Abstract
Adhesion of 19 Bifidobacterium strains to native maize, potato, oat, and barley starch granules was examined to investigate links between adhesion and substrate utilization and to determine if adhesion to starch could be exploited in probiotic food technologies. Starch adhesion was not characteristic of all the bifidobacteria tested. Adherent bacteria bound similarly to the different types of starch, and the binding capacity of the starch (number of bacteria per gram) correlated to the surface area of the granules. Highly adherent strains were able to hydrolyze the granular starches, but not all amylolytic strains were adherent, indicating that starch adhesion is not a prerequisite for efficient substrate utilization for all bifidobacteria. Adhesion was mediated by a cell surface protein(s). For the model organisms tested (Bifidobacterium adolescentis VTT E-001561 and Bifidobacterium pseudolongum ATCC 25526), adhesion appeared to be specific for α-1,4-linked glucose sugars, since adhesion was inhibited by maltose, maltodextrin, amylose, and soluble starch but not by trehalose, cellobiose, or lactose. In an in vitro gastric model, adhesion was inhibited both by the action of protease and at pH values of ≤3. Adhesion was not affected by bile, but the binding capacity of the starch was reduced by exposure to pancreatin. It may be possible to exploit adhesion of probiotic bifidobacteria to starch granules in microencapsulation technology and for synbiotic food applications.
Probiotics are live microorganisms that are used as dietary supplements with the aim of benefiting the health of consumers by positively influencing the intestinal microbial balance (12). Bifidobacterium and Lactobacillus species have been the focus of probiotic interest since a large population of these bacteria in the intestinal tract is generally considered to be indicative of a healthy microbiota (2, 11, 21). These bacteria are increasingly being included as functional ingredients, particularly in dairy products such as yogurts and fermented milks, as evidence accumulates that they have beneficial effects on human health (20).
In addition to the probiotic approach involving directly introducing live bacteria into the colon through dietary supplementation, another approach to increase the number of bifidobacteria in the intestinal microbiota is through the use of prebiotics. Prebiotics are nondigestible dietary components that pass through the digestive tract to the colon and selectively stimulate proliferation and/or activity of populations of desirable bacteria in situ (13, 27). Due to the potential synergy between probiotics and prebiotics, foods containing a combination of these ingredients are often referred to as synbiotics (6, 13).
The prebiotics identified thus far are nondigestible carbohydrates, including lactulose, inulin, and a range of oligosaccharides (7). Some starches also escape complete digestion during passage through the human small intestine and arrive in the colon as fermentable carbohydrate sources for intestinal bacteria (8, 9). Granular starches synthesized by a number of food plants provide examples of such resistant starches, and they are incompletely digested due to their size and molecular conformation (28). A range of human intestinal bacteria can ferment soluble starch; the most numerically dominant of these bacteria are members of the genera Bacteroides, Bifidobacterium, Fusobacterium, and Butyrivibrio (19). However, in animal models, inclusion of resistant starches in the diet has been shown to increase the population of bifidobacteria in the intestinal tract (4, 5, 16, 25, 29). Resistant starches have therefore also been proposed as potential prebiotics.
It has been shown previously that some intestinal bacteria can adhere to starch in vitro and that adhesion is sometimes required for efficient utilization of the substrate (1, 23, 26). However, the role of adhesion in starch metabolism by bifidobacteria is currently unknown. The aims of the present study were to investigate the diversity among bifidobacteria of starch adhesion to different types of starch granules, to examine if there is a correlation between starch utilization and adhesion, and to obtain a preliminary understanding of the mechanisms involved in adhesion.
Bacterial adhesion to starch may also provide advantages in new probiotic technologies that enhance delivery of viable and metabolically active probiotics to the intestinal tract. Workers have recently developed microencapsulation technology that involves encasing bacteria in the hollow core of partially hydrolyzed granular starch, which is then encapsulated in an outer coating of amylose (10, 22). This technology is designed to protect the probiotic bacteria from adverse environmental conditions during processing, in products during storage, and during passage through the upper gastrointestinal tract. Therefore, another goal of the present study was to evaluate the potential for exploiting the ability of bifidobacteria to adhere to starch for use in this microencapsulation technology and for synbiotic applications.
MATERIALS AND METHODS
Microorganisms and growth conditions.
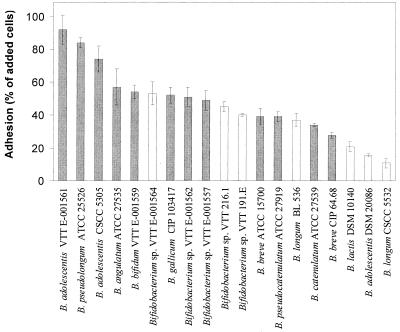
The 19 Bifidobacterium strains used in this investigation are shown in Fig. 1. The bacteria were revived from frozen glycerol stocks stored at −70°C and inoculated into 10 ml of broth medium containing 10 g of beef extract liter−1, 10 g of pancreatic digest of casein liter−1, 3 g of yeast extract liter−1, 5 g of sodium chloride liter−1, and 10 g of glucose liter−1 (pH 6.8). The bacteria were passaged twice in fresh medium before they were used in the starch adhesion and hydrolysis experiments. Each time, the bacteria were grown for 24 h at 37°C under an anaerobic atmosphere containing 85% nitrogen, 10% hydrogen, and 5% carbon dioxide. Unless otherwise stated, the bacteria were in the stationary phase when they were used in the adhesion experiments.
FIG. 1.
Adhesion of bifidobacteria to Hylon VII maize starch granules. ░⃞, amylolytic strain; □, nonamylolytic strain. Error bars indicate ±1 standard deviation from the mean (n =3 ). Means differing by more than 25% are statistically different (P < 0.05). VTT, VTT Biotechnology, Espoo, Finland (human intestinal isolates); ATCC, American Type Culture Collection, Manassas, Va.; CSCC, CSIRO Starter Culture Collection, Melbourne, Australia; CIP, Collection de Bactéries de l'Institut Pasteur, Paris, France; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany. Strain BL 536 was isolated from a freeze-dried commercial product from Wisby, Germany.
Starch samples.
The following types of starch granules were used in this investigation: maize (Hylon VII; high-amylose maize starch; National Starch and Chemical Co., Bridgewater, N.J.), oat (native oat starch; Primalco, Koskenkorva, Finland), potato (native potato starch; Järviseudun Peruna, Vimpeli, Finland), and barley (native barley starch; Primalco). The average granule diameter for each starch type was determined by using a laser diffraction particle size analyzer (LS 230; Coulter, Fullerton, Calif.). Typically, 500,000 particles were analyzed. The number of particles per gram was determined by counting the number of starch granules in three samples of a suspension containing 2 g liter−1 with a hemocytometer. Surface area was calculated by assuming that the granules were spherical.
Starch utilization.
Hydrolysis of the starch granules by the bacteria was examined qualitatively by using agar plates containing the various types of starch at a concentration of 5 g liter−1. The agar medium contained the same constituents as the broth medium described above, as well as 10 g of agar liter−1. To minimize starch gelatinization, the granular starch was added to the medium only after the medium had cooled to 45°C after autoclaving. To produce uniform colonies approximately 5 mm in diameter, the bacteria were inoculated onto the agar surface by adding 5 μl of broth culture. The bacteria were then incubated for 3 days at 37°C under anaerobic conditions. Following incubation, the agar plates were flooded with a solution of 1% iodine in 2% potassium iodide for 1 min to stain the starch dark blue. Starch hydrolysis was indicated by zones of clearing surrounding the bacterial colonies.
Starch utilization was also examined in fermentations of granular maize starch (Hylon VII) by the 19 strains of bifidobacteria. The bacteria were grown in broth culture as described above, except that glucose was replaced by starch as the carbon source. The concentrations of starch at the start of each fermentation and after 3 days of growth were estimated by measuring the concentrations of glucose liberated from the starch following acid hydrolysis of 2-ml fermentation samples. The starch was hydrolyzed by adding 1 ml of 1 M H2SO4 to the samples, which were incubated in a boiling water bath for 2 h. The samples were then neutralized with 1 M NaOH. The glucose concentrations in the samples were measured by high-performance liquid chromatography (Waters, Milford, Mass.) by using an Animex HPX-87C column (Bio-Rad, Hercules, Calif.) at 85°C. The mobile phase was water at a flow rate of 0.5 ml min−1. The internal standard was fructose. Sugars were detected with a Waters 410 differential refractometer.
Adhesion to starch granules.
Adhesion to starch granules was measured by a cosedimentation assay. Cells were first washed twice with 10 ml of 0.1 M phosphate buffer (pH 7.0) before they were resuspended in the same buffer at a concentration of approximately 107 cells ml−1. Two milliliters of the bacterial suspension was thoroughly mixed in a 1-cm-diameter test tube with an equal volume of a suspension of starch granules (10 g liter−1) in 0.1 M phosphate buffer (pH 7.0). The bacterium-starch suspension was allowed to stand at room temperature for 1 h to allow the starch to sediment. Two 150-μl samples were then taken from 0.5 cm below the liquid surface, and the optical density at 540 nm (OD540) was measured with a microplate reader (Multiscan EX; Labsystems, Helsinki, Finland). Phosphate buffer was used as a blank for all readings from the microplate. In order to calculate the percentage of cells that adhered to the starch and then cosedimented to bottom of the test tube, the OD540 was compared to the OD540 of similar samples from two control tubes containing (i) bacteria but no starch and (ii) starch but no bacteria. The OD540 for the bacterial control (1) was between 0.15 and 0.25, while the OD540 for the starch control was always less than 0.01. The proportion of added bacteria that adhered to the starch granules was calculated as follows: percentage of cells adhering to starch = 100% − {[(a − b)/c] · 100%}, where a is the OD540 of a sample from the tube containing starch plus bacteria, b is the OD540 of a sample from a control tube containing starch but no bacteria, and c is the OD540 of a sample from a control tube containing bacteria but no starch. Strains in which more than 70% of the cells adhered to the starch granules were considered highly adherent. Moderate adhesion was defined as adhesion in which between 40 and 70% of the cells adhered in the assay, while less than 40% adhesion was considered poor adhesion.
Three strains, Bifidobacterium adolescentis VTT E-001561 (highly adherent), Bifidobacterium sp. strain VTT E-001562 (moderately adherent), and Bifidobacterium longum CSCC 5532 (poorly adherent), showed a tendency to form bacterial aggregates. However, the aggregates sedimented slowly compared to the starch granules and did not interfere with the starch-binding assay.
Effect of growth phase on adhesion.
The effect of growth phase on adhesion to starch was examined with three Bifidobacterium strains. The strains examined were B. adolescentis VTT E-001561 (highly adherent), Bifidobacterium sp. strain VTT E-001557 (moderately adherent), and Bifidobacterium lactis DSM 10140 (poorly adherent). The bacteria were grown in broth medium as described above. The growth phases of the bacteria were monitored throughout the fermentations by measuring the OD540. Adhesion of the bacteria to Hylon VII high-amylose maize starch was examined when the cells were in the late lag phase, the exponential phase, and the stationary phase.
Study of adhesion mechanisms.
Preliminary investigations of the mechanisms involved in adhesion were undertaken to determine if adhesion was due to hydrophobic or electrostatic interactions, growth medium components, or specific cellular or extracellular proteinaceous adhesive factors produced by the bacteria. Additionally, the nature of the receptor sites in the adhesion reaction was investigated by using a range of potential inhibitors of adhesion, including glucose, maltose, maltodextrin, amylose, amylopectin, and soluble starch. The treatments used in the adhesion assays are described in Table 1. For each treatment, the bacterial cells were first washed twice in 0.1 M phosphate buffer (pH 7.0). The experiments were performed with two adherent strains (B. adolescentis VTT E-001561 and Bifidobacterium pseudolongum ATCC 25526) and two poorly adhering strains (Bifidobacterium breve CIP 64.68 and B. lactis DSM 10140). Additionally, adhesion of three other strains showing strong adhesion to starch (B. adolescentis CSCC 5305, Bifidobacterium angulatum ATCC 27535, and Bifidobacterium bifidum VTT E-001559) was examined following treatment of the cells with proteinase K as described in Table 1. Hylon VII high-amylose maize starch was used as the starch source in the mechanism studies. Each experiment was performed three times. The viable cell count was determined before and after each pretreatment and after the adhesion assays. Tenfold serial dilutions of the bacterial suspensions were plated onto reinforced clostridial agar (Oxoid, Basingstoke, United Kingdom). Following inoculation, the agar plates were incubated anaerobically for 2 days at 37°C.
TABLE 1.
Treatments used in the investigation of mechanisms of adhesion of bifidobacteria to starch granules
| Treatment | Description |
|---|---|
| Phosphate buffer | No cell pretreatment; the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) |
| Fresh medium | No cell pretreatment; the adhesion experiment was performed in fresh growth medium (pH 6.8) |
| Spent medium | No cell pretreatment; the adhesion experiment was performed in spent supernatant from a 24-h culture of the bacterial strain (pH 4.5) |
| Pepsin-treated spent medium | No cell pretreatment; the adhesion experiment was performed in spent medium treated with 30 U of pepsin A (EC 3.4.23.1; Sigma Chemical Co., St. Louis, Mo.) ml−1 for 6 h at 37°C; for pepsin treatment, the pH of the medium was reduced to 2.0 by using 0.1 M HCl and then readjusted prior to the adhesion experiment to 4.5 by using 0.1 M NaOH |
| Pepsin-treated starch | No cell pretreatment; the adhesion experiment was performed by using Hylon VII that had been treated with 30 U of pepsin ml−1 (at pH 2.0) for 6 h at 37°C; the Hylon VII was washed and resuspended in phosphate buffer (pH 7.0) for the adhesion experiment |
| Proteinase K-treated cells | Cells (108 CFU ml−1) were pretreated with 10 U of proteinase K (Roche Molecular Biochemicals, Basel, Switzerland) ml−1 at pH 7.0 for 6 h at 37°C; the cells were then washed twice, and the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) |
| NaCl | No cell pretreatment; the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) containing 0.5 M NaCl |
| Tween 80 | No cell pretreatment; the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) containing 3.0 g of Tween 80 liter−1 |
| Pepsin-treated cells | Cells (108 CFU ml−1) were pretreated with 30 U of pepsin ml−1 at pH 2.0 for 6 h at 30°C; the cells were then washed twice, and the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) |
| Heat-treated cells | Cells (108 CFU ml−1) were heat treated at 65°C for 30 min at pH 7.0; the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) |
| Effect of potential inhibitors | No cell pretreatment; the adhesion experiment was performed in 0.1 M phosphate buffer (pH 7.0) containing 5 g of one of the following carbohydrates per liter: glucose, maltose, lactose, trehalose, cellobiose, maltodextrin 18.5, maltodextrin 22, amylose, amylopectin, and soluble starch |
Cell surface hydrophobicity.
The cell surface hydrophobicities of the strains shown in Fig. 1 were measured to establish if there was a correlation between hydrophobicity and adhesion of the bacteria to starch granules (Hylon VII). Hydrophobicity was measured by using the test for bacterial adhesion to hydrocarbon (18). Briefly, bacteria in the stationary phase were washed twice in sterile phosphate-buffered saline (pH 7.4) and resuspended in phosphate-buffered saline at an OD540 of approximately 0.4 (A0). For each strain, 1 ml of hexadecane (Aldrich, Milwaukee, Wis.) was added to 4 ml of bacterial suspension in a test tube, which was then vortexed for 20 s and equilibrated for 30 min at 37°C to allow phase separation. After incubation, the OD540 of a sample from the aqueous lower layer was measured (Af). The percentage of bacterial adhesion to the hydrocarbon was calculated as follows: (1 − Af/A0) · 100%. The correlation between cell surface hydrophobicity and adhesion of the bacteria to Hylon VII was calculated by linear regression analysis employing the least-squares method.
Adhesion under conditions that simulated the upper gastrointestinal tract conditions.
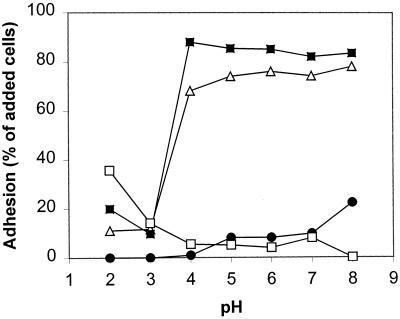
To determine the effect of passage through the stomach and small intestine on adhesion of bifidobacteria to starch granules, adhesion experiments were performed in vitro under conditions simulating the physiological conditions in the upper gastrointestinal tract. The experiments were performed with two adherent strains (B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526) and two poorly adhering strains (B. breve CIP 64.68 and B. lactis DSM 10140). The effect of pH on adhesion was examined over a wide range of values, pH 2.0 to 8.0. Additionally, the effects of pepsin, bile, and pancreatin on adhesion were examined by using the protocols described in Table 2.
TABLE 2.
Treatments used to simulate conditions for adhesion during passage through the upper gastrointestinal tract
| Treatment | Description |
|---|---|
| Effect of pH | No cell pretreatment; the adhesion experiments were performed in 0.1 M citrate-phosphate buffers at pH 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, and 8.0 |
| Effect of acid plus pepsin | No cell pretreatment; the adhesion experiments were performed in 0.2 M HCl-KCl buffer (pH 3.0) containing 30 U of pepsin A (EC 3.4.23.1; (Sigma Chemical Co.) ml−1 |
| Effect of bile | No cell pretreatment; the adhesion experiments were performed in 0.1 M phosphate buffer (pH 7.0) containing 3.0 g of porcine bile (Sigma Chemical Co.) liter−1 |
| Effect of pancreatin | No cell pretreatment; Hylon VII was pretreated for 6 h at 37°C with 0.01 g of porcine pancreatin (Sigma Chemical Co.) g−1; the starch was washed twice, and the adhesion experiments were performed in 0.1 M phosphate buffer (pH 7.0) |
Statistical methods.
Statistical differences in the levels of adhesion of the bacteria to starch were analyzed in each experiment by using one-way analysis of variance and Scheffé post hoc analysis.
RESULTS
Diversity in adhesion to starch by bifidobacteria.
The abilities of a range of Bifidobacterium strains to adhere to starch were examined and compared to their abilities to hydrolyze starch by using maize, potato, barley, and oat starch granules. The same strains were observed to hydrolyze the starch in both the liquid and solid media. The data in Fig. 1 for high-amylose maize starch granules is representative of the pattern of adhesion and starch hydrolysis observed for all starch types. A decline in the ability to adhere to starch was observed for the group of strains tested; the values ranged from more than 90% of the added cells adhering for B. adolescentis VTT E-001561 to approximately 10% adhesion for B. longum CSCC 5532. All of the highly adherent strains were also able to hydrolyze the starch granules. Three B. adolescentis strains were examined, and the amylolytic strains (VTT E-001561 and CSCC 5305) were both adherent, whereas the nonamylolytic strain (DSM 20086) adhered relatively poorly to the starch (P < 0.01). However, there was not a general association between amylolytic activity and starch adhesion for the Bifidobacterium isolates examined, since not all of the amylolytic isolates displayed high adhesion to starch.
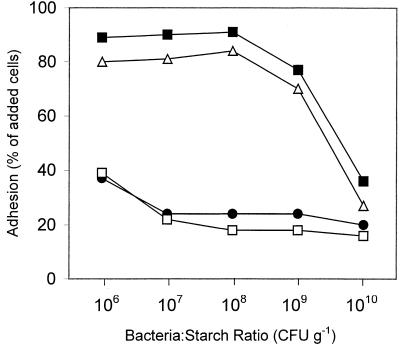
The binding capacity (number of bacteria per gram of starch) of the maize starch was approximately 108 cells g−1 for adherent strains such as B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526 adhering to Hylon VII (Fig. 2). In contrast, strains that adhered poorly in the adhesion assay at a concentration of 107 cells per ml, such as B. lactis DSM 10140 and B. breve CIP 64.68, remained relatively poorly adherent even when the cell concentration was decreased 10-fold (Fig. 2).
FIG. 2.
Effect of cell concentration on adhesion of bifidobacteria to Hylon VII maize starch granules. ▪, B. adolescentis VTT E-001561; ▵, B. pseudolongum ATCC 25526; ●, B. breve CIP 64.68; □, B. lactis DSM 10140.
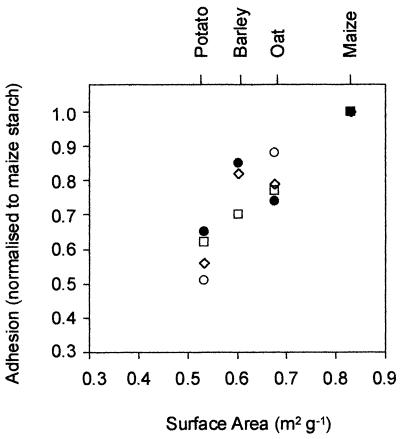
There was a strong correlation between the binding capacities of the various types of starch and the average surface areas that the different granules, which were different sizes, provided for cell adsorption (Fig. 3). The Hylon VII maize starch granules, which had the highest ratio of average surface area to mass, had the highest binding capacity.
FIG. 3.
Correlation between adhesion of bifidobacteria to different types of starch granules and granule surface area. □, B. pseudolongum ATCC 25526 (r2 = 0.992); ●, B. adolescentis VTT E-001561 (r2 = 0.734); ○, B. angulatum ATCC 27535 (r2 = 0.904); ⋄, B. adolescentis CSCC 5305 (r2 = 0.839). The global r2 value for all points is 0.865.
Effect of bacterial growth phase on adhesion.
The effect of bacterial growth phase on adhesion was investigated with the highly adherent strain B. adolescentis VTT E-001561, the moderately adherent strain Bifidobacterium sp. strain VTT E-001557, and the poorly adherent strain B. lactis DSM 10140. The three strains had similar growth curves, and they were in the lag phase until 4 to 5 h after inoculation and reached the stationary phase after 14 to 17 h of fermentation. Lag-phase cells were harvested after 4 h; exponential-phase cells were harvested at 9 h; and stationary-phase cells were harvested after 24 h of growth. The growth phase during batch fermentation did not influence the degree of adhesion to the starch granules. For all three strains, the percentages of cells adhering to the starch granules did not differ significantly (P > 0.05) for cells harvested in the lag phase, the exponential phase, and the stationary phase.
Adhesion mechanism.
The cell surface hydrophobicities of the bifidobacterial strains shown in Fig. 1, as measured by bacterial adhesion to hexadecane, varied widely (data not shown). However, no linear correlation between cell surface hydrophobicity and adhesion to the starch granules was observed (r2 = 0.023).
Further investigations were conducted to elucidate the adhesion mechanism involved in adsorption of the adherent strains B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526 to Hylon VII. The results obtained with the two strains were similar, and therefore only the results obtained with B. adolescentis VTT E-001561 are shown in Fig. 4.
FIG. 4.
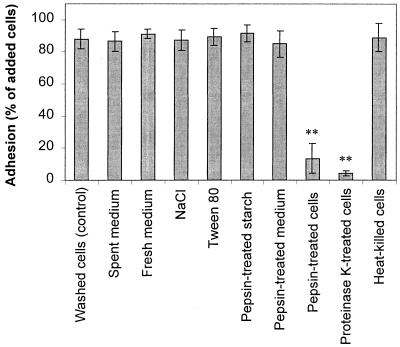
Investigation of the adhesion mechanisms of B. adolescentis VTT E-001561 binding to Hylon VII maize starch. Adhesion was prevented when the bacterial cells were treated with protease, indicating that cell surface proteins are involved in bacterium-starch binding. Error bars indicate ±1 standard deviation from the mean (n = 3). Double asterisks indicate results that are statistically different from the control results (P < 0.01).
Adhesion factors in fresh or spent extracellular medium did not play a role in adhesion, since washing the cells and resuspending them in buffer, fresh medium, or pepsin-treated spent medium did not inhibit adhesion (Fig. 4). Adding 0.5 M NaCl to provide counterions to block electrostatic interactions between the starch and the bacteria also failed to inhibit adhesion, as did adding the detergent Tween 80 to similarly reduce hydrophobic interactions. However, treatment of the bacteria with pepsin at pH 2.0 completely inhibited adhesion to the starch. Adhesion of B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526 was also pH sensitive and was inhibited at pH 2.0 and 3.0, but it remained consistently high at higher pH values (Fig. 5). The loss of adhesion at low pH was irreversible (data not shown). To determine if the reduction in adhesion observed after treatment of the cells with pepsin at pH 2.0 was due to protease activity or simply to low pH, the cells were also treated with proteinase K at pH 7.0. This treatment also resulted in a considerable reduction in adhesion (Fig. 4). Proteinase K treatment had a similar effect on adhesion of the adherent strains B. adolescentis CSCC 5305, B. angulatum ATCC 27535, and B. bifidum VTT E-001559 (data not shown).
FIG. 5.
Effect of pH on adhesion of bifidobacteria to Hylon VII maize starch granules. ▪, B. adolescentis VTT E-001561; ▵, B. pseudolongum ATCC 25526; ●, B. breve CIP 64.68; □, B. lactis DSM 10140.
Pretreatment of the bacteria with both proteinase K and pepsin destroyed cell viability, as did heat treatment at 65°C for 30 min. For both B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526, the viable cell count was approximately 107 CFU ml−1 prior to treatment but fell to below the detection limit of 102 CFU ml−1 following heat treatment or incubation with proteinase K. However, the cells killed by heat treatment adhered as well as viable cells at pH 7.0 (Fig. 4), indicating that cell viability was not a prerequisite for adhesion.
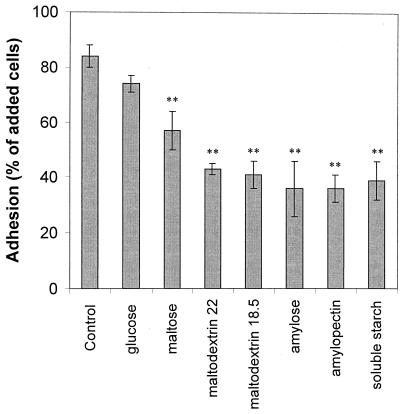
Treatment of the starch granules with pepsin did not affect adhesion of the bacteria (Fig. 4), suggesting that the cells were not adhering to any proteins or peptides associated with the starch granules. Soluble starch and starch hydrolysis products, including glucose, maltose, and maltodextrin, inhibited adhesion of both B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526, with the degree of inhibition increasing with molecular size. The results obtained for B. adolescentis VTT E-001561 (Fig. 6) were similar to those obtained for B. pseudolongum ATCC 25526. Both amylose and amylopectin inhibited adhesion to approximately the same extent. Adhesion was not significantly inhibited by other glucose-containing disaccharides, such as cellobiose, trehalose, and lactose, even at concentrations up to 50 g/liter (data not shown).
FIG. 6.
Inhibition of adhesion of B. adolescentis VTT E-001561 to Hylon VII maize starch granules by soluble starch polysaccharides and starch hydrolysis products. Each inhibitor was added to the adhesion assay mixture at a concentration equivalent to the granular starch concentration (5 g liter−1). Error bars indicate ±1 standard deviation from the mean (n = 3). Results that are statistically different from the control results are indicated by a single asterisk (P < 0.05) or double asterisks (P < 0.01).
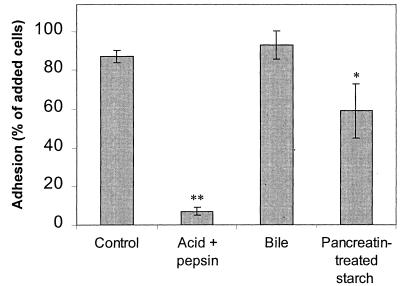
Adhesion in the gastrointestinal tract.
The results of the investigations of adhesion during passage through the stomach and small intestine were also similar for the two strains investigated. Therefore, only the results obtained for B. adolescentis VTT E-001561 are presented in Fig. 7. The sensitivity of adhesion to low pH and protease observed in the mechanism studies suggested that adhesion would not be maintained during passage through the stomach. Indeed, in an in vitro simulation of conditions in the stomach, both B. adolescentis VTT E-001561 and B. pseudolongum ATCC 25526 failed to adhere. The presence of bile did not influence adhesion. However, treatment of the Hylon VII starch granules with pancreatin resulted in a reduction in the binding capacity of the starch. The effect was less pronounced for B. adolescentis VTT E-001561, for which the level of adhesion decreased to approximately 60% of the cells added, than for B. pseudolongum ATCC 25526, for which pancreatin treatment of the starch reduced adhesion to only 40% of the cells added.
FIG. 7.
Influence of upper gastrointestinal conditions on adhesion of B. adolescentis VTT E-001561 to Hylon VII maize starch granules. Adhesion was prevented in an acidic and protease-rich environment simulating conditions in the stomach. Bile did not affect adhesion. The binding capacity of the starch granules was reduced by treatment of the granules with pancreatin. Error bars indicate ±1 standard deviation from the mean (n = 3). Double asterisks indicate results that are statistically different from the control results (P < 0.01).
DISCUSSION
Adhesion to granular starch has previously been reported for two amylolytic strains of Bifidobacterium isolated from humans (29). However, it was not known previously if adhesion to starch is a common characteristic of bifidobacteria or if there is a link between adhesion to starch and utilization of the starch as a carbon substrate. Additionally, the mechanisms used by bifidobacteria to adhere to starch had not been explored. The results of the present investigation indicate that strong adhesion of bifidobacteria to granular starch is not a trait of all strains in this genus. The strains examined could not be separated into distinct adherent and nonadherent groups. Rather, a graduated cline in adhesion capacity was observed for strains belonging to this genus. Adherent strains were able to bind to similar degrees to starch granules from a variety of different plant sources, although the specific binding capacity of the starch granules (number of bacteria per gram of starch) was dependent on the granule surface area.
The starch-binding mechanisms of the adherent Bifidobacterium strains appeared to involve a specific cell surface protein(s) rather than nonspecific hydrophobic or electrostatic interactions. Treatment of the starch granules with pancreatin (amylolytic activity) significantly reduced adhesion of the bacteria, whereas protease pretreatment of the granules did not affect adhesion. Additionally, the α-1,4-linked glucose disaccharide maltose inhibited adhesion, but no effect was observed with the β-1,4-linked glucose disaccharide cellobiose or the α,α-1,1-linked glucose disaccharide trehalose. This suggests that the binding protein(s) has a specific affinity for starch-like carbohydrates and that adhesion does not involve proteins or peptides associated with the starch granules. Although adhesion was inhibited to a small degree by glucose and maltose, the increasing degree of inhibition with increasing starch polymer length suggests that the adhesive proteins may have a higher affinity for larger molecules. However, the observed effect may also reflect greater steric hindrance by the larger starch polymers that more effectively block interactions between the surface of the bacteria and the starch granules.
Specific starch-binding proteins have been observed in another intestinal bacterium, Bacteroides thetaiotaomicron (1), which produces a number of noncatalytic outer membrane proteins involved in starch adhesion (23, 26). In this organism, adhesion to starch is a prerequisite for starch utilization since amylase activity is only cell associated and is not secreted into the extracellular medium. However, this does not appear to be the case for all bifidobacteria since a number of starch-hydrolyzing strains used in the present study adhered relatively poorly to starch granules. Additionally, extracellular amylase activity has been reported in bifidobacteria (15, 17), including the highly adherent strain B. pseudolongum (ATCC 25526) used in the present investigation (30). The ability of bifidobacteria to adhere to starch may correlate to the proportion of starch-degrading enzymes that are cell associated in particular strains. Cell-bound amylases may themselves be involved in bacterium-starch adhesion since starch-binding domains are common in starch-degrading enzymes (14). It was interesting to observe that the nonamylolytic B. adolescentis strain used in this investigation did not adhere to the starch, whereas the two amylolytic strains of this species adhered very well. Amylase-associated adhesion may be involved in this species. However, the nature of the proteins involved in starch adhesion in this and other Bifidobacterium species remains unknown. Although not all amylolytic bifidobacteria were adherent, all of the highly adherent strains did utilize the starch. Adhesion may therefore play a role in efficient utilization of starch for some strains.
In some cellulolytic bacteria, adhesion to the insoluble substrate is a prerequisite for efficient substrate hydrolysis and occurs via cell surface protein complexes called cellulosomes that include binding proteins and hydrolyzing enzymes (3). In mixed-culture competition studies involving cellulolytic ruminal bacteria, the more adherent organisms were observed to have a selective advantage over less adherent bacteria due to their physical proximity to the substrate (24). It is possible that physical association with starch may also provide adherent bifidobacteria with a competitive advantage for utilization of resistant starch as a carbon and energy source in the human colon and that this could be exploited in the development of resistant starch-Bifidobacterium synbiotics.
The presence of high-amylase maize resistant-starch has been reported to increase survival of bifidobacteria at low pH, in bile, and during passage through the intestinal tract of mice (29). Adhesion to the starch was considered a possible mechanism for increased bacterial survival (29). However, the acid- and protease-sensitive nature of adhesion to starch observed in the present study suggests that it is unlikely that bifidobacteria remain bound to starch granules in the stomach. Viable and metabolically active bifidobacteria eluting from the stomach concurrently with resistant starch may be able to reattach to the starch in the small intestine. However, the binding capacity (number of bacteria per gram) of the starch granules was substantially reduced following treatment with pancreatin, suggesting that amylase in the small intestine reduces adhesion of bifidobacteria to the starch.
One possible solution to ensure physical association between bifidobacteria and resistant starch during passage through the gastrointestinal tract is coencapsulation of the probiotic and prebiotic. Technology to encapsulate probiotics within starch granules that are then coated with amylose has been developed with the aim of protecting the bacteria during processing, storage, and passage through the gastrointestinal tract (22). Binding of adherent strains to the resistant starch core may facilitate encapsulation of the bacteria when this technology is used. In terms of providing the largest possible bacterium/encapsulation material ratio, the results of the present investigation indicate that the plant origin of the granular starch itself is not as critical as the size of the starch granules used, with smaller granules providing the largest surface area for bacterial adhesion. Hylon VII maize starch provided a binding capacity of approximately 108 CFU g−1 for adherent strains. At higher cell concentrations the proportion of bacteria that adhered to the starch dropped, presumably due to steric hindrance of available adhesion sites.
In conclusion, not all bifidobacteria adhere to granular starch, and adhesion does not appear to be a requirement for starch utilization by all strains in this genus. Cell surface proteins that specifically bind to α-1,4-linked glucose saccharides are involved in adhesion of the bacteria to the starch. The nature of these proteins and their role in starch catabolism by bifidobacteria remain to be explored. Further investigations are necessary to determine the degrees of adhesion of bifidobacteria and other bacteria to different types of resistant starch in the gastrointestinal tract and the impact of adhesion on substrate utilization, colonization, and competition in the oligotrophic colonic environment.
ACKNOWLEDGMENTS
We thank Jaana Lehtinen for technical assistance and Martin Playne from Melbourne Biotechnology, Melbourne, Australia, for his kind donation of CSIRO Starter Culture Collection strains.
REFERENCES
- 1.Anderson K L, Salyers A A. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1989;171:3199–3204. doi: 10.1128/jb.171.6.3199-3204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballongue J. Bifidobacteria and probiotic action. In: Salminen S, von Wright A, editors. Lactic acid bacteria: microbiology and functional aspects. New York, N.Y: Marcel Dekker Inc.; 1998. pp. 519–597. [Google Scholar]
- 3.Bayer E A, Morag E, Shoham Y, Tormo J, Lamed R. The cellulosome: a cell surface organelle for the adhesion to and degradation of cellulose. In: Fletcher M, editor. Bacterial adhesion: molecular and ecological diversity. New York, N.Y: Wiley-Liss; 1996. pp. 155–182. [Google Scholar]
- 4.Brown I, Warhurst M, Arcot J, Playne M, Illman R J, Topping D L. Fecal numbers of bifidobacteria are higher in pigs fed Bifidobacterium longumwith a high amylose cornstarch than with a low amylose cornstarch. J Nutr. 1997;127:1822–1827. doi: 10.1093/jn/127.9.1822. [DOI] [PubMed] [Google Scholar]
- 5.Brown I L, Wang X, Topping D L, Playne M J, Conway P L. High amylose maize starch as a versatile prebiotic for use with probiotic bacteria. Food Aust. 1998;50:603–610. [Google Scholar]
- 6.Collins M D, Gibson G R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 7.Crittenden R G. Prebiotics. In: Tannock G W, editor. Probiotics: a critical review. Wymondham, United Kingdom: Horizon Scientific Press; 1999. pp. 141–156. [Google Scholar]
- 8.Cummings J H, Macfarlane G T. Colonic microflora: nutrition and health. Nutrition. 1997;13:476–478. doi: 10.1016/s0899-9007(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J H, Macfarlane G T. Role of intestinal bacteria in nutrient metabolism. Clin Nutr. 1997;16:3–11. [Google Scholar]
- 10.Forssell, P., P. Myllärinen, J. Heinämäki, P. Palviainen, J. Yliruusi, and K. Poutanen. October 1999. Coated starch capsules and a process for producing them. World patent WO9952512A1.
- 11.Freter R. Factors affecting the microecology of the gut. In: Fuller R, editor. Probitoics: the scientific basis. London, United Kingdom: Chapman and Hall; 1992. pp. 111–144. [Google Scholar]
- 12.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 13.Gibson G R, Roberfroid M B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 14.Janecek S, Sevcik J. The evolution of starch-binding domain. FEBS Lett. 1999;456:119–125. doi: 10.1016/s0014-5793(99)00919-9. [DOI] [PubMed] [Google Scholar]
- 15.Ji G E, Han H K, Yun S W, Rhim S L. Isolation of amylolytic Bifidobacteriumsp. Int-57 and characterization of amylase. J Microbiol Biotechnol. 1992;2:85–91. [Google Scholar]
- 16.Kleessen B, Stoof G, Proll J, Schmiedl D, Noack J, Blaut M. Feeding resistant starch affects fecal and cecal microflora and short-chain fatty acids in rats. J Anim Sci. 1997;75:2453–2462. doi: 10.2527/1997.7592453x. [DOI] [PubMed] [Google Scholar]
- 17.Lee S K, Kim Y B, Ji G E. Note: purification of amylase secreted from Bifidobacterium adolescentis. J Appl Microbiol. 1997;83:267–272. doi: 10.1046/j.1365-2672.1997.00145.x. [DOI] [PubMed] [Google Scholar]
- 18.Li J, McLandsborough L A. The effects of surface charge and hydrophobicity of Escherichia coliand its adhesion to beef muscle. Int J Food Microbiol. 1999;53:185–193. doi: 10.1016/s0168-1605(99)00159-2. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane G T, Englyst H N. Starch utilization by the human large intestinal microflora. J Appl Bacteriol. 1986;60:195–201. doi: 10.1111/j.1365-2672.1986.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 20.Mattila-Sandholm T, Blum S, Collins J K, Crittenden R, de Vos W, Dunne C, Fondén R, Grenov B, Isolauri E, Kiely B, Marteau P, Morelli L, Ouwehand A, Reniero R, Saarela M, Salminen S, Saxelin M, Schiffrin E, Shanahan F, Vaughan E, von Wright A. Probiotics: towards demonstrating efficacy. Trends Food Sci Technol. 1999;10:393–399. [Google Scholar]
- 21.Mikelsaar M, Mändar R, Sepp E. Lactic acid microflora in the human microbial ecosystem and its development. In: Salminen S, von Wright A, editors. Lactic acid bacteria: microbiology and functional aspects. New York, N.Y: Marcel Dekker Inc.; 1998. pp. 279–342. [Google Scholar]
- 22.Myllärinen, P., P. Forssell, A. von Wright, M. Alander, T. Mattila-Sandholm, and K. Poutanen. October 1999. Starch capsules containing microorganisms and/or polypeptides and/or proteins and a process for producing them. World patent WO9952511A1.
- 23.Reeves A R, Wang G R, Salyers A A. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1997;179:643–649. doi: 10.1128/jb.179.3.643-649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Odt C L, Weimer P J. Competition for cellulose among three predominant ruminal cellulolytic bacteria under substrate-excess and substrate-limited conditions. Appl Environ Microbiol. 1997;63:734–742. doi: 10.1128/aem.63.2.734-742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silvi S, Rumney C J, Cresci A, Rowland I R. Resistant starch modifies gut microflora and microbial metabolism in human flora-associated rats inoculated with faeces from Italian and UK donors. J Appl Microbiol. 1999;86:521–530. doi: 10.1046/j.1365-2672.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- 26.Tancula E, Feldhaus M J, Bedzyk L A, Salyers A A. Location and characterization of genes involved in binding of starch to the surface of Bacteroides thetaiotaomicron. J Bacteriol. 1992;174:5609–5616. doi: 10.1128/jb.174.17.5609-5616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Loo J, Cummings J, Delzenne N, Englyst H, Franck A, Hopkins M, Kok N, Macfarlane G, Newton D, Quigley M, Roberfroid M, van Vliet T, van den Heuvel E. Functional food properties of non-digestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94-1095) Br J Nutr. 1999;81:121–132. doi: 10.1017/s0007114599000252. [DOI] [PubMed] [Google Scholar]
- 28.Vonk R J, Hagedoorn R E, de Graaff R, Elzinga H, Tabak S, Yang Y X, Stellaard F. Digestion of so-called resistant starch sources in the human small intestine. Am J Clin Nutr. 2000;72:432–438. doi: 10.1093/ajcn/72.2.432. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Brown I L, Evans A J, Conway P L. The protective effects of high amylose maize (amylomaize) starch granules on the survival of Bifidobacteriumspp. in the mouse intestinal tract. J Appl Microbiol. 1999;87:631–639. doi: 10.1046/j.1365-2672.1999.00836.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Conway P L, Brown I L, Evans A J. In vitro utilization of amylopectin and high-amylose maize (amylomaize) starch granules by human colonic bacteria. Appl Environ Microbiol. 1999;65:4848–4854. doi: 10.1128/aem.65.11.4848-4854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]