Abstract
Sanchuan ham is a kind of popular fermented meat product in China. To understand the role of microorganisms in reducing the accumulation of Biogenic amine (BA) during ham fermentation. Biogenic amine oxidase-producing strains were screened and identified using color development method on double-layered plate, oxidase test, high-performance liquid chromatography (HPLC), physiological, biochemical methods, and 16 S rDNA. And then a model for simulated fermentation of Sanchuan ham was developed using the strains as single or mixed starter cultures. The results showed that two biogenic amine oxidase-producing strains were identified as Enterococcus faecium and Enterococcus faecalis from Sanchuan ham by compared to the NCBI database. And the mixed starter cultures showed a more remarkable effect on the decreased production of BA compared to single starter cultures, especially cadaverine and tyramine. The cadaverine was decreased from 92.74 ± 2.44 mg/Kg to 53.95 ± 2.69 mg/Kg and tyramine was decreased from 94.23 ± 3.42 mg/kg to 57.24 ± 3.51 mg/kg in mixed starter cultures than the control group. These results indicate exist biogenic amine oxidase-producing strains could decrease the accumulation of BA in Sanchuan ham. This study reveals important findings for improving the safety and health of Sanchuan ham and other fermented meat products.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05419-y.
Keywords: Sanchuan ham, Biogenic amine oxidase-producing strains, Starter culture, Biogenic amine degradation, Meat products
Highlights:
1. There are two kinds of strains Enterococcus faecium and Enterococcus faecalis was screed from Sanchuan ham which could decrease biogenic amines produce.
2. The microorganisms from Sanchuan ham could decrease biogenic amines produce and effectively reduce the accumulation of biogenic amines.
Introduction
Biogenic amine (BA) is a group of low molecular nitrogen-containing organic compounds which exist in the body(Santos 1996). Taken in appropriate amounts, BA can contribute to physiology and metabolism. However, with excessive intake, BA can trigger a toxic bodily response, especially histamine and tyramine which are the most toxic(Rabie et al., 2011). Recent studies have shown that autochthonous starter cultures may control the accumulation of BA in fermented meat products while retaining their sensory characteristics(Dias et al., 2020). Biogenic amine oxidase (BAO) is an enzyme present in microorganisms and involved in the biogenic amine metabolic pathway and responsible for the catalytic oxidation of biogenic amine into aldehydes, ammonia, and hydrogen peroxide(Yamada et al., 2002). In recent years, strains which produce BAO have been widely used to prevent and reduce the accumulation of BA in food, especially fermented foods(Zeng et al., 2021, Yujia 2019, Li and Lu 2020).
Sanchuan ham is a kind of soft ham produced in Lijiang, Yunnan province, China, well known for its superior quality and unique flavor, with over 600 years of history(Chen et al., 2016). It has a low content of salt, nitrite, and peroxide, which is related to the production process of Sanchuan ham(Hu 2017). Sanchuan ham is produced during the coldest period of the year in Sanchuan dam (local temperature around 5℃) using fresh pig hind legs that are left to dry for 24 h, after which the surface of the meat is covered with salt. The applied salt penetrates the meat by spraying the surface of the meat with corn wine, which results in a unique aroma and also contributes to preservation. Salt curing usually occurs for 20 days, after which the material undergoes air drying for two months and moisture content is significantly reduced. Following drying, the ham is placed into large bamboo baskets with good air permeability and covered with plant ashes and left to ferment for eight months. In this process, plant ashes can retain an important amount of water present in the ham, hence playing a role in pest control. When the study of the change rule of BA during the processing of Sanchuan ham found that the eight months in fermentation the concentration of BA was decreased from 158.08 ± 31.35 mg/kg to 112.52 ± 24.45 mg/kg in one month(Hu et al., 2018). There may exist biogenic amine oxidase-producing strains in Sanchuan ham during fermentation to degrade BA.
Therefore, the aim of this study was to screened biogenic amine oxidase-producing strains from Sanchuan ham and provide identification of strains using molecular techniques. A simulated ham fermentation processing was carried out using single or mixed strains as starter cultures. Physicochemical properties, microecological system, and BA content in each fermentation process were detected. Inoculation of meat with mixed starter cultures effectively decreases the production of BA. This study provides evidence of the safety of Sanchuan ham and serves as a reference for the investigation of biogenic amine oxidase-producing strains in other fermented hams.
Materials and methods
Screening method
Sanchuan ham was purchased in the market in Sanchuan dam, Yongsheng county, Lijiang city, Yunnan province, China, the number of samples is three. The screening method uses a color development method on a double-layered plate by Lu(Lu et al., 2015). To isolate biogenic amine oxidase-producing strains, 20 g of Sanchuan ham was sliced and placed in a 250 ml conical flask with 180 mL of NaCl 0.9%, followed by incubation for 1 h at room temperature under agitation (200 rpm). The bacterial initial inoculum was adjusted to 10− 1 CFU/mL. An aliquot of 1 mL of bacterial suspension was inoculated in MRS and MSA (LuQiao Co., Beijing, China) incubated at 37 ℃ for 24 h. The enrichment culture was serially diluted in triplicate (1:10) in saline, and 1mL of each dilution was inoculated onto the lower screen medium. After cultivation at 37 ℃ for 72 h, the upper color development medium (50℃) was layered onto the lower detection medium under aseptic conditions and the result was recorded as alterations in color within 5 min of incubation. The sterile medium was used as a negative control. Visual interpretation of color changes was as follows: yellow or no color change means negative and purple means positive for the production of biogenic amines. Bacterial isolates that did not produce biogenic amine were further submitted to the oxidase test.
Assessment of oxidase production
The method for the oxidase test has been described by Liu(Liu et al., 2016). Colonies showing negative results in the biogenic amines production test were selected and tested for their ability to produce oxidase. Briefly, colonies were transferred to a white filter paper and Gordon and McLeod’s reagent (1% dimethyl p-phenylenediamine dihydrochloride) was added dropwise. The development of pink color indicated a positive result. Likewise, after the addition of a drop of 1% α-naphthol ethanol solution, the development of dark blue color within 30 s and no discoloration was regarded as positive, whereas the result was considered negative if blue color developed within 2 min. Bacterial isolates positive to the oxidase test were included in the subsequent tests.
Identification of biogenic amines oxidase-producing strains by PCR
Biogenic amines oxidase-producing strains were initially identified based on morphology and Gram staining, but further identification was conducted by 16S ribosomal DNA (rDNA) sequencing. Amplification of the 16S rDNA of biogenic amine oxidase-producing strains was performed using universal primers (upstream primer 27F: 5’-AGAGTTTGATCCTGGCTCAG-3, downstream primer 1492R: 5’-GGTTACCTTGTTACGACTT-3’)(Mishra et al., 2017, Tajabadi et al., 2012, Iruene et al., 2021, Lim 2020). Bacterial cells were cultured overnight in 2 mL of MRS at 30 ℃ and then submitted to centrifuged at 8000 rpm for 10 min. The cell pellet was washed, resuspended in 0.5 mL of TE-buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0), and lysed by 20% sodium dodecyl sulfate (SDS). The solution was boiled for 20 min and cellular debris was discarded following centrifugation at 13,000 g for 3 min. Total DNA in the supernatant was precipitated with 70% ethanol and used as template DNA in PCR.
PCR amplification was performed in 20 uL reaction mixture containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 20 pmol of each primer, a 0.2 mM of each deoxynucleotide triphosphates (Tiangen, Beijing City, China), 1 U of Taq DNA polymerase (Tiangen, Beijing City, China), and 10 ng template DNA. PCR amplifications were performed in 25 cycles (95℃ for 30 s, 56℃ for 30 s, and 72 ℃ for 2 min) in a GeneAmp PCR 2400 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) with an initial denaturation at 95 ℃ for 5 min and a final extension at 72 ℃ for 10 min. PCR amplicons were revealed by horizontal electrophoresis on a 1.5% agarose gel followed by staining with ethidium bromide solution (0.5 µg/mL). Purification of PCR amplicons was performed with QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) and elution in Tris–HCl (10 mM, pH 8.5). Amplicons were prepared with the ABI Taq Dye Deoxy™ Terminator Cycle sequencing kit (Applied Biosysterns) and directly sequenced in an ABI Model 377 automated DNA sequencer (Applied Biosystems). DNA sequences were analyzed using the Basic Local Alignment Search Tool [National Center for Biotechnology Information (NCBI), Bethesda, MD] for the identification of biogenic amines oxidase-producing strains.
Simulate the fermentation of Sanchuan ham
Four batches of simulating the fermentation of Sanchuan ham were manufactured: a control batch without starter cultures (Control group); a batch inoculated with OX4 (OX4 group); a batch inoculated with L5 (L5 group); and a batch inoculated with OX4 and L5 (OX4 and L5 co-culture group). Buy meat from the market, sterilization with spray alcohol and irradiation under an ultraviolet lamp for 30 min. The starter cultures were suspended in 500mL of cold water achieving a final concentration of 107 colony forming units (CFU) per gram of meat. The fermented program consisted of two days of fermentation at 22℃ and 90% relative humidity, followed by twelve days of ripening at 15℃ and 75% relative humidity. Sample were collected at 0, 1, 3, 5, 7, 10, 14 d. 0 day-1 day was curing period, 1 day-3 day was fermentation period and 3 day-14 day was mature period. Moisture content, water activity, pH, and concentration of NaCl were determined according to China national standard(2008a, 2008b, 2009, 2010).
Quantification of biogenic amines
The method of quantification of biogenic amine production has been described previously by Roseiro(Roseiro et al., 2006). 10 g of Sanchuan ham was added to 20 mL 0.1 mol/L HCL and homogenized in a Waring Blender for 3 min. Homogenates were centrifuged (10,000 g, 15 min, 4 ℃) and supernatants were filtered through 0.45 μm filter paper. Filtrates were placed in volumetric flasks, and 0.1 mol/L HCL was added to a final volume of 50 ml. Samples of standard biogenic amine solutions and 1 mL aliquots of ham extracts were derivatized with dansyl-chloride (10 mg/mL) according to the previously described method. One milliliter of each bacterial culture was derivatized with dansyl-chloride using the same procedure used for dansylation of standard biogenic amine solutions. Aliquots of 20 µL of dansylated derivatives were used for HPLC injection. Biogenic amines were determined by High Ultra Performance Liquid Chromatography (U3000, Thermo, Fisher, Waltham, MA, USA) using a ZORBA×Eclipse×DB-C18 reversed-phase column (4.6 mm × 250 mm, 5 μm, Thermo Fisher). The column temperature was 30 ℃ and the detection wavelength was 254 nm. Gradient elution program stared with 60:40 (v/v) acetonitrile: water at a flow rate of 1.0 mL/min, followed by a linear increase to 65:35 (v/v) acetonitrile: water (1.0 mL/min) during the next 5 min, and then a linear increase to 100% acetonitrile (1.0 mL/min) for 15 min, maintaining 100% acetonitrile (1.0 mL/min) for 4.0 min, and the ratio of acetonitrile: water was decreased to 60:40 (1.0 mL/min) for the last 10 min.
Statistical analysis
Pearson correlation was performed to determine the relationships among pH, water content, and biogenic amine content in all four samples. All statistical analyses were performed using the Statistical Package for Social Sciences, (SPSS) version 19.0 (SPSS Inc., Chicago, IL, USA). P values < 0.05 were statistically significant.
Results
Screening and identification of biogenic amine oxidase-producing strains from Sanchuan ham
To investigate whether the presence of biogenic amine oxidase-producing strains in Sanchuan ham, nine strains of lactic acid bacteria which come from Sanchuan ham was screened using a color development method on a double-layer plate and the oxidase test. The results showed that four of the nine strains were positive strains (OX3, L1, L2, and L3), and five strains were negative (OX1, OX2, OX4, L4, L5). The five strains were identified for their ability to degrade biogenic amines, the result showed that OX4, L4, and L5 showed greater ability to decrease the production of BA compared with OX1 and OX2 (Table 1). In particular, L5 had the highest capacity to degradation rates for tryptamine, cadaverine, tyramine, and spermine of 33.49%, 42.76%, 34.21%, 32.43%, respectively. OX4 could have degradation rates for phenethylamine, putrescine, histamine, and spermine of 43.44%, 39.43%, 45.20%, and 39.91%, respectively. These results indicate that OX4, L4, and L5 have a better ability to degrade biogenic amines was selected to use in subsequent experiments.
Table 1.
The biogenic amines degradation rate of bacterial strains in Sanchuan hams
| TRY | PHE | PUT | CAD | HIS | TYR | SPD | SPM | |
|---|---|---|---|---|---|---|---|---|
| OX1 | 14.39% | 9.22% | 11.97% | 12.35% | 16.10% | 9.13% | 7.98% | 4.38% |
| OX2 | 12.69% | 10.90% | 14.74% | 9.88% | 14.34% | 7.45% | 15.81% | 9.70% |
| OX4 | 31.47% | 43.44% | 39.43% | 39.23% | 45.20% | 29.83% | 39.91% | 28.53% |
| L4 | 23.32% | 34.90% | 27.28% | 27.02% | 31.37% | 25.52% | 12.02% | 12.43% |
| L5 | 33.49% | 39.92% | 35.05% | 42.76% | 38.30% | 34.21% | 28.48% | 32.43% |
Colonial morphology is an important characteristic of bacteria identification. To determine colony morphology, OX4, L4, L5 were streaked onto MRS agar and plates were incubated at 37 ℃ for 24 h. Colony morphology of all three bacterial isolates was round, milky, opaque, moist, clear edges, and raised enter. After microscopic evaluation, OX4, L4, L5 appeared as Gram-positive bacteria, bulbous rods, and short chains.
To identified the species of strains using the biochemical identification experiments, the results showed that L4 and L5 were identified as Enterococcus faecium, and OX4 was identified as Enterococcus faecalis (Table 2). Furthermore, these strains were identified by 16 S rDNA sequencing. Compared with the NCBI database, L4 and L5 sequences showed 100% similarity to Enterococcus faecium (OZH61256.1), and the OX4 sequence showed 98% to Enterococcus faecalis (EGG54264.1). Collectively, these results showed that two species of biogenic amine-producing strains were screened from Sanchuan ham.
Table 2.
Biochemical characteristics of LAB
| Strain | D-Ribose | Lactose | Galactose | Glucose | Sorbose | Melezitose | Cellobiose | Mannose | Arabinose | Maltose | Sucrose | Fructose | Raffinose | Xyloxe | Mannitol | Salicylicacid | Rhamnose | Esculoside | Amygdalin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OX4 | + | + | + | + | - | - | + | + | + | + | + | + | + | - | + | + | - | + | + |
| L4 | + | + | + | + | - | - | - | + | + | + | + | + | + | - | + | + | - | + | - |
| L5 | + | + | + | + | - | - | - | + | + | + | + | + | + | - | + | + | - | + | - |
Physicochemical parameters
To assess the changes in physicochemical parameters of biogenic amine oxidase-producing strains, the moisture contents, water activity (Aw), pH, and NaCl concentration of experimentally fermented Sanchuan ham were determined.
The result showed that the moisture content of the four groups (Control group, OX4 group, L5 group, OX4 and L5 co-culture group) of fermented meat decreased with the increase of time (Fig. 1 A). The initial moisture content was 79.9% in the experimentally fermented Sanchuan ham. During curing (day 0–1), the moisture content of all four fermented Sanchuan ham samples decreased gradually. During fermentation (day 1–3), the moisture content of Sanchuan ham decreased significantly resulting in significant water leakage. During maturation (day 3–14), the moisture content of all four experimental groups decreased progressively, reaching the lowest value on day 14. At the last day of maturation (14th), Sanchuan ham fermented with OX4 showed the highest moisture content (42.8%), while ham fermented with OX4 and L5 co-culture showed the lowest moisture content (41.3%) (Fig. 1 A).
Fig. 1.
Physical and chemical properties of Sanchun ham during processing
A - moisture content; B - water activity; C - pH; D - NaCl content; L5 is the Enterococcus faecium, OX4 is the Enterococcus faecalis; OX4 + L5 as Enterococcus faecium and Enterococcus faecalis mixture
Aw decreased progressively throughout manufacturing in four groups (Fig. 1B). Initial aw in experimentally fermented Sanchuan ham was 0.909. During curing (day 0–1) and fermentation (day 1–3), aw in all groups gradually decreased. During maturation (day 3–14), aw in all groups decreased significantly, reaching the lowest value on day 14. At the last day of maturation (14th), the highest aw was found in the control group (0.798), while ham fermented with the OX4 and L5 co-culture had the lowest aw (0.763). This might be due to close to the isoelectric point of the protein, resulting in a decrease in the ability of the protein to bind water(Mejri et al., 2017). Lower aw could extend and improve the shelf life and safety of food(Kamil and Khalid 2018).
The pH value in the control group was higher compared to the other three starter culture groups throughout the manufacturing period (Fig. 1 C). The initial pH of freshly fermented Sanchuan ham was 5.98. During the curing (day 0–1), the pH of the control group increased slightly, while pH of starter culture groups was decreased. During fermentation (day 1–3), pH of the control group did not significantly decrease, but pH of starter culture groups was significantly decreased. During maturation (day 3–14), pH of all four groups did not change significantly. This finding could be due to protein decomposition were induced by bacterial proteases to produce an alkaline substance, which can potentially neutralize the lactic acid produced by inoculated lactic acid bacteria(Ruiz-Moyano et al., 2011).
NaCl content in four groups increased with time (Fig. 1D). Initial NaCl content in fresh Sanchuan ham was 0.21%. During curing, NaCl content in four experimental groups increased significantly. During fermentation and maturation, NaCl content in four experimental groups gradually increased, reaching the highest level on day 14. This could be due to the increase of time, NaCl gradually penetrated into meat, and moisture content decreased, increasing salt content. The NaCl content was no significant in the four groups during the processing (P > 0.05).
Microbial analysis
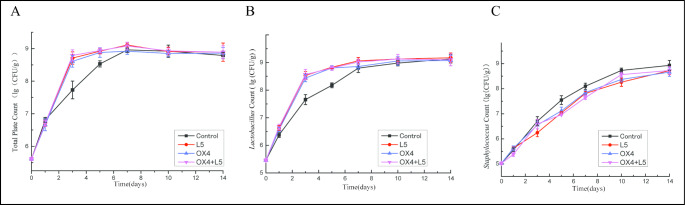
Changes in bacterial population dynamics could affect biogenic amine formation during ham fermentation. To investigate the role of bacteria in Sanchuan ham, total plant count, Lactobacillus count, and Staphylococcus count were enumerated in four groups.
Total plant count and Lactobacillus count in four groups initially increased and then stabilized (Fig. 2 A and Fig. 2B). During curing and fermentation, total plant count and Lactobacillus count in the three starter culture groups were significantly higher compared to the control group. After maturation, total plant count and Lactobacillus count in the three starter culture groups remained stable. Total plant count and Lactobacillus count in the control group increased significantly from day 3 to 7 and stabilized from day 7 to 14. Total plant count and Lactobacillus count in the control group increased time was later than starter culture groups. This showed that inoculated strains rapidly dominated the microbiota of fermented meat. Staphylococcus count in four groups increased with time (Fig. 2 C). Initial number of Staphylococcus spp. in fresh Sanchuan ham was 5.02 ± 0.054 log10 CFU/g. Staphylococcus count in four groups did not expand significantly during the curing. During fermentation, Staphylococcus count in four groups increased significantly and then remained stable during maturation. Throughout the production process, Staphylococcus count in the control group was higher than in the three starter culture groups. This result could be due to the inoculated strains is lactic acid bacteria having the capacity to produce bacteriocin, which might have inhibited the growth of Staphylococcus(Huichao et al., 2019, Essid and Hassouna 2013).
Fig. 2.
Microbial changes during Sanchuan ham meat processing
A - variation of total plate count; B - variation of Lactobacillus count; C - variation of Staphylococcus count; L5 is the Enterococcus faecium, OX4 is the Enterococcus faecalis; OX4 + L5 as Enterococcus faecium and Enterococcus faecalis mixture
Biogenic amines Analysis
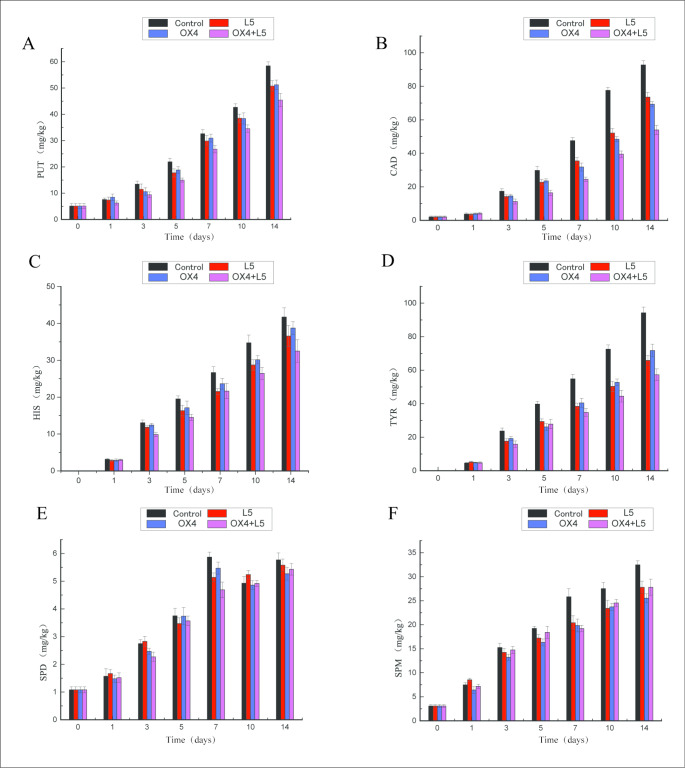
The accumulation of biogenic amines in fermented meats during processing was determined by HPLC (Fig. 3). And detected six kinds of biogenic amines, that is putrescine (PUT), cadaverine (CAD), histamine (HIS), tyramine (TYR), spermine (SPM), and spermidine (SPD).
Fig. 3.
The change of biogenic amine content in Sanchuan ham samples
A-F is the variation of Putrescine, Cadaverine, Histamine, Tyramine, Spermidine, Spermine amount in Sanchuan ham samples; L5 is the Enterococcus faecium, OX4 is the Enterococcus faecalis; OX4 + L5 as Enterococcus faecium and Enterococcus faecalis mixture
CAD and PUT were often used as important indicators of red meat and food hygiene(Wang et al., 2021). As shown in Fig. 3 A and Fig. 3B, the content of PUT and CAD in all groups increased during the processing. During the curing, the content of PUT and CAD in four groups did not increase significantly. During fermentation, the content of PUT and CAD in all groups increased gradually. During maturation, the accumulation of putrescine accelerated, the content of PUT and CAD in the control group was higher than in the other starter culture groups. On day 14, the OX4 + L5 co-culture group had significantly lower levels of putrescine (45.42 ± 2.48 mg/Kg) compared to the control group (58.45 ± 1.52 mg/Kg), and the content of cadaverine in the OX4 + L5 co-culture group was significantly lower (53.95 ± 2.69 mg/Kg) than that in the control group (92.74 ± 2.44 mg/Kg). This indicates that mixed starter cultures positively affected the putrescine and cadaverine production in Sanchuan ham fermented with a single starter culture.
HIS is the most toxic BA and is related to the presence of other amines, such as CAD, PUT, and TYR(Shalaby 1996). As shown in Fig. 3 C and Fig. 3D, content of HIS and TYR in fermented meats increased during production. HIS and TYR was not detected in freshly fermented meats. During curing, HIS and TYR content in four groups did not increase significantly. During fermentation, HIS and TYR content in all groups increased rapidly. During maturation, HIS and TYR content in the control group was higher than the starter cultures groups, indicating that starter cultures had biogenic amine degrading ability. On day 14, histamine content in the OX4 + L5 co-culture group was 32.51 ± 3.04 mg/Kg, histamine content in the control group was 41.75 ± 2.48 mg/Kg, and histamine content in L5 group and OX4 group was 36.58 ± 2.88 mg/Kg and 38.75 ± 1.74 mg/Kg, respectively. On day 14, tyramine content in the OX4 + L5 co-culture group was significantly lower than that in the control group (94.23 ± 3.42 mg/Kg) and lower compared to the other single starter culture groups. This showed that mixed starter cultures positively affected the histamine and tyramine production in Sanchuan ham fermented with a single starter culture.
As shown in Fig. 3E F, the content of SPD and SPM was generally increased with time. The initial content of SPM was 1.08 ± 0.11 mg/Kg and the initial content of SPD was 3.08 ± 0.31 mg/Kg in fresh meat. During the curing and fermentation stages, no significant increase in the content of SPM. During curing, no significant increase in spermidine content was observed. During fermentation, spermidine content increased significantly. During processing, no significant differences were found in the content of SPM and SPD among the starter culture groups and the control group. Microorganisms could use SPD and SPM as nitrogen and carbon sources, and SPD and SPM present in raw materials(HERNÁNDEZ-JOVER et al., 1997).
Discussion
In the present study, two species of biogenic amine oxidase-producing strains found in Sanchuan ham were screened and their activity characterized. Moreover, the ability of these strains to decrease the production of biogenic amines in an experimentally fermented Sanchuan ham was measured. During Sanchuan ham fermentation, six biogenic amines were detected, and tryptamine and phenethylamines were not detected. Moreover, the use of strains in the fermentation of Sanchuan ham revealed a positive effect on the degradation of biogenic amines. Remarkably, the use of mixed starter cultures showed a greater degradation effect on biogenic amines than the use of single starter cultures; in particular, the mixed starter cultures composed of OX4 and L5 had a pronounced degradation effect on putrescine, cadaverine, and tyramine. Sun(Sun 2016) investigated the traditional method of natural fermentation of Sichuan sausage and isolated three strains (Enterococcus faecium, Enterococcus faecalis, and Staphylococcus sciuri) with biogenic amine degradation ability; Enterococcus faecium and Enterococcus faecalis were used as starter cultures in Sichuan sausage fermentation, and levels of cadaverine, histamine, and tryptamine in sausages inoculated with starter cultures were significantly lower compared with the control group.
Anli(Anli et al., 2004) indicated that tyramine content over 100 mg/kg is likely to cause adverse effects on human health. On day 14, the tyramine content in the control group (uninoculated Sanchun ham) was close to 100 mg/kg, which posed a potential safety hazard. In this study, the use of a mixed starter culture had a significant effect on tyramine degradation. Lu(Lu et al., 2010) showed that the microorganism mainly involved in the fermentation of sausage had a similar effect on the degradation of tyramine as that described herein. Furthermore, as above reported, the degradation of spermine and spermidine by an inoculated bacterial starter was not significant, as previously described by Komprda(Komprda et al., 2004). The present study also revealed that the spermine content remained relatively constant throughout processing and storage, a finding that is corroborated by Zhang(Zhang 2015).
Conclusions
In this study, we screened and identified two kinds of biogenic amine strains from Sanchuan ham, it was Enterococcus faecium and Enterococcus faecalis, respectively. These strains were used to ferment Sanchuan ham and found mixed starter cultures have a better biogenic amine degrading activity which indicated that Enterococcus faecium and Enterococcus faecalis could degrade biogenic amines in Sanchuan ham. The effect of the mixed starter cultures on the degradation of putrescine, cadaverine, and tyramine in fermented meat was remarkable, whereas contents of spermine and spermidine remained constant during the processing of Sanchuan ham. Counts of total bacteria and lactic acid bacteria in all four groups increased initially and then remained stabilized. The pH value of Sanchuan ham inoculated with the starter cultures decreased rapidly during fermentation and was significantly lower than that of the control group. In this study, we elucidate the control effect of starter culture on biogenic amines accumulation in Sanchuan ham, which is considered as a potential mixed starter for fermented meat products.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mogoedit for its linguistic assistance during the preparation of this manuscript.
Abbreviations
- BA
Biogenic Amine.
- BAO
Biogenic Amine Oxidase.HPLC:High-Performance Liquid Chromatography.
- TRY
Tryptamine.
- PHE
Phenylethylamine.
- PUT
Putrescine.
- CAD
Cadaverine.
- HIS
Histamine.
- TYR
Tyramine.
- SPD
Spermidine.
- SPM
Spermine.
Authors’ contributions
ZY, HY, and GJ designed this study. ZY and LZ conducted the experiments. ZY and LZ performed the data analyses, HY and GJ contributed reagents and materials. ZY, LZ HJ, and GJ drafted and revised the manuscript. All authors read and approved the final version of this manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (31460445) and Yunnan University Key Laboratory of Food Microbial Resources and Utilization (Yunjiaofa [2018] No. 135).
Availability of data and material-
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not Applicable.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethic approval
Not Applicable.
Consent for publication
Not Applicable.
Consent to participate
Not Applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunhe Zhang, Email: 765191027@qq.com.
Zezhong Li, Email: 674687723@qq.com.
Yongjin Hu, Email: huyjin@126.com.
Jiashun Gong, Email: gong199@163.com.
References
- 2008a). Determination of pH of meat and meat products. In: GB/T9695.5. China
- 2008b). Determination of sodium chloride in food. In: GB/T 12457. China
- (2009). Determination of moisture activity in food. In: GB/T 23490. China
- (2010). Determination of moisture content in food. In: GB/T 5009.3. China
- Anli RE, Vural N, YiLmaz S, Vural H. The determination of biogenic amines in Turkish red wines. J Food Compos Anal. 2004;17:53–62. doi: 10.1016/S0889-1575(03)00104-2. [DOI] [Google Scholar]
- Chen L, Chen H, Hu Y. Changes of main physical and chemical indexes during the maturity process of Sanchuan ham. J food Saf Qual inspection. 2016;6:2205–2209. [Google Scholar]
- Dias I, Laranjo M, Potes ME, Agulheiro-Santos AC, Elias M. Autochthonous Starter Cultures Are Able to Reduce Biogenic Amines in a Traditional Portuguese Smoked Fermented Sausage. Microorganisms. 2020;8:686. doi: 10.3390/microorganisms8050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essid I, Hassouna M. Effect of inoculation of selected Staphylococcus xylosus and Lactobacillus plantarum strains on biochemical, microbiological and textural characteristics of a Tunisian dry fermented sausage. Food Control. 2013;32:707–714. doi: 10.1016/j.foodcont.2013.02.003. [DOI] [Google Scholar]
- HERNÁNDEZ-JOVER T, Izquierdo-Pulido M, VECIANA-NOGUÉS MT, MARINÉ-FONT A, Vidal-Carou MC. Effect of Starter Cultures on Biogenic Amine Formation during Fermented Sausage Production. J Food Prot. 1997;60:825–830. doi: 10.4315/0362-028X-60.7.825. [DOI] [PubMed] [Google Scholar]
- Hu Y, Xue Q, Li Z, Zhang Y, Li S. The Change Rule of Biogenic Amines During the Processing of Sanchuan Ham. J Light Ind. 2018;033:1–8. [Google Scholar]
- Hu YJ, Chen H, Xue QL, Kui MY, Fu XP, Zhu RJ, Pu YH, Huang QC. Study on the changes law of microbial flora during Yunnan Sanchuan ham processing. J Light Ind. 2017;32:8–15. [Google Scholar]
- Huichao Z, Binbin L, Lili Z, Qingling W, Baokun L, Shiling L. The effects of amine oxidase-producing starter culture on biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. J Food Saf. 2019;39:1–7. doi: 10.1111/jfs.12638. [DOI] [Google Scholar]
- Iruene IT, Wafula EN, Kuja J, Mathara JM. African Journal of Food Science Phenotypic and genotypic characterization of lactic acid bacteria isolated from spontaneously fermented vegetable amaranth. Afr J Food Sci. 2021;15:254–261. doi: 10.5897/AJFS2021.2107. [DOI] [Google Scholar]
- Kamil E, Khalid OA. The determination of some biogenic amines in Turkish fermented sausages consumed in Van. Toxicol Rep. 2018;5:639–643. doi: 10.1016/j.toxrep.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komprda T, Smela D, Pechova P, Kalhotka L, Stencl J, Klejdus B. Effect of starter culture, spice mix and storage time and temperature on biogenic amine content of dry fermented sausages. Meat Sci. 2004;67:607–616. doi: 10.1016/j.meatsci.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Li B, Lu S. The importance of amine-degrading enzymes on the biogenic amine degradation in fermented foods: A review. Process Biochem. 2020;99:331–339. doi: 10.1016/j.procbio.2020.09.012. [DOI] [Google Scholar]
- Lim E-S. Isolation and Identification of Probiotic Bacillus strain Forming Amine Oxidase from Traditional Fermented Soybean Paste. J Korean Appleed Sci Technol. 2020;37:1535–1544. [Google Scholar]
- Liu YH, Lu SL, Lu J, Zhang YQ, Feng L. Screening of amine oxidase-producing strains from lactic acid bacteria. Mod Food Sci Technol. 2016;32:106–113. [Google Scholar]
- Lu S, Xu X, Zhou G, Zhu Z, Meng Y, Sun Y. Effect of starter cultures on microbial ecosystem and biogenic amines in fermented sausage. Food Control. 2010;21:444–449. doi: 10.1016/j.foodcont.2009.07.008. [DOI] [Google Scholar]
- Lu SL, Jiang CH, Xu XL, Xu CJ, Li KX, Shu RH. Improved screening procedure for biogenic amine production by lactic acid bacteria and Enterobacteria. Czech J Food Sci. 2015;33:19–26. [Google Scholar]
- Mejri L, Ziadi A, Adab SE, Boulares M, Essid I, Hassouna M (2017) Effect of commercial starter cultures on physicochemical, microbiological and textural characteristics of a traditional dry fermented sausage reformulated with camel meat and hump fat. J Food Meas Charact 11: 758–767
- Mishra BK, Hati S, Das S, Patel K. Identification and Characterization of Lactobacillus isolates from fermented soya food Tungrymbai, Meghalaya, India. Int J Curr Microbiol Appl Sci. 2017;6:1103–1112. doi: 10.20546/ijcmas.2017.602.124. [DOI] [Google Scholar]
- Rabie MA, Siliha H, El-Saidy S, El-Badawy AA, Malcata FX. Reduced biogenic amine contents in sauerkraut via addition of selected lactic acid bacteria. Food Chem. 2011;129:1778–1782. doi: 10.1016/j.foodchem.2011.05.106. [DOI] [Google Scholar]
- Roseiro C, Santos C, Sol M, Silva L, Fernandes I. Prevalence of biogenic amines during ripening of a traditional dry fermented pork sausage and its relation to the amount of sodium chloride added. Meat Sci. 2006;74:557–563. doi: 10.1016/j.meatsci.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moyano S, Martin A, Benito MJ, Hernandez A, Casquete R, Cordoba MDG. Application of Lactobacillus fermentum HL57 and Pediococcus acidilactici SP979 as potential probiotics in the manufacture of traditional Iberian dry-fermented sausages. Food Microbiol. 2011;28:839–847. doi: 10.1016/j.fm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Santos MHS. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Shalaby AR. Significance of biogenic amines to food safety and human health. Food Res Int. 1996;29:675–690. doi: 10.1016/S0963-9969(96)00066-X. [DOI] [Google Scholar]
- Sun X (2016) Sichuan sausage biogenic amine degradation bacteria screening and preliminary applications. Sichuan agricultural university
- Tajabadi N, Mardan M, Mustafa S, eizabadi FF, Manap M. Weissella sp.Taj-Apis, a novel lactic acid bacterium isolated from honey. J Food Agric Environ. 2012;10:263–267. [Google Scholar]
- Wang D, Hu G, Wang H, Wang L, Zhang Y, Zou Y, Zhao L, Liu F, Jin Y. Effect of Mixed Starters on Proteolysis and Formation of Biogenic Amines in Dry Fermented Mutton Sausages. Foods. 2021;10:2939. doi: 10.3390/foods10122939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Adachi O, Ogata K. Amine Oxidases of Microorganisms. Agric Biol Chem. 2002;29:864–869. [Google Scholar]
- Yujia Z (2019) Effect of Lactobacillus Plantarum on the Formation of Biogenic Amines in Mutton Fermented Sausage. Inner Mongolia Agricultural University
- Zeng J, Wu J, Chen H, Ni S. Review on biological degradation of biogenic amines in food. Int J Agricultural Sci Food Technol. 2021;7:331–334. [Google Scholar]
- Zhang H, Lu S, Ma Y, Li X, Jiang C, Wang S. Effects of biogenic amine oxidase producing strains during the maturation of smoked horsemeat sausages. Mod food Sci Technol. 2015;31:122–128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not Applicable.