Abstract
The tocotrienols (T3) are the vitamin E family members with excellent antioxidant properties and prevent autocatalytic lipid peroxidation. Palm Fatty Acid Distillate (PFAD) is a volatile organic material obtained during palm oil refining and consists of tocol (α-tocopherol/T, α, γ, β, and δ- tocotrienol/T3) up to 0.8% and represents a potential source for recovering these valuable compounds. Various methods have been studied to extract vitamin E; however, only a few are commercially available. To fulfil the need for technology for recovering these minor components, leaching the tocotrienol from solidified PFAD was carried out. In this study, the fatty acid of PFAD was saponified using NaHCO3/Ca(OH)2 and extracted the unsaponifiable fraction using solvent. Further, fraction was purified by low temperature solvent based crystallization using hexane at 5 ± 1.0 °C for 24 h and removed sterol. The best determined parameters like reaction time (30 min), the ratio of PFAD to Ca(OH)2 (1,w/w), reaction temperature (30 °C) and stirring speed 300 rpm were yielded up to 98.6% fatty acid conversion to calcium soap and extraction with hexane obtained higher concentration of tocol (28.1%). The sterol separated from the tocol rich fraction by low temperature solvent crystallization contains up to 37.1% of phytosterol (β-sitosterol) concentration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05402-7.
Keywords: Calcium soap, Extraction, Palm fatty acid distillate, Palm oil, Tocotrienol
Introduction
Palm oil is one of the widely used vegetable oil that is rich in phytonutrients, and about 1% of that comprises of vitamin E, carotenoids, phytosterols, squalene, phospholipids (Estiasih et al. 2013). Palm oil contains almost 70–80% tocotrienol, of which 43% is γ-tocotrienol, 24% is α-tocotrienol, 11% is δ-tocotrienol, and remaining is 21% is α-tocopherol of vitamin E (Ahsan et al. 2015; Liu et al. 2008). Palm Fatty Acid Distillate (PFAD) is the volatile organic material obtained during palm oil deodorization, consisting of 0.8% tocotrienol and tocopherol, which is a potential source for recovering these valuable compounds (Posada et al. 2007). PFAD mainly contains 96.1% fatty acids and glycerides and other minor bioactive compounds such as tocopherols and tocotrienols, phytosterols, squalene, and other hydrocarbons (Estiasih et al. 2013). The tocotrienols (T3) are the members of vitamin E family with an excellent antioxidant property that prevent autocatalytic lipid peroxidation process, and also possesses various special properties like anti-carcinogenic, neuroprotective, and plasma cholesterol-lowering properties which are different from tocopherols (Ahsan et al. 2015; Liu et al. 2008; Seppanen et al. 2010). Nowadays, industry refines enormous amounts of crude palm oil (CPO), wherein, 100 tons of CPO produces about 3.66 ton PFAD. Usually, PFAD has been used for nonfood industries, mainly oleochemical industries, to make soap, feed, and oleochemicals (Ahsan et al. 2015). Therefore, several methods have been used for the extraction of vitamin-E such as solvent extraction, supercritical fluid CO2 extraction (SFE) (Nagesha et al. 2003), enzymatic method, chemical (saponification, transesterification) method, adsorption (Chu et al. 2003), molecular distillation (Posada et al. 2007), microwave-assisted extraction (Duvernay 2005), dry fractionation (Ngoc Doan et al. 2021), and membrane technology (Maarasyid et al. 2014). Malaysian Palm Oil Board (MPOB) has developed a product on vitamin E called “Palm Vitee” (Kasim et al. 2010; Nagao et al. 2005).
Nowadays molecular distillation is used for separation and purification of materials of different molecular weights. In this method, transesterification is carried out with alcohol in a suitable catalyst and distilled at a higher temperature. This process requires an extensive vacuum, water washing, and low recoveries of Vitamin E (15–57%) are reported. Process modification by using five molecular distillation stages or chemical modification has been done to improve this vitamin E recovery up to 80–90% (Nagao et al. 2005). Posada et al. carried out extraction of tocotrienol from palm fatty acid by molecular distillation, and the final product was obtained contains tocol 8.83% where it contains 6.63% of tocotrienol and 2.23% tocotrienol. The chemical modification like saponification and esterification with the three step distillation at high temperatures may degrade antioxidants or diminish their functionality (Posada et al. 2007). The bases used for the saponification are highly reactive can react with the both hydroxyl group of tocol and fatty acid and form water soluble soap, which is forms, micelles or emulsions would cause problems in separation of tocol fraction from the soap mixtures (Liu et al. 2008). Molecular distillation has some commercial production problems as it requires strict operational conditions coupled with multistage distillation, ultimately leading to higher investment costs. Another technology reported a dry fractionation process in which fractional crystallization was done at controlled temperature for separating solid fraction (stearin) and a liquid fraction (olein) to concentrate vitamin E from PFAD in olein fraction achieve 8% concentration of Vit E (Ngoc Doan et al. 2021). As this is a green process due to no solvent involvement, it requires controlled cooling programs and further purification, which may involve the chemicals. Extraction of high-purity concentrate of tocotrienols and tocopherols usually requires a series of physical and chemical treatment steps that are difficult to perform due to their complexity, inefficiency, or high cost (Posada et al. 2007; Yamamoto et al. 2015). Therefore, there is a need to develop a technology that is effective in recovering these minor but crucial components, improve time efficiency with safety products and cost-effectiveness, as well as environmentally friendly.
Other processes include the formation of complexes with the fatty acids present in the PFAD and converting the fatty acid salts, which are insoluble in organic solvents. This complex does not entrap the sterols, tocotrienols, tocopherols, and other bulky molecules present in the deodorizer sludge or distillate (Sampathkumar 1986). Using this fact, PFAD treated with calcium hydroxide/sodium bicarbonate or other bases like NaOH, KOH in the presence of a solvent medium containing water or an inert organic solvent to form calcium salts/ soap of the naturally occurring free fatty acids. These salts were precipitated and removed. After removing the calcium salts, the liquid phase's material contained the natural tocopherols and tocotrienols in a far more concentrated form than that present in the starting material. The saponification carried out with bases like NaOH & KOH leads to the formation of soapstock, which requires water washing, has high energy requirement, is time-consuming, and the production of a large amount of byproducts that pollute the environment. The water washing for soap removal can lead to the loss of unsaponifiable fractions and ultimately the tocols present. On the other hand, saponification of PFAD by using NaHCO3/Ca(OH)2, where the NaHCO3 is week base and that can selectively react with –OH of the carboxylic group, converts the PFAD to soap. Calcium ion precipitation of fatty acid to calcium salt gives hard, white or yellowish-white, semi-solids, or white powder structure. These salts have various applications as stabilizers, lubricants, dust removing agents, waterproofing agents, and auxiliary agents in diverse industries like plastic, paper, engineering, ceramic, and cosmetic industries. (Yamamoto et al. 2015; Handojo et al. 2018). Furthermore, it has been recently found that the calcium salts of fatty acids are useful for increasing the milk yield and the fat content in the milk. So it is now being used as an additive to the feed for cows (Handojo et al. 2018). Calcium salts are insoluble in water, ethanol, and ether. Due to this, the unsaponifiable matter present in a fatty substance can be extracted by some specified solvent, remains non-volatile under defined conditions of the test (Yamamoto et al. 2015; Ladole et al. 2018).
This study aims to develop a process for extraction and purification of the valuable tocotrienols from PFAD using solid–liquid extraction. The objective includes converting the fatty acid present in PFAD to calcium salt/ soap, which is insoluble in organic solvents and extracting the unsaponifiable matter using food-grade solvents. This study aims to identify the effect of different bases on converting fatty acid to salt/ soap with a minimum requirement of reagents to get the more tocotrienol containing fraction. Further purification of tocol fraction includes low temperature crystallization using hexane to separate sterols. β-sitosterol can be recovered as a by-product with good yield and high purity.
Materials and methodology
Materials
Palm fatty acid distillate, discharged from physical refining of palm oil, was provided by Muez Hest India Pvt. Ltd (Mumbai, Maharashtra) and Tocotrienol (40% pure) was kindly provided by Corotino SDN BHD (Malaysia) as a free sample. Sterol standards (Cholesterol, Stigmasterol, β- Sitosterol) were procured from m/s Sigma-Aldrich Chemical Company, India. Other chemicals used for the extraction were of extra pure grade and HPLC grade and were procured from m/s Thomas Baker Chemicals Pvt. Ltd. (Mumbai, Maharashtra).
Methodology
Physio-chemical properties and fatty acid composition of PFAD
Acid Value (AV) [Te 1a-64], free fatty acid content (FFA) [Ca 5a-40], saponification value (SV) [Cd 3–25], unsaponifiable matter (UM) [Ca 6a-40] and moisture and volatile matter [Ca 2c-25], were determined using standard methods of AOCS (Firestone 2009). Ce 1a-13 (AOCS) was used to analyze the fatty acid composition using Thermo scientific (GC1000) system. Fatty acid methyl esters were prepared according to the procedure Ce 2–66 (AOCS). The analysis was carried out using a capillary gas chromatograph equipped with a capillary column (BPX-70; 50 m × 0.22 mm, 0.2 μm film thickness). The oven temperature and detector temperature was maintained at 240 °C. Injector temperature: 185 °C, detector temperature: 200 °C; carrier gas: nitrogen; split ratio 1:50; injection volume: 1.0 μL. The qualitative composition was obtained by comparison of peak retention times with the respective fatty acids standards.
Identification and quantification of tocotrienol from PFAD
Standard stock solution of tocotrienol was prepared by dissolving 100 mg of tocotrienol standard in 100 mL of methanol and subsequently diluted to obtain a series of working standards, ranging from 10 to 200 ppm to construct a calibration curve. PFAD was heated to 40˚C in a water bath and stirred to make a homogenized mixture in a semi-solid state at room temperature (25 ± 2 °C). A known amount of homogenized PFAD/ UF/ TRF was diluted in methanol, mixed, degassed, and filtered (Nylon filters, 0.22 μm pore size) before the analysis.
The estimation of tocotrienol was carried out using an HPLC system of Thermo Fisher Scientific, connected to an injector with a 20 μL sample loop. The standard tocotrienol and sample preparation was used for HPLC analysis. The sample (20 μL) was injected into an HPLC column for the quantification of tocotrienol. The column used was a Hypersil Gold C18 (250 × 4.6 mm, 5 μm). The mobile phase was methanol (MeOH): Water (H2O) in the ratio 95:5 (v/v), respectively, which had a run time of 25 min with a flow rate of 1 mL/min. The tocotrienol content was detected at a wavelength of 295 nm using a UV detector and quantified based on peak area at particular retention time. The standard stock solution with variable dilution was analyzed by the HPLC method as described above, and calibration curves were made by plotting the area under the curve against the concentration (Ali and Nazzal 2009; Ko et al. 2008). The tocol concentration present in PFAD was calculated by correcting the concentration of tocol standard to 100%.
Determination of phytosterols
For the HPLC analysis, standard solution of sterols was prepared by dissolving 30–50 mg of tocotrienol standard in 100 mL of mobile phase (30:70, v/v, methanol: acetonitrile) and subsequently diluted to obtain a series of working standards, ranging from 10 to 200 ppm to construct a calibration curve. A known amount of PFAD/sterol rich fraction/ tocol rich fraction obtained after low temperature crystallization was diluted in mobile phase, mixed, degassed, and filtered (Nylon filters, 0.22 μm pore size) before the analysis. The HPLC–UV system of Thermo Fisher Scientific, connected to an injector with a 20 μL sample loop and a UV detector. Separation was performed in column Hypersil Gold C18 (250 × 4.6 mm, 5 μm), with 30:70 (v/v) methanol (MeOH): acetonitrile (ACN) at 1.2 mL/min as mobile phase and run time of 25 min. The sterol content was detected at a wavelength of 205 nm and quantified based on peak area at particular retention time. The standard stock solution with variable dilution was analyzed by the HPLC method as described above, and calibration curves were made by plotting the area under the curve against the concentration (Sanchez-Machado et al. 2004).
Conversion of fatty acid to salt/soap using calcium hydroxide (Ca(OH)2) or Sodium bicarbonate (NaHCO3)
In this study PFAD was heated to 40 °C to convert it into homogeneous liquid mixture, and then solvent ratio of 3.3, Hexane: PFAD (w/w) was added to dissolve all the fatty acid material into it. The conversion of fatty acid material to the salt/soap was carried out by adding the reactant, NaHCO3 in aqueous form or Ca(OH)2 added directly as solid in the PFAD mixture, with specified reaction parameters like reaction time (60 min), the ratio of alkali:PFAD (0.5, w/w), reaction temperature (30 °C), and stirring speed (500 rpm) as initially assumed parameters. The acid value change measured the conversion of the fatty acid to salt/soap before and after the reaction. After completion of the reaction the liquid fraction (unsaponifiable matters that dissolve in solvent) was separated from the mixture using centrifugation. Three times washing with adequate solvent was given to solid layer and collected liquid fraction containing tocols together. The solvent was evaporated from liquid fraction at 40 °C under vacuum and were analyzed for the tocol content in extracted unsaponifiable matter. In the case of reaction carried out with NaHCO3 as reactant, mixture after reaction was washed with water (1:10, PFAD:water, w/w) to remove water soluble soaps from mixture and get unsaponifiable matter in the solvent. For this study, only the tocols in the unsaponifiable matter were determined because the solid fraction is insoluble in methanol and tocol level is of unsaponifiable matter were consistently higher than those in solid fraction.
The effect of ratio of PFAD:Ca(OH)2, reaction time, temperature, and mixing speed on saponification by Ca(OH)2
Various reaction parameters like, ratio of reactant to PFAD (0.5 to 1.5, w/w), temperature (30–70 °C), time (10–50 min) and stirring speed (100-500 rpm) of reaction, which affects conversion of fatty acid to soap/salt and the extraction of tocotrienol with different solvents were studied to determine best conditions by varying a single parameter at a time. In this study, changed the ratio by considering the raw material contains 97% of FFA and glycerides and determined best parameters that affect the conversion of fatty acid and glycerides present in PFAD to salt.
Effect of various parameters on the extractability of tocol component from the calcium salt
Calcium hydroxide used as a reactant for converting the fatty acid present in the PFAD to salt is mainly from a strong base group compound, which can be reacting with hydroxide group present in the tocol components. So to identify the best ratio at which the calcium hydroxide gives higher conversion with a higher yield of the tocol in the product, varied the ratios to 0.5, 1.0 and, 1.5 with (Ca(OH)2:PFAD) (w/w). In this study, all best determined parameters were taken constant and only varied the ratio, as studied before and determined the tocotrienol content in the final product.
The solvents' effect on conversion and extraction of the tocotrienol was also studied by selecting different solvents that compatible in terms of solubility parameter, polarity index, and extractability from previous reports (Ko et al. 2008). The solvents used for the extraction were Ethyl acetate (EA), Methanol (MeOH), Acetone (AC), Acetonitrile (ACN), Ethanol (EtOH), and Hexane (He) with ratio of 3.3, solvent: PFAD (w/w). The same procedure of three-time washing, separation of two phases using centrifuge, and the evaporation of solvent followed by analysis of tocotrienol content in unsaponifiable matter using HPLC method were performed. Further, carried out extraction using hexane with different ratios 1.32, 2.64, 3.95, 5.27, and 6.59 of solvent:PFAD (w/w) and washing with same ratio for three times to identify an optimum solvent ratio which gives the highest yield of tocotrienol from the calcium salt. The solvent was then evaporated in a rotary vacuum evaporator and residue was analyzed for tocotrienol content using HPLC.
Low temperature solvent crystallization
The unsaponifiable fraction (UF) obtained after extraction with hexane was dissolved in hexane at different ratios hexane:UF of 3.30, 6.59, 9.89,13.2, and 16.5 (w/w), and added 10% water into it (based on the weight of UF) and mixed well using a stirrer for 30 min and then cooled at 5 ± 1.0 °C for 72 h. The crystallized product was separated by filtration and solvent traces removed by air drying to get crystalline phytosterols mixture (Khatoon et al. 2010). The filtrate obtained was collected, the solvent was evaporated in a rotary vacuum evaporator and residue was analyzed for tocotrienol content and sterol content. The sterol and tocol content in the crystals obtained also analyzed using HPLC.
Statistical analysis
The entire experiments were performed in triplicates and the results are expressed in mean ± standard errors. The data was analyzed by using SPSS version 16, One-way ANOVA was applied to test the mean differences and the statistical significance differences between mean values was established at P < 0.05 and Duncan’s new multiple range test.
Result and discussion
Physio-chemical properties and fatty acid composition of PFAD
PFAD is a dark yellow colored hard solid at room temperature, containing 81.4 ± 2.2 % of free fatty acids and 3.67 ± 0.1% unsaponifiable material which contains the various minor phytonutrients as shown in Table 1. The fatty acid composition of PFAD shows that palmitic acid is the major fatty acid (49.1%), followed by oleic acid (37.2%) present in PFAD (Posada et al. 2007). Estiasih et al. (2018) found that the tocol fraction of PFAD contains about 73% of tocotrienol, and major phytosterol is beta sitosterol which is about 89%.
Table 1.
Physio-chemical properties and typical composition of PFAD
| Parameter | Value |
|---|---|
| Physical appearance | Dark yellow, solid at RT |
| Acid value (mg KOH/g) | 171.0 ± 2.0a |
| Saponification value (mg KOH/g) | 198.4 ± 2.0a |
| Free fatty acid (%)(as palmitic acid) | 81.4 ± 2.2a |
| Glycerides content (%) | 14.4 ± 2.0b |
| Moisture and volatile matter (%) | 0.959 ± 0.01a |
| Unsaponifiable matter (%) | 3.67 ± 0.1a |
| Tocol (tocotrienol + tocopherol), (%) | 0.82 ± 0.2a, 0.5–0.8b |
| Phytosterols (%) | 0.4b, 0.35 ± 0.02a |
| Squalene (%) | 0.8b |
aExperimentally determined, bReported byTop (2010)
Identification and quantification of tocotrienol from PFAD
Standard calibration curve of tocol and phytosterols components
Plotted the standard curve by total concentration taken to the area obtained of tocol and phytosterols components separately and obtained their regression equations with coefficient (R2) of 0.952–0.999. The peaks obtained in HPLC in the sequence of δ, ϒ, α, β- tocotrienol, α- tocopherol are correlate with the results reported by Ali and Nazzal (2009) and the peaks obtained of sterol by HPLC method correlate to the results reported by Sánchez‐Machado et al. (2010).
Conversion of fatty acid to salt/ soap using calcium hydroxide (Ca(OH)2) or sodium bicarbonate (NaHCO3)
In some approaches, the authors carried out the saponification of PFAD using KOH and extracted the unsaponifiable matter using hexane. The UF is further purified by low temperature crystallization with hexane and obtained 18.31% of phytosterols (Ahmadi et al. 2018), and 21.81% tocotrienol (Ahmadi and Estiasih 2010, and Estiasih et al. 2013). Also the reaction carried out in present study of fatty acids with NaHCO3 converts PFAD to water soluble soap. The saponification carried out with these leads to the formation of soapstock, which requires water washing, has high energy requirement, is time-consuming, and the production of a large amount of byproducts that pollute the environment. The treatment carried out with 1 ratio of PFAD: NaHCO3 this treatment converts the 95–98% of fatty acid to soap and extracted the tocol fraction with hexane able to extract only 32–35 mg/g (3.2–3.5%) which seems the tocotrienol loss during the water washing. While the calcium hydroxide treatment makes the insoluble salts and due to its solid nature unsaponifiable matter can able to extract easily. In this study observed that the tocol concentration after both Ca(OH)2 and NaHCO3 treatment was 128 ± 0.4 mg/g (12.8%) and 32.2 ± 0.5 mg/g (3.22%) respectively. So from this observations obtained, taken Ca(OH)2 treatment for the further study.
Effect of various parameters on the conversion of fatty acid to calcium salt such as molar ratio, time of reaction, speed of mixing, temperature
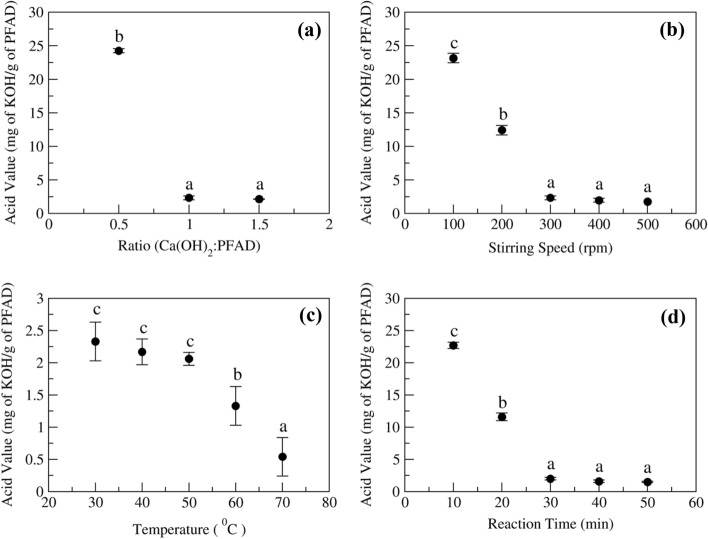
The optimization of reaction parameters was done by one factor at a time method, where firstly changed stoichiometric ratio to (Ca(OH)2: PFAD) 0.5, 1 and 1.5 (w/w) and other parameters taken as time, temperature and, speed constant to 60 min, 30 °C and, 500 rpm respectively. Figure 1a showed that at 0.5 ratios the acid value can be reduced to 24.26 ± 0.3 mg of KOH/g of PFAD which was initially 171.0 ± 2.0 mg of KOH/g of PFAD (Table 1) and proportional to 15.6 ± 0.1% and 81.4 ± 2.2% fatty acid (FA) as palmitic acid respectively. The changing in ratio to 1 and 1.5 the acid value gets reduced to 2.33 ± 0.3 and 2.14 ± 0.4 mg of mg of KOH/g of PFAD, 1.5 ± 0.3 and 1.4 ± 0.05% fatty acid respectively. which seems that these are very effective ratios for the conversion of fatty acid to salts and relates with reported previously (Handojo et al. 2018). A lower acid number was preferred and indicated a higher reaction conversion. The calcium salt prepared with 1.5 ratio requires more reactant and product containing unreacted calcium hydroxide, which reduces the product's quality for further application. On the other hand, the 1 ratio also shows a good conversion near 1.5 ratio with less reactant. On the basis of these observations and product requirements selected 1 ratio as best ratio (Handojo et al. 2018).
Fig. 1.
Effect of reaction parameters on conversion of fatty acid to calcium salt a Ratio (Ca(OH)2: PFAD, w/w); Fixed parameters: stirring speed- 500 rpm, temperature- 30 °C, reaction time- 60 min, b Stirring speed; Fixed parameters: ratio- 1 (w/w), temperature- 30 °C, reaction time- 60 min, c Reaction temperature; Fixed parameters: ratio- 1 (w/w), stirring speed- 300 rpm, reaction time- 60 min, and d Reaction time; Fixed parameters: ratio- 1 (w/w), stirring speed- 300 rpm, temperature- 30 °C. Means pursued by the same letter(s) within treatment do not differ significantly (p < 0.005) according to Duncan’s new multiple range test
In further study, changed another parameter which is agitation/mixing speed of the reaction. Figure 1b, shows that as with increase in the agitation can increase the rate of reaction to convert the fatty acid to calcium soap/salt. So carried out the reactions by varying the agitation from 100 to 500 rpm and other parameter are kept constant to ratio 1, reaction temperature 30 °C and time 60 min. The reaction carried out at 100 and 200 rpm showed incomplete conversion as it only reduce the acid value to 23.7 ± 0.7 and 12.41 ± 0.7 mg of KOH/g of PFAD, proportional to 14.6 ± 0.7 and 8.5 ± 0.2% fatty acid respectively. Further, increase in the speed to 300, 400 and 500 rpm reduced the acid value to 2.29 ± 0.2, 1.97 ± 0.3 and 1.76 ± 0.02 mg of KOH/g of PFAD, (1.4 ± 0.2, 1.3 ± 0.1 and 1.1 ± 0.1%, FA) respectively, where 300 rpm showed the maximum conversion of fatty acid to salt and above that speed increase in the speed not significantly differ the conversion.
Temperature is also a major factor that affects the reaction rate, so further considered temperature and determined best temperature for reaction. Figure 1c showed that, reaction at 30 °C converts maximum fatty acids material to salts as it reduces the acid value to 2.31 ± 0.4 mg of KOH/g of PFAD (1.5 ± 0.2%, FA), which on further increased in temperature decreased the acid value to 0.54 ± 0.3 mg of KOH/g of PFAD (0.35 ± 0.01%, FA) at 70 °C temperature. But reaction carried out at 30 °C shows 128.3 ± 4.0 mg/g (12.8%) of tocol concentration and on higher temperature showed only 34.3 ± 2.1 mg/g (3.43%) tocol concentration after extraction. It is because there are chances of thermal degradation at higher temperatures, and calcium hydroxide can react with a hydroxide group, reducing the tocotrienol yield (Kasim et al. 2010; Hiromori et al. 2016). A lower temperature (30 °C) is desirable to concentrate tocol in the final product as it minimizes the degradation of bioactive compounds and converts 98.6% of fatty acids to salt.
Reaction time is another factor that can affect the reaction rate, so the determination of best reaction time is carried out varying reaction time intervals from 10 to 50 min and using all other three parameters constant to their best determined values. The reaction carried out at 10 and 20 min showed low conversion of fatty acid to salt as it reduced the acid value to 22.7 ± 0.5 (14.6 ± 0.3%, FA) and 11.6 ± 0.6 mg of KOH/g of PFAD (7.5 ± 0.2%, FA), respectively. The 30 min time reaction effectively converts fatty acid to calcium salt as it reduces the acid value of product to 2.0 ± 0.2 mg of KOH/g of PFAD, (1.3 ± 0.2% FA) and further increase in time at 40, 50 min didn’t show significant reduction in acid value than 30 min reaction (Fig. 1d) shows shorter time required for the conversion.
Effect of various parameters on the extractability of tocol component from the calcium salt
Effect of ratio (Ca(OH)2:PFAD) (w/w) on tocol extraction
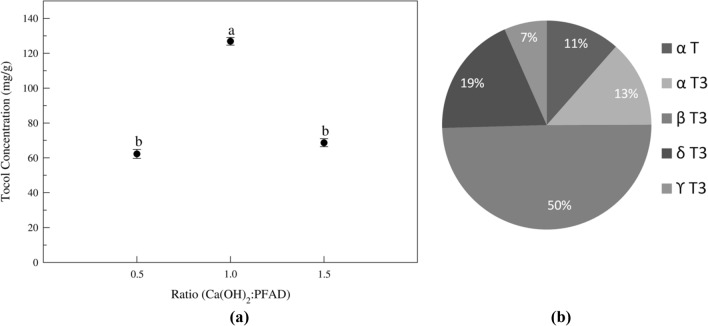
The stoichiometric ratio of calcium hydroxide to PFAD contributes to converting fatty acid to salt and the tocol concentration in the unsaponifiable matter. Figure 2a shows that at 0.5 ratio, it shows less extraction as all the fatty acid and glycerides present in the PFAD are not converted into the calcium salt and that unreacted fatty material get extracted with tocotrienol in the final product. The 1 ratio converts maximum fatty acids and glycerides to salt and due to this the concentration of the tocol in the final product increased to 128.3 ± 4.0 mg/g (12.8%) which was initially 8.2 ± 0.1 mg/g (0.82%). When further increased the ratio to 1.5 it converts nearly all the fatty acid and glycerides to salt. Still, as T3 structurally consists of phenolic –OH group and strong bases at higher concentration can react with phenolic –OH group which converts it to salts, due to these it showing decrease in the tocol content at 1.5 ratio. This implies that the alkali amount significantly affected the contents and recoveries of tocopherols and free phytosterols (Kasim et al. 2010; Ramamurthi and McCurdy 1993). The pie chart in Fig. 2b represents the percentage of component present in the extracted tocol fractions. The tocol rich fraction extracted and purified consist of 80% tocotrienols of total tocols concentration.
Fig. 2.
a Effect of (Ca(OH)2:PFAD) (w/w) ratio on tocol concentration. b Percent component in tocol fraction. Fixed parameters: stirring speed- 300 rpm, temperature- 30 °C, reaction time- 60 min. Means pursued by the same letter(s) within treatment do not differ significantly (p < 0.005) according to Duncan’s new multiple range test
As per the literature report, the presence of peroxyl and hydroperoxyl radicals, which are formed in the presence of oxygen, UV radiation, higher alkali and temperature, free radicals can oxidize/degrades the tocopherol (Kasim et al. 2010; Lushchak and Semchuk 2012). In this study, loss of tocol was reduced by carrying out the reaction in a short time, low temperature and adequate quantity of reactant.
Effect of solvents on the extraction of tocotrienol from the calcium salt prepared
As shown in Fig. 3a, studied 6 solvents which are suitable solvents for the extraction and showing all properties compatible like EA, MeOH, AC, ACN, EtOH and, HE. As shown in Fig. 3a, the hexane as solvent gives 130.4 ± 4.6 mg/g (13.04%) of total tocol components higher than other solvents as it has more extractability, hydrophobicity and non-polar than other solvents. The other solvents like acetonitrile and methanol also show good extraction capacity toward tocol fraction and ethyl acetate, showing the least extractability as it cannot penetrate the salt and provide for complete tocol extraction. The hexane results show more extractability of tocol fraction as it is more suitable solvent to extract lipid fractions than other solvents, and tocol are the lipid soluble fraction.
Fig. 3.
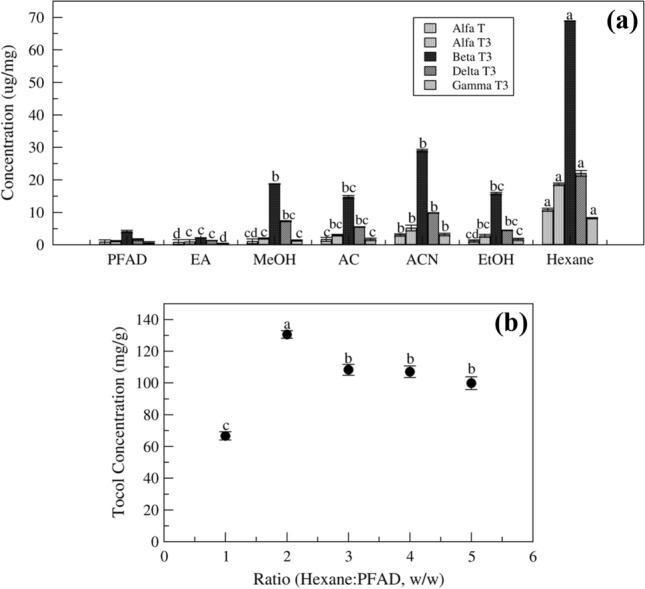
Effect of parameters on extraction of tocol a Type of solvent. b Hexane: PFAD (w/w) ratio; Fixed parameters; ratio- 1 (w/w), stirring speed- 300 rpm, temperature- 30 °C, reaction time- 60 min. Means pursued by the same letter(s) within treatment do not differ significantly (p < 0.005) according to Duncan’s new multiple range test
Effect of the solvent concentration on the extraction of tocotrienol from the calcium salt
In the previous solvent screening study, selected hexane as the best extraction solvent and used for further study. As per Fig. 3b, it can confirm that the increase the solvent to an adequate ration then only extractability of the tocol component increases above that it will decreases, where the hexane at 2.64 ratio give maximum (128.4 ± 3.4 mg/g, 12.8%) tocol extraction, and increase in solvent gives less extraction, it can be due to the more solvent tends to extract the salt or unreacted material. The 2.64 ratio of solvent is best for extracting tocol from salt and has less solvent requirement for the extraction.
Low temperature solvent crystallization
In this study, crystallization of sterols present in the unsaponifiable fraction (UF) was carried out at lower temperature (5 ± 1.0 °C) in presence of hexane. The strategic approach to purify T3 from the unsaponifiable fraction (UF) is based on differential solubility and polarity as the basis for solvent crystallization. As per the literature reports, sterols are more soluble in hexane at higher temperature but are less soluble at lower temperature, while the tocols are more soluble in hexane than sterols at lower temperature (Wei et al. 2010). The nature and composition of the solvent and cosolvent is the most important, which strongly influences both the separation/crystallization of sterol and tocol. Hexane with some amount water suited to give good crystallization as sterols tend to form hydrates with water which reduces their solubility in hexane (Moreira and Baltanas 2004).
Figure 4a–d, shows the effect of solvent ratio on the crystallization of sterol and tocol concentration at variable hexane ratios, where Fig. 4a gives the tocol concentration in the filtrate obtained after crystallization and filtration, it shows at hexane to UF ratio of 3.3 (w/w) gives 223.9 ± 2.2 mg/g (22.4%) tocol concentration in TRF, and increases to 280.9 ± 2.4 mg/g (28.%) which was initially up to 128 ± 4.0 mg/g (12.8%) in UF. On further increase in the solvent the tocol concentration in fraction decreases as presence of more solvent slower the crystallization of sterol, by solubilizing more sterol and other component.
Fig. 4.
Concentration of tocol and sterol after low temperature solvent crystallization with different solvent ratio (hexane:UF), a Concentration of tocols in tocol rich fraction (TRF), b Concentration of tocols in phytosterol rich fraction (PRF), c Concentration of phytosterol (β-sitosterol) in phytosterol rich fraction (PRF), d, Concentration of phytosterol (β-sitosterol) in tocol rich fraction (TRF). Means pursued by the same letter(s) within treatment do not differ significantly (p < 0.005) according to Duncan’s new multiple range test
Figure 4b shows the tocol present in the residue/crystals obtained after low temperature solvent crystallization followed by filtration. Low temperature solvent crystallization carried out at 3.3 ratio of hexane: UF shows more tocotrienol present in the sterol fraction (34.2 ± 0.7 mg/g, 3.42%), and further increase in the solvent ratio shows decrease in concentration at 6.6 ratio (5.7 ± 0.6 mg/g, 0.57%) but further increase in the ratio of solvent slightly increases tocol in crystals. From the Fig. 4a and b can say that adequate ratio of hexane to UF is 6.6, at which the tocol concentration in tocol rich fraction (TRF) is much higher; where in sterol crystals it is very less.
Figure 4c gives the phytosterols concentration in the crystals obtained, where it showing at 6.59 ratio gives more concentration 371.7 ± 7.7 mg/g (37.2%) which is showing maximum extraction of sterol from UF. It also showing the maximum extraction at moderate solvent ratio, as it reduces the tocol and sterol concentration with low solvent or more solvent ratios with UF and it is mainly due to the solubility’s of the tocol and sterol in the hexane, and their polarities. The crystallized product obtained after low temperature solvent crystallization followed by filtration did not have any bad odor and various useful properties because of that it can be used in foods, pharmaceuticals and cosmetics. This sterol fraction can be used in various applications in different food products such as spreads, yoghurt, fruit juices including fruit nectars and tomato juice, healthy functional foods, beverages and also cooking oils have been reported (Khatoon et al. 2010).
Figure 4d shows the concentration of sterol in the tocol rich fraction (TRF), which may shows that maximum sterol were removed from UF at 6.6 ratio of hexane to UF, as it shows 34.8 ± 1.6 mg/g (3.48%) concentration this is initially 171.7 ± 0.7 mg/g (17.2%). The hexane ratio 3.3 shows the higher sterol is present in the TRF (107.8 ± 1.6 mg/g, 10.8%), and the hexane ratio increase to 9.9 and above not reducing the sterol significantly than the ratio of 6.6, shows that the solvent: UF ratio of 6.6 (w/w) is moderate and giving maximum crystallization/ separation of sterol fraction from UF.
On the other hand, for any given ratios and obtained concentrations of tocol and sterol in TRF and sterol crystals vice versa, the use of a higher hexane:UF ratio (w/w) (9.9 or more) was not convenient, as recovery did not improve compared with lower ratio of 6.6. The use of surplus amounts of solvent increases the cooling and evaporation costs, which is having disagreeable for economic considerations (Moreira and Baltanas 2004). The final product obtained had 25.76% tocotrienols, 2.26% tocopherol, and 0.366% sterols. Despite the saponification of FFA to calcium salt, the product represents a concentration of 25.34%. The other components, which represent 46.27% of the product, corresponded probably to squalene and mono- and di-glycerides. And in the case of the by-product (PRF) obtained, it contains 37.06% of sterols, 0.65% tocol, and the remaining compounds correspond to FFA, squalene, and mono- and di-glycerides (Estiasih and Ahmadi 2018).
Conclusion
Extraction and purification of tocotrienol were carried out from PFAD using a solid–liquid extraction technique by converting PFAD into commercially important calcium salt. The saponification method was implemented using calcium hydroxide. The tocol fraction was extracted using hexane and further purified by low temperature solvent crystallization. The best determined conditions for tocol extraction was found at 1 ratio of (Ca(OH)2: PFAD), 300 rpm, 30 °C and 30 min of reaction time. The sterol was effectively removed from tocol fraction using low temperature solvent crystallization at 5 °C. The total concentration of tocol obtained after the removal of sterol was 280.9 ± 2.4 mg/g (28.1%) which contain about 80% tocotrienols and by-product obtained showed 371 ± 7.7 mg/g (37.1%) of phytosterol (β-sitosterol). The tocotrienols (T3) with enriched antioxidant properties are recovered from PFAD by simple, time efficient, cost-effective and environmentally friendly techniques.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to M/s. Muez-Hest India Ltd., Mumbai and Corotino SDN BHD, Malaysia for their generosity providing the palm fatty acid deodorized distillate and tocotrienol samples. The authors are grateful to M/s. Marico Ltd. and Prime Minister’s Fellowship Scheme for Doctoral Research, a public-private partnership between Science & Engineering Research Board (SERB), Department of Science & Technology (DST), Government of India (GOI) and Confederation of Indian Industry (CII) for funding this research.
Author contributions
Amol J. Gore and Sunil S. Bhagwat designed the experimental scheme and ideas; Amol J. Gore finished the experiment, data curation, formal analysis, methodology, validation, and the first draft of the article; Sunil S. Bhagwat supervised all the experiments and data; Amol J. Gore and Sunil S. Bhagwat revised and improved the article.
Authors declare that (i) the work described has not been published before (except in the form of an abstract, a published lecture or academic thesis), (ii) it is not under consideration for publication elsewhere, (iii) its submission to JFST publication has been approved by all authors as well as the responsible authorities tacitly or explicitly at the institute where the work has been carried out, (iv) if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder, and (v) JFST will not be held legally responsible should there be any claims for compensation or dispute on authorship.
Funding
There was no funding for this work.
Declarations
Conflict of interest
The authors declare there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadi K, Oktafa H, Estiasih T. The effect of phytosterol-rich fraction from palm fatty acid distillate on blood serum lipid profile of dyslipidaemia rats. J Diet Suppl. 2018;15:728–739. doi: 10.1080/19390211.2017.1406025. [DOI] [PubMed] [Google Scholar]
- Ahmadi K, Estiasih T (2010) Optimizing crystallization conditions for obtaining tocotrienol enriched fraction from palm fatty acids distillate. International Oil Palm Conference, Yogyakarta, Indonesia pp 1–3
- Ahsan H, Ahad A, Siddiqui WA. A review of characterization of tocotrienols from plant oils and foods. J Chem Biol. 2015;8(2):45–59. doi: 10.1007/s12154-014-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H, Nazzal S. Development and validation of a reversed-phase HPLC method for the simultaneous analysis of simvastatin and tocotrienols in combined dosage forms. J Pharm Biomed Anal. 2009;49(4):950–956. doi: 10.1016/j.jpba.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Chu BS, Baharin BS, Quek SY, Man YBC. Separation of tocopherols and tocotrienols from palm fatty acid distillate using hydrolysis-neutralization-adsorption chromatography method. J Food Lipids. 2003;10(2):141–152. doi: 10.1111/j.1745-4522.2003.tb00011.x. [DOI] [Google Scholar]
- Duvernay WH, Assad JM, Sabliov CM, Lima M, Xu Z. Microwave extraction of antioxidant components from rice bran. Pharm Eng. 2005;25(4):126. [Google Scholar]
- Estiasih T, Ahmadi K. Bioactive compounds from palm fatty acid distillate and crude palm oil. IOP Conf Ser: Earth Environ Sci. 2018;131:012–016. doi: 10.1088/1755-1315/131/1/012016. [DOI] [Google Scholar]
- Estiasih T, Ahmadi K, Widyaningsih TD, Maligan JM, Mubarok AZ, Zubaidah E, Mukhlisiyyah J, Puspitasari R. Bioactive compounds of palm fatty acid distillate (PFAD) from several palm oil refineries. Adv J Food Sci Technol. 2013;5:1153–1159. doi: 10.19026/ajfst.5.3074. [DOI] [Google Scholar]
- Firestone D (2009) Official methods and recommended practices of the AOCS. AOCS.
- Handojo LA, Indarto A, Shofinita D, Meitha A, Nabila R, Triharyogi H. Calcium soap from palm fatty acid distillate (PFAD) for ruminant feed: Quality of calcium source. MATEC Web Conf. 2018;156:10–13. doi: 10.1051/matecconf/201815602007. [DOI] [Google Scholar]
- Hiromori K, Shibasaki-Kitakawa N, Nakashima K, Yonemoto T. Novel simple process for tocopherols selective recovery from vegetable oils by adsorption and desorption with an anion-exchange resin. Food Chem. 2016;194:1–5. doi: 10.1016/j.foodchem.2015.07.137. [DOI] [PubMed] [Google Scholar]
- Kasim NS, Gunawan S, Yuliana M, Ju YH. A simple two-step method for simultaneous isolation of tocopherols and free phytosterols from soybean oil deodorizer distillate with high purity and recovery. Sep Sci Technol. 2010;45(16):2437–2446. doi: 10.1080/01496391003789171. [DOI] [Google Scholar]
- Khatoon S, Rajan RR, Krishna AG. Physicochemical characteristics and composition of Indian soybean oil deodorizer distillate and the recovery of phytosterols. J Am Oil Chem Soc. 2010;87(3):321–326. doi: 10.1007/s11746-009-1499-8. [DOI] [Google Scholar]
- Ko SN, Lee SM, Kim IH. The concentration of tocols from rice bran oil deodorizer distillate using solvent. Eur J Lipid Sci Technol. 2008;110(10):914–919. doi: 10.1002/ejlt.200700310. [DOI] [Google Scholar]
- Ladole MR, Nair RR, Bhutada YD, Amritkar VD. Ultrasonics - Sonochemistry Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason Sonochem. 2018;48:453–462. doi: 10.1016/j.ultsonch.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Liu D, Shi J, Posada LR, Kakuda Y, Xue SJ. Separating tocotrienols from palm oil by molecular distillation. Food Rev Int. 2008;24(4):376–391. doi: 10.1080/87559120802303840. [DOI] [Google Scholar]
- Lushchak VI, Semchuk NM. Tocopherol biosynthesis: chemistry, regulation and effects of environmental factors. Acta Physiol Plant. 2012;34(5):1607–1628. doi: 10.1007/s11738-012-0988-9. [DOI] [Google Scholar]
- Maarasyid C, Muhamad II, Supriyanto E. Potential source and extraction of Vitamin E from palm-based oils: A review. J Teknol. 2014;69(4):43–50. [Google Scholar]
- Moreira EA, Baltanás MA. Recovery of phytosterols from sunflower oil deodorizer distillates. J Am Oil Chem Soc. 2004;81(2):161–167. doi: 10.1007/s11746-004-0875-x. [DOI] [Google Scholar]
- Nagao T, Kobayashi T, Hirota Y, Kitano M, Kishimoto N, Fujita T, Shimada Y. Improvement of a process for purification of tocopherols and sterols from soybean oil deodorizer distillate. J Mol Catal B Enzym. 2005;37(1–6):56–62. doi: 10.1016/j.molcatb.2005.09.005. [DOI] [Google Scholar]
- Nagesha GK, Manohar B, Udaya Sankar K. Enrichment of tocopherols in modified soy deodorizer distillate using supercritical carbon dioxide extraction. Eur Food Res Technol. 2003;217(5):427–433. doi: 10.1007/s00217-003-0781-5. [DOI] [Google Scholar]
- Ngoc Doan P, Tan T, Siow LF, Tey BT, Chan ES, Tang T, Abdul Karim NA, Phuah E, Lee Y. Dry fractionation approach in concentrating tocopherols and tocotrienols from palm fatty acid distillate: a green pretreatment process for Vitamin E extraction. J Am Oil Chem Soc. 2021;98:609–620. doi: 10.1002/aocs.12488. [DOI] [Google Scholar]
- Posada LR, Shi J, Kakuda Y, Xue SJ. Extraction of tocotrienols from palm fatty acid distillates using molecular distillation. Sep Purif Technol. 2007;57(2):220–229. doi: 10.1016/j.seppur.2007.04.016. [DOI] [Google Scholar]
- Ramamurthi S, McCurdy AR. Enzymatic pretreatment of deodorizer distillate for concentration of sterols and tocopherols. J Am Oil Chem Soc. 1993;70(3):287–295. doi: 10.1007/BF02545310. [DOI] [Google Scholar]
- Sampathkumar (1986) Process for recovering tocopherols from deodorizer sludge, U. S. Patent 4594437, 1073–1078.
- Sánchez-Machado DI, López-Hernández J, Paseiro-Losada P, López-Cervantes J. An HPLC method for the quantification of sterols in edible seaweeds. Biomed Chromatogr. 2004;18(3):183–190. doi: 10.1002/bmc.316. [DOI] [PubMed] [Google Scholar]
- Seppanen CM, Song Q, Saari Csallany A. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J Am Oil Chem Soc. 2010;87(5):469–481. doi: 10.1007/s11746-009-1526-9. [DOI] [Google Scholar]
- Top AGM. Production and utilization of palm fatty acid distillate (pfad) Lipid Technol. 2010;22(1):11–13. doi: 10.1002/lite.200900070. [DOI] [Google Scholar]
- Wei D, Wang L, Liu C, Wang B. β-Sitosterol solubility in selected organic solvents. J Chem Eng Data. 2010;55(8):2917–2919. doi: 10.1021/je9009909. [DOI] [Google Scholar]
- Yamamoto Y, Kawamura Y, Yamazaki Y, Kijima T, Morikawa T. Palmitoleic acid calcium salt: A lubricant and bactericidal powder from natural lipids. J Oleo Sci. 2015;64(3):283–288. doi: 10.5650/jos.ess14176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.