Abstract
Dahi is widely used fermented milk product in India. Low Density Polyethylene (LDPE) is the most extensively used packaging material for Dahi in India. The present study was conducted to develop the analytical methods for extraction and migration of chemical additives from LDPE into dahi. Characterization of dahi packaging materials collected from five different firms was done by Fourier Transform Infrared Spectroscopy. Focused ultrasound solid liquid extraction method was observed to be better as compared to solid liquid extraction method as the former extracted maximum additives from the LDPE. Out of total 76 chemical additives extracted from LDPE, only eight (10.52%) matched with the existing positive list of polyolefins prescribed by Bureau of Indian Standads (BIS). The overall migration of chemical additives from all the LDPE samples was below their maximum limit as given by BIS standards. Chemical additives which migrated into the simulants included the antioxidants, fatty acids and their derivatives, unreacted hydrocarbons, plasticizers, lubricants and surfactant etc.
Keywords: Dahi, Low density polyethylene, Chemical additives, FULSE, FTIR, GC–MS
Introduction
Dahi, also known as curd, is an ancient Indian fermented milk product that has maintained its appeal in the Indian diet despite changing lifestyles and eating patterns. Dahi is produced from heat treated milk after inoculation with starter culture. It represents about 7% of India's total milk production (Prajapati and Sreeja 2014) and this sector is displaying a growth rate of more than 20% per annum (Veena et al. 2017). Dahi represents 90% of all the cultured milk products being produced in India (Behare et al. 2009).
Packaging is the 5th largest sector with an approximate valuation of USD 50.5 billion and is expected to reach USD 204.81 billion by 2025 (Global Flexible Plastic Packaging Market Report 2020). Packaging has been defined as a socioscientific discipline that works within society to guarantee that products are delivered to end customers in the best possible condition for usage (Lockhart 1997). Containment, protection, preservation, communication, usefulness, and performance are all functions of packaging (Robertson 2005). Earlier, the packaging of Dahi was done in the earthenware pot with a loose cover of greaseproof paper. But, earthenware pots were very heavy, highly fragile and the inside product develop shrinkage cracks due to the oozing of water from its body. Now a days, various packaging materials are being used for packaging of dahi. Injection molded polystyrene and polypropylene (PP) cups with aluminium foil based peelable lids have recently been launched. The plastic cups are light in weight, simple to handle and hygienic. Polyethylene (PE), polypropylene, polystyrene packaging material are also used for packaging of dahi (Saint-Eve et al. 2008). PE is the polymer of ethylene obtained under a high pressure of 1000–3000 atm and a temperature of 100–350 °C. Low density polyethylene (LDPE) is a strong barrier to water vapour (Miltz et al. 1992). India's overall polymer usage is 20 million tons and compared to other polymers; the use of PE and polypropylene are highest (FICCI 2016). Presence of additives such as antioxidants, plasticizers, heat and light stabilizers, lubricants, UV printing inks components or slip additives (Cooper and Tice 1995) etc. in the polymer matrix allows the modification or improvement of the polymer properties and performance. However, the migration of such additives to food causes adverse health effects on the human body such as elevated cholesterol level, reproductive problems like low infant birth weights, some immunological problems such as lowering of immune functions, liver and kidney damage, thyroid functions, and cancer in some cases (Simoneau 2008; Singh et al. 2020).
The term "migration" refers to the process by which chemical additives diffuse from a zone of greater concentration (food kackaging material) to a zone of lower concentration (food) (Arvatoyannis and Bosnea 2004). Now a days, the food processing industries as well as the consumers are more concerned about safety and the quality of food products. In a packaged food, the hermeticity and permeation provide the assurance of package integrity, while migration provides the assurance towards safety (Barnes et al. 2007).
Studies related to the characterization of the different packaging materials used for manufacturing dahi is lacking in the literature. There is also a need to optimize the conditions for the extraction of these additives from the packaging materials for their further analysis, identification and final quantification. LDPE being the most used packaging material for the dahi was selected for the present study. The present study was aimed for characterization of LDPE based dahi packaging materials collected from five different manufacturers by automated thickness gauge and Fourier Transform Infrared Spectroscopy. The conditions for extraction of chemical additives from LDPE using solid liquid extraction (SLE) and focused ultrasound solid liquid extraction (FULSE) were optimized. Further, protocols were developed for quantification of migrated chemical additives from the LDPE into the simulants and dahi using gas chromatography mass spectrometry (GC–MS).
Materials and methods
Packaging materials
The unused packaging films labelled as LDPE with different containment capacities (180 g, 200 g, 400 g, 500 g, 1 kg and 5 kg) were collected from five different manufactures in India.
Preparation of dahi
Dahi was prepared from buffalo milk collected from Livestock Research Centre, National Dairy Research Institute, Karnal city, India. Starter culture used for dahi was collected from National Collection ofDairy Cultures (NCDC, Culture No. 167), National Dairy Research Institute, Karnal.
Methods
Characterization of LDPE Dahi packaging materials
The dahi packaging materials obtained from five different firms labelled as LDPE were characterized by two methods; thickness measurement by automated thickness gauge and polymer identification by ATR-FTIR spectrophotometer.
Thickness measurement by automated thickness gauge
Thickness measurement of packaging material was done using digital automated thickness gauge (Mitutoyo Cooperation, Kanagawa, Japan) by setting the scale to zero on screw gauge followed by placing the sample into it. Readings displayed were recorded.
Characterization of dahi packaging materials by FT-IR spectrophotometer
The chemical charachterization of packaging materials was carried out using Attenuated Total Reflection-Fourier transform infrared spectroscopy (ATR-FTIR) spectrophotometer. Spectra of Dahi packaging material was attained using ATR-FTIR. ATR diamond crystal was cleaned with 70 (%) 2-propanol solution and a background scan was conducted between samples. To ensure good contact between the sample and crystal, it was compressed against the same with a force of minimum 80 N. The IR-solution software's peak height algorithm was used to identify absorption bands. Minimum four matching absorption bands were used for identification. The packaging films were cleaned with warm water and cut into small pieces. Spectra of samples were obtained using FT-IR (IR Affinity-I 206-73500-38 Shimadzu Corporation, Kyoto, Japan) spectrophotometer with diamond crystal cell ATR and inbuilt IR-Solution software at 4 cm−1 resolution. Thirty scans of each sample were taken from region 4500–450 cm−1 against air as background. Spectra of packaging material (henceforth called as LDPE) obtained were observed to be of LDPE as given by the inbuilt reference library in the FTIR spectrophotometer. Same was also compared with spectra of LDPE available in the literature.
Determination of overall migration of additives from LDPE into simulants
Minimum three samples of LDPE from each brands representing a particular batch were used. The samples were rinsed with water (25–30 °C) to remove extraneous materials. Dahi pouches were filled up with the simulant B (3 (%) acetic acid) and D (n-heptane) and exposed to nominal filling capacity or at least 1 ml/cm2 of contact area or exposed area 450 cm2 at 40 °C for 10 days and 38 °C for 30 min, respectively in a temperature controlled incubator. Overall migration of chemical additives from packaging material into the simulants was determined as per method prescribed by BIS ( IS 9845:1998).
Optimization of conditions for extraction of chemical additives from LDPE using solid liquid extraction (SLE) and their detection by GC–MS
SLE was used for extraction of chemical additives from LDPE. In SLE method, both the extracting solvent and time period needed for complete extraction were simultaneously optimized. Six different solvents/solvent combinations, viz. acetonitrile, n-hexane, n-heptane, acetonitrile: methanol (1:1), hexane: methanol (1:1) and chloroform: methanol (1:1) were used in order to extract both polar and non-polar additives from LDPE for six different time periods such as 0, 6, 12, 18, 24 and 48 h. LDPE films were then exposed to these optimised conditions and amount of extractives obained was determined using the method of Moreta and Tena (2015). Finally, extracts were mixed with 5 mL of methanol and filtered through a 0.22 µm nylon filter and analyzed by GC–MS (TQ8030, Shimadzu Cooperation, Japan).
Optimization of conditions for extraction of chemical additives from LDPE using FULSE method and their detection by GC–MS
FULSE conditions were optimized by using Box-Behnken design (BBD) using Design-Expert software (version 13.0). Moreta and Tena (2015) method was used for extraction of additives with slight modification. A 25 mm2 slice was cut out of LDPE packaging material. Sample, 0.50 g was placed into a 10 ml glass vial, 0.2 ml of 25 µg/ml 2,4-toluenediamine (TDA) in methanol and 4.5 ml of chloroform: methanol (1:1) were added and mixed. Then, the probe was immersed in the mixture. The extractions were carried out in an ice-water bath and the sample was subjected to a 62% ultrasonic irradiation power (466 watts) for 535 s, during which the greatest number of additives were extracted. The extract obtained was reconstituted into 5 mL of methanol, filtered through a 0.22 µm nylon filter and analysed by GC–MS.
Determination of migrated chemical additives from LDPE into food simulants
Initial steps for determination of migrated additives from LDPE into food simulants were the same as was in overall migration. The obtained extract in the overall migration study was reconstituted with 5 mL methanol and mixed using vortex mixture for 1 min. Solution was then filtered through 0.22 µm size syringe filter and analysed by GC–MS.
Extent of migration of additives from LDPE into dahi at different time intervals
Dahi was prepared in lab using the standard protocol and sealed. After that it was exposed for 160 h at 4 °C and analysed at regular time intervals of 16, 32, 48, 64, 80, 96, 112, 128, 144 and 160 h. Migrated chemical additives from LDPE into dahi was determined using the method of Sorenson (2006). All of the extracted solutions were analysed by GC–MS under the following conditions: Carrier gas, helium; flow, 1 ml/min; mode, splitless; column, SLB-5MS; Dia, 30X0.25 mm; thickness, 0.25 μm; Injection volume, 1 ml; Injector temperature, 280 °C. Oven program: initial temperature 70 °C (2 min.), final temperature 300 °C; run for 20 min at 300 °C with ramp rate 10 °C/min. The GC column was washed with hexane in between samples to avoid previous sample contamination. Using the above conditions, the retention time obtained from standard solutions was 45 min. All samples were filtered through 0.22 µm syringe filter and analysed by GC–MS. Total chemical additives identified by GC–MS (TQ8030, Shimadzu Cooperation, Japan) in the extraction process from LDPE used for packaging of dahi were compared with the existing positive list of constituents of polyethylene as per IS 16738: 2018.
Statistical analysis
FULSE conditions were optimized by using BBD (Box-Behnken design) using Design-Expert software (version 13.0). The optimal conditions for the extraction of chemical additives from packaging materials were determined using the Duncan's Multiple Range test on the data obtained from the GC–MS analysis. The mean concentrations of the chemicals extracted from SLE and FULSE samples were compared using paired t-test.
Results and discussion
Thickness measurement of LDPE used for packaging of dahi
The results of thickness measurement of dahi packaging films are presented in Table 1. Results varied from 50.66 to 110.00 μm for 180 g to 5 kg containment capacity of dahi packaging films, respectively. It was observed that with increase in the capacities of packaging material, the thickness increased (Table 1). As per IS 16738: 2018, thickness of PE pouches used for packaging of liquid milk should not be less than 50 μm and 40 μm for 1 kg and 500 g pouches, respectively. According to Plastic Waste Management Rules (PWMR) (2016), thickness of plastic packaging films used for packaging of food commodities should have minimum thickness of 50 μm for facilitating collection and recycling of plastic waste. The results indicated that the overall thickness of the packaging material used in the study was within ± 10 (%) of the declared value. As per obtained data, the thickness of all the LDPE films of different containment capacities procured from different manufactures were complying with the standards.
Table 1.
Thickness and overall migration of chemical additives from LDPE dahi packaging films of different capacities procured from different brands into simulants B and D
| Brands | Containment Capacity | Thickness (µm) | Overall migration Simulant ‘B’ (mg/dm2) |
Overall migration Simulant ‘D’ (mg/dm2) |
|---|---|---|---|---|
| Brand 1 | 5 kg | 110.00 ± 1.00A | 1.85 ± 0.11a | 0.51 ± 0.09b |
| Brand 1 | 1 kg | 62.67 ± 1.53B | 1.95 ± 0.30a | 0.21 ± 0.00b |
| Brand 2 | 1 kg | 60.67 ± 2.08H | 1.80 ± 0.23a | 0.31 ± 0.04b |
| Brand 3 | 1 kg | 62.66 ± 0.58B | 1.38 ± 0.07a | 0.23 ± 0.00a |
| Brand 4 | 1 kg | 61.33 ± 1.53I | 3.80 ± 0.78a | 0.35 ± 0.02b |
| Brand 5 | 1 kg | 60.33 ± 1.41I | 3.50 ± 0.48a | 0.32 ± 0.14b |
| Brand 1 | 500 g | 57.67 ± 2.58G | 3.11 ± 0.63a | 0.26 ± 0.01b |
| Brand 1 | 400 g | 53.67 ± 2.52D | 2.15 ± 2.14a | 0.27 ± 0.16b |
| Brand 2 | 400 g | 54.33 ± 1.15E | 1.56 ± 0.65a | 0.33 ± 0.05b |
| Brand 3 | 400 g | 56.66 ± 1.13F | 1.52 ± 0.11 a | 0.15 ± 0.02 b |
| Brand 1 | 200 g | 52.53 ± 1.53C | 0.72 ± 0.07a | 0.32 ± 0.06b |
| Brand 2 | 200 g | 51.66 ± 0.58C | 2.31 ± 1.17a | 2.01 ± 1.17b |
| Brand 3 | 200 g | 52.00 ± 2.00 J | 2.49 ± 1.00a | 0.26 ± 0.07b |
| Brand 1 | 180 g | 50.66 ± 1.15C | 0.89 ± 0.05a | 0.17 ± 0.01b |
Data is presented as Mean ± SE. Values with different uppercase superscript letters within a column are significantly (p < 0.05) different from each other. Values with different lowercase superscript letters within a row are significantly (p < 0.05) different from each other
FTIR spectra of dahi packaging materials
The FTIR spectra of packaging materials from five different brands, viz. 1, 2, 3, 4, 5 are shown in Fig. 1. These packaging materials were identified as of LDPE on comparing the obtained spectra with the inbuilt library in the FTIR spectrophometer. All indicated peaks in Fig. 1 were also matched with the standard spectra of LDPE (Asensio et al. 2009). The peaks at 2915 and 2848 cm−1 in LDPE polymer were due to the presence of alkanes of asymmetric and symmetric stretching vibrations, respectively. The peak at 1467, 1462 and 1366 cm−1 in LDPE were due to the presence of C-H bending of alkanes. There were also the additional peaks at 730 and 716 cm−1 in LDPE which were due the presence of aliphatic ethers having CH2 rocking deformation.
Fig. 1.
Spectra produced from dahi packaging films procured from five different brands (Brand 1, 2, 3, 4 and 5), letters represent characteristic absorption bands (cm−1) used to identify each polymer
Overall migration of chemical additives from LDPE into simulants
Overall migration is the sum of all substances that can migrate from the food contact materials into food (or food simulants). Rather than assessing the toxicological effects, the overall migration limit concept is basically used for monitoring the total amount of additives or materials migrating into food as well as to reduce the list of trials for specific migration (Arvanitoyannis and Bosnea 2004). The plastic materials manufactured or prepared for the purpose of food packaging need to be in compliance with EU regulation with respect to migration (Schaefer 2007; Veraart and Coulier 2007). Two types of simulants were used for determination of overall migration, simulant B (3 (%) acetic acid) and simulant D (n-heptane) as specified by the BIS because dahi contains both fat and also it is acidic in nature. Overall migration of chemical additives from LDPE films procured from different firms was below the maximum acceptable limit given by BIS (IS: 9845 (1898) (R2015)) and all were complying with the standards. The statistically significant (p < 0.05) difference was observed between the migration values of chemical additives in simulant B and simulant D as determined by paired sample t-test at 95 (%) level of confidence. It was observed that the thickness of the packaging material showed significant effect on the migration values of chemical additives in both simulants B and D. Overall migration in simulant B and simulant D were significantly (p < 0.05) different from each other as shown in Table 1. All LDPE samples showed the overall migration of chemical additives below the maximum limit (10 mg/dm2) given by BIS (IS: 9845 (1898) (R2015)). Significant (p < 0.05) difference was observed between the overall migration of chemical additives in simulant B and D from all the LDPE samples procured from different brands.
Table 2 presents the migrated chemical additives from different LDPE films into simulant B and simulant D, respectively. Total 15 and 24 chemical additives were migrated from LDPE packaging materials into simulant B and D, respectively which contributed to about 19.73 and 31.57 (%) of total 76 chemical additives. It was observed that thickness of the packaging materials showed no relation with the overall migration in both the simulant B and simulant D. Czerniawski and Pogorzelska (1997) determined the overall migration from various packaging materials such as high density polyethylene (HDPE), linear low density polyethylene (LLDPE), polyethylene terephthalate (PET) and reported very low migration values (0.2–1.0 mg/dm2) into aqueous food simulants. They also concluded that there is no significant differences between the migration values in distilled water and 3 (%) aqueous acetic acid. Bradley et al. (2009) reported that determination of specific migration using overall migration methods is appropriate for polymers which have low intrinsic migration. The method is incompatible with polymers with high specific migration limits and polymers with a greater intrinsic migration rate. Goulas (2001) found that the migration rates of additives from coextruded five-layer packaging films into distilled water (0.11–0.72 mg/dm2) and 3 (%) aqueous acetic acid (0.2–0.79 mg/dm2) were much less than the EU prescribed migration limit of 10 mg/dm2. Additionally, no statistically significant differences existed in total migration values across identical five-layered films of varying thicknesses. Garde et al. (2001) also determined the overall migration from PP films of varying thicknesses (50, 100 and 200 µm) into distilled water and 3 (%) aqueous acetic acid and reported that the migration values into both aqueous simulants were extremely low (0.2–0.4 mg/dm2) and were not dependent on film thickness. They also stated that there was no statistically significant difference in migration results while employing these solvents.
Table 2.
Migrated chemical additives from LDPE films into simulants B and D detected using GC–MS
| Type of simulants | Additives |
|---|---|
| Simulants “B” |
Phenol, 3,5-bis(1,1-dimethylethyl)- Dibutyl phthalate Methyl tetradecanoate Hexadecanoic acid, methyl ester Methyl 10-trans,12-cis-octadecadienoate Methyl stearate Octadecane, 3-ethyl-5-(2-ethylbutyl)- Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester Phthalic acid, hexyl oct-3-yl ester [1,1'-Biphenyl]-2,3'-diol, 3,4',5,6'-tetrakis(1,1-dimethylethyl)- 1-Dodecanol 9-Octadecenoic acid, methyl ester, (E)- Methyl 10-trans,12-cis-octadecadienoate Di-2,6-dimethyl-4-heptyl phthalate 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester |
| Simulants “D” |
Methyl 10-trans,12-cis-octadecadienoate Hexadecanoic acid, methyl ester Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester 1-Nonadecene 1-Heptacosanol Octacosanol 13-Docosenamide, (Z)- Hexanedioic acid, bis(2-ethylhexyl) ester Tetrapentacontane, 1,54-dibromo- Bis(2-ethylhexyl) phthalate Octacosanol Squalene Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) Tris(2,4-di-tert-butylphenyl) phosphate n-Tetracosanol-1 9-Octadecenamide, (Z)- 1-Hexadecanol Tributyl acetylcitrate Hexanedioic acid, bis(2-ethylhexyl) ester Eicosane 9-Octadecenenitrile, (Z)- Dotriacontane 2-Methyltetracosane Benzenamine, 4,4'-methylenebis |
Determination of chemical additives extracted from LDPE using solid liquid extraction (SLE) by GC–MS
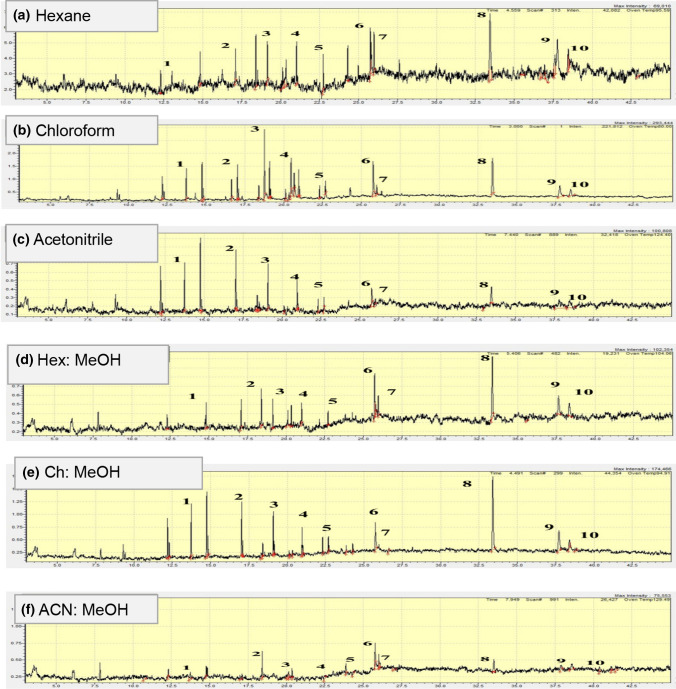
To extract plastic additives from the packaging material, a variety of solvents are available. Non-polar solvents such as tetra hydrofurane, chloroform, dichloromethane, hexane, heptane or diethyl ether have traditionally been described in the literature for extracting additives from plastic because they expand the polymer and therefore promote additive extraction. As the plastic packaging materials contain both polar and non-polar additives, the mentioned non-polar solvents extracts limited additives which are only having affinity towards the non-polar solvents. In order to overcome this problem, we tried six different combinations of polar and non-polar solvents with different polarity index. Based on the literature, the following solvents and solvent mixtures (v/v) were tested: acetonitrile: methanol (1:1), chloroform: methanol (1:1), n-hexane: methanol (1:1), acetonitrile, chloroform and n-hexane. Chloroform and hexane as non-polar solvents and acetonitrile as polar solvent were used. For each kind of solvent, two blanks were performed and tests were carried out in triplicate. Five milliliters of solvent was used for extraction of additives from 0.2 g LDPE sample for six different dynamic extraction times (0, 6, 12, 18, 24 and 48 h) at room temperature. No increase was observed in the amount of additives extracted after 18 h, so 18 h was selected for further work. Figure 2 shows the chromatograms of ten common additives observed in each solvent. In general, chloroform: methanol (1:1) extracted the highest amount of additives and acetonitrile: methanol (1:1) the lowest, as shown in Fig. 2e and f, respectively. Duncan’s Multiple Range test was also applied on the data obtained from GC–MS after extraction of chemical additives from LDPE. It was observed that the solvent combination of chloroform: methanol (1:1) with a time period of 18 h was ranked first for extraction of chemical additives from LDPE, so used for further study. The same solvent and time combination was further used for the extraction of additives from LDPE.
Fig. 2.
Chromatograms of chemical additives extracted using different sets of solvents a hexane, b chloroform, c acetonitrile, d hexane:methanol, e chloroform:methanol, f acetonitrile:methanol after 18 h. 1. butylated hydroxytoluene, 2. hexadecanoic acid, methyl ester, 3. 9-octadecenoic acid, methyl ester, (E)-, 4. methyl stearate, 5. hexanedioic acid, bis (2-ethylhexyl) ester, 6. 13-docosenamide, (Z)-, 7. squalenes, 8. phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1), 9. tris (2,4-di-tert-butylphenyl) phosphate, 10. benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester
Additives extracted in chloroform: methanol (1:1) was quantified by using a GC–MS system. Due to hazardous nature of chloroform, it is not suitable for GC–MS analysis, so the solvent after extraction of additives from packaging materials was evaporated to dryness (up to 0.1 mL) and further reconstituted with 5 mL methanol. Total eleven samples of dahi packaging films made up of LDPE were extracted using optimized SLE method. In all these samples, total 76 chemical additives were extracted and were classified into total 13 categories on the basis of their function in plastic polymers. Lubricants and surfactant and unreacted hydrocarbons were the major additive categories which contributed to about 18 and 17 (%), respectively while the anti-adhesive and slip agents were the lowest contributing about 1 and 3 (%), respectively in LDPE. The common additives used in manufacturing of PE packaging materials as has been reported in literarture are as follows: dipentyl phthalate, di-(2-ethylhexyl) adipate, diethyl phthalates, di-octyladipate, diisobutyl phthalate, and di-n-butyl phthalate (Sablani and Rahman 2007). Commonly used stabilizers, UV stabilizers and antioxidants are Bisphenol A (BPA); Cadmium and Lead compounds; Octylphenol; Nonylphenol compounds; 1,3,5-Tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione (TGIC)/1,3,5-tris[(2S and 2R)-2,3-epoxypropyl]-1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (-TGIC), Butylated hydroxytoluene, 2- and 3-t-butyl-4 hydroxyanisole (Kattas et al. 2000; Crompton 2007; Hahladakis et al. 2018). Similar type of compounds have been reported in our study also.
Moreta and Tena (2015) worked on estimation of different additives (Irgafos 168, Erucamide, oxidized Irgafos 168, Irganox 1010 and Irganox 1076) from plastic (Multilayer packaging PE) used in packaging by LC–MS method within a run time of 10 min. They used various extractions technique such as pressurized liquid extractions, FULSE and SLE techniques to evaluate additives. They reported that all extraction methods showed good extraction efficiency for slip agents, except for the FULSE which did not show significant speed up in the extraction process and hence was rejected in their work. The extraction efficiency reported to be more dependent on the swelling of packaging material than on the extraction force. As a result, despite the fact that a 10 h SLE is a lengthy procedure, it was chosen due to its inexpensive cost, ease of use, and high recovery values.
Determination of chemical additives extracted from LDPE using focused ultrasound solid liquid extraction method (FULSE) by GC–MS
The FULSE method was optimized for conditions where maximum extraction of chemical additives from LDPE was observed. This was for the first time when the FULSE method was utilized to extract additives from the packaging material of a dairy product (Dahi). All FUSLE tests were carried out at 5 °C in an ice-water bath at 50 (%) pulsed cycle. LDPE films were cut to an area of around 0.5 cm2 before extraction. The influence of volume of extracting solvent, extraction time and ultrasonic irradiation power were optimized by using a BBD. BBD often has fewer design points; it can be less expensive compared to Central Composite Design (CCD) with the same number of factors. Ultrasonic irradiation power was studied from 40 to 90 (%), extraction time between 30 and 560 s and volume of extracting solvent from 3 to 8 mL. In this study, independent variables were: (i) volume of extracting solvent (mL), (ii) extraction time (s) and (iii) irradiation power (%).
Based on the experimental results, the five best combinations of the independent variables for the extraction of chemical additives from LDPE (60 μm) were used. Best combination among these five was with a solvent volume of 4.5 mL, extraction time of 535 s and ultrasonic irradiation power of 62 (%) (466 Watts) because the difference between predicted and recorded values for this combination was the least as compared to the other combinations. The ten common chemical additives observed in the results of all the combination employed for the extraction of the additives using FULSE considered as responses for each combination were as follows; 2,4-Di tert-butylphenol, hexadecanoic acid, methyl ester, 9-octadecenoic acid, methyl ester, methyl stearate, hexanedioic acid, bis(2-ethylhexyl) ester, 13-docosenamide, squalene, phenol, 2,4-bis(1,1-dimethylethyl)-phosphite(3:1), tris(2,4-di-tert-butylphenyl) phosphate and benezepropanoic acid, 3,5-bis(1,1 dimethylethyl)-4-hydroxy-,octadecyl ester.
Comparison of extraction efficiency of SLE and FULSE extraction methods
Extraction efficiency of SLE and FULSE methods were compared based on the number of peaks and their obtained area %. Both in SLE and FULSE methods, the common ten chemical additives were selected and their (%) area of concentration from both the methods were determined. Paired t-test showed the significant (p < 0.05) difference between the extraction efficiency of the two methods. FULSE method was observed to be more efficient over SLE method for extraction of chemical additives from LDPE packaging materials as it extracted more number of additives as compared to the former and it also requires lower time for extraction.
Comparing extracted additives from LDPE with its existing positive list of additives
The chemical additives extracted from LDPE used for the packaging of dahi by using SLE method were matched with its existing positive list given by BIS (IS: 16738 (2018). Out of total 76 chemical additives extracted from LDPE, only eight (10.52 (%)) were matched with the existing positive list of polyolefin. Around 70–90 (%) of the extracted chemical additives from all the LDPE packaging materials were not listed in their existing positive list of additives given by BIS.
Real time analysis in dahi samples
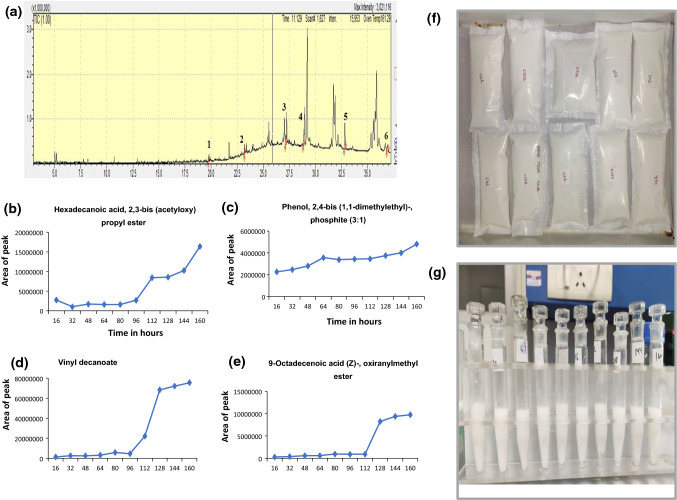
In real time analysis, the migration of chemical additives from LDPE into dahi was determined (Table 3). Total 12 categories of chemical additives were observed to be migrated into dahi which included antioxidants, plasticizers, fatty acids and their derivatives, lubricants and surfactant, unreacted hydrocarbon etc. Figure 3a shows the typical GC–MS chromatograms of the additives (heptacos-1-ene (RT 19.75), hexadecanoic acid, methyl ester (RT 18.26), hexanedioic acid, bis (2-ethylhexyl) ester (RT 22.48), vinyl decanoate (RT 28.80), Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) (RT 32.85), tris (2,4-di-tert-butylphenyl) phosphate (RT 36.95)) with peak numbers 1, 2, 3, 4, 5 and 6, respectively which got migrated into the dahi at the time interval of 16 h. Hexadecanoic acid, methyl ester, phenol, 2,4-bis(1,1-dimethylethyl)-phosphite (3:1), 9-octadecenoic acid, methyl ester, (E)- and hexanedioic acid, bis (2-ethylhexyl) ester were the four chemical additives which were commonly observed at all the intervals of time. Extracted concentrations of these additives were constant up to a period of 96 h and 112 h for first three additives and last additive, respectively and then increased steadily up to 160 h (Fig. 3 b, c, d, e). Sorensen (2006) determined phthalates [dibutyl phthalate, benzyl butyl phthalate, di-2-ethylhexyl phthalate, di-isononyl phthalate and di-isodecyl phthalate] which were spiked in milk and milk products using LC–MS. But they did not carry out the real time analysis.
Table 3.
Classification of chemical additives extracted from LDPE films
| Category | Additives |
|---|---|
| Hydrocarbon |
Undecane 1-Tridecene Tetradecane Tridecane, 7-methylene- 3-Hexadecene, (Z)- Dodecane, 2-methyl- 1-Tetracosene Squalene 9-Octadecene, (E)- 1-Nonadecene Hexadecane Cyclopentane, 3-hexyl-1,1-dimethyl- Cetene Triacontane, 1-bromo- Tetrapentacontane, 1,54-dibromo- 2-Methyltetracosane |
| Lubricants and Surfactants |
Tetradecane 1-Decanol, 2-hexyl- Dodecane, 2-methyl- 1-Tetracosene Methyl stearate Hexadecane Behenic alcohol Glycidyl palmitate 1-Dodecanol Eicosane/Heneicosane Octacosanol Triacontane, 1-bromo- Tetrapentacontane, 1,54-dibromo- Benzene, (2,3-dimethyldecyl)- Dotriacontane -Methyltetracosane Heptacosane/Pentacosane/Hexacosane |
| Antioxidants |
Phenol, 3,5-bis(1,1-dimethylethyl)- Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester 1,1'-Biphenyl]-2,3'-diol, 3,4',5,6'-tetrakis(1,1-dimethylethyl)- Squalene Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) Tris(2,4-di-tert-butylphenyl) phosphate Hexanedioic acid, bis(2-ethylhexyl) Octadecane, 3-ethyl-5-(2-ethylbutyl)- 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione |
| UV/ Thermal Stabilizers |
Methyl stearate Tris(2,4-di-tert-butylphenyl) phosphate Phthalic acid, hexyl oct-3-yl ester |
| Plasticizers |
Bis(2-ethylhexyl) phthalate 1-Propene-1,2,3-tricarboxylic acid, tributyl ester Tributyl acetylcitrate Hexanedioic acid, bis(2-ethylhexyl) Eicosane/Heneicosane Phthalic acid, hexyl oct-3-yl ester |
| Adhesive and Sealants |
2-Propenoic acid, tridecyl ester Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester |
| Fatty acids and their derivatives |
Methyl tetradecanoate 9,12-Octadecadienoic acid, methyl ester Hexadecanoic acid, methyl ester 16-Hentriacontanol cis-11-Eicosenamide 1-Heptacosanol/1-Hexacosanol Decanoic acid, methyl ester 9-Octadecenoic acid, methyl ester, (E)- Methyl 10-trans,12-cis-octadecadienoate Methyl myristoleate n-Tetracosanol-1 Undec-10-ynoic acid, heptadecyl ester Sulfurous acid, dodecyl 2-propyl ester |
| Ingredients of dyes and pigments |
n-Tridecylcyclohexane 9-Octadecenoic acid, methyl ester, (E)- Benzenamine, 4,4'-methylenebis- Cyclohexane, octyl- Hexadecyl isopropyl ether Behenic alcohol Octacosanol |
| Polymers modifier |
2-Propenoic acid, tridecyl ester 1-Hexadecanol |
| Starting materials/Intermediate/Breakdown products |
Methyl tetradecanoate Hexadecanoic acid, methyl ester 9,12-Octadecadienoic acid, methyl ester 1-Heptacosanol/1-Hexacosanol Benzenamine, 4,4'-methylenebis- 9-Octadecenenitrile, (Z)- Hexadecyl isopropyl ether Behenic alcohol Glycidyl palmitate 10-Benzyloxytricyclo[4.4.0.0(3,8)]decan-4-ol Undec-10-ynoic acid, heptadecyl ester Carbonic acid, eicosyl vinyl ester Acetophenone cis-11-Eicosenamide |
| Slip agents |
9-Octadecenamide, (Z)- 13-Docosenamide, (Z)- 1-Decanol, 2-hexyl- |
| Others | 9-Octadecenamide, (Z)- |
| Anti-adhesive | Octadecenamide |
Fig. 3.
a GC–MS chromatogram of additives migrated from LDPE into dahi after 16 h 1. heptacos-1-ene, 2. 5,5-diethylpentadecane, 3. hexadecanoic acid, methyl ester 4. vinyl decanoate 5. phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) 6. tris(2,4-di-tert-butylphenyl) phosphate, b hexadecanoic acid, 2,3-bis (acetyloxy) propyl ester, c phenol, 2,4 bis(1,1-dimethylethyl)-phosphite (3:1), d vinyl decanoate, e 9-octadecenoic acid (Z)-, oxiranylmethyl ester; Migration of additives, b hexadecanoic acid, 2,3-bis (acetyloxy) propyl ester, c phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1), d vinyl decanoate, e 9-octadecenoic acid (Z)-, oxiranylmethyl ester during different time intervals (16, 32, 48, 64, 80, 96, 112, 128, 144 and 160 h) from LDPE into dahi, f sealed pouches for real time analysis in dahi, g extracted additives at different intervals of time
Conclusion
Packaging films procured from five dfferent firms labelled as LDPE was observed to be of same material by ATR-FTIR. Results indicated that the thickness of all the LDPE films procured from different manufactures were complying with the BIS standards. Migration of chemical additives from LDPE packagining matrial indicated that the overall migration into both simulant B (3 (%) acetic acid) and simulant D (n-heptane) were below the maximum limit (10 mg/dm2) as recommended by BIS. Antioxidants, fatty acids and their derivatives, unreacted hydrocarbons, plasticizers, lubricants and surfactant etc. were the chemical additives which migrated into the simulants. Solvent combination of chloroform: methanol (1:1) appeared to be suitable for extraction of chemical additives from LDPE. SLE and FULSE methods for extraction of chemical additives from LDPE films were compared and it was observed that the FULSE method was able to extract all chemical additives within 9 min vis-à-vis SLE method which took 18 h. Further, FULSE method was observed to be more efficient as compared to that of the SLE method as it extracted more number of chemical additives from LDPE films. Chemical additives, viz. antioxidants, plasticizers, fatty acids and their derivatives, lubricants and surfactant, unreacted hydrocarbon etc. were migrated into dahi during real time analysis. It was observed that many of the extracted chemical additives from LDPE packaging material did not match with the positive list prescribed by BIS. It appears that positive list needs to be extended as these additives were used by all manufactures which may be a process requirement. Study could help the regulatory bodies to monitor the additives being used in the packaging materials and also fixing the extent of migration from the LDPE films into dahi.
Acknowledgements
The authors acknowledge the financial assistance from the Ministry of Food Processing Industries with Project Code: 4001/530/1011837/40010.
Abbreviations
- BBD
Box–Behnken design
- BOD
Bio-oxygen demand
- FTIR
Fourier transform infrared spectroscopy
- FULSE
Focused ultrasound solid liquid extraction
- GC–MS
Gas chromatography mass spectrometry
- HDPE
High density polyethylene
- IS
Indian Standard
- LCMS
Liquid chromatography mass spectrometry
- LDPE
Low density polyethylene
- LLDPE
Linear low density polyethylene
- NCDC
National collection of dairy cultures
- PP
Polypropylene
- PE
Polyethylene
- PET
Polyethylene terephthalate
- PLE
Pressurized liquid extractions
- PWMR
Plastic waste management rule
- RT
Retention time
- SLE
Solid liquid extraction
Author’s contributions
KC: Characterization and overall migration study. KG: Assisted in the interpretation of the data and writing of the manuscript. KL: Determined the chemical additives in the extracts using GCMS. RS: Conceived and planned the experiments, writing of the manuscript. BM: Assisted in RSM using BBD design, optimisation of conditions of solid liquid extraction. NS: optimisation of conditions of focused ultrasound solid liquid extraction method and RSM.
Funding
Ministry of Food Processing Industries with Project Code: 4001/530/1011837/40010.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Krushna Chapke, Email: krushnachapke17@gmail.com.
Kamal Gandhi, Email: kamalgandhindri@gmail.com.
Kiran Lata, Email: kiranlatahisar@gmail.com.
Rajan Sharma, Email: rajansharma21@gmail.com.
Bimlesh Mann, Email: bimleshmann@gmail.com.
Nishi Singh, Email: nishisinghndri1807@gmail.com.
References
- Arvanitoyannis IS, Bosnea L. Migration of substances from food packaging materials to foods. Crit Rev Food Sci Nutr. 2004;44(2):63–76. doi: 10.1080/10408690490424621. [DOI] [PubMed] [Google Scholar]
- Asensio RC, Moya MS, de la Roja JM, Gomez M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal Bioanal Chem. 2009;395(7):2081–2096. doi: 10.1007/s00216-009-3201-2. [DOI] [PubMed] [Google Scholar]
- Barnes K, Sinclair R, Watson D, editors. Chemical migration and food contact materials. Cambridge: Woodhead Publishing; 2007. pp. 1–12. [Google Scholar]
- Behare P, Singh R, Singh RP. Exopolysaccharide-producing mesophilic lactic cultures for preparation of fat-free Dahi—an Indian fermented milk. J Dairy Res. 2009;76(1):90–97. doi: 10.1017/S0022029908003865. [DOI] [PubMed] [Google Scholar]
- Bradley EL, Castle L, Jickells SM, Mountfort KA, Read WA. Use of overall migration methodology to test for food-contact substances with specific migration limits. Food Addit Contam. 2009;26(4):574–582. doi: 10.1080/02652030802477947. [DOI] [PubMed] [Google Scholar]
- Cooper I, Tice PA. Migration studies on fatty acid amide slip additives from plastics into food simulants. Food Addit Contam. 1995;12(2):235–244. doi: 10.1080/02652039509374298. [DOI] [PubMed] [Google Scholar]
- Crompton TR. Additive migration from plastics into foods: a guide for analytical chemists. Shrewsbury: Smithers Rapra Publishing; 2007. pp. 31–39. [Google Scholar]
- Czerniawski B, Pogorzelska Z. Investigations on overall migration of various plastic materials and articles used in contact with foodstuffs. Packag Technol Sci Int J. 1997;10(5):261–270. doi: 10.1002/(SICI)10991522(199709/10)10:5<261::AIDPTS407>3.0.CO;2-E. [DOI] [Google Scholar]
- FICCI (2016) Plastic packaging report. https://ficci.in/spdocument/20690/plastic-packaging-report.pdf
- Garde JA, Catala R, Gavara R, Hernandez RJ. Characterizing the migration of antioxidants from polypropylene into fatty food simulants. Food Addit Contam. 2001;18(8):750–762. doi: 10.1080/02652030116713. [DOI] [PubMed] [Google Scholar]
- Goulas AE. Overall migration from commercial coextruded food packaging multilayer films and plastics containers into official EU food simulants. Eur Food Res Technol. 2001;212(5):597–602. doi: 10.1007/s002170000294. [DOI] [Google Scholar]
- Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- IS: 9845 (1898) (R2015). Determination of overall migration of constituents of plastics materials and articles intended to come in contact with foodstuffs—method of analysis. Bureau of Indian Standards, Manak Bhawan, New Delhi, pp 1–5
- IS: 16738 (2018) Positive list of constituents for polypropylene, polyethylene and their copolymers for its safe use in contact with foodstuffs and pharmaceuticals. Bureau of Indian Standards, Manak Bhawan, New Delhi, pp 1–9
- Kattas L, Gastrock F, Levin I, Cacciatore A. Plastic additives. In: Harper CA, editor. Modern plastics handbook. 1. New York: McGraw-Hill; 2000. pp. 4–1. [Google Scholar]
- Lockhart HE. A paradigm for packaging. Packag Technol Sci Int J. 1997;10(5):237–252. doi: 10.1002/(SICI)1099-1522(199709/10)10:5<237::AID-PTS395>3.0.CO;2-(%)23. [DOI] [Google Scholar]
- Miltz J, Passy N, Mannheim CH. Mass transfer from and through packaging materials. Packag Technol Sci. 1992;5(1):49–56. doi: 10.1002/pts.2770050110. [DOI] [Google Scholar]
- Moreta C, Tena MT. Determination of plastic additives in packaging by liquid chromatography coupled to high resolution mass spectrometry. J Chromatogr A. 2015;1414:77–87. doi: 10.1016/j.chroma.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Packaging Industry in India (2020)-Growth, Trends, Forecasts (2020–2025) https://www.reportlinker.com/p05867471/?utm_source=GNW
- Prajapati JB, Sreeja V, (2014) Industry point of view update. In: Dahi and Related Products. (Ed. 1st). New Delhi publisher, New Delhi, India, pp 88
- Robertson GL. Food packaging, principles and practice. 2. New York: CRC Press; 2005. [Google Scholar]
- Sablani SS, Rahman MS (2007) Food packaging interaction. In: Handbook of food preservation. CRC Press, Washington State University Pullman, pp 957–974
- Saint-Eve A, Lévy C, Le Moigne M, Ducruet V, Souchon I. Quality changes in yogurt during storage in different packaging materials. Food Chem. 2008;110(2):285–293. doi: 10.1016/j.foodchem.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Schafer A. Regulation of food contact materials in the EU. In: Barnes KA, Sinclair CR, Watson DH, editors. Chemical migration and food contact materials. Boca Raton: CRC Prss; 2007. pp. 43–63. [Google Scholar]
- Simoneau C. Food contact materials. Compr Anal Chem. 2008;51:733–773. doi: 10.1016/S0166-526X(08)00021-4. [DOI] [Google Scholar]
- Singh N, Sharma R, Mann B, Gandhi K (2020) Migration of chemical additives from packaging materials into dairy products: safety and regulatory perspectives. Indian Dairyman pp 46–54
- Sorensen LK. Determination of phthalates in milk and milk products by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(7):1135–1143. doi: 10.1002/rcm.2425. [DOI] [PubMed] [Google Scholar]
- The plastic waste management rules (2016) https://pib.gov.in/newsite/printrelease.aspx?relid=138144
- Veena N, Nath BS, Srinivas B, Balasubramanyam BV. Quality attributes of dahi prepared from milk fortified with omega-3 fatty acids, phytosterols and polydetxrose. J Food Sci Technol. 2017;54(7):1765–1775. doi: 10.1007/s13197-017-2596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart R, Coulier L. Compliance testing of chemical migration from food contact materials. Chem Migrat Food Contact Mater. 2007;56:87–121. doi: 10.1533/9781845692094.2.87. [DOI] [Google Scholar]