Abstract
The study focuses on development of modified atmosphere packaging for fresh-cut minimally processed cauliflower to increase its shelf-life and enhancing the on-farm value addition by utilizing perforated packaging films along with pretreatments with ozonated water and antimicrobials. The samples were treated with ozonated water, oregano essential oil (antimicrobial), and cinnamon essential oil (antimicrobial). Based on preliminary treatments, the number of perforations made by specially prepared tool was kept as 6 and 12, which represented 5.1 × 10–5 and 1.02 × 10–4% of the total surface area of packages. These perforated packaged samples were stored in cold room at 4 °C temperature for 28 days. The shelf life and quality of treated cauliflower samples were compared with untreated samples. The quality analysis of the samples was carried up to 28 days at 7 days intervals based on their weight loss, headspace gas composition, texture, pH, total soluble solids, ascorbic acid, total phenolics, total microbial count, and sensory characteristics. The quality analysis revealed that ozonated water-treated samples at 12 perforations and stored at 4 °C for 28 days were most efficient in keeping it fresh without any significant reduction in quality as compared to the cauliflower stored for one week at normal conditions.
Keywords: Cauliflower, Modified atmosphere packaging, Perforations, Shelf-life enhancement, Quality analysis, Ozonated water treatment
Introduction
Cauliflower is one of several vegetables in the species Brassica oleracea, from family Brassicaceae. There is a great challenge for post-harvest management as it is highly perishable. The short shelf-life and fast quality deterioration are the main challenges confronted during the production and marketing of fresh minimally processed products. It should be notable that the quality of fresh produce after its harvest cannot be enhanced; nevertheless, the rate of undesirable changes can be slowed down and quality can be maintained for a longer time (Arah et al. 2015). Cold storage is an option to preserve such types of commodities for a longer period, but due to the high initial and operating costs, it is often not within the reach of common farmers. Preserving techniques like modified atmosphere packaging (MAP) including active packaging can be used for extending the shelf-life of fresh or minimally processed produce (Robertson 2016). Also, the MAP methods are combined with different pre-treatments for different fruits and vegetable products (Khazaei et al. 2011; Jouki and Khazaei 2012; Dadashpour et al. 2014; Tayyari et al. 2017). In the last decade, so many new techniques have been studied, which include the different non-thermal technologies such as ozone treatment (Galanakis 2020) and gamma irradiation (Jouki and Khazaie 2013, 2014), which can sustain the bioavailability of food components and improve their functional properties (Galanakis 2021). These technologies help to maintain the quality of products and can be adequately fitted into the domain of industry (Galanakis et al. 2021).
The use of perforated packaging films for creating MAP to extend the shelf life of perishables has now become very popular and thus is a promising option for on-farm value addition and extension of shelf life of such commodities. This type of film tends to deliver suitable mass exchange rates, which appropriately allows the entry and exit of oxygen and carbon dioxide, respectively. Perforation-mediated MAP works on the principle of permitting the perforations to regulate the exchange of O2 and CO2 and create the desired atmosphere inside an otherwise gas-tight package (Fonseca et al. 2000). It is of prime importance to include low-density packaging films with micro-perforations in hot climatic developing countries to combine MAP with cheap adiabatic cooling methods (Khan and Patel 2010). Recently, a study by Pinheiro et al. (2021) have used different gas mixtures, Ar (30% CO2/70% Ar), N2O (30% CO2/70% N2O), N2 (30% CO2/70% N2), and air (20.8% O2/79.2% N2) in MAP of sardine fillets stored at 3 °C for 12 days and the results indicated decrease in the hydrolysis of lipids and sensory properties enhancement in packages stored with Ar. The perforation-mediated MAP does not need very high technical skill, however shows sufficient potential for storage of minimally processed produces.
Most of the literature reported different pretreatment and packaging methods for increase the shelf life of the cauliflower. In the current study, ozone, cinnamon, and oregano essential oils (EOs) treatments, as an antimicrobial solution, were used to find out their effect on MAP. It was considered that the pretreatments with ozonated water and antimicrobials, with non-conventional preservation technologies, could improve the shelf life of minimally processed cauliflower (Deng et al. 2015). Cinnamon and oregano essential oils having anti-microbial action could be used to improve the shelf life of minimally processed cauliflower. Ozone has shown potential use for food processing and has been declared safe in many countries. The application of ozone and essential oil for the preservation of fresh-cut minimally processed vegetables is a recent area and limited literature of its application on cauliflower is available. The application of ozonated water to fresh-cut vegetables for sanitation purposes has extended the shelf life and reduced microbial populations for different kinds of vegetables (Sarron et al. 2021). Keeping this in view, the present research work was undertaken to study the effects of ozone and antimicrobial pretreatment with perforation mediated MAP on the shelf life of cauliflower.
Material and methods
Raw materials
Freshly harvested cauliflower (Brassica oleracea var. botrytis L.) was received in one batch from the local market (Bhubaneswar, India) and brought to the laboratory. The cauliflower samples with leaves were brought from the trusted local vendor directly from the field according to our requirement in plastic crates provided with shock-absorbing cushions to maintain the freshness and quality. The unwanted leafy portions were separated before further processing. The cauliflower of combined florets and stem were sorted into small buds so that they would be easy to pack and ready to eat. Stems were dissected into upper and lower regions of almost equal length and width. The separated buds were washed using potable water and the extra moisture on the surface was removed using forced air.
Pre-treatments and storage conditions
The three treatments viz. ozonated water, cinnamon, and oregano essential oils (EOs) were applied. An ozonator (KENT ozone vegetable purifier) was used to give ozonated water treatment. The samples were treated with ozonated water for 30 min. The cinnamon and EOs were used as antimicrobial solutions through the dipping method. Solutions were prepared by dissolving 10 mL of oregano essential oil and 10 mL of cinnamon essential oil separately in 1 L of water. The samples were kept in a walk-in type cold room (Ozone Biotech, New Delhi) at 4 °C. The cold room was capable of maintaining the temperature with a variation of ± 1 °C. Samples are drawn at designated intervals of 7, 14, 21, and 28 days for quality analysis.
Design of packaging and sample preparation
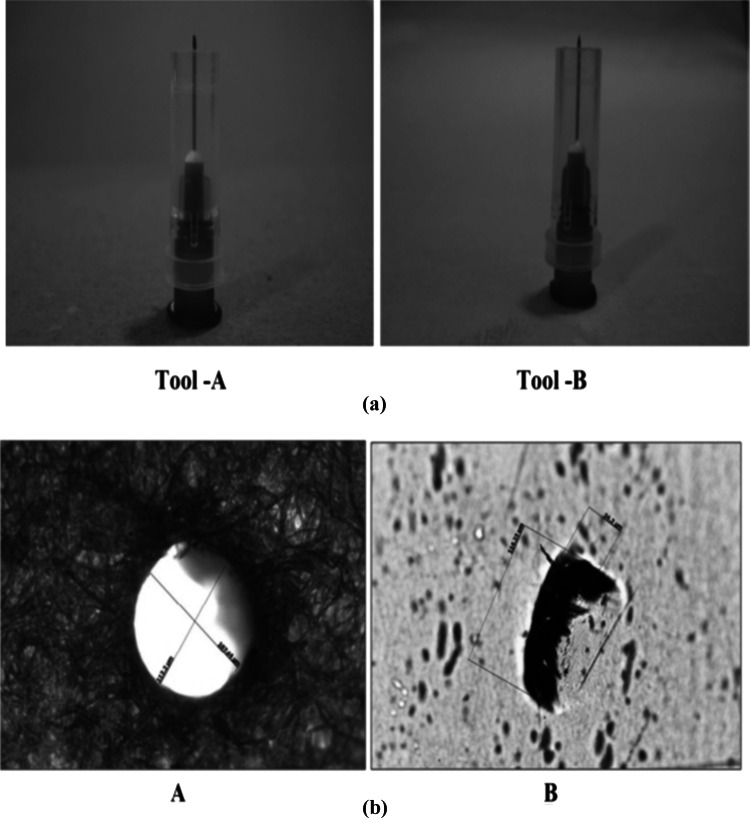
Commercially available polypropylene (PP) film (thickness: 35 μm) was used for the study. A sealing machine of hand-operated type (DMT, Impulse sealer, India) was employed for the preparation of pouches. The package dimension was kept as 150 × 150 mm2 for holding 150 g cauliflowers. The perforations size was kept uniform by using small tools equipped with surgical needles of 0.45 mm diameter as shown in Fig. 1a (Dawange et al. 2016). After the withdrawal of the needle, the perforations observed on paper were circular, however, rectangular-shaped perforations were observed on plastic films, demonstrating contraction of the holes as shown in Fig. 1b (Dawange et al. 2016). The size of perforations was measured with a trinocular research microscope (Zeiss, Primostar, Germany) and the area was calculated. The average length and width of the perforations on the PP sheet were 0.126 mm and 0.023 mm, respectively. Thus, the area of each perforation was taken as 0.002898 mm2 (Dawange et al. 2016).
Fig. 1.
a Images of designed tools using 0.45 mm diameter surgical needles with suitable stoppers, b microscopic view of the perforation
The number (area) of perforations on packaging films used for the study was decided based on preliminary experiments on the resultant gas composition within packages. The numbers of perforations were kept as 6 and 12 in the packages after preliminary experiments. Thus, the perforation area was 5.1 × 10–5% of the packaging area in case of 6 perforations and 1.02 × 10–4% for 12 perforations.
Physiological weight loss (PLW)
The weight of the samples was measured by a digital weighing balance (Citizen, CY 510, India). Before opening the sample packages on days of analysis, the weights were noted and the physiological loss in weight (PLW%) was determined.
Headspace gas analysis
Prior to opening the packages for physico-chemical analysis, the headspace oxygen, carbon dioxide, and nitrogen concentrations within package headspace were measured by Checkmate II headspace analyzer (PBI Dansensor, Ringsted, Denmark) as per Ayhan et al. (2008). Headspace gas in the sample packages was measured by a hypodermic needle inserted through a septum adhered to the pouches. Three measurements were taken from three replications and the mean was taken as the final value.
Texture analysis
The texture analyzer (Model: TA-XT Plus, Make: Stable Micro Systems, UK) was used to determine the cutting force of cauliflower samples by a Warner/Blatzer (HDP/BS) blade set (Ayhan et al. 2008). Minimally processed cauliflower buds of uniform thickness were placed on the platform of the texture analyzer. The experiments were operated at a pre-test speed (5.0 mm/s), test speed (50 mm/min), post-test speed (8.3 mm/s), and penetration distance (30 mm) as per Simon et al. (2008). Kramer standard shear cell was used for measurement of texture with approximately 45 g of cauliflower on the Kramer cell for each experiment. The maximum shear force was measured in newton (N) and the shear press displacement speed was 50 mm/min. The highest value of force was taken as a measurement for hardness.
pH
Cauliflower samples of 25 g were blended with 25 mL distilled water (pH 7) using mortar pestle as per Ayhan et al. (2008). A microprocessor-based pH meter (ESICO-1013) was used for pH measurement. Three readings from each sample were recorded and their mean was presented as final data.
Total soluble solids
Total soluble solids (%) were measured by a refractometer which measures refractive index (°Brix) as per Ayhan et al. (2008). A 5 g of cauliflower sample was taken from a package and blended with 25 mL of distilled water using a mortar pestle. The mixture was filtered through Whatman filter paper (#1) for removing suspended solids and to obtain a clear filtrate. A 3–4 mL filtered sample was placed on the lens of the pocket refractometer (PAL 3, ATAGO, Japan).
Ascorbic acid
A titrimetric method was used for the determination of ascorbic acid using AOAC Official Method 967.21 (AOAC 1999) based on the reduction of 2,6-dichlorophenol indophenols by ascorbic acid and those based on the reduction of dehydroascorbic acid with 2,4-dinitrophenyl hydrazine and expressed as mg 100 g−1 fresh weight (FW).
Total phenolics
Total phenolic content was assessed by the Folin-Ciocalteu reagent (FCR), and the extract absorbance was measured at 765 nm. FCR is a commercial-grade phenol reagent (Fisher scientific, product no 35953) which was diluted by 10 times for the purpose. Colorimetric method using gallic acid was employed (Singleton and Rossi 1965). In brief, 2 g of samples were dissolved in methanol (80%) at room temperature for 15 h on an orbital shaker. After centrifuging and homogenizing (10,000 rpm for 20 min), the supernatant was saved and the residue was reextracted twice with the same procedure. One mL filtered sample and 7 mL of distilled water were taken. After adding Folin reagent and saturated sodium carbonate, the extract absorbance was measured with the UV–visible spectrophotometer (Perkin-Elmer, Massachusetts, USA). The total phenol content is stated in terms of milligrams of gallic acid equivalent per 100 g (mg GAE 100 g−1) dry weight.
where X = ppm for sample from standard curve, M = Moisture content (%).
Microbiological analysis
Microbiological analysis in terms of the total bacterial count was studied immediately after the processing, and during the storage period on days of sample analysis (Simon et al. 2004). Ten grams of cauliflower sample was homogenized with a mortar pestle. Samples were serially diluted (1:10) and total aerobic bacteria were enumerated using the spread plate with nutrient agar (NA) media incubated at 37 °C for 24 h. On each day of analysis, the samples were examined and the results were stated in terms of colony-forming units per gram (CFU g−1).
Sensory characteristics
A 9-point hedonic scale with a consumer test panel consisting of 9 semi-trained members was employed for the sensory analysis as per the method described by Alipoorfard et al. (2020) with minor modifications. The sensory attributes included taste, appearance or color, texture, flavor, and overall acceptability of minimally processed cauliflower. The panelists have evaluated the quality and ranked each cauliflower sample by using the 9-point hedonic scale (1 = Dislike Extremely, 2 = Dislike Very Much, 3 = Dislike Moderately, 4 = Dislike Slightly, 5 = Neither Like Nor Dislike, 6 = Like Slightly, 7 = Like Moderately, 8 = Like Very Much, 9 = Like Extremely). For simulating consumers’ acceptability to cauliflower samples on the shelf of a supermarket, the color/appearance of samples was observed prior to package opening.
Statistical analysis
The experiments were planned using a completely randomized full factorial design (4 × 2 × 3) with three replications. The data obtained were analyzed for the statistically significant difference at a 5% level of probability using Analysis of Variance (ANOVA).
To find out the best possible conditions for storage of cauliflower, the qualitative analyses of the sample were carried up for 28 days at 7 days intervals. A total of twelve replicates per treatment (packaging) were included in this study. At designated intervals (7, 14, 21, and 28 days). The number (area) of perforations on packaging films used for the study was decided based on preliminary experiments on the resultant gas composition within packages. The numbers of perforations were kept as 6 and 12 in the packages after preliminary experiments. Thus, the perforation area was 5.1 × 10–5 percent of the packaging area in case of 6 perforations and 1.02 × 10–4 percent for 12 perforations.
Results and discussion
Physiological loss in weight (PLW)
The weight loss (%) of minimally processed cauliflower during the storage period is shown in Fig. 2. It was observed that the weight loss was almost of the same magnitude in 6 and 12 perforation packages for untreated and ozonated water-treated samples. Similarly, the samples treated with oregano and cinnamon showed almost similar PLW, which were lower than the untreated and ozonated water treated samples. In all the cases, the PLW was much below the permissible limit of 10% and was acceptable.
Fig. 2.
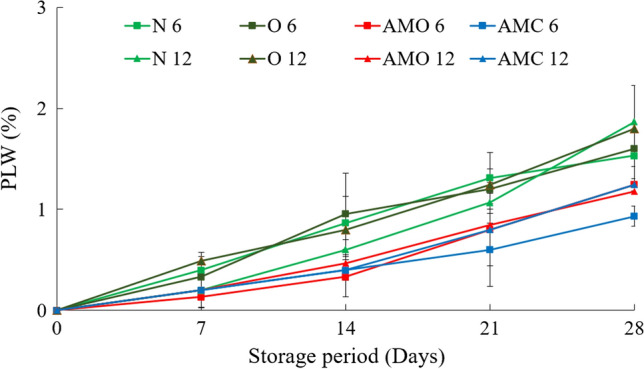
Variation of physiological loss in weight (PLW) of cauliflower with storage period. (N6: Untreated- 6 perforation package; O6: Ozonated water treated- 6 perforation package; AMO 6: Oregano treated- 6 perforation package; AMC 6: Cinnamon treated- 6 perforation package; N12: Untreated sample- 12 perforation package; O12: Ozonated water treated- 12 perforation package; AMO 12: Oregano treated- 12 perforation package; AMC 12: Cinnamon treated- 12 perforation package) (Values are mean of three analyses ± standard deviation)
The loss of moisture by evaporation of water and transpiration affected by the water vapor transmission rate of the film was the main reason for the loss of sample weight (Goswami and Mangaraj 2011). However, in the present situation, it was observed that the variations in the perforations did not cause significant differences in the PLW, indicating that 6 perforations were adequate for the exchange of moisture released by the cauliflower.
It shows that the minimum PLW of 0.6 ± 0.36% after 21 days was in the case of the cinnamon-treated samples held in 6 perforation packs. Though, no significant difference was observed between the 6 and 12 perforation pack samples treated with cinnamon and oregano. The ozonated water treated and the untreated samples showed 1–1.3% PLW after 21 days of storage in the 6 and 12 perforation packs which were also acceptable.
After 28 days of storage also the cinnamon and oregano treated samples in 6 and 12 perforation packs showed lower PLW as compared to the untreated and ozonated water treated samples (Fig. 2). However, the maximum PLW for all the treatments was 1.87 ± 0.2%, which was much below the permissible limit for the PLW. Oregano and cinnamon treated samples showed lower PLW, which could be due to the lower amount of moisture lost by the cauliflower during the treatment. There was no significant difference between the PLW of samples treated with ozonated water in 6 and 12 perforation packs and the untreated sample in 12 perforation packs. The perforations did not significantly affect the PLW though the effect of the treatments was marked. A study done on spinach by Mersinli et al. (2021) reported the weight losses of the samples were delayed by ozone treatments, but mostly affected by transpiration from the product surface, respiration rate, and electrolyte leakage.
Headspace gas composition
The changes in concentrations of oxygen (O2) and carbon dioxide (CO2) in different packages during the storage period are shown in Fig. 3. It was observed that the oxygen concentration in all packages constantly decreased during the experiment. The residual oxygen levels in packages were higher for packages with more perforations. In the perforated packages, the concentrations of carbon dioxide increased steadily due to oxygen consumption and release of CO2. The responses observed for the accumulation of carbon dioxide in the packages were just in the opposite pattern as that of oxygen (Dhalsamant et al. 2015).
Fig. 3.
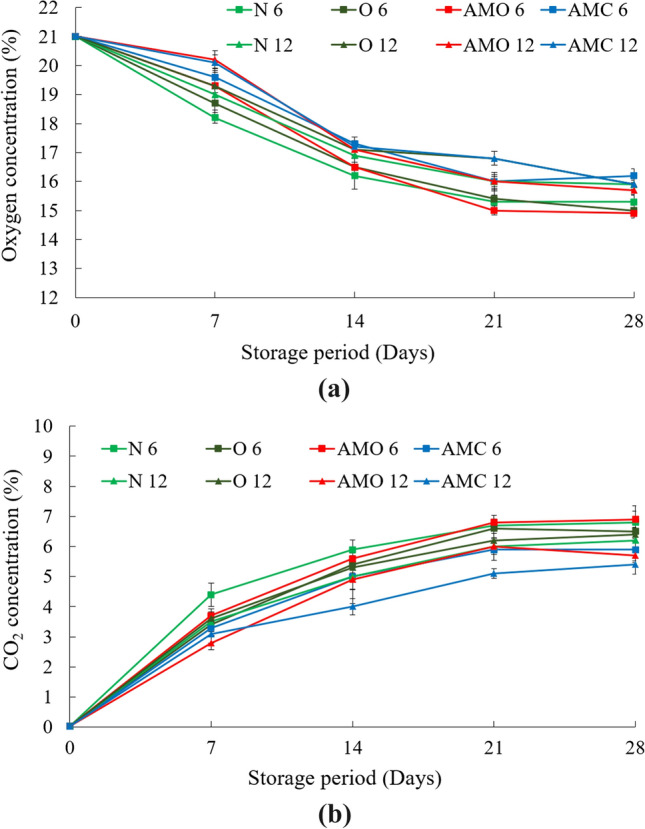
Variation in a oxygen concentration, b carbon dioxide concentration in different packages of cauliflower with storage period. (N6: Untreated- 6 perforation package; O6: Ozonated water treated- 6 perforation package; AMO 6: Oregano treated- 6 perforation package; AMC 6: Cinnamon treated- 6 perforation package; N12: Untreated sample- 12 perforation package; O12: Ozonated water treated- 12 perforation package; AMO 12: Oregano treated- 12 perforation package; AMC 12: Cinnamon treated- 12 perforation package) (Values are mean of three analyses ± standard deviation)
The statistical analysis indicated that the minimum oxygen concentration of 15.0 ± 0.15% was obtained for samples treated with oregano in 6 perforation package and the maximum value of 16.8 ± 0.24% was for cinnamon treated samples in 12 perforation packages. No significant difference was observed between the oxygen concentrations in cinnamon treated samples and samples treated with ozonated water in 12 perforation packs. The observation on the 28th day was almost in a similar trend as that on the 21st day. There was no significant difference in the O2 and CO2 content in packages for treated and untreated samples for the same perforations.
The reduction in oxygen level below 21%, specifically below 10% leads to a substantial reduction in respiration rate. In addition, at a low O2 level the ethylene (C2H4) production and activities that play a vital part in fruit ripening can be inhibited (Dash 2015). The lower O2 limit values vary with the commodity, cultivar, and temperature within 0.15–5%. The classification of commodities has also been done as per their tolerance to lower O2 and higher CO2 concentrations. In general, for most produces 2–5% O2 and 3–8% CO2 levels are considered appropriate (Dash 2015). However, auxiliary controls are required during storage as the change in O2 level inevitably adjusts CO2 level, which may not be the recommended level of CO2 (Dash 2015). In our situation, as the basic objective was to find a low-cost storage device for on-farm storage and distribution of cauliflower, the addition of further active agents was not considered.
Texture
The shear strength of the cauliflower floret (the lower portion) and the stem were measured with the help of a texture analyzer and the observations are shown in Fig. 4. The initial values of the shear strength were observed to be 33.30 ± 8.70 N and 28.35 ± 9.79 N for floret and stem, respectively. It was observed that for untreated and ozonated water treated samples the strength of the cauliflower floret and stem decreased up to 7 days after which there was a slight increase, and thereafter it remained almost constant up to 28 days. The natural aging of the product could attribute to the decrease in shear strength during the first 7 days (Simon et al. 2008). Additionally, the loss of moisture from the product causing dryness could attribute to the increase of shear strength after the 7th day. The reduced water loss during storage could attribute to the observed lower increment in shear strength in MAP packed cauliflower. The floret (flower) required a higher force for cutting as compared to the stem for all untreated and treated samples.
Fig. 4.
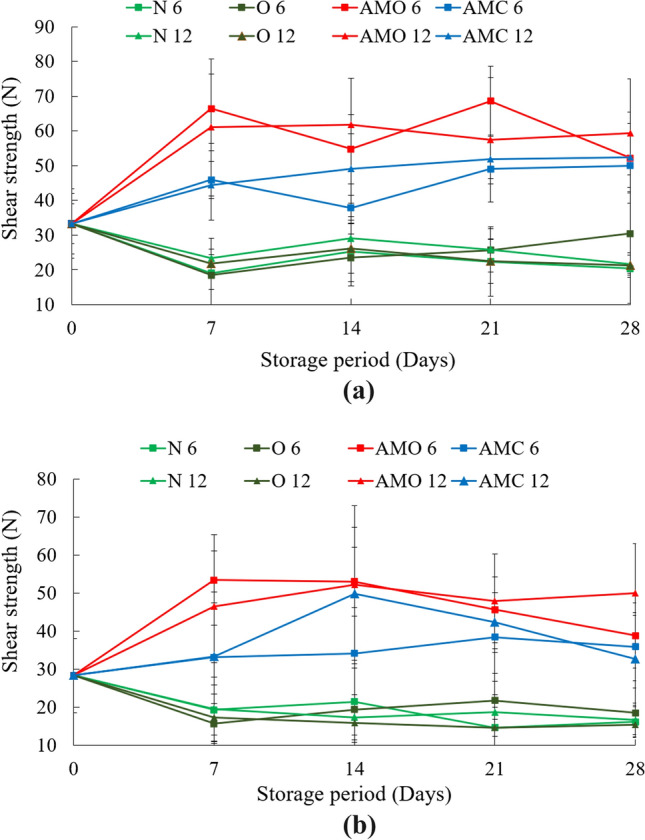
Variation in shear strength (N) of cauliflower a floret, b stem during the storage period. (N6: Untreated- 6 perforation package; O6: Ozonated water treated- 6 perforation package; AMO 6: Oregano treated- 6 perforation package; AMC 6: Cinnamon treated- 6 perforation package; N12: Untreated sample- 12 perforation package; O12: Ozonated water treated- 12 perforation package; AMO 12: Oregano treated- 12 perforation package; AMC 12: Cinnamon treated- 12 perforation package) (Values are mean of three analyses ± standard deviation)
The higher shear strength of the samples indicates that they were more elastic, which would not be acceptable to the consumers, thus the lower shear strength would be preferred. In terms of textural attributes, the untreated samples with 6 perforations were the best ones having the values of 22.41 ± 5.24 N after 21 days of storage. However, no significant difference was observed between the untreated samples and samples treated with ozonated water in 6 and 12 perforation packages. Similarly, Gao et al. (2014) observed a reduction in firmness from 17.32 N to 13 N after 16 days of storage at 4 °C.
After 28 days, the minimum shear strength of 20.40 ± 4.02 N was detected for the florets in 6 perforation packs untreated samples and for the stems, the minimum value was 15.44 ± 2.54 N for the ozonated water treated samples in 12 perforation packs. However, as mentioned above, no significant difference was observed between the untreated and samples treated with ozonated water in 6 and 12 perforation packs for both flowers and stems. The effects of change in perforations were not significant from the statistical analysis though there was a difference with the pretreatments. The untreated and ozonated water treated samples required minimum shear force while oregano and cinnamon treated samples required the maximum force. The retention of moisture in both oregano and cinnamon-treated samples made them chewy. Due to its chewiness, the texture was changed and ultimately higher force was required.
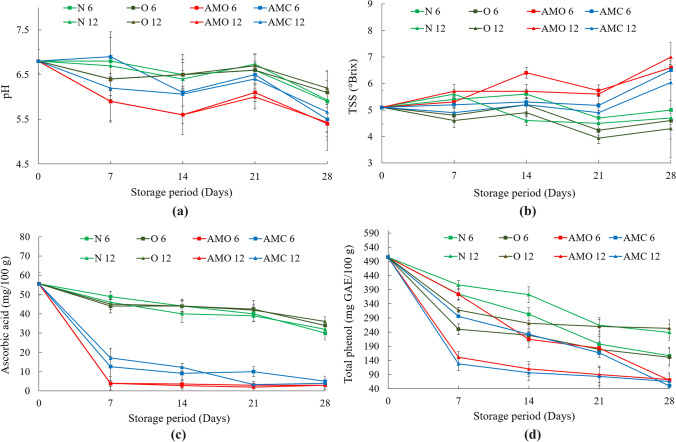
pH
The initial value of the pH was observed to be 6.8 ± 0.26. The pH of the samples usually decreased during the storage period, though the pattern of decrease was not uniform. The minimum value of pH was detected in the samples treated with the oregano, followed by the cinnamon treated samples as shown in Fig. 5a. The pH in these treated samples for both 6 and 12 perforation packages ranged between 6.0 ± 0.26 and 6.5 ± 0.30 after 21 days. The untreated and ozonated water treated samples in both perforation conditions ranged between 6.6 ± 0.20 and 6.7 ± 0.21.
Fig. 5.
Variation in a pH, b TSS, c ascorbic acid, d total phenol content of cauliflower during the storage period. (N6: Untreated- 6 perforation package; O6: Ozonated water treated- 6 perforation package; AMO 6: Oregano treated- 6 perforation package; AMC 6: Cinnamon treated- 6 perforation package; N12: Untreated sample- 12 perforation package; O12: Ozonated water treated- 12 perforation package; AMO 12: Oregano treated- 12 perforation package; AMC 12: Cinnamon treated- 12 perforation package) (Values are mean of three analyses ± standard deviation)
After 28 days of storage also the oregano and cinnamon treated samples showed minimum pH values which ranged between 5.4 ± 0.36 and 5.67 ± 0.57. The untreated and ozonated water treated samples had a pH in between 5.9 ± 0.69 and 6.20 ± 0.36 after 28 days of storage. The statistical analysis also revealed that the perforation areas did not significantly affect the difference in pH of the samples.
Total soluble solids (TSS)
Figure 5b shows changes in total soluble solids (TSS) of cauliflower during the storage period. The initial value of the TSS was observed to be 5.1 ± 0.62°Brix. The TSS of the cauliflower samples were observed to have increased slightly during the storage, though the trend was not very similar for all samples. The untreated and ozonated water treated samples in both 6 and 12 perforation packs showed a decrease in the TSS values, whereas the samples treated with the cinnamon and oregano showed an increase in TSS values. The reason could not be explained. There was not much variation in the TSS values of untreated and ozonated water treated samples in both 6 and 12 perforations.
After 21 days, the samples treated with oregano in 6 perforation packs showed the maximum TSS, which was 5.73 ± 0.21°B and it was not significantly different from the 12 perforation packs. The ozonated water treated samples had the minimum TSS in both 6 and 12 perforation packs. The trend of change of TSS was also the same for the observations on the 28th day. The ozonated water treated sample in 12 perforation packs showed the minimum TSS (4.3 ± 0.40°B), which was not significantly different from the samples stored in 6 perforation packs.
The use of sugar as a respiration substrate could have been the cause for the decrease in sugars during storage. The polypropylene film and non-perforated PVC film creating the modified atmospheres may affect the respiration rate reducing the decrease in sugar as compared to perforated PVC film (Mertens and Tranggono 1989). On the contrary, Hodges et al. (2006) have reported the atmosphere with 3% O2 and 5% CO2 does not affect the sugar content.
Ascorbic acid
The initial value of the ascorbic acid in the cauliflower samples was 55.58 ± 5.74 mg 100 g−1. It was observed that the untreated samples and samples treated with ozonated water had better retention of ascorbic acid, which was much higher than the oregano and cinnamon treated samples as shown in Fig. 5c. After 21 days of storage, the ozonated water treated samples in 6 perforation packs showed the highest retention of ascorbic acid which was 42.5 ± 4.4 mg 100 g−1. However, it was not significantly different from the untreated sample in 6 and 12 perforation packs and the ozonated water treated samples in 12 perforation packs. A remarkable reduction in the ascorbic acid content was observed in the samples treated with cinnamon and oregano.
The maximum ascorbic acid retention of 36 ± 2.6 mg 100 g−1 was observed in the case of ozonated water treated sample in 12 perforation packs after 28 days of storage. These results are very close with the ascorbic content of 39–36.94 mg 100 g−1 reported by Mashabela et al. (2019) for fresh-cut cauliflower stored in MAP. Ascorbic acid content was not significantly different from ozonated water treated sample in 6 perforation pack and the untreated sample in 12 perforation packs. The ascorbic acid content in the cinnamon and oregano treated samples were much lower as compared to the untreated and ozonated water treated samples. The cruciferous vegetables show higher ascorbic acid retention and vitamin C loss during vegetable storage depends on the vegetable type (Lee and Kader 2000). An ascorbic acid retention level of 96.8% in cauliflower stored for 3 weeks at 2 °C was reported by Albrecht et al. (1990). However, in our case, the retention was 76%, which might have been due to the higher temperature during storage and the perforation and treatment.
Total phenols
The total phenol content gradually decreased during the storage period as shown in Fig. 5d. The initial phenol content of the samples was 504.33 ± 9.07 mg GAE 100 g−1. After 21 days of storage, the maximum total phenol was in the case of untreated samples in 12 perforation packs, which was 264 ± 21.17 mg GAE 100 g−1. The total phenol content of the sample was not significantly different from that of the ozonated water treated samples in 12 perforation packs.
Similarly, after 28 days of storage, the ozonated water treated samples in 12 perforation packs showed 252.73 ± 30.8 mg GAE 100 g−1 total phenols and it was not significantly different from untreated samples in 12 perforation packs. The obtained results are nearly equal to the Olive kernel reported 255 mg GAE 100 g−1 (Rosello-Soto et al. 2015). It was observed that the oregano and cinnamon treated samples showed much lower total phenols in comparison to the untreated and samples treated with ozonated water. The total phenols of cauliflower in packages were not significantly affected by the number of perforations.
Total bacteria count
Microbiological control is vital in fruits and vegetables for avoiding poisoning of food and other health risks. Chaturvedi et al. (2013) have reported that pathogenic microorganism contamination of vegetables is possible during their growth in field, harvesting, post-harvesting practices, and distribution. Thus, host/consumers are susceptible to infections from vegetables due to the colonization of microorganisms in them. In our experiment, low initial mesophiles count i.e. 2.94 log CFU g−1 in cauliflower was observed, which was slightly more the value reported by Simon et al. (2008) i.e. 2.76 log CFU g−1 and lower than the count reported by Berrang et al. (1990) i.e. 5 log CFU g−1, although a mesophile count between 3.6–5.4 log CFU g−1 for packed cauliflower was reported by Garg et al. (1990).
After 21 days of storage, a minimum of 3.7 ± 0.16 log CFU g−1 and 3.8 ± 0.22 log CFU g−1 were found in ozonated and cinnamon treated samples for 6 and 12 perforation packs, respectively. The total bacteria count of the 28th day was slightly increased as compared to the 21st day of storage. Figure 6 shows the total bacterial load in the samples on the 28th day of storage. The maximum colonies were observed as 6.9 ± 0.08 log CFU g−1 and 6.7 ± 0.14 log CFU g−1 for the untreated samples in 12 and 6 perforations, respectively. The ozonated water treated samples in 06 perforation packs showed 4.9 ± 0.22 log CFU g−1 and that for 12 perforation packs was 5.2 ± 0.08 log CFU g−1. These were lower than the untreated sample. A total microbial counts of 5 log CFU g−1 was seen in cauliflower packaged in perforated film, whereas the recommended limit is 8 log CFU g−1 (Licciardello et al. 2012).
Fig. 6.
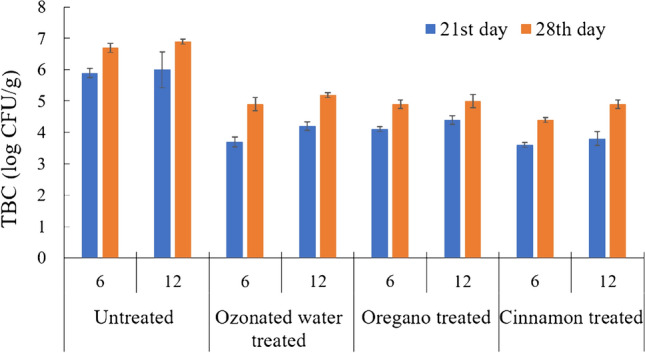
Change in total bacterial counts of cauliflower after 28 days of storage. (N6: Untreated- 6 perforation package; O6: Ozonated water treated- 6 perforation package; AMO 6: Oregano treated- 6 perforation package; AMC 6: Cinnamon treated- 6 perforation package; N12: Untreated sample- 12 perforation package; O12: Ozonated water treated- 12 perforation package; AMO 12: Oregano treated- 12 perforation package; AMC 12: Cinnamon treated- 12 perforation package) (Values are mean of three analyses ± standard deviation)
The value of TBC in oregano treated samples was slightly more than cinnamon treated samples. Lopez et al. (2007) reported that for a relatively resistant gram-negative, the thyme and cinnamon EOs generally had a higher minimum inhibitory concentration (MICs) than the oregano EO. These higher initial counts or contamination during processing could have been the reason for higher counts observed in the packed cauliflower. Additionally, a slight difference was observed in 6 and 12 perforation packs in all treatments.
Sensory analysis
The sensory evaluation of the samples was conducted on the 21st day and 28th day in terms of taste, color, flavor, texture, and overall acceptability. The quality was judged by the consumer panel team consisting of nine members. The samples were rated on a nine-point hedonic scale as shown in Table 1. It was observed that the samples treated with ozonated water in 12 perforation packs received the highest score followed by 6 perforations. No significant difference was observed between the 6 and 12 perforation packs for individual treatments for all the quality parameters. The minimum scores were obtained by the samples treated with cinnamon and oregano as there was a foul smell from the stored products. Tirkey et al. (2014) also reported similar results with fresh-cut unripe papaya in MAP storage.
Table 1.
Sensory analysis of cauliflower after 21 and 28 days of storage
| Treatment | Package perforations | Taste | Color | Flavor | Texture | Overall acceptability | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 days | 28 days | 21 days | 28 days | 21 days | 28 days | 21 days | 28 days | 21 days | 28 days | ||
| Ozonated water | 06 | 7.4 ± 0.40ab | 7.2 ± 0.43ab | 7.8 ± 0.42a | 7.1 ± 0.40a | 7.3 ± 0.26ab | 7 ± 0.35ab | 7.4 ± 0.25b | 7 ± 0.35b | 7.8 ± 0.22ab | 7.6 ± 0.29ab |
| Ozonated water | 12 | 7.7 ± 0.52a | 7.5 ± 0.30a | 8.0 ± 0.40a | 7.4 ± 0.25a | 7.7 ± 0.60a | 7.2 ± 0.26a | 7.8 ± 0.51a | 7.5 ± 0.49a | 8 ± 0.51a | 7.9 ± 0.42a |
| Untreated | 06 | 7.1 ± 0.42b | 6.9 ± 0.28b | 7.1 ± 0.38b | 6.6 ± 0.22b | 7 ± 0.38b | 6.8 ± 0.55b | 7.2 ± 0.28b | 6.9 ± 0.51b | 7.5 ± 0.29b | 7 ± 0.55c |
| Untreated | 12 | 7.5 ± 0.64b | 7 ± 0.64b | 7.4 ± 0.49b | 6.7 ± 0.48b | 7.3 ± 0.64b | 6.9 ± 0.4b | 7.4 ± 0.52b | 7.2 ± 0.49ab | 7.7 ± 0.46b | 7.2 ± 0.49bc |
| Oregano | 06 | 6.3 ± 0.40c | 6.1 ± 0.43c | 6 ± 0.42c | 5.4 ± 0.40c | 6.3 ± 0.25c | 5.4 ± 0.35d | 6 ± 0.51c | 5.4 ± 0.35 cd | 5 ± 0.22d | 5 ± 0.37d |
| Oregano | 12 | 6.1 ± 0.52 cd | 5.8 ± 0.48 cd | 5.7 ± 0.31 cd | 5.3 ± 0.25 cd | 6.1 ± 0.28c | 5.8 ± 0.37c | 5.9 ± 0.41 cd | 5.7 ± 0.49c | 5.5 ± 0.50c | 5.2 ± 0.51d |
| Cinnamon | 06 | 5.8 ± 0.25de | 5.6 ± 0.33de | 5.6 ± 0.23d | 5.1 ± 0.12 cd | 5.6 ± 0.32d | 5.1 ± 0.16de | 5.7 ± 0.36 cd | 5.1 ± 0.16de | 4.3 ± 0.41e | 3.8 ± 0.66e |
| Cinnamon | 12 | 5.7 ± 0.22e | 5.4 ± 0.22e | 5.5 ± 0.30d | 5.1 ± 0.31d | 5.7 ± 0.22d | 5 ± 0.33e | 5.5 ± 0.15d | 5 ± 0.33e | 4.5 ± 0.5e | 4 ± 0.27e |
a,b,c,d,eMeans in the same column with different letters represent significant differences (p < 0.05)
Given the sensory and other parameters it is suggested to store the cauliflower after treating it with ozonated water for 30 min in 12 perforation packs (area 1.02 × 10–4% of the total package surface area). The minimally processed cauliflower stored by such means can be stored for about 4 weeks to acceptable consumer preference.
Conclusion
From the current research, it was concluded that fresh-cut cauliflower pretreated with ozonated water for 30 min followed by packing in 35 µm PP pouches with 12 perforations (1.02 × 10–4% perforated area) gained maximum sensory acceptability when stored at 4 °C. The pretreatment with perforation mediated MAP helped in enhancing the shelf life of minimally processed cauliflower for 28 days without any significant reduction in essential quality attributes with retaining maximum ascorbic acid content of 36 ± 2.6 mg 100 g−1 and total phenols content of 252.73 ± 30.8 mg GAE 100 g−1. Thus, it could be inferred that ozonated water can be a promising treatment in improving the shelf-life of minimally processed cauliflower under perforation-mediated modified atmosphere packaging.
Author contributions
VF: Conceptualization, Methodology, Resources, Visualization, Writing-original draft. SKD: Conceptualization, Methodology, Resources, Supervision, Visualization. KD: Conceptualization, Methodology, Writing- review and editing. NRS: Conceptualization, Methodology, Resources, Visualization. USP: Conceptualization, Methodology, Resources, Visualization.
Funding
Not applicable.
Declarations
Conflict of interest
On behalf of all the authors, the corresponding author declares that there is no conflict of interest.
Consent for publication
The authors approve the consent for publication.
Ethical approval
The authors approve the ethics for submission.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albrecht JA, Schafer HW, Zottola EA. Relationship of total sulfur to initial and retained ascorbic acid in selected cruciferous and noncruciferous vegetables. J Food Sci. 1990;55:181–183. doi: 10.1111/j.1365-2621.1990.tb06047.x. [DOI] [Google Scholar]
- Alipoorfard F, Jouki M, Tavakolipour H. Application of sodium chloride and quince seed gum pretreatments to prevent enzymatic browning, loss of texture and antioxidant activity of freeze dried pear slices. J Food Sci Technol. 2020;57:3165–3175. doi: 10.1007/s13197-020-04265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC Official Methods of Analysis (1999) Washington, DC, USA: AOAC International. Association of Official Analytical Chemists. Official method 985. 33. vitamin c, (reduced ascorbic acid) in ready-to-feed milk based infant formula 2, 6-dichloroindophenol titrimetric method; pp 1108–1109
- Arah IK, Amaglo H, Kumah EK, Ofori H. Preharvest and postharvest factors affecting the quality and shelf life of harvested tomatoes: a mini review. Int J Agron. 2015;2015:1–6. doi: 10.1155/2015/478041. [DOI] [Google Scholar]
- Ayhan Z, Esturk O, Tas E. Effect of modified atmosphere packaging on the quality and shelf life of minimally processed carrots. Turk J Agric for. 2008;32:57–64. [Google Scholar]
- Berrang ME, Brackett RE, Beuchat LR. Microbial, color and textural qualities of fresh asparagus, broccoli, and cauliflower stored under controlled atmosphere. J Food Prot. 1990;53:391–395. doi: 10.4315/0362-028X-53.5.391. [DOI] [PubMed] [Google Scholar]
- Chaturvedi M, Kumar V, Singh D, Kumar S. Assessment of microbial load of some common vegetables among two different socioeconomic groups. Int Food Res J. 2013;20:2927. [Google Scholar]
- Dadashpour A, Rahemi M, Jouki M. Effect of modified atmosphere packaging on persimmon fruit (cv. Karaj) physical, chemical and mechanical properties. Agro Food Ind Hi Tech. 2014;25:214–216. [Google Scholar]
- Dash SK. Modified atmosphere packaging of food. In: Alavi S, Thomas S, Sandeep KP, Kalarikkal N, Varghese J, Yaragalla S, editors. Polymers for packaging applications. CRC Press; 2015. pp. 337–378. [Google Scholar]
- Dawange SP, Dash SK, Bal LM, Panda MK. Quality of minimally processed carrots in perforation-mediated modified-atmosphere packaging (PM-MAP) J Food Meas Charact. 2016;10:746–754. doi: 10.1007/s11694-016-9359-3. [DOI] [Google Scholar]
- Deng Q, Zinoviadou KG, Galanakis CM, Orlien V, Grimi N, Vorobiev E, Lebovka N, Barba FJ. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng Rev. 2015;7:357–381. doi: 10.1007/s12393-014-9104-9. [DOI] [Google Scholar]
- Dhalsamant K, Dash SK, Bal LM, Panda MK. Effect of perforation mediated MAP on shelf life of mushroom (Volvariella volvacea) Sci Hortic. 2015;189:41–50. doi: 10.1016/j.scienta.2015.03.027. [DOI] [Google Scholar]
- Fonseca SC, Oliveira FA, Lino IB, Brecht JK, Chau KV. Modelling O2 and CO2 exchange for development of perforation-mediated modified atmosphere packaging. J Food Eng. 2000;43:9–15. doi: 10.1016/S0260-8774(99)00122-3. [DOI] [Google Scholar]
- Galanakis CM. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9:523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis CM. Functionality of food components and emerging technologies. Foods. 2021;10:128. doi: 10.3390/foods10010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis CM, Rizou M, Aldawoud TM, Ucak I, Rowan NJ. Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci Technol. 2021;110:193–200. doi: 10.1016/j.tifs.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Feng L, Jiang T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014;149:107–113. doi: 10.1016/j.foodchem.2013.10.073. [DOI] [PubMed] [Google Scholar]
- Garg N, Churey JJ, Splittstoesser DF. Effect of processing conditions on the microflora of fresh-cut vegetables. J Food Prot. 1990;53:701–703. doi: 10.4315/0362-028X-53.8.701. [DOI] [PubMed] [Google Scholar]
- Goswami TK, Mangaraj S (2011) Advances in polymeric materials for modified atmosphere packaging (MAP). Multifunct nanoreinf polym food packag. Woodhead Publishing, pp 163–242
- Hodges DM, Munro KD, Forney CF, McRae KB. Glucosinolate and free sugar content in cauliflower (Brassica oleracea var. botrytis cv. Freemont) during controlled-atmosphere storage. Postharvest Biol Technol. 2006;40:123–132. doi: 10.1016/j.postharvbio.2005.12.019. [DOI] [Google Scholar]
- Jouki M, Khazaei N. The effect of modified atmosphere packaging and calcium chloride dripping on the quality and shelf life of Kurdistan strawberries. Int J Food Process Technol. 2012;3:184. [Google Scholar]
- Jouki M, Khazaei N. Effects of low-dose γ-irradiation and modified atmosphere packaging on shelf-life and quality characteristics of saffron (Crocus Sativus Linn) in Iran. Food Sci Biotechnol. 2013;22:687–690. doi: 10.1007/s10068-013-0132-7. [DOI] [Google Scholar]
- Jouki M, Khazaei N. Effect of low-dose gamma radiation and active equilibrium modified atmosphere packaging on shelf life extension of fresh strawberry fruits. Food Packag Shelf Life. 2014;1:49–55. doi: 10.1016/j.fpsl.2013.12.001. [DOI] [Google Scholar]
- Khan KA, Patel MB. Evaluation of physico-chemical and microbiological parameters in carrot stored under modified atmospheric packaging. SAARC J Agric. 2010;8:29–38. [Google Scholar]
- Khazaei N, Jouki M, Jouki A. Effects of modified atmosphere packaging on physico-chemical characteristics and sensory evaluation of bitter orange (Citrus aurantium) Indian J Agric Sci. 2011;81:1014–1018. [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Licciardello F, Muratore G, Spagna G, Branca F, Ragusa L, Caggia C, Randazzo C, Restuccia, C (2012) Evaluation of some quality parameters of minimally processed white and violet-pigmented cauliflower curds. In: VI International symposium on brassicas and XVIII crucifer genetics workshop, vol 1005, pp 301–308
- Lopez P, Sanchez C, Batlle R, Nerin C. Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J Agric Food Chem. 2007;55:4348–4356. doi: 10.1021/jf063295u. [DOI] [PubMed] [Google Scholar]
- Mashabela M, Mahajan PV, Sivakumar D. Influence of different types of modified atmosphere packaging films and storage time on quality and bioactive compounds in fresh-cut cauliflower. Food Packag Shelf Life. 2019;22:100374. doi: 10.1016/j.fpsl.2019.100374. [DOI] [Google Scholar]
- Mersinli E, Koyuncu MA, Erbas D. Quality retention of minimally processed spinach using low-dose ozonated water during storage. Turk J Agric for. 2021;45:133–143. doi: 10.3906/tar-2004-75. [DOI] [Google Scholar]
- Mertens H, Tranggono M. Ethylene and respiratory metabolism of cauliflower (Brassica oleracea L. convar. botrytis) in controlled atmosphere storage. Acta Hort. 1989;258:493–501. doi: 10.17660/ActaHortic.1989.258.55. [DOI] [Google Scholar]
- Pinheiro ACAS, Tappi S, Patrignani F, Lanciotti R, Romani S, Rocculi P. Effects of novel modified atmosphere packaging on lipid quality and stability of sardine (Sardina pilchardus) fillets. Int J Food Sci Technol. 2021;56:945–953. doi: 10.1111/ijfs.14747. [DOI] [Google Scholar]
- Robertson GL. Food packaging: principles and practice. CRC Press; 2016. [Google Scholar]
- Rosello-Soto E, Barba FJ, Parniakov O, Galanakis CM, Lebovka N, Grimi N, Vorobiev E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioproc Tech. 2015;8:885–894. doi: 10.1007/s11947-014-1456-x. [DOI] [Google Scholar]
- Sarron E, Gadonna-Widehem P, Aussenac T. Ozone treatments for preserving fresh vegetables quality: a critical review. Foods. 2021;10:605. doi: 10.3390/foods10030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Gonzolez-Fandos E, Obar VT. Influence of washing and packaging on the sensory and microbiological quality of fresh peeled white asparagus. J Food Sci. 2004;69:FMS6–FMS12. doi: 10.1111/j.1365-2621.2004.tb17869.x. [DOI] [Google Scholar]
- Simon A, Gonzalez-Fandos E, Rodriguez D. Effect of film and temperature on the sensory, microbiological and nutritional quality of minimally processed cauliflower. Int J Food Sci Technol. 2008;43:1628–1636. doi: 10.1111/j.1365-2621.2007.01672.x. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Tayyari F, Khazaei J, Rajaei P, Jouki M. Effects of modified atmosphere packaging systems, low temperature and storage time on the quality of fresh minimally processed pomegranate arils. Carpathian J Food Sci Technol. 2017;9:16–26. [Google Scholar]
- Tirkey B, Pal US, Bal LM, Sahoo NR, Bakhara CK, Panda MK. Evaluation of physico-chemical changes of fresh-cut unripe papaya during storage. Food Packag Shelf Life. 2014;1:190–197. doi: 10.1016/j.fpsl.2014.02.002. [DOI] [Google Scholar]