Abstract
Intensification in synthesis of triglycerides of octanoic acid using a heterogeneous amberlyst-15 catalyst has been investigated with the application of ultrasound under solvent-free conditions. Further, the frying characteristics of medium-chain triglycerides (tricaprin) are evaluated by deep frying of French fries in various combinations of palm oil and tricaprin. Understanding into the effect of parameters such as the temperature of the reaction (over the range of 40 °C–80 °C), loading of amberlyst-15 (1%–5%) and molar ratio of fatty acid to glycerol (3:1–3:5) along with the ultrasound conditions as duty cycle (40%–90%) and time on the conversion (%) has also been developed. Based on the outcomes of the study, optimum reaction conditions seen are 3:4 as the molar ratio of fatty acid (C8): glycerol, amberlyst-15 loading of 3% and a reaction temperature of 50 °C. It was further observed that the optimum ultrasound conditions required for maximum conversion of 99.8% were 240 W power, 80% duty cycle and 15 min as the ultrasound irradiation time. Under similar conditions, the conventional synthesis resulted in only 20% conversion in 15 min. Reusability studies also established that the acid–resin catalyst was effectively reused for 8 times. The PV, p-AV and TPC of frying oil combination containing higher tricaprin (50:50–palm oil: MCT) was 8.4 ± 0.8, 23.1 ± 0.01 and 29.8 ± 0.01 respectively, which were lowest as compared with other blends indicating the beneficial effects of MCT in frying applications. The work clearly shows that the ultrasound application for the synthesis of triglyceride of octanoic acid gives higher conversion (%) in a less time and also demonstrates that MCT could be a good alternative for deep frying of foods in combination with palm oil to enhance the shelf-life of food.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05379-3.
Keywords: Octanoic acid, Ultrasound, Amberlyst-15, Medium chain triglyceride, Deep-fat frying, Palm oil
Introduction
Octanoic acid is a medium chain fatty acid and the triglycerides of octanoic acid are known as medium chain triglycerides. Nowadays researchers are attracted towards medium chain triglycerides (MCT) due to its health friendly characteristics. MCT is believed as a chief source of energy and is used in infant foods. It is popularly used for weight loss and is also used in various clinical nutritions (Marten et al. 2006). Most of the fat we eat contain long chain triglycerides, though these long chain triglycerides are often related to increased health risk, and hence there is demand for fat/oil which is health friendly. MCT are triglycerides containing medium chain fatty acids esterified to glycerol at all the three positions and considered as a neutraceutical fat with various health benefits. Medium chain triglycerides are metabolized in human body, absorbed easily and it provides immediate energy and also not stored in body as a fat (You et al. 2008) (Marten et al. 2006).
MCT are used in food formulation as a blend with other oils. Medium chain triglycerides are also used in synthesis of interesterified fat, to improve the physicochemical characteristics and stability of interesterified lipid towards oxidation (Jadhav et al. 2021b,c, 2022). The only natural source of medium chain fatty acids is coconut oil, palm kernel oil and to some extent bovine milk. Fractionation of coconut oil is typically done to separate the fatty acids which are further processed to synthesize medium chain triglycerides (Marten et al. 2006). Synthetically medium chain triglycerides are obtained by esterification reaction between glycerol and fatty acid using chemical catalyst or using enzyme. Many studies have shown activity of enzymes for enzymatic production of medium chain triglycerides. Kim and Rhee (1991) reported slow activity of immobilized lipase enzyme for production of medium chain triglyceride without using any solvent at 40 °C. Kwon et al. (1996) reported activity of 11 lipases from different microbial origin for synthesis of triglyceride of capric acid in organic solvent. It was found that enzymatic synthesis of MCT is slow and takes much time, and greatly influenced by the reaction temperature. Some enzymes showed highest activity at particular temperature whereas other enzymes did not show such activity at that temperature. It was also reported that Lipases from Chromobactarium viscosum and Rhizomucor miehei showed highest activity for production of tricaprin synthesis in 18 h of reaction, while lipases from Aspergillus niger, Rhizopus javanivus and Rhizopus delemar showed highest activity for production of dicaprin.
Enzymes used for enzymatic synthesis of medium chain triglycerides are very much specific to reaction conditions and function only in narrow range of temperature (Kwon et al. 1996; Jadhav and Annapure 2021). In place of organic solvent, ionic liquids are also reported to be used for production of medium chain triglycerides to increase the activity of enzyme (Pan et al. 2013). Due to high cost and slow reaction of enzyme, another pathway to overcome this is using chemical catalyst. The chemical catalysts include metal catalysts like calcium oxide or zinc oxide (Boulos 2013). Medium chain triglycerides are also synthesized using acid catalyst like sulphuric acid and hydrochloric acid though at higher temperature. There are many limitations associated with use of metal catalyst as they are expensive, need purification step for removal from reaction mixture and show higher activity only at higher temperature. Similarly, acid catalyst like sulphuric acid gives dark product which additionally requires bleaching of reaction mass and also the removal of acid catalyst from reaction is more troublesome. The present study focuses on use of green, energy efficient and environment friendly process based on the heterogeneous reusable catalyst as amberlyst-15 for synthesis of triglyceride of octanoic acid without solvent. Amberlyst-15 is being reported as one of the effective heterogeneous catalysts for esterification reaction (Sirsam et al. 2016) though it offers mass transfer resistances giving slow processes and hence importance of ultrasound applied as process intensification approach is established.
Ultrasound are sound waves which exceeds audible frequency range (more than 20 kHz). It is a kind of vibrational energy which is dissipated by an transducer having capability of transforming electrical energy into acoustic energy (Li et al. 2021). The acoustic wave transmits through a reaction medium and generates compressions and rarefaction cycles in the medium. This process releases huge amount of energy on collapse of generated cavities and generates high turbulence in the medium, thus increasing rate of mass transfer and also pushing reaction in forward direction (Chatel and Colmenares 2017). Based on frequency, ultrasound is classified as power ultrasound (20–100 kHz), high-frequency ultrasound (100 kHz–1 MHz) and diagnostic ultrasound (1–500 MHz). Ultrasound with frequency range from 20–100 kHz is usually preferred in food processing and also in organic synthesis. In the case of lower frequency ultrasound, there is formation of number of cavities which collapse violently and generate dominant shock waves and turbulence as the physical effects (Jadhav et al. 2021a; Singla and Sit 2021) facilitating applications limited by mass transfer.
Immersed frying or deep fat frying requires large amount of oil, which is absorbed in food while frying of food, accounting for as high as 40% of total weight. The oil used for frying mainly includes palm oil, cottonseed oil, sunflower oil, groundnut oil, soyabean oil etc. and all these forms usually contains long chain triglycerides. When foods are subjected to frying in such oil at high temperature, it results in thermal deterioration of oil or fat. Many efforts are made to minimize this deterioration and minimize the amount of oil absorption in food. MCT having numerous health benefits can be used as a good frying oil in combination with other oil like palm oil. Consumption of Medium chain Triglycerides is also associated with the high satiety value (Kinsella et al. 2017). MCT cannot be used as a complete substitute to frying oil because of presence of medium chain fatty acids which have shorter chain length which tend to form foam (Takeuchi et al. 2008) and hence a combination of MCT and oil is generally recommended. In summary, the main intention of the current research is to formulate a greener and environment friendly process for synthesis of medium chain triglycerides by using reusable heterogeneous catalyst, energy efficient ultrasound process to decrease the reaction time and temperature and to get maximum conversion of reactants. In addition, frying characteristics of medium chain triglycerides in combination with palm oil have been evaluated.
Materials and methods
Materials
Octanoic acid (C8), glycerol and amberlyst-15 were purchased from S. D. Fine chemicals Pvt. Ltd. Mumbai, India. Palm oil and potatoes were purchased from local market in Mumbai.
Experimental methodology
Setup for conventional approach
The experimental setup consists of 100 mL three-neck flask, magnetic needle for uniform mixing of reaction mixture, vacuum pump to remove water formed during reaction, water cooled condenser to condense vapours, chiller to supply chilled water to condenser, stopper and heater for heating the flask to achieve the desired temperature.
Setup for ultrasound based approach
Ultrasonic horn working at 22 kHz and 240 W (calorimetric efficiency of 7% meaning actual power dissipated into solution is 16.8 W) was obtained from M/S Dakshin, Mumbai, India. The tip diameter of horn used in synthesis was 2 cm. The actual reaction between octanoic acid and glycerol was carried out in a 250 mL beaker with the horn inserted at the centre of the vessel. Beaker containing reaction mixture along with magnetic needle was immersed in water bath and magnetic stirrer with agitation speed of 300 rpm was applied for mixing of immiscible liquid phases.
Experimental procedure
Octanoic acid was taken in 250 mL reaction vessel followed by addition of glycerol on the basis of selected molar ratio of reactant. Initial sample was withdrawn to estimate initial acid value. The desired temperature was obtained by heating and catalyst amberlyst-15 added to reaction mixture under stirring. The beaker containing reaction mixture was kept in water bath to maintain constant temperature. Magnetic stirrer was used to stir the reaction mixture so that uniform mixing is achieved. In the case of ultrasound assisted operation, horn tip was immersed in the reaction mixture so that the tip of horn is 10 mm away from the point of intersection between two layers. An aliquot was withdrawn from reaction mixture at regular intervals for both conventional and ultrasound assisted process to calculate acid value and monitor the progress of reaction. The influence of various reaction parameters such as temperature over the range of 40 °C–80 °C, molar ratio of octanoic acid (C8) to glycerol over the range of 3:1 to 3:6 (moles:moles) and amberlyst-15 loading over 1% to 5% (by weight of fatty acid) was investigated.
For the reusability studies, after completion of reaction, the acid resin catalyst was separated from the product by filtration through whatmann filter paper no 1. The separated acid resin catalyst was washed with hexane and after washing, it was kept in oven (70 °C for 3 min) to remove the traces of solvent that could be present. Subsequently, catalyst was used for another reaction and then the process repeated in multiple cycles to check the reusability of the catalyst.
Frying of French fries
To check frying characteristics of medium chain triglyceride, French fries were fried in four different palm oil and MCT combinations with proportions (palm oil: MCT) as 70:30, 50:50, 30:70. Initially for preparation of French fries, potatoes were thoroughly washed with water and then the outer skin was peeled followed by slicing of potatoes in a proper shape of 1 cm thick and 6 cm long. 150 mL frying oil was added in fryer and heated up to 180 ± 5 °C. During the deep frying, 50 g of French fries were deep fried for 4 min. Fried oil after single frying cycle was stored at -18 °C for further analysis. Oil extracted from food subjected to single frying cycle was extracted by Soxhlet extraction and also stored at -18 °C for further analysis. All frying trials were conducted in triplicates.
Analysis
Estimation of presence of free fatty acids in reaction mixture
American Oil Chemists' Society (AOCS) official method was used to estimate presence of free fatty acid in the reaction mixture. The method involves taking 1 g of sample to which 30 mL neutralized ethyl alcohol (95%) and about 1 mL of phenolphthalein indicator is added and then titrated against standard KOH solution shaking the beaker vigorously during titration. The end point is reported from colourless to light pink. Calculations are done using formula:
where W = weight of sample in grams; N = Normality of KOH solution; V = Volume of KOH used during titration in mL.
Fourier transform infrared analysis
Fourier transform infrared spectra was obtained using FTIR-8400S Shimadzu spectrometer, Japan. The FTIR was equipped with miracle 10 single reflection attenuated total reflectance accessory for sampling. Before injection of sample, the sample injection port was cleaned thoroughly with ethyl alcohol to remove impurities and then sample diluted in acetone was placed on the sample holder cell for IR determination.
HPLC analysis
The reaction proceeds with formation of mono and di glycerides initially which are then converted to triglycerides. The formation of the triglyceride was checked using Shimadzu HPLC2050, Japan equipped with RI detector. The column used for analysis of triglycerides was HiQSil C18 having dimensions of 4.6 mm as the ID and 250 mm as Length. The flow rate of mobile phase was maintained constant at 1 mL/minute. Acetic acid and acetonitrile (6:94 V/V) solution was the applied mobile phase. Quantity of sample introduced for the analysis was 2µL.
Estimation of rate constant
The esterification of octanoic acid with glycerol in the presence of amberlyst-15 forms triglycerides of octanoic acid with removal of water as a co-product. In the present work one of the reactants is used in excess and thus we assume pseudo second order kinetic model for calculation of rate constant as given below:
| 1 |
where CA is the concentration of octanoic acid in mol/L, t is the reaction time in min, rA is the reaction rate in mol/(L min) and k is the rate constant in L/(mol min). When we integrate Eq. (1), we get following equation:
| 2 |
Substituting the definition of conversion and rearranging of the above equation gives Eq. (3)
| 3 |
where CA0 is initial concentration of octanoic acid in mol/L and X is conversion of octanoic acid at given time t. The straight line is obtained, if the assumed model works for the esterification reaction when we plot X/(1 − X) versus t. The slope of the obtained plot is used to obtain the value for rate constant.
Analysis of fried French fries and residual used oil
Total absorbed oil in French fries
The fried French fries were crushed into small pieces. About 10 g of crushed French fries was kept in a paper thimble. The thimble was then loaded in the main chamber of soxhlet extractor. 250 mL petroleum ether was placed in a 500 mL round bottom flask (RBF). The RBF was then attached to extractor and condenser. The solvent in RBF was heated using heating mantle. After heating, the solvent vapours travel up a distillation arm and falls on thimble. The oil is extracted in the solvent, and the process of soxhlet extraction was carried out for 8 h. The solvent was separated from extracted oil using rotary evaporator at 40 °C. The extracted oil was used for further analysis.
Peroxide value of extracted oil
Peroxide value gives an indication of rancidity in sample of oil. Peroxide value was determined as per the AOCS standard procedure. 5 g of oil sample was taken in a conical flask and then mixed with 3:2 acetic acid: Chloroform solution along with 0.5 mL saturated potassium iodide. The resultant mixture was kept in dark for 1 min for liberation of iodine. Subsequently the solution was mixed with 30 mL distilled water and then titrated with 0.01 N Sodium Thiosulphate in the presence of starch indicator. The following formula was used to calculate the peroxide value of the titrated sample of oil.
Viscosity
Viscosity of all combinations of palm oil: MCT and palm oil was measured after first frying cycle. Viscosity was measured using Brookfield viscometer DV II model, Mumbai with LV 2 Spindle under shear rate range of 0–100 s−1.
Total polar compounds
Total polar compounds in oil extracted from French fries and residual used fried oil after first frying were determined using chromatographic techniques. The oil sample extracted using Soxhlet technique was used for analysis performed using technique reported in literature (Bhattacharya et al. 2008). The chromatographic column was 450 mm long and 21 mm in internal diameter. The column was packed with 30 mL solvent which was mixture of petroleum ether and diethyl ether in the ratio of 80:20. The percentage TPM was evaluated using following equation
p-Anisidine value
Using the AOCS Official Method Cd 18-90 (1998), p-Anisidine value of oil extracted from French fries and left-over fried oil was estimated. 0.5 g of oil sample was diluted with iso-octane in a 25 mL volumetric flask. Two test tubes were taken i.e., one for sample and one for blank. Sample test tube was filled with 5 mL sample and to blank test tube, only solvent was added. This was followed by addition of p-Anisidine reagent to both the test tubes. The reagent was mixed thoroughly by shaking test tubes. The absorbance of solutions in both the test tubes was measured at 350 nm after 10 min. The p-Anisidine value was calculated using following equation.
Colour of oil
Colour of oil was evaluated using AOCS Official Method Cc 13b-45 (1989). Tintometer Model F, Lovibond, Mumbai was used for colour analysis of oil. All the oil samples were heated at 45 °C and mixed well. 12 mL of oil sample was poured in sample vial and sample vial was fixed at sample holding cell. The same process was used for all the oil samples. All the oil samples were analysed using red and yellow scale.
Reproducibility and statistical analysis
In order to check the repeatability of data obtained in the experiments, the synthesis of octanoic acid was repeated at least three times. The data presented in graphs and given in result and discussion sections is average of three values obtained. It was seen that the variation in the values was ± 2% of the reported values as also represented in figures in terms of error bars. Statistical analysis of data was also done using single factor ANOVA using Minitab 16. The results were considered significant for P < 0.05.
Results and discussion
Effect of molar ratio
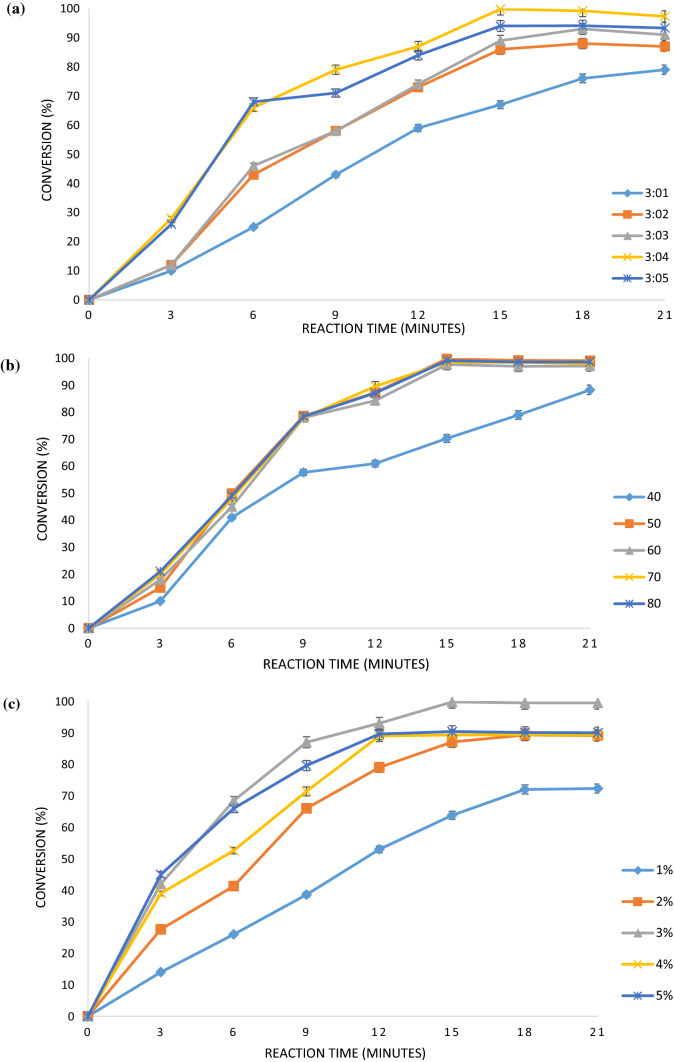
Ideally one mole of glycerol requires three moles of fatty acid to be esterified properly at three positions in glycerol under the stoichiometric proportions. In present study, different molar ratios of octanoic acid: glycerol (3:1–3:5) were applied to investigate its effect on rate of reaction. While studying effect of molar ratio, the temperature and amberlyst-15 loading was kept constant at 50 °C and 3% respectively. The obtained results are graphically represented in Fig. 1a where it is observed that the conversion of reactant is affected by change in molar ratio of reactant till an optimum ratio of octanoic acid: glycerol as 3:4, beyond which no appreciable increase is observed. From the data represented in Fig. 1a, it is clear that initially there is increase in conversion with increase in molar ratio because of increase in rate of forward reaction due to presence of glycerol in excess. Increase in molar ratio of one of the reactants i.e., glycerol results is absorbing water formed during reaction. This phenomenon reduces chances of reaction to be reversible and equilibrium shifts towards right and conversion of fatty acid increases. In earlier studies, Deshmane et al. (2008) described that conversion of esterification reaction between lauric acid and glycerol was 98.5% using glycerol in optimum excess at 90 °C and in 6 h treatment. Another study by Mohod and Gogate (2017) also reported 97.8% conversion for the reaction between lauric acid and glycerol with use of excess glycerol in the reaction mixture in 1:10 (lauric acid: glycerol) proportions in 120 min and at 90 °C.
Fig. 1.
Effect of Operating parameters on the conversion (%) of the esterification process a molar ratio of octanoic acid: glycerol b reaction temperature and c amberlyst-15 loading
Effect of reaction temperature
Esterification reaction temperature effect was studied over the range of 40 °C–80 °C. During this study, molar ratio and catalyst loading was kept constant as 3:4 (octanoic acid: glycerol) and 3% respectively. The impact of temperature on conversion of reaction is illustrated in Fig. 1b. It can be observed from Fig. 1b that a rise in temperature increases rate of reaction and also equilibrium conversion. Highest conversion is observed at 50 °C which may be due to the fact that increase in temperature enhanced the ability of glycerol to absorb water and reaction goes in forward direction. The rise in reaction temperature also favours good mixing of two phases of glycerol and fatty acid thereby increasing the rate of reaction. Increasing in temperature beyond 50 °C showed marginal decrease in the conversion which may be due to occurrence of excessive cavitation events and generation of vaporous cavities that collapse with lower intensity. Mohod and Gogate (2017) also described similar effect of reaction temperature on yield of reaction for production of medium chain triglyceride of lauric acid with the maximum conversion demonstrated at 80 °C. The observed difference in the optimum confirmed the imperative nature of the reported results in the current work.
Effect of catalyst loading
Effect of amberlyst-15 loading was also studied (range of 1% to 5%) at constant temperature of 50 °C and molar proportions of octanoic acid: glycerol as 3:4. The results for the impact of amberlyst-15 loading on the esterification reaction is illustrated in Fig. 1c. From the figure, it is found that for a positive change in the amberlyst-15 loading from 1 to 3%, conversion enhanced dominantly for both the approaches of conventional and ultrasound assisted. It has been seen from the study that the maximum conversion is observed under conditions of temperature as 50 °C, molar ratio as 3:4 and catalyst loading 3% in the case of ultrasound process. In the case of conventional process, the maximum conversion of 89% is seen with same Amberlyst loading of 3%, but in higher reaction time of 29 h, whereas in ultrasound assisted process conversion of 99.8% is seen only in 15 min of reaction time. Initially, the catalyst provides more active sites to substrate and hence the conversion dominantly increases up to optimum concentration of 3%. Beyond 3%, there is no observable effect on the rate of conversion. More et al. (2017) studied effect of sulphuric acid and hydrochloric acid loading on the rate of reaction. It was demonstrated that the increase in catalyst concentration increases rate of reaction for synthesis of triglyceride of caprylic acid. About 96.6% yield of tricaprylin was reported with 1% optimum loading of sulphuric acid at 170 °C and 540 min of reaction.
Effect of ultrasonic parameters
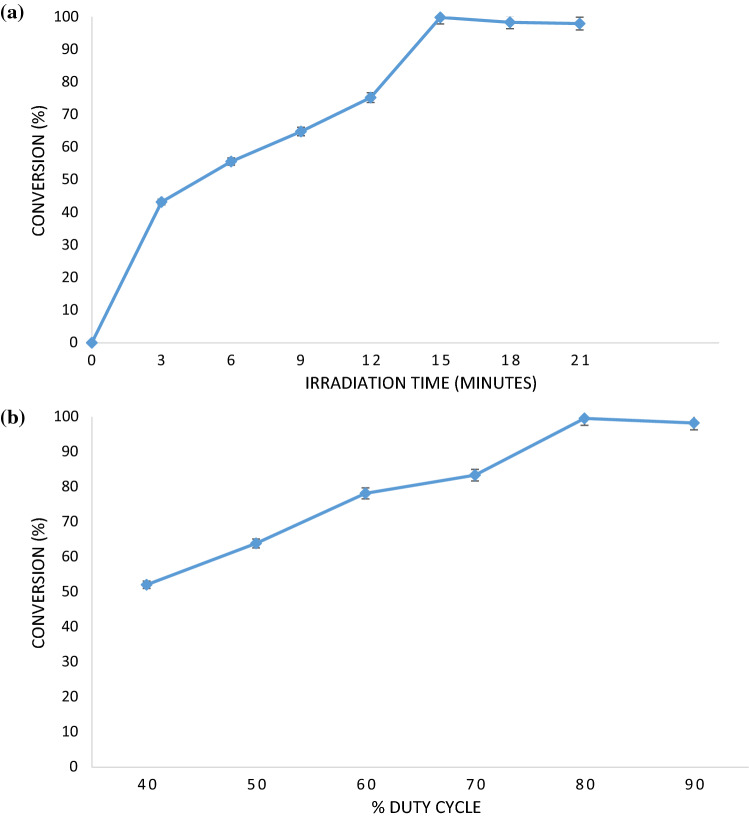
Ultrasound parameters are also responsible in deciding the maximum conversion (%) of esterification reaction. The parameters which were studied in the work were irradiation time and duty cycle. It was observed from the showed results in Fig. 2a that the irradiation time of 15 min gave maximum conversion (%) of esterification reaction. After 15 min, the ultrasound application did not show any appreciable increase in the rate of reaction. During the initial stages of ultrasonic irradiation, cavitational effects have dominant effect on the catalyst activity as well as elimination of the mass transfer resistances leading to increase in the conversion significantly. Longer exposure of catalyst to ultrasound has a negative effect on catalyst leading to almost constant value or no increase in conversion (%) after a specific time of reaction. There is possibility of structural damage to catalyst on being exposure to ultrasound intensity for longer time. Based on the obtained results in the current work, irradiation time was optimized as 15 min.
Fig. 2.
Effect of ultrasound parameters on the conversion (%) of esterification reaction a Irradiation time b duty cycle
Duty cycle of ultrasound is equally important in speeding up the reaction. Duty cycle of ultrasound refers to on and off time of irradiation. Different values of duty cycles used in the present study were 40%, 50%, 60%, 70%, 80% and 90%. 40% duty cycle means in a cycle, irradiation time is 4 s and then there is 6 s off time. The results are represented in Fig. 2b. Duty cycle of 80% i.e., 8 s on and 2 s off cycle was found to be the best which showed maximum conversion (%) of 99.6%. Duty cycle beyond 80% did not show any appreciable increase in the conversion. Keeping in mind economics of overall process, 80% duty cycle was considered to be optimized. Increase in the duty cycle means reaction mixture is exposed to sonication effect for more time which may also affect the reusability of catalyst and hence it not good to use very high duty cycle. More et al. (2017) also reported similar trend for effect of ultrasound on yield (%) of triglyceride of octanoic acid with use of sulphuric acid catalyst. 80% duty cycle was also reported as most effective in their study giving maximum yield (%) in 9 h of reaction.
Reusability of catalyst
One of the main aims of this research was to develop a novel process for synthesis of medium chain triglyceride using heterogeneous catalyst which can be reused again for same reaction. The obtained results for reusability illustrated in Fig. 3 establish the excellent recyclability of acid resin catalyst for eight cycles. After eight cycles, there was decrease in the activity of acid resin and hence the conversion (%) was also affected. Zare et al. (2020) studied recyclability of amberlyst-15 for synthesis of artificial banana flavour and authors reported in the study that the Amberlyst-15 was effectively recycled for six cycles. Beyond six cycles the catalyst showed decline in the catalytic performance, thus affecting the rate of reaction. The decline in performance of the amberlyst-15 may be due to deactivation during the exposure to the reaction conditions and possible unfavourable changes in the structure caused due to sonication.
Fig. 3.
Reusability of acid-resin catalyst (Reaction temperature 50 °C, Molar ratio of reactant 3:4, amberlyst-15 loading 3%, ultrasound duty cycle 80%, irradiation time 15 min)
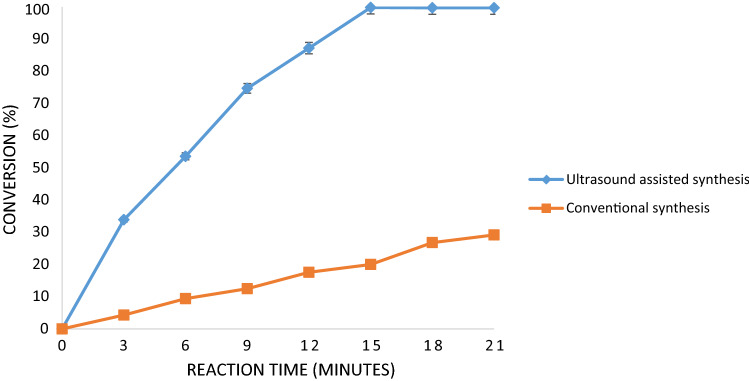
Comparison with conventional synthesis method
Conventional process of esterification reaction was also studied in the work to compare the efficacy with the ultrasonic method of synthesis of triglyceride of octanoic acid. The result is represented in Fig. 4 where it is seen that novel process of ultrasound gave 99.8% conversion (%) under conditions of 15 min as reaction time, temperature of 50 °C, amberlyst-15 loading of 3% and molar ratio of 3:4 (octanoic acid: glycerol). With the conventional method only 20% conversion (%) was observed at same conditions clearly confirming that conventional esterification process for synthesis of medium chain triglyceride takes much more time. Ataide et al. (2007) studied synthesis of tricaproin, tricaprylin and trienentin using the conventional process for 20 h as reaction time at 200 °C. Author reported yield (%) of tricaproin, tricaprylin and trienentin as 17%, 97% and 79% respectively. Wong et al. (2000) also studied synthesis of triglycerides of medium chain fatty acid using the conventional approach at 37 °C for 24 h and reported highest yield of 17.28% for tricaprylin. Lu et al. (2017) also studied synthesis of long and medium chain triglycerides using interesterification reaction with 74.9% yield in 6 h. Boulos (2013) reported best results for conversion of medium chain triglycerides using metal salt catalyst in 22 h. Study of literature revealed that conventional synthesis of medium chain triglycerides requires more time and higher temperature for obtaining high conversion. In the present study also, conventional process of esterification gave 20% conversion (%) in 15 min at 50 °C whereas high conversion of 96% was obtained in 29 h. Conventional method is thus found to be energy intensive operation, whereas in ultrasound assisted process cavitational effects releases high energy in short time (Chatel and Colmenares 2017) leading to enhanced heat and mass transfer which helps in achieving intensification. Mohod and Gogate (2018) studied intensified synthesis of medium chain triglyceride using sonication. Author reported maximum conversion of 77.5% using ultrasonic horn at 90 °C using homogeneous catalyst. Thus, it clearly demonstrates that the ultrasonication is a great intensification approach for synthesis of medium chain triglycerides though the extent of intensification depends on the specific system.
Fig. 4.
Comparison between ultrasound assisted synthesis and conventional synthesis of triglyceride of octanoic acid
Kinetic study
The acid–resin catalysed synthesis of triglyceride of octanoic acid was studied at various temperatures from 40 °C to 80 °C. Second order kinetic model was used to calculate reaction rate constants at different temperatures. The plot of X/(1 − X) vs time was used to estimate the values of rate constant at different temperature as summarized in Table 1. It was observed that values of rate constant for esterification of octanoic acid increased from 0.0097 to 0.0369 l/(mol min) with an increase in temperature from 40 °C to 80 °C. Mohod and Gogate (2017) also reported kinetic study for synthesis of medium chain triglyceride using sulphuric acid. Author reported that with a rise in the reaction temperature, the values for rate constant also increased from 0.0391 to 0.4630 l/mol min, similar to that seen in the present work.
Table 1.
Obtained results for the reaction rate constant
| Temperature (°C) | Rate Constant (L/mol min) | Correlation factor (R2) |
|---|---|---|
| 40 | 0.0097 | 0.9723 |
| 50 | 0.023 | 0.9958 |
| 60 | 0.026 | 0.9895 |
| 70 | 0.03 | 0.9946 |
| 80 | 0.0369 | 0.9823 |
FTIR analysis
The characterization of medium chain triglyceride of octanoic acid was obtained by IR Spectra as shown in Figure S1. Confirmation to formation of tricaprylin is obtained from the presence of peak at 1731.86 cm−1 which refers to carbonyl group with strong intensity. 2926.01 cm−1 peak corresponds to C–H bond whereas peaks at 1450.47 cm−1and 1365.60 cm −1 are attributed to medium carbon- hydrogen bending and peak at 725.23 cm−1 is attributed to C–H strong bending. More et al. (2017) also showed peak for carbonyl stretching at 1741 cm−1 in the case of synthesis of tricaprylin. Ataide et al. (2007) also reported the existence of IR analysis peak at 1730 cm−1 for the synthesis of tricaprylin. The IR spectra result observed in the current research work are indeed matching with the IR studies of other researches dealing with tricaprylin synthesis.
HPLC analysis
The formation of triglyceride of octanoic acid was also established by HPLC analysis as per the result depicted in Figure S2, which also shows the comparison with chromatogram of standard tricaprylin. Triglyceride of octanoic acid showed retention time at 6.7 min very similar to the standard. More et al. (2017) also reported results for the HPLC analysis of Tricaprylin with retention time at 6.7 min. Based on the obtained results of the chromatograms for standard as well as synthesized tricaprylin and comparison with literature, we confirm the formation of medium chain triglyceride of octanoic acid in the present work.
Characteristics of oil extracted from fries and frying medium
The quality characteristics of oil extracted from French fries and that of left-over oil in fryer have been shown in Table 2. The French fries were deep fried for 4 min at 180 °C. The oil extracted from the French fries was inferior in quality in comparison with the oil left over in fryer. The values were higher in the case of palm oil and lower in 50:50 combination of palm oil: MCT as shown in Table 2. The frying oil combination with 30:70 palm oil: MCT showed formation of foam during frying, which can be attributed to the presence of higher quantity of medium chain triglyceride in combination. The presence of medium chain fatty acid having shorter chain length in the medium chain triglyceride tend to form foam at higher temperature. As seen from Table 2a, there is formation of FFA in oil extracted from fried foods. Higher amount of FFA were reported in palm oil then the palm oil blends. Similar trend was observed for formation of FFA in residual used oil (Table 2b) though the quantum of FFA were lower in residual used oil as compared with the oil extracted from foods. The peroxide value indicating the quantum of polar oxidized compounds was more in oil extracted from French fries fried in palm oil and it decreased with increase in concentration of MCT in frying oil combination. The combination of 50:50 (Palm oil: MCT) showed best results attributed to presence of saturated fatty acid in MCT, which shows higher stability during frying process (Sanibal 2004). The quality of left-over oil in fryer was better than the oil extracted from French fries. The values of peroxide value were much higher in the case of palm oil as compared with palm: MCT combinations. During frying the water liberated from food comes in contact with the triglyceride molecules and brings about hydrolysis of these triglycerides, thus forming diglyceride, monoglyceride, free fatty acids etc. These freely present fatty acids could undergo thermal and oxidative deterioration easily as compared with the fatty acids esterified to glycerol backbone (Choe and Min 2007). Thus, the lipid profile of fried food contains more absorbed oxidized products in comparison with the residual frying oil. According to studies reported in the literature, the deep-fried food takes up more oxidized fat by the process of sorption from frying oil. Most of the oil is confined to the surface region of the fried products and it is mostly absorbed during the cooling process. Large amount of oil is pulled in the food product when it is removed from the fryer because of vacuum effect. Oil uptake in food is a surface phenomenon, and along with this oil, many oxidative products generated during deep frying of oil also penetrates in the food (Narayan and Kummerow 1963). The color of fried oil changed with the palm oil, 70:30 and 50:50 showing reading of 78 ± 0.45, 53.9 ± 0.77, 38.4 ± 0.32 Lovibond units on red scale and 8.9 ± 0.63, 8.4 ± 0.11, 7.9 ± 0.98 Lovibond units on yellow scale respectively. Same trend of decrease in Lovibond unit on red and yellow scale was observed in oil extracted from fries. The highest Lovibond unit for palm oil and blend with higher palm oil content on red scale could be due to light red color of palm oil. The viscosity of frying medium changes during frying due to accumulation of non-volatile oxidation products in the frying medium, which increases the viscosity of frying medium (Table 2a and b). Frying oil blend containing more MCT concentration showed lower viscosity i.e., 93 ± 0.6 cPs whereas highest value was shown by palm oil i.e., 109 ± 1.6 after first frying cycle. The blend with more MCT contains more saturation and hence formation of non-volatile oxidation products was less giving lower viscosity change. Among all four frying oil combinations i.e., pure palm oil, 70:30, 50:50 and 30:70 (palm: MCT), the combination of 50:50 (palm: MCT) showed good frying characteristics with a smaller number of polar oxidized compounds formed and less peroxide value indicating that it was less susceptible to damage because of the optimum presence of MCT which are less affected during frying. It is also important to note that a subsequent increase in the concentration of MCT in frying oil i.e., 30:70 (palm: MCT) leads to higher foam during frying and this is not suitable for the processing. The foam generation hampers the rate of heat transfer and the French fries are not fried properly. Thus, MCT could be effectively used in proper combination at optimum loading with other traditional frying oil to reduce the oxidative and thermal damage to food and make fried food healthier.
Table 2.
Quality of oil in different processing approaches
| Parameters | Palm oil | 70:30 (palm:MCT) | 50:50 (palm:MCT) |
|---|---|---|---|
| (A) Oil extracted from fried fries using different combinationsa | |||
| FFA | 0.91 ± 0.1 | 0.67 ± 0.1 | 0.27 ± 0.2 |
| PV | 14.1 ± 1.3 | 11.6 ± 1.2 | 8.4 ± 0.8 |
| Viscosity (cPs at 25 °C) | 115 ± 2.1 | 112 ± 1.7 | 97 ± 0.43 |
| TPC | 39.1 ± 0.1 | 33.7 ± 1.3 | 29.8 ± 0.01 |
| p-Anisidine value | 33.6 ± 0.2 | 28.4 ± 0.03 | 23.1 ± 0.02 |
| Colour | |||
| R-scale | 82 ± 0.51 | 57.9 ± 0.73 | 40.4 ± 0.27 |
| Y-scale | 7.3 ± 0.32 | 6.4 ± 0.21 | 5.9 ± 0.81 |
| (B) Oil left over in fryer after first frying cycleb | |||
| FFA | 0.83 ± 0.2 | 0.55 ± 0.01 | 0.13 ± 0.01 |
| PV | 9.2 ± 0.01 | 8.6 ± 0.02 | 6.4 ± 0.01 |
| Viscosity (cPs at 25 °C) | 109 ± 1.6 | 103 ± 1.3 | 93 ± 0.6 |
| TPC | 31.4 ± 1.1 | 27.5 ± 1.2 | 22.8 ± 1.1 |
| p-Anisidine value | 28.2 ± 0.67 | 26 ± 0.33 | 19.8 ± 0.98 |
| Colour | |||
| R-scale | 78 ± 0.45 | 53.9 ± 0.77 | 38.4 ± 0.32 |
| Y-scale | 8.9 ± 0.63 | 8.4 ± 0.11 | 7.9 ± 0.98 |
Analysis was done in triplicate and the values are reported as mean ± SD. Statistical analysis of data was done using single factor ANOVA using Minitab 16. The results were considered significant for P < 0.05. (R- red scale on tintometer, Y- Yellow scale on tintometer)
a30:70 (palm: MCT)—Due to formation of large amount of foam, there was poor heat transfer and fries were not fried properly. Hence this combination was not considered
b30:70 (palm: MCT)—Due to formation of large amount of foam in the frying oil, it was not considered for analysis
Conclusion
In the present work, enhanced conversion of octanoic acid for the synthesis of triglyceride is demonstrated using ultrasound assisted process based on amberlyst-15 as a reusable heterogeneous catalyst. With the application of ultrasound, the reaction time decreased from 24 h for the conventional process to only 15 min due to creation of local hot spots and enhanced mass transfer in the reaction mixture. Maximum conversion (%) of 99.8% was obtained at 50 °C with a molar ratio of 3:4 (octanoic acid: glycerol) and catalyst loading of 3% using the ultrasound assisted approach under conditions of 80% duty cycle and 15 min irradiation time. Amberlyst-15 showed reusability for eight cycles and thereafter the conversion decreased with the use of the same catalyst again. Amberlyst-15 showed higher activity compared to other acid or metal catalysts which are expensive and dangerous to handle at large scale. It was further demonstrated that the MCT (tricaprin) can be blended with traditional frying oil in proper combination. 50:50 (palm: MCT) combination demonstrated good frying characteristics and also the quality of oil extracted from food and left-over oil in fryer was much better for the combination as compared with palm oil. The combination containing higher amount of MCT (30:70–Palm: MCT) failed due to formation of large amount of foam confirming that optimum blending of MCT and palm oil is recommended. Overall, the present study clearly demonstrated the use of ultrasound as the intensifying option for production of triglyceride of octanoic acid with maximum conversion (%) in minimum time using heterogeneous reusable catalyst and also the application of MCT as a frying medium in combination with traditional oil at optimum loading.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
HBJ is thankful to Department of Science and Technology, Government of India for providing DST-Inspire fellowship for doctoral research.
Authors' contributions
HBJ: Methodology; Investigation; Original draft preparation, PRG: Reviewing and Editing; Supervision, USA: Supervision; Project administration.
Funding
Department of Science and Technology, New Delhi IN, DST Inspire Fellowship, Harsh Jadhav.
Declaration
Conflict of interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parag Gogate, Email: pr.gogate@ictmumbai.edu.in.
Uday Annapure, Email: us.annapure@ictmumbai.edu.in.
References
- Ataide R, Raquel M, De Lima F et al (2007) Chemical synthesis of tricaproin, trienantin and tricaprylin. Int J Food Sci Technol 1504–1508. 10.1111/j.1365-2621.2006.01376.x
- Bhattacharya AB, Sajilata MG, Singhal RS. Lipid profile of foods fried in thermally polymerized palm oil. Food Chem. 2008;109:808–812. doi: 10.1016/j.foodchem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Boulos Z (2013) Method for preperation of triglycerides of medium chain fatty acids. Int Pat Treaty 1–20
- Chatel G, Colmenares JC. Sonochemistry: from basic principles to innovative applications. Top Curr Chem. 2017;375:1–4. doi: 10.1007/s41061-016-0096-1. [DOI] [PubMed] [Google Scholar]
- Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72:R77–86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Deshmane VG, Gogate PR, Pandit AB. Process intensification of synthesis process for medium chain glycerides using cavitation. Chem Eng J. 2008;145:351–354. doi: 10.1016/j.cej.2008.08.012. [DOI] [Google Scholar]
- Jadhav HB, Annapure U. Designer lipids-synthesis and application—a review. Trends Food Sci Technol. 2021;116:884–902. doi: 10.1016/j.tifs.2021.08.020. [DOI] [Google Scholar]
- Jadhav HB, Annapure US, Deshmukh RR. Non-thermal technologies for food processing. Front Nutr. 2021;8:1–14. doi: 10.3389/fnut.2021.657090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav HB, Gogate PR, Waghmare JT, Annapure US. Intensified synthesis of palm Olein designer lipids using sonication. Ultrason Sonochem. 2021;73:105478. doi: 10.1016/j.ultsonch.2021.105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav HB, Gogate P, Annapure U. Process intensification of acidolysis reaction catalysed by enzymes for synthesis of designer lipids using sonication. Chem Eng J. 2022;428:131374. doi: 10.1016/j.cej.2021.131374. [DOI] [Google Scholar]
- Jadhav HB, Gogate PR, Annapure US (2021b) Intensification of enzymatic synthesis of corn oil designer lipids using sonication. Arab J Sci Eng 46 (in press). 10.1007/s13369-021-06255-6
- Kim SM, Rhee JS. Production of medium-chain glycerides by immobilized lipase in a solvent-free system. J Am Oil Chem Soc. 1991;68:499–503. doi: 10.1007/BF02663821. [DOI] [Google Scholar]
- Kinsella R, Maher T, Clegg ME. Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav. 2017;179:422–426. doi: 10.1016/j.physbeh.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Kwon DY, Song HN, Yoon SH. Synthesis of medium-chain glycerides by lipase in organic solvent. J Am Oil Chem Soc. 1996;73:1521–1525. doi: 10.1007/BF02523519. [DOI] [Google Scholar]
- Li W, Gamlath CJ, Pathak R et al (2021) Ultrasound—the physical and chemical effects integral to food processing. In: Knoerzer K, Juliano P, Smithers G (eds) Innovative food processing technologies. Cambridge, pp 329–358
- Lu J, Jin Q, Wang X, Wang X. Preparation of medium and long chain triacylglycerols by lipase-catalyzed interesterification in a solvent-free system. Process Biochem. 2017;54:89–95. doi: 10.1016/j.procbio.2016.12.015. [DOI] [Google Scholar]
- Marten B, Pfeuffer M, Schrezenmeir J (2006) Medium-chain triglycerides. Int Dairy J 16:1374–1382. 10.1016/j.idairyj.2006.06.015
- Mohod AV, Gogate PR. Intensified synthesis of medium chain triglycerides using novel approaches based on ultrasonic and microwave irradiations. Chem Eng J. 2017;317:687–698. doi: 10.1016/j.cej.2017.02.102. [DOI] [Google Scholar]
- Mohod AV, Gogate PR. Intensified synthesis of medium chain triglycerides using ultrasonic reactors at a capacity of 4L. Ultrason Sonochem. 2018;42:347–355. doi: 10.1016/j.ultsonch.2017.11.044. [DOI] [PubMed] [Google Scholar]
- More SB, Gogate PR, Waghmare JS. Intensification of acid catalyzed synthesis of tricaprylin using ultrasound pretreatment. Chem Eng Process Process Intensif. 2017;120:317–329. doi: 10.1016/j.cep.2017.07.027. [DOI] [Google Scholar]
- Narayan KA, Kummerow FA. Factors influencing the formation of complexes between oxidized lipids and proteins. J Am Oil Chem Soc. 1963;40:339–342. doi: 10.1007/BF02631552. [DOI] [Google Scholar]
- Pan Q, Yang L, Meng X. Optimization of enzymatic synthesis of tricaprylin in ionic liquids by response surface methodology. J Am Oil Chem Soc. 2013;90:501–509. doi: 10.1007/s11746-012-2186-8. [DOI] [Google Scholar]
- Sanibal EAA. Frying oil and fat quality measured by chemical, physical, and test kit analyses. J Am Oil Chem Soc. 2004;81:847–852. doi: 10.1007/s11746-004-0990-8. [DOI] [Google Scholar]
- Singla M, Sit N. Application of ultrasound in combination with other technologies in food processing: a review. Ultrason Sonochem. 2021;73:105506. doi: 10.1016/j.ultsonch.2021.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirsam R, Hansora D, Usmani GA. A Mini-Review on solid acid catalysts for esterification reactions. J Inst Eng Ser E. 2016;97:167–181. doi: 10.1007/s40034-016-0078-4. [DOI] [Google Scholar]
- Takeuchi H, Sekine S, Kojima K, Aoyama T. The application of medium-chain fatty acids: edible oil with a suppressing effect on body fat accumulation. Asia Pac J Clin Nutr. 2008;17:320–323. doi: 10.6133/apjcn.2008.17.s1.79. [DOI] [PubMed] [Google Scholar]
- Wong WC, Basri M, Razak CNA, Salleh AB. Synthesis of medium-chain glycerides using lipase from Candida rugosa. J Am Oil Chem Soc. 2000;77:85–88. doi: 10.1007/s11746-000-0013-9. [DOI] [Google Scholar]
- You YQN, Ling PR, Zhensheng QuJ, Bistrian BR. Effects of medium-chain triglycerides, long-chain triglycerides, or 2-monododecanoin on fatty acid composition in the portal vein, intestinal lymph, and systemic circulation in rats. J Parenter Enter Nutr. 2008;32:169–175. doi: 10.1177/0148607108314758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare M, Golmakani MT, Sardarian A. Green synthesis of banana flavor using different catalysts: a comparative study of different methods. Green Chem Lett Rev. 2020;13:82–91. doi: 10.1080/17518253.2020.1737739. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.