Abstract
The study highlights the impact of vacuum (13.33 kPa) and atmospheric (101.325 kPa) pressure on the Physico-chemical stability of rice bran oil (RBO) during continuous frying and heating at equivalent thermal driving force (∆T = 45 °C). Reduced operating pressure played a major role in retaining the Physico-chemical quality of RBO. Results show that the PV, FFA, p-An value, IV, TOTOX value, total polar compound (TPC), saturated fatty acids, CIE color values, and viscosity of RBO increased significantly (p < 0.05) at a higher rate during frying and heating (22.24 h) under atmospheric pressure as compared to vacuum pressure. TPC and total saturated fatty acids were formed 34.37% and 32.76%, and 7.33% and 2.23% more, respectively, whereas, total unsaturated fatty acids were found to be 3.34% and 1.04% less during frying and heating at atmospheric pressure as compared to vacuum pressure condition. In general, vacuum frying technology is suitable for making papaya chips with extended reuse of RBO.
Keywords: Frying, Rice bran oil (RBO), Vacuum, Oxidation, Fatty acid profile, Viscosity
Introduction
According to a report of Research and Markets (2020), India is the largest RBO producer in the world followed by China and Japan. RBO is one of the highly preferred mediums for deep-fat frying of snacks in several countries (India, China, Japan, Korea, Indonesia) due to its high smoke point, delicate flavor, and rich phytonutrients composition such as tocopherols, tocotrienols, γ-oryzanol, phytosterols, polyphenols, squalene, and some amount of omega 3 fatty acids. The concentration of tocopherol (vitamin E) and γ-oryzanol in RBO ranges from 0.10 to 0.14% and 0.9 to 2.9%, respectively (Silva et al. 2006). The presence of γ-oryzanol, tocopherols, and tocotrienols leads to high antioxidant activity and oxidative stability of RBO even at high processing temperatures. According to World Health Organization (WHO), National Institute of Nutrition (NIN), and Indian Council of Medical Research (ICMR), RBO contains closest to a recommended fatty acid composition i.e., SFA (27–33%), MUFA (33–40%) and PUFA (27–33%) as compared to other edible oils as well as effective in reducing bad cholesterol and increasing the level of good cholesterol (Pali 2013). It is also considered as suitable edible oil for hyperlipidemia patients due to its hypocholesterolemic activity (Wilson et al. 2000).
High oxidative, as well as thermal stability, makes RBO preferred oil for frying, baking, and other applications (Choudhary and Grover 2013). However, several factors are affecting the rate of oil decomposition during frying and heating such as temperature, time, oil composition, antioxidants, type of frying material, frying load, type of frying, intermittent or continuous frying/heating, and replenishment of fresh oil. The decomposition of oil forms undesirable compounds that affect the color, flavor, taste, and shelf life of fried products. Hydrolysis, oxidation, and polymerization are some of the major chain reactions that occur in oil during heating and frying operations. Moreover, the formation of undesirable complex compounds during heating and frying of oil also poses a severe health risk in humans (Sanibal and Filho 2004).
Reddy et al. (2013) reported that RBO has good cooking quality, oxidative stability, shelf life, nutritive value, and sensory attributes as compared to other edible oils. Latha and Nasirullah (2014) found a significant (p < 0.05) decrease in PV, FFA, tocopherol, and linoleic acid content, but, the Physico-chemical characteristics of RBO remained fairly good after static heating (180 ± 2 °C) up to 8 h. Fan et al. (2013) also reported a 50% higher stability of RBO as compared to palm oil after continuous frying at 185 ± 5 °C for 6 h per day for up to 5 days. Crosa et al. (2014) studied the effect of vacuum and traditional frying on the stability of refined sunflower oil (SFO). They observed a significant decrease in the rate of deterioration reaction in SFO during vacuum frying, whereas the combined effect of vacuum and added synthetic antioxidant further significantly improved the stability of SFO.
Papaya (Carica papaya L.) is one of the most nutritious fruits consumed worldwide as such fresh (Sivakumar and Wall 2013). India is the largest papaya producer shares about 48% of the world's total production (FAO 2019). But, the processed products of papaya available in the market are very less such as tutti-frutti, fruit leather, Osmo-dehydrated chunks, and papaya juice. In our earlier studies (Pandey and Chauhan 2018; Pandey et al. 2020) papaya chips were developed using vacuum frying technology but the effect of papaya frying on the quality of frying oil remained unanalyzed. Furthermore, most of the studies in the literature are limited to the effect of traditional frying of potatoes on the quality of fried oil. Therefore, the objective of the present work was to evaluate the influence of vacuum and atmospheric frying of papaya on the Physico-chemical (peroxide value, free fatty acid value, p-anisidine value, iodine value, totox value, total polar compounds, oryzanol content, fatty acid composition, color, and viscosity) properties of RBO.
Materials and methods
Materials
Fresh semi-ripe papaya fruits (Carica papaya L.) (yellow color strips on 1/3rd of fruit skin) and refined rice bran oil (RBO) of Sunpure brand (MK Agrotech Pvt. Ltd., Srinrangapatna, Karnataka) were procured from the local market of Mysore (India) and stored < 5 °C until used. All the chemicals and solvents used in this study were procured from SD Fine Chem. Ltd. and fatty acid standards were procured from Sigma-Aldrich Co., St. Louis, USA.
Equivalent thermal driving force
To compare the effect of frying and heating on the quality of RBO at vacuum and atmospheric pressure, the concept of equivalent thermal driving (ETD) force was applied in the study. ETD force can be defined as the difference between the oil temperature and the boiling point of water at any specified working pressure. The boiling point of water is 100 °C at atmospheric pressure, whereas, at the operating pressure of 13.33 kPa water starts boiling at 55 °C (Mariscal and Bouchon 2008).
Sample preparation
Papaya slices (dimension 4.0 cm × 2.0 cm × 3.1 mm) were fried in a commercial deep fat fryer (Future Tech Foods Pvt. Ltd., Pune, India), where RBO temperature was kept 100 ± 2 °C at vacuum (13.33 kPa) and 145 ± 2 °C at atmospheric (101.325 kPa) pressure, separately. The sample to oil ratio was kept constant (1:60) throughout the frying experiments to avoid temperature fluctuation. However, on the other hand, the heating experiment of RBO was performed in a heating oven (Pathak Electrical Works Pvt. Ltd., Mumbai, India), fitted with a hydraulic vacuum pump, at the same temperature and pressure as used for the frying experiment. The oil samples obtained after vacuum frying, vacuum heating, atmospheric frying, and atmospheric heating process were coded as VFO, VHO, AFO, and AHO, respectively.
All frying and heating experiments were performed up to a maximum duration of 22.24 h (6 frying × 28 min × 8 days) without replacing the oil and the samples were collected at a specified time interval of 2.48 h (6 frying × 28 min). The frying/heating of oil up to 2.48 h/day was considered as one frying/heating cycle and a total of 8 frying/heating cycles were performed in 22.24 h. After each frying/heating cycle samples were cooled to room temperature (25 ± 4 °C) before packing in 4 layers metalized polypropylene pouches and stored (5 °C) for further analysis.
Physico-chemical analysis
Peroxide value (PV)
The peroxide value (PV) of RBO was analyzed as the method described by AOCS Official Method (AOCS 1998). 4 g of oil sample was weighed in an iodine flask and 20 ml of chloroform was added before keeping for 1 h mechanical shaking. Later, 30 ml of glacial acetic acid (acidic medium) followed by 1 ml of saturated potassium iodide (KI) solution was added to the 20 ml of sample extract and the flask was kept in dark for the incubation period of 30 min. After incubation, 50 ml of distilled water was added to the flask followed by 1 ml of starch indicator (0.1%). The peroxide content in the oil was determined quantitatively by titration with standard sodium thiosulphate solution (0.02 N) using the following formula:
Free fatty acids (FFA)
The free fatty acid value (FFA) was analyzed by the AOCS Official Method (AOCS 1998). 1 g of oil sample weighed accurately in a 100 ml titration flask, separately and 50 ml of neutralized alcohol-benzene mixture (1:1) was added and mixed thoroughly. Samples were titrated against standard methanolic potassium hydroxide (0.02 N) using phenolphthalein as an indicator. The FFA value was calculated using the following formula:
p-Anisidine value (AnV)
The p-Anisidine value was measured as the method described by List et al. (1974). 0.5 g of oil sample was weighed accurately in a volumetric flask and volume was made up to 50 ml using iso-octane. The optical density of the solution was measured at 3 nm in a spectrophotometer against a reagent blank (Reading A). Later, 5 ml of sample solution was mixed with 1 ml of p-anisidine solution (0.25% in glacial acetic acid) and kept for incubation up to 10 min at room temperature. After incubation, absorbance was measured at 350 nm against a reagent blank (Reading B). The p-anisidine value was calculated using the following formula:
Iodine value (IV)
The iodine value was analyzed as described in the AOAC official method (AOCS 1998). 0.3 g of oil sample was mixed with 20 ml of carbon tetrachloride followed by 25 ml of iodine monochloride (1.65%). The reaction mixture was kept for incubation in dark for 30 min at room temperature. After incubation, 100 ml of degassed water was added to the reaction mixture followed by 20 ml of potassium iodide (15%) solution. 1 ml of 0.1% freshly prepared starch indicator was added to the reaction mixture and titrated against standard sodium thiosulphate (0.1 N) until the color disappears.
The iodine value was calculated using the following formula:
Total oxidation
Totox value of the sample represents the total amount of intermediate polar compounds (peroxides and aldehydes) that forms during lipid oxidation (Latha and Nasirullah 2014). Totox value was calculated using the following formula:
where, AV = Anisidine value and PV = Peroxide value.
Total polar compounds (TPC)
The total polar compound of the sample was measured by following the method described in AOCS (1998). 1 g of oil sample was mixed with the solvent mixture (petroleum ether: diethyl ether; 87:13) and loaded into a chromatographic column packed with activated silica gel. The sample mixture was eluted drop by drop followed by adding 150 ml of the solvent mixture from the top of the column. The eluted sample mixture was collected in a pre-weighed conical flask and solvent was evaporated in a hot air oven (70 °C) until constant weight was achieved. The total polar compound was calculated using the following formula:
Oryzanol content
The oryzanol content of RBO was estimated as a method given by Latha and Nasirullah (2014). 10 mg of oil sample was dissolved in hexane and volume was made up to 10 ml. The optical density (OD) of the solution was measured using a 1 cm quartz cell at 314 nm in a spectrophotometer (Shimadzu model 1609, Tokyo, Japan). The oryzanol content in the sample was calculated using the following formula:
Fatty acid profile
The fatty acid profile of rice bran oil (RBO) was determined following the method given by Padmashree et al. (2012). The fatty acid composition was estimated after saponifying 0.3 g of RBO with methanolic KOH (0.5 M). Esterification of the samples was performed using 140 ml of methanolic boron trifluoride. The sample volume of 0.5 µl was injected for analysis with a split ratio of 1:3 using Hydrogen gas as a carrier (40 cm/s). The fatty acid methyl esters were separated using Gas Chromatography (1000HR, Chemito, Chennai, India) equipped with BPX-70 Column (60 m × 0.25 mm id) and flame ionization detector. The temperature of the injector and detector were stabilized at 240 and 250 °C, respectively. The program parameter used was oven temperature range 50–220 °C with an initial holding time of 2 min and heating rate of 4 °C/min.
CIE color coordinates
The CIE color coordinates of oil samples were measured by tri-stimulus colorimeter (Miniscan XE plus, Model number 45/0-S, Hunter Associates Laboratory Inc., Reston, VA, USA) using D-65 illuminant and 10° observer. The instrument was calibrated using white and black standard ceramic tiles and the readings were recorded with inbuilt software (Easy Match QC, Hunter Associates Laboratory, Inc., Reston, VA, USA). The color change was expressed in terms of CIE L* value (lightness-darkness), a* value (redness-greenness), and b* value (yellowness-blueness).
Viscosity
The viscosity of oil was measured using MCR100 controlled stress rheometer (Paar Physica, Anton Paar, Gmbh, Germany) equipped with coaxial cylinders (CC 27) and the radii ratio of coaxial cylinders was 1.08477. The rheometer was equipped with an electric temperature-controlled Peltier system (TEZ-15P-C) to control the experimental temperature and to maintain a constant temperature, a circulating water bath was used (Viscotherm VT-2, Paar Physica, Anton Paar Gmbh, Austria). The rheological parameter shear stress (Pa) was measured linearly increasing up to a shear rate of 300 s−1 with 20 s per each point and 20 shear stress-shear rate data points were collected and analyzed using universal software US200 (Paar Physica, Anton Paar Gmbh, Germany). The rheological measurements were carried out at a constant temperature of 25 °C.
Statistical analysis
The significance of differences between Physico-chemical changes in differently processed RBO samples was analyzed through analysis of variance (ANOVA). SPSS statistical software Version 20 (IBM SPSS statistics, USA) was used to perform the ANOVA analysis of data. The significance of data was analyzed at a 95% level of confidence (p < 0.05), and Duncan's test was used for further statistical analysis.
Results and discussion
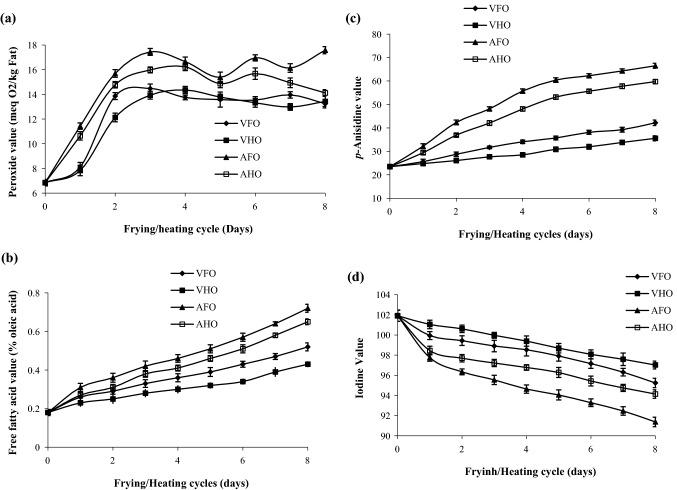
Figure 1 shows the effect of operating pressure (13.33 and 101.325 kPa) and samples moisture content (86.73%, wb) during continuous frying under vacuum and atmospheric conditions on the quality characteristics of RBO.
Fig. 1.
Changes in quality attributes of RBO during continuous frying of papaya at vacuum and atmospheric conditions
Peroxide value (PV)
Figure 2a shows that the PV of fresh RBO was found to be 6.85 meq O2/kg. However, the PV of VFO and AFO was increased from 8.12 to 13.25 meq O2/kg and 11.43–17.58 meq O2/kg after 1–8 frying cycles, respectively. The PV of RBO heated at vacuum (VHO) and atmospheric pressure (AHO) was also found to be increased from 7.83 to 13.43 and 10.64 to 14.12 meq O2/kg in 1 to 8 heating cycles, respectively. There was a 15.64%, 12.52%, 40.07%, and 35.62% increase in PV for VFO, VHO, AFO, and AHO, respectively, after the first frying and heating cycle. However, the PV value was further increased up to 48.30%, 48.99%, 61.04%, and 51.49% in VFO, VHO, AFO, and AHO, respectively, after 8 frying and heating cycles (22.24 h). The exposure of RBO to moisture-containing samples during frying might be responsible for high PV in VFO and AFO as compared to VHO and AHO, respectively (Mishra and Sharma 2014). However, the low oxygen content during frying and heating under vacuum conditions reduced the formation and decomposition of hydroperoxides which leads to low PV in VFO and VHO as compared to AFO and AHO, respectively (Shyu et al. 1998). Fan et al. (2013) also reported an inconsistent increase in PV of RBO during atmospheric frying. Debnath et al. (2012) reported that the PV of RBO was increased markedly during the first and second frying and heating cycles, but, later it became steady between 3 to 6 frying and heating cycles. They found a significant (p < 0.05) difference between PV of RBO obtained from frying and heating to the initial 2 cycles which later became non-significant (p > 0.05). The formation of secondary oxidation products might be responsible for the inconsistent increase in PV of RBO with the progress of frying and heating cycles. The transformation of peroxides into carbonyl compounds and aldehydes due to thermal decomposition might be also responsible for the inconsistent increase in PV (Shahidi and Wanasundara 2002). The spontaneous decomposition of hydroperoxides to secondary oxidation products makes PV an unreliable tool for measuring the deterioration of oil quality during frying (Tarmizi and Niranjan 2013).
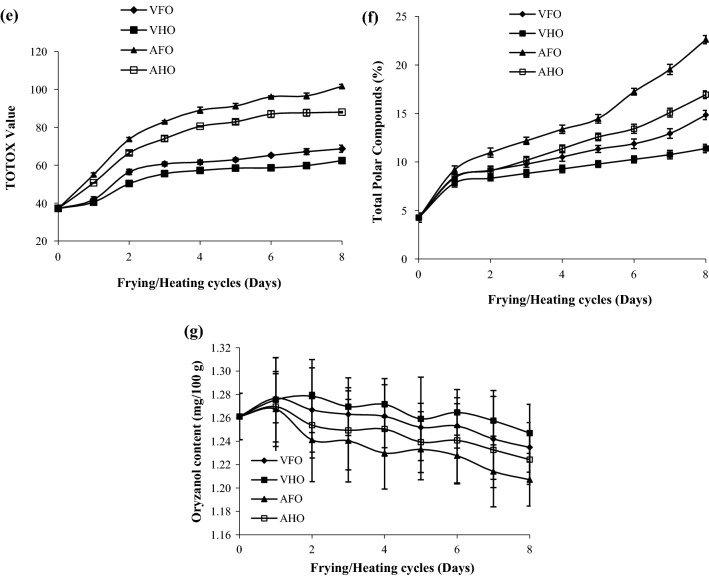
Fig. 2.
Chemical changes in RBO during frying and heating at vacuum and atmospheric pressure conditions a Peroxide value, b free fatty acid, c p-Anicidine value, d iodine value, e TOTOX value, f total polar compound and g oryzanol content. VFO vacuum fried oil, VHO vacuum heated oil, AFO atmospheric fried oil, AHO atmospheric heated oil
Free fatty acid value
The FFA content in fresh RBO was found to be 0.18%, which increased during frying and heating under vacuum and atmospheric pressure conditions (Fig. 2b). The FFA content in VFO and AFO ranged from 0.26 to 0.52% and 0.31 to 0.72%, respectively, after 1 to 8 consecutive frying cycles. However, in VHO and AHO, the FFA content was ranging from 0.23 to 0.43% and 0.27 to 0.65%, respectively, after 1 to 8 consecutive heating cycles. All the samples showed a significant (p < 0.05) increase in FFA content after the first frying and heating cycle, i.e., 28%, 41.94%, 21.74%, and 33.33% (oleic acid) for VFO, AFO, VHO, and AHO, respectively. However, the FFA content increased up to 65.38%, 75.0%, 58.14%, and 72.31% (oleic acid) in VFO, AFO, VHO, and AHO, respectively, after 8 frying and heating cycles. The absence of oxygen during frying and heating under vacuum conditions might have resulted in low FFA in VFO and VHO as compared to AFO and AHO, respectively (Crosa et al. 2014). However, a steady increase in FFA content in RBO was observed during consecutive frying or heating cycles. The frying of moisture-containing samples might have resulted in high FFA value in VFO and AFO as compared to VHO and AHO, respectively (Mishra and Sharma 2014). FFA values increased significantly (p < 0.05) among the samples during 5–8 frying and heating cycles. Debnath et al. (2012) also reported similar results where FFA of RBO increased markedly up to 3 frying and heating cycles, but, later the increase in FFA was steady with the progress of frying and heating cycles. They reported a significant (p < 0.05) difference in FFA of RBO after 3 frying and heating cycles. Fan et al. (2013) reported a significant (p < 0.05) increase in FFA content in RBO after frying French fries for up to 5 consecutive cycles. The oxidation and cleavage of double bonds formed carbonyl compounds, which get oxidized to low molecular weight fatty acid resulting in a continuous increase in FFA content of oil with the progress of frying and heating cycles. Sample moisture content also played a major role in accelerating the hydrolysis of oil and the formation of mono and diacylglycerol, glycerol, and FFA during frying (Irwandi et al. 2000; Ramadan et al. 2006).
p-Anicidine value (p-An value)
The p-An value of fresh RBO was found to be 23.47, which was increased due to frying and heating under vacuum and atmospheric conditions (Fig. 2c). The p-An value of VFO, VHO, AFO, and AHO was found to be 25.60, 24.79, 32.18, and 29.40, respectively, after the first frying and heating cycles. The p-An value was increased from 8.32 to 44.36%, 5.32 to 34.0%, 27.04 to 64.70%, and 20.17 to 60.71% in VFO, VHO, AFO, and AHO, respectively after 1 to 8 frying or heating cycles as compared to fresh RBO. The significant (p < 0.05) difference in p-An value was might be due to the high stability of RBO under vacuum conditions. The low availability of oxygen reduced the rate of hydroperoxide decomposition and formation of secondary oxidation products under vacuum as compared to atmospheric conditions (Belkova et al. 2018). The reduction in secondary oxidation products resulted in a significantly (p < 0.5) low p-An value in VFO and VHO as compared to AFO and AHO, respectively. Crosa et al. (2014) also reported that the reduction in secondary oxidation products was responsible for the low p-An value of oil during vacuum frying as compared to traditional frying. Latha and Nasirullah (2014) reported a 72.68% increase in p-An value of RBO during traditional frying for 8 h at 180 °C. However, Fan et al. (2013) reported a 51.05% increase in p-An value of RBO during traditional frying for 30 h at 185 °C. The difference in processing conditions among the studies such as frying temperature, time, and moisture content of samples might be responsible for the difference in p-An value of RBO.
Iodine value (IV)
The IV of fresh RBO was found to be 101.91, which was reduced up to 6.54%, 4.80%, 10.34%, and 7.62% in VFO, VHO, AFO, and AHO, respectively, after 8 frying and heating cycles under vacuum and atmospheric conditions (Fig. 2d). A significantly (p < 0.05) less reduction in IV of VFO and VHO as compared to AFO and AHO throughout the frying and heating cycles was noticed which might be due to less exposure to oxygen. However, the higher reduction in IV of VFO and AFO as compared to VHO and AHO was might be due to the high rate of oxidation and polymerization of oil during frying of the moisture-containing sample (Mishra and Sharma 2014). These results are following the fatty acid profile of RBO (Table 1) where the polyunsaturated fatty acids decreased to a higher extent during frying as compared to heating. Fan et al. (2013) also reported similar results where IV of RBO was decreased up to 28.10% during traditional frying for 30 h at 185 °C. However, Latha and Nasirullah (2014) reported a 5.18% decrease in IV of RBO during traditional frying for 8 h at 180 °C.
Table 1.
Changes in the fatty acid composition of RBO during frying/heating under vacuum and atmospheric conditions
| Fatty acid | Fresh RBO | Samples name | Changes during frying/heating of RBO (days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
|
Total saturated fatty acids (C:8:0; C:12:0 C14:0; C16:0; C18:0; C20:0; C21:0: C22:0; C24:0) |
22.74 | VFO | 23.58aA | 25.91bC | 26.52cC | 26.87cdC | 27.23deC | 27.64efC | 28.03fC | 28.57gC |
| VHO | 23.46aA | 24.12bA | 24.4bcA | 24.75cdA | 25.06dA | 25.43eA | 25.86fA | 26.25gA | ||
| AFO | 24.14aB | 26.72bD | 27.81cD | 28.44dD | 28.93eD | 29.58fD | 30.10gD | 30.83hD | ||
| AHO | 23.58aA | 24.67bB | 24.91bB | 25.31cB | 25.68 dB | 26.06eB | 26.41fB | 26.85gB | ||
| Unsaturated fatty acids | ||||||||||
|
Monounsaturated fatty acid (C14:1; C16:1; C18:1; C20:1) |
40.8 | VFO | 40.66gB | 40.07fB | 39.91fB | 39.26eB | 38.87 dB | 38.24cB | 37.69bB | 37.11aB |
| VHO | 40.76gB | 40.59gC | 40.25fC | 39.79eC | 39.38dC | 38.86cC | 38.41bC | 38.02aC | ||
| AFO | 40.28hA | 39.69gA | 39.26fA | 38.59eA | 37.92dA | 37.45cA | 36.98bA | 36.39aA | ||
| AHO | 40.67gB | 40.55afC | 40.23fC | 39.61eC | 39.18dC | 38.73cC | 38.22bC | 37.84aC | ||
|
Polyunsaturated fatty acids (C18:2; C18:3) |
32.1 | VFO | 31.33hA | 30.69gA | 30.18fB | 29.76eB | 29.21 dB | 28.79cB | 28.24bB | 27.83aB |
| VHO | 31.88gA | 31.49fA | 31.05eC | 30.47eC | 29.96dC | 29.41cC | 28.9bC | 28.47aC | ||
| AFO | 31.31hA | 30.62gA | 29.65fA | 28.63eA | 28.04dA | 27.53cA | 26.91bA | 26.38aA | ||
| AHO | 31.56hA | 30.79gA | 30.39fB | 29.83eB | 29.37 dB | 28.92cB | 28.38bB | 27.96aB | ||
| Total unsaturated fatty acids | 72.9 | VFO | 71.99hB | 70.76gAB | 70.09fB | 69.02eB | 68.08 dB | 67.03cB | 65.93bB | 64.94aB |
| VHO | 72.64gB | 72.08fBC | 71.3fD | 70.26eC | 69.34dD | 68.27cD | 67.31bD | 66.49aD | ||
| AFO | 71.59hA | 70.31gA | 68.91fA | 67.22eA | 65.96dA | 64.98cA | 63.89bA | 62.77aA | ||
| AHO | 72.23hB | 71.34gC | 70.62fC | 69.45eB | 68.56dC | 67.66cC | 66.60bC | 65.80aC | ||
*The first different superscript letters (a–h) shows the values are significantly different at p < 0.05 in a row
*The second different superscript letters (A–D) shows the values are significantly different at p < 0.05 in a column
Tortox value (TV)
The TV is a sum of 2 PV and p-An value, which gives an actual picture of the overall oxidative status of oil (Wai et al. 2009). The TV of fresh RBO was found to be 37.17, which increased drastically during frying and heating (Fig. 2e). The TV was found to be ranging from 41.83 to 68.67 and 40.44 to 62.42 in VFO and VHO, respectively, however in AFO and AHO it was ranging from 55.03 to 101.66 and 50.67 to 87.99, respectively, after 1 to 8 cycles. There was a significant (p < 0.05) difference observed in the TV of samples, except in the first frying and heating cycle under vacuum conditions. The TV of VFO, VHO, AFO, and AHO increased up to 45.87%, 40.45%, 63.44%, and 57.76%, respectively, after 8 cycles. This shows that the presence of oxygen as well as moisture significantly (p < 0.05) increased the overall oxidation and polymerization as well as the breakdown of unsaturated fatty acids during frying and heating of oil. The results are following the fatty acid profile of RBO, where unsaturated fatty acids decreased at a significantly (p < 0.05) higher rate during frying as compared to heating and during atmospheric conditions as compared to vacuum conditions.
Total polar compound (TPC)
The level of TPC (25–27%) has been suggested as a threshold point beyond which the frying oil should be discarded (Shyu et al. 1998; Sanibal and Filho 2004). Figure 2f shows the effect of frying or heating under vacuum and atmospheric conditions on the TPC of RBO. The TPC of fresh RBO was found to be 4.26%, which increased 71.29, 62.60, 81.16, and 74.85 times more in VFO, VHO, AFO, and AHO, respectively, after 8 frying and heating cycles. The TPC in VFO, VHO, AFO, and AHO was found to be ranging from 8.37 to 14.84%, 7.83 to 11.39%, 9.19 to 22.61%, and 8.45 to 16.94%, respectively, from 1 to 8 cycles. The high TPC in AFO and AHO was due to the presence of air during frying and heating which increased the rate of polar compounds formation as compared to VFO and VHO, respectively. However, the high TPC in VFO and AFO as compared to VHO and AHO, respectively, was associated with the high rate of peroxide and hydroperoxide formation due to the frying of the moisture-containing sample. Shyu et al. (1998) also reported similar results where the TPC in palm oil and soybean oil increased less than 20% and 14%, respectively after vacuum frying of carrot for 48 h at 105 °C. Debnath et al. (2012) reported the high rate of TPC formation in RBO during traditional frying as compared to heating. Crosa et al. (2014) also reported similar results where TPC of high oleic sunflower oil was found to be significantly higher during traditional frying as compared to vacuum frying. Termizi and Niranjan (2013) reported a significant (p < 0.05) difference in TPC of palm olein oil after 40 h of vacuum frying at 180 °C when oil drainage was performed under vacuum and atmospheric conditions.
Oryzanol content
Figure 2g shows that the oryzanol content in fresh RBO was found to be 1.26 mg/100 g which reduced to a higher extent in AFO followed by AHO, VFO, and VHO after 8 frying and heating cycles but the reduction was non-significant (p > 0.05). However, Debnath et al. (2012) reported a 13% and 15% reduction in DPPH radical scavenging activity of RBO after heating and frying, respectively. The reduction in DPPH radical scavenging activity of RBO was might be due to the loss of tocopherol and tocotrienol content during heating and frying because they together with oryzanol content participate in radical scavenging activity (Gopal Krishna 2002). Mishra and Sharma (2014) also reported a significant (p < 0.05) loss of oryzanol content of RBO during frying of potato at 210 °C for 32 s. They found that the oryzanol content of RBO reduced to a greater extent after frying the high moisture-containing sample. However, Latha and Nasirullah (2014) reported marginal losses in oryzanol content of RBO during traditional frying due to its high thermal stability.
Fatty acid profile
The nutritional value of oil mainly depends on its fatty acid composition. RBO usually has a balanced composition of saturated to monounsaturated to polyunsaturated fatty acids (i.e., 23:44:33). Table 1 shows the relative changes produced in the fatty acid profile of fresh RBO during frying and heating under vacuum and atmospheric conditions. The total saturated fatty acid of RBO was found to be increased from 22.74 to 28.57, 26.25 30.83, and 26.85% in VFO, VHO, AFO, and AHO, respectively, from 1 to 8 frying and heating cycles. However, the total unsaturated fatty acid of RBO has been affected adversely during frying and heating. The mono, poly, and total unsaturated fatty acid content of fresh RBO decreased from 40.8 to 37.11, 38.02, 36.39, and 37.84, from 32.1 to 27.83, 28.47, 26.38, and 27.96% and from 72.81 to 64.94, 66.49, 62.77 and 65.80% in VFO, VHO, AFO, and AHO, respectively, from 1 to 8 frying or heating cycles. The highest relative change was observed in polyunsaturated fatty acids as compared to monounsaturated fatty acid contents of samples. The higher relative change in polyunsaturated fatty acids was due to their higher reactivity as compared to monounsaturated fatty acids (linolenic acid > linoleic acid > oleic acid) (Roman et al. 2013; Martin-Polvillo et al. 2004).
The results show that presence of moisture and air during heating and frying positively affected the saturated fatty acid content; whereas, the unsaturated fatty acids were affected negatively. The relative increase in saturated fatty acid content was may be due to higher oxidation, isomerization, and polymerization of the unsaturated fatty acid content of oil during frying or heating (Bruhl 2014). The absence of air during frying or heating relatively reduced the change in the rate of saturated fatty acid formation as well as degradation of unsaturated fatty acid contents in VFO and VHO as compared to AFO and AHO. However, the frying of moisture-containing samples resulted in a relatively higher increase in the saturated fatty acid ratio and a decrease of unsaturated fatty acid ratio in VFO and AFO as compared to VHO and AHO. After 8 frying and heating cycles the relative change in SFA:MUFA:PUFA was from about 23:41:32 to 29:37:27, 26:38:28, 31:36:26, and 27:38:28 for VFO, VHO, AFO, and AHO, respectively. Similar reports also exist in literature where the saturated fatty acid content in oil was relatively increased and unsaturated fatty acid content decreased with the progress of frying or heating cycles (Crosa et al. 2014; Latha and Nasirullah 2014; Debnath et al. 2012; Sharoba and Ramandan 2012).
Instrumental color values
The quality of oil has been widely determined by its color, as a subjective or objective index (Maskan 2003). Table 2 shows the changes in L*, a*, and b* value of RBO after frying and heating. The L*, a*, and b* values of all the samples were found to increase with the progress of frying and heating cycles. However, after the 6th and 7th frying cycles, the L* value of AFO and VFO decreased, but the reduction was found to be non-significant (p > 0.05). The changes in the ratio of redness and yellowness due to polymerization during frying might be responsible for an increased lightness of RBO. Fan et al. (2013) also reported similar results where the lightness, as well as redness, and yellowness of the RBO were increased after traditional frying for 30 h at 180 °C. In the AFO sample, the a* value increased at a higher rate, whereas, the b* value increased at a slower rate as compared to other samples. The higher loss of green color pigments and formation of Maillard reaction products due to interaction between sugar and amino acids of papaya chips during frying might be responsible for the higher a* value in AFO as compared to other samples. However, the formation of chroman-5,6-quinones at a higher rate due to partial oxidation might be responsible for the highest b* value in AFO as compared to other samples (Maskan 2003). Latha and Nasirullah (2014) also observed similar results where the redness and yellowness of RBO increased during frying. The transfer of food components such as carbohydrates, sulfur, phosphate, trace metals, etc. in oil during frying might be also responsible for the increase in redness and yellowness of samples. Moreover, the sample moisture content played a major role in the darkening of the color index during frying. Mishra and Sharma (2014) reported a higher increase in the color index of oil during frying of the high moisture-containing sample as compared to sample with low moisture content.
Table 2.
Changes in physical attributes of RBO during frying/heating at vacuum and atmospheric pressure
| Parameters | Fresh | Samples name | Frying/heating cycles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
| Color | L* value | 4.13 ± 0.07 | VFO | 4.26 ± 0.08aA | 4.33 ± 0.05abA | 4.4 ± 0.06abA | 4.48 ± 0.06bcB | 4.59 ± 0.05cdBC | 4.75 ± 0.06deC | 4.96 ± 0.06fC | 4.88 ± 0.04efB |
| VHO | 4.16 ± 0.08aA | 4.21 ± 0.06abA | 4.28 ± 0.05abcA | 4.36 ± 0.05bcdA | 4.41 ± 0.05cdeA | 4.48 ± 0.04defAB | 4.53 ± 0.04efA | 4.61 ± 0.04fA | |||
| AFO | 4.29 ± 0.07aA | 4.36 ± 0.06aA | 4.45 ± 0.07abA | 4.58 ± 0.04bcB | 4.72 ± 0.06cdC | 5.01 ± 0.06eD | 4.88 ± 0.06deC | 4.73 ± 0.06cdAB | |||
| AHO | 4.21 ± 0.05aA | 4.28 ± 0.07abA | 4.35 ± 0.06abA | 4.44 ± 0.05bcAB | 4.53 ± 0.05cdAB | 4.62 ± 0.05deB | 4.71 ± 0.05efB | 4.79 ± 0.04fB | |||
| a* value | − 0.58 ± 0.04 | VFO | − 0.41 ± 0.05aAB | − 0.06 ± 0.04bAB | 0.21 ± 0.04cA | 0.36 ± 0.06dBC | 0.51 ± 0.04eB | 0.66 ± 0.04fB | 0.75 ± 0.04fgB | 0.84 ± 0.04gB | |
| VHO | − 0.43 ± 0.05aA | − 0.14 ± 0.04bA | 0.08 ± 0.05cA | 0.16 ± 0.05cdA | 0.26 ± 0.06deA | 0.37 ± 0.05efA | 0.42 ± 0.05fA | 0.49 ± 0.04fA | |||
| AFO | − 0.14 ± 0.05aC | 0.15 ± 0.04bC | 0.34 ± 0.03cB | 0.49 ± 0.05dC | 0.68 ± 0.06eC | 0.92 ± 0.04fC | 1.07 ± 0.06gC | 1.14 ± 0.05gC | |||
| AHO | − 0.26 ± 0.05aBC | 0.09 ± 0.06bBC | 0.17 ± 0.05bcA | 0.28 ± 0.04cAB | 0.42 ± 0.05dAB | 0.57 ± 0.05eB | 0.63 ± 0.06eB | 0.70 ± 0.05eB | |||
| b* value | 1.37 ± 0.12 | VFO | 1.84 ± 0.12aB | 2.26 ± 0.16bBC | 2.53 ± 0.12bcBC | 2.87 ± 0.13cdB | 3.24 ± 0.13deC | 3.61 ± 0.15efC | 3.72 ± 0.12fC | 3.76 ± 0.16fB | |
| VHO | 1.97 ± 0.15aB | 2.42 ± 0.10bC | 2.92 ± 0.14cC | 3.33 ± 0.11dC | 3.65 ± 0.14deD | 3.87 ± 0.13efC | 4.06 ± 0.13fC | 4.17 ± 0.14fC | |||
| AFO | 1.42 ± 0.16aA | 1.5 ± 0.13abA | 1.85 ± 0.11bcA | 2.04 ± 0.12cdA | 2.27 ± 0.15deA | 2.56 ± 0.15efA | 2.72 ± 0.13fA | 2.94 ± 0.16fA | |||
| AHO | 1.63 ± 0.14aAB | 2.04 ± 0.15bB | 2.35 ± 0.12bcB | 2.63 ± 0.12cdB | 2.82 ± 0.13deB | 3.04 ± 0.14efB | 3.28 ± 0.12fgB | 3.42 ± 0.14gB | |||
| Viscosity | 71.31 ± 0.18 | VFO | 72.62 ± 0.10aB | 73.71 ± 0.16bB | 74.34 ± 0.25cB | 74.99 ± 0.20 dB | 75.74 ± 0.26eB | 76.89 ± 0.14fB | 78.25 ± 0.22gB | 79.83 ± 0.19hB | |
| VHO | 72.03 ± 0.09aA | 72.49 ± 0.14aA | 73.05 ± 0.21bA | 73.44 ± 0.25bA | 74.36 ± 0.25cA | 75.15 ± 0.14dA | 75.87 ± 0.19eA | 76.95 ± 0.20fA | |||
| AFO | 73.85 ± 0.21aC | 75.87 ± 0.17bD | 78.57 ± 0.10cD | 81.42 ± 0.09dD | 84.00 ± 0.20eD | 87.26 ± 0.20fD | 90.56 ± 0.22gD | 94.25 ± 0.18hD | |||
| AHO | 72.89 ± 0.25aB | 74.36 ± 0.21bC | 75.92 ± 0.16cC | 76.81 ± 0.14dC | 77.73 ± 0.19eC | 79.03 ± 0.15fC | 80.23 ± 0.17gC | 82.36 ± 0.18hC | |||
*The first different superscript letters (a–h) shows the values are significantly different at p < 0.05 in a row
*The second different superscript letters (A–D) shows the values are significantly different at p < 0.05 in a column
Viscosity
The viscosity of oil had a major role in determining the amount of oil sticking on the cavities of food crust during frying (Maskan 2003; Tarmizi and Niranjan 2013). Table 2 shows that the viscosity of fresh RBO (71.31 mP s) was increased by 10.67%, 7.32%, 24.33%, and 13.41% in VFO, VHO, AFO, and AHO, after 8 frying and heating cycles, respectively. The rate of increase in viscosity was higher in AFO followed by AHO, VFO, and VHO. The higher increase in viscosity of AFO and AHO as compared to VFO and VHO, respectively, was due to the high rate of oxidation and polymerization of oil during heating and frying under atmospheric conditions. However, the high viscosity of VFO and AFO as compared to AFO and AHO, respectively, was due to the frying of the moisture-containing sample which increased the rate of degradation and polymerization of RBO. Moreover, the formation of water in oil-type emulsion might be also responsible for the increase in viscosity of RBO during frying. Tarmizi and Niranjan (2013) also reported similar results where the viscosity of palmolein oil was increased 15.96% and 18.74% after 40 h of vacuum frying at 180ºC and drainage under vacuum and atmospheric conditions, respectively. According to Shyu et al. (1998), the viscosity of palm oil, lard, and soybean oil increased 13.64%, 17.24%, and 18.60%, respectively, after 48 h of vacuum frying at 180ºC. The increase in peroxide value, carbonyl value, TPC, and dielectric constant of oil during heating and frying might be responsible for the increase in viscosity (Shyu et al. 1998; Tarmizi and Niranjan 2013). Kalogianni et al. (2011) reported that the viscosity of oil increased to a higher extent during frying as compared to the heating of oil under similar conditions. The higher rate of heat and mass transfer and saturation of oil due to oxidation, polymerization, hydrolysis, and fission products during frying might be responsible for the higher viscosity of oil after frying as compared to heating.
Conclusion
Rice bran oil (RBO) was found to be fairly stable during frying and heating at vacuum and atmospheric pressure conditions. It can be concluded that the oxidative stability of RBO was highly affected during frying and heating under atmospheric conditions as compared to under vacuum conditions. The peroxide value, free fatty acid value, p-anisidine value, total oxidation, total polar compound, and total saturated fatty acid were found to be highest, whereas, iodine value and total unsaturated fatty acid were found to be lowest after frying under atmospheric pressure conditions. Polymerization during frying and heating caused a significant (p < 0.05) increase in viscosity and CIE L*, a*, and b* values of RBO under both vacuum (13.33 kPa) and atmospheric (101.325 kPa) pressure conditions.
Acknowledgements
The authors wish to acknowledge Defence Research and Development Organization, Ministry of Defence, Government of India for providing Junior Research Fellowship and required facilities for conducting the study.
Abbreviations
- RBO
Rice bran oil
- VFO
Vacuum fried oil
- AFO
Atmospheric fried oil
- VHO
Vacuum heated oil
- AHO
Atmospheric heated oil
- TPC
Total polar compound
- TOTOX
Total oxidation
- SFA
Saturated fatty acid
- MUFA
Mono-unsaturated fatty acid
- PUFA
Poly-unsaturated fatty acid
- N
Normality
- TV
Titer value
- Meq
Milli equivalent
Author’s contributions
Mr. PAK Conceptualization, Methodology, Investigation, Formal analysis and writing—original draft. Dr. COP: Supervision and guidance, Resources, Data curation, Formal analysis, Review and editing of original draft. Ms. RN Conceptualization, Methodology and Investigation. Ms. PA Methodology and Investigation. Mr. MSS Mehodology, Investigation, Formal analysis, Review and editing. Dr. SAD Resources, Guidance and supervision.
Funding
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding work reported in this paper.
Consent to participate
Yes.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOCS (1998) In: Firestone D (ed) Official methods and recommended practices of the American Oil Chemists Society. AOCS Press, Champaign
- Belkova B, Hradecky J, Hurkova K, Forstova V, Vaclavik L. Impact of vacuum frying on quality of potato chips and frying oil. Food Chem. 2018;241:51–59. doi: 10.1016/j.foodchem.2017.08.062. [DOI] [PubMed] [Google Scholar]
- Bruhl L. Fatty acid alterations in oils and fats during heating and frying. Eur J Lipid Sci Technol. 2014;106:707–715. doi: 10.1002/ejlt.201300273. [DOI] [Google Scholar]
- Choudhary M, Grover K. Effect of deep fat frying on physicochemical properties of rice bran oil blends. IOSR J Nurs Health Sci. 2013;1:1–10. doi: 10.9790/1959-0140110. [DOI] [Google Scholar]
- Crosa MJ, Skerl V, Cadenazzi M, Olazabal L, Silva R, Suburu G, Torres M. Changes produced in oils during vacuum and traditional frying of potato chips. Food Chem. 2014;146:603–607. doi: 10.1016/j.foodchem.2013.08.132. [DOI] [PubMed] [Google Scholar]
- Debnath S, Rastogi NK, Gopal Krishna AG, Lokesh BR. Effect of frying cycles on physical, chemical and heat transfer quality of rice bran oil during deep-fat frying of poori: an Indian traditional fried food. Food Biopro Process. 2012;90:249–256. doi: 10.1016/j.fbp.2011.05.001. [DOI] [Google Scholar]
- Fan HY, Hasmadi MS, Chew HM. Frying stability of rice ban oil and palm olein. Int Food Res J. 2013;20:2173–2177. [Google Scholar]
- FAO (2019) Major tropical fruits—Statistical Compendium 2018. Rome
- Gopala Krishna AG. Nutritional components of rice bran oil in relation to processing. Lipid Technol. 2002;14:80–84. [Google Scholar]
- Irwandi J, Che Man YB, Kitts DD. Synergistic effect of rosemary and sage extracts and citric acid on fatty acid retention of RBD palm olein during deep-fat frying. J Am Oil Chem Soc. 2000;77:527–533. doi: 10.1007/s11746-000-0149-7. [DOI] [Google Scholar]
- Kalogianni EP, Karapantsios TD, Miller R. Effect of repeated frying on the viscosity, density and dynamic interfacial tension of palm and olive oil. J Food Eng. 2011;105:169–179. doi: 10.1016/j.jfoodeng.2011.02.021. [DOI] [Google Scholar]
- Latha RB, Nasirullah DR. Physico-chemical changes in rice bran oil during heating at frying tempearature. J Food Sci Technol. 2014;51:335–340. doi: 10.1007/s13197-011-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List GR, Evans CD, Kwolek WF, Warner K, Boundy RK. Oxidation and quality of soybean oil: a preliminary study of the anisidine test. J Am Oil Chem Soc. 1974;51:17–21. doi: 10.1007/BF02545207. [DOI] [Google Scholar]
- Mariscal M, Bouchon P. Comparison between atmospheric and vacuum frying of apple slices. Food Chem. 2008;107:1561–1569. doi: 10.1016/j.foodchem.2007.09.031. [DOI] [Google Scholar]
- Martin-Polvillo M, Mrquez-Ruiz G, Dobarganes MC. Oxidative stability of sunflower oils differing in unsaturation degree during long-term storage at room temperature. J Am Oil Chem Soc. 2004;81:577–583. doi: 10.1007/s11746-006-0944-1. [DOI] [Google Scholar]
- Maskan, Change in colour and rheological behaviour of sunflower seed oil during frying and after adsorbent treatment of used oil. Eur Food Res Technol. 2003;218:20–25. doi: 10.1007/s00217-003-0807-z. [DOI] [Google Scholar]
- Mishra R, Sharma HK. Effect of frying conditions on the physico-chemical properties of rice bran oil and its blended oil. J Food Sci Technol. 2014;51:1076–1084. doi: 10.1007/s13197-011-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashree A, Sharma GK, Srihari KA, Bawa AS. Development of shelf stable protein rich composite cereal bar. J Food Sci Technol. 2012;49:335–341. doi: 10.1007/s13197-011-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pali V. Rice bran oil—unique gift of nature: a review. Agri Rev. 2013;34:288–294. doi: 10.5958/j.0976-0741.34.4.014. [DOI] [Google Scholar]
- Pandey AK, Chauhan OP. Process optimization for development of vacuum fried papaya (Carica papaya L.) chips using response surface methodology. Agric Res. 2018;8:364–373. doi: 10.1007/s40003-018-0375-x. [DOI] [Google Scholar]
- Pandey AK, Sunny K, Ravi N, Chauhan OP, Patki PE. Use of partial drying and freezing pre-treatments for development of vacuum fried papaya (Carica papaya L.) chips. J Food Sci Technol. 2020;57:2310–2320. doi: 10.1007/s13197-020-04269-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan MF, Sharanabasappa G, Seetharam YN, Seshagiri M, Moersel JT. Profile and levels of fatty acids and bioactive constituents in mahua butter from fruit-seeds of buttercup tree [Madhuca longifolica (Koenig)] Eur Food Res Technol. 2006;222:710–718. doi: 10.1007/s00217-005-0155-2. [DOI] [Google Scholar]
- Reddy KJ, Jayathilakan K, Pandey MC, Radhakrishana, Evaluation of the physico-chemical stability of rice bran oil and its blends for the development of frunctional meat products. Int J Food Nutri Sci. 2013;2:46–53. [Google Scholar]
- Research and Markets (2020) Rice bran oil market: global industry trends, share, size, growth, opportunity and forecast 2019–2024. https://www.researchandmarkets.com/research/bxc2n4/global_rice_bran?w=12. Accessed on 31 Dec, 2020
- Roman O, Heyd B, Broyart B, Castillo R, Maillard M. Oxidative reactivity of unsaturated fatty acids from sunflower, high oleic sunflower and rapeseed oils subjected to heat treatment, under controlled conditions. Food Sci Technol. 2013;52:49–59. [Google Scholar]
- Sanibal EAA, Filho JM. Frying oil and fat quality measured by chemical, physical and test kit analysis. J Am Oil Chem Soc. 2004;81:847–852. doi: 10.1007/s11746-004-0990-8. [DOI] [Google Scholar]
- Shahidi F, Wanasundara UN. Methods for measuring oxidative rancidity in fats and oils. In: Akoh CC, Min DB, editors. Food lipids: chemistry, nutrition and biotechnology. 2. New York: Marcel Dekker Inc.; 2002. pp. 465–482. [Google Scholar]
- Sharoba AM, Ramadan MF. Impact of frying on fatty acid profile and rheological behavior of some vegetable oils. J Food Process Technol. 2012;3:161–169. [Google Scholar]
- Shyu SL, Hau LB, Hwang LS. Effect of vacuum frying on the oxidative stability of oils. J Am Oil Chem Soc. 1998;75:1393–1398. doi: 10.1007/s11746-998-0188-3. [DOI] [Google Scholar]
- Silva MA, Sanches C, Amante ER. Prevention of hydrolytic rancidity in rice bran. J Food Eng. 2006;75:487–491. doi: 10.1016/j.jfoodeng.2005.03.066. [DOI] [Google Scholar]
- Sivakumar D, Wall MM. Papaya fruit quality management during the postharvest supply chain. Food Rev Int. 2013;29:24–48. doi: 10.1080/87559129.2012.692138. [DOI] [Google Scholar]
- Tarmizi AH, Niranjan K. Post-frying oil drainage from potato chips and French fries: a comparative study of atmospheric and vacuum drainage. Food Bioprocess Technol. 2013;6:489–497. doi: 10.1007/s11947-011-0685-5. [DOI] [Google Scholar]
- Wai WT, Saad B, Lim BP. Determination of TOTOX value in palm oleins using a FI-potentiometric analyzer. Food Chem. 2009;113:285–290. doi: 10.1016/j.foodchem.2008.06.082. [DOI] [Google Scholar]
- Wilson TA, Ausman LM, Lawton CW, Hegsted DM, Nicolosi RJ. Comparative cholesterol lowering properties of vegetable oils: beyond fatty acids. J Am College Nutr. 2000;19:601–607. doi: 10.1080/07315724.2000.10718957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.