Abstract
Fermented camel milk provides many health benefits like antidiabetic activity, anti-hypertensive activity etc. Fermented camel milk contains IPP or VPP rich ACE inhibitory peptides. The aim of this study was to spot the novel Angiotensin I-Converting Enzyme inhibitory peptides liberated by the potent proteolytic Lactobacillus acidophilus NCDC-15 from camel milk (Indian breed). NCDC-15 had exhibited maximum PepX activity (0.655) and ACE-inhibitory activity (78.33%) at 12 and 48 h of incubation at 37 °C respectively. Proteolytic activity was measured using o-phthaldialdehyde method and observed maximum (0.976 OD) at 2% of inoculation for 12 h of incubation at 37 °C. Water soluble extracts derived from fermented camel milk were ultrafiltered through 3 kDa, 5 kDa and 10 kDa membrane filters from which 3 kDa permeates (48.01% peptides production & 49.46% ACE-inhibition) and 10 kDa permeates (55.04% peptides production & 42.40% ACE-inhibition) had shown maximum peptides production and ACE-inhibitory activity. Overall, 24 peptides were identified from the samples of 3 kDa permeates [6 fractions (K1, L1, M1, N1, O1 and P1)] and 10 permeates [5 fractions (S, T, U, V and W)]. Novel peptide (AIGPVADLHI) was matched with k-casein in AHTPDB database and other peptides were also found matched with α and β-caseins of camel milk.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05357-9.
Keywords: Camel milk, Angiotensin I-Converting Enzyme (ACE) inhibitory, Proteolytic activity, Lactobacillus acidophilus, Bioactive peptides
Introduction
Cardiovascular diseases are the leading cause of the death globally. A World Health Organization (WHO) report showed that every year more than 17 million people die from cardiovascular diseases (Wang et al. 2016). High blood pressure, smoking, diabetes, lack of exercise, obesity, high cholesterol in the blood, poor diet, and excessive alcohol consumption can induce these diseases. One of them is high blood pressure (hypertension) and related problems are prevalent in rural and urban areas of the world. According to a study, about 33% urban and 25% rural Indians are hypertensive (Anchala et al. 2014). Globally, a study conducted by Imperial College London and WHO on hypertension between 1990 and 2019 suggested that the number of people living with hypertension has doubled to 1.28 billion since 1990 (Zhou et al. 2021).
As per medical reports, hypertension leads to diseases such as stroke (heart attack), coronary heart disease, kidney disability, kidney dysfunction and in extreme cases death of the patient (López-Fandiño et al. 2006). ACE enzyme is also familiar as “Peptidyl Dipeptide Hydrolase” (EC 3.4.15.1), and it plays a critical function in maintaining the blood pressure in human body. The enzyme releases a vasoconstrictor called Angiotensin II after cleaving at the dipeptide angiotensin at the C-terminal that plays a key function in the rennin angiotensin system (RAS) raising the blood pressure (Skeggs et al. 1956). Varieties of ACE-inhibitory agents (drugs) are available in the market, such as, temocapril, enalapril, lisinopril and captopril which when used for a prolonged period causes negative effect on human health. Such problems are encouraging the researchers to find novel ACE-inhibitory agent that is a natural, economical, and safe for human consumption with no side effects. Milk proteins are digested by the lactic cultures which release health promoting physiologically active peptides. Thus, is related with the proteolytic system the lactic cultures (Padghan et al. 2017). Bacterial cell-wall associated proteinases generate oligopeptides from the milk proteins that are transported into the cells and further broken down into various peptides by intracellular peptidases (Nielsen et al. 2009). Lactobacillus acidophilus is a major fermenting and health supporting agent in the acidophilus milk and many other milk-based products (Kongo and Malcata 2016). Lactobacillus acidophilus NCDC-15 was widely studied by various researchers for its probiotic and proteolytic properties in milk and skim milk products (Padghan et al. 2017; Tripathi and Jha 2004). It was also used in buttermilk preparation which was further tested to produce ACE-inhibitory peptides (Padghan et al. 2017). This lactic culture was selected for this study after reviewing its overall therapeutic potential and probiotic properties in other milk-based mediums (Padghan et al. 2017; Tripathi and Jha 2004; Selwal and Selwal 2014). ACE-inhibitory peptides were derived from various types of milk and milk products. However, camel milk is also searched for the presence of ACE-inhibitory peptides in different parts of the world (Al haj et al. 2018).
It is defined that; intact camel milk protein is encrypted with sequences of amino acids which are once released from milk proteins shows health benefit to the human. These are released during in vivo digestion or milk fermentation using lactic cultures or derived using purified enzymes. Peptides isolated from camel milk shows various health benefits which include anti-microbial, anti-oxidative, immunomodulatory, anti-hypertensive and anti-inflammatory (Mati et al. 2017). Thus, camel milk is selected for this study to determine the bio-functional property of fermented camel milk (Dromedary camel with one hump) and establishing novel food for the future.
In the literature, only the presence of bioactivity is reported in camel milk (Indian dromedary breed) fermented with specific lactic culture. But there was a scarcity of well-planned experiment which used a proteolytic strain (Lactobacillus acidophilus) and established rate of inoculation and incubation period for the higher ACE-inhibitory activity in camel milk. However, this experiment was planned to explore the new anti-hypertensive peptides from the dromedary camel (one hump) milk fermented with potent probiotic Lactobacillus. Thus, the purpose of this study was to produce and identify the novel peptides with ACE inhibitory activity from camel milk fermented with Lactobacillus acidophilus NCDC-15.
Materials and methods
Materials
Collection of camel milk
Camel milk for this experiment was acquired from a same camel and breed nurtured under a farm situated near Ankalav village, Anand, Gujarat, India. Initial pH of the camel milk was 6.60. To remove the impurities from the milk, muslin cloth was used, and further pasteurized (90 °C for 10 min) and further storage done at and 5 ± 2 °C. pH of raw camel milk was determined as per the procedure described in Indian Standards (1961) and suggested by Shariati et al (2020) with a calibrated pH meter at 25 ± 1° (Oaklon pH 700, Eutech instrument).
Chemicals and reagents
All the reagents and chemicals used in this analysis were purchased from the Sigma-Aldrich Corporation (Sigma, USA).
Lactic culture
Proteolytic lactic strain Lactobacillus acidophilus NCDC-15 was procured from National Dairy Research Institute, Karnal, India. The procured lactic strain in the lyophilized from was firstly activated in skim milk medium (10% total solids) and further re-activated anaerobically in camel milk medium (Hati et al. 2018; Jouki et al. 2021). During the activation, inoculation rate was 2% (v/v) and further incubated at 37 °C for 24 h and stored at 5 ± 2 °C until further use. Inoculation of the lactic culture was carried out in the laminar airflow in an aseptic conditions and further incubation was done in the incubator maintained at 37 °C (Hati et al. 2018). Lactic culture was inoculated in to skim milk or camel milk for obtaining approximately 108 colony forming units (CFU) per ml. During the experiment, its activity and purity were analysed prior to transfer in another medium (Parmar et al. 2018).
Methods
Fermentation of camel milk
The lactic strain was activated as previously mentioned and the fermentation of camel milk was carried out at the inoculation rate of 2% (v/v) and incubating the milk at 37 °C for 24 h which was stored at 5 ± 2 °C until further use (Parmar et al. 2018). pH of fermented camel milk was estimated as 4.47 using the method described in Indian Standards (1961) suggested by Shariati et al (2020) with a calibrated pH meter (Oaklon pH 700, Eutech instrument).
X-prolyl-dipeptidyl aminopeptidase (PepX) activity determination
PepX activity was analysed through the method of Donkor et al. (2007a). This enzymatic method depends upon the reaction between the substrate (Glycyl-prolyl p-nitroanilide) and supernatant of the test culture. Here in this method, the lactic strain was activated (2% (v/v) for 24 h at 37 °C) in broth (De Man, Rogosa and Sharpe (MRS) broth) and further samples were prepared based on the incubation time (0, 3, 6, 9, and 12 h) at 37 °C. Then, after completion of specific incubation periods, the samples were subjected to the centrifugation which provides cell pellets. These pellets were sonicated agreeing to the method of Solanki et al. (2017). In this method, Gly-Pro-pNA was used as the substrate and sample obtained after sonication was used as an enzyme. Mixture of sample (100 μL), substrate (50 μL) (6.4 mmol/L of glycyl-prolyl p-nitroanilide) and tris-buffer [50 mmol L−1 of Tris–HCl buffer (pH 7.0)] was used according to the method of Donkor et al. (2007a). This spectrophotometric reaction was measured as the degree of hydrolysis. It gives idea about the capacity of lactic strain to produce proline specific peptides from the medium (Donkor et al. 2007a).
Water soluble extract
Camel milk fermented with the NCDC-15 was incubated for particular incubation periods and further subjected to the centrifugation at 13,000 rpm for 20 min at 4 °C (HERMLE centrifuge, Germany) to obtain the water-soluble extracts (WSE) following (Donkor et al. 2007a). These samples were assessed for the ACE-inhibitory activity (Donkor et al. 2007a).
Angiotensin-I-inhibitory activity
Angiotensin-I-inhibitory is a measurement of the release of hippuric acid due to catalytic activity on the substrate N-Hippuryl-Histidine-Leucine (Hip-His-Leu)) by ACE as described (Al haj et al. 2018). Calculation of ACE inhibition was done using the formula given by Hati et al. (2015).
Assessment of proteolysis using o-phthaldialdehyde (OPA)
O-phthaldialdehyde (OPA) method was used to determine the proteolytic activity of NCDC-15. It was conducted with the same method used by Hati et al (2015) in which the test culture was added in three different rates of inoculation (1.0%, 1.5%, and 2.0%) and further incubation done for 0, 3, 6, 9, and 12 h at 37 °C. Maximum proteolytic activity was determined with the respective incubation period and inoculation rate. The best combination was further used for further study.
Assessment of relative proteolytic (peptides production) activity and fractionation of peptides
Relative proteolytic activity (Rpa) was determined through HPLC after comparing the area of peak of the fermented samples and unfermented samples agreeing the method of Vasiljevic and Jelen (2002). Hence, the samples prepared under the superlative inoculation rate and incubation time along with unfermented milk were centrifuged and further the supernatants were ultra-filtered using 3 kDa, 5 kDa and 10 kDa membrane filters (Amicon® ultra-4 ml cellulose membrane filter, Merck Millipore Ireland) at 6000 rpm for 20 min at 4 °C (Hermle centrifuge, Germany). Samples derived as permeates and retentate were analysed for ACE-inhibitory activity and subjected to the RP-HPLC to determine the percentage of peptides production comparatively to the unfermented samples by conferring the method followed by Vasiljevic and Jelen (2002). Later, comparing the total peak area before and after the fermentation, Rpa was estimated using the formula given by Vasiljevic and Jelen (2002).
Reversed-phase liquid chromatography mass spectrometry (RP-LC/MS)
RP-LC/MS was carried out to identify and characterise the novel bioactive peptides. Samples of permeates or retentates (3 kDa, 5 kDa, 10 kDa) were tested for ACE-inhibitory activity and relative proteolytic activity (% rpa). Further, the samples with highest amount of peptides and ACE-inhibitory activity were injected into the RP-HPLC to collect different fractions. After that, the fractions were further injected in RP-LC/MS system following the method Solanki et al. (2017). Identification and characterization of peptides was carried out by using the method conferred by Solanki and Hati (2018) which includes peptides identification through using enhanced mass spectra (EMS) followed by three Enhanced Product Ion (EPI) scan using Information Dependent Analysis (IDA) survey scan.
LC–MS/MS
In this study, the LC-100 system was used with the same specifications and temperature conditions used by Solanki and Hati (2018). It was composed of degasser, quaternary pump, C18 column (2.1 × 50 mm, 1.7 µm), auto-sampler, and column oven. Peptides identification was carried out by using the previously identified IDA parameters according to the method of Parmar et al. (2018). Raw data collection and processing was done using the Mascot Script (Matrix Science, UK) reported by Tagliazucchi et al. (2015). Minor adjustments were carried in the analysing parameters as MS/MS tolerance (0.6) and peptide mass tolerance (1.2). All the peptides were searched in UniProtKB/Swiss-Prot database for checking likeness to already discovered peptides. The significant score achieved after the Mascot analysis was taken into consideration and searched for identity match in NCBI (Al haj et al. 2018) and Protein Information Resource (PIR) (Barker et al. 2001). Antihypertensive activity of the peptides was explored online in AHTPDB (Kumar et al. 2015).
SDS-PAGE profile of fermented camel milk samples
In this experiment, protein profiles of fermented and unfermented camel milk were studied using SDS-PAGE described by Carrasco-Castilla et al. (2012). Here, for this study the separating gel (15%), Staking gel (4%) and ultra-low range molecular weight marker (mol. weight 1.06–26.6 kDa) were used.
Statistical examination
All the enzymatic experiments in this study were carried out in triplicates. Completely randomized design was used for the analysis of PepX and ACE-inhibitory activity, and factorial completely randomized design was used in proteolytic activity. The software developed by Sheoran et al. (1998) was used through the link http://14.139.232.166/opstat/ with the analysis of variance at 5% level of significance. In this study, F test was used to determine the significant difference between the treatments and methods suggested by Ghafarloo et al. (2020).
Results and discussion
PepX activity
X-prolyl dipeptidyl aminopeptidases is also known as PepX and derived from bacteria. Proline or an alanine (Xaa-Pro, Xaa-Ala) (dipeptides) can be released by this enzyme by catalysing at the penultimate spot of the polypeptide sequence. There is a proved relationship which explains the liberation of bioactive peptides and PepX activity of the lactic cultures (Pan et al. 2005). Both the samples (extracellular and intracellular samples) were examined for PepX activity after the specific incubation periods (0, 3, 6, 9, and 12 h) at 37 °C. Intracellular extracts obtained from sonication treatment had shown higher PepX activity as compared to the extracellular extracts. Thus, this study was carried out with intracellular extracts, instead of extracellular extracts of NCDC-15. Table 1 shows the PepX activity of NCDC-15 tested for 0, 3, 6, 9, and 12 h at 37 °C with the rate of inoculation 2%. Observations were taken at 410 nm in the spectrophotometer. The absorbance of all the samples differs with the time of incubation period significantly (p < 0.05) which was in concurrence with the study of Solanki et al. (2017) and Parmar et al. (2020). PepX activity was observed lower (0.093) at 0 h of incubation while maximum at the 12 h of incubation (0.655) at 37 °C. Similar reports were submitted by the researchers, where they determined the PepX activity of Lactobacillus rhamnosus MTCC 5945 (NS4) along with significant difference in the activity with the increasing incubation periods (Solanki and Hati 2018). Similar results were obtained during the study of PepX activity of L. fermentum TDS030603 (LBF) and L. bulgaricus NCDC (09) by using similar method, the same trend was also observed by Solanki et al. (2017) where the increasing trend of PepX activity was also found during the analysis of five Lactobacillus cultures i.e., L. casei (NK9) (KR732325), L. rhamnosus (NK2) (KR080695), L. fermentum (M5) (KU366365), L. fermentum TDS030603 (MTCC 25067) (LF), and L. paracasei (M16) (KU366368) in the same medium. Some researchers also reported the existence of proteolytic enzymes or systems in the lactic acid bacteria (Savijoki et al. 2006). Multiple types of proteolytic enzymes were reported from Lactobacillus group of bacteria which also include the PepX and compose different proteolytic enzymes including proline-specific aminopeptidase (PepR). Existence of PepX in the intracellular extract of L. helveticus JCM1004 was reported by Pan et al. (2005) and the same researchers also reported the hydrolysis of skimmed milk proteins by PepX from the intracellular extract of L. helveticus JCM1004. Presence of PepX was reported in all the species of lactic acid bacteria (Kunji et al. 1996). Some other researchers also identified the presence of various proline specific peptidases in the L. casei ssp. casei IFPL 731 (cell-free extract) and further mentioned that the enzyme usually cleaves N terminal X-Pro dipeptides from tri- and oligo peptides (Donkor et al. 2007a). Presence of extracellular cell-associated aminopeptidase, peptidyl-dipeptidase, X-prolyl dipeptidyl-peptidase (PepX) and carboxypeptidase activities were reported in Streptococcus thermophilus LMD-9 and CNRZ1066 (Hafeez et al. 2013, 2015). The authors conferred the potential implication of intracellular PepX in the hydrolysis of proline-rich peptides extracellularly and a single PepX might be responsible for the concurrent intra- and extracellular enzyme activity (Hafeez et al. 2019). Thus, it can be said that the amino peptidase activity of lactic culture can support to the growth of lactic acid bacteria by supplying free amino acids with different fragments of bioactive peptides (Pan et al. 2005).
Table 1.
PepX activity of camel milk fermented with NCDC-15
| Incubation periods (h) | Absorbance at 410 nma |
|---|---|
| 0 | 0.093 ± 0.001d |
| 3 | 0.162 ± 0.003c |
| 6 | 0.409 ± 0.017b |
| 9 | 0.619 ± 0.060a |
| 12 | 0.655 ± 0.005a |
aAbsorbance Avg ± SD
ACE-inhibitory activity
In this experiment, ACE-inhibition is measured as an absorbance at 228 nm. Variability observed in ACE-inhibitory activity along with the different incubation periods. Along with growing incubation periods, inhibition of Angiotensin-I-converting enzyme was significantly increased (p < 0.05) in this study which was found like the study of Solanki et al. (2017). Initially, higher inhibitory activity (56.22%) was estimated at 0 h of the incubation period (Table 2) which was matching with the study on bovine milk (unfermented) (Donkor et al. 2007b) and camel milk (unfermented) (Solanki and Hati 2018). ACE-inhibitory activity at 0 h of incubation was may be due to the existing heat resistant enzymes of the milk (Gobbetti et al. 2004) or may be due to the peptide’s generation due to the bacterial enzymes in raw milk (Otte et al. 2007) or due to heat treatment (Haque and Chand 2006). Subsequently, Al haj et al. (2018) reported the ACE-inhibitory activity in their fermented camel milk samples S1 (IC50 144, 3.12 µg/mL) (camel milk fermented with Streptococcus thermophilus and Lactobacillus acidophilus and S2 (IC50 144, 3.12 µg/mL) (S. thermophilus and L. helveticus) at 0 h of inoculation which was found in accordance with this study as the initial incubating samples shows ACE-inhibition in fermented camel milk.
Table 2.
ACE-inhibitory activity of camel milk fermented with NCDC 15
| Period (in hours) | ACE-inhibitory activity (%)a |
|---|---|
| 0 | 56.22 ± 1.07c |
| 12 | 70.1 ± 0.80b |
| 24 | 78.02 ± 2.55a |
| 48 | 78.33 ± 2.51a |
a% ACE-inhibitory activity ± SD
Samples have shown ACE-inhibitory activity between 56.22 and 78.33% (Table 2) during the time of incubation from 0 to 48 h. After 48 h of incubation, test samples showed the highest ACE-inhibition (78.33%). During this study, at the incubation of 12 h and 24 h, ACE-inhibition was observed as 70.19% and 78.02% respectively. The same lactic culture along with other cultures such as Lactobacillus bulgaricus (NCDC 009), Lactobacillus helveticus (NCDC 288), Lactobacillus rhamnosus (NCDC 024), Lactobacillus plantarum (NCDC 417), Lactobacillus paracasei subsp. paracasei (NCDC 022), and Lactobacillus casei (NCDC 297), were examined to produce peptides from the goat milk which was fermented at 3% of inoculation rate for 14 h at 37 °C (Sathya et al. 2017). Coincidently, the ACE-inhibition of the same lactic culture in goat milk was (76.36%) after 14 h of incubation, which was slightly higher than this study (70.19%) after 12 h of incubation at 37 °C.
The same lactic culture was used as an adjunct culture for preparing fermented milk product “buttermilk” and studied the generation ACE-inhibitory peptides under the different time–temperature combinations. The sample which consists of “Lactobacillus acidophilus NCDC-15” as an “adjunct culture” has shown minimum IC50 value as 36.62 ± 0.051 mg of protein/ml. It was also reported that heating of milk samples prior to culture addition raised ACE-inhibitory activity. Production of ACE-inhibitory peptides increased when NCDC-15 wad added as an adjunct culture (Padghan et al. 2017). Soleymanzadeh et al. (2019) reported the ACE-inhibitory and antioxidative activity of camel milk fermented using Leuconostoc lactis PTCC1899 at 37 °C for 24 h. Their data shows that the unfermented camel milk exhibited lower activities as compared to fermented samples. The increase in antioxidant activity (ABTS, 454.57 in control to 899.34 µM TE mg−1 protein) and ACE-inhibitory activity (no activity in control to 3.48 ± 0.12 mg mL−1 IC50 in fermented sample) in fermented whey fraction as compared to unfermented fraction defines the ability of lactic culture in liberating the peptides during the fermentation through proteolytic activity (Soleymanzadeh et al. 2019.
Assessment of proteolysis using o-phthaldialdehyde (OPA)
In proteolysis, it was determined that the rate of proteolysis was significantly (p < 0.05) increased along with the increasing trend of incubation time as reported by Solanki et al. (2017). Similar trend of rising proteolytic activity over the different incubation periods (0, 6, 12, 24 and 48 h at 37 °C) was also reported by Parmar et al. (2018) during their study on goat milk using L. casei (NK9) and L. fermentum (MTCC 25067) (LF). Proteolytic characteristic of Leuconostoc lactis PTCC1899 was analyzed in camel milk fermented at 37 °C for 24 h. Proteolytic activity of fermented samples (whole whey fraction) was also reported higher (4.52 ± 0.00 mg mL−1) as compared to unfermented whey fraction (control) (1.25 ± 0.097 mg mL−1) found in accordance with this study. Subsequently, degree of proteolysis was increasing from 0 h of incubation to 12 h of incubation at 37 °C (Soleymanzadeh et al. 2019).
Maximum proteolysis was observed after the incubation of 12 h which was significantly higher than other incubation periods (Table 3). 0.883 was the periodic mean during the study, which indicates the highest proteolysis after the incubation for 12 h. Finally, after studying the statistical data, we found that the best addition rate of lactic culture was 2% and incubation period was 12 h. So, this was the best combination of incubation period and inoculation rates rather than other combinations. Coincidently, the final growth conditions of this study were matched with one study, where the best growth conditions for L. fermentum TDS030603 (LBF) and L. bulgaricus NCDC (09) by measuring the proteolytic activity (Solanki et al. 2017).
Table 3.
Proteolytic activity (absorbance at 340 nm) of camel milk fermented with NCDC-15
| Rate of inoculation (%) | Incubation times (h) | ||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | |
| Proteolytic activity (O.D. at 340 nm) | |||||
| 1% | 0.605 ± 0.013a,b | 0.649 ± 0.009a | 0.667 ± 0.006b | 0.689 ± 0.010b | 0.710 ± 0.009b |
| 1.5% | 0.614 ± 0.065a | 0.651 ± 0.008a | 0.658 ± 0.002b | 0.702 ± 0.007b | 0.962 ± 0.044a |
| 2% | 0.571 ± 0.025b | 0.601 ± 0.026b | 0.775 ± 0.008a | 0.952 ± 0.007a | 0.976 ± 0.019a |
aProteolytic activity (absorbance): mean ± SD. Values with different superscripts differ significantly (p < 0.05)
As the different growth phases of the lactic culture changes, the amount of free amino acid and nourishment required for the lactic acid bacteria changes (Donkor et al. 2007a). A similar trend of increasing proteolytic activity in the camel milk (fermentation carried out using with Streptococcus thermophilus and Lactobacillus acidophilus and (S1) or Streptococcus thermophiles and Lactobacillus helveticus (S2)) was reported by Al haj et al. (2018). The degree of proteolysis was found to be increasing g from 0 to 24 h at 40 °C in camel milk fermented with both S1 and S2, which was found in accordance with this study (Al haj et al. 2018).
Fractionation of peptides and determination of relative proteolytic activity (peptides production)
Ultra-filtration membranes were used for the separation of samples bases on the molecular weight. Samples were prepared as permeates and retentate were subjected in the RP-HPLC for separation of the peptides and fractionation as well as for the examination of relative proteolytic activity. ACE-inhibitory activity of all the samples was tested and it was observed that the activity of all the samples vary significantly with respect to the molecular weight cut off membranes (Table 4) (Hati et al. 2017; Solanki et al. 2017).
Table 4.
ACE-inhibitory activity and peptides production of permeates and retentates of camel milk fermented with NCDC-15 under the optimized conditions
| Lactic culture (NCDC-15) | % ACE-inhibitory activity | Peptides production (%) |
|---|---|---|
| 3 kDa P | 49.46 ± 1.022a | 48.01 ± 0.737b |
| 3 kDa R | 39.75 ± 1.708b | 61.57 ± 1.089a |
| 5 kDa P | 19.12 ± 1.663b | 18.06 ± 0.304b |
| 5 kDa R | 24.33 ± 0.759a | 38.28 ± 0.587a |
| 10 kDa P | 42.40 ± 1.781a | 55.04 ± 1.376a |
| 10 kDa R | 42.25 ± 1.893b | 36.79 ± 1.684b |
Here, P = Permeate, R = Retentate, kDa = kilo Dalton, % ACE-inhibitory activity ± SD
Here, 3, 5 and 10 KDa are the fractionated samples derived through ultra-filtration (UF)
From all the samples, 3 kDa permeate expressed excessive ACE-inhibitory inhibition (49.46%) than the other permeates which was found like the studies of Gonzalez-Gonzalez et al. (2013). While on the other hand, 42.25% of ACE-inhibitory activity showed by the 10 kDa retentate samples during the study. 3 kDa permeates showed highest peptides production (48.01%) in case of permeates and was found like the results of Rodriguez-Figueroa et al. (2012). Peptides with low molecular weight expressed maximum ACE-inhibition, which was like the study of Rahimi et al. (2016). Same researchers examined highest inhibition of ACE enzyme using the 3 kDa permeate and retentate of camel milk casein samples rather than 5 kDa samples. Similarly, camel milk was fermented with Leuconostoc lactis PTCC1899 and the whey fraction was subjected to ultrafiltration through 3 kDa, 5 kDa and 10 kDa membrane filters. The authors found the presence of highest ACE-inhibitory peptides in < 3 kDa fractions (IC50 = 1.61 ± 0.18 mg mL−1) as compared to other fractions from fermented and camel milk samples (5–10 kDa and 3–5 kDa) (Soleymanzadeh et al. 2019). Using the same method, Parmar et al. (2018) had conducted the study on goat milk using the Lactobacillus cultures i.e., L. casei (NK9) and L. fermentum (MTCC 25067) (LF) and reported significant difference in the percentage of peptides production of various permeates and retentate samples of 3 kDa, 5 kDa and 10 kDa samples which also supports this study.
Peptides identification and characterization: RP-LC/MS based analysis of ACE-inhibitory peptides
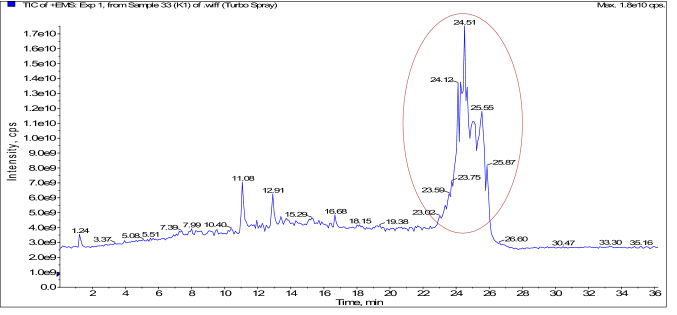
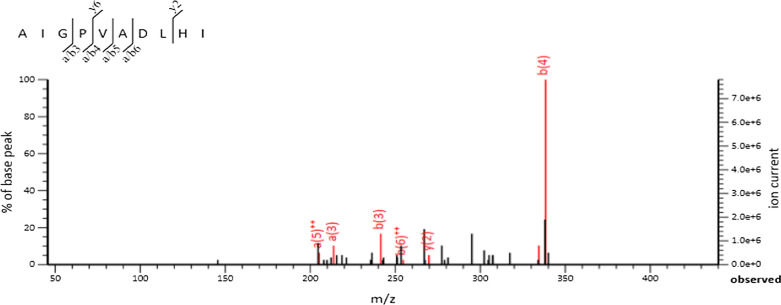
Samples with superior ACE-inhibitory activity (3 kDa and 10 kDa permeates) were analysed with the RP-LC/MS to identify and characterize the novel peptides. Total 24 peptides were identified from 6 fractions (K1, L1, M1, N1, O1 and P1) of 3 kDa permeates. On the other hand, from the 10 kDa permeates, 5 (S, T, U, V and W) fractions were tested and in total 12 peptides were characterized. Figure 1, showing the total ion chromatogram of 3 kDa sample (permeate). For the determination of homology with the camel milk protein, characterized sequences of both the permeates (3 kDa and 10 KDa) were subjected to tow different databases, i. e., BLAST/P (Supplementary Table 1(A) and (B)) in NCBI (Al haj et al. 2018) and PIR (Supplementary Table 2(A) and (B)) database of protein information resource (Barker et al. 2001). Database of antihypertensive peptides (AHTPDB) was used to search the bio-functionality of the individual sequence (Supplementary Table 3(A) and (B) (Kumar et al. 2015; Parmar et al. 2018, 2020; Panchal et al. 2021). In this study, using BLAST/P, our identified sequences were matched with camel milk caseins as k-casein (k-CN) and β-casein (β-CN), and alpha-lactalbumin protein. On the other hand, peptides in PIR were matched with α-S1-CN, α-S2-CN, β-casein, and k-casein. Finally, the results from AHTPDB, suggest that our sequences were matched with already identified sequences from different sources. Sequence AIGPVADLHI (Fig. 2) was matched with k-casein in the database of Antihypertensive peptides (AHTPDB) database, so the novelty of the peptide was confirmed. Previously, it was matched in NCBI and PIR, where found similarity with camel milk protein. Coincidentally, the peptides AIGPVADLHI derived from fraction K1 was matched GPV in the similar sequence proposed by Dziuba et al. (2009). A similar sequence was proposed as the ACE-inhibitory sequence by Martínez-Maqueda et al. (2012). Camel milk caseins exhibit lower homology match with the bovine proteins (caseins). This was explained as the camel milk caseins are more prone to proteolysis during the process of fermentation as compared to bovine milk due to the presence of more cite for enzymatic activity in the protein sequences. Thus, it may be estimated that the peptides generated from camel milk differ to that of bovine caseins (El-Hatmi et al. 2016).
Fig. 1.
Total ion chromatogram of 3 kDa permeate (fraction K1) produced by NCDC-15 [spectra of enhanced mass spectra scan (EMS) to enhanced product ion (EPI) scan in reverse phase-HPLC/MS and highlighted portion exhibiting the spectra of the derived peptide AIGPVADLHI from K1 fraction]
Fig. 2.
MS/MS spectrum of 3 kDa permeate (fraction K1) matched with National Center for Biotechnology Information (NCBI), and Protein Information Resource (PIR) databases [sequneces identified from β-casein and k-casein fraction of camel milk proteins]
Some of the potent precursors of peptides liberated in fermented milk may also serves as the sources of smaller bioactive peptides which would be liberated in intestine with the action of proteolytic enzymes which further shows health beneficial functions (anti-hypertensive, anti-inflammatory, mineral binding) (El-Hatmi et al. 2016). Thus, some peptides in this study were mentioned as the precursor of different proteins as in Supplementary Table 3(A and B). Further, Soleymanzadeh et al. (2019) mentioned that the accessibility of the peptide to the active site of ACE is substantially related to the hydrophobic amino acids that are present in the peptide sequence. Peptides were identified in the study also possess hydrophobic amino acids in their sequence which supports that data published for the structure of ACE-inhibitory peptides (Solanki and Hati 2018). Aliphatic chained amino acids like Val in the peptide sequence could enhance ACE-I activity of the peptide (Soleymanzadeh et al. 2019).
However, GPPYQPLVPR identified from 3 kDa permeate sample, (K1 fraction) (Supplementary Table 3A) had similarity with AVPYPQR reported by Soleymanzadeh et al. (2019) from 3 kDa permeate samples from fermented camel milk fraction with residues 192–198 of β-casein in Bos Taurus (AVPYPQR). Moreover, the previous research reported that the presence of Proline in every three positions of the C terminal residues could positively affect the ACE-I activity of the peptide (Girgih et al. 2014). Besides, it has been demonstrated that Proline in the antepenultimate position in the peptide sequence could enhance peptide binding to the enzyme (Xue et al. 2018). Peptide identified from this study i.e. PPPGSKSPGT (3 kDa permeate, fraction M1) found in accordance with the Xue et al. (2018). These peptides also matched with the ACE-inhibitory peptides derived from milk and maize proteins. Getting proline specific peptides form fermented camel milk is more than bovine milk proteins and the presence of higher proline content in the primary structure of camel milk caseins were observed than bovine milk (Moslehishad et al. 2013). Moreover, the presence of positively charged amino acids in C terminal of the peptide such as Arg could contribute in ACE-I activity and cause a substantial increase in inhibitory potency (Solanki and Hati 2018). Thus, some peptides from the study were also mentioned as the precursor of different proteins in Supplementary Table 3(A and B).
SDS-PAGE analysis
In this study, under the optimized growth conditions camel milk was fermented and subjected to the centrifuge to recover the water-soluble extracts. This analysis was conducted using the water-soluble extracts as samples along with marker of ultra-low range molecular weights (M.W. 1.06–26.6 kDa) from Sigma (Fig. 3). It was observed that, in the SDS-PAGE analysis of camel milk without test cultures, extra protein bands were observed than the fermented camel milk with the test culture. In fermented camel milk samples, bands of molecular weight ranging from 1.06 to 6.5 kDa were identified (Fig. 3). Protein band with molecular weight of 14.2 kDa was observed only in camel milk without the test culture but not in NCDC-15 fermented milk. The presence of a protein band with a high molecular weight in unfermented milk represents intact proteins (α-lactalbumin). According to prior research, the protein band of 14.2 kDa corresponds to α-lactalbumin (Lisak et al. 2013). Presence of lower molecular weight bands (10 kDa) in the SDS profile has increased the possibility of finding the bioactive peptides from fermented camel milk (Meisel 2005). A decrease in the concentration of α-lactalbumin (ALA) was observed SDS- PAGE gel electrophoresis. After analysis of milk composition of both camel milk and bovine milk showed camel milk lacks the β-lacto globulin protein while bovine milk contained 3500 mg/ml (Singh et al. 2006). The decrease in the concentration of casein like milk proteins leads to fading of protein bands due to proteolytic activity of LAB in fermented milk samples as shown in the protein bands of NCDC-15 (Vithana et al. 2012). In the present study, the peptides obtained with the lactic culture NCDC-15 with low molecular weight bands of 1.06 kDa showed fermentation of total protein which is in the agreement of earlier worker (González-Olivares et al. 2014). Vithana et al. (2012) has fermented deer milk and cow milk with Streptococcus salivarius sub spp. thermophilus, Lactobacillus casei strain Shirota and Lactobacillus delbrueckii sub spp. bulgaricus at 37 °C for 24 h. The protein decomposition of fermented samples by all the three strains was studied using SDS-PAGE and compared with the samples of unfermented deer and cow milk. They observed that after 24 h of incubation casein degradation was visible in the form of fading bands which confirmed by the SDS-PAGE analysis. NCDC-15 fermented sample showed a protein band with 1 kDa peptide (bradykinin) which might have ACE-inhibitory activity. Bradykinin was known as a natural ACE-inhibitory peptide.
Fig. 3.
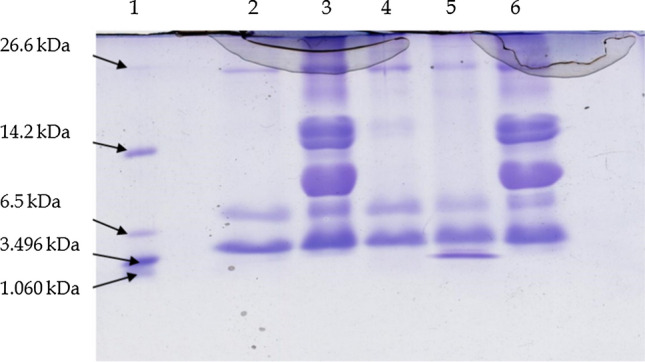
Protein profile of fermented camel milk revealed by SDS-PAGE [Lane 1, Marker; Lane 2-T1; Lane 3-T2; Lane 4-T3; Lane 5-T4 (NCDC-15); Lane 6-unfermented camel milk (Control) and T2, T3, and T are from different test cultures]
Conclusion
The L. acidophilus NCDC-15 used in this experiment was evaluated for the generation of ACE inhibitory peptides from camel milk by optimizing the growth conditions. This lactic culture has exhibited maximum PepX activity (0.655) and ACE-inhibitory activity (78.33%) at 12 h and 48 h of incubation at 37 °C respectively. Optimum proteolytic activity (0.976) was observed at 2% of inoculation for 12 h of incubation at 37 °C. 3 kDa permeates (48.01% peptides and 49.46% ACE-inhibition) and 10 kDa permeates (55.04% peptides and 42.40% ACE-inhibition) had produced maximum peptides and ACE-inhibitory activity. Total 10 fractions were obtained from RP-HPLC of 3 kDa (fractions (K1, L1, M1, N1, O1 and P1)) and 10 kDa permeates (5 fractions (S, T, U, V and W)). Overall, 24 peptides were identified from the samples of 3 kDa permeates (6 fractions) and 10 permeates. Novel peptide i.e. AIGPVADLHI was matched with k-casein in AHTPDB database, and GPV matched with the previously reported peptides with ACE inhibitory activity. Fermented camel milk with L. acidophilus NCDC-15 could be used for the preparation of camel milk-based beverages, nutraceuticals, or functional foods with anti-hypertensive activity. A combination of the culture and camel milk could be a novel food or therapeutic product with anti-hypertensive activity. Further, in vivo validation is required before claiming the health benefits.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Authors thankfully acknowledge the Plant Biotechnology Department, Anand Agricultural University, Anand, India for providing the LCMS facility for peptide sequencing.
Abbreviations
- ACE
Angiotensin I-Converting Enzyme
- AHTPDB
Database of antihypertensive peptides
- BLAST
Basic local alignment search tool
- EMS
Enhanced mass spectra scan
- EPI
Enhanced product ion
- ESI
Electron spray ionization
- Gly-Pro-pNA
Glycyl-prolyl p-nitroanilide
- HA
Hippuric acid
- HHL
N-hippuryl-histidne-leucine
- HL
Histydyl-leucine
- HPL
High performance liquid chromatography
- IDA
Information dependent analysis
- NCBI
National Centre for Biotechnology Information
- NCDC
National Collection of Dairy Culture
- OD
Optical density
- RP-LC/MS
Reversed phase liquid chromatography mass spectrometry
- PIR
Protein information resources
Authors' contribution
DS: Methodology, investigation, formal analysis, data curation, writing—original draft preparation. AS: data curation; formal analysis; methodology; project administration; resources; software; SP: data curation; formal analysis; writing—review and editing; methodology; SH: supervision, conceptualization, funding acquisition, investigation, project administration, resources, validation.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alhaj OA, Metwalli AA, Ismail EA, Ali HS, Al-Khalifa AS, Kanekanian AD. Angiotensin converting enzyme-inhibitory activity and antimicrobial effect of fermented camel milk (Camelus dromedarius) Int J Dairy Technol. 2018;71:27–35. doi: 10.1111/1471-0307.12383. [DOI] [Google Scholar]
- Anchala R, Kannuri NK, Pant H. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32(6):1170–1177. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker WC, Garavelli JS, Hou Z, Huang H, Ledley RS, Mc Garvey PB, Mewes HW, Orcutt BC, Pfeiffer F, Tsugita A, Vinayaka CR, Xio C, Yeh LSL, Wu C. Protein information resource: a common resource for expert annotation protein data. Nucleic Acids Res. 2001;29:29–32. doi: 10.1093/nar/29.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco-Castilla J, Hernández-Álvarez AJ, Jiménez-Martínez C, Jacinto-Hernández C, Alaiz M, Girón-Calle J, Vioque J, Dávila-Ortiz G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012;131:1157–1164. doi: 10.1016/j.foodchem.2011.09.084. [DOI] [PubMed] [Google Scholar]
- Donkor ON, Henriksson A, Singh TK, Vasiljevic T, Shah NP. ACE-inhibitory activity of probiotic yoghurt. Int Dairy J. 2007;17:1321–1331. doi: 10.1016/j.idairyj.2007.02.009. [DOI] [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin converting enzyme inhibitory activity in fermented milk. Dairy Sci Technol. 2007;86:21–38. doi: 10.1051/lait:2006023. [DOI] [Google Scholar]
- Dziuba M, Dziuba B, Iwaniak A. Milk proteins as precursors of bioactive Peptides. Acta Sci Pol Technol Aliment. 2009;8:71–90. [Google Scholar]
- El-Hatmi H, Jrad Z, Khorchani T, Jardin J, Poirson C, Perrin C, Cakir-Kiefer C, Girardet JM. Identification of bioactive peptides derived from caseins, glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1), and peptidoglycan recognition protein-1 (PGRP-1) in fermented camel milk. Int Dairy J. 2016;56:159–168. doi: 10.1016/j.idairyj.2016.01.021. [DOI] [Google Scholar]
- Girgih AT, He R, Aluko RE. Kinetics and molecular docking studies of the inhibitions of angiotensin converting enzyme and renin activities by hemp seed (Cannabis sativa L.) peptides. J Agric Food Chem. 2014;62:4135–4144. doi: 10.1021/jf5002606. [DOI] [PubMed] [Google Scholar]
- Gobbetti M, Minervini F, Rizzello CG. Angiotensin I-converting enzyme- inhibitory and antimicrobial bioactive peptides. Int J Dairy Technol. 2004;57:173–188. doi: 10.1111/j.1471-0307.2004.00139.x. [DOI] [Google Scholar]
- Gonzalez-Gonzalez C, Gibson T, Jauregi P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int J Food Microbiol. 2013;167:131–137. doi: 10.1016/j.ijfoodmicro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- González-Olivares LG, Añorve-Morga J, Castañeda-Ovando A, Contreras-López E, Jaimez-Ordaz J. Peptide separation of commercial fermented milk during refrigerated storage. Food Sci Technol (campinas) 2014;34:674–679. doi: 10.1590/1678-457X.6415. [DOI] [Google Scholar]
- Hafeez Z, Cakir-Kiefer C, Girardet J-M. Hydrolysis of milk-derived bioactive peptides by cell-associated extracellular peptidases of Streptococcus thermophilus. Appl Microbiol Biotechnol. 2013;97:9787–9799. doi: 10.1007/s00253-013-5245-7. [DOI] [PubMed] [Google Scholar]
- Hafeez Z, Cakir-Kiefer C, Girardet J-M. New insights into the proteolytic system of Streptococcus thermophilus: use of isracidin to characterize cell-associated extracellular peptidase activities. J Agric Food Chem. 2015;63:7522–7531. doi: 10.1021/acs.jafc.5b01647. [DOI] [PubMed] [Google Scholar]
- Hafeez Z, Cakir-Kiefer C, Lecomte X. The X-prolyl dipeptidyl-peptidase PepX of Streptococcus thermophilus initially described as intracellular is also responsible for peptidase extracellular activity. J Dairy Sci. 2019;102:113–123. doi: 10.3168/jds.2018-14823. [DOI] [PubMed] [Google Scholar]
- Haji Ghafarloo M, Jouki M, Tabari M. Production and characterization of synbiotic Doogh, a yogurt-based Iranian drink by gum arabic, ginger extract and B. bifidum. J Food Sci Technol. 2020;57:1158–1166. doi: 10.1007/s13197-019-04151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque E, Chand R (2006) Milk protein derived bioactive peptides. https://www.dairyscience.info/index.php/exploitation-of-anti-microbial-proteins/111-milk-protein-derived-bioactive-peptides.html. Accessed 14 Sept 2021
- Hati S, Sreeja V, Solanki J, Prajapati JB. Influence of proteolytic lactobacilli on ACE inhibitory activity and release of bioactive peptides. Indian J Dairy Sci. 2015;68:584–591. [Google Scholar]
- Hati S, Sakure A, Mandal S. Impact of proteolytic Lactobacillus helveticus MTCC5463 on Production of bioactive peptides derived from honey based fermented milk. Int J Pept Res Ther. 2017;23:297–303. doi: 10.1007/s10989-016-9561-5. [DOI] [Google Scholar]
- Hati S, Patel N, Mandal S. Comparative growth behaviour and biofunctionality of lactic acid bacteria during fermentation of soy milk and bovine milk. Probiotics Antimicrob Prot. 2018;10:277–283. doi: 10.1007/s12602-017-9279-5. [DOI] [PubMed] [Google Scholar]
- Indian Standards Methods of test for dairy industry part-II chemical analysis of milk. Indian Stand Inst New Delhi. 1961;18:1479. [Google Scholar]
- Jouki M, Khazaei N, Rezaei F, Taghavian-Saeid R. Production of synbiotic freeze-dried yoghurt powder using microencapsulation and cryopreservation of L. plantarum in alginate-skim milk microcapsules. Int Dairy J. 2021;122:105–133. doi: 10.1016/j.idairyj.2021.105133. [DOI] [Google Scholar]
- Kongo JM, Malcata FX. Acidophilus milk. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of food and health. New York: Academic Press; 2016. pp. 6–14. [Google Scholar]
- Kumar R, Chaudhary K, Sharma M, Nagpal G, Chauhan JS, Singh S, Gautam A, Raghava GPS. AHTPDB: a comprehensive platform for analysis and presentation of antihypertensive peptides. Nucleic Acids Res. 2015;43:956–962. doi: 10.1093/nar/gku1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunji ERS, Mierau I, Hagting A, Poolman B, Konings WN. The proteotytic systems of lactic acid bacteria. Anton Leeuw. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- Lisak K, Toro-Sierra J, Kulozik U, Bozanic R, Cheison SC. Chymotrypsin selectively digests β-lactoglobulin in whey protein isolate away from enzyme optimal conditions: potential for native α-lactalbumin purification. J Dairy Res. 2013;80:14–20. doi: 10.1017/S0022029912000416. [DOI] [PubMed] [Google Scholar]
- López-Fandiño R, Otte J, van Camp J. Physiological, chemical, and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J. 2006;6:1277–1293. doi: 10.1016/j.idairyj.2006.06.004. [DOI] [Google Scholar]
- Martínez-Maqueda D, Miralles B, Recio I, Hernández-Ledesma B. Antihypertensive peptides from food proteins: a review. Food Funct. 2012;3:350–361. doi: 10.1039/c2fo10192k. [DOI] [PubMed] [Google Scholar]
- Mati A, Senoussi-Ghezali C, Ahmed Zennia SS, Almi-Sebbane D, El-Hatmi H, Girardet JM. Dromedary camel milk proteins, a source of peptides having biological activities—a review. Int Dairy J. 2017;73:25–37. doi: 10.1016/j.idairyj.2016.12.001. [DOI] [Google Scholar]
- Meisel H. Biochemical properties of peptides encrypted in bovine milk proteins. Curr Med Chem. 2005;12:1905–1919. doi: 10.2174/0929867054546618. [DOI] [PubMed] [Google Scholar]
- Moslehishad M, Ehsani MR, Salami M. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int Dairy J. 2013;29:82–87. doi: 10.1016/j.idairyj.2012.10.015. [DOI] [Google Scholar]
- Nielsen MS, Martinussen T, Flambard B, Sorensen KI, Otte J. Peptide profiles and angiotensin-I-converting enzyme inhibitory activity of fermented milk products: effect of bacterial strain, fermentation pH, and storage time. Int Dairy J. 2009;19:155–165. doi: 10.1016/j.idairyj.2008.10.003. [DOI] [Google Scholar]
- Otte J, Shalaby SM, Zakora M, Pripp AH, El-Shabrawy SA. Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: effect of substrate, enzyme and time of hydrolysis. Int J Dairy Technol. 2007;17:488–503. doi: 10.1016/j.idairyj.2006.05.011. [DOI] [Google Scholar]
- Padghan PV, Mann B, Sharma R, Bajaj R, Saini P. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks (Lassi) fermented by Lactobacillus acidophillus with consideration of incubation period and simmering treatment. Int J Pept Res Ther. 2017;23:69–79. doi: 10.1007/s10989-016-9540-x. [DOI] [Google Scholar]
- Pan D, Luo Y, Tanokura M. Antihypertensive peptides from skimmed milk hydrolysate digested by cell-free extract of Lactobacillus helveticus JCM1004. Food Chem. 2005;91:123–129. doi: 10.1016/j.foodchem.2004.05.055. [DOI] [Google Scholar]
- Panchal G, Sakure A, Hati S. Peptidomic profiling of fermented goat milk: considering the fermentation-time dependent proteolysis by Lactobacillus and characterization of novel peptides with antioxidative activity. J Food Sci Technol. 2021 doi: 10.1007/s13197-021-05243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H, Hati S, Sakure A. In vitro and in silico analysis of novel ACE-inhibitory bioactive peptides derived from fermented goat milk. Int J Pept Res Ther. 2018;24:441–453. doi: 10.1007/s10989-017-9630-4. [DOI] [Google Scholar]
- Parmar H, Hati S, Panchal G, Sakure AA. Purification and production of novel angiotensin I-converting enzyme (ACE) inhibitory bioactive peptides derived from fermented goat milk. Int J Pept Res Ther. 2020;26:997–1011. doi: 10.1007/s10989-019-09902-7. [DOI] [Google Scholar]
- Rahimi M, Ghaffari MS, Salami M, Mousavy SJ, Niasari-Naslaji A, Jahanbani A, Yousefinejad S, Khalesi M, Moosavi-Movahedi AA. ACE- inhibitory and radical scavenging activities of bioactive peptides obtained from camel milk casein hydrolysis with proteinase K. Dairy Sci Technol. 2016;96:489–499. doi: 10.1007/s13594-016-0283-4. [DOI] [Google Scholar]
- Rodriguez-Figueroa JC, González-Córdova AF, Torres-Llanez MJ, Garcia HS, Vallejo-Cordoba B. Novel angiotensin I-converting enzyme inhibitory peptides produced in fermented milk by specific wild Lactococcus lactis strains. J Dairy Sci. 2012;95:5536–5543. doi: 10.3168/jds.2011-5186. [DOI] [PubMed] [Google Scholar]
- Sathya P, Radha K, Sathian CT, Srinivasan C. Angiotensin converting enzyme inhibitory activity from fermented goat milk produced with different lactic acid bacteria. Int J Curr Microbiol Appl Sci. 2017;6:1670–1676. doi: 10.20546/ijcmas.2017.612.188. [DOI] [Google Scholar]
- Savijoki K, Ingmer H, Varmanen P. Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol. 2006;71:394–406. doi: 10.1007/s00253-006-0427-1. [DOI] [PubMed] [Google Scholar]
- Selwal KK, Selwal MK. Evaluation of viability and “in vitro” probiotic properties of Lactobacillus acidophilus NCDC 15 culture before and after freeze drying. Minerva Biotecnol. 2014;26:137–142. [Google Scholar]
- Shariati Z, Jouki M, Rafiei F. Flavored functional drinking yogurt (Doogh) formulated with Lactobacillus plantarum LS5, cress seed gum, and coriander leaves extract. Food Sci Nutr. 2020;8:894–902. doi: 10.1002/fsn3.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran OP, Tonk DS, Kaushik LS, Hasija RC, Pannu RS (1998) Statistical software package for agricultural research workers. Recent advances in information theory, statistics & computer applications by D.S. Hooda & R.C. Hasija, Department of Mathematics Statistics, CCS HAU, Hisar (139–143). http://14.139.232.166/opstat/
- Singh R, Ghorui SK, Sahani MS. Camel milk: properties and processing potential. In: Sahani MS, editor. The Indian camel. Bikaner: NRCC; 2006. pp. 59–73. [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki D, Hati S. Considering the potential of Lactobacillus rhamnosus for producing Angiotensin I-Converting Enzyme (ACE) inhibitory peptides in fermented camel milk (Indian breed) Food Biosci. 2018;23:16–22. doi: 10.1016/j.fbio.2018.03.004. [DOI] [Google Scholar]
- Solanki D, Hati S, Sakure A. In silico and in vitro analysis of novel angiotensin I-converting enzyme (ACE) inhibitory bioactive peptides derived from fermented camel milk (Camelus dromedarius) Int J Pept Res Ther. 2017;23:441–459. doi: 10.1007/s10989-017-9577-5. [DOI] [Google Scholar]
- Soleymanzadeh N, Mirdamadi S, Mirzaei M, Kianirad M. Novel β-casein derived antioxidant and ACE-inhibitory active peptide from camel milk fermented by Leuconostoc lactis PTCC1899: Identification and molecular docking. Int Dairy J. 2019;97:201–208. doi: 10.1016/j.idairyj.2019.05.012. [DOI] [Google Scholar]
- Tagliazucchi D, Martini S, Bellesia S, Conte A. Identification of ACE-inhibitory peptides from Phaseolus vulgaris after in vitro gastrointestinal digestion. Int J Food Sci Nutr. 2015;66:774–782. doi: 10.3109/09637486.2015.1088940. [DOI] [PubMed] [Google Scholar]
- Tripathi V, Jha YK. Development of whey beverage with antagonistic characteristics and probiotics. Int J Food Prop. 2004;7:261–272. doi: 10.1081/JFP-120030037. [DOI] [Google Scholar]
- Vasiljevic T, Jelen P. Lactose hydrolysis in milk as affected by neutralizers used for the preparation of crude β-galactosidase extracts from Lactobacillus bulgaricus 11842. Innov Food Sci Emerg. 2002;3:175–184. doi: 10.1016/S1466-8564(02)00016-4. [DOI] [Google Scholar]
- Vithana ONL, Mason SL, Bekhit AEA, Morton JD. In vitro digestion of Red Deer (Cervus elaphus) and Cow (Bos taurus) milk. Int Food Res J. 2012;19:1367–1374. [Google Scholar]
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Wang X, Hu Z. Identification and characterization of an angiotensin-converting enzyme inhibitory peptide derived from bovine casein. Peptides. 2018;99:161–168. doi: 10.1016/j.peptides.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Zhou B, Carrillo R, Danaei G, Riley L, Paciorek C, Stevens G, Gregg E, Bennett J, Solomon B, Singleton R, Sophiea M, Iurilli M, Lhoste V, Cowan M, Savin S, Woodward M, Balanova Y, Damasceno A. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.