness and yellowness of samples (P < 0.001). TPA parameters increased. The count of Lactobacillus acidophilus increased during the storage (P < 0.05) but Bifidobacterium lactis spp. animalis count increased first 14 days of storage and later decreased (P < 0.05). The samples produced from buffalo milk by adding probiotic bacteria had the highest sensory scores.
Keywords: Mozzarella cheese, Probiotics, Buffalo milk, Quality, Texture
Introduction
The Mozzarella cheese that is among these mentioned ones comes from pasta filata type cheese family and is consumed before it is ripened. The Mozzarella cheese is a renowned Italian cheese which is an indispensable part of pizza production in various varieties, from the yellow colored blocks produced from cow milk to the white colored wheels made of buffalo milk, produced in almost all over the world (Francolino et al. 2010). Mozzarella cheese, primarily produced with buffalo milk, today, can be produced with mixtures of cow and buffalo milk or can be produced solely with cow milk (Pisano et al. 2016).
Probiotics are defined as very important live microorganisms that resides inside the human colon and have a symbiotic relationship with their host, showing great potential for the development of the immune system and the healing of inflammatory disease (Li et al. 2019; Liao et al. 2020). Milk and dairy products are the food kinds, in which probiotic microorganisms are mostly added. Cheese has a stiffer structure and suitable pH, fat content and solid texture; it can protect probiotic microorganisms more efficiently and for longer periods compared to other fermented dairy products (Mazinani et al. 2016). It has been reported that many probiotic cheeses have been produced (Murtaza et al.2017; De Almeida et al. 2018; Silva et al. 2018).
The main purpose of this study was to evaluate the survival of probiotic bacteria of Mozzarella cheese during storage and the effect of the addition of probiotic bacteria on the change of physicochemical, textural and sensory properties of high moisture Mozzarella cheeses during storage.
Material and methods
Materials
The cow milk (density 1.028 g/mL, protein 3.22%, fat 3.30% and dry matter 12.3%) and water buffalo milk (density 1.037 g/mL, protein 4.82%, fat 7,60%, dry matter 14.56%) were obtained from farmers in Afyonkarahisar, Turkey. Thermophilic cheese culture consists of Streptococcus thermophilus (Cryofast ST051), Lactobacillus delbrueckii spp. bulgaricus (Lyofast SP5), Lactobacillus delbrueckii spp. lactis (Cryofast SL 083 F) were obtained from Sacco, Istanbul, Turkey. Probiotic cultures (Lactobacillus acidophilus (Pro Lafti L10) and Bifidobacterium animalisspp. lactis (Pro Lafti B-94) were obtained from DSM, Istanbul, Turkey and calf rennet (Ren-na®) was obtained from Mayasan, Istanbul, Turkey.
Cheese making
Cheese production was carried out according to Akarca et al. (2016) and Nguyen et al. (2017) (Fig. 1). Milks were pasteurized at 75 °C for 15 s. and then cooled at 36 °C. Later starter cultures and probiotic bacteria were added. After 45 min. waiting, calcium chloride (0.2 g/L milk) and calf rennet having a coagulation strength of 1:16 000 MCU/mL were added, and the milk allowed to set for approximately 60 min. until an appropriate curd firmness. Then curd was cut into small cubes when it attained firmness sufficient to withstand cutting and left to heal for 10 min. and the curd heated to 37 °C until the pH of the curd decreased to the desired pH. After whey drainage, until pH 5.2, the curds were cut up and milled and dry-salted with 2.5% w/w salt. Then the curd was submerged in 82 °C water (2.5 kg of water/kg of curd) and hand stretched until forming a smooth, bright and homogeneous mass. The curd was then molded into small balls (80–100 g). After this process, the cheese balls were placed into the water at 0 °C. The cheese samples had been kept in water for about 10 min. and stored at the 4 °C.
Fig. 1.
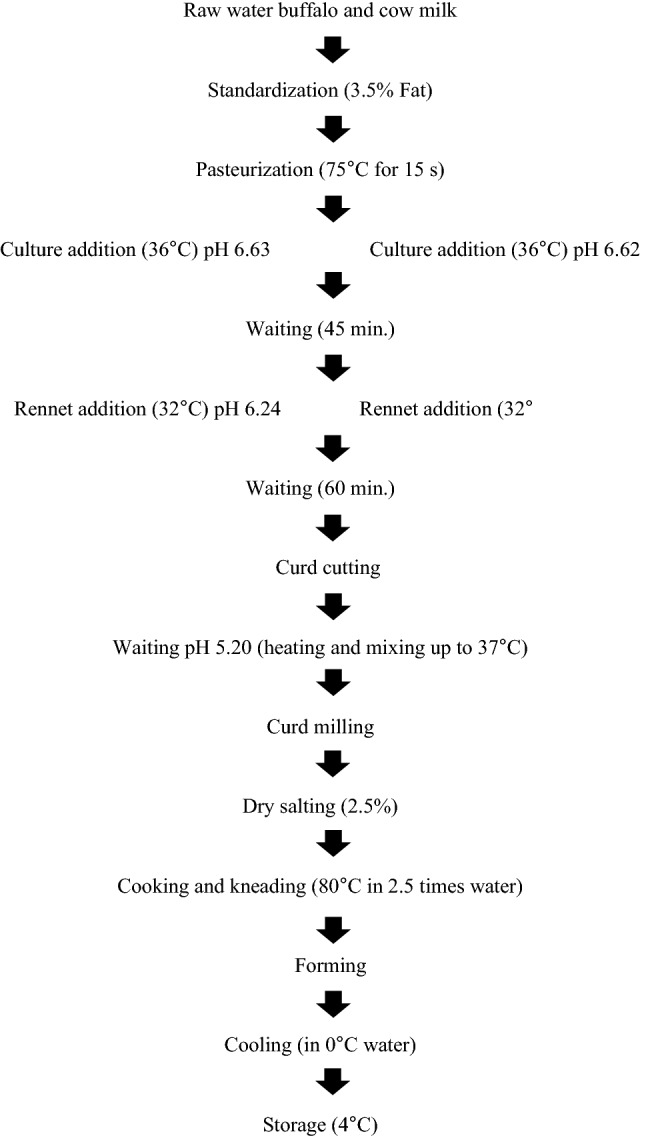
Flow diagram of Mozzarella cheeses production
Chemical analysis
The titratable acidity of the Mozzarella cheese was analyzed as milk acidity according to AOAC 942.15 (AOAC 2016a). Changes the dry matter of the samples for 21 days ripening period were evaluated during the according to official analytical chemists’ methods AOAC 930.15 (AOAC 2016b). The ripening index and amount of protein of Mozzarella cheese were determined as a described by Kurt et al. (2007).
Color values
Color values of cheese measurements were performed using a colorimeter (Minolta Chroma Meter CR-400, Osaka). The lightness (L*), redness (a*) and yellowness (b*) values of samples were determined according to Akarca et al. (2016).
The texture profile analysis
The texture profile analysis (TPA) of the Mozzarella samples was performed at room temperature using a texture analyzer (TA-XT2i; Stable Microsystems Ltd. Surrey, UK) with a 5 kg load cell. The cheese samples were cut in the form of cylinders with 22 ± 0.5 mm in diameter and 20 ± 0.5 mm in length. The TPA conditions fitted with a P/N 0.5probe (10 mm diameter) moving at a speed of 1 mm s1 for 8 mm using cycle or normal programs.
The values for hardness (N), springiness, cohesiveness, gumminess (N), adhesiveness (g,s) and chewiness (N) were determined according to texture analyzer manual (Dai et al. 2018). The analyses were conducted in triplicate.
Microbiological methods
The Lactobacillus acidophilus count was measured with de Man, Rogosa and Sharpe agar (Merck, Germany, 1.10660) and Bifidobacterium animalisspp. lactis count was measured Tryptic Soy Agar (Merck, Germany, 105458) which were incubated at 37ºC for 48 h (ISO 2006, 2010).
Sensory analysis
In the sensory assessment of the cheese, samples were analyzed by a 20 trained member panel of the department of food engineering. The score cards were used for sensory evaluation. The hedonic scale used a 9-point scale, where 1–3 was not acceptable, 4–5 was fairly acceptable, 6–7 was well acceptable, 8–9 was very good for taste and odor, flavor, texture, color and general appreciation (Akarca et al. 2016).
Experimental design and statistical analysis
The design of research was completely randomized having a factorial structure (4 × 4). The factors were storage time (0, 7, 14 and 21 days) and cheese samples (cow milk Mozzarella, cow milk Mozzarella with probiotic bacteria, water buffalo milk Mozzarella and water buffalo milk Mozzarella with probiotic bacteria). Analysis of two-way variance was used to determine the differences (P < 0.05) between the samples during storage time. The results of the analysis were subjected to the ANOVA procedure followed by Duncan’s multiple range tests (SPSS, 17.0.1 version). Design was completely randomized with replications.
Results and discussion
Storage time (P < 0.0001) had a significant effect on acidity, dry matter, amount of protein and ripening index of Mozzarella cheese samples. Also, samples significant effect on acidity, amount of protein (P < 0.01) and dry matter content (P < 0.0001). Besides storage time x samples interactions influenced the °SH value, dry matter content, ripening index (P < 0.0001) and amount of protein (P < 0.01) of the samples (Table 1).
Table 1.
Ls means values for storage time, samples and storage time x samples interaction on °SH, dry matter content (%), amount of protein (%) and ripening index
| Source of variation | °SH | Dry matter content | Amount of protein | Ripening index |
|---|---|---|---|---|
| Samples | ||||
| CM-M | 23.48 ± 2.94b | 51.30 ± 0.68b | 27.32 ± 2.76b | 26.77 ± 6.34a |
| CM-PM | 27.95 ± 5.86ab | 52.28 ± 0.31ab | 29.40 ± 4.61ab | 30.94 ± 9.00a |
| WM-M | 27.08 ± 2.52ab | 57.52 ± 0.55ab | 30.58 ± 3.73ab | 32.17 ± 8.69a |
| WM-PM | 31.03 ± 6.80a | 58.78 ± 0.55a | 32.57 ± 4.52a | 34.85 ± 10.57a |
| P value | .023 | < .0001 | .006 | .338 |
| Storage time | ||||
| 0 | 22.45 ± 2.16b | 51.01 ± 2.99b | 26.42 ± 2.31d | 26.54 ± 2.26d |
| 7 | 25.65 ± 2.47b | 53.47 ± 4.03ab | 27.74 ± 3.11c | 26.83 ± 1.55c |
| 14 | 28.30 ± 3.46ab | 56.63 ± 3.88ab | 31.29 ± 2.44b | 36.40 ± 4.01b |
| 21 | 35.40 ± 5.89a | 58.75 ± 3.83a | 34.44 ± 3.69a | 40.97 ± 5.69a |
| P value | < .0001 | < .0001 | < .0001 | < .0001 |
| ST × S | ||||
| CM-M × 0 | 20.10 ± 0.43n | 48.22 ± 1.27g | 24.92 ± 2.23e | 18,09 ± 1.07k |
| CM-PM × 0 | 21.30 ± 0.85mn | 49.13 ± 1.58fg | 25.16 ± 1.59e | 21.11 ± 0.56ıj |
| WM-M × 0 | 24.90 ± 0.99ıjk | 52.75 ± 1.79def | 27.21 ± 2.71de | 19.51 ± 1.27jk |
| WM-PM × 0 | 23.50 ± 0.43kl | 55.09 ± 0.27cd | 28.38 ± 2.44cde | 23.46 ± 0.93hı |
| CM-M × 7 | 22.50 ± 0.57lm | 48.81 ± 1.26g | 25.31 ± 1.81e | 25,04 ± 1.37gh |
| CM-PM × 7 | 26.10 ± 0.57ghı | 51.03 ± 1.44efg | 26.41 ± 2.85de | 28.12 ± 0.25ef |
| WM-M × 7 | 25.50 ± 0.85hıj | 53.68 ± 1.89de | 29.32 ± 4.53bcde | 26.54 ± 1.20fg |
| WM-PM × 7 | 28.50 ± 0.28ef | 55.61 ± 1.84cd | 29.90 ± 2.51bcde | 27.60 ± 1.54efg |
| CM-M × 14 | 24.20 ± 0.57jk | 52.85 ± 1.60def | 28.95 ± 1.55cde | 30.43 ± 0,89e |
| CM-PM × 14 | 29.10 ± 0.28de | 56.02 ± 2.68cd | 30.95 ± 2.57bcde | 37.51 ± 0.82c |
| WM-M × 14 | 27.40 ± 1.13fg | 59.95 ± 1.73ab | 31.86 ± 2.90bcd | 37.56 ± 2.37c |
| WM-PM × 14 | 32.50 ± 0.57c | 61.25 ± 1.50ab | 38.59 ± 1.88a | 40.11 ± 1.29bc |
| CM-M × 21 | 27.10 ± 0.71fgh | 54.18 ± 2.19cde | 30.11 ± 1.49bcde | 33.53 ± 1.64d |
| CM-PM × 21 | 35.30 ± 0.57b | 57.71 ± 1.74bc | 35.11 ± 2.70ab | 41.97 ± 1.25b |
| WM-M × 21 | 30.50 ± 0.98d | 60.15 ± 1.30ab | 33.96 ± 2.95abc | 40.15 ± 1.41bc |
| WM-PM × 21 | 39.60 ± 1.41a | 63.06 ± 0.93a | 32.57 ± 4.51abc | 48.21 ± 1.20a |
| P value | < .0001 | < .0001 | .002 | < .0001 |
CM-M Cow Milk Mozzarella, CM-PM Cow Milk Mozzarella with probiotic bacteria, WBM-M Water buffalo Milk Mozzarella, WBM-PM Water buffalo Milk Mozzarella with probiotic bacteria, ST Storage time, S Samples. a–n (↓): Values with the same capital letters in the same column for each analysis differ significantly (p < .05)
The acidity values increased in all the cheese samples during storage (P < 0.05). The increase in the °SH values in all the cheese samples throughout the storage period was a result of the lactic acid produced by the growth of the starter and probiotic bacteria found in the Mozzarella cheese, which could ferment lactose.
The highest °SH value was determined that, the sample produced with water buffalo milk added probiotic bacteria WBM-PM (39.6°SH) and CM-PM (35.3°SH). The °SH values were more increased probiotic bacteria added samples than no added probiotic bacteria. This can be explained by the addition of probiotic bacteria to increase acid production. Similarly, Pignata et al. (2015) and Akarca et al. (2016) have reported in their studies that the acidity value of Mozzarella cheese increased during the storage period.
The dry matter content increased in all the Mozzarella samples during storage (P < 0.05). At the end of storage, it was figured out that the highest increase was in the samples (WBM-PM 63.06% and WBM-M 60.15% respectively) produced out of the water buffalo milk. The reason for this increase in the amount of dry matter content is thought to have emerged from the moisture loss during the storage of the cheeses.
It was also detected that cheese samples produced from water buffalo milk had a higher ratio of dry matter increase compared to cheese samples produced from cow milk. This current difference is thought to have existed because the amount of starting dry matter of water buffalo milk used in the production was high. Akarca et al. (2016) stated that the dry matter content of Mozzarella cheese increased during the storage.
Amount of protein increased in all the cheese samples during the 21-day storage (P < 0.05). It was determined that the sample with the lowest amount of protein content was CM-M with 33.53% (Table 1). Yet, highest increase in the amount of protein was achieved in samples that included probiotic bacteria culture (CM-PM %41.97 and WBM-PM 48.21%).
In the research by Smith (2013) it was determined that the amount of the protein in low moisture and partially fat-free Mozzarella cheese was 26.1%. Moreover, in a research by Pignata et al. (2015) on Mozzarella cheese produced from water buffalo and cow milk, determined the amount of the protein values of the samples produced from water buffalo and cow milk as 23.82% and 20.87% respectively. Also, Ayyash and Shah (2011) carried out a similar study and they determined the protein ratios of Mozzarella cheese samples were between 26.53% and 27.93%. The reason for the mentioned difference between the results of other studies and the results obtained in this research is believed to have come from the amount of dry matter which goes up depending on the losses in moisture during storage period of the cheese.
During the storage time, the ripening index of the Mozzarella cheeses were increased (P < 0.05). At the end of the storage period, it was determined that the sample with the highest ripening index was the control sample. Additionally, it was found out that the highest increase over the storage period was in the case of WBM-PM with 48.21. It is considered that this difference may have turned up because the proteolytic activity of the probiotic cultures that were added during production was higher.
Akarca et al. (2016), have similarly indicated in their studies that there was an increase in the ripening index throughout the storage period.
Color is one of the most critical visual quality criteria in foods. Quality evaluations of color may include analytical and sensory measurements. Storage time (P < 0.0001) and samples (only a* value, P < 0.01) had a significant effect on the color values of the Mozzarella cheeses. Also, there was a significant storage time x probiotic culture interaction color values of the samples (P < 0.0001).
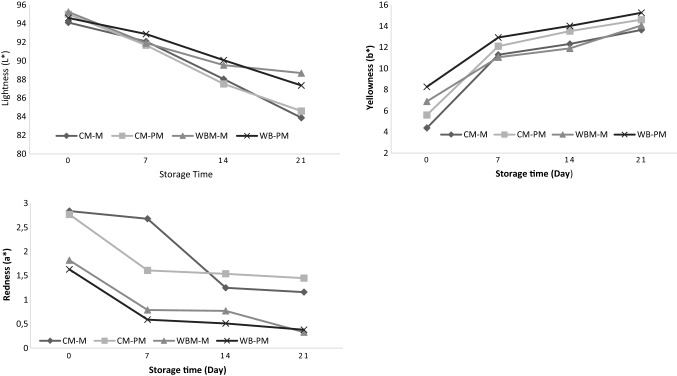
The changes in L*, a* and b* values of Mozzarella cheeses during storage are shown in Figs. 2, 3 and 4. L*(Lightness) values of all the samples decreased during storage (P < 0.05; Fig. 2). It was resolved that the decrease in L* values during the storage period in cheese produced with cow milk was found to be higher compared to the other samples. Almost all of the carotene in the water buffalo milk is converted to vitamin A, and as the vitamin A is also colorless, the color of the milk also gets whiter. It is thought that the reason why the Mozzarella cheese produced from water buffalo milk has higher L*value is due to fact that the water buffalo milk is whiter (Ozsunar 2010). The highest decrease was in the sample of CM-PM. In a similar manner, Johnston and Darcy (2000) have reported that L* value decreased during storage period. It is evaluated that, the decrease in L* values showed up due to the fact the Mozzarella cheese has water loss, partial oxidation throughout the ripening period and the increase of the microorganism counts in the cheese samples (Johnston and Darcy 2000; Ozsunar 2010; Akarca et al. 2016).
Fig. 2.
Effect of probiotic culture and storage time on lightness (L*), redness (a*) and yellowness (b*) values of Mozzarella cheese
It was determined that during the storage period, a* values decreased all samples by an average value of 1.43 during the 21-day storage period (P < 0.05; Fig. 2). The highest decrease was determined in cheese sample produced with cow milk with 1.68. Cheese samples that produced with water buffalo milk followed this value with 1.49 (P < 0.05). Compared to other samples, a* values of the Mozzarella cheese produced by adding probiotic culture have been found to be lower. It has been reported that reducing the fat in Mozzarella cheese gives a greenish color (Ozsunar 2010). Because a* values were gained positively in the study, this is contemplated to be indicative of redness. Thus, it was concluded that the produced cheese types included the proper fat ratio. Parallel to the results that were achieved in this study, Solak (2013) also stated that a* value decreases during the storage period. Johnston and Darcy (2000) pointed out that the changes in a* value during the ripening period in Mozzarella cheese should not be considered as meaningful.
It has been found out that the b* values (yellow–blue color) of all Mozzarella cheese samples used in the study have increased throughout the storage period. At the beginning of storage, b* values were determined to be between 8.27 and 4.38, whereas these values, with an average increase by 8.34, ranged between 15.77 and 13.65 at the end of the 21 day storage period (P < 0.05; Fig. 2). In a similar way, Johnston and Darcy (2000), Akbulut (2007) and Akarca et al. (2016) reported that b* value in Mozzarella cheese ripened at 4 °C went upwards during the ripening period. The increase in b*values in cheese was associated with the intensity of the heat treatment applied in the production of the cheese and the loss of moisture and oxidation during the storage of the cheese (Akarca et al. 2016). It was also determined at the end of storage that, the b* values of cheeses with no probiotic bacteria added had higher than the probiotic bacteria added samples. The highest value in yellowness might be due to the acidity of the cheese.
In their study, Sert et al. (2014) stated that as the acidity value increased, the value of yellowness decreased. The reason why the probiotic added samples included lower b* values compared to other samples is thought to have emanated from probiotic bacteria, especially from Lactobacillus acidophilus bacteria, which produces more acid than the others.
Texture is a basic parameter of cheese quality. Furthermore, texture and general acknowledgment are more significant than taste ingredient of cheese. These components have precedence for consumer (Aday and Karagul Yuceer 2014).
The storage time (P < 0.0001) and sample difference (P < 0.01) had effects on hardness (the maximum force required to compress cheese) values on cheese samples (Table 2). Also, storage time × samples (P < 0.0001) interactions influenced the hardness of the samples (Table 2). Hardness values of the Mozzarella samples decreased during the storage (P < 0.05). Like those reported by Ozsunar (2010) and Smith (2013). It was ascertained at the end of the storage period that the highest value belongs to WBM-M sample with 1379.7 N. On the other hand, the lowest value belongs to WBM-PM sample with 911.7 N. It was thought that decreased of the hardness was caused by proteolysis, which occurs during storage period. The hardness values of cheese samples that included probiotic culture have decrease more in comparison with the other samples. This situation shows that probiotic microorganisms cause more proteolysis.
Table 2.
Ls means values for storage time, samples and storage time x samples interaction on texture profile of Mozzarella cheese
| Source of variation | Hardness | Springiness | Cohesiveness | Gumminess | Adhesiveness | Chewinnes |
|---|---|---|---|---|---|---|
| Samples | ||||||
| CM-M | 1450.48 ± 221.99b | 0.84 ± 0.06a | 0.78 ± 0.06a | 1144.67 ± 257.54a | −6.91 ± 3.57a | 965.9242 ± 231.17a |
| CM-PM | 1056.15 ± 141.65c | 0.80 ± 0.06ab | 0.73 ± 0.08ab | 776.26 ± 148.66b | −8.07 ± 4.10a | 626.9044 ± 154.96b |
| WBM-M | 1753.28 ± 369.01a | 0.81 ± 0.06ab | 0.73 ± 0.06ab | 1278.98 ± 329.96a | −34.95 ± 30.69b | 1042.885 ± 297.68a |
| WBM-PM | 1187.23 ± 250.51bc | 0.75 ± 0.06b | 0.70 ± 0.04b | 834.68 ± 209.92b | −43.94 ± 24.02b | 634.5613 ± 200.24b |
| P value | < .0001 | .045 | .096 | .0001 | .0001 | .0001 |
| Storage time | ||||||
| 0 | 1690.93 ± 421.32a | 0.83 ± 0.06a | 0.77 ± 0.07a | 1297.65 ± 364.79a | −1.53 ± 0.76a | 1081.082 ± 306.91a |
| 7 | 1396.25 ± 312.03ab | 0.82 ± 0.06ab | 0.75 ± 0.06a | 1042.83 ± 247.18ab | −21.64 ± 16.25ab | 856.5927 ± 243.59ab |
| 14 | 1234.18 ± 239.74b | 0.80 ± 0.06ab | 0.73 ± 0.07a | 907.32 ± 205.79b | −29.63 ± 22.21ab | 730.3551 ± 192.49b |
| 21 | 1125.78 ± 232.58b | 0.76 ± 0.06b | 0.70 ± 0.06a | 786.81 ± 202.62b | −41.08 ± 32.44b | 602.2446 ± 189.20b |
| P value | .007 | .126 | .206 | .008 | .007 | .003 |
| ST × S | ||||||
| CM-M × 0 | 1725.40 ± 252.72bc | 0.88 ± 0.08a | 0.82 ± 0.08a | 1425.55 ± 353.63ab | −1.96 ± 0.25a | 1239.48 ± 190.24ab |
| CM-PM × 0 | 1238.90 ± 78.77efg | 0.84 ± 0.04ab | 0.80 ± 0.03a | 951.73 ± 9.43cdef | −6.49 ± 0.93ab | 1009.69 ± 199.54bcd |
| WBM-M × 0 | 2274.30 ± 94.61a | 0.83 ± 0.02ab | 0.78 ± 0.05a | 1709.74 ± 263.94a | −8.41 ± 1.76ab | 872.26 ± 154.19cde |
| WBM-PM × 0 | 1525.10 ± 129.82cd | 0.78 ± 0.08ab | 0.73 ± 0.06a | 1103.58 ± 222.88bcde | −10.81 ± 1.03b | 742.27 ± 56.67cdef |
| CM-M × 7 | 1458.50 ± 118.37de | 0.86 ± 0.07ab | 0.77 ± 0.03a | 1168.47 ± 135.95bcd | −2.37 ± 0.74a | 799.25 ± 32.46cdef |
| CM-PM × 7 | 1080.80 ± 113.99fgh | 0.81 ± 0.08ab | 0.75 ± 0.09a | 804.96 ± 21.51def | −7.37 ± 0.92ab | 652.93 ± 85.72def |
| WBM-M × 7 | 1827.50 ± 48.22b | 0.82 ± 0.05ab | 0.73 ± 0.14a | 1335.78 ± 164.43bc | −10.19 ± 1.38b | 585.03 ± 202.79ef |
| WBM-PM × 7 | 1218.20 ± 100.27efg | 0.77 ± 0.04ab | 0.68 ± 0.08a | 862.09 ± 2.28def | −12.36 ± 1.70b | 470.40 ± 72.83f |
| CM-M × 14 | 1327.60 ± 161.50def | 0.84 ± 0.01ab | 0.75 ± 0.08a | 1040.09 ± 201.07cde | −0.74 ± 0.13a | 1415.35 ± 170.71a |
| CM-PM × 14 | 983.60 ± 69.16gh | 0.80 ± 0.07ab | 0.73 ± 0.07a | 722.92 ± 189.59ef | −40.97 ± 3.18d | 1099.99 ± 210.39abc |
| WBM-M × 14 | 1531.60 ± 82.45cd | 0.81 ± 0.08ab | 0.72 ± 0.03a | 1101.59 ± 16.04bcde | −54.45 ± 5.26e | 891.60 ± 80.48bcde |
| WBM-PM × 14 | 1093.90 ± 73.11fgh | 0.75 ± 0.09ab | 0.70 ± 0.10a | 764.69 ± 2023ef | −79.60 ± 6.31g | 764.59 ± 236.11cdef |
| CM-M × 21 | 1290.40 ± 90.93def | 0.79 ± 0.05ab | 0.72 ± 0.08a | 944.56 ± 139.37def | −1.07 ± 0.13a | 870.25 ± 267.49cde |
| CM-PM × 21 | 921.30 ± 24.18h | 0.75 ± 0.04ab | 0.71 ± 0.05a | 625.46 ± 61.73f | −31.75 ± 4.38c | 663.76 ± 34.82def |
| WBM-M × 21 | 1379.70 ± 61.66de | 0.78 ± 0.09ab | 0.70 ± 0.03a | 968.84 ± 179.74cdef | −45.47 ± 5.43d | 572.52 ± 60.52ef |
| WBM-PM × 21 | 911.70 ± 86.83h | 0.71 ± 0.07b | 0.67 ± 0.06a | 608.05 ± 6.60f | −61.54 ± 6.12f | 431.72 ± 38.33f |
| P value | < .0001 | .640 | .832 | < .0001 | < .0001 | < .0001 |
CM-M Cow Milk Mozzarella, CM-PM Cow Milk Mozzarella with probiotic bacteria, WBM-M Water buffalo Milk Mozzarella, WBM-PM Water buffalo Milk Mozzarella with probiotic bacteria, ST Storage time, S Samples, TAMB Total Aerobic Bacteria Count. a–h (↓) Values with the same capital letters in the same column for each analysis differ significantly (p < .05)
The interactions between proteins and polysaccharides in the cheese are very important in terms of improving the structure and stability of the product and the polysaccharide types and the charge carried by these is responsible for managing these interactions (Hosseini et al. 2013). Therefore, high protein values affected the hardness values of the cheeses (Sahan et al. 2008).
The storage time (P < 0.05) had effects on springiness (mm) (elasticity of the cheese after the release of force) values on Mozzarella cheeses (Table 2). Springiness (mm) values of cheese samples decreased during storage (P < 0.05). The most decreased springiness values during the storage were samples with produced by adding probiotic culture. At the end of the storage period the most decrease of the samples was found in the WBM-PM with 0.71 mm. However, the least decrease of cheeses was detected in the CM-M with 0.79 mm.
As a result of the studies by Jeewanthi et al. (2015) and Bunker (2016), it was similarly revealed that the springiness values in Mozzarella cheese decreased during storage period. It is believed that depending on the high proteolysis, intermolecular bonds in Mozzarella cheese showed less interaction. Based on this action, the bonds between casein molecules unfolded less interaction with each other especially in the samples produced by adding probiotic culture. Thus, it caused the springiness values of the cheese to fall further. In addition to that, it was determined that the acidity levels of cheese samples (Table 1) which was obtained by adding probiotic bacteria increased more during the storage period when it is compared to other cheese samples. It is predicted that this increase in the acidity ratios has made a detrimental effect on the springiness values of the cheese samples.
Cohesiveness (the resistance values that the cheese samples display against the second compression) values decreased throughout the storage period in all cheese samples (P > 0.05; Table 3). It was determined that the highest cohesiveness value at the end of the 21-day storage period in CM-M with 0.73 whereas the lowest value was found in WBM-PM case with 0.67. It was defined that the decrease of cohesiveness value was close to each other in all samples. However, it was also found that cheese samples produced from water buffalo milk decreased more than other samples. Accordingly, our results, Jeewanthi et al. (2015) and Pignata et al. (2015) determined in their studies that the cohesiveness values of the Mozzarella cheese decreased during the storage period. It is believed that the reason for the decrease of the cohesiveness values in cheese samples depending on the storage period was due to the increased acidity of milk and proteolysis.
Table 3.
Ls means values for storage time, samples and storage time x samples interaction on sensory properties
| Source of variation | Color | Odor | Texture | Flavor | General appreciation |
|---|---|---|---|---|---|
| Samples | |||||
| CM-M | 5.82 ± 0.86b | 6.59 ± 0.97b | 7.96 ± 0.23a | 6.18 ± 0.48b | 6.70 ± 0.28b |
| CM-PM | 6.73 ± 1.54ab | 6.85 ± 0.70b | 8.16 ± 0.21a | 6.68 ± 0.57b | 6.94 ± 0.30b |
| WBM-M | 7.40 ± 1.41a | 7.98 ± 1.05a | 7.80 ± 0.18a | 8.19 ± 0.25a | 8.04 ± 0.31a |
| WBM-PM | 7.83 ± 1.49a | 8.25 ± 0.90a | 6.61 ± 0.14b | 8.22 ± 0.26a | 8.35 ± 0.30a |
| P value | .033 | .002 | < .0001 | .002 | < .0001 |
| Storage time | |||||
| 0 | 7.77 ± 1.01a | 7.99 ± 0.88a | 6.99 ± 0.17c | 7.91 ± 0.32a | 8.04 ± 0.31a |
| 7 | 7.89 ± 0.95a | 8.04 ± 0.85a | 7.49 ± 0.27bc | 8.11 ± 0.27a | 8.17 ± 0.27a |
| 14 | 7.22 ± 0.99a | 7.64 ± 0.71a | 7.79 ± 0.23ab | 7.61 ± 0.25a | 7.55 ± 0.25a |
| 21 | 4.89 ± 0.55b | 6.00 ± 0.72b | 8.26 ± 0.29a | 5.63 ± 0.59b | 6.27 ± 0.59b |
| P value | < .0001 | < .001 | .008 | < .0001 | < .0001 |
| ST × S | |||||
| CM-M × 0 | 6.30 ± 0.27cd | 7.15 ± 0.38c | 7.22 ± 0.28fg | 6.74 ± 0.31g | 7.01 ± 0.20de |
| CM-PM × 0 | 7.81 ± 0.28b | 7.23 ± 0.15c | 7.63 ± 0.17def | 7.53 ± 0.45def | 7.51 ± 0.40cde |
| WBM-M × 0 | 8.15 ± 0.38ab | 8.65 ± 0.30ab | 8.18 ± 0.20c | 8.71 ± 0.17ab | 8.72 ± 0.18a |
| WBM-PM × 0 | 8.83 ± 0.04a | 8.92 ± 0.10a | 8.84 ± 0.18ab | 8.66 ± 0.08ab | 8.93 ± 0.07a |
| CM-M × 7 | 6.54 ± 0.20cd | 7.18 ± 0.33c | 7.36 ± 0.18efg | 7.12 ± 0.37efg | 7.27 ± 0.33cde |
| CM-PM × 7 | 7.85 ± 0.37b | 7.35 ± 0.23c | 8.16 ± 0.16cd | 7.83 ± 0.41cde | 7.67 ± 0.13cd |
| WBM-M × 7 | 8.35 ± 0.33ab | 8.72 ± 0.14ab | 8.23 ± 0.30c | 8.81 ± 0.06a | 8.78 ± 0.13a |
| WBM-PM × 7 | 8.85 ± 0.16a | 8.92 ± 0.07a | 8.88 ± 0.10a | 8.71 ± 0.31ab | 8.95 ± 0.04a |
| CM-M × 14 | 5.92 ± 0.23de | 6.96 ± 0.18c | 7.13 ± 0.27h | 6.84 ± 0.17fg | 7.01 ± 0.35de |
| CM-PM × 14 | 6.91 ± 0.24c | 7.05 ± 0.44c | 7.83 ± 0.43cde | 7.21 ± 0.38efg | 6.91 ± 0.23de |
| WBM-M × 14 | 7.94 ± 0.45b | 8.22 ± 0.27b | 7.94 ± 0.31cd | 8.01 ± 0.31bcd | 7.84 ± 0.39bc |
| WBM-PM × 14 | 8.12 ± 0.57ab | 8.32 ± 0.21b | 8.31 ± 0.24bc | 8.41 ± 0.28abc | 8.45 ± 0.24ab |
| CM-M × 21 | 4.52 ± 0.23gh | 5.06 ± 0.24f | 6.25 ± 0.23ı | 4.02 ± 0.27h | 5.53 ± 0.53f |
| CM-PM × 21 | 4.35 ± 0.20h | 5.77 ± 0.08e | 6.34 ± 0.33hı | 4.16 ± 0.38h | 5.67 ± 0.24f |
| WBM-M × 21 | 5.17 ± 0.06fg | 6.34 ± 0.27d | 6.81 ± 0.11gh | 7.26 ± 0.52efg | 6.84 ± 0.51e |
| WBM-PM × 21 | 5.52 ± 0.49ef | 6.83 ± 0.18cd | 7.04 ± 0.31g | 7.11 ± 0.34efg | 7.05 ± 0.55de |
| P value | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 |
CM-M Cow Milk Mozzarella, CM-PM Cow Milk Mozzarella with probiotic bacteria, WBM-M Water buffalo Milk Mozzarella, WBM-PM Water buffalo Milk Mozzarella with probiotic bacteria, ST Storage time, S Samples. a–h (↓): Values with the same capital letters in the same column for each analysis differ significantly (p < .05)
The storage time (P < 0.01) and sample difference (P < 0.01) had effects on gumminess adhesiveness and chewiness values on Mozzarella cheeses (Table 2). In addition, storage time × samples (P < 0.0001) interactions influenced the gumminess, adhesiveness and chewiness of the samples (Table 2). Gumminess (the force required to swallow cheese) of all cheese samples decreased the during storage period (P < 0.05). At the end of 21-day storage, the highest value was determined with 968.84 at WBM-M sample. This value was followed by CM-M sample with 944.56. Like happened in the hardness value, it is thought that the proteolysis has been effective on the reduction of gumminess value. Therefore, it was found out that the samples with the most reduction rate in gumminess value were the ones that included probiotic culture throughout the storage period. In the studies performed by Jeewanthi et al. (2015), it was discovered that there was a decrease in the gumminess values of the cheese during the storage period, which is in parallel with the results achieved in this study.
Adhesiveness (the force required to remove the cheese from the prob) values decreased by an average value of − 35.23 samples with added probiotic bacteria and − 43.86 non-added probiotic bacteria during storage period (P < 0.05). It is thought that there is a significant effect of proteolysis and moisture content, which are the biochemical events that occur in cheese, on the increase in the adhesiveness values observed in the cheese samples. In particular, the increase in moisture content significantly increases the adhesiveness values (Solak 2013).
In all of the samples, a decrease in adhesiveness values was detected throughout the storage period, although the highest decrease in Mozzarella cheese samples was resulted in the ones produced from water buffalo milk (WBM-M-78.86 and WBM-PM-60.47) (P < 0.05). It is evaluated that this difference appeared because the proteolysis observed in cheeses produced from water buffalo milk is more than that produced from cow milk and due to the increased water retention capacity of proteins denatured as a result of proteolysis.This result is due to the increase in small peptides due to proteolysis. Because these small peptides may increase the binding forces within the cheese matrix by increased absorption of moisture (Tunic et al.1997).
In their studies carried out before, Bhaskaracharya (2000) have indicated that the adhesiveness values of the cheese samples decreased depending on the storage, which is similar to one of the results of this research.
The chewiness (the force required to make a solid food ready for swallowing) value decreased in all samples depending on the storage period (P < 0.05). It was figured out at the end of the storage period that the maximum decrease was in WBM-M sample with 650.76 kg (mm) while the minimum decrease was in the CM-PM sample with 328.85 kg (mm). It was detected at the end of the storage period that the samples presenting the highest decrease in chewiness values were the Mozzarella cheese types produced by adding probiotic bacteria. It is thought that the reason for this is that the addition of probiotic bacteria increases the proteolysis observed in cheese types and it disrupts the network structure of casein and its interaction with other components of casein. According to their research by Bunker (2016), this study obtained similar results as theirs, which displays that the chewiness values of Mozzarella cheese decreased during the storage period.
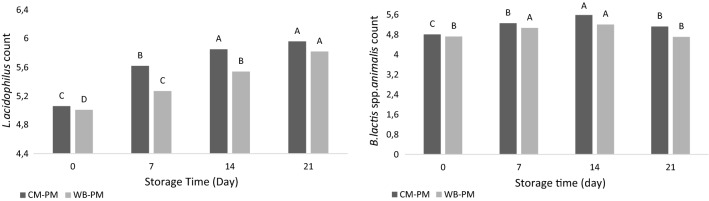
Storage time (P < 0.0001) had a significant effect on the Lactobacillus acidophilus count of Mozzarella cheeses. Lactobacillus acidophilus count of the Mozzarella samples increased during storage period (P < 0.05; Fig. 3). At the beginning of the study, Lactobacillus acidophilus was inoculated in equal amounts to both milk samples (6 log CFU ml−1 on average). However, in the analyzes carried out following the production, it was determined that this count dropped to an average of 5 log CFU g−1. It is believed that this decline was due to the heat treatment applied during the production of the cheese samples.
Fig. 3.
Lactobacillus acidophilus and Bifibacterium animalis spp. lactis counts of Mozzarella cheese during the storage
When Lactobacillus acidophilus exchange is examined in the cheese samples made from both milk types depending on the storage period, it was determined that the increase in the number of Lactobacillus acidophilus was found to be similar. In other words, it was determined that there was no significant effect of milk variety used in production on the number of Lactobacillus acidophilus throughout the storage period (P > 0.05).
Storage time (P < 0.01) had a effect on the Bifidobacterium animalis spp. lactis count of samples. Although Bifidobacterium lactis spp. animalis counts of the samples increased in the first 14 days of storage, it began to decrease after the14th day (P < 0.05; Fig. 3).
As a result of 21 days of storage, it was determined that the highest decrease between the two samples was in the WBM-PM sample with a value of 4.71 ± 0.04 log CFU g−1. In a similar way, in the research by Yalcin (2016), it was reported that an increase in Bifidobacterium bifidum numbers in unsalted quark cheese until day 21 has been observed whereas a decrease occurred after 21st day.
Even though some cheese varieties have been studied as vehicles for probiotic microorganisms (De Almeida et al. 2018) there are very few studies in which the incorporation of probiotic microorganisms to fresh pasta filata cheese was evaluated (Angiolillo et al. 2014).
Sensory analysis results regarding the color and appearance, taste and aroma, texture, aroma and general acceptance values on the 0th, 7th, 14th and 21th days of the storage are shown in Table 3. Samples difference affected the color (P < 0.05), odor (P < 0.01), texture (P < 0.0001), flavor (P < 0.01) and general acceptability of the cheese samples (P < 0.0001; Table 3). Also, storage time affected the color, odor, flavor, general acceptability (P < 0.001) and texture (P < 0.01) of the Mozzarella cheese samples (Table 3). Moreover, storage time x sample difference interaction affected color, odor, texture flavor and general acceptability (P < 0.0001) scores (Table 3). In general, all the sensory evaluation scores increased first 7 days, but then decreased and all sensory scores of cheeses produced from cow's milk are lower than those produced from water buffalo milk (P < 0.05; Table 3). Similarly, Yazici et al. (2010) and Akarca et al. (2016) state that storage time had a negative effect on flavor scores, and all scores significantly decreased during storage.
The highest color scores were given to samples WBM-PM (5.52) and WBM-M (5.17) however lowest color scores was given to samples CM-PM (4.35) and CM-M (4.52). Traditional Italian Mozzarella is made of water buffalo milk and its color is quite white. Cow milk contains more beta carotene than water buffalo milk. Therefore, its color is yellow. As a result, cheese produced from cow's milk is more yellow in comparison to those produced from water buffalo milk. In their research Fasale et al. (2017) found that the color scores of Mozzarella cheeses produced from water buffalo milk were higher than those produced from cow's milk.
In the sensory evaluation tests carried out during the 21 days storage period, the highest odor and flavor score was given to samples produced from water buffalo milk by adding probiotic bacteria (P < 0.05; Table 3). Cosentino et al. (2016) state that type of milk used in Mozzarella cheese production were significant effect on odor and flavor scores of Mozzarella cheeses. According to the authors, milk, cooked milk, acid milk, and ripened cheese are among the most common attributes for describing the odor and flavor of cheese.
The WBM-PM samples had the highest texture scores while CM-M had the lowest scores in the sensory evaluation (P < 0.0001). Likewise, Murtaza et al. (2017) reported that the texture scores of Cheddar cheeses produced from water buffalo milk were higher than those produced from cow milk. According to the general acceptance values, the most favored sample was produced from water buffalo milk by adding probiotic bacteria (P < 0.05; Table 3). So, Mozzarella cheeses produced with water buffalo milk are more popular than those produced with cow's milk.
According to these results, Mozzarella cheese is a suitable food matrix for probiotic bacteria. Similarly, various researchers have reported that cheese is a well-established food for probiotic bacteria due to its nutritional composition (De Almeida et al. 2018).
Conclusion
In this study, probiotic bacteria were added to Mozzarella cheese which is one of the most preferred and consumed cheese in the world. The Mozzarella cheese produced from water buffalo milk by adding probiotic bacteria had the highest sensory scores, whereas cheese produced from cow milk without adding probiotic bacteria had the lowest sensory score.
Although the count of probiotic bacteria inoculated during the production phase decreases with scalding, the increase in storage time is a positive result. In order for the count of viable probiotic bacteria to be 7–8 log CFU g−1or higher, developer research should be done on the study.
Today, the increasing trend to consume organic and healthy foods in the world promotes the daily and regular consumption of probiotics. Mozzarella cheese should be considered a helpful carrier to allow the daily consumption of doses of viable probiotic bacteria at sufficiently high levels to benefit the host and Mozzarella cheese (especially produce from water buffalo milk) with improved functional properties with the addition of probiotic bacteria will attract attention from the consumers.
Acknowledgements
This research was supported by Afyon Kocatepe University Research Fund (Project Number: 12.FEN.BİL.26).
Authors' contribution
GA Planning the research, conducting the analysis, writing and editing the manuscript. GY Planning the research, conducting the analysis, writing and editing the manuscript.
Funding
This research was supported by Afyon Kocatepe University Research Fund (Project Number: 12.FEN.BİL.26).
Declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethic approval
We declare that the writing of the letter does not violate any code of ethics.
Consent to participate
All the authors featured in this research and manuscript have participated of their own free will and will.
Consent for publication
We approve the use of all data and information belonging to us in the publication of our article.
Availability of data and material
All materials and data used in this research and manuscript are available to all researchers.
Code availability
No special software application and code were used in the writing of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aday S, Karagul Yuceer Y. Physicochemical and sensory properties of Mihalic cheese. Int J Food Prop. 2014;17(10):2207–2227. doi: 10.1080/10942912.2013.790904. [DOI] [Google Scholar]
- Akarca G, Çağlar A, Tomar O. The effects spicing on quality of Mozzarella cheese. Mljekarstvo: Časopis Za Unaprjeđenje Proizvodnje i Prerade Mlijeka. 2016;66(2):112–121. doi: 10.15567/mljekarstvo.2016.0203. [DOI] [Google Scholar]
- Akbulut Ç (2007) The effect of whey draining pH on some physicochemical and sensory properties of Mozzarella cheese made from water buffalo milk. (Master’s thesis, Ondokuz Mayıs University, Graduate School of Natural and Applied Science, Samsun, Turkey). Retrieved from https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp
- Angiolillo L, Conte A, Faccia M, Zambrin AV, Del Nobile MA. A new method to produce synbiotic Fiordilatte cheese. Innov Food Sci Emerg Technol. 2014;22:180–187. doi: 10.1016/j.ifset.2013.09.010. [DOI] [Google Scholar]
- AOAC (2016a) Official methods of analysis. 978.18., 20th edn. Washington
- AOAC (2016b) Official methods of analysis. 981.12., 20th edn. Washington
- Ayyash MM, Shah NP. Proteolysis of low-moisture Mozzarella cheese as affected by substitution of NaCl with KCl. J Dairy Sci. 2011;94(8):3769–3777. doi: 10.3168/jds.2010-4104. [DOI] [PubMed] [Google Scholar]
- Bhaskaracharya RK (2000) The texture and microstructure of Mozzarella cheese as affected by fat content, EPS producing starter culture and fat replacers. Doctoral thesis, Victoria University of Technology. http://vuir.vu.edu.au/15412/
- Bunker HS (2016) The effect of freezing rate on quality attributes of low-moisture part-skim mozzarella. Doctoral thesis, The Ohio State University. https://etd.ohiolink.edu/apexprod/rws_olink/r/1501/10?clear=10&p10_accession_num=osu1468872007
- Cosentino C, Faraone D, Paolino R, Freschi P, Musto M. Sensory profile and acceptability of a cow milk cheese manufactured by adding jenny milk. J Dairy Sci. 2016;99(1):228–233. doi: 10.3168/jds.2015-10107. [DOI] [PubMed] [Google Scholar]
- Dai S, Jiang F, Corke H, Shah NP. Physicochemical and textural properties of Mozzarella cheese made with konjac glucomannan as a fat replacer. Food Res Int. 2018;107:691–699. doi: 10.1016/j.foodres.2018.02.069. [DOI] [PubMed] [Google Scholar]
- deAlmeida JDSO, Dias CO, Pinto SS, Pereira LC, Verruck S, Fritzen-Freire CB, et al. Probiotic Mascarpone-type cheese: characterisation and cell viability during storage and simulated gastrointestinal conditions. Int J Dairy Technol. 2018;71:195–203. doi: 10.1111/1471-0307.12457. [DOI] [Google Scholar]
- Fasale AB, Patil VS, Bornare DT. Process optimization for mozzarella cheese from cow and buffalo milk. Int J Food Ferment Technol. 2017;7(1):165–173. doi: 10.5958/2277-9396.2017.00018.6. [DOI] [Google Scholar]
- Francolino S, Locci F, Ghiglietti R, Iezzi R, Mucchetti G. Use of milk protein concentrateto standardize milk composition in Italian citric Mozzarella cheese making. LWT-Food Sci Technol. 2010;43(2):310–314. doi: 10.1016/j.lwt.2009.08.007. [DOI] [Google Scholar]
- Hosseini SMH, Emam-Djomeh Z, Razavi SH, Moosavi-Movahedi AA, Saboury AA, Atri MS, Van der Meeren P. β-Lactoglobulin–sodium alginate interaction as affected by polysaccharide depolymerization using high intensity ultrasound. Food Hydrocoll. 2013;32(2):235–244. doi: 10.1016/j.foodhyd.2013.01.002. [DOI] [Google Scholar]
- ISO . Milk products enumeration of presumptive Lactobacillus acidophilus on a selective medium Colony-count technique at 37 °C (20128:2006) Geneva: Switzerland; 2006. [Google Scholar]
- ISO . Milk products enumeration of presumptive bifidobacterial Colony count technique at 37 °C (29981:2010) Geneva: Switzerland; 2010. [Google Scholar]
- Jeewanthi RK, Lee NK, Lee KA, Yoon YC, Paik HD. Comparative analysis of improved soy-Mozzarella cheeses made of ultra filtratedand partly skimmed soy blends with other Mozzarella types. J Food Sci Technol. 2015;52(8):5172–5179. doi: 10.1007/s13197-014-1542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DE, Darcy PC. The effects of high pressure treatment on immature Mozzarella cheese. Milchwissenschaft. 2000;55(11):617–620. [Google Scholar]
- Kurt A, Çakmakçı S, Çağlar A. A guidebook of analysis methods of milk and milk products. New York: Faculty of Agriculture Publications; 2007. p. 18. [Google Scholar]
- Li W, Liu L, Tian H, Luo X, Liu S. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr Polym. 2019;223:115065. doi: 10.1016/j.carbpol.2019.115065. [DOI] [PubMed] [Google Scholar]
- Liao N, Pang B, Jin H, Xu X, Yan L, Li H, et al. Potential of lactic acid bacteria derived polysaccharides for the delivery and controlled release of oral probiotics. J Control Release. 2020 doi: 10.1016/j.jconrel.2020.04.022. [DOI] [PubMed] [Google Scholar]
- Mazinani S, Fadaei V, Khosravi-Darani K. Impact of Spirulina platensis on physicochemical properties and viability of Lactobacillus acidophilus of probiotic UF feta cheese. J Food Process Preserv. 2016;40(6):1318–1324. doi: 10.1111/jfpp.12717. [DOI] [Google Scholar]
- Murtaza MA, Huma N, Shabbir MA, Murtaza MS, Anees-ur-Rehman M. Survival of micro-organisms and organic acid profile of probiotic Cheddar cheese from buffalo milk during accelerated ripening. Int J Dairy Technol. 2017;70(4):562–571. doi: 10.1111/1471-0307.12406. [DOI] [Google Scholar]
- Nguyen HT, Ong L, Lopez C, Kentish SE, Gras SL. Micro structure and physico chemical properties reveal differences between high moisture buffalo and bovine Mozzarella cheeses. Food Res Int. 2017;102:458–467. doi: 10.1016/j.foodres.2017.09.032. [DOI] [PubMed] [Google Scholar]
- Ozsunar A (2010) The effect of mixture with water buffalo and cow milk to Mozzarella like cheese in physicochemica lproperties and aroma profile. Doctoral thesis, Trakya University, Graduate School of Natural and Applied Science, Tekirdağ, Turkey. Retrieved from: https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp
- Pignata MC, Ferrão S, Oliveira CP, Faleiro AS, Bonomo RC, Silva WS, et al. Mechanical parameters of the Mozzarella from water buffalo with inclusion levels of the cow's milk: preliminary Study at the Lab Scale. J Bioanal Biomed. 2015 doi: 10.4172/1948-593X.1000143. [DOI] [Google Scholar]
- Pisano MB, Scano P, Murgia A, Cosentino S, Caboni P. Metabolomics and microbiological profile of Italian Mozzarella cheese produced with buffalo and cow milk. Food Chem. 2016;192:618–624. doi: 10.1016/j.foodchem.2015.07.061. [DOI] [PubMed] [Google Scholar]
- Sahan N, Yasar K, Hayaloglu AA, Karaca OB, Kaya A. Influence of fatreplacers on chemical composition, proteolysis, texture profiles, meltability and sensory properties of low-fat Kashar cheese. J Dairy Res. 2008;75(1):1. doi: 10.1017/S0022029907002786. [DOI] [PubMed] [Google Scholar]
- Sert D, Akin N, Aktumsek A. Lipolysis in Tulum cheese produced from raw and pasteurized goats milk during ripening. Small Rumin Res. 2014;121(2–3):351–360. doi: 10.1016/j.smallrumres.2014.06.006. [DOI] [Google Scholar]
- Silva HL, Balthazar CF, Esmerino EA, Neto RP, Rocha RS, Moraes J, et al. Partial substitution of NaCl by KCl and addition of flavor enhancers on probiotic Prato cheese: a study covering manufacturing, ripening and storage time. Food Chem. 2018;248:192–200. doi: 10.1016/j.foodchem.2017.12.064. [DOI] [PubMed] [Google Scholar]
- Smith JR (2013) Assessment of structure and component mobility within Mozzarella cheese. Doctoral thesis, Massey University of Food Technology, New Zealand. Retrieved from https://mro.massey.ac.nz/bitstream/handle/10179/5209/02_whole.pdf?isAllowed=y&sequence=3
- Solak B (2013) Effect of processing parameters applied during the production of melting type cheese produced using different types of cheese on some characteristics of cheese. Doctoral thesis, Selcuk University, Graduate School of Natural and Applied Science, Konya, Turkey. Retrieved from http://acikerisimarsiv.selcuk.edu.tr:8080/xmlui/bitstream/handle/123456789/1315/335287.pdf?sequence=1&isAllowed=y
- Tunick MH, Cooke PH, Malin EL, Smith PW, Holsinger VH. Reorganisation of casein submicelles in Mozzarella cheese during storage. Int Dairy J. 1997;7:149–155. doi: 10.1016/S0958-6946(96)00057-X. [DOI] [Google Scholar]
- Yalcin O (2016) Determination of Probiotic Bacteria Addition on Microbial and Sensory Quality of Lor Whey Cheese. Master’s thesis, Balıkesir University, Graduate School of Natural and Applied Science, Balıkesir, Turkey. Retrieved from https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp
- Yazici F, Dervisoglu M, Akgun A, Aydemir O. Effect of whey pH at drainage on physicochemical, biochemical, microbiological, and sensory properties of Mozzarella cheese made from buffalo milk during refrigerated storage. J Dairy Sci. 2010;93(11):5010–5019. doi: 10.3168/jds.2009-2908. [DOI] [PubMed] [Google Scholar]