Abstract
The purpose of this study was to investigate the effects of white ginseng addition (1%, 1.5%, 2%, 2.5% and 3% of meat weight) on the physical and chemical properties of roast chickens. The parameters studied were basic characteristics (salting absorptivity, texture, shear force, pH and sensory evaluation), lipid and protein oxidation, volatile compounds and ginsenoside content. Headspace solid phase micro-extraction and gas chromatography-mass spectrometry (GC–MS) were used to identify the flavor compounds of samples. The changes in physical and chemical properties showed that white ginseng had a positive effect on the quality of roast chickens. The oxidation rate of lipid and protein decreased with the increase of white ginseng addition. In addition, the contents of Ginsenoside Rg1 (Rg1), Ginsenoside Re (Re) and Ginsenoside Rb1 (Rb1) in samples were 5.763 μg/g, 6.047 μg/g and 8.447 μg/g, respectively. Obtained data evidenced the possibility of improvement of the quality characteristics and enrichment of the flavor of roast chickens by adding white ginseng.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05394-4.
Keywords: White ginseng, Roast chicken, Lipid oxidation, Protein oxidation, Volatile flavor
Introduction
Chicken is a high-protein, low-fat, and phospholipid-rich food, second only to pork in global meat consumption and it is expected to exceed pork in global production in the future (FAS/USDA, 2021). Roast chicken is deeply loved by consumers because of its unique flavor. Roast chicken has the characteristics of beautiful appearance, bright color, fresh and tender meat and easy to chew (Zhou et al. 2019).
With the improvement of the modern economic level, people's attention on health is also increasing. In recent years, the functionality of food has received great attention from the scientific community, consumers and food manufacturers (Gul et al. 2016). Natural extracts often have certain functionalities, such as polysaccharides, polyphenols and flavonoids which often have excellent hygroscopicity and antioxidant properties. Therefore, natural functional substances applied to foods can better satisfy modern people’s pursuit of healthier food. Grape seed extract can reduce the content of thiobarbituric acid, improve the brightness value and prolong the storage period of roast chicken was proved by Guo et al. (2020). Herbs had a strong alleviating effect on reducing the content of mycotoxins during cooking chicken breast muscle (Sobral et al. 2019). Hence, the combination of natural active substances and roast chickens will be a new choice for the meat processing industry in the future.
Panax ginseng C. A. Meyer (P. ginseng) is a perennial herb belonging to the genus Panax L and family Araliaceae Juss, which is widely distributed and has been used in cooking and traditional medicine for the treatment of various diseases since ancient times (Ratan et al. 2021). Fresh ginseng can be directly made into white ginseng by sun drying, and the water content of white ginseng is less than 14%. White ginseng has the function of enhancing immunity, anti-tumor, anti-atherosclerosis and protecting myocardium (Hussain et al. 2021). Especially, ginsenoside in white ginseng can enhance immunity, improve hematopoietic function and reduce blood lipid (Lee et al. 2015). In addition, white ginseng polysaccharides have immunomodulatory, anti-tumor, anti-radiation and hypoglycemic activities, and pectin in white ginseng polysaccharides has hypoglycemic and antioxidant effects (Guo et al. 2021).
Although natural functional substances are beneficial to the human body, large doses of them will cause the food to produce a strong bitter flavor, which masks the taste of the food itself. Therefore, it is necessary to select an appropriate quantity of ginseng through experiments. By analyzing the composition of volatile flavor compounds, it is possible to explore which components affect the flavor of roast chickens, to be controlled in industrial production. In addition, ginseng is mainly used in beverage and dairy products at present, and the effects of ginseng on meat quality are rarely reported (Jung et al. 2017; Tárrega et al. 2012). Therefore, it is very necessary to study the addition of ginseng into roast chickens.
In this research, the effects of white ginseng on the basic characteristics of roast chickens were studied from the aspects of salting absorptivity, pH, texture, shear force and sensory evaluation. And the effects of white ginseng on lipid and protein oxidation were studied with the value of thiobarbituric acid and the content of protein sulfhydryl and carbonyl. In addition, the optimal addition amount of white ginseng was selected by sensory evaluation. Finally, the compositions of volatile flavor components were analyzed and compared with the control group. Therefore, the main purpose of this study was to evaluate the effect of white ginseng on the quality and flavor of roast chickens.
Materials and methods
Materials
Sanhuang chickens were purchased from Xiaohelong farmers market (Changchun, China). Roosters with a weight of 1500 ± 50 g at 120 days of age were selected for experiments. The chickens were stored at − 20 °C before the test. White ginseng (Dried ginseng with a growing period of fewer than 5 years) were purchased from the Middle East big market Xingwang ginseng shop (Changchun, China). Spices (clove, cinnamon, etc.) were purchased from the Dachang seasoning market (Changchun, China). Soybean oil (Golden Arowana, China).
Samples treatment
Roast chickens production process
1) Chicken pretreatment
Sanhuang chickens were selected in line with national food hygiene and safety standards. Cleaned the whole chicken to make sure it was free of bloodstain and other impurities.
2) Pickled liquid production
According to the weight of the meat, 1.5% green onion, 1.5% ginger, 1.5% garlic, 1.5% salt, 1.5% soy sauce, 1.5% cooking wine, 1% granulated sugar, 0.6% monosodium glutamate, 0.3% compound phosphate, 0.3% cinnamon, 0.3% radix angelicae, 0.3% anise, 0.3% rhizoma kaempferiae, 0.3% galangal, 0.3%sichuan pepper, 0.2% grass cardamom, 0.2% amomum kernel, 0.2% tangerine Peel, 0.2% dried Lemon, 0.2% dried Pepper, 0.2% fragrance amomum, 0.2% licorice, 0.2% myrcia, 0.2% cumin and 0.1% clove were added all to 30% water and cooked for 45 min. Filter the pickled liquid with three layers of sterile gauze to remove filtrate residue. After filtration, the filtrate was saved at 4 °C for further study.
3) Tumbled marinating
The cooled pickled liquid was evenly injected into the chest, leg, wing and joint of the whole chicken in a small amount at a fixed dose several times, and then was vacuumed storage at 4 ± 1 °C for 4 h. Following, sealed the pickled liquid with the chicken and make it vacuum-tumbled (GR-30, Zhucheng Ruiyang Machinery Co., Ltd, Shandong, China). Tumbling for 15 min and pausing for 15 min, the total effective tumbling time was 120 min and the vacuum degree was at 0.04 MPa.
4) Roasting
The whole chicken was brushed with edible soybean oil and roast in a far-infrared rotary roasting oven (KJL-08, Furong Food Machinery Factory, Shanghai, China) at 160 °C for 30 min, 180 °C for 20 min, and 120 °C for 30 min, successively. Finally, brush with honey for 10 min before the end of roasting to brighten the chicken. 6 chickens were roasted at the same time.
5) Cooling and Packing
The roast chicken was cooled to room temperature (25 °C) in a vacuum cooler (JSM-SF1M, JSIMa Machinery Co., LTD., Shandong, China) and vacuum-packed through a vacuum packing machine (DZ-600/2S, Xiaokang Machinery Co., LTD, Shandong, China), suction time 15 s, vacuum 0.06 MPa, using high-temperature heating sealing under aseptic conditions.
Extraction of white ginseng
Ginseng was crushed into powder through a pulverizer (FW135, Tester Instrument Co., LTD, Tianjin, China), and the powder was sifted through a 60-mesh sieve to ensure uniformity of the powder. Ginseng was extracted by distilled water (the solid to liquid ratio was 1:10). Ultrasonic-microwave combined reaction extraction technology (XO-SM50, Shunliu Instrument Co., LTD, Nanjing, China) was used to extract white ginseng in this experiment. The reaction system adopted the SAMSUNG microwave generation system from Korea. Mixed ginseng powder with water, under the microwave power of 250 W and ultrasonic frequency of 40 kHz, maintain the temperature at 40 °C and extracted for 40 min.
Treatment group and control group
The control group did not add white ginseng with pickling liquid. For the treatment group, the extract solutions of white ginseng (1%, 1.5%, 2%, 2.5% and 3% of meat weight) were added into the pickled liquid.
Physical analysis
Salting absorptivity
The total weight (m1) of the chicken was weighed before marinating. After vacuum tumbling, the chicken was removed from the tumbler and gently wiped off excess brine before weighing (m2). The calculation formula was as follow:
pH
The pH values of roast chicken were measured using a digital pH-meter (PHSJ-4A, Leici Instrument Factory, Shanghai, China).
Texture and shear force
The roast chicken sample was cut into square meat pieces (1 cm × 1 cm × 1 cm) along the vertical direction of myofibrils. The texture profile was measured and analyzed in the "secondary compression mode" (CT3-50 kg, Brookfield Co., USA) with the probe of TA39, the fixture of TA-BT, the number of cycles at 2, and the test speed at 1 mm/s. Hardness, cohesiveness, springiness, gumminess and chewiness were selected as measurement indexes.
Sensory evaluation
Sensory evaluation was conducted at the College of Food Science and Engineering of Jilin University by 10 trained team members (five females and five males, aged between 22 and 27) with prior experience and familiarity with meat products. At the beginning of the experiment, the panelists had a sensory evaluation of common roasted chicken products in the market, and were familiar with the flavor and aroma of roasted chicken and had reference to the experiment. The roast chicken breast samples were cut into cubes (15 mm × 15 mm × 15 mm) and coded with three random numbers. To avoid biasing responses, each panelist was provided with water and plain crackers for palate cleansing before tasting the samples. 6 groups of samples were provided for each sensory evaluation, with 3 parallel samples in each group. Before tasting, panelists were familiar with the aroma, flavor and taste of ginseng. A 9-point hedonic scale (1 = dislike extremely, 5 = neither like nor dislike, 9 = like extremely) was used to access the samples by considering appearance, aroma, texture, flavor and overall acceptability (Zhou et al. 2021; Samant et al. 2016).
Chemical analysis
Lipid oxidation assay
Lipid oxidation was determined by the thiobarbituric acid (TBA) method. The samples (10 g) were shaken with 50 mL of trichloroacetic acid (7.5%, w/v, 1 g/L EDTA) for 35 min. The filtrates (5 mL) were then mixed with 5 mL of TBA (0.02 M) and boiled in a water bath at 100 °C for 45 min. The cooled filtrate was centrifuged at 1600 rpm for 5 min, and then 5 mL of chloroform was added to the supernatant. After cooling, the absorbance of the supernatant was measured at 532 nm and 600 nm by an ultraviolet spectrophotometer (TU-1810, Huishi Biochemical Reagents Co., Ltd, Shanghai, China). The content of TBA reaction substances was calculated according to the standard curve of 1,1,3,3-tetraethoxypropane solution. The TBARs was calculated as follows:
where A532 nm and A600 nm are absorbances of the supernatant at wavelengths of 532 nm and 600 nm, respectively; 155 is the molar absorbance coefficient.
Protein sulfhydryl group content
The sulfhydryl group content was measured using 5,5′-dithiobis-2,2-nitrobenzoic acid (DTNB). 1 mL of 1 mg/L protein solution was mixed with 9 mL of 50 mM phosphate buffer (pH 8.0), and then 0.02 mL of 2 mM DTNB was vortexed and homogenized. After incubation for 1 h at 25 °C in the dark, the absorbance was measured at 412 nm. The sulfhydryl concentration was calculated using an extinction coefficient of 13.6 mM−1 ∙cm−1 and was expressed as nmol sulfhydryl/mg protein.
Protein carbonyl group content
The total carbonyls content was measured using 2,4-dinitrophenylhydrazine (DNPH). 3 mL of 5 mg/mL protein solution added 1 mL of 10 mM DNPH was used as the experimental group, and 3 mL of 5 mg/mL protein solution added 1 mL 2 M HCl was used as the control group. Both samples were stirred at room temperature for 1 h and 1 mL of 20% TCA was added. The samples were centrifuged at 8000 g for 5 min. The precipitate was washed three times with 2 mL of ethanol/ethyl acetate (1:1) solution to eliminate DNPH. After complete removal of DNPH residues, the precipitate was dissolved in 6 M guanidine hydrochloride and centrifuged at 4000 g for 10 min to remove insoluble fragments. The absorbance was measured at 412 nm. The carbonyl concentration was calculated using an extinction coefficient of 22.0 mM−1∙cm−1 and was expressed as nmol carbonyl/mg protein.
Volatile compounds analysis
Headspace solid phase micro-extraction combined with gas chromatography-mass spectrometry (5977A, Agilent Inc., USA) was used. Exactly 2 g of sample per treatment group was weighed into 20 mL SPME vials. The PDMS-100 solid-phase micro-extraction needle was inserted for extraction for 50 min. The extraction tip was recovered and the extraction needle was placed in a gas phase injector for thermal analysis. The analysis time was 30 s and the analysis temperature was 260 °C. Volatile flavor compounds were searched and matched by the NIST 11 mass spectrometry library, and the relative quantification was carried out by the peak area normalization method.
GC conditions: The type of chromatographic column was HP-1 Methyl Siloxane (30 m × 250 μm, 0.10 μm) and the injection temperature was 280 °C. The heating procedure was as follows: the initial column temperature was 50 °C and maintained for 3 min, and then increased to 260 °C at a rate of 10 °C/min and maintained for 10 min. The detection temperature was 280 °C. The non-shunt method was used, and the flow rate of carrier gas He (purity 99.99%) was 1 mL/min.
MS conditions: The energy of the electron ionization source was 70 eV. The temperature of the transmission line temperature was 280 °C, the temperature of the ion source was 230 °C, and the temperature of the quaternary bar was 150 °C. The full scanning mode was used, and the scanning range was 20–600 m/z.
Statistical analysis
The determinant was performed at least 3 replicates, and the results were expressed as the mean ± standard errors (SE). The statistical analysis and mean comparison were conducted using the statistical software package SPSS 19.0 (SPSS Company of Chicago, Illinois, USA). All data were analyzed by ANOVA. Statistical significance was defined at p < 0.05.
Results and discussion
Basic characteristics
As shown in Table 1, with the increase of the addition amount of white ginseng, the salting absorptivity of roast chicken presented upward trends (P < 0.05). This was because the polysaccharides in white ginseng extract solution reduced the moisture loss during the marinating and roasting. White ginseng extracts contain polysaccharides, the main components of which are amyloid glucan and pectin (Guo et al. 2021). Pectin is a hydrophilic plant gum with good moisture adsorption capacity (Einhorn-Stoll 2018). In this experiment, ultrasound-microwave combined reaction extraction technology was used to maintain the activity of macromolecular pectin and to improve the viscosity of the pickled liquid. The extraction rate of ginseng pectin with different chemical structures had been proved to be improved by extruding the extracted ginseng fiber (Ma, et al. 2019). In addition, white ginseng extracted at 40 °C in this experiment could retain more neutral sugars with higher bioactivity and thickening gel function (Chen et al., 2020). In conclusion, the increased salting absorptivity can improve the texture of the roast chicken and enhance the flavor, thus improving the overall quality.
Table 1.
Effects of adding amount of white ginseng on basic characteristics of roast chickens
| White ginseng (%) | Salting absorptivity (%) | pH | Hardness (N) | Cohesiveness | Springiness (mm) | Gumminess (N) | Chewiness (mJ) |
|---|---|---|---|---|---|---|---|
| 0 | 16.75 ± 0.73c | 5.465 ± 0.004e | 4.69 ± 0.15a | 0.55 ± 0.04a | 2.26 ± 0.03ab | 2.56 ± 0.03a | 5.81 ± 0.03a |
| 1 | 17.08 ± 0.17c | 5.513 ± 0.010d | 4.64 ± 0.59a | 0.55 ± 0.03a | 2.27 ± 0.03a | 2.52 ± 0.06a | 5.77 ± 0.02a |
| 1.5 | 17.79 ± 0.57c | 5.639 ± 0.007c | 4.55 ± 0.39a | 0.51 ± 0.04a | 2.27 ± 0.03a | 2.32 ± 0.03b | 5.27 ± 0.01b |
| 2 | 18.95 ± 0.92b | 5.736 ± 0.006b | 3.92 ± 0.14ab | 0.55 ± 0.04a | 2.24 ± 0.04ab | 2.15 ± 0.02c | 4.82 ± 0.03c |
| 2.5 | 20.27 ± 0.16a | 5.726 ± 0.006b | 3.98 ± 0.88ab | 0.52 ± 0.07a | 2.26 ± 0.02ab | 2.07 ± 0.02d | 4.67 ± 0.02d |
| 3 | 20.03 ± 0.61a | 5.751 ± 0.002a | 3.41 ± 0.35b | 0.56 ± 0.01a | 2.21 ± 0.04b | 1.91 ± 0.04e | 4.23 ± 0.03e |
Note: Results are expressed as means ± standard deviation, and different letters in the same column are significantly different (p < 0.05)
With the increase in the addition amount of white ginseng, the pH of roast chickens increased from 5.465 ± 0.004 to 5.751 ± 0.002 compared to the control group (P < 0.05). This is because white ginseng contains alkaloids, amines and alkaline inorganic salts, and its extract is weakly alkaline (Hyun et al. 2020). Therefore, the addition amount of white ginseng was positively correlated with the pH of roast chicken.
The increase of white ginseng content decreased the hardness of roast chicken (P < 0.05). When the amount of ginseng added accounted for 3% of the meat weight, the hardness decreased from 4.69 ± 0.15 to 3.41 ± 0.35 compared to the control. This was due to the combination of polysaccharides in ginseng extracts with water, which increased the salting absorptivity and led to the decrease of hardness (Einhorn-Stoll 2018). Similarly, with the increase in the addition amount of white ginseng, the gumminess and chewiness of roast chickens all showed decreasing trends (P < 0.05), and these indicators were positively correlated with hardness. In addition, there were no significant changes in cohesiveness and springiness (P > 0.05).
Texture characteristics affect sensory quality, and better texture leads to better product acceptability. According to the analysis of texture in the sensory evaluation, the product could have a better taste by reducing the hardness, viscosity and chewiness in a certain range. The experimental results showed that adding 1–3% white ginseng could improve the texture of roast chicken.
Sensory analysis
Figure 2 showed the results of sensory evaluation of roasted chicken with different white ginseng additions. There was no significant difference in appearance among each treatment (P > 0.05). This was probably because the addition of white ginseng did not affect the color of the skin of roast chickens. When the addition amount of white ginseng added up to 2.5% of meat weight, the aroma evaluation reached the maximum value of 7.82 ± 0.41. The results indicated that white ginseng could improve the aroma of roast chicken. In addition, the texture score increased from 6.35 ± 0.03 to 7.59 ± 0.06 with the increase of white ginseng in the range of 0–2.5% (P < 0.05). This was because the polysaccharides in the white ginseng extract combined with water to make the chicken meat softer in texture. A previous study showed that the interaction between polysaccharides, polyphenols and protein could improve the sensory quality of food, which may be the reason for this change in roast chicken (Foegeding et al. 2017).
Fig. 1.
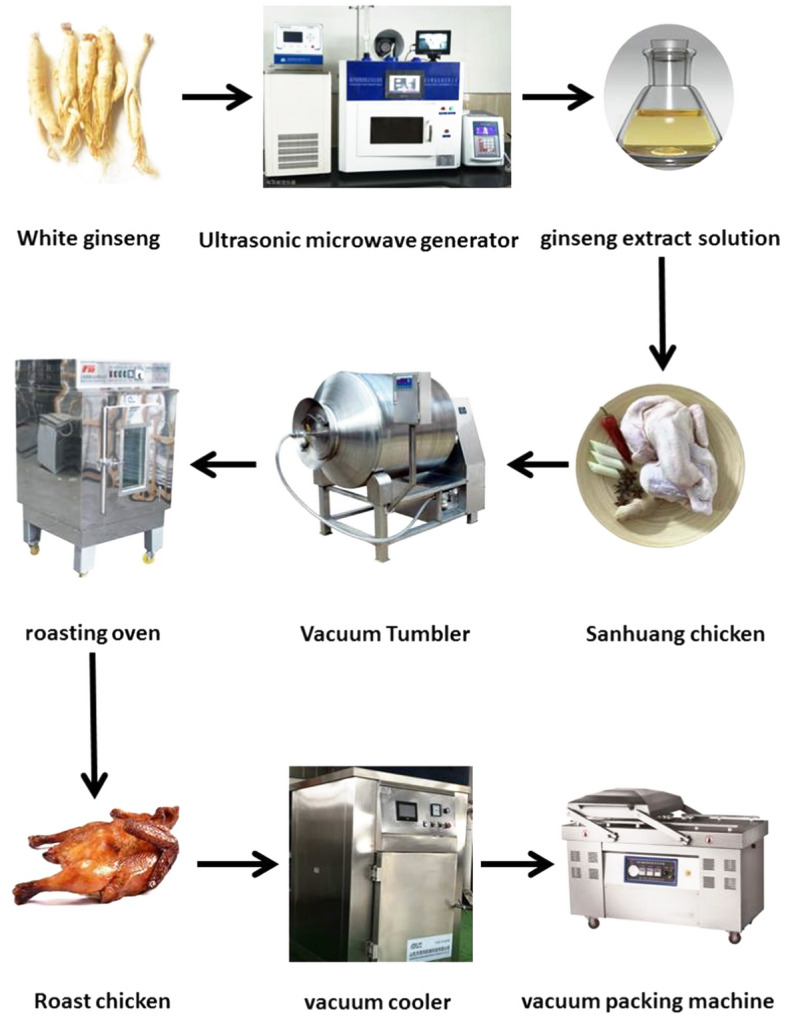
Production process flowchart of roast chicken
Fig. 2.
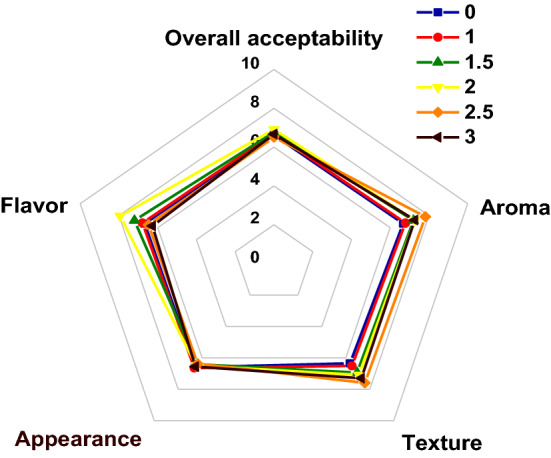
Effects of adding amount of white ginseng on sensory evaluation of roast chickens
The obtained results showed that the difference in the flavor of white ginseng treatment was significant (P < 0.05). With the increase of the addition amount of white ginseng, the flavor of roast chicken showed a trend of increasing and then decreasing (P < 0.05). When the amount of white ginseng added up to 2% of the meat weight, the best flavor evaluation (7.94 ± 0.74) was obtained. This may be since a certain amount of white ginseng increased the flavor of roast chicken, while excessive white ginseng will increase the bitterness. The bitterness of ginseng was attributed to the presence of triterpenoid peptides (Tárrega et al. 2012). The triterpenoid peptide is the main component of ginsenosides. With the increase of ginseng supplemental level, the bitter taste in roast chicken increased obviously, which was due to the increase of triterpenoid peptide content. Both the presence of ginsenosides and their thermal treatment affected some sensory properties of roast chickens, most notably an increase in bitterness and metallic taste. Our experimental study found that if more than 2% of ginseng is added to roasted chicken, the acceptable taste threshold of bitter taste in the human mouth will be exceeded and the sensory quality will be decreased. Furthermore, the overall acceptability was the highest when the white ginseng was added at a level of 2% of meat weight.
Lipid oxidation
The results of TBARS value were presented in Fig. 3(A), with the increase in the level of white ginseng addition, and the TBARS value of roast chickens significantly decreased from 0.748 ± 0.007 mg/kg to 0.243 ± 0.030 mg/kg (P < 0.05). This may be due to the inhibition of lipid oxidation by flavonoids, polysaccharides and ginsenosides in white ginseng through the inhibition of free radical initiated chain reactions and metal ion catalyzed oxidation reactions. In addition, the active components of white ginseng can also reduce the production of TBARS by reacting with lipid peroxides (Ratan et al. 2021). Ginseng polysaccharides exhibited high antioxidant activity in DPPH radical scavenging system with a dose–response relationship (Chen et al. 2019). In addition, ginseng polysaccharides can scavenge hydroxyl radicals and superoxide anion radicals were proved by Wang et al. (2012).
Fig. 3.
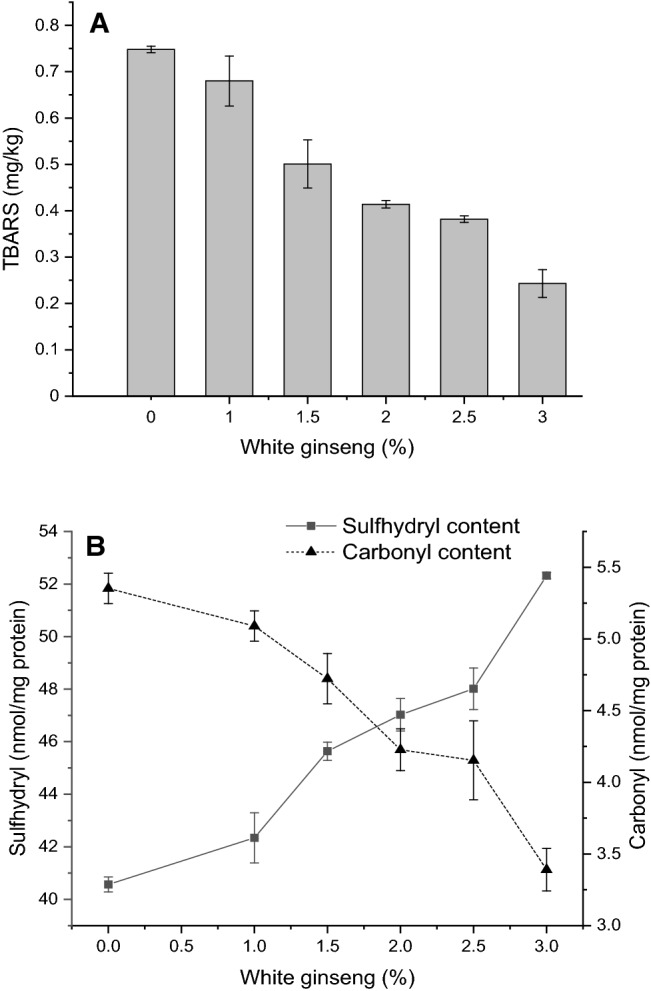
Effects of adding amount of white ginseng on lipid oxidation (TBARS, A) and protein oxidation (the content of Sulfhydryl and Carbonyl, B) in roast chickens
Lipid is the precursor of most volatile flavor components. Excessive oxidation of lipid will produce a rancidity odor, accelerate the deterioration of chicken, and affect the storage period. The results showed that the addition of white ginseng could inhibit lipid oxidation of roast chicken to prolong the flavor and quality.
Protein oxidation
As shown in Fig. 3(B), the content of sulfhydryl groups in roast chickens gradually increased from 40.567 ± 0.288 nmol/mg prot to 52.320 ± 0.121 nmol/mg prot with the increase of white ginseng addition (P < 0.05), which may be due to the competition of ginsenoside, polysaccharides and other active substances in the extract of white ginseng with sulfhydryl groups for free radicals to protect sulfhydryl groups from oxidation (Zainudin et al. 2021; Cheng et al. 2017). Moreover, plant extracts containing phenolic substances could reduce the oxidation of protein sulfhydryl groups in meat and scavenge free radicals (Martinez et al., 2020).
With the increase of white ginseng addition amount, the content of carbonyl groups in roast chickens showed a decreasing trend (from 5.353 ± 0.106 nmol/mg prot to 3.390 ± 0.148 nmol/mg prot; P < 0.05), which may be due to the antioxidant active substances in ginseng extracts that weakened the oxidation rate of amino acids in the protein side-chain (Morcuende et al. 2020; Soglia et al. 2020; Estévez et al. 2011). Mutton pie treated with chrysanthemum as a natural antioxidant could increase the content of the sulfhydryl group and inhibit protein carbonylation, which was consistent with the results of this experiment (Khan et al. 2020). In conclusion, the results showed that white ginseng could inhibit the oxidation of lipid and protein in roast chicken.
Protein oxidation can accelerate the spoilage of roast chicken, affecting the tenderness, moisture and color of meat. In addition, amino acids in proteins and carbonyl compounds undergo Maillard reactions to produce flavor compounds. Reducing the protein oxidation rate, on the one hand, can improve the quality and prolong the shelf life, on the other hand, can reduce the change of the flavor of roast chicken during storage.
Volatile compounds
Based on the above experimental results, roast chicken supplemented with 2% white ginseng was used as the treatment group. The volatile flavor compounds of the control and treatment groups were analyzed by ion mass spectrometry (ION) using GC–MS. There were significant differences in the volatile flavor components between the two groups of samples. They had some of the same flavor components, but their relative contents were different.
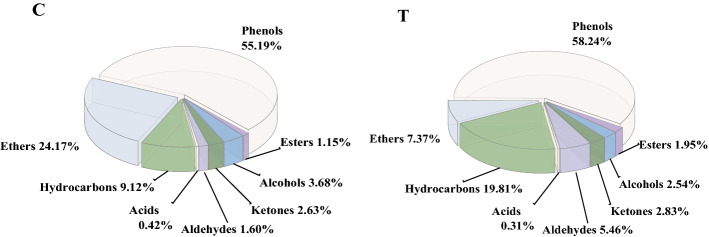
The relative content of each substance was analyzed by the area normalization method as shown in Table 2. A total of 87 volatile flavor compounds were identified in the samples of both groups, including 46 kinds of hydrocarbons, 6 kinds of aldehydes, 3 kinds of acids, 6 kinds of esters, 9 kinds of ketones, 3 kinds of ethers, 10 kinds of alcohols and 4 kinds of phenols. 39 and 70 kinds of volatile flavor compounds were identified in the control group and the experimental group, respectively. The volatile flavor compounds in the treatment group increased by 31 species and were more complex than those in the control group. The types and relative contents of volatile compounds in the control group and treatment group were shown in Fig. 4. The varieties and relative contents of hydrocarbons, aldehydes, esters, ketones and phenols in the treatment group were higher than those in the control group. It was stated that the addition of white ginseng could increase the variety of volatile flavor substances in roast chicken. The previous study has shown that lipid oxidation plausibly affected the change of aroma in jerky chicken (Silva et al. 2018). In this study, the addition of 2% white ginseng changed the rate of lipid oxidation in roast chicken, which may affect the composition of volatile flavor compounds. In addition, the volatile oil in white ginseng can also impart some aroma to roast chicken (Hyun et al. 2020).
Table 2.
Effects of white ginseng on the volatile compounds of roast chickens
| Code | Compound | Chemical formula | Control group | Treatment group |
|---|---|---|---|---|
| Relative content (%) | ||||
| Hydrocarbons | ||||
| A1 | (Z)-3-Heptene* | C7H14 | ND | 0.11 |
| A2 | 3-Thujene | C10H16 | 0.09 | ND |
| A3 | (1S)-(-)-alpha-Pinene | C10H16 | 0.40 | ND |
| A4 | Camphene | C10H16 | 0.08 | ND |
| A5 | beta-Phellandrene | C10H16 | 0.13 | ND |
| A6 | beta-Pinene | C10H16 | 0.24 | 0.08 |
| A7 | beta-Myrcene | C10H16 | 0.65 | ND |
| A8 | alpha-Phellandrene | C10H16 | 0.12 | 0.18 |
| A9 | 1R-.alpha.-Pinene | C10H16 | 0.07 | ND |
| A10 | a-Terpinene | C10H16 | 0.32 | 0.21 |
| A11 | D-Limonene | C10H16 | 3.94 | 0.73 |
| A12 | (Z)-13,7-dimethyl-3,6-octatriene | C10H16 | 0.21 | ND |
| A13 | g-Terpinene | C10H16 | 0.59 | 0.12 |
| A14 | D2-Carene | C10H16 | 0.24 | 0.03 |
| A15 | Cyclofeuchene* | C10H16 | ND | 0.08 |
| A16 | γ-Pyronene* | C10H16 | ND | 0.19 |
| A17 | 1-Methylnaphthalene | C11H10 | 0.13 | ND |
| A18 | 1-Undecene* | C11H22 | ND | 0.09 |
| A19 | 2,3,4,5-Tetramethyltricyclo[3.2.1.02,7]oct-3-ene* | C12H18 | ND | 0.36 |
| A20 | Dodecane | C12H26 | 0.18 | 0.16 |
| A21 | Cyclododecene, 1-methyl-* | C13H24 | ND | 0.20 |
| A22 | Tridecane* | C13H28 | ND | 0.17 |
| A22 | Tetradecane | C14H30 | 0.69 | ND |
| A24 | Himachala-2,4-diene* | C15H24 | ND | 0.16 |
| A25 | 1,4-Dimethyl-8-isopropylidenetricyclo[5.3.0.0(4,10)]decane* | C15H22 | ND | 0.20 |
| A26 | Bicyclo[5.3.0]decane,2-methylene-5-(1-methylvinyl)-8-methyl-* | C15H24 | ND | 1.98 |
| A27 | Caryophyllene-(I1) * | C15H24 | ND | 0.15 |
| A28 | 1,4,7,-Cycloundecatriene,1,5,9,9-tetramethyl,Z,Z,Z-* | C15H24 | ND | 1.22 |
| A29 | Isocaryophillene* | C15H24 | ND | 0.77 |
| A30 | 1H-Cycloprop[e]azulene,decahydro-1,1,7-trimethyl-4-methylene-* | C15H24 | ND | 0.62 |
| A31 | Bicyclogermacrene* | C15H24 | ND | 1.61 |
| A32 | Caryophyllene | C15H24 | 0.49 | 0.28 |
| A33 | b-Farnesene | C15H24 | 0.14 | 3.36 |
| A34 | alpha-Farnesene | C15H24 | 0.29 | ND |
| A35 | d-Cadinene | C15H24 | 0.12 | 0.27 |
| A36 | b-Chamigrene* | C15H24 | ND | 0.11 |
| A37 | Calarene* | C15H24 | ND | 1.11 |
| A38 | a-Gurjunene* | C15H24 | ND | 2.17 |
| A39 | g-Gurjunene* | C15H24 | ND | 0.65 |
| A40 | a-Elemene* | C15H24 | ND | 0.11 |
| A41 | a-Neoclovene* | C15H24 | ND | 1.43 |
| A42 | beta.-Neoclovene* | C15H24 | ND | 0.44 |
| A43 | α-Ethenyl-α,3-dimethyl-6-(1-methylethylidene)-3-cyclohexene-1-ethanol* | C15H24O | ND | 0.06 |
| A44 | 1α-(Cyclohexylmethyl)-4α-ethylcyclohexane* | C15H28 | ND | 0.13 |
| A45 | Hexadecane* | C16H34 | ND | 0.18 |
| A46 | Tetratriacontane* | C34H70 | ND | 0.09 |
| Aldehydes | ||||
| B1 | 2,3-Epoxy-4,4-dimethylpentane | C7H14O | 0.21 | ND |
| B2 | 3-Cyclohexen-1-carboxaldehyde, 3,4 -dimethyl-* | C9H14O | ND | 0.12 |
| B3 | (E,E)-2,4-decadienal* | C10H16O | ND | 0.12 |
| B4 | Hexadecanal | C16H32O | 1.39 | 4.93 |
| B5 | 9-Octadecenal, (Z)- * | C18H34O | ND | 0.19 |
| B6 | Octadecanal* | |||
| Acid | C18H36O | ND | 0.10 | |
| C1 | Acetic acid | C2H4O2 | 0.31 | 0.15 |
| C2 | Tetradecanoic acid* | C14H28O2 | ND | 0.16 |
| C3 | Propanoic acid,2-methyl-,1-(1,1-dimethylethyl)- 2-methyl-1,3-propanediyl ester | C16H30O4 | 0.11 | ND |
| Esters | ||||
| D1 | Phosphinic acid, dipropyl-, propyl ester* | C4H6O3 | ND | 0.45 |
| D2 | Ethyl p-methoxycinnamate | C12H14O3 | 0.38 | 0.47 |
| D3 | Terpinyl acetate | C12H20O2 | 0.54 | ND |
| D4 | Phthalic acid, isopentyl 3-methylp henyl ester* | C14H20O3 | ND | 0.14 |
| D5 | Diisobutyl phthalate | C16H22O4 | 0.23 | 0.55 |
| D6 | Propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl) -2-methyl-1,3-propanediyl ester* | C16H30O4 | ND | 0.34 |
| Ketones | ||||
| E1 | 4-Hydroxy-2-butanone* | C4H8O2 | ND | 0.05 |
| E2 | Coumarin | C9H6O2 | 0.62 | ND |
| E3 | Fenchon | C10H16O | 0.14 | 0.09 |
| E4 | Dihydrocarvone | C10H16O | 0.23 | ND |
| E5 | 3-methyl-6-(1-methylethyl)-2-Cyclohexen-1-one | C10H16O | 0.34 | ND |
| E6 | Xanthoxylin | C10H12O4 | 1.30 | 0.26 |
| E7 | Ethanone,1-(2-hydroxy-4,6-dimethoxyphenyl)- * | C14H10O2 | ND | 2.23 |
| E8 | D( +)-Carvone* | C10H14O | ND | 0.09 |
| E9 | 4'-tert-Butyl-2',6'-dimethylacetophenone* | C14H20O | ND | 0.11 |
| Ethers | ||||
| F1 | Allyl methyl sulfide* | C4H8S | ND | 0.04 |
| F2 | Estragole | C10H12O | 3.05 | 0.70 |
| F3 | Anethole | C10H12O | 21.12 | 6.63 |
| Alcohols | ||||
| G1 | Allyl mercaptan* | C3H6S | ND | 0.02 |
| G2 | Hinokitiol* | C10H12O2 | ND | 0.25 |
| G3 | 1,3-Methano-5bH-cyclobuta[cd]pentalen-5b-ol, octahydro-* | C10H14O | ND | 0.14 |
| G4 | Eucalyptol | C10H18O | 1.67 | 0.47 |
| G5 | Linalool | C10H18O | 0.94 | 0.27 |
| G6 | l-4-Terpineol | C10H18O | 0.94 | 0.27 |
| G7 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha., 5-trimethyl-, cis- | C10H18O2 | 0.13 | ND |
| G8 | Espatulenol* | C15H24O | ND | 0.65 |
| G9 | cis-nerolidol* | C15H26O | ND | 0.19 |
| G10 | Ginsenol* | C15H26O | ND | 0.28 |
| Phenols | ||||
| H1 | Eugenol | C10H12O2 | 55.19 | 57.84 |
| H2 | trans-m-Propenyl guaiacol* | C10H12O2 | ND | 0.14 |
| H3 | Durenol* | C10H14O | ND | 0.12 |
| H4 | Apiol* | C12H14O4 | ND | 0.14 |
Note: ND: Not detected; Treatment group: Roast chicken with adding 2% white ginseng;
"*" indicates the new flavor compound obtained by adding 2% white ginseng
Fig. 4.
Types and relative contents of volatile compounds in roast chickens. Note: C. The control group; T. The treatment group
The hydrocarbons are mainly produced by homolysis of alkoxy radicals in fatty acids. There were more kinds of hydrocarbons in both groups of samples, but their higher threshold had little effect on the flavor of roast chicken. Saturated alkanes have little direct contribution to flavor, while terpenes have a strong flavor. Beta-Pinene, alpha-Phellandrene, D-Limonene, Caryophyllene and so on were detected in samples from both groups with citrus and lemon aroma, mainly derived from spices (Zhou et al. 2021). In the treatment group, b-Farnesene, a-Gurjunene, g-Gurjunene and a-Elemene were all detected. They belong to the sesquiterpenoids in the essential oil of ginseng and have the aroma and biological activity of ginseng (Hyun et al. 2020; Lee et al. 2011).
Aldehydes have a low threshold and are an important component of the flavor of roast chicken fat. Samples from both groups contained high levels of hexadecanal and had fruit and lasting fragrance. (E, E) -2, 4-decadienal was detected in the treatment group as a major oxide of linoleic acid (Sun et al. 2014), which is considered one of the key characteristic volatiles of grease and fried flavored chicken. Three kinds of acid compounds were detected with relatively small contents in both groups of samples. Acetic acid was detected in both groups of samples. It is the main component of vinegar, but most of the acetic acid in the vinegar evaporates after roasting at high temperatures, and the detected acetic acid may be caused by microbial fermentation of organic matter. Tetradecanoic acid detected in the treatment group had creamy, fatty and cocoa flavors. In addition, a total of 6 ester compounds were detected in the samples of both groups, but at relatively low levels. The Ethyl p-methoxycinnamate in both groups was mainly derived from cinnamon. Especially, disobutyl phthalate was detected in both groups of samples, which is slightly soluble in water and mainly used as a plasticizer, probably related to the packaging materials of the raw materials (Chen et al. 2019).
Ketones are produced by lipid oxidation and Maillard reaction and have pleasant odors such as fragrance, cream and fruit. However, the threshold value of ketones was much higher than their isomeric aldehydes and ketones contributed less to the flavor of the chicken. In the two volatile groups, ketones accounted for 2.63% and 2.83% of the total volatiles, respectively. Fenchon and Xanthoxylin were found in both sample groups, mainly from cumin and pepper in the spices. The ethers were representative of the important components of the flavor of roasted chicken. Anethole was an important component of the main flavor of roasted chicken and was found in high levels in both groups of samples, accounting for 21.12% and 6.63% of the relative content, respectively. It is mainly derived from star anise spice and can increase the richness and flavor level of products, inhibit lipid oxidation, and stabilize the color and luster (Sun et al. 2014). Allyl methyl sulfide was detected in both groups of samples, mainly from garlic added during the marinating process.
Alcohols are produced by lipid oxidation and Scott reaction. Three identical alcohol compounds were detected in both groups of samples. Eucalyptus may be derived from ginger, Linalool may be derived from cinnamon and l-4-Terpenol is the main component of volatile oil. Eucalyptus and Linalool are important aroma active components in meat flavors. Hinokitiol, Espatulenol, cis-Nerolidol and Ginsenol were detected in the treatment group, which may be derived from white ginseng (Hyun et al. 2020). Hinokitiol is a natural compound of monoterpenes with an aromatic smell. Espatulenol is sesquiterpenes. cis-Nerolidol has a neroli flavor and woody fragrance with a long-lasting fragrance, and has the function of setting aroma. Ginsenol is specific alcohol of ginseng.
Phenolic compounds all have a special aromatic odor. The relative content of Eugenol in the two groups of samples accounted for 55.19% and 58.24% of the total volatile substances, respectively. Eugenol is mainly extracted from spices. It has the odor of cloves and has antibacterial and stomach-strengthening effects. There were 3 more phenolic compounds in the treatment group than the control group, among which trans-m-Propenyl guaiacol and Durenol have a smoky flavor, and Apiol might be derived from spices.
The sensory parameters of flavor and aroma were correlated with the flavor compounds measured by GC–MS. Compared with the control group without ginseng, the sensory evaluation of the treatment group supplemented with 2% ginseng was better, and more volatile flavor substances were detected by GC–MS.
Ginsenoside content
The content of ginsenoside in roast chicken supplemented with 2% white ginseng was determined according to the results of the quality characteristic test. The contents of Rg1, Re and Rb1 in the roast chicken were 5.763 μg/g, 6.047 μg/g and 8.447 μg/g, respectively. Ginsenosides can affect the sensory quality of roast chicken, and the increase of ginsenosides can increase the bitter taste of roast chicken. In addition, as the main functional component of ginseng, ginsenosides have the effects of improving immunity, anti-fatigue and anti-oxidation (Lee et al. 2015). Determination of ginsenoside content in roast chicken can provide a reference for similar products.
Conclusions
The obtained results showed that the addition of white ginseng could improve the salting absorptivity of roast chicken, resulting in more tender, soft and fragrant meat. White ginseng could improve the texture of roast chicken, enhance the flavor level, and significantly inhibit the oxidation of fat and protein. Compared with the control group, roasted chickens supplemented with 2% white ginseng increased 31 volatile flavor compounds. Ginsenoside was detected in the final product, which proved that the addition of white ginseng improved the nutritional value of roast chicken. With more and more consumers pursuing the natural health care of food and paying attention to the nutritional value of food, the inclusion of white ginseng in roast chicken has a potential market prospect.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks for the support of the staff of the Laboratory in Food Science and Engineering and the support from the Changchun Key Research Development Program (21ZGN37).
Author contributions
Conceptualization, Methodology, Supervision, YZ; Formal nalysis, Investigation, Experiment, Writing–Original Draft Preparation, XG; Investigation, Material, ZL; Investigation, Material, QM; Writing–Review & Editing, Visualization, LW.
Funding
Funding information is not applicable/No funding was received.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not Applicable.
Declarations
Conflict of interest
The authors have declared no conflict of interest for this article.
Ethics approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- FAS/USDA. 2021. Livestork and poultry: world markets and trade. Accessed Apr. 2021. http://www.fas.usda.gov/data/livestock-and-poultry-world-markets-and-trade
- Chen F, Huang G. Antioxidant activity of polysaccharides from different sources of ginseng. Int J Biol Macromol. 2019;125:906–908. doi: 10.1016/j.ijbiomac.2018.12.134. [DOI] [PubMed] [Google Scholar]
- Chen Y, He Q, Shen D, Jiang Z, Eremin SA, Zhao S. Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the diisobutyl phthalate in yoghurt. Food Control. 2019;105:38–44. doi: 10.1016/j.foodcont.2018.11.052. [DOI] [Google Scholar]
- Chen J, Cheng H, Zhi Z, Zhang H, Linhardt RJ, Zhang F, Chen S, Ye X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocolloids. 2021;112:106160. doi: 10.1016/j.foodhyd.2020.106160. [DOI] [Google Scholar]
- Cheng J, Liu X, Zhang Y, Zhang Y, Chen Z, Tang D, Wang J. Protective effects of momordica grosvenori extract against lipid and protein oxidation-induced damage in dried minced pork slices. Meat Sci. 2017;133:26–35. doi: 10.1016/j.meatsci.2017.04.238. [DOI] [PubMed] [Google Scholar]
- Einhorn-Stoll U. Pectin-water interactions in foods – from powder to gel. Food Hydrocolloids. 2018;78:109–119. doi: 10.1016/j.foodhyd.2017.05.029. [DOI] [Google Scholar]
- Estévez M, Ventanas S, Heinonen M, Puolanne E. Protein carbonylation and water-holding capacity of pork subjected to frozen storage: effect of muscle type, premincing, and packaging. J Agric Food Chem. 2011;59(10):5435–5443. doi: 10.1021/jf104995j. [DOI] [PubMed] [Google Scholar]
- Foegeding EA, Plundrich N, Schneider M, Campbell C, Lila MA. Protein-polyphenol particles for delivering structural and health functionality (reprinted from food hydrocolloids, vol 72, pg 163–173, 2017) Food Hydrocolloids. 2018;78:15–25. doi: 10.1016/j.foodhyd.2018.02.047. [DOI] [Google Scholar]
- Gul K, Singh AK, Jabeen R. Nutraceuticals and functional foods: the foods for the future world. Crit Rev Food Sci Nutr. 2016;56(16):2617–2627. doi: 10.1080/10408398.2014.903384. [DOI] [PubMed] [Google Scholar]
- Guo Y, Huang J, Chen Y, Hou Q, Huang M. Effect of grape seed extract combined with modified atmosphere packaging on the quality of roast chicken. Poult Sci. 2020;99(3):1598–1605. doi: 10.1016/j.psj.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Shao S, Wang D, Zhao D, Wang M. Recent progress in polysaccharides from panax ginseng C. A Meyer Food & Function. 2021;12(2):494–518. doi: 10.1039/d0fo01896a. [DOI] [PubMed] [Google Scholar]
- Hussain F, Akram A, Hafeez J, Shahid M. Biofunctional characterization of red, black and white ginseng (panax ginseng meyer) root extracts. Revista Mexicana De Ingenieria Quimica. 2021;20(1):173–184. doi: 10.24275/rmiq/Bio1735. [DOI] [Google Scholar]
- Hyun SH, Kim SW, Seo HW, Youn SH, Kyung JS, Lee YY, In G, Park C, Han C. Physiological and pharmacological features of the non-saponin components in korean red ginseng. J Ginseng Res. 2020;44(4):527–537. doi: 10.1016/j.jgr.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Lee N, Paik H. Bioconversion, health benefits, and application of ginseng and red ginseng in dairy products. Food Sci Biotechnol. 2017;26(5):1155–1168. doi: 10.1007/s10068-017-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Xu W, Wang D, Yun A, Khan A, Zongshuai Z, Ijaz MU, Yiqun C, Hussain M, Huang M. Antioxidant potential of chrysanthemum morifolium flower extract on lipid and protein oxidation in goat meat patties during refrigerated storage. J Food Sci. 2020;85(3):618–627. doi: 10.1111/1750-3841.15036. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee HJ, Yu HJ, Ju DW, Kim Y, Kim C, Kim C, Cho Y, Kim N, Choi S, Suh HJ. A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng (panax ginseng CA meyer) J Sci Food Agric. 2011;91(8):1466–1473. doi: 10.1002/jsfa.4334. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong J, Eun S, Kim D. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. 2015;762:333–343. doi: 10.1016/j.ejphar.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Ma X, Jin Z, Jin T. Effects of extrusion conditions on chemical properties of extruded white ginseng root hair. J Sci Food Agric. 2019;99(6):3186–3191. doi: 10.1002/jsfa.9535. [DOI] [PubMed] [Google Scholar]
- Martínez L, Jongberg S, Ros G, Skibsted LH, Nieto G. Plant derived ingredients rich in nitrates or phenolics for protection of pork against protein oxidation. Food Res Int. 2020;129:108789–108789. doi: 10.1016/j.foodres.2019.108789. [DOI] [PubMed] [Google Scholar]
- Morcuende D, Vallejo-Torres C, Ventanas S, Martinez SL, Ruiz SC, Estevez M. Effectiveness of sprayed bioactive fruit extracts in counteracting protein oxidation in lamb cutlets subjected to a high-oxygen MAP. Foods. 2020;9(11):1. doi: 10.3390/foods9111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan ZA, Haidere MF, Hong YH, Park SH, Lee J, Lee J, Cho JY. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45(2):199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samant SS, Crandall PG, O’Bryan CA, Lingbeck JM, Martin EM, Tokar T, Seo H. Effects of smoking and marination on the sensory characteristics of cold-cut chicken breast filets: a pilot study. Food Sci Biotechnol. 2016;25(6):1619–1625. doi: 10.1007/s10068-016-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FAP, Estévez M, Ferreira VCS, Silva SA, Lemos LTM, Ida EI, Shimokomaki M, Madruga MS. Protein and lipid oxidations in jerky chicken and consequences on sensory quality. Food Sci Technol. 2018;97:341–348. doi: 10.1016/j.lwt.2018.07.022. [DOI] [Google Scholar]
- Sobral MMC, Cunha SC, Faria MA, Martins ZE, Ferreira IMPLVO. Influence of oven and microwave cooking with the addition of herbs on the exposure to multi-mycotoxins from chicken breast muscle. Food Chem. 2019;276:274–284. doi: 10.1016/j.foodchem.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Soglia F, Baldi G, Petracci M. Effect of the exposure to oxidation and malondialdehyde on turkey and rabbit meat protein oxidative stability. J Food Sci. 2020;85(10):3229–3236. doi: 10.1111/1750-3841.15403. [DOI] [PubMed] [Google Scholar]
- Sun L, Chen J, Li M, Liu Y, Zhao G. Effect of star anise (illicium verum) on the volatile compounds of stewed chicken. J Food Process Eng. 2014;37(2):131–145. doi: 10.1111/jfpe.12069. [DOI] [Google Scholar]
- Tárrega A, Salvador A, Meyer M, Feuillère N, Ibarra A, Roller M, Terroba D, Madera C, Iglesias JR, Echevarría J, Fiszman S. Active compounds and distinctive sensory features provided by american ginseng (panax quinquefolius L.) extract in a new functional milk beverage. J Dairy Sci. 2012;95(8):4246–4255. doi: 10.3168/jds.2012-5341. [DOI] [PubMed] [Google Scholar]
- Wang R, Chen P, Jia F, Tang J, Ma F. Optimization of polysaccharides from panax japonicus C.A. meyer by RSM and its anti-oxidant activity. Int J Biol Macromol. 2012;50(2):331–336. doi: 10.1016/j.ijbiomac.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Zainudin MAM, Jongberg S, Lund MN. Combination of light and oxygen accelerates formation of covalent protein-polyphenol bonding during chill storage of meat added 4-methyl catechol. Food Chem. 2021;334:127611–127611. doi: 10.1016/j.foodchem.2020.127611. [DOI] [PubMed] [Google Scholar]
- Zhou R, Grant J, Goldberg EM, Ryland D, Aliani M. Investigation of low molecular weight peptides (<1 kDa) in chicken meat and their contribution to meat flavor formation. J Sci Food Agric. 2019;99(4):1728–1739. doi: 10.1002/jsfa.9362. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang X, Chen Y, Yuan B. Effects of different paprikas on the quality characteristics and volatile flavor components of spiced beef. J Food Process Preserv. 2021 doi: 10.1111/jfpp.15353. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Not Applicable.