Abstract
The effect of replacement of wheat flour with buckwheat flour at levels of 10, 20, 30, 40, and 50% on nutritional, texture, and physicochemical characteristics of bread was studied. Among others, parameters such as amino acid profile, antioxidant properties, and inositol phosphate content were determined. Amino acid score was calculated in order to evaluate the biological value of the bread protein. The breads with buckwheat flour were characterized by significantly lower whiteness of the crumb, compared to wheat bread. A positive effect of 10, 20, and 30% buckwheat flour content on the reduction of the crumb hardness, gumminess, chewiness was observed in comparison to other bread samples. A positive effect of buckwheat flour in the amount of 10–30% on the texture parameters and slowing down the process of bread staling was observed. The antioxidant properties and inositol phosphates increased with the share of buckwheat flour in the formula. A significant increase in protein was observed in bread from 20% share of buckwheat flour. The limiting amino acid of the protein of the tested flours and breads was lysine. For wheat bread, the amino acid score was 44.71% and for those with buckwheat flour it ranged from 45.67 to 75.38%.
Keywords: Wheat–buckwheat bread, Amino acid profile, Nutritional value of protein, Texture properties of the crumb, Inositol phosphates
Introduction
Grain products, including bread, are perhaps the most common food products in the world. Bread takes different forms, resulting from composition and baking methods, depending on the region of origin. For years, there has been a tendency in the bakery industry to develop different products using cereals and so-called pseudo cereal with health-promoting properties. Raw materials with potential functional ingredients are of great interest, particularly buckwheat as well as oats, millet, amaranth, quinoa, etc. Therefore, the production of various bakery products, not only based on buckwheat flour, containing several health-promoting ingredients, such as fiber, antioxidants, minerals, has gained a lot of interest in recent years. Consumption of these products can contribute to the improvement of health, additionally reducing the risk of dietary diseases (Zhang et al. 2007). Skrabanja et al. (2001) showed that the addition of buckwheat to bread significantly lowers the postprandial blood glucose response and the level of insulin compared to white wheat bread. In this regard, buckwheat, which is increasingly used as a gluten-free additive in bread, pastry products, pasta, and extruded products (Giménez-Bastida et al. 2015; Wójtowicz et al. 2013) receives particular attention. From a nutritional perspective, buckwheat is a valuable source of protein, presenting high biological value, micronutrients, dietary fibers, and flavonoids, e.g., rutin and many antioxidant compounds such as tocopherols, kaempferol, quercetin and phenolic acids (Giménez-Bastida et al. 2015; Sindhu and Khatkar 2019). It should be emphasized that buckwheat flour has no allergic prolamins, allowing it to be used in the production of food suitable for people sensitive to gluten proteins (Wronkowska et al. 2013).
Studies carried out by Zhang et al. (2007) show that the long-term consumption of buckwheat and buckwheat-based products can be a factor preventing diseases such as hypertension, elevated blood sugar levels, cardiovascular diseases, and dyslipidemia.
Appreciating the potential nutritional value of buckwheat flour as an additive in bread production, it was decided to study the possibility of using this flour for the enrichment of bread products, and the influence of this flour on the chemical, nutritional as well as the technological quality of bread with its different percentage content. The aim of the study was to analyze and compare selected chemical, nutritional and texture features of the crumb in bread with 10, 20, 30, 40 and 50% content of buckwheat flour, on the day of baking and after 3-day storage period. Most of the studies published so far concern the addition of buckwheat flour for food production, most often up to a share not exceeding 30 (Chłopicka et al. 2012; Gavurniková et al. 2011; Lin 2009) or 40% (Selimović et al. 2014; Wójtowicz et al. 2013; Wronowska et al. 2013). Our research takes into account and extends the current view on the issues related to the use of buckwheat flour in the production of bread. The current work should be a valuable extension of the previous achievements with regard to the biological value of protein and the presence of phytates in buckwheat flour and the bread obtained with it.
Materials and methods
Materials
The study material consisted of wheat bread (WS), obtained from refined wheat flour (PZZ Krakow, Poland) and bread in which a part of the wheat flour was replaced by buckwheat flour (Melvit S.A. Warsaw, Poland), in quantities of 10 (WB10), 20 (WB20), 30 (WB30), 40 (WB40) and 50 percent (WB50), regarding the wheat flour weight basis. The recipe consisted of 10.5 kg of refined wheat flour, 6.41 dm3 of water, 0.32 kg of yeast and 0.21 kg of salt. Thirty loaves (500 g each) of each type of bread were obtained.
Bread preparation
The dough was prepared using a single-phase method in a fast-rotating spiral mixer Ibis MS 130 (IBIS, Szubin, Poland). After initial fermentation (60 min, 21 ºC, 60% relative humidity), the dough was divided into pieces, formed, and placed in metal molds. After the fermentation (45 min, 37 ºC, 90% relative humidity), the bread was baked in a MIWE IDEAL (MIWE Michael Wenz GmbH, Arnstein, Germany) batch oven at 230 °C for 40 min.
Methods
Analysis of basic features
Two hours after baking, the following characteristics were noted: total baking loss (in %) calculated as the difference between the mass of the raw roll and the mass of the roll one hour after baking; the specific volume of bread (in cm3), which was evaluated by the AACC-approved Method 10–05.01 (AACC 2000); and crumb moisture (in %) estimated using the gravimetric method (AOAC 2006, Method No. 925.10).
Color analysis
Color was determined by the instrumental method according to the CIElab (L*, a*, b*) system using a Konica Minolta CM-3500d spectrophotometer (Konica Minolta Sensing, Osaka, Japan) as described previously (Mikulec et al. 2019).
Antioxidant properties
Total phenolic content
Total phenolic content was estimated using Folin–Ciocalteu reagent (Meda et al. 2005). Samples were prepared at 0.05 g/mL concentration using methanol/water solution (1:1 v/v) and filtered through a disc filter, size 0.45 µm. The filtrate was mixed with Folin–Ciocalteu reagent solution (1:10 v/v) and sodium carbonate solution (75 mg/mL) in the proportion 1:25:25 and mixed. After 1 h of incubation at ambient temperature in the dark, the absorbance was measured spectrophotometrically (Spectro UV–VIS Dual Beam UVS-2800, Labomed, Inc. USA) at a wavelength of 760 nm. A standard curve was made for gallic acid in the range from 14.0 to 112.0 mg/L (R2 = 0.9981) with limits of detection and quantification of 6.38, and 19.34 mg/L, respectively. Results were expressed as mg of gallic acid equivalent (GAE) per kg of product. Measurements were done in duplicate.
Free radical scavenging ability
Antioxidant activity against the DPPH• free radical was done according to Turkmen et al. (2006). Sample solutions (0.05 g/mL) were filtered and then 5.0 mL of filtrate was mixed with 1.5 mL of FRAP solution and incubated at 37 °C for 0.5 h. Subsequently, absorbance was measured spectrophotometrically (Spectro UV–VIS Dual Beam UVS-2800, Labomed, Inc. USA) at a wavelength of 593 nm. FRAP consisted of acetate buffer (pH 3.6) with 2,4,6-tri(2-pyridyl)-s-triazine (10 mM in 40 mM HCl) and FeCl3 (20 mM aqueous solution) in 10:1:1 proportion.
A standard curve was made for FeSO4 in the range from 0.01 to 0.08 mM (R2 = 0.9995) with limits of detection and quantification of 0.002 and 0.006 mM, respectively. Results were expressed as µM of Fe2+ per kg of the product. Measurements were conducted in duplicate.
Ferric ion reducing capability
Ferric ions reducing ability was established according to Benzie and Strain (1996). Sample solutions (0.05 g/mL) were filtered and then 5.0 mL of filtrate was mixed with 1.5 mL of FRAP solution and incubated at 37 °C for 0.5 h. Subsequently absorbance was measured spectrophotometrically (Spectro UV–VIS Dual Beam UVS-2800, Labomed, Inc. USA) at a wavelength of 593 nm. FRAP consisted of acetate buffer (pH 3.6) with 2,4,6-tri(2-pyridyl)-s-triazine (10 mM in 40 mM HCl) and FeCl3 (20 mM aqueous solution) in 10:1:1 proportion.
A standard curve was made for FeSO4 in the range from 0.01 to 0.08 mM (R2 = 0.9995) with limits of detection and quantification of 0.002 and 0.006 mM, respectively. The results were expressed as µM of Fe2+ per kg of the product. Measurements were conducted in duplicate.
Protein content and amino acid composition
Protein content (N × 6.25) was determined by the Kjeldahl method (AOAC 950.36). Amino acid analysis was performed according to the method as described by Cieślik et al. (2017). Lyophilized samples were hydrolyzed using 6 M HCl containing 0.5% phenol at 110 °C for 24 h under an argon atmosphere. The hydrolysates were lyophilized, dissolved in buffer solution (sodium citrate buffer pH 2.2) and filtered through a 0.45 μm syringe filter before being applied to the amino acid analyzer. Amino acids were determined by ion-exchange chromatography, with a strong cation ion exchanger and sodium-citrate elution buffer system followed by post-column derivatization with ninhydrin and spectrophotometric detection at 570 and 440 nm, according to the standard protocol of manufacturer. Sulfur-containing amino acids were analyzed as oxidation products obtained by performic acid oxidation followed by a standard hydrolysis procedure with HCl. For calibration of the amino acid analyzer, the amino acid standard solution was used (Sigma, USA). Evaluation of the acquired data was performed using the software of the chromatographic device (Chromulan, Pikron, Czech Republic). Tryptophan was not determined as it decomposed during acid hydrolysis, and asparagine and glutamine turned to aspartic acid and glutamic acid and were determined in these forms.
Protein nutritional quality
Based on the amount and type of amino acids, the nutritional value of protein amino acid Score (AAS) was calculated (Eq. 1):
| 1 |
*recommended amino acid scoring patterns for adolescents and adults, according to FAO (2013).
Total phytate and inositol phosphate determination
Extraction of inositol phosphates from samples was conducted according to Sandberg et al. (1987). Briefly, 0.5 g of samples was extracted in 20 mL of 0.5 M hydrochloric acid at 20 °C for 3 h. Extracts were centrifuged for 10 min at 6000×g, frozen overnight, centrifuged again, and separated from the extract by ion exchange chromatography using 2 g of AG 1 × X8 anion-exchanger (200–400 mesh, Bio-Rad). Eluates were evaporated and redissolved in deionized water and injected into an HPLC/RI system (Knauer, Germany). The separation of inositol phosphates was done using an Aquasil C18 column (with guard column) working at 40 °C and at the flow rate of 0.8 mL/min. The mobile phase consisted of 0.05 M formic acid: methanol (HPLC grade) 49:51 and 1.5 mL/100 mL of tertbutylammonium hydroxide (40%, Sigma Aldrich) were added. The pH was adjusted to 4.3 by addition of sulfuric acid. Calibration of the HPLC/RI system was done using sodium phytate (Sigma Aldrich) as the external standard and standard solutions containing 0.05–1.0 μmol/mL of phytate were prepared. Correction factors for differences in detector response of inositol hexa- (IP6), penta- (IP5), tetra- (IP4) and triphosphates (IP3) were calculated as described previously (Sandberg et al. 1987).
Texture analysis
Selected mechanical features of the crumb, such as hardness, chewiness, gumminess, springiness, and resilience according to Szcześniak (2002), were determined with the penetration test, using a single-arm TA.XT2.Plus texture analyzer (Stable Micro System Ltd, Godalming, Surrey, United Kingdom). A P/36R aluminum probe was used with a diameter of 36 mm. The measurement was performed with the following settings: probe movement rate: 2 mm·s−1, 50% deformation of the entire sample height, 5 s intermission between the first and the second compression. Measurements were conducted in eight repetitions.
Statistical analysis
Statistical analysis was carried out using Statistica 13.3 (StatSoft, Tulsa, USA). Analysis of variance and the statistical significance of differences was performed using Duncan’s test at p ≤ 0.05. A two-way ANOVA for percentage of substitution, day of storage and their interaction was used to test texture features at a significant level p ≤ 0.05. When the Levene test indicated significant differences the post-hoc Fisher’s least significant difference test was performed. The results were presented as mean ± standard deviation.
Response surface analysis was carried out at p < 0.05. Experimental data were fitted to a second-order polynomial model (Eq. 2), as follows:
| 2 |
where Y is the dependent variable; β0 is a constant; βi, βii, and βij are coefficients estimated by the model (linear, quadratic and the coefficient for the interaction effect) and Xi and Xj are independent variables.
Results and discussion
Basic features
Wheat bread was characterized by a slightly lower, although not significant, baking weight loss (10.45%), compared to bread supplemented with buckwheat flour which ranged from 10.9 to 12.02% (Table 1). The bread with 40 and 50% content of buckwheat flour was characterized by a significantly smaller volume of loaves compared to the other samples (Table 1). The reason for this phenomenon can be seen in the change in the gluten protein content after replacing part of the wheat flour with glutenfree buckwheat flour as well as in the increased content of fiber which reduces the ability to retain fermentation gases (Gavurniková et al. 2011). Similar results were obtained by Psodorov et al. (2014), who observed changes in the volume of bread with buckwheat flour, starting from its 20% share in relation to wheat flour. Moisture content on the day of baking was significantly higher for wheat bread (46.52%), compared to those supplemented with buckwheat flour. The value of this parameter decreased significantly with the higher share of buckwheat flour (Table 1).
Table 1.
Selected quality parameters of wheat bread supplemented with buckwheat flour
| Kind of bread | WS | WB10 | WB20 | WB30 | WB40 | WB50 |
|---|---|---|---|---|---|---|
| Physico-chemical parameters of bread | ||||||
| Total baking loss [%] | 10.45ab ± 1.34 | 12.02cb ± 1.51 | 10.90ab ± 1.09 | 11.63ba ± 1.18 | 11.44ba ± 1.85 | 11.38ba ± 0.69 |
| Volume [cm3] | 412.51d ± 3.54 | 401.12c ± 5.07 | 400.05c ± 1.01 | 397.52c ± 4.75 | 337.54b ± 3.55 | 325.11a ± 3.04 |
| Moisture of bread crumb [%] | 46.52f ± 0.02 | 46.43e ± 0.01 | 46.23d ± 0.03 | 46.13c ± 0.02 | 45.81b ± 0.03 | 45.35a ± 0.02 |
| The values of total colour difference and parameters L a and b for colour of bread crumb | ||||||
| L | 73.18*f ± 0.62 | 67.72e ± 0.76 | 64.01d ± 0.61 | 61.02c ± 0.88 | 60.39b ± 0.58 | 57.06a ± 0.46 |
| a | 0.85a ± 0.09 | 1.41b ± 0.11 | 2.11c ± 0.13 | 2.70d ± 0.14 | 3.13e ± 0.15 | 3.57f ± 0.13 |
| b | 18.88e ± 0.45 | 16.22d ± 0.24 | 15.45c ± 0.33 | 15.17c ± 0.29 | 14.64b ± 0.28 | 13.90a ± 0.23 |
| ∆E | – | 4.86a ± 0.80 | 8.36b ± 0.33 | 11.40c ± 0.29 | 11.79c ± 0.66 | 15.03d ± 0.50 |
| Antioxidant properties of wheat–buckwheat breads | ||||||
| TPC mg GAE/kg | 197.28a ± 9.60 | 343.91b ± 26.92 | 508.19c ± 48.00 | 668.25d ± 45.88 | 868.47e ± 24.97 | 902.88fe ± 25.50 |
| FRAP µMFe2+/kg | 795.04b ± 136.65 | 3433.07c ± 142.11 | 5558.39d ± 278.80 | 7584.62e ± 366.59 | 9444.39f ± 386.21 | 11,009.80g ± 243.92 |
| DPPH mg Trolox/kg | 230.10a ± 17.68 | 468.98b ± 30.65 | 725.05c ± 15.51 | 872.11d ± 6.70 | 1016.50e ± 32.56 | 1236.76f ± 29.60 |
WS wheat bread, WB10 bread with 10% addition of buckwheat flour, WB20 bread with 20% addition of buckwheat flour, WB30 bread with 30% addition of buckwheat flour, WB40 bread with 40% addition of buckwheat flour, WB50 bread with 50% addition of buckwheat flour values in the same row marked with different letters are statistically significantly different at p ≤ 0.05 ± SD
Color analysis
As expected, the addition of buckwheat flour affected the color of resulting loaves. Breads with buckwheat flour were characterized by significantly lower brightness (L parameter in the CIElab system) of the crumb, within the range 67.72–57.06, compared to wheat bread (73.18). The value of this parameter decreased significantly with the higher content of buckwheat flour (Table 1). As the share of buckwheat flour increased, a significant increase in redness (a) was observed. Values of these parameter ranged from 1.41 for WB10 to 3.57 for WB50, along with a significant decrease of yellowness (b) in bread crumb—from 16.22 for WB10 to 13.9 for WB50 (Table 1). The obtained results are partly consistent with those obtained by Selimović et al. (2014) who observed an increase in the content of both red and yellow pigment, and a decrease in bread brightness with an increase in the proportion of buckwheat flour. The largest total color difference (∆E) calculated in comparison to wheat bread differed significantly among different breads. The value of this parameter increased with the share of buckwheat flour. The lowest value was found for WB10 (4.86) and the highest for WB50 (15.03) (Table 1).
Antioxidant properties
Antioxidant activity is an especially important quality of food. Zieliński and Kozłowska (2000) assessed the antioxidant activity of 80% of methanol extracts from whole cereal grains. They found that buckwheat had the greatest antioxidant capacity compared to barley, oats, wheat, or rye.
The antioxidant properties of the raw materials (flours) and obtained breads were determined by spectroscopic methods. The addition of buckwheat flour clearly contributed to increasing the antioxidative potential of the obtained bread products (Table 1). The highest total polyphenol content (2298.25 mg GAE/kg), ferric ion reducing capability (19,382.6 µMFe2 + /kg) and free radical scavenging ability (4250.77 mg Trolox/kg) were found in buckwheat flour for wheat flour the values were 496.63 mg GAE/kg, 117.39 µMFe2 + /kg and 206.73 mg Trolox/kg respectively. The total polyphenol content, ferric reducing ability and free radical scavenging ability in bread obtained with the addition of buckwheat flour increased because of the substitution of wheat flour with buckwheat flour more abundant in polyphenols (Table 1). Similar results were obtained by Selimović et al. (2014), who observed an increase in the total phenol content and antioxidant activity in bread with 15% to 40% addition of buckwheat flour. Chłopicka et al. (2012) observed a higher content of phenols and antioxidant activity in bread with 15 and 30% buckwheat, compared to wheat bread enriched with amaranth or quinoa.
Protein content and amino acid composition
Buckwheat flour was characterized by significantly higher protein content (12.29%) compared to wheat flour (11.52%) (Table 2). From 20% addition (Table 2) of buckwheat flour, a significant increase in protein was observed in bread supplemented with buckwheat flour. The protein fraction of the analyzed buckwheat flour was particularly rich in such amino acids as glutamic acid, aspartic acid, arginine, leucine, and lysine. Such amino acids as cysteine, methionine, and histidine were found in smaller amounts (Table 2). The obtained results are consistent with other authors who determined similar amino acid contents in the tested buckwheat grains or flours (Mota et al. 2016). In contrast to the pure buckwheat flour in buckwheat flour supplemented breads glutamic acid, proline, leucine, aspartic acid, arginine, serine and phenyloalanine dominated in the amino acid profile. The limiting amino acids of cereal products may be, depending on the variety, growing and storage conditions, lysine, threonine, or leucine (Ufaz and Galili 2008). It should be emphasized that buckwheat proteins, compared to those of wheat, were characterized by a significantly higher content of exogenous lysine (Table 2), an amino acid that limits the protein nutritional value of cereals. Because the methods of chemical determination of amino acids only allow their content to be determined in the product, they cannot be the main criterion for assessing protein quality. They do not consider the protein digestibility, the degree of its availability or the demand for individual amino acids, especially essential ones. For a long time, it was believed that buckwheat proteins have low digestibility, due to the presence of polyphenols. These ingredients are naturally present in buckwheat, but their interactions with proteins slow down the process of protein digestion in the small and large intestine (Ikeda et al. 1986). It is worth emphasizing that the fermentation processes taking place in the intestines with the participation of specific intestinal microbiota are the subject of increasing interest of researchers. However more recent studies indicate that, microbial fermentation in the colon may increase the digestibility of proteins blocked by polyphenols (Skrabanja et al. 2001). On the other hand, it is known that the presence of peptides resistant to digestion contributes to the elimination of steroids from the gastrointestinal tract, which reduces the formation of gallstones and also contributes to the reduction of mammary carcinogenesis and colon carcinogenesis (Luthar et al. 2021).
Table 2.
Amino acid (AA) profile of wheat and wheat–buckwheat bread
| Wheat flour | Buckwheat flour | WS | WB10 | WB20 | WB30 | WB40 | WB50 | |
|---|---|---|---|---|---|---|---|---|
| Protein [%] | 11.52a ± 0.02 | 12.29d ± 0.11 | 11.47a ± 0.04 | 11.44a ± 0.01 | 11.51b ± 0.01 | 11.58c ± 0.01 | 11.66d ± 0.01 | 11.81e ± 0.04 |
| Amino acid | mg g−1 of protein Essential amino acids (EAA) | |||||||
| Histidine | 24.46cb ± 0.64 | 23.57bac ± 0.92 | 23.98bac ± 1.30 | 23.04ab ± 0.57 | 22.84a ± 0.66 | 22.39a ± 0.95 | 21.94a ± 0.40 | 21.75a ± 0.99 |
| Isoleucine | 37.74f ± 1.24 | 31.78a ± 0.75 | 37.99ef ± 0.62 | 36.36ed ± 0.96 | 35.12d ± 0.58 | 34.54d ± 0.64 | 34.74c ± 0.36 | 33.74b ± 0.56 |
| Leucine | 73.51g ± 1.49 | 56.63a ± 1.25 | 73.07g ± 1.61 | 70.50f ± 1.15 | 68.58e ± 0.95 | 64.48d ± 0.72 | 62.78c ± 0.27 | 61.76b ± 0.48 |
| Lysine | 21.60a ± 0.70 | 52.15d ± 1.35 | 21.46a ± 1.44 | 21.92a ± 0.58 | 26.39b ± 2.21 | 31.89c ± 0.77 | 33.76c ± 0.77 | 36.18c ± 0.46 |
| Methionine | 18.75c ± 0.53 | 19.27d ± 0.26 | 16.86a ± 0.33 | 16.72a ± 0.48 | 17.10b ± 0.29 | 17.64b0.34 | 17.72b ± 0.39 | 17.58b ± 0.36 |
| Phenylalanine | 53.24f ± 1.43 | 39.08a ± 0.53 | 51.09f ± 1.06 | 50.22e ± 0.80 | 50.15e ± 0.42 | 49.85d ± 0.53 | 48.90c ± 0.37 | 47.53b ± 0.77 |
| Threonine | 26.88a ± 1.03 | 32.74e ± 0.76 | 27.99ab ± 0.72 | 27.55a ± 1.08 | 28.71b ± 0.88 | 30.14c ± 1.01 | 31.46d ± 0.51 | 32.47de ± 0.66 |
| Valine | 43.72cb ± 1.49 | 43.22cb ± 0.89 | 43.69cb ± 1.14 | 42.43ba ± 0.83 | 42.38bac ± 1.81 | 42.28bac ± 2.60 | 41.77ab ± 1.39 | 41.77ab ± 1.15 |
| Total EAA | 299.90 | 298.44 | 296.13 | 288.74 | 291.27 | 293.21 | 293.07 | 292.78 |
| Non-essential amino acids (non-EAA) | ||||||||
| Alanine | 31.17a ± 1.12 | 39.43d ± 0.93 | 31.69a ± 1.15 | 32.99b ± 071 | 33.44b ± 0.96 | 36.47c ± 1.57 | 36.41c ± 1.15 | 35.86c ± 0.94 |
| Arginine | 39.93a ± 1.22 | 80.60g ± 2.03 | 38.60a ± 0.97 | 41.95b ± 0.96 | 46.82c ± 0.43 | 53.49d ± 0.82 | 56.74e ± 1.76 | 58.96f ± 1.74 |
| Aspartic acid | 45.29a ± 1.81 | 82.48f ± 1.88 | 45.10a ± 0.93 | 47.27b ± 1.77 | 53.02c ± 0.64 | 60.19d ± 0.85 | 62.42e ± 1.76 | 64.25e ± 1.79 |
| Cysteine | 22.49e ± 0.47 | 20.67bc ± 0.39 | 21.48d ± 0.13 | 21.41d ± 0.28 | 20.98c ± 0.11 | 20.24ba ± 0.21 | 19.61a ± 0.34 | 19.48ab ± 0.72 |
| Glutamic acid | 402.67g ± 14.40 | 154.21a ± 4.19 | 386.60f ± 12.89 | 356.87e ± 8.54 | 331.12d ± 9.07 | 292.68c ± 7.64 | 291.35c ± 5.82 | 259.94b ± 7.12 |
| Glycine | 36.73a ± 1.33 | 52.83d ± 1.24 | 36.77a ± 1.15 | 38.21a ± 0.75 | 40.51b ± 1.22 | 46.84c ± 3.12 | 44.96c ± 1.40 | 44.72c ± 1.27 |
| Proline | 132.61g ± 3.16 | 32.76a ± 0.82 | 129.93g ± 4.19 | 115.72f ± 1.76 | 105.33e ± 1.97 | 98.29d ± 4.38 | 89.14c ± 1.56 | 75.22b ± 2.94 |
| Serine | 50.98d ± 1.89 | 41.97a ± 1.12 | 50.48dc ± 2.09 | 48.77cd ± 0.66 | 47.29bc ± 1.65 | 49.30d ± 2.14 | 48.56cd ± 1.35 | 45.85b ± 1.25 |
| Tyrosine | 32.23e ± 1.27 | 27.53b ± 0.58 | 29.55d ± 0.86 | 28.05c ± 0.30 | 27.98b ± 0.72 | 27.61b ± 0.52 | 26.34b ± 0.72 | 25.12a ± 0.72 |
| Total non-EAA | 794.10 | 532.48 | 770.20 | 730.24 | 706.49 | 685.11 | 675.53 | 629.40 |
Meaning of the symbols as in Table 1
Values at the same row marked with different letters are statistically significantly different at p ≤ 0.05 ± SD
Therefore, the content of amino acids in the studied protein was compared with the model proposed by the FAO/WHO (2013). Based on the amino acid score (AAS), an indicator of protein quality in the diet, it was calculated that the amino acid limiting the biological value of the protein of the tested flours and breads was lysine. The AAS for wheat bread was 44.71% and for those with buckwheat flour it ranged from 45.67 for WB10 to 75.38% for WB50 (Table 3). Due to the high content of lysine and a well-balanced level of other amino acids, this protein has a high biological value. This is particularly important because the human body cannot produce lysine; therefore, it should be obtained from food. From a nutritional point of view, lysine is particularly important among buckwheat proteins, as it can contribute to lowering cholesterol level as well as increasing the fecal excretion of steroids or removing bile acid, which contributes to reduced gallstone formation (Ge and Wang 2020; Skrabanja et al. 2001).
Table 3.
Nutritional value of protein of wheat and wheat –buckwheat bread
| EAA | Protein [mg·g−1] | FAO 2011 reference | AAS [%] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WF | BF | WS | WB10 | WB20 | WB30 | WB40 | WB50 | WF | BF | WS | WB10 | WB20 | WB30 | WB40 | WB50 | ||
| Val | 43.72 | 43.22 | 43.69 | 42.43 | 42.38 | 42.28 | 41.77 | 41.77 | 40.00 | 109.30 | 108.05 | 109.23 | 106.08 | 105.95 | 105.70 | 104.43 | 104.43 |
| Thr | 26.88 | 32.74 | 27.99 | 27.55 | 28.71 | 30.14 | 31.46 | 32.47 | 25.00 | 107.52 | 130.96 | 111.96 | 110.20 | 114.84 | 120.56 | 125.84 | 129.88 |
| Ile | 37.74 | 31.78 | 37.99 | 36.36 | 35.12 | 34.54 | 34.74 | 33.74 | 30.00 | 125.80 | 105.93 | 126.63 | 121.20 | 117.07 | 115.13 | 115.80 | 112.47 |
| His | 24.46 | 23.57 | 23.98 | 23.04 | 22.84 | 22.39 | 21.94 | 21.75 | 16.00 | 152.88 | 147.31 | 149.88 | 144.00 | 142.75 | 139.94 | 137.13 | 135.94 |
| Leu | 73.51 | 56.63 | 73.07 | 70.5 | 68.58 | 64.48 | 62.78 | 61.76 | 61.00 | 120.51 | 92.84 | 119.79 | 115.57 | 112.43 | 105.70 | 102.92 | 101.25 |
| Lys | 21.6 | 52.15 | 21.46 | 21.92 | 26.39 | 31.89 | 33.76 | 36.18 | 48.00 | 45.00 | 108.65 | 44.71 | 45.67 | 54.98 | 66.44 | 70.33 | 75.38 |
| AAA | 85.47 | 66.61 | 80.64 | 78.27 | 78.13 | 77.46 | 75.24 | 72.65 | 41.00 | 208.45 | 162.47 | 196.68 | 190.89 | 190.56 | 188.92 | 183.51 | 177.19 |
| SAA | 41.24 | 39.94 | 38.34 | 38.13 | 38.08 | 37.88 | 37.32 | 37.07 | 23.00 | 179.30 | 173.66 | 166.70 | 165.79 | 165.56 | 164.69 | 162.27 | 161.19 |
Meaning of the symbols as in Table 1
SAA sulphur-containing AA, AAA aromatic AA
Total phytate and inositol phosphates
The greatest number of individual forms of inositol phosphates (IP3-IP6) was determined in buckwheat flour (2.122 g/100 g dry basis [d.b.]), wheat flour contained 0.149 (g/100 g d.b.), and the dominant form in the inositol phosphate profile, both in flours and breads, was inositol hexaphosphate (IP6). In the WS, WB10, WB20, WB30, WB40 and WB50 breads, their content was 0.011, 0.073, 0.157, 0.278, 0.678 and 1.016 g/100 g d.b., respectively. There was a significant increase in inositol phosphates with an increase in the share of buckwheat flour in breads. The properties of inositol phosphates depend on the degree of their phosphorylation. Derivatives with a higher degree of phosphorylation (IP4, IP5, IP6) can form insoluble complexes with multivalent cations, e.g., calcium, copper, iron, cobalt, manganese, and zinc, thus limiting the amount of these elements absorbed from the gastrointestinal tract and are called mineral absorption inhibitors. On the other hand, the high content of this fraction may explain the high values obtained in the FRAP analysis based on the reducing properties of iron ions. In contrast, inositol derivatives with a lower degree of phosphorylation (IP1, IP2 and IP3) do not form the above-mentioned complexes. The effect on the organism of inositol hexaphosphate IP6 (phytic acid), which is present in the highest amounts in legume seeds and cereal grains, is the most thoroughly known. In clinical trials, it was found that a high content of this component in the diet was negatively correlated with the occurrence of colorectal cancer, and its effectiveness increased with the simultaneous administration of inositol. In addition to the anti-carcinogenic effect of IP6, the effect of this component on the reduction of the tendency for the formation of kidney stones, reduction of the risk of developing cardiovascular diseases, and a hypocholesterolemic effect were also found (Fox and Erbel 2003; Vučenik and Stains 2010). It is worth emphasizing that the analyzed buckwheat bread was most abundant in this fraction.
Texture analysis
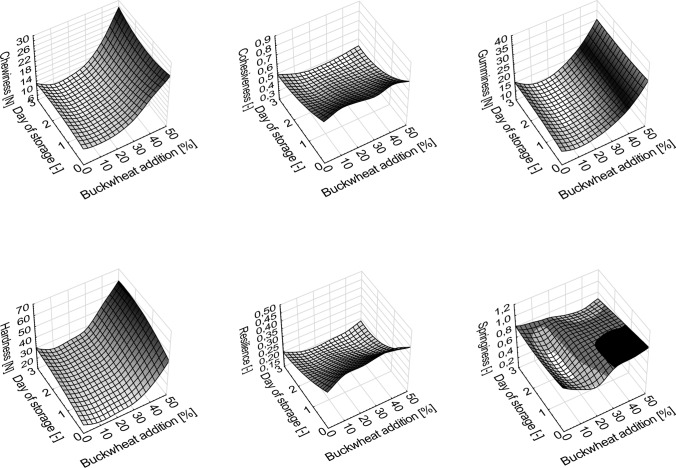
The hardness of the crumb is the main determinant of the quality of the bread and is closely related to the perception of fresh bread by consumers. The parameters of the bread texture on the day of baking (fresh bread) as well after 3-day storage are presented in Table 4 and Fig. 1. Changes in textural parameters as a function of flour share and time of storage are presented in Fig. 1 and Table 5. During storage, an increase in crumb hardness was observed in all tested breads. In the case of hardness, all coefficients of the polynomial equation were statistically significant. It proves that buckwheat flour shares as well as time of storage and interaction of these parameters affect this textural feature. The fresh bread and bread after storage, WB10, WB20 and WB30 showed significantly lower hardness compared to the other breads. On the other hand, WB50 demonstrated the highest value of this parameter. In addition, a positive effect of 10, 20 and 30% buckwheat flour content on the reduction of the crumb hardness can be observed compared to wheat bread. Standard bread immediately after baking was characterized by significantly lower springiness of the crumb compared to the other bread, with a value of 0.85. During storage, it was observed that the springiness of the crumb in all the breads was reduced. Considering the values of the polynomial equation, only the quadratic coefficient for the share of the buckwheat flour was statistically significant (Table 5). On the day of baking WB10 and WB20 were characterized by the significantly lowest gumminess and chewiness, compare to the other bread. The values of these parameters for WB40 and WB50 were about 2–3 times higher than for standard bakery products and those with 10% and 20% buckwheat flour content, respectively. During storage, a positive effect of 10—30% of buckwheat flour on the reduction of gumminess and chewiness of crumb was observed in comparison to other bread (Table 4). The influence of buckwheat flour on these parameters was proved by the polynomial model which revealed no interaction between share of the buckwheat flour and time of bread storage (Table 5) Fresh standard bread was characterized by a significantly lower crumb resilience, 0.34, in comparison to fresh buckwheat bread, and additionally a decrease in resilience was observed during storage. There was a statistically significant interaction between time of storage and share of buckwheat flour, which influenced values of crumb resilience (Table 4).
Table 4.
Textural parameters of wheat and buckwheat breads
| Bread | Day of storage | Hardness [N] | Springiness [−] | Cohesiveness [−] | Gumminess [−] | Chewiness [−] | Resilience [−] |
|---|---|---|---|---|---|---|---|
| WS | 0 | 18.44bc ± 1.66 | 0.85ghi ± 0.03 | 0.66k ± 0.03 | 12.46efg ± 0.80 | 10.46cde ± 0.57 | 0.34k ± 0.03 |
| WS | 1 | 24.59efg ± 1.46 | 0.84ghi ± 0.05 | 0.60j ± 0.05 | 15.89i ± 0.84 | 12.85fg ± 0.40 | 0.27i ± 0.05 |
| WS | 2 | 27.34h ± 1.23 | 0.80defg ± 0.05 | 0.51f ± 0.02 | 14.32h ± 1.01 | 11.43ef ± 1.10 | 0.24gh ± 0.04 |
| WS | 3 | 30.42i ± 2.68 | 0.77cdef ± 0.04 | 0.49ef ± 0.03 | 15.60i ± 1.69 | 11.36def ± 0.97 | 0.19ef ± 0.01 |
| WB10 | 0 | 13.96a ± 0.67 | 0.89ijk ± 0.03 | 0.77m ± 0.06 | 10.84bcd ± 0.91 | 10.35cde ± 0.24 | 0.43mn ± 0.02 |
| WB10 | 1 | 17.63bc ± 0.96 | 0.89ijk ± 0.02 | 0.56ghi ± 0.04 | 9.79abc ± 0.53 | 9.65bcd ± 0.53 | 0.31jk ± 0.05 |
| WB10 | 2 | 22.13de ± 1.32 | 0.80defg ± 0.02 | 0.40abc ± 0.01 | 9.34ab ± 1.18 | 7.58a ± 0,61 | 0.16cd ± 0.01 |
| WB10 | 3 | 20.42cd ± 1.87 | 0.76bcde ± 0.04 | 0.41bcd ± 0.03 | 9.72abc ± 0.99 | 7.38a ± 0.68 | 0.15abc ± 0.02 |
| WB20 | 0 | 16.09ab ± 0.69 | 0.91jk ± 0.01 | 0.73l ± 0.01 | 11.60def ± 0.70 | 10.55cde ± 0.62 | 0.41lm ± 0.02 |
| WB20 | 1 | 21.98de ± 0.62 | 0.87hijk ± 0.05 | 0.58ij ± 0.04 | 13.41gh ± 1.26 | 11.53ef ± 0.90 | 0.29ij ± 0.03 |
| WB20 | 2 | 22.39def ± 1.08 | 0.85fghij ± 0.04 | 0.46de ± 0.02 | 8.09a ± 1.06 | 8.62b ± 0.77 | 0.23fg ± 0.01 |
| WB20 | 3 | 24.95fgh ± 2.91 | 0.73bc ± 0.01 | 0.37a ± 0.01 | 9.70abc ± 1.12 | 8.49b ± 0.45 | 0.13a ± 0.01 |
| WB30 | 0 | 17.04b ± 1.74 | 0.89ijk ± 0.03 | 0.73l ± 0.04 | 15.04hi ± 1.13 | 13.64g ± 1.23 | 0.43n ± 0.03 |
| WB30 | 1 | 22.67defg ± 1.98 | 0.86ghijk ± 0.02 | 0.57hij ± 0.06 | 13.27fgh ± 0.86 | 11.23cdef ± 1.68 | 0.28ij ± 0.02 |
| WB30 | 2 | 23.79efg ± 2.47 | 0.77cde ± 0.05 | 0.43cd ± 0.03 | 11.22cde ± 1.03 | 10.73cde ± 1.69 | 0.18cde ± 0.02 |
| WB30 | 3 | 24.50efgh ± 1.98 | 0.61a ± 0.02 | 0.39abc ± 0.06 | 13.42gh ± 0.86 | 9.59bc ± 1.68 | 0.15ab ± 0.02 |
| WB40 | 0 | 25.41gh ± 2.31 | 0.91jk ± 0.03 | 0.78m ± 0.03 | 20.08j ± 2.06 | 20.77i ± 1.76 | 0.45n ± 0.02 |
| WB40 | 1 | 36.95j ± 2.34 | 0.92k ± 0.05 | 0.53fgh ± 0.01 | 18.79j ± 0.75 | 15.90h ± 0.85 | 0.26ghi ± 0.01 |
| WB40 | 2 | 42.01k ± 1.88 | 0.82efgh ± 0.04 | 0.43cd ± 0.04 | 18.57j ± 1.55 | 16.63h ± 1.17 | 0.16abcde ± 0.02 |
| WB40 | 3 | 46.06l ± 3.40 | 0.69b ± 0.02 | 0.38ab ± 0.02 | 19.53j ± 0.74 | 15.46h ± 1.00 | 0.14ab ± 0.03 |
| WB50 | 0 | 43.55kl ± 2.42 | 0.91jk ± 0.06 | 0.69k ± 0.04 | 31.06l ± 1.55 | 24.90k ± 1.89 | 0.39l ± 0.03 |
| WB50 | 1 | 50.75m ± 0.70 | 0.81defgh ± 0.05 | 0.52fg ± 0.02 | 27.49k ± 1.06 | 22.51j ± 0.64 | 0.27hi ± 0.01 |
| WB50 | 2 | 61.16° ± 3.67 | 0.74bcd ± 0.04 | 0.42bcd ± 0.02 | 32.31l ± 1.33 | 25.18k ± 1.37 | 0.18cde ± 0.02 |
| WB50 | 3 | 57.79n ± 1.47 | 0.70bc ± 0.03 | 0.43bcd ± 0.04 | 28.25k ± 2.63 | 27.56l ± 1.30 | 0.19de ± 0.01 |
| Statistical parameters [p values] | |||||||
| Share | < 0.0001 | 0,0589 | 0.0002 | 0.0253 | 0.0006 | 0.0001 | |
| Day | 0.1245 | 0.2302 | 0.5322 | 0.7937 | 0.2368 | 0.3435 | |
| Share * day | < 0.0001 | < 0.0001 | 0.0124 | 0.0002 | 0.0116 | 0.0002 | |
Meaning of the symbols as in Table 1
0-baking day. 1–3-day after baking
Małe litery w obrębie rodzaju chleba; duże litery dla dni—twardość 10, 20,30, standard, 40, 50
Fig. 1.
Changes in texture properties after 3 days of storage
Table 5.
Parameters of second-order polynomial model describing textural changes of breads during storage
| Hardness | Springiness | Cohesiveness | Gumminess | Chewiness | Resilience | P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable coefficient | SE | P value | Variable coefficient | SE | P value | Variable coefficient | SE | P value | Variable coefficient | SE | P value | Variable coefficient | SE | P value | Variable coefficient | SE | ||
| β0 | 20.22 | 0.95 | < 0.0001 | 0.7320 | 0.0675 | < 0.0001 | 0.7298 | 0.0178 | < 0.0001 | 14.57 | 0.58 | < 0.0001 | 12.71 | 0.54 | < 0.0001 | 0.3929 | 0.0106 | < 0.0001 |
| βi | − 0.96 | 0.06 | < 0.0001 | − 0.0043 | 0.0046 | 0.3575 | − 0.0012 | 0.0012 | 0.3386 | − 0.54 | 0.04 | < 0.0001 | − 0.36 | 0.04 | < 0.0001 | 0.0010 | 0.0007 | 0.1891 |
| βii | 0.03 | 0.00 | < 0.0001 | 0.0002 | 0.0001 | 0.0573 | 0.0000 | 0.0000 | 0.3183 | 0.02 | 0.00 | < 0.0001 | 0.01 | 0.00 | < 0.0001 | 0.0000 | 0.0000 | 0.5295 |
| βj | 6.29 | 0.98 | < 0.0001 | 0.0344 | 0.0696 | 0.6224 | − 0.1894 | 0.0183 | < 0.0001 | − 0.83 | 0.60 | 0.1649 | − 1.75 | 0.55 | 0.0020 | − 0.1442 | 0.0110 | < 0.0001 |
| βjj | − 1.30 | 0.30 | < 0.0001 | − 0.0034 | 0.0213 | 0.8723 | 0.0318 | 0.0056 | < 0.0001 | 0.28 | 0.18 | 0.1330 | 0.35 | 0.17 | 0.0411 | 0.0235 | 0.0034 | < 0.0001 |
| βi βj | 0.06 | 0.02 | 0.0002 | − 0.0021 | 0.0011 | 0.0656 | − 0.0005 | 0.0003 | 0.1231 | − 0.01 | 0.01 | 0.2382 | 0.00 | 0.01 | 0.7737 | − 0.0004 | 0.0002 | 0.0116 |
| R2 | 0.9691 | 0.2860 | 0.9085 | 0.9613 | 0.9525 | 0.9450 | 0.9455 | |||||||||||
| R2 adj | 0.9365 | 0.0419 | 0.8178 | 0.9209 | 0.9032 | 0.8894 | 0.8894 | |||||||||||
β0 is a constant; βi—share of buckwheat flour linear coefficient, βii—share of buckwheat flour quadratic coefficient, βj—day of bread storage linear coefficient, βjj—day of bread storage quadratic coefficient and βi·βj coefficient for the interaction effect
Texture changes during storage can be considered as an indicator of the degree of staling of the breadcrumb, which results mainly from the retrogradation of the amylose fraction in both wheat and buckwheat starch (Ribotta et al. 2003). Based on these changes, it is possible to note a positive effect of buckwheat flour in the amount of 10–30% on the texture parameters and slowing down the process of bread staling. The changes in the mechanical features of the bread crumb resulted mainly from the decrease in the amount of gluten while adding the buckwheat flour, an ingredient with a higher dietary fiber content (Collar et al. 2007). The obtained results are different fromthose obtained by Lin et al. (2013), who observed an increase in hardness, gumminess, and chewiness with 15% buckwheat flour content in bread.
Conclusion
The share of buckwheat flour contributed to the lowering of bread quality—a reduction of the volume of bread from its 40% share. Bread with buckwheat flour was characterized by the significantly lower whiteness of the crumb, in comparison to wheat bread. A positive effect of 10, 20 and 30% buckwheat flour content on the reduction of the crumb hardness, gumminess and chewiness was observed in comparison to other bread. The antioxidant properties and inositol phosphates increased with the increase in the proportion of buckwheat flour in the bread. Starting with a 20% addition of wheat flour, a significant increase in protein was observed in bread with buckwheat flour. The limiting amino acid of the protein of the tested flours and breads was lysine. On the other hand, it is worth to emphasize that incorporation of buckwheat flour into wheat bread formulation brought many health benefits. Antioxidant properties as well as biological value of bread supplemented with buckwheat flour increases compared to wheat bread. First, the values of indicators such as AAS increased, which is associated with the introduction of buckwheat protein with a better biological value.
Based on the obtained results, it can be concluded that the 30% share of buckwheat flour in the production of bread is sufficient.
Funding
This Research was finance by the Ministry of Science and Higher Education of the Republic of Poland.
Declaration
Conflict of interest
we declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stanislaw Kowalski, Email: rrkowals@cyf-kr.edu.pl.
Anna Mikulec, Email: amikulec@pwsz-ns.edu.pl.
Barbara Mickowska, Email: barbara.mickowska@urk.edu.pl.
Krzysztof Buksa, Email: krzysztof.buksa@urk.edu.pl.
References
- AACC, American Association of Cereal Chemists (2000) Approved methods of the AACC, 10th ed. St Paul, MN, USA
- AOAC, Association of Analytical Chemists International (2006) Official Methods of Analysis, 18th ed. Gaithersburg
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Cieślik I, Migdał W, Topolska K, Mickowska B, Cieslik E. Changes of amino acid and fatty acid profile in freshwater fish after smoking. J Food Process Preserv. 2017 doi: 10.1111/jfpp.13357. [DOI] [Google Scholar]
- Chłopicka J, Pasko P, Gorinstein S, Jedryas A, Zagrodzki P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci Technol. 2012;46:548–555. doi: 10.1016/j.lwt.2011.11.009. [DOI] [Google Scholar]
- Collar C, Santos E, Rosell CM. Assessment of the rheological profile of fibre-enriched bread doughs by response surface methodology. J Food Eng. 2007;78:820–826. doi: 10.1016/j.jfoodeng.2005.11.026. [DOI] [Google Scholar]
- FAO (2013) Dietary protein quality evaluation in human nutrition (Report of an FAO Expert Consultation, FAO Food and Nutrition Paper no.92). Rome, Italy. Food and Agriculture Organization of the United Nations [PubMed]
- Fox Ch, Eberl M. Phytic acid (IP6), novel broad spectrum anti-neoplastic agent: a systematic review. Complement Ther Med. 2003;10(4):229–234. doi: 10.1016/S0965-2299(02)00092-4. [DOI] [PubMed] [Google Scholar]
- Gavurniková S, Havrlentová M, Mendel L, Čičova I, Bieliková M, Kraic J. Parameters of wheat flour, dough and bread fortified by buckwheat and millet flours. Agriculture (poľnohospodárstvo) 2011;57(4):144–153. [Google Scholar]
- Ge RH, Wang H. Nutrient components and bioactive compounds in Tartary buckwheat bran and flour as affected by thermal processing. Int J Food Prop. 2020 doi: 10.1080/10942912.2020.1713151. [DOI] [Google Scholar]
- Giménez-Bastida JA, Piskuła MK, Zieliński H. Recent advances in processing and development of buckwheat derived bakery and non-bakery products—a review. Pol J Food Nutr Sci. 2015;65(1):9–20. doi: 10.1515/pjfns-2015-0005. [DOI] [Google Scholar]
- Ikeda K, Oku M, Kusano T, Yasumoto K. Inhibitory potency of plant antinutrients towards the in vitro digestibility of buckwheat protein. J Food Sci. 1986;51:1527–1530. doi: 10.1111/j.1365-2621.1986.tb13851.x. [DOI] [Google Scholar]
- Lin LY, Hsieh YJ, Liu HM, Lee CC, Mau JL. Flavor components in buckwheat bread. J Food Process Preserv. 2009;33:814–826. doi: 10.1111/j.1745-4549.2008.00313.x. [DOI] [Google Scholar]
- Lin LY, Wang HE, Lin SD, Liu HM, Mau JL. Changes in buckwheat bread during storage. J Food Process Preserv. 2013;37:285–290. doi: 10.1111/j.1745-4549.2011.00647.x. [DOI] [Google Scholar]
- Luthar Z, Zhou M, Golob A, Germ M. Breeding buckwheat for increased levels and improved quality of protein. Plants. 2021;10:14. doi: 10.3390/plants10010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Mikulec A, Kowalski S, Sabat R, Skoczylas Ł, Tabaszewska M, Wywrocka-Gurgul A. Hemp flour as a valuable component for enriching physicochemical and antioxidant properties of wheat bread. LWT Food Sci Technol. 2019 doi: 10.1016/j.lwt.2018.12.028. [DOI] [Google Scholar]
- Mota C, Santos M, Mauro R, Samman N, Matos AS, Torres D, Castanheira I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016 doi: 10.1016/j.foodchem.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Psodorov DB, Vujić DN, Ačanski MM, Pastor KA, Razmovski RN, Kravić SZ. The content of buckwheat flour in wheat bread. Acta Period Technol. 2014;45:79–87. doi: 10.2298/APT1445079P. [DOI] [Google Scholar]
- Ribotta PD, Leon AE, Aňon MC. Effect of freezing and frozen storage on the gelatinization and retrogradation of amylopectin in dough baked in a differential scanning calorimeter. Food Res Int. 2003;36:357–363. doi: 10.1016/S0963-9969(02)00227-2. [DOI] [Google Scholar]
- Sandberg AS, Andersson H, Carlsson NG, Sandstrom B. Degradation products of bran phytate formed during digestion in the human small intestine: effect of extrusion cooking on digestibility. J Nutr. 1987;117(12):2061–2065. doi: 10.1093/jn/117.12.2061. [DOI] [PubMed] [Google Scholar]
- Selimović A, Miličević D, Jašić M, Selimović A, Ačkar D, Pešić T. The effect of baking temperature and buckwheat flour addition on the selected properties of wheat bread. Croat J Food Sci Technol. 2014;6(1):43–50. [Google Scholar]
- Sindhu R, Khatkar BS. Pseudocereals nutritional composition functional properties and food applications. In: Deka SC, Seth D, Hulle NRS, editors. Food bioactives: functionality and applications in human health. Florida: Apple Academic Press; 2019. pp. 129–148. [Google Scholar]
- Skrabanja V, Liljeberg Elmstahl HG, Kreft I, Björck IM. Nutritional properties of starch in buckwheat products: studies in vitro and in vivo. J Agric Food Chem. 2001;49:490–496. doi: 10.1021/jf000779w. [DOI] [PubMed] [Google Scholar]
- Szczesniak AS. Texture is a sensory property. Food Qual Prefer. 2002;13(4):215–222. doi: 10.1016/S0950-3293(01)00039-8. [DOI] [Google Scholar]
- Turkmen N, Sari F, Poyrazoglu ES, Velioglu YS. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006;95:653–657. doi: 10.1016/j.foodchem.2005.02.004. [DOI] [Google Scholar]
- Ufaz S, Galili G. Improving the content of essential amino acids incrop plants: goals and opportunities. Plant Physiol. 2008;147:954–961. doi: 10.1104/pp.108.118091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vučenik I, Stains J. Cancer preventive and therapeutic properties of IP6: efficacy and mechanisms. Period Biol. 2010;112:451–458. [Google Scholar]
- Wójtowicz A, Kolasa A, Mościcki L. Influence of buckwheat addition on physical properties, texture and sensory characteristics of extruded corn snacks. Pol J Food Nutr Sci. 2013;63:239–244. doi: 10.2478/v10222-012-0076-2. [DOI] [Google Scholar]
- Wronkowska M, Haros M, Soral-Śmietana M. Effect of starch substitution by buckwheat flour on gluten-free bread quality. Food Bioproc Technol. 2013;6(7):1820–1827. doi: 10.1007/s11947-012-0839-0. [DOI] [Google Scholar]
- Zhang HW, Zhang YH, Lu MJ, Tong WJ, Cao GW. Comparison of hypertension, dyslipidemia and hyperglicaemia between seed-consuming and non-consuming mongolianchinese populations in Inner Mongolia, China. Clin Exp Pharmacol Physiol. 2007;34:838–844. doi: 10.1111/j.1440-1681.2007.04614.x. [DOI] [PubMed] [Google Scholar]
- Zielińśki H, Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]