Abstract
The study was evaluated the impact of cannabidiol (CBD) on thyroid hormones by modulation cannabinoid receptor-2 (CB2) and vitamin D receptor (VDR) in rats fed with vitamin D3 deficiency diet (VDD). CB2-receptors were analyzed by RT-PCR method and others biomarkers by ELISA. The relative expression of CB2 (thyroid ~ 4 folds), VDR protein (liver, 151.72%), and (kidney, 66%) was significantly increased in CBD-60 compared to VDD. Vitamin D3 metabolites were significantly increased serum (189.42%), kidney (73.84%), and liver (58.11%) in CBD-60 than VDD. Increased thyroxine (59.9%) and calcitonin (213.59%); while decreased thyroid-stimulating hormone (36.15%) and parathyroid hormone (38.64%) was observed CBD treatment in VDD rats. In conclusion, CBD treatment improves CB2 and VDR expression and the level of vitamin D3 metabolites, along with improved thyroid hormones, including calcitonin. This is the first report with an improved CB2 and VDR expression after CBD treatment in VDD induced animals. Thus, CBD can be considered to use in hypothyroidism conditions and to maintain bone health.
Keywords: Cannabinoid receptor, Vitamin D3 deficiency diet, Cannabidiol, Vitamin D3– metabolite, VDR, Hypothyroidism
Introduction
The non-psychotropic cannabinoids, cannabidiol (CBD) is derived from the Cannabis sativa plant (Borrelli 2009). The human body has an endocannabinoid system (ECS), which is responsible for maintaining the energy balance (homeostasis) in response to stress (Komorowski and Stepień 2007). An endocannabinoid system is composed of three components: endocannabinoids, receptors, and enzymes. To carry out specific functions, endocannabinoids bind to specific receptors in the body. Once the endocannabinoids have performed their functions, enzymes break them down. ECS to a number of processes within the body, including metabolism, chronic pain, motor control, sleep, muscle formation, and skin and nerve formation. Each of these functions contributes to homeostasis, or stability, in the body’s internal environment (Saponaro et al. 2021; Lu and Mackie 2016). CBD research for thyroid disorders is still in its early stages, however, the existence of ECS receptors on the thyroid gland and in the brain region that controls thyroid function is promising. It was also reported that vitamin D3 receptor (VDR), cannabinoid receptor (CB1R), and CB2R are expressed in thyroid glands. One research finding suggests a possible role of cannabinoids in regulating rat thyroid hormonal activity (Porcella et al. 2002), and especially CB2 receptor could serve as a useful biomarker and potential therapeutic target in thyroid neoplasia (Lakiotaki et al. 2015). It has been observed that CBD exerted its pharmacological effects through interacting with the cannabinoid receptors (CB1 and CB2). Both CB1 and CB2 receptors are expressed in the kidneys, and the endocannabinoids (e.g., anandamide), may influence the renal hemodynamics and tubular sodium reabsorption by using the mechanism of CB1 receptor activation (Avraham et al. 2011; Ritter et al. 2016). Therefore, CBD may act on the specific targets present in the liver and kidneys through its receptor system. There is across talk between VDR and thyroid receptors. Therefore modulation the expression of VDR and vit. D3 metabolites may alter thyroid hormone levels and functions.
Numerous studies shown that CBD can be helpful in managing symptoms commonly associated with thyroid disorders. The molecular mechanisms underlying some pharmacological effects of CBD are poorly understood (Boggs et al. 2018) and still have to be fully investigated, keeping in mind that the pharmacological targets for CBD are numerous and not fully established, some being controversial. Furthermore, the mechanisms by which CBD and vitamin D3 exert their effects are different and are more complicated when delivered in combination (Petrescu et al. 2020). As of now, there are scanty of scientific literature existed related to CBD responses under vitamin D3 deficiency diet (VDD) induced rats. Hence, the study was designed to investigate the impact of cannabidiol (CBD) on the levels of VDRs and its metabolites (25-OH-vit D3, 1,25-(OH)2-vit D3) in serum, liver, and kidney tissues. Simultaneously, it was also evaluated the effect of CBD on the level of thyroid and parathyroid hormones in VDD-induced abnormalities in male Sprague Dawley rats.
Materials and methods
Reagents and chemicals
Cannabidiol (CBD; 99.9%) was obtained from Standard Hemp Company, USA. Standard normal chow diet and VDD were obtained from Altromin Spezialfutter GmbH & Co. Germany.
Animals and VDD Model
Adult male Sprague Dawley rats (age 10–12 weeks; weight 250–300 g; Vivo Bio-Tech, India) were used in this study. Experiments were conducted under Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA) guidelines. The test facility (Dabur Research Foundation, India) was registered (Registration No. 64/PO/br/s/99/CPCSEA) for an experiment of animals with the CPCSEA, Ministry of Environment and Forest, Govt. of India. The Institutional Animal Ethical Committee (IAEC) approved the study protocol (IAEC/41/506) dated 17th January 2018. The rats were housed under environmentally controlled conditions in a 12 h light/dark cycle at 25 °C and were provided with food and water. Efforts were made to reduce animal suffering and minimize the number of animals used for these experiments. Except for normal control, all the animals were fed with VDD for three weeks. Three weeks after initiating VDD induction, a circulatory level of 25(OH) vitamin D3 was estimated (data not shown). Reduction of the level of 25(OH) vitamin D3 about 50–60% in the disease control group (VDD) compared to the normal control animals considered as VDD induced animals and was selected for further treatment.
Experimental
The 36 adult rats were randomly assigned into six groups (n = 6): normal control with 0.5% CMC, VDD model with 0.5% CMC, VDD + calcitriol (0.5 µg/kg body weight; b.w.) treatment, VDD + CBD (15 mg/kg; b.w.) treatment, VDD + CBD (30 mg/kg; b.w.) treatment, and VDD + CBD (60 mg/kg; body weight) treatment groups. CBD suspension was prepared in 10% sesame oil with 0.5% CMC followed by sonication and vortex for 10 min. This suspension was administered via oral gavage (per oral) at a volume of 10 mL/kg. All the treatment groups’ animals were treated orally as per groups mentioned after induction of the VDD model for successive 56 days (eight weeks). At 8th week, after overnight fasting, all the animals were bled from retro-orbital plexus under general anesthesia (isoflurane-3%) in a pre-coated heparinized centrifuge tubes. The serum samples were subjected for vitamin D3 metabolites and thyroid biomarker analysis. After blood collection, animals were humanely sacrificed by a CO2 asphyxiation followed by exsanguinations. Thyroid, liver, and kidney tissues were collected, homogenized, and stored at − 80 °C for estimation of cannabinoid receptor-2 (CB2-R), vitamin D3 metabolites, and VDR expression.
CB2-R Expression by RT-PCR
RNA was extracted from frozen thyroid tissues using TRIZOL reagent (Invitrogen Corp.), as per manufacturer’s instructions. After extraction, purity was confirmed by spectrophotometry (UV–Vis-NIR Spectrophotometer UV-3600 Plus; Shimadzu, Japan). cDNA was synthesized using SuperScript II first-strand synthesis system for PCR (Invitrogen Corp.) at 42 °C for 60 min. RT-PCR was performed using TaqMan Gene Expression Assays (Applied Biosystems, USA), using 1 µL of cDNA as per manufacturer's instructions. The forward (5ꞌ-GGGTCGACTCCAACGCTATC-3ꞌ) and reverse (5ꞌ-AGGTAGGCGGGTAACACAGA-3ꞌ) primers used in PCR for Cnr2 (the gene for CB2Rs). Primers for GAPDH were also used as a loading control (forward, (5ꞌ-TGACCACAGTCCATGCCATC-3ꞌ); backward (5ꞌ-GGATAGGGCCTCTCTTGCTC-3ꞌ). Cnr2-CT values were obtained from PCR amplification. Relative quantification (RQ) was calculated from the Cnr2-CT and IC-CT values for the different test samples. Relative quantification (RQ) of Cnr2 gene in the treatment groups was calculated with respect to the normal control using the following formula:
where N is the relative Threshold Cycle (CT) value of the treatment groups with respect to the normal control (Navarrete et al. 2018).
Estimation of Vitamin D receptor (VDR) and metabolites
About 100 mg of the liver and kidney tissues were rinsed with 1X PBS, homogenized in 1 mL of 1X PBS, and stored overnight at − 20 °C. To break the cell membrane, two freeze–thaw cycles were performed. Then, the homogenates were centrifuged at 5000 g for 5 min at 2 to 8 °C. The supernatant was removed and assayed immediately. Subsequently, an aliquot was stored at − 20 °C or − 80 °C. Liver and kidney homogenate was subjected to estimate VDR and metabolites (25 hydroxy vit.D3, 1, 25 dihydroxy vit.D3); while serum sample was subjected for metabolites using ELISA as per manufacturer’s instruction.
Evaluation of thyroid biomarkers
After the experimental period, all the animals were sacrificed and blood was collected from the retro-orbital plexus. Serum was separated from the blood to estimate thyroid biomarkers (TSH, T4, calcitonin, and PTH) using ELISA as per the standard manufacturer’s protocol.
Statistics
The obtained data are shown (mean ± standard error of the mean) and subjected to statistical analysis using Sigma-Plot (V11.0). For multiple comparisons, one-way analysis of variance (ANOVA) followed by post-hoc analysis by Tukey’s test and for between two groups comparison Student’s t-test was performed. P ≤ 0.05 was considered as statistically significant.
Results
CB2-R expression in thyroid tissues
The relative expression of cannabinoid 2 receptor (CB2-R) in thyroid tissues was reduced in the VDD compared to NC. Further, it was significantly (F(2,15) = 6.761, p = 0.008) increased ~ 3 and 4 folds in calcitriol and CBD-60, respectively, compared to VDD. Additionally, it was significantly (F(2,15) = 4.544, p = 0.029) increased ~ 3 folds in CBD-30 compared to VDD (Fig. 1).
Fig. 1.
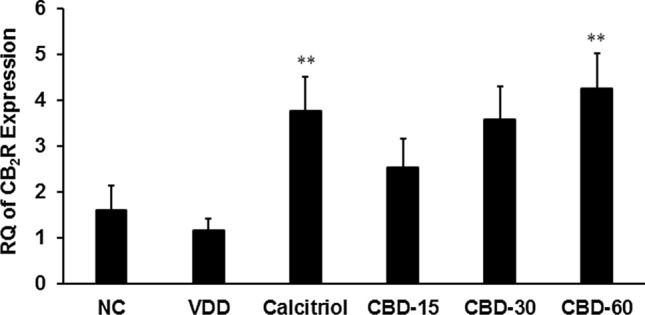
Effects of 56 days successive treatment of cannabidiol (CBD) on cannabinoid 2 receptor (CB2R) expression in thyroid tissue of Sprague Dawley rats fed with vitamin D3 deficiency diet (VDD). NC: Normal control; calcitriol (0.5 µg/kg as positive control); CBD @ 15, 30, and 60 mg/kg body weight per oral. Data shows mean ± SEM (n = 6) of relative quantification (RQ). **p ≤ 0.01 vs. VDD
Vitamin D3 receptor (VDR) expression
The VDR protein expression in liver tissue was significantly (CI = 37.604 to 93.476; t = 5.227, p ≤ 0.001) decreased by 50.37% in VDD than NC. Pair t-test revealed that it was significantly (CI = − 194.680 to − 1.280; t = − 2.258, p = 0.048) increased by 151.72% in CBD-60 than VDD (Fig. 2a). Additionally, expression of VDR protein in kidney tissue was significantly (CI = 5.304 to 18.656, t = − 3.999, p = 0.003) decreased by 53.22% in VDD than NC. Pair t-test data showed that it was significantly (CI = − 12.330 to − 1.570; t = − 2.878, p = 0.016) increased by 66% in CBD-60 than VDD (Fig. 2b).
Fig. 2.
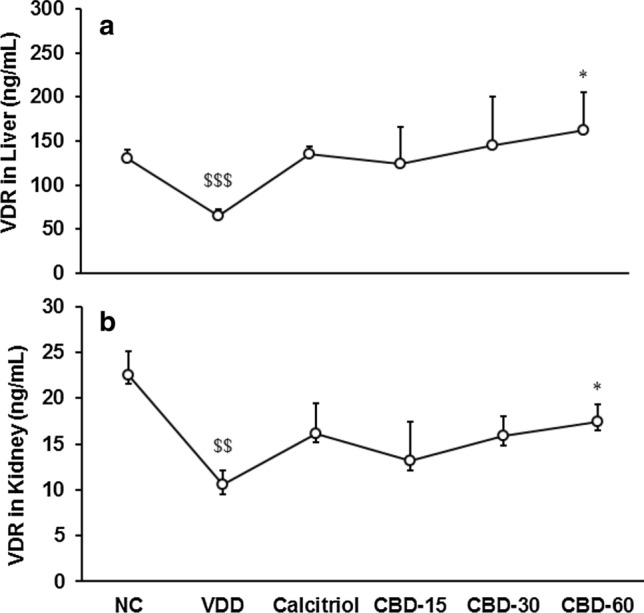
Effects of 56 days successive treatment of cannabidiol (CBD) on vitamin D3 receptor (VDR) protein expression (ng/mL) in Sprague Dawley rats fed with vitamin D3 deficiency diet (VDD); a liver and b kidney tissues. NC Normal control; calcitriol (0.5 µg/kg as positive control); CBD @ 15, 30, and 60 mg/kg body weight per oral. Data shows mean ± SEM (n = 6). $$p ≤ 0.01 and $$$p ≤ 0.001 vs. NC; *p < 0.05 vs. VDD
Vitamin D3 metabolites in serum
The level of 25-OH vitamin D3 in serum was significantly (CI = 17.447 to 36.833; t = 6.239, p ≤ 0.001) decreased by 60.31% in disease control (VDD) group than normal control (NC). Tukey's post hoc test revealed that revealed that it was significantly (F(4,25) = 8.043, p ≤ 0.001) increased by 198.66 and 189.42% in calcitriol and CBD-60, respectively, compared to VDD (Fig. 3a). Further, 1, 25-(OH)2 vitamin D3 in serum was significantly (CI = 100.928 to 488.052; t = 3.390, p = 0.007) decreased by 65.88% in VDD group than NC. Tukey's post hoc test analysis showed that it was significantly (F(4,25) = 2.854, p = 0.045) increased by 205.45 and 200.75% in calcitriol and CBD-60, respectively, compared to VDD (Fig. 3a).
Fig. 3.
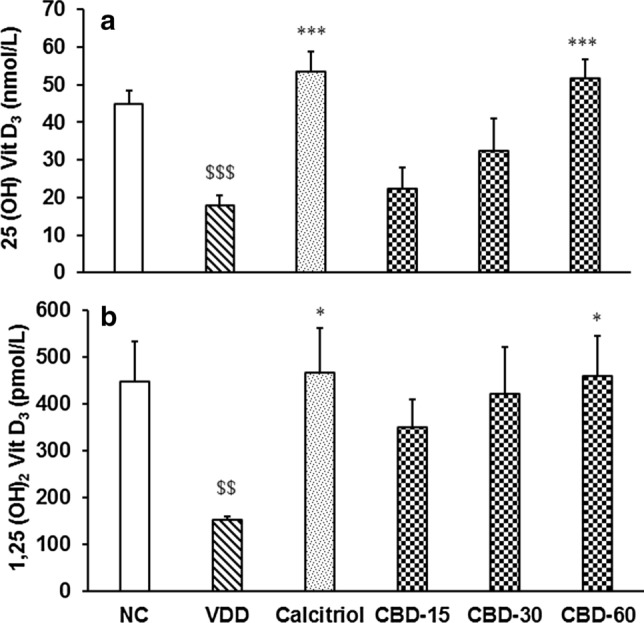
Response to vitamin D3 metabolites (a) 25-hydroxy vitamin D3 and (b) 1,25-dihydroxy vitamin D3 in serum after treatment with cannabidiol (CBD) for 56 days in vitamin D3 deficient diet (VDD)-induced Sprague Dawley rats. NC Normal control; calcitriol (0.5 µg/kg as positive control); CBD @ 15, 30, and 60 mg/kg body weight per oral. Data shows mean ± standard error of mean (n = 6). $$p ≤ 0.01 and $$$p ≤ 0.001 vs. NC; *p ≤ 0.05 and ***p ≤ 0.001 vs. VDD
Vitamin D3 metabolites in tissues
The level of 25-OH vitamin D3 in liver tissue was significantly (CI = 1223.828 to 2576.172; t = 6.261, p ≤ 0.001) decreased by 36.54% in disease control (VDD) group than normal control (NC). Tukey's post hoc test showed that it was significantly (F(2,15) = 6.4, p = 0.010) increased by 24.97% in CBD-60 compared to VDD. Tukey's post hoc test revealed that in kidney tissue 25-OH vitamin D3 was significantly (F(4,25) = 27.241, p ≤ 0.001) increased by 50.51, 43.46, 68.05, and 73.84% in calcitriol, CBD-15, CBD-30, and CBD-60, respectively, compared to VDD (Fig. 4a). Additionally, the level of 1, 25-(OH)2 vitamin D3 in liver tissue was significantly (CI = 3.143 to 6.599; t = 6.281, p ≤ 0.001) decreased by 42.26% in VDD compared to NC. It was significantly (F(4,25) = 5.597, p = 0.002) increased by 54.07 and 58.11% in calcitriol and CBD-60, respectively, compared to VDD. Further, in kidney tissue it was significantly (CI = 0.423 to 5.354; t = 2.610, p = 0.026) decreased by 25.61% in VDD compared to NC. Pair t-test assessment showed it was significantly (CI = − 6.181 to − 0.315; t = − 2.467, p = 0.033) increased by 38.74% compared to VDD (Fig. 4b).
Fig. 4.
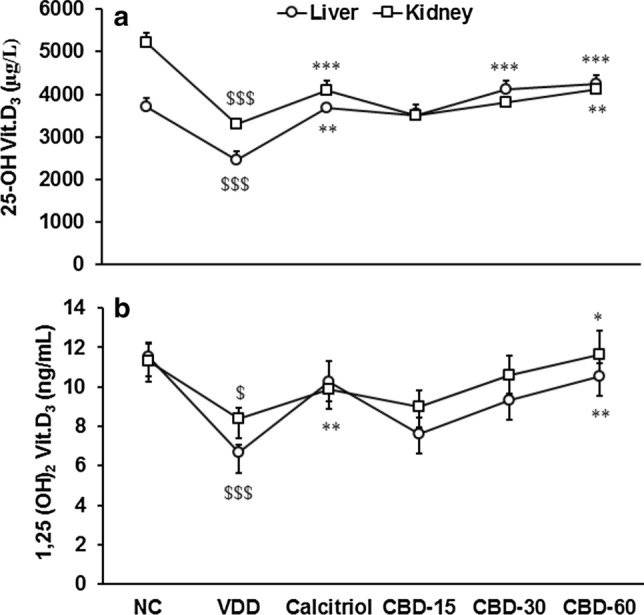
Response to vitamin D3 metabolites (a) 25-hydroxy vitamin D3 and (b) 1,25-dihydroxy vitamin D3 in liver and kidney tissues after treatment with cannabidiol (CBD) for 56 days in vitamin D3 deficient diet (VDD)-induced Sprague Dawley rats. NC Normal control; calcitriol (0.5 µg/kg as positive control); CBD @ 15, 30, and 60 mg/kg body weight per oral. Data shows mean ± standard error of mean (n = 6). $p ≤ 0.05 and $$$p ≤ 0.001 vs. NC; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. VDD
Analysis of thyroid hormones in serum
Thyroid stimulating hormone (TSH) was significantly (CI = -4.073 to -0.951; t = -3.585, p = 0.005) increased by 143.95% in VDD than NC. It was decreased by 38.43, 30.88, and 36.15% in calcitriol, CBD-30, and CBD-60, respectively than VDD (Fig. 5a). Thyroxine (T4) was significantly (CI = 3.834 to 28.951; t = 2.908, p = 0.016) decreased by 30.54% in VDD than NC. Tukey's post hoc test revealed that it was significantly (F(4,25) = 6.771, p ≤ 0.001) increased by 59.90% in CBD-60 than VDD (Fig. 5b).
Fig. 5.
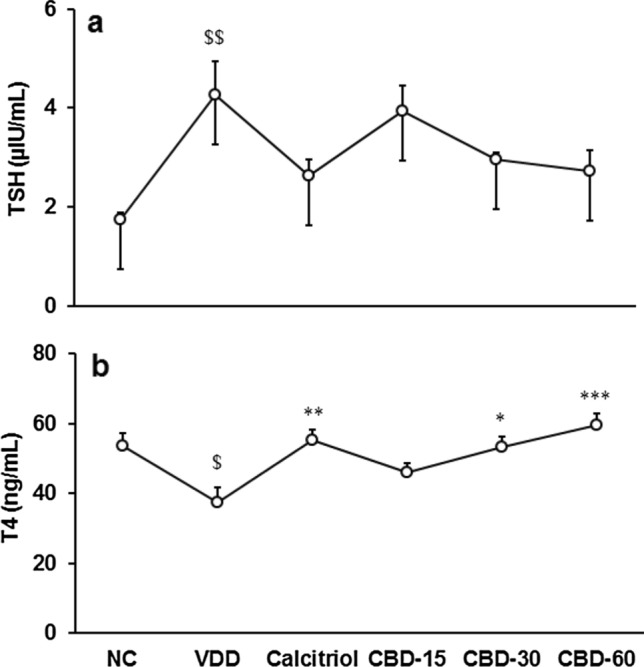
Response to thyroid hormones (a) thyroid stimulating hormone (TSH) and (b) thyroxine (T4) in serum after treatment with cannabidiol (CBD) for 56 days in vitamin D3 deficient diet (VDD)-induced Sprague Dawley rats. NC Normal control; calcitriol (0.5 µg/kg as positive control); CBD @ 15, 30, and 60 mg/kg body weight per oral. Data shows mean ± standard error of mean (n = 6). $p ≤ 0.05 and $$p ≤ 0.01 vs. NC; *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 vs. VDD
Analysis of calcitonin and parathyroid hormones in serum
Calcitonin was significantly (CI = 42.818 to 94.970; t = 5.887, p ≤ 0.001) decreased by 63.98% in VDD than NC. Tukey's post hoc analysis revealed that it was significantly (F(4,25) = 8.917, p ≤ 0.001) increased by 213.59% in CBD-60 than VDD (Fig. 6a). Parathyroid hormone (PTH) was significantly (CI = − 14.199 to − 4.276; t = − 4.148, p = 0.002) increased by 65.36% in VDD than NC. Tukey's post hoc test revealed that it was significantly (F(4,25) = 8.249, p ≤ 0.001) decreased by 38.64% in CBD-60 compared to VDD (Fig. 6b).
Fig. 6.
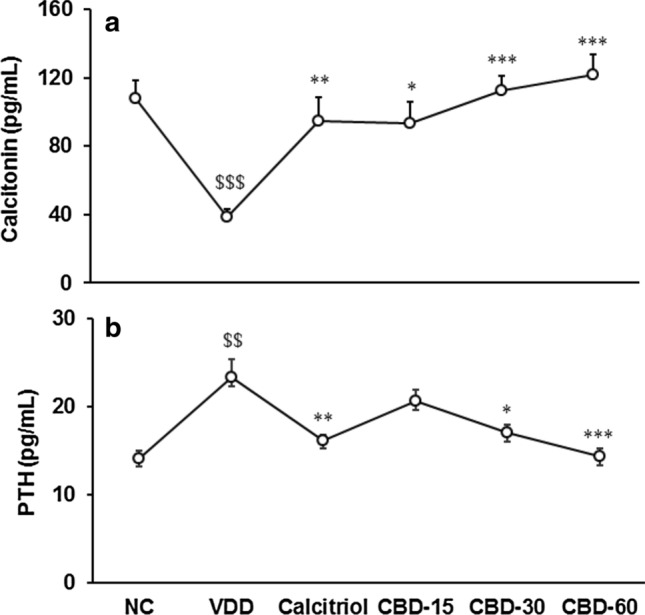
Response to (a) calcitonin and (b) parathyroid hormone (PTH) in serum after treatment with cannabidiol (CBD) for 56 days in vitamin D3 deficient diet (VDD)-induced Sprague Dawley rats. NC Normal control; calcitriol (0.5 µg/kg as positive control); CBD @ 15, 30, and 60 mg/kg body weight per oral. Data shows mean ± standard error of mean (n = 6). $$p ≤ 0.01 and $$$p ≤ 0.001 vs. NC; *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 vs. VDD
Discussion
CBD’s safety profile is already established in a plethora of ways. However, additional studies are needed to close some knowledge gaps detailed above for a well-tested, complete pharmaceutical compound. This is the right time for CBD research. If we look recent past, Epidiolex® (CBD 98%) was approved by the FDA to treat intractable epilepsy in patients with Dravet’s or Lennox-Gastaut syndromes (www.fda.gov). Kidneys are the major site of vitamin D metabolites mainly 1, 25(OH)2D3 formation. CBD has been reported to protect the kidney against many inflammations and oxidative damage. It also been reported to have a protective effect on kidney damage and decrease lipid levels. However, CBD action was found to be independent of the cannabinoid receptor 1 and 2 (CB1 and CB2) expression (Soderstrom et al. 2017).
Disturbances in vitamin D cause the development of chronic kidney disease mineral and bone disorders (CKD-MBD). Even vitamin D deficiency causes renal secondary hyperparathyroidism (RSHPT), the most common mineral disorder in later stages of CKD (Chacar et al. 2020). In CKD, renal production of 25-hydroxyvitamin D3 and 1, 25-dihydroxyvitamin D3 is reduced (Bosworth and de Boer, 2013). Thus, CBD has a significant role in decreased kidney oxidative damage, while the level of vitamin D3 metabolites was significantly improved after treatment. Vitamin D-binding protein (DBP) is needed for vitamin D3 metabolites transportation in circulation. The major vitamin D3 metabolite, 25(OH)D3 is formed in the liver and converted into 1, 25-dihydroxyvitamin D3 [1,25(OH)2D3] or calcitriol by 1α-hydroxylation process. The pharmacology and molecular target mechanism of cannabidiol (CBD) at receptor level is very complex and variable (Castillo et al. 2010). Vitamin D3 and CBD are known to stimulate bone regeneration. Both cannabinoid receptors (CB1 and CB2) were detected in human osteoblasts and osteoclasts. Numerous studies have confirmed that CB2 participates in bone metabolism and regulates bone mass by stimulating osteoblasts and inhibiting osteoclasts. Moreover, CBD at low dose (0.75 µM) induced osteogenic gene expression (Navarrete et al. 2018; Kamali et al. 2019). It was suggested to inhibit osteoclast and stimulate osteoblast differentiation by blocking G-protein-coupled receptor 55 (GPR55) (Ligresti et al. 2016). In this experiment, CBD treatment significantly increased VDR expression in both liver and kidney tissues, which could be through osteoblast stimulation and inhibition of osteoclast differentiation by blocking GPR55 (novel cannabinoid receptors) and regulate bone mass. Hence, it could be used as a standard osteogenesis promoter in order to measure the effects of CBD, which can be directly related to an improved level of vitamin D3 metabolites (25 (OH) D3, 1, 25(OH)2D3) in VDD induced animals. However, an improved level of VDR after treatment with the CBD was reported first time in VDD induced animals, which is a novel finding of CBD in liver functioning.
CBD treatment significantly improved the levels of thyroid hormones, which in turn regulates the vitamin D3 metabolism. In addition, it was reported that cannabinoid receptors are involved in healing of malignant and benign thyroid lesions and relief from inflammation, hormonal imbalance, depression, etc. In 2017, a researchers group reported a significant role of marijuana in thyroid function and autoimmunity, also reduced the level of TSH after treatment with marijuana (Malhotra et al. 2017). Besides, CBD was also related to the alteration in calcitonin gene-related protein (CGRP) release (Hammell et al. 2016). In hypothyroidism due to ~ 21 days deficiency of vitamin D3, significantly reversed after treatment with CBD in a dose-dependent manner. Disturbed thyroid function may lead to fatigue, sudden weight gain, constipation, hair loss, dry skin, high cholesterol, and cold intolerance, which can be maintained after treatment with CBD (Shahid et al. 2021). In this study, VDR expression was found higher in CBD treatment groups compared to the disease control (VDD) group (Fig. 2). CBD treatment also increased the levels of vitamin D3 metabolites in serum, liver, and kidney tissues (Figs. 3 and 4). Additionally, chronic doses of CBD led to an increased levels of thyroxine (T4) (Fig. 5b) and calcitonin (Fig. 6a). Therefore, it is assumed that chronic CBD regimen improved thyroid hormone levels by complex interactions between CB2 receptor and VDR pathways. Hope so, a positive relationship exists between CBD treatment and the expression levels of VDR and vitamin D3. These findings conceivably reflect a cross-talk between endocannabinoid receptors such as CB2R and VDR. Thus, the results indicate that the thyroid functions in rat can dynamically change after chronic treatment with CBD. These findings provide evidence for significant changes in thyroid hormone levels that led to the probable functional consequences of long-term use of CBD in the VDD model.
The mechanisms leading to the changes in VDR expression and vitamin D3 metabolites are presently not known. However, this can conceivably originate via multiple mechanisms, likely triggered initially via activation of CB2 cannabinoid receptors by CBD. It is known that the CBD and VDR systems might interact through nuclear receptor pathways at different levels. The effects of CBD were found due to the interactions and modulation by endogenous cannabinoids (eCBs) via CB2 receptor, followed by interaction between the two systems at the receptor level, notably between VDR and CB2R (Carriba et al. 2007; Navarro et al. 2008). Our hypothesis is that repeated activation of CB2R by long-term CBD treatment leads to epigenetic modifications through VDR activation and/or inhibition of transcription factors. These modifications resulted in alterations in the expression patterns of the CB2 and VDR and vitamin D3 metabolites, resulting in increased calcitonin and thyroid hormone levels. This hypothesis warrants further experiments to decipher the different steps that led to these dramatic changes.
The present study provides an evidences that CBD can be used against various thyroid disorders. This stands as an important milestone paving the way for possible repurposing of this CBD-based medicine for treating thyroid disorders, as well as other thyroid-related conditions. There was no evidence related to the direct effect of CBD on thyroid hormones, but some information is available related to the response of endocannabinoids on thyroid function. Since CBD affects endocannabinoids, we can only speculate about the effects. Therefore, details mechanistic aspect still requires more research.
Conclusions
In conclusion, the administration of oral CBD significantly improved vitamin D receptor (VDR) expression, vitamin D3 metabolites, and thyroid functions in vitamin D3 deficiency rats. This improvement after treatment with the CBD was reported first time in VDD-induced animals, which is a novel finding of CBD. Thus, these findings suggested that CBD can be used among people with hypothyroidism under vitamin D3 deficient conditions.
Acknowledgements
The authors extend their sincere thanks and gratitude to Dabur Research Foundation, India, for providing the facilities and support that enabled the successful completion of the work.
Abbreviations
- CBD
Cannabidiol
- ECS
Endocannabinoid system
- VDR
Vitamin D3 receptor
- CB1R
Cannabinoid receptor
- VDD
Vitamin D3 deficiency diet
- RQ
Relative quantification
- CT
Threshold cycle
- T4
Thyroxine
- TSH
Thyroid stimulating hormone
- PTH
Parathyroid hormone
- CKD
Chronic kidney disease
- MBD
Mineral and bone disorders
- CGRP
Calcitonin gene-related protein
Author contributions
MKT: Conceptualization, Project administration, Resources, SM: Writing—review & editing, Formal analysis; Software, Methodology, SJ: Conceptualization, Project administration, Supervision, Writing—review & editing, Validation.
Funding
Not applicable.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
(IAEC/41/506) dated 17th January 2018.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Avraham Y, Grigoriadis N, Poutahidis T, Vorobiev L, Magen I, Ilan Y, Mechoulam R, Berry E. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162(7):1650–1658. doi: 10.1111/j.1476-5381.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, Schnakenberg Martin AM, Thurnauer H, Davies A, D'Souza DC, Ranganathan M. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology. 2018;235(7):1923–1932. doi: 10.1007/s00213-018-4885-9. [DOI] [PubMed] [Google Scholar]
- Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di Marzo V, Izzo AA. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (berl) 2009;87(11):1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Semin Nephrol. 2013;33(2):158–168. doi: 10.1016/j.semnephrol.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Müller C, Woods AS, Hope BT, Ciruela F, Casadó V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32(11):2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37(2):434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Chacar FC, Kogika MM, Zafalon RVA, Brunetto MA. Vitamin D metabolism and its role in mineral and bone disorders in chronic kidney disease in humans, dogs and cats. Metabolites. 2020;10(12):499. doi: 10.3390/metabo10120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL, Westlund KN. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20(6):936–948. doi: 10.1002/ejp.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Oryan A, Hosseini S, Ghanian MH, Alizadeh M, Baghaban Eslaminejad M, Baharvand H (2019) Corrigendum to "Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects" [Mater. Sci. Eng. C. 101C (2019) pages 64–75]. Mater Sci Eng C Mater Biol Appl 126:112179. doi:10.1016/j.msec.2021.112179 [DOI] [PubMed]
- Komorowski J, Stepień H. The role of the endocannabinoid system in the regulation of endocrine function and in the control of energy balance in humans. Postepy Hig Med Dosw (online) 2007;61:99–105. [PubMed] [Google Scholar]
- Lakiotaki E, Giaginis C, Tolia M, Alexandrou P, Delladetsima I, Giannopoulou I, Kyrgias G, Patsouris E, Theocharis S. Clinical significance of cannabinoid receptors CB1 and CB2 expression in human malignant and benign thyroid lesions. Biomed Res Int. 2015;2015:839403. doi: 10.1155/2015/839403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiat. 2016;79(7):516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Heptulla RA, Homel P, Motaghedi R. Effect of marijuana use on thyroid function and autoimmunity. Thyroid. 2017;27(2):167–173. doi: 10.1089/thy.2016.0197. [DOI] [PubMed] [Google Scholar]
- Navarrete F, García-Gutiérrez MS, Aracil-Fernández A, Lanciego JL, Manzanares J. Cannabinoid CB1 and CB2 receptors, and monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinson’s disease. Neurotherapeutics. 2018;15(2):459–469. doi: 10.1007/s13311-018-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Carriba P, Gandı´a J, Ciruela F, Casado´ V, Corte´ s A, Mallol J, Canela EI, Lluis C, and Franco R. (2008) Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. Sci World J 8: 1088-1097. doi:10.1100/tsw.2008.136 [DOI] [PMC free article] [PubMed]
- Petrescu NB, Jurj A, Sorițău O, Lucaciu OP, Dirzu N, Raduly L, Berindan-Neagoe I, Cenariu M, Boșca BA, Campian RS, Ilea A. Cannabidiol and vitamin D3 impact on osteogenic differentiation of human dental mesenchymal stem cells. Medicina (kaunas) 2020;56(11):607. doi: 10.3390/medicina56110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcella A, Marchese G, Casu MA, Rocchitta A, Lai ML, Gessa GL, Pani L. Evidence for functional CB1 cannabinoid receptor expressed in the rat thyroid [published correction appears in Eur J Endocrinol 2002 Sep; 147(3):434] Eur J Endocrinol. 2002;147(2):255–261. doi: 10.1530/eje.0.1470255. [DOI] [PubMed] [Google Scholar]
- Ritter JK, Li G, Xia M, Boini K. Anandamide and its metabolites: what are their roles in the kidney? Front Biosci (schol Ed) 2016;8:264–277. doi: 10.2741/s461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponaro F, Ferrisi R, Gado F, Polini B, Saba A, Manera C, Chiellini G. The role of cannabinoids in bone metabolism: a new perspective for bone disorders. Int J Mol Sci. 2021;22(22):12374. doi: 10.3390/ijms222212374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid MA, Ashraf MA, Sharma S. (2021) Physiology, Thyroid Hormone. [Updated 2021 May 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500006/ [PubMed]
- Soderstrom K, Soliman E, Van Dross R. Cannabinoids modulate neuronal activity and cancer by CB1 and CB2 receptor-independent mechanisms. Front Pharmacol. 2017;8:720. doi: 10.3389/fphar.2017.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Not applicable.


