Abstract
Plant-based milk products are gaining attention since it has been demonstrated that the consumption of animal-derived foods had to be reduced to combat global climate change. The production of plant-based milk includes a starch hydrolysis step for raw materials with high starch content such as cereals and pulses, since the gelatinized starch forms a thick slurry which causes an unsuitable consistency for a drinkable product. The objectives of this work were to investigate the effects of slurry concentration (solid to solvent ratio), enzyme including temperature, enzyme amount and mixing (rotation) speed on the pasting properties especially final viscosity of a crude chickpea milk and also to investigate the potential use of Micro Visco Amylo-Graph for monitoring starch hydrolysis. Response surface methodology, based on Box Behnken Design, was used to assess the parameters and to optimize the hydrolysis conditions for the minimum final viscosity. In conclusion, it was observed that slurry concentration and enzyme including temperature were the most critical factors that affect either the pasting properties or the final viscosity of the crude chickpea milk. Briefly, lower final viscosities were obtained from samples which were prepared at lower beginning concentrations and treated with higher enzyme amounts at lower temperatures.
Keywords: Box-Behnken design, Chickpea milk, Micro visco-amylo-graph, Response surface methodology, Starch hydrolysis
Introduction
Plant-based milk products are non-dairy alternatives/ milk substitutes/ milk analogs which were derived from the water extraction of plant materials and imitates cow’s milk in appearance and consistency, but free from animal-based ingredients. Plant-based milks are categorized in 5 groups based on raw material as follows: (1) cereal-based (i.e. oat milk, rice milk), (2) pseudo cereal-based (i.e. quinoa milk, teff milk), (3) legume-based (i.e. soy milk, chickpea milk), (4) nut-based (i.e. almond milk, coconut milk), (5) seed-based (i.e. flax milk, hemp milk) (Sethi et al. 2016). There is a growing demand for plant-based milk products owing to many reasons, including lactose intolerance, cow’s milk allergies, cholesterol issues, phenylketonuria, lifestyle choices such as vegan or vegetarian/flexitarian diets, concerns about animal welfare, antibiotic residues, growth hormones, environmental pollution, etc. (Haas et al. 2019; Mäkinen et al. 2016; Paul et al. 2020; Sethi et al. 2016). Furthermore, plant-based milks are often enriched with minerals, vitamins and/or proteins and therefore accepted as a functional food and have been extensively utilized in various recipes as an ingredient, especially in western countries (Sethi et al. 2016). The global plant milk market reached an estimated size of US $8.51 billion in 2016 and is forecasted to achieve a market volume of US $24.6 billion in 2025 (Haas et al. 2019).
Plant-based milk analogs are typically produced by grinding the raw material, extraction, separation of coarse particles, standardization or formulation, homogenization, and heat treatment for microbial stability. Some of the raw materials such as nuts contain low amounts of starch, however when using raw materials such as cereals, pseudo cereals or some legumes which have notably high amounts of starch, gelatinization emerges as a problem during the heat treatment especially for sterilization. The product loses its drinkable consistency and tends to show a pudding-like structure as a result of gelatinization that occurs during heat treatment, which is an inevitable step to produce a long-lasting product (Mäkinen et al. 2016; Sethi et al. 2016; Silva et al. 2020). Therefore, starch hydrolysis arises as a crucial step for plant-based milk that produced from raw materials with especially high starch content. Various enzyme or enzyme combinations, mainly amylases, have been suggested to hydrolyze starch in a cost- efficient and timely manner (Gugger et al. 2016; Lindahl et al. 1997; Triantafyllou 2002). These starch hydrolysis procedures involve an incubation step, mostly aiming to reach a certain final viscosity depending on the target plant-based product (milk, yogurt, ice cream, etc.). However, there is no data available related to the fate of starch during incubation. In this study, chickpea flour was used due to being a promising plant-based milk raw material since it is a rich source of protein, dietary fiber and minerals, contains no registered allergens as there are for soybeans and also has an acceptable mild flavor among the raw materials used for legume-based milk analogs (Cabanillas et al. 2018; Rincon et al. 2020).
Micro Visco-Amylo-Graph (MVAG) is used for measuring the gelatinization and pasting properties of starch and provides information about enzyme activity by testing the viscosity of the suspensions. It allows monitoring temperature-dependent and time-dependent viscosity properties of flours and starches. Briefly, flour–water or starch–water suspension is heated and cooled down in a rotating bowl under control conditions. Since cereal or pulse-based plant milks have high amounts of starch and therefore require a starch hydrolysis step to maintain fluidity or beverage-like consistency in the final heat-treated product, to monitor the variation of viscosity under controlled starch hydrolysis conditions will provide a powerful tool for production of these products at industrial scale. Therefore, the aims of this study were to investigate the potential use of MVAG for monitoring the liquefaction behavior of a crude chickpea milk and to find the optimum hydrolysis condition for a final product (heated and cooled) that has a viscosity under 20 cP (10 BU). To our knowledge, this is the first attempt to model the influence of starch hydrolysis conditions on final viscosity of a plant-based milk using MVAG.
Material and methods
Material
Three kg of chickpea (Cicer arietinum L., variety of Koçbaşı) (19.5% protein, 4.6% fat, 42.8% total starch, 2.6% ash) was purchased from supermarket. Dry chickpea seeds, which were produced in 2019 in Turkey, were finely ground with a laboratory type mill (Karaerler Makine, Ostim, Ankara) and sieved through 300 μm standard sieves (Retsch, Haan, Germany). Chickpea flour < 300 μm (~ 75% of the total weight) was used as the material of the present study.
Method
Moisture content
The moisture content of the flour was measured with a moisture analyzer (Ohaus MB27, New Jersey, USA) in 5 replications. The moisture content of the flour was 7.65% after milling and it was measured each time before the MVAG analysis.
Micro visco-amylo-graph analysis
Designed amounts of chickpea flour (14% moisture basis) were transferred to an Erlen-Mayer and mixed with fixed amount (115 mL) of distilled water. The content was transferred to the rotating bowl of the Micro Visco-Amylo-Graph, after mixing it manually for 15–20 s, and the test was started. The slurry was mixed at different speeds (rpm), treated with different amounts of enzyme (α-amylase) at different temperatures for the test with the aim of imitating starch hydrolysis conditions. The range of these independent variables was determined according to preliminary analysis. The gelatinization and pasting properties of the chickpea slurries were measured with Micro Visco-Amylo-Graph (MVAG, Brabender, Germany). The content was heated from 30 to 90 °C at 7.5 °C/min rate, held at 90 °C for 5 min, cooled down to 50 °C at 7.5 °C/min rate and held at 50 °C for 2 min. Total test time was 20.2 min. Enzyme (α-amylase) was directly included into the content externally and manually at specific slurry temperatures (70, 80, and 90 °C) during the test. The α-amylase enzyme (Spezyme LT 300, liquid form, minimum activity of 30 000 RAU/g, working pH range 5.5–7.5; inactivated rapidly around 90 °C or higher) was kindly provided by DuPont (Wilmington, Delaware, USA). The following data was recorded: beginning of gelatinization temperature (°C), beginning of gelatinization viscosity (BU), maximum viscosity (peak viscosity) (BU), start of holding period viscosity (BU), start of cooling period viscosity (hot paste viscosity) (BU), end of cooling period viscosity (cold paste viscosity) (BU), end of final holding period viscosity (final viscosity) (BU), breakdown viscosity (BU), and setback viscosity (BU).
Experimental design and statistical analysis
Factors affecting starch hydrolysis of crude chickpea milk production were analyzed with response surface methodology using Box-Behnken Design. This design led to studying the effects of 4 factors, namely slurry concentration (10–15%), enzyme concentration (5–20 µL), enzyme including temperature (70–90 °C) and rotation speed (200–300 rpm), in a single block of 29 sets of test conditions and 5 central points. The order of the experiments was fully randomized. Each factor (independent variable) has high, central, and low levels. The relationship between the factors and the response was investigated using a full quadratic polynomial model as it was the best fitted model to demonstrate the effects of the factors on the responses. The experimental data were fitted using the following second-order polynomial equation:
| 1 |
where Y was the response (final viscosity, peak viscosity, breakdown or setback); β0 is the constant coefficient; βi is the linear coefficient; βii is the quadratic coefficients; βij is the two-factor interaction coefficient and Xi and Xj are the independents variables (slurry concentration, enzyme concentration, enzyme including temperature, and rotation speed). For optimization purposes, solely final viscosity was chosen as the response variable. Statistical analyses were performed with Design-Expert ver. 13 (StatEase, Inc., Minneapolis, USA) and SPSS (IBM SPSS Statistics 23, WA, USA) software packages.
Results and discussion
Pasting properties of chickpea slurries without enzyme addition
Preliminary analyses were conducted to determine the range of independent variables. Although the final viscosity of a drinkable suspension is reported to be between 2000 and 50,000 cP, (Vykhodtsev 2010) the viscosity of a standard cow’s milk is around 2 cP (1 BU) and commercial plant-based milk products have a viscosity ≤ 20 cP at room temperature. Therefore, in this study, it was aimed to obtain a final viscosity below 10 BU (~ 20 cP). The minimum enzyme amount required for starch hydrolysis was selected as 5 μL for analytical reasons since the repeatability of measurements made in volumes less than 5 μL may be low. It was observed that slurry (chickpea flour–water) concentrations below 10% showed a viscosity around 10 BU at the end of the final holding period even with 5 μL of α-amylase addition. On the other hand, it was speculated that raw material concentrations above 15% may not be cost-effective for a commercial plant-based milk product. Therefore, the slurry concentration was selected in a range of 10–15%. Also, using rotation speeds slower than 200 rpm resulted in inconsistent results for samples which has a concentration of 15% and treated with 5 μL enzyme concentration due to the lumps occurred inside the rotating bowl which indicates that the enzyme could not be homogeneously distributed throughout the slurry at the noted conditions. Therefore, the lowest rotating speed was selected as 200 and 300 rpm was the maximum possible level for MVAG instrument. Gelatinization and pasting properties of the chickpea slurries without enzyme addition were shown in Table 1. Pasting temperature is the temperature at which the first detectable viscosity is measured by the MVAG instrument and is strongly associated with the ability of starch granules to absorb water. Pasting temperature of the chickpea slurries mixed at the same rotation speed significantly decreased with increasing concentration (p < 0.05). It was reported that higher starch concentrations lead to lower pasting temperatures and the presence of monosaccharides and oligosaccharides have been reported to lead to an upward shift of pasting temperature (Colonna et al. 1992). Peak viscosity, which is the highest viscosity attained by the slurry during the run, increased with increasing slurry concentration, as expected (p < 0.05). Moreover, higher peak viscosities were observed in samples which were stirred at higher rotation speeds in the bowl in accordance with the findings of Suh and Jane who also reported that faster stirring speed resulted in higher peak viscosity values (Suh and Jane 2003). This tendency was more prominent at higher concentrations (Table 1). Higher rotation speeds may have increased the interaction of starch molecules with water and with other components of the flour such as protein. Breakdown is a measure of stability or susceptibility of cooked starch granules to disintegrate during continued stirring and heating. No breakdown was observed for samples prepared at 10% concentration regardless of the rotation speed. In other words, the viscosity of the content did not change during the holding/cooking period (at 90 °C for 5 min). However, more concentrated chickpea slurries had breakdown values between 3 and 20 BU. Breakdown value showed an increasing trend with increasing slurry concentration. Setback is the difference between hot and cold paste viscosities meaning the amount of increase in viscosity during the cooling period. Higher setback is associated with higher retrogradation tendency and more syneresis. Setback values significantly increased with increasing either slurry concentration or rotation speed (p < 0.05). Increasing the slurry concentration from 10 to 15% resulted in almost threefold increase in setback values. Cold paste viscosity is the viscosity attained as the cooked paste is cooled down to 50 °C. Generally, the cooling is ended either at 30 °C or 50 °C. In this study, more repeatable results were obtained with cooling the content from 90 to 50 °C with respect to cooling to 30 °C. Afterwards, the content was held at 50 °C for an additional 2 min to observe the variation in viscosity. End of final holding period viscosity is the final viscosity after cooling and holding periods. As can be seen from Table 1, cold paste viscosity and final viscosity followed the same trend and significantly increased with increasing slurry concentration and rotation speed (p < 0.05). Also, it was observed that final viscosity values were lower than cold paste viscosity and the difference between them increased with increasing slurry concentration. Although both of the noted parameters were measured at the same temperature (50 °C), continuous stirring lead to a disintegration in starch granules and decrease in viscosity. In this study, it was aimed to obtain a cooked chickpea slurry or a crude chickpea milk which had a final viscosity below 10 BU at the end of the experiment (50 °C). The final viscosity values of the chickpea slurries without enzyme addition ranged between 145 and 525 BU and were far from the target viscosity. In general, final viscosity increased with increasing slurry concentration and higher rotation speed (p < 0.05) (Table 1).
Table 1.
Pasting properties of chickpea slurries without enzyme addition
| Concentration (%) | Rotation speed (rpm) | Pasting temperature (°C) | Peak viscosity (BU) | Breakdown (BU) | Setback (BU) | Cold paste viscosity (BU) | Final viscosity (BU) |
|---|---|---|---|---|---|---|---|
| 10 | 200 | 73.50 ± 0.20 Aa | 99.67 ± 1.67 Cb | 0.00 ± 0.00 | 49.00 ± 0.57 Cc | 148.67 ± 2.19 Cc | 145.00 ± 2.52 Cc |
| 250 | 73.66 ± 0.03 Aa | 108.00 ± 0.01 Cb | 0.00 ± 0.00 | 56.00 ± 0.57 Cb | 164.00 ± 0.57 Cb | 159.67 ± 0.33 Cb | |
| 300 | 73.53 ± 0.03 Aa | 126.33 ± 0.88 Ca | 0.00 ± 0.00 | 63.66 ± 0.33 Ca | 190.33 ± 0.66 Ca | 183.67 ± 0.33 Ca | |
| 12.5 | 200 | 70.70 ± 0.05 Ba | 215.33 ± 0.33 Bb | 10.00 ± 0.57 Ba | 65.33 ± 0.33 Bc | 271.00 ± 0.57 Bc | 257.67 ± 0.88 Bc |
| 250 | 70.33 ± 0.14 Ba | 220.33 ± 1.45 Bb | 3.66 ± 0.33 Bc | 79.00 ± 0.57 Bb | 296.33 ± 0.66 Bb | 280.00 ± 1.15 Bb | |
| 300 | 70.76 ± 0.03 Ba | 244.00 ± 0.57 Ba | 6.66 ± 0.33 Bb | 88.33 ± 0.33 Ba | 325.67 ± 0.66 Ba | 310.33 ± 0.33 Ba | |
| 15 | 200 | 69.23 ± 0.14 Ca | 350.00 ± 5.69 Ac | 15.66 ± 0.33 Ab | 134.00 ± 1.53 Ac | 468.33 ± 6.89 Ac | 441.00 ± 6.56 Ac |
| 250 | 68.60 ± 0.05 Cb | 387.00 ± 2.08 Ab | 19.00 ± 0.57 Aa | 155.67 ± 0.88 Ab | 523.67 ± 3.48 Ab | 492.00 ± 4.04 Ab | |
| 300 | 68.86 ± 0.21 Cab | 414.64 ± 2.73 Aa | 20.33 ± 0.66 Aa | 163.33 ± 4.18 Aa | 557.67 ± 6.57 Aa | 525.33 ± 6.64 Aa |
Means followed by different capital letters for the same rotation speed are significantly different (p < 0.05). Means followed by different small cases for the same concentration are significantly different (p < 0.05)
Pasting properties of chickpea slurries with enzyme addition
It has been clearly demonstrated in the literature that high amounts of starch pose a problem during the heat processing of plant-based milks, and hydrolysis of starch should be employed to maintain the liquid state of the product (Deswal et al. 2014; Mäkinen et al. 2016; Sethi et al. 2016; Silva et al. 2020). However, data related to the conditions of starch hydrolysis are still lacking. Timing of enzyme treatment is suggested to be during or after gelatinization since starch molecule becomes more suitable for digestion by amylases (Tester et al. 2006). However, too little information is available about the incubation time or the duration of the hydrolysis process. Deswal et al. (2014) suggested an optimum incubation (liquefaction) time of 49 min with α-amylase to produce oat milk. In this study, it was observed that the viscosity sharply decreased immediately following α-amylase addition, and there is certainly no need for long incubation times for liquefaction purposes to produce chickpea milk. However, incubation with certain enzymes or enzyme combinations for a specific period of time may be the case in order to alter the viscosity and/or sugar composition of a cereal or pulse suspension (Triantafyllou 2002).
The MVAG analyses were conducted on 29 starch hydrolysis conditions which were created by the Box-Behnken design. Gelatinization and pasting properties of enzyme (α-amylase) added chickpea slurries according to the Box-Behnken design were shown in Table 2. Four different responses (peak viscosity, breakdown, setback, and final viscosity) were selected from the MVAG output. Since these responses are interrelated and the aim of the study was to find the optimum hydrolysis condition to obtain the lowest final viscosity (≤ 10 BU), solely final viscosity was used for optimization purposes. The quadratic model was significant for all responses when each response was assessed individually (Table 3), while it was insignificant when all of the responses were evaluated together (data not shown). Lack of fit value was significant except for the final viscosity when the responses were evaluated individually (Table 3). Lack of fit value was also significant when all the responses were evaluated together (data not shown). Insignificant “lack of fit” value is desirable as we want a model that fits. The relationship between the independent and dependent variables was modelled and the estimated coefficients for the fitted polynomial was presented in Table 4. It is evident from the table that all responses are significantly affected from the independent variables of enzyme including temperature (A) and slurry concentration (B) (p < 0.05). On the other hand, rotation speed (C) had no significant effect on any of the responses (p > 0.05). The effect of enzyme amount (D) was merely significant on setback and final viscosity values while it was insignificant for peak viscosity and breakdown (Table 4).
Table 2.
Pasting properties of chickpea slurries with enzyme addition
| Run | Concentration (%) | Enzyme including temperature (°C) | Enzyme amount (μL) | Rotation speed (rpm) | Peak viscosity (BU) | Breakdown (BU) | Setback (BU) | Final viscosity (BU) |
|---|---|---|---|---|---|---|---|---|
| 1 | 12.5 | 80 | 12.5 | 250 | 111.33 ± 0.33 | 102.00 ± 1.00 | 4.33 ± 0.33 | 13.00 ± 0.01 |
| 2 | 15 | 80 | 12.5 | 300 | 278.67 ± 3.48 | 257.00 ± 3.06 | 16.33 ± 0.66 | 37.00 ± 1.53 |
| 3 | 12.5 | 70 | 12.5 | 200 | 15.33 ± 0.88 | 5.66 ± 0.33 | 3.00 ± 0.01 | 13.00 ± 0.57 |
| 4 | 15 | 70 | 12.5 | 250 | 33.00 ± 1.73 | 18.50 ± 1.44 | 5.50 ± 0.29 | 19.50 ± 0.28 |
| 5 | 12.5 | 70 | 5 | 250 | 19.00 ± 0.01 | 8.50 ± 0.28 | 3.00 ± 0.01 | 13.50 ± 0.28 |
| 6 | 12.5 | 80 | 12.5 | 250 | 111.00 ± 0.57 | 100.33 ± 0.66 | 3.66 ± 0.33 | 14.00 ± 0.57 |
| 7 | 15 | 80 | 5 | 250 | 256.00 ± 1.15 | 229.50 ± 2.60 | 21.50 ± 0.28 | 47.00 ± 1.15 |
| 8 | 10 | 80 | 12.5 | 200 | 34.00 ± 1.15 | 24.67 ± 1.45 | 1.66 ± 0.33 | 10.66 ± 0.33 |
| 9 | 12.5 | 90 | 5 | 250 | 187.33 ± 3.67 | 129.67 ± 6.33 | 23.67 ± 1.67 | 74.00 ± 0.01 |
| 10 | 12.5 | 80 | 12.5 | 250 | 111.67 ± 0.33 | 100.67 ± 0.33 | 4.33 ± 0.33 | 14.66 ± 0.33 |
| 11 | 12.5 | 70 | 20 | 250 | 12.66 ± 0.66 | 4.67 ± 1.86 | 3.66 ± 0.33 | 15.00 ± 0.01 |
| 12 | 12.5 | 80 | 5 | 200 | 109.33 ± 8.33 | 99.00 ± 8.50 | 3.33 ± 0.33 | 14.00 ± 0.57 |
| 13 | 12.5 | 70 | 12.5 | 300 | 16.00 ± 2.08 | 9.67 ± 3.28 | 4.66 ± 0.33 | 11.00 ± 0.57 |
| 14 | 12.5 | 80 | 20 | 300 | 124.67 ± 0.33 | 117.00 ± 0.57 | 4.00 ± 0.01 | 12.00 ± 0.57 |
| 15 | 15 | 90 | 12.5 | 250 | 340.00 ± 12.10 | 278.30 ± 14.70 | 28.33 ± 3.18 | 85.00 ± 0.57 |
| 16 | 15 | 80 | 20 | 250 | 250.67 ± 5.49 | 235.33 ± 3.48 | 10.00 ± 0.57 | 25.00 ± 0.57 |
| 17 | 10 | 80 | 20 | 250 | 35.00 ± 1.73 | 33.00 ± 2.52 | 1.66 ± 0.88 | 5.00 ± 0.57 |
| 18 | 12.5 | 80 | 5 | 300 | 117.33 ± 0.66 | 103.33 ± 0.66 | 6.33 ± 0.33 | 19.66 ± 0.33 |
| 19 | 12.5 | 90 | 20 | 250 | 197.00 ± 0.57 | 179.67 ± 2.03 | 12.66 ± 0.33 | 33.00 ± 0.57 |
| 20 | 12.5 | 80 | 20 | 200 | 92.00 ± 6.43 | 88.00 ± 6.08 | 3.33 ± 0.33 | 8.50 ± 0.28 |
| 21 | 12.5 | 90 | 12.5 | 300 | 213.67 ± 0.66 | 176.33 ± 1.33 | 13.66 ± 0.66 | 48.00 ± 0.01 |
| 22 | 10 | 70 | 12.5 | 250 | 33.33 ± 0.33 | 17.66 ± 0.33 | 2.33 ± 0.33 | 17.00 ± 0.01 |
| 23 | 12.5 | 80 | 12.5 | 250 | 110.67 ± 1.20 | 100.33 ± 0.88 | 4.00 ± 0.01 | 14.66 ± 0.33 |
| 24 | 12.5 | 80 | 12.5 | 250 | 105.67 ± 0.33 | 92.33 ± 1.45 | 4.00 ± 0.01 | 19.50 ± 0.01 |
| 25 | 10 | 80 | 12.5 | 300 | 40.66 ± 0.33 | 30.66 ± 0.33 | 3.66 ± 0.33 | 14.00 ± 0.57 |
| 26 | 10 | 90 | 12.5 | 250 | 88.00 ± 1.15 | 78.66 ± 0.33 | 3.00 ± 0.57 | 11.50 ± 0.29 |
| 27 | 10 | 80 | 5 | 250 | 36.00 ± 1.15 | 26.00 ± 0.57 | 2.33 ± 0.33 | 12.00 ± 0.57 |
| 28 | 15 | 80 | 12.5 | 200 | 247.00 ± 2.65 | 225.67 ± 1.76 | 13.66 ± 0.33 | 36.00 ± 0.57 |
| 29 | 12.5 | 90 | 12.5 | 200 | 193.33 ± 1.67 | 175.00 ± 3.00 | 15.00 ± 1.00 | 42.00 ± 0.01 |
Table 3.
Analysis of variance (ANOVA) results of the response surface quadratic model for the individual responses
| Source | Sum of square | df | Mean square | F value | p value Prob > F | ||
|---|---|---|---|---|---|---|---|
| Peak viscosity (BU) | Model | 2.320E + 005 | 14 | 16,571.79 | 33.92 | < 0.0001 | Significant |
| Residual | 6838.97 | 14 | 488.50 | ||||
| Lack of fit | 6814.21 | 10 | 681.42 | 110.10 | 0.0002 | Significant | |
| Pure error | 24.76 | 4 | 6.19 | ||||
| Total | 2.388E + 005 | 28 | |||||
| Breakdown (BU) | Model | 1.856E + 005 | 14 | 13,255.37 | 20.53 | < 0.0001 | Significant |
| Residual | 9037.46 | 14 | 645.53 | ||||
| Lack of fit | 8977.77 | 10 | 897.78 | 60.16 | 0.0006 | Significant | |
| Pure error | 59.69 | 4 | 14.92 | ||||
| Total | 1.946E + 005 | 28 | |||||
| Setback (BU) | Model | 1402.02 | 14 | 100.14 | 23.01 | < 0.0001 | Significant |
| Residual | 60.94 | 14 | 4.35 | ||||
| Lack of fit | 60.63 | 10 | 6.06 | 77.96 | 0.0004 | Significant | |
| Pure error | 0.31 | 4 | 0.078 | ||||
| Total | 1462.96 | 28 | |||||
| Final viscosity (BU) | Model | 10,049.99 | 14 | 717.86 | 24.96 | < 0.0001 | Significant |
| Residual | 402.69 | 14 | 28.76 | ||||
| Lack of fit | 377.36 | 10 | 37.74 | 5.96 | 0.0501 | Not significant | |
| Pure error | 25.33 | 4 | 6.33 | ||||
| Total | 10,452.68 | 28 |
Table 4.
Estimated coefficients for the fitted polynomial representing the relationship between the individual responses and independent variables
| Source | Peak viscosity (BU) | Breakdown (BU) | Setback (BU) | Final viscosity (BU) | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient estimate | p value | Coefficient estimate | p value | Coefficient estimate | p value | Coefficient estimate | p Value | |
| Intercept | 110.07 | 99.13 | 4.07 | 15.17 | ||||
| A* | 90.83 | < 0.0001 | 79.42 | < 0.0001 | 6.18 | < 0.0001 | 17.04 | < 0.0001 |
| B* | 94.86 | < 0.0001 | 86.14 | < 0.0001 | 6.72 | < 0.0001 | 14.94 | < 0.0001 |
| C* | 8.33 | 0.2126 | 6.33 | 0.4024 | 0.72 | 0.2504 | 1.46 | 0.3622 |
| D* | − 1.08 | 0.8676 | 5.14 | 0.4950 | − 2.07 | 0.0040 | − 6.81 | 0.0006 |
| AB | 63.08 | < 0.0001 | 49.71 | 0.0016 | 5.54 | 0.0001 | 17.75 | < 0.0001 |
| AC | 4.92 | 0.6632 | − 0.67 | 0.9589 | − 0.75 | 0.4840 | 2.00 | 0.4681 |
| AD | 4.00 | 0.7228 | 13.46 | 0.3074 | − 2.92 | 0.0143 | − 10.63 | 0.0014 |
| BC | 6.25 | 0.5806 | 6.33 | 0.6258 | 0.17 | 0.8753 | − 0.58 | 0.8309 |
| BD | − 1.08 | 0.9233 | − 0.29 | 0.9820 | − 2.71 | 0.0211 | − 3.75 | 0.1837 |
| CD | 6.17 | 0.5856 | 6.17 | 0.6349 | − 0.58 | 0.5849 | − 0.54 | 0.8428 |
| A2 | − 10.20 | 0.2595 | − 20.59 | 0.0581 | 4.18 | 0.0002 | 14.26 | < 0.0001 |
| B2 | 30.26 | 0.0036 | 26.00 | 0.0207 | 3.12 | 0.0019 | 6.40 | 0.0088 |
| C2 | 6.47 | 0.4685 | 8.12 | 0.4293 | 0.45 | 0.5892 | − 0.33 | 0.8790 |
| D2 | 0.84 | 0.9241 | 0.83 | 0.9349 | 1.31 | 0.1329 | 1.28 | 0.5537 |
p-value ≤ 0.05 is statistically significant
*A Enzyme including temperature, B slurry concentration, C rotation speed, D enzyme amount
The highest peak viscosity, breakdown, setback, and final viscosity values were observed in the same sample which was coded as 15 (15% slurry concentration, 90 °C enzyme including temperature, 12.5 μL enzyme amount, and 250 rpm rotation speed). Peak viscosity is the highest measured value of viscosity attained by the slurry during the heating cycle. All of the samples in the top five in terms of peak viscosity have a concentration of 15%. Moreover, the common feature of the five samples with the lowest peak viscosity was that the enzyme including temperature of them was 70 °C. Adding 5 or 20 μL α-amylase into a chickpea slurry which has a concentration of 15% at 80 °C or into a chickpea slurry which has a concentration of 12.5% at 70 °C gave similar results in terms of peak viscosity value (Table 2). Similarly, mixing the slurry at a rotation speed of 200 or 300 rpm resulted in similar peak viscosities for samples which had the same concentration and were treated with the same amount of enzyme at the same temperature. These results explain the insignificance of the effects of enzyme amount and rotation speed on peak viscosity. Furthermore, it was observed that a hydrolysis condition could resulted in both the lowest peak viscosity and a moderate final viscosity and vice versa (Table 2). Therefore, peak viscosity should not be considered as an indicator of final viscosity in the case of external enzyme addition.
The effects of enzyme including temperature, slurry concentration, and their interaction were significant on breakdown value (p < 0.05) (Table 4). Breakdown followed a similar trend with peak viscosity. Higher breakdown values were observed in samples which had higher concentration and treated with α-amylase at higher temperatures. Samples which treated with the enzyme at 70 °C showed lower breakdown values (Table 2). The effects of the added enzyme amount and rotation speed were insignificant on breakdown (p > 0.05). The breakdown values of enzyme treated samples ranged between 4.67 and 278.30 BU (Table 2). However, the highest breakdown value observed in the samples without enzyme addition was 20.33 BU. Remarkably higher breakdown values of samples with added α-amylase compared to their counterparts without added α-amylase was attributed to the continuation of the enzyme inactivation process during the holding period at 90 °C.
Setback value varied between 1.66 and 28.33 BU in enzyme treated samples, while it ranged between 49.00 and 163.33 BU in samples without enzyme addition. Amylases are used extensively to retard retrogradation. Lower retrogradation tendency of α-amylase treated starch was attributed to the formation of low molecular weight dextrins which reduce the ability of residual starch to retrograde by interfering in the reassociation of starch chains (Fu et al. 2015; Wang et al. 2015). All of the independent variables except for rotation speed significantly affected the setback value of α-amylase treated samples (Table 4). Setback values tended to decrease with decreasing slurry concentration and enzyme including temperature and increasing enzyme amount.
Cold paste viscosity results (data not shown) of the enzyme added samples was almost equal to their final viscosity results unlike the samples without enzyme addition. The MVAG program was set to hold the content at 50 °C for 2 more min after the cooling step (from 90 to 50 °C). No remarkable viscosity change in enzyme-treated samples during the 2 min with continuous stirring was attributed to the complete inactivation of the enzyme. Final viscosity of α-amylase treated chickpea slurries ranged between 5 and 85 BU (Table 2). The effects of enzyme including temperature (A), slurry concentration (B), enzyme amount (D), the two-level interactions of enzyme including temperature x slurry concentration (AB) and enzyme including temperature x enzyme amount (AD) and the second-order effect of enzyme including temperature (A2) and slurry concentration (B2) were significant on final viscosity (p < 0.05) (Table 4). Briefly, lower final viscosities were obtained from samples which were prepared at lower concentrations and treated with higher enzyme amounts at lower temperatures.
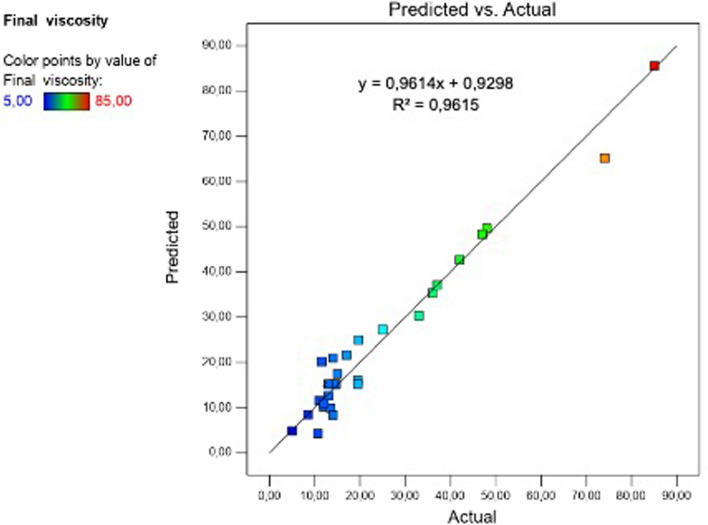
In this study, it was aimed to put forward the optimum condition that provides a crude chickpea milk viscosity below 10 BU at the end of the experiment (50 °C). Among the experienced 29 hydrolysis conditions, solely 3 combinations resulted in a final viscosity of 10 BU or less (Run no: 8, 17, and 20) (Table 2). The response surface quadratic model was the best fitted model to demonstrate the effects of the factors on the final viscosity. The model had a high F value and was statistically significant (p < 0.05). Besides, the p value of lack of fit was insignificant indicating that the model fits the experimental data accurately. The R2 (coefficient of determination) value was 0.9615. The R2 value of ≥ 0.6 is considered as a valid model (Gong et al. 2007). This coefficient, which ranges from 0 to 1, represents the part of the response variation that is attributable to variations of the factors and their interactions used in the model. The closer the R2 value is to 1 the higher the predictive power of the model (Lahlali et al 2008). The relationship between the actual and predicted final viscosity values was shown in Fig. 1. The predicted R2 of 0.7883 is in reasonable agreement with the adjusted R2 of 0.9229. The difference between predicted and adjusted R2 values is less than 0.2. The adjusted R2 is the corrected form of R2 according to the sample size and number of the terms in the model. Large differences between the two values may be interpreted as sign for a model with too many terms or a too small sample size (Haaland 1989). Adequate precision measures the signal to noise ratio and a ratio greater than 4 is desirable. The adequate precision value was 21.078 for the model which indicates an adequate signal. In conclusion, statistical parameters showed that the relationship between independent variables and final viscosity is adequately represented by the model.
Fig. 1.
The relationship between the actual and predicted final viscosity values
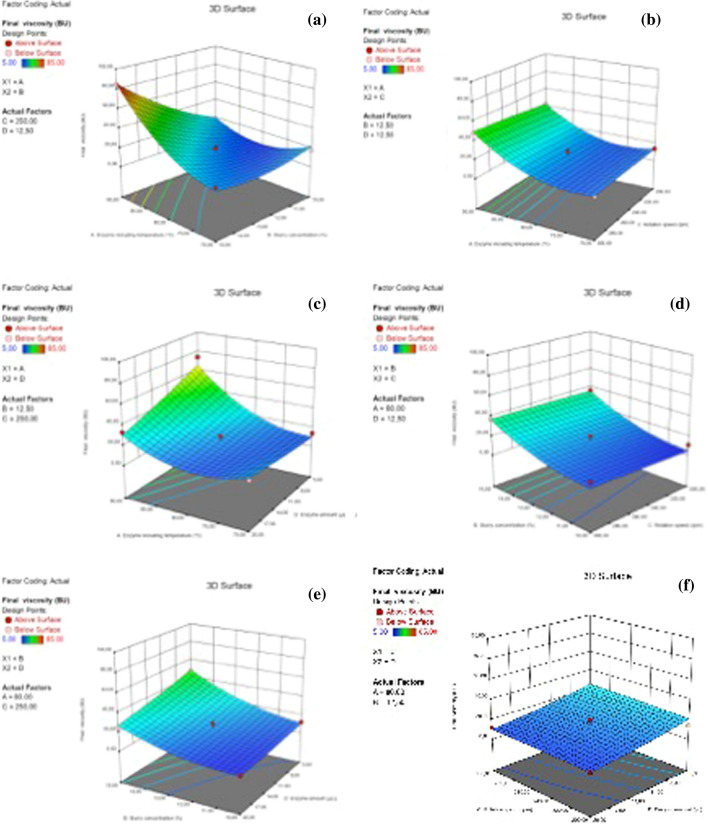
The 3D surface graphs for the combined effects of two factors on final viscosity of chickpea milks were shown in Fig. 2. There were two significant two-level interactions in the model: enzyme including temperature × slurry concentration (AB) (Fig. 2a) and enzyme including temperature x enzyme amount (AD) (Fig. 2c). The effect of the interactions of other factors was statistically insignificant on final viscosity (p > 0.05). It can be clearly seen that final viscosity remarkably increased when the enzyme including temperature was increased especially for samples with higher slurry concentration (Fig. 2a).
Fig. 2.
3D surface graphs for the combined effects of two factors on final viscosity
The quadratic term of enzyme amount had a negative effect on final viscosity indicating that the final viscosity decreased with increasing enzyme amount, as expected. Lower amounts of enzyme included at higher temperatures into the chickpea slurry resulted in higher final viscosity (Fig. 2c). The target for optimization was a final viscosity value between 0 and 10 BU. Optimization was performed using a multiple response method called desirability. Desirability value is an objective function that ranges from zero outside of the limits to one at the goal. For several responses and factors, all goals get combined into one desirability function (StateEase 2021) The higher the desirability value, the more appropriate the proposed parameters and targeted results become (Corzo and Gomez 2004). As a result of the MVAG experiments, numerous optimum hydrolysis conditions that resulted in a final viscosity lower than 10 BU and a desirability value of 1 were determined. Among them, 2 starch hydrolysis conditions may have a high potential to be used as the optimum condition. One is the optimum condition that predicted to be resulted in the lowest final viscosity (5.7 BU) which suggests preparing a slurry at a concentration of 10.0% and adding 12.9 µL of α-amylase at 79.8 °C while mixing the content at 230 rpm throughout the process. The actual final viscosity observed at the noted conditions was 9 BU, on average. The other optimum condition may be selected as the condition which enables minimum amounts of α-amylase contribution. This other optimum condition suggests preparing a slurry at a concentration of 11.4% and adding 5.2 µL of α-amylase at 75.6 °C while mixing the content at 205 rpm throughout the process. The actual final viscosity observed at the noted conditions was 9.5 BU, on average while it was predicted to be 8.8 BU. Besides, it should be noted that none of the optimum hydrolysis combinations had a slurry concentration higher than 12.7% and an enzyme including temperature higher than 85.5 °C.
Conclusion
To conclude, the effects of starch hydrolysis parameters on gelatinization and pasting properties of a crude chickpea milk were investigated using Box-Behnken response surface design. It was observed that slurry concentration and enzyme including temperature were the most critical factors that affect the pasting properties especially final viscosity of a crude chickpea milk. Optimization using response surface methodology, based on Box-Behnken approach, was a successful tool for determining the optimal starch hydrolysis conditions to obtain a suitable final viscosity. Moreover, the generated model predicted the actual data with a remarkably high degree of accuracy (R2 = 0.9615). The MVAG instrument apparently has the potential to be used for monitoring starch hydrolysis. However, external, and manual enzyme addition to the MVAG bowl may cause repeatability problems. Therefore, a modification on the instrument bowl that will allow the addition of enzymes from outside may be suggested for measuring not only the activity of intrinsic amylases but also the activity of external amylases or other enzyme sources.
Acknowledgements
The authors would like to thank to Brabender/Anamed & Analytic Group and to Melek Malkoç for their technical assistance.
Abbreviations
- MVAG
Micro visco-amylo-graph
- BU
Brabender unit
- ANOVA
Analysis of variance
- RAU
Reference amylase unit
- cP
Centipoise (mPa s)
Authors' contributions
NYT contributed to conception and design, acquisition of data, analysis and interpretation of data; FK carried out the experiments and contributed to the first draft of the manuscript, HP contributed to statistical analysis and NBT was second reader of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Neşe Yılmaz Tuncel, Email: neseyilmaz@comu.edu.tr.
Fatma Korkmaz, Email: fyilmaz@comu.edu.tr.
Havva Polat, Email: havva.polat@comu.edu.tr.
Necati Barış Tuncel, Email: baristuncel@comu.edu.tr.
References
- Cabanillas B, Jappe U, Novak N. Allergy to peanut, soybean, and other legumes: Recent advances in allergen characterization, stability to processing and IgE cross-reactivity. Mol Nutr Food Res. 2018;62(1):1700446. doi: 10.1002/mnfr.201700446. [DOI] [PubMed] [Google Scholar]
- Colonna P, Leloup V, Buléon A. Limiting factors of starch hydrolysis. Eur J Clin Nutr. 1992;46(2):17–32. [PubMed] [Google Scholar]
- Corzo O, Gomez ER. Optimization of osmotic dehydration of cantaloupe using desired function methodology. J Food Eng. 2004;64:213–219. doi: 10.1016/j.jfoodeng.2003.09.035. [DOI] [Google Scholar]
- Deswal A, Deora NS, Mishra HN. Optimization of enzymatic production process of oat milk using response surface methodology. Food Bioproc Tech. 2014;7:610–618. doi: 10.1007/s11947-013-1144-2. [DOI] [Google Scholar]
- Fu Z, Chen J, Luo SJ, Liu CM, Liu W. Effect of food additives on starch retrogradation: a review. Starch/staerke. 2015;67(1–2):69–78. doi: 10.1002/star.201300278. [DOI] [Google Scholar]
- Gong WJ, Zhang YP, Xu GR, Wei XJ, Lee KP. Optimization strategies for separation of sulfadiazines using BoxBehnken design by liquid chromatography and capillary electrophoresis. J Cent South Univ Technol. 2007;14(2):196–201. doi: 10.1007/s11771-007-0039-7. [DOI] [Google Scholar]
- Gugger ET, Galuska P, Tremaine A. United states patent application US20160309732A1: Legume-based dairy substitute and consumable food products incorporating same. USA: General Mills, Inc; 2016. [Google Scholar]
- Haaland PD. Statistical problem solving. In: Deeker M, editor. Experimental design in biotechnology. New York: CRC Press; 1989. pp. 1–18. [Google Scholar]
- Haas R, Schnepps A, Pichler A, Meixner O. Cow milk versus plant-based milk substitutes: a comparison of product image and motivational structure of consumption. Sustainability. 2019;11(18):5046. doi: 10.3390/su11185046. [DOI] [Google Scholar]
- Lahlali R, Massart S, Serrhini MN, Jijakli MH. A Box-Behnken design for predicting the combined effects of relative humidity and temperature on antagonistic yeast population density at the surface of apples. Int J Food Microbiol. 2008;122(1–2):100–108. doi: 10.1016/j.ijfoodmicro.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Lindahl L, Ahlden I, Öste R, Sjöholm I. US. 1997;5(686):123. [Google Scholar]
- Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr. 2016;56(3):339–349. doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- Paul AA, Kumar S, Kumar V, Sharma R. Milk analog: plant based alternatives to conventional milk, production, potential and health concerns. Crit Rev Food Sci Nutr. 2020;60(18):3005–3023. doi: 10.1080/10408398.2019.1674243. [DOI] [PubMed] [Google Scholar]
- Rincon L, Botelho RBA, de Alencar ER. Development of novel plant-based milk based on chickpea and coconut. LWT—Food Sci Technol. 2020;128:109479. doi: 10.1016/j.lwt.2020.109479. [DOI] [Google Scholar]
- Sethi S, Tyagi SK, Anurag RK. Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol. 2016;53(9):3408–3423. doi: 10.1007/s13197-016-2328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva ARA, Silva MMN, Ribeiro BD. Health issues and technological aspects of plant-based alternative milk. Food Res Int. 2020;131:108972. doi: 10.1016/j.foodres.2019.108972. [DOI] [PubMed] [Google Scholar]
- StateEase (2021) https://www.statease.com/software/design-expert/. Accessed 20 June 2021
- Suh D, Jane J. Comparison of starch pasting p roperties at various cooking conditions using the micro visco-amylo-graph and the rapid visco analyser. Cereal Chem. 2003;80:745–749. doi: 10.1094/CCHEM.2003.80.6.745. [DOI] [Google Scholar]
- Tester RF, Qi X, Karkalas J. Hydrolysis of native starches with amylases. Anim Feed Sci Technol. 2006;130(1–2):39–54. doi: 10.1016/j.anifeedsci.2006.01.016. [DOI] [Google Scholar]
- Triantafyllou AÖ (2002) United states patent application US6451369B1: Non-dairy, ready-to-use milk substitute, and products made therewith. Oatly AB
- Vykhodtsev SV (2010) United states patent application US 2010/0098805 A1: Probiotic oat-based food product and process for making the same
- Wang S, Li C, Copeland L, Niu Q, Wang S. Starch retrogradation: a comprehensive review. Compr Rev Food Sci Food Saf. 2015;14(5):568–585. doi: 10.1111/1541-4337.12143. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.