Abstract
The main aim of this study was to deliver green tea catechins with enhanced bioavailability using niosomal system. Catechins-loaded niosomes were prepared using food grade surfactant, Tween 60 and membrane stabilizers namely, lauryl alcohol, cetyl alcohol and cholesterol by thin film hydration technique. Catechins-loaded niosomes exhibited a hydrodynamic diameter of 58.48 nm with a narrow size distribution (PDI = 0.13) and zeta potential of − 31.75 mV, suggestive for homogeneity and good stability. Niosomes entrapped about 85.82% of catechin and showed sustained release under simulated GI conditions. Morphology of niosomal vesicles were carried out using scanning electron microscopy-energy X-ray dispersion spectroscopy, transmission electron microscopy and atomic force microscopy. Fourier-transform infrared spectroscopy and High-performance liquid chromatography analysis confirmed successful encapsulation of catechins. Antioxidant activity of catechins was retained in the niosomal form. Fortification of milk with catechins loaded niosomes showed no significant changes on sensory, physicochemical properties and exhibited higher antioxidant property.
Keywords: Green tea catechins, Milk, Entrapment efficiency, Fortification, Thin film hydration, Antioxidant property
Introduction
Catechins, belonging to flavonoids known as flavan-3-ols family, are abundantly present in green tea. They possess numerous health benefits such as antioxidative, anti-inflammatory, anti-carcinogenic, anti-proliferative, anti-hypertensive, anti-thrombogenic and lipid-lowering effects (Liang et al. 2016). Green tea catechins have drawn considerable interest in researchers, manufacturers and consumers because of their promising nutraceutical properties and have already been used in many fields including cosmetic, pharmaceutical and food industry (Song et al. 2014). However, they have a low bioavailability, poor absorption, low aqueous solubility, unstable in the digestive tract and readily get degraded in alkaline pH, which restrict in realizing their potential health benefits (Ahmad et al. 2018). To overcome these hurdles, various nanoencapsulation strategies have been used, which included embedding of Epigallocatechin gallate (EGCG) in polysaccharide matrix such as gum arabic and maltodextrin, with EGCG being released in 3 h in intestinal tract (Rocha et al. 2011). Similarly, it was reported that green tea catechins and EGCG liposomes incorporated in low fat hard cheese effectively retained the antioxidant activity after digestion (Rashidinejad et al. 2016). Also, bioactive caseinophosphopeptide and chitosan nanoparticles manifested controlled release and enhanced antioxidative property (Hu et al. 2013), while β-lactoglobulin nanoparticles (Li et al. 2012a, b) and catechins loaded in starch-based nanoparticles showed enhanced bioavailability and stability (Ahmad et al. 2018).
Niosomes are novel kind of vesicular system with a closed bilayer structure formed by self-assembly of non-ionic surfactants and stabilizers, with their hydrophilic end towards the aqueous phase between the bilayers and the hydrophobic ends face each other within the surfactant bilayer. These vesicles can entrap both hydrophilic and hydrophobic compounds. The main advantages of niosomes when compared with other encapsulation techniques include low cost, high stability and biodegradability, besides being nontoxic and can deliver the entrapped bioactive to the target site in a sustained and controlled manner so that their bioavailability is improved (Bhardwaj et al. 2020). Resveratrol and quercetin loaded niosomes using non-ionic surfactants and fatty alcohol by using thin film hydration and sonication methods exhibited controlled release under simulated gastrointestinal (GI) conditions, lower toxicity and high intracellular reactive oxygen species (ROS) scavenging activity when compared to free resveratrol (Vankayala et al. 2018). Iron-entrapped niosomes formulated with Span 80 and lauryl alcohol by ethanol injection method showed high encapsulation efficiency (72–84%) and stability, and when fortified in yoghurt, the textural and rheological properties were similar to regular yoghurt (Gutiérrez et al. 2016). EGCG niosomes prepared using Tween 60 and cholesterol improved the chemical stability and antioxidant activity of EGCG in GI tract (Liang et al. 2016). Catechin and EGCG-loaded niosomes enhanced the cellular uptake and transport across human intestinal Caco-2 cell monolayer, provided chemical stability and exhibited lower toxicity as compared to free catechin and EGCG (Song et al. 2014).
With this aim the present study, catechin entrapped niosomes were prepared to improve its bioavailability and circumvent metabolic degradation by reducing the particle size to less than 100 nm using thin film hydration technique. Nanoparticles are prone to hold the advantage of uptake of first pass metabolism of bioactives. Nanoparticles with smaller size of 100—400 nm facilitate the cellular uptake (El-Sherbiny et al. 2015), preferentially bypass via “M cells” in Peyer patches by the mechanism of lymphoid uptake (endocytosis), consequently delivering the bioactive nanoparticles directly into lymphatic systematic circulation and circumventing metabolic degradation (Mittal et al. 2007; Managuli et al. 2018). They improve the therapeutic performance by delayed clearance from the transmission and protect the bioactives from biological environment (Khindri et al. 2015; Ravalika and Sailaja 2017). Tween 60, a common food grade non-ionic surfactant approved by Food and Drug Administration (FDA), lauryl alcohol, cetyl alcohol and cholesterol (as membrane stabilizer) were selected to prepare niosomes. The formulated niosomes were characterized for mean hydrodynamic diameter, size distribution, zeta potential, entrapment efficiency, morphology, simulated GI release and antioxidant activity. The main objective of this study was to utilize them for fortification of milk.
Materials and methods
Materials
Green tea catechins of > 97% purity, Tween 60, lauryl alcohol, cetyl alcohol and cholesterol were purchased from TCI Chemicals Pvt. Ltd. Chennai, India. Trifluroacetic acid (TFA) and methanol were obtained from Merck (Merck, Germany). Dihexadecyl phosphate (DCP), ethanol and phosphate buffered saline were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). Triton X-100 and 2,2, diphenyl-1-picrylhydrazyl radical (DPPH) of analytical reagent grade were purchased from HiMedia Laboratories Pvt. Ltd., (Mumbai, India). Milli-Q water of 18.2 Ω was used in preparation and analysis of catechins-loaded niosomes.
HPLC chromatographic conditions
The analytical High performance liquid chromatography (HPLC) (model e2695, Waters Corporation, Milliford, MA, USA) comprising of autosampler, temperature control module and photodiode array detector (model 2998) was used for detection of catechins by using E Pro and Empower 3 Software. The chromatographic separation was performed using C18 column (length of 250 × 4.6 mm and particle size of 5 μm; Phenomenex, USA) fitted with guard column (10 × 3 mm). About 20 µL of the sample was injected into the column at a flow rate of 0.8 mL/min. The catechin derivatives were eluted at 25 °C, and were detected at UV wavelength of 273 nm.
Preparation of standard catechins solution for HPLC analysis
A stock solution containing catechins was prepared by dissolving a known amount in a mixture of 20% methanol in water (v/v) to get a concentration of 250 μg/mL. Standard solution with a concentration of 50 μg/mL was prepared by diluting the stock solution using same solvent and it was filtered through a 0.22 μm syringe filter. The concentration of catechin niosomes was maintained same as that of free catechins and the entrapped catechin in the nanovesicles was disrupted using 20% Triton X-100 in methanol.
Preparation of catechins-loaded niosomes
Catechins loaded niosomes were prepared by thin film hydration method with slight modifications. Song et al. (2014). Tween 60 at 250 μM concentration and lauryl alcohol was used as non-ionic surfactant (NIS) and membrane stabilizer. The NIS and stabilizer, taken in 1.5:1 ratio in rotary flask along with dihexadecyl phosphate (0.005 mg), were dissolved in 100 mL of ethanol and the solvent was rotary-evaporated (Rota Vapor R3, Buchi AG, Flawil, Switzerland) at 55 °C and vacuum at 600 mg, until a thin dry film was formed on the inner wall of the flask. The film was further dried in an oven at 50 °C for an hour. Later, 100 mL of phosphate buffer saline (pH 7.4) containing catechins (0.25 mg/mL) was added to hydrate the film for 2 h to form catechins-loaded niosomes. The niosome solution was heated at 70 °C and subjected to high shear homogenization (Unidrive X1000D, CAT GmbH, Germany) at 27,670 g for 15 min. The resultant niosomal suspension was set aside at room temperature to allow for the vesicle membrane to anneal and for the foam to escape. Catechins loaded niosomes were also prepared using other two stabilizers such as cetyl alcohol and cholesterol in similar manner. During preparation, the working solutions were protected from exposure to light. Blank niosomes were also prepared using the same method without incorporation of catechins.
Characterization of catechins-loaded niosomes
Hydrodynamic diameter and zeta potential
Hydrodynamic diameter, polydispersity index (PDI) and zeta potential (ZP) of blank, catechins loaded niosomes were determined by dynamic light scattering (DLS) technique using Litesizer TM 500 (Anton Paar, GmbH, Pvt. Ltd., Austria). The niosomal solution was diluted with Milli-Q water (1:10) to get transmittance above 85%, and it was homogenized for 10 min at 27,670 g to get uniform suspension before measurement. Measurements were done in triplicate at 25 °C using the polystyrene cuvette at a back-scattering angle of 173°.
Entrapment efficiency
The unentrapped free catechins were separated from the niosomes by dialysis method with minor modifications (Vankayala et al. 2018). Exactly 3 mL of niosomal suspension was loaded into the dialysis membrane bag (molecular weight cut off was 12.4 kDa), clipped at both ends, and it was dialyzed using 500 mL of milli-Q-water for 2 h with gentle stirring on a magnetic stirrer. Later, 3 mL of the dialysate was collected and the free catechin was determined by measuring the optical density using UV–VIS spectrophotometer (UH5300, Hitachi High Technologies Corporation, Tokyo, Japan) at 273 nm. The concentration of catechins present in the samples was calculated from the standard curve with the concentration range of 0–40 µg/mL. The linear regression equation y = 0.025x + 0.004 with R2 = 0.999 was obtained from the standard curve (where y = absorbance at 273 nm, x = concentration of catechin µg/mL). The entrapment efficiency (EE) was calculated using Eq. 1.
| 1 |
where CT is the total amount of catechins remaining in the dialysis bag and CF is free or unentrapped catechins. The amount of entrapped catechin nanovesicles remaining in the dialysis bag was collected by disrupting the niosomes using 20% Triton X-100 in methanol, and the concentration was determined as per the above-mentioned spectrophotometric method.
Morphological characterization
Scanning electron microscopy- energy X-ray dispersion spectroscopy
The surface morphology of niosomes was monitored using field emission scanning electron microscope (FESEM) (Zeiss Ultra 55 with patented GEMINI column technology, Carl Zeiss AG, GmbH, Oberkochen, Germany). The silicon wafers, placed on aluminium stub with the help of a double-sided adhesive carbon tape, were cleaned with nitrogen flushing. The samples were placed on silicon wafer and dried under room temperature. The vesicles were sputter-coated with gold and palladium (80:20) in single cycle under vacuum using Q150R ES Turbo, pumped with a top-of-the-range sputter coater (Quorum Technologies Ltd, Laughton, Sussex, UK). Morphological features were observed and recorded at 5 kV and aperture size of 30 μm.
The Energy dispersion X-ray spectroscopy (EDS) (Zeiss GEMINI Ultra 55, Carl Zeiss AG, GmbH, Oberkochen, Germany) coupled to FESEM was used to get the detailed information on the spectra and elemental composition from the niosomes.
Transmission electron microscopy
The niosomal suspension was also observed through transmission electron microscopy (HRTEM – JEOL – JEM2100, Japan). A drop of the niosomal formulation was placed on a carbon-coated copper grid, and the excess sample was removed with filter paper. Later, a drop of 1% (w/v) phosphotungstic acid solution was applied to the carbon grid and left to stand for 2 min. Once the excess staining agent was removed with filter paper, the sample was air-dried and observed in the transmission electron microscope.
Atomic force microscopy
The morphological characteristics of catechins-loaded niosomes were analysed using atomic force microscope(AFM) (Park NX20, Park system Inc, Santa Clara, California, USA) with slight modifications (Peres et al. 2011). A drop of niosome samples was drop-casted on 0.17 mm cover glass and it was dried in vacuum desiccator for 96 h. The two- and three-dimensional images of the catechin niosomes was scanned with silicon probe with a constant spring of 0.4 Nm−1. The surface roughness (Ra) and square root thickness (Rb) were calculated using the following Eqs. 2 and 3, respectively.
| 2 |
| 3 |
where N is the number of sampling evaluation lengths, Zi and Zaverage are the ith highest peak, and lowest valley, respectively.
Fourier-transform infrared spectroscopy
A drop of sample was added using a Pasteur pipette on the crystal plate such that it covers the crystal for measurement. The Fourier-transform infrared spectroscopy (FTIR) spectrum was recorded in a spectrophotometer (Perkin Elmer Frontier, TL8000 TG-IR interface, USA) at a resolution of 4 cm−1 and 16 scans/min in the wavenumber region of 400–4000 cm−1. The FTIR spectra were recorded for all the encapsulants used in the formulations.
HPLC analysis of catechins before and after encapsulation into niosomes
HPLC was used to separate and identify the components of catechins used for preparation of niosomes and to confirm whether the catechins were successfully loaded into niosomes. Prior to analysis, all the solvents and solutions (mobile phase, standards and samples) were filtered through 0.22 µm syringe filter and then degassed in a sonicator (GT Sonic, China) for 15 min. Once a stable baseline was obtained, 20 µL of sample was injected and run with isogradient for 30 min. The flow rate was adjusted to 0.8 mL/min and the catechins were eluted from the column at room temperature (~ 30 °C). The compounds were identified by comparison with the standards and their elution times.
In-vitro release of catechins-loaded niosomes
In-vitro release studies under simulated GI tract pH were conducted by dialysis method developed by Liang et al. (2016) with slight modifications. Five mL of niosomal suspension was loaded into a cellulose membrane dialysis bag, clipped at both ends and immersed in 150 mL of solution maintained at pH 1.2 using 0.1 N hydrochloric acid containing 3.2 mg of pepsin for 2 h and later in 150 ml of phosphate buffer solution containing 3.2 mg of pancreatin at a pH 7.4 for 22 h representing the gastric and small intestinal environmental conditions, respectively with gentle stirring at 37 °C. In addition, the release studies were also carried out for free catechins taken at concentration similar to those of niosomes. Free catechin was diluted in a solution containing 12% (v/v) of ethanol to maintain the sink conditions. Aliquots of the samples in the dialysis bags were collected at predetermined time intervals (0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h) and, the volume drawn was replaced with the same amount of fresh solution. Concentration of catechins in the samples was determined from standard curve as discussed before.
Antioxidant activity
The antioxidant activity of free catechins and catechins-loaded niosomes was determined by DPPH radical scavenging assay according to the method reported by (Zaiter et al. 2016) with some modifications. Exactly 0.4 mL of each sample solution in the concentration range of 20–100 μg/mL was added to 2 mL of anhydrous ethanol, and the contents were vigorously mixed for 3 min. Later, 1.6 mL of 100 μM DPPH solution in ethanol was added. The reaction mixture was incubated in the dark for 30 min, and the reduction in absorbance was recorded at 517 nm against the blank using the UV–Vis spectrophotometer. For positive control, 1.6 mL of DPPH solution in ethanol was mixed with 2 mL of ethanol, whereas the blank was taken as anhydrous ethanol. The percentage DPPH radical scavenging activity (RSA) was calculated using Eq. 4.
| 4 |
where A is the absorbance of control after reaction, B is the absorbance of samples after the reaction and C is the absorbance of the blank solution.
Fortification of milk with catechins loaded niosomes
Exactly 12 mg of catechins in the form of niosomes was added to 240 mL of pasteurized toned milk which contains 30 g of fat kg−1and 85 g of solid not fat kg−1. Control sample of milk was prepared without incorporation of niosomes. The sensory evaluation of milk samples was performed using 9-point hedonic scale (where 1 = dislike extremely, 5 = neither like nor dislike, 9 = like extremely) by selected group of panelists consisting of 10 members (six males and four females) among scientists, technical officers/assistants and graduate students of the institute. Physico-chemical characteristics of milk such as pH was determined according to AOAC method No. 981.12, titratable acidity (TA, AOAC No. 947.05) was assessed using the titration method and viscosity was measured as per AOAC method No. 974.07 (AOAC 2019). In-vitro release of catechin and antioxidant activity from fortified milk was determined similar procedure as described above. The colour parameters of the fortified milk were derived using image analysis. The samples (10–15 g) were accurately weighed and spread uniformly over a sterile petridish (50 × 15 mm); placed on the flat bed of the scanner (Canoscan 9000F mark II) and the scanned images of the samples were acquired following the protocol standardized by (Wasnik et al. 2019). The images were imported to Adobe Photoshop and measured as whitening index.
Statistical analysis
Statistical analysis was performed using SPSS.23.0 (IBM SPSS, Chicago, USA) software, and the data were assessed using one-way or two-way analysis of variance (ANOVA) and the treatment means were compared using Post hoc Duncan’s multiple range test at 0.05 significance level. The results are presented as means ± standard deviation. All the experiments were performed in triplicate.
Results and discussion
Hydrodynamic diameter, polydispersity index and zeta potential
Hydrodynamic diameter, polydispersity index and zeta potential of catechins loaded niosomes prepared using lauryl alcohol as stabilizer showed lowest hydrodynamic diameter 58.48 nm than those prepared with cetyl alcohol (449.86 nm) and cholesterol (265.8 nm) (Table 1). Lauryl alcohol, a transparent liquid might have interacted to a higher extent with non-ionic surfactant and hydrophilic catechins than cetyl alcohol, which is a white crystalline solid. Bandyopadhyay and Neeta (2007), observed formation of spontaneous bilayer vesicles of bioactives was prepared using 1:1 ratio of Span 60 and fatty alcohols such as stearyl alcohol, cetyl alcohol and lauryl alcohol in ethanol. Li et al. (2012a, b) prepared catechins and EGCG niosomes with 150 µM concentration of Span 60 and cholesterol at 10:9 molar ratio using thin film hydration showed a particle size of 204 and 235 nm, respectively. Catechins and EGCG loaded niosomes with Span 60 and cholesterol using thin film hydration technique, and achieved the particle size of 100 nm (Song et al. 2014). These results clearly suggested that the particle size of the niosomes depend upon the type of stabilizer used. Polydispersity index of all the blank and catechin samples was less than 0.3 which indicates size of vesicles was relatively homogeneous and monodispersity. Zeta potential of all catechins loaded niosomes was found to be negative, indicated good stability and less agglomeration between the niosomal vesicles. The negative charge on the niosomes might be due to the interaction of Tween 60, stabilizer and catechins themselves (Mazyed et al. 2021). Ahmad et al. (2018), observed negative zeta potential on catechin nanoparticles prepared using different types of starch.
Table 1.
Effect of fatty alcohol on hydrodynamic diameter, PDI*, zeta potential and entrapment efficiency of blank and catechin loaded niosomes
| Characteristics | Sample | Lauryl alcohol | Cetyl alcohol | Cholesterol |
|---|---|---|---|---|
| Hydrodynamic diameter (nm) | Blank | 77.98 ± 4.31a | 382.69 ± 7.25b | 286.38 ± 3.60c |
| Catechin | 58.48 ± 8.39a | 449.86 ± 3.76b | 265.8 ± 8.91c | |
| PDI | Blank | 0.17 ± 2.12a | 0.25 ± 1.35b | 0.26 ± 1.38b |
| Catechin | 0.13 ± 1.43a | 0.30 ± 1.99b | 0.28 ± 2.26b | |
| Zeta potential (mV) | Blank | − 26.49 ± 0.75a | − 24.92 ± 1.89a | − 27.70 ± 1.42a |
| Catechin | − 31.75 ± 3.05a | − 26.53 ± 1.30a | − 28.29 ± 1.05a | |
| Entrapment efficiency (%) | Blank | – | – | – |
| Catechin | 85.82 ± 1.85a | 70.00 ± 2.00b | 64.55 ± 4.76c |
*Polydispersity Index, Values in rows with different superscripts with small letters (a, b and c) differ significantly (p < 0.05). Results are expresses as (mean ± SD, n = 4)
Entrapment efficiency
Entrapment efficiency of catechins-loaded niosomes is summarized in (Table 1). Higher entrapment efficiency of 85.82% was observed for catechin niosomes prepared using lauryl alcohol followed by cetyl alcohol (70.00%) and cholesterol (64.55%). These results clearly suggest that entrapment efficiency depended on the type of stabilizer used for preparation of niosomes. Li et al. (2012a), reported encapsulation efficiency of 60 − 70% for β-Lg-EGCG nanoparticles. Li et al. (2012b) observed, catechin or EGCG niosomes prepared with Span 60 using cholesterol by thin film hydration showed encapsulation efficiency of 49.48 or 53.05%, respectively. Based on the particle size and entrapment efficiency data, catechins-loaded niosomes prepared using Tween 60 and lauryl alcohol, was selected for further characterization.
Morphological characterization
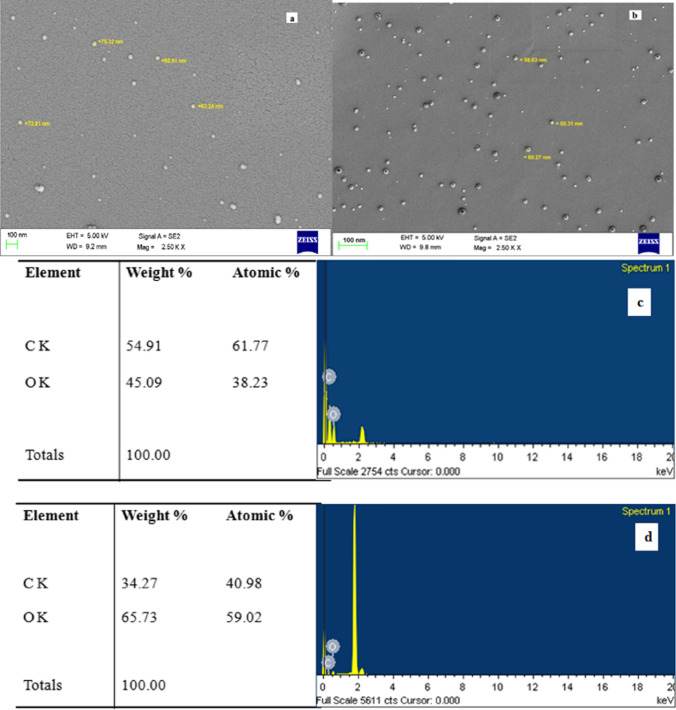
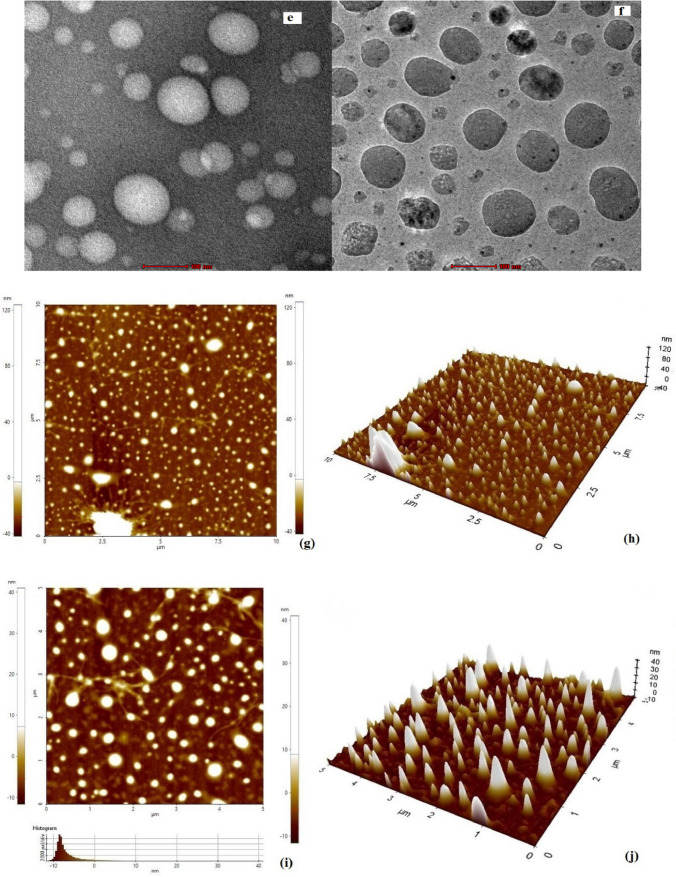
The SEM images of blank and catechins-loaded niosomes prepared using Tween 60 and lauryl alcohol are shown in Fig. 1a, b. It is evident that the vesicles were monodispersed, exhibiting spherical structure, smooth shape and narrow particle size distribution. The surface was apparently free from visible pores and cracks. Mean particle size of the niosomes prepared using Tween 60 and lauryl alcohol was 58 nm. The SEM images also showed the distribution of catechins (hydrophilic and darker area) embedded by non-ionic surfactant and stabilizer (lighter area). The EDS spectra of blank and catechins-loaded niosomes prepared with Tween 60 and lauryl alcohol are illustrated in Fig. 1c, d. The EDS images revealed the major elements were carbon and oxygen in blank and catechin niosomes. Higher oxygen and lower carbon were observed in catechin niosomes as expected that might be due to 5 hydroxyl groups of catechins attributed to interaction between the functional groups of catechins and encapsulants. The TEM image (Fig. 1e, f) of catechin niosomes prepared with Tween 60 and lauryl alcohol also showed that vesicles were homogeneous and monodisperse in nature. Liang et al. (2016) also reported based on the TEM image of EGCG niosomes were monodisperse and the size correlated with DLS results.
Fig. 1.
Images of Scanning electron microscopy (a, b), Scanning electron microscopy- Energy X-ray dispersion spectroscopy (c, d), Transmission electron microscopy (e, f) and two dimensional (g, i) and three dimensional (h, j) of Atomic force microscopy of blank and catechin loaded niosomes
Surface morphology of catechins-loaded niosomes was also obtained by atomic force microscopy. The two dimensional and three-dimensional images (Fig. 1g–j) of AFM represent the shape and size of catechin niosomes The AFM images showed that vesicles of catechin niosomes were spherical and uniformly distributed, with a hydrodynamic diameter range of 40–60 nm. The roughness (Ra) value for blank and catechin niosomes were 75.1 and 67 nm, while the corresponding thickness (Rb) values were 65 and 53.2 nm respectively. These findings are consistent with the results obtained by both dynamic light scattering and scanning electron microscopy, suggesting that stable, catechins-loaded niosomes could be successfully prepared using lauryl alcohol and Tween 60. Zou et al. (2014) prepared EGCG nanoliposomes using ethanol injection method combined with dynamic high-pressure microfluidization, and reported that the vesicles were spherical-shaped with diameter of 100 nm. Peres et al. (2011) also observed that the carbohydrate coated EGCG nanoparticles showed similar diameters when analyzed with DLS and AFM. Hu et al. (2013) studied the surface morphology of the EGCG-loaded chitosan-caseinophosphopeptide nanoparticles. The particle size obtained with AFM was smaller than that with DLS method, which might be due to the process involved and the physical state of samples used for analysis.
Fourier-transform infrared spectroscopy
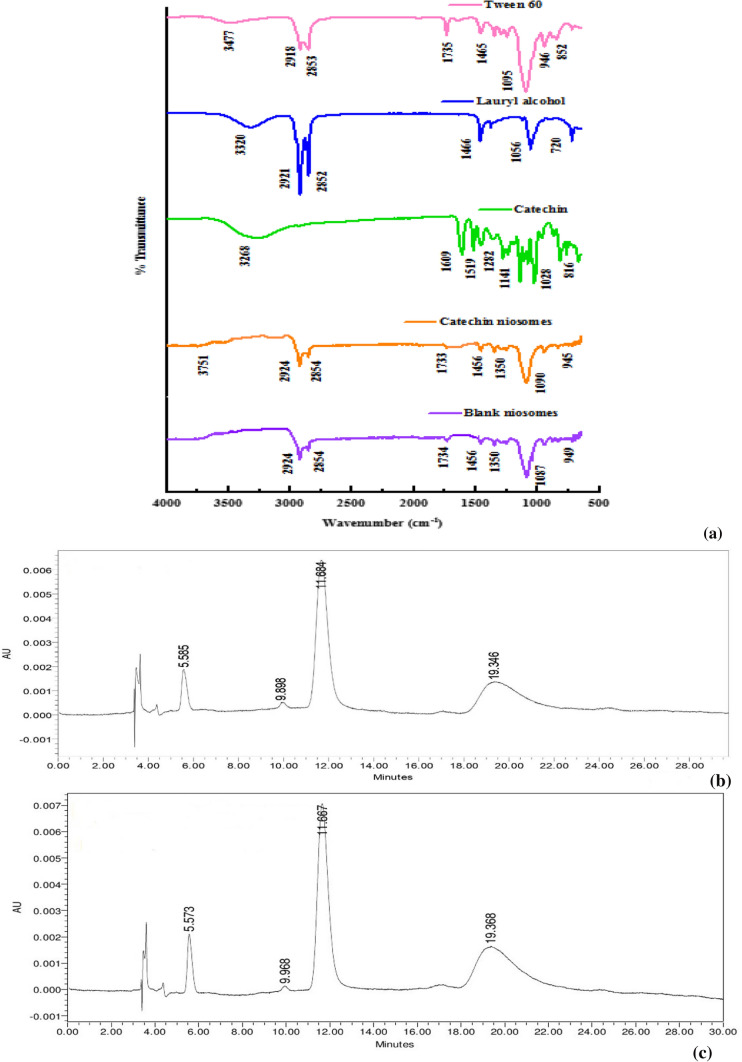
Fourier-transform infrared spectroscopy (FTIR) was used to ascertain the loading of catechins into niosomes and to understand the possible interactions among the non-ionic surfactant, stabilizer and catechins. The FTIR spectra of the encapsulants such as Tween 60 and lauryl alcohol, and those of catechins, blank niosomes and catechins-loaded niosomes (CNT60) were depicted (Fig. 2a). The finger print regions of Tween 60 were observed at 3477 and 1641; 2918 and 2853, 1735, 1095, 946 and 852 cm−1, which corresponded to the presence of its -OH, -C–H, -C=O, -C–O–O–C- and -C–O–C- bond stretchings, respectively. The FTIR spectra of lauryl alcohol clearly showed a broad band at 3320 cm−1, representing the stretching of hydroxyl group, while the two main characteristic peaks at 2921 and 2852 cm−1 belonged to asymmetric and symmetric –C–H- stretching vibrations of methylene group, respectively. A strong characteristic peak was observed at 1466 cm−1 and a weak characteristic peak at 1378 cm−1, which represented the –C–H- asymmetric bending vibration of methylene group. The absorption peak at 1056 cm−1 was assigned to -C–O- symmetric stretching vibration of –C–O–C, while the peak at 721 cm−1 was related to rocking vibration of methylene group (Huai et al. 2013; Memon et al. 2013).
Fig. 2.
Fourier transform-infrared spectroscopy of catechin loaded niosomes (a) HPLC chromatogram of catechin derivatives before (b) and after (c) encapsulation into niosomes prepared with Tween 60 and lauryl alcohol
The spectrum of catechins exhibited characteristic peaks related to the -OH stretching at 3600–3200 cm−1 and the presence of aromatic ring quadrant at 3268 cm−1, -OH deformation of aromatic alcohol at 1609 cm−1, -C–C- stretching at 1600–1500 cm−1, -C–O stretching of aromatic and aliphatic secondary alcohol in the oxygen ring at 1282 and 1141 cm−1, -C–O stretch at 1078 cm−1 and aromatic ring between 1200 and 800 cm−1 characteristic bands (Pool et al. 2012).. In blank and catechins-loaded niosomes, the characteristic peaks of Tween 60 at 2854, 1456, 1087, 949 and 845 cm−1, and that of lauryl alcohol at 1456, 2852, 2924 cm−1 were observed, which indicated that both Tween 60 and lauryl alcohol were present in the niosomes. However, in catechins-loaded niosomes, -OH stretching band (3600–3200 cm−1) was slightly shifted to 3751 cm−1 with increased energy absorption, confirming that catechins were associated with lauryl alcohol by hydrogen bonds. The missing –C=O peaks of Tween 60 and lauryl alcohol at 1735 and 1056 cm−1, and shifting of the strong characteristic peak of -CH bending vibration of methylene group of lauryl alcohol at 1466 cm−1 to 1456 cm−1, demonstrated the new hydrogen bonds were formed in the vesicles.
HPLC analysis of catechin before and after encapsulation into niosomes
The catechins used for preparation of niosomes were analysed before and after encapsulation using HPLC. It was observed from Fig. 2b, c that the catechins used for preparation of niosomes consisted of four main derivatives such as C, EC, EGCG and ECG, and their peaks were observed at retention times of 5.585, 9.898, 11.684 and 19.346 min, respectively. The results also showed that catechins were successfully loaded into niosomes, and their peaks after encapsulation were observed with a slight shift in the retention times to 5.573, 9.968, 11.667 and 19.368 min, respectively.
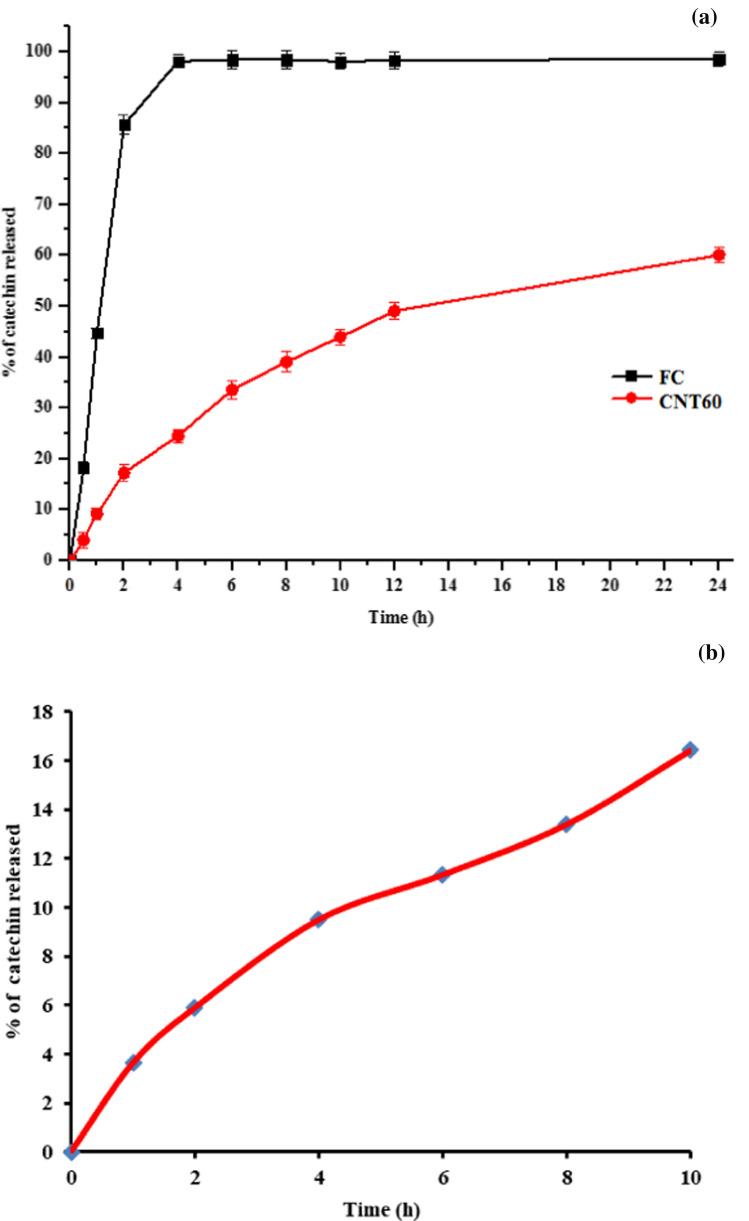
In vitro release studies of catechins niosomes under simulated GI conditions
In vitro release studies of free catechins and catechins-loaded niosomes were conducted using 12 kDa dialysis membrane under simulated gastric and intestinal conditions at pH 1.2 and 7.4, respectively for 24 h. The release pattern of free catechins and catechins from loaded niosomes are presented in Fig. 3a. During the first 2 h at simulated gastric pH (1.2), rapid or ‘burst’ release of > 85% occurred with free catechins. Li et al. (2012a, b) observed fast diffusion of > 90% of free catechins in 2 h. Similarly, Liang et al. (2016) also reported only 10% of EGCG remained after 3.5 h at pH 2. Later, under simulated intestinal pH (7.4), the total catechin released was 98.47% in 6 h.
Fig. 3.
In-vitro release of free catechin, catechin loaded niosomes (a) and fortified milk (b)
In comparison to its free form, only 17.22% of catechins were released from the niosomes in 2 h at pH 1.2, and only 24.52% of catechins were released from the niosomes at intestinal pH in 4 h. Thereafter, the release was controlled and sustained, and only 39.05, 49.07 and 60.08% of catechins was released after 8, 12 and 24 h, respectively (Fig. 3a). The sustained release indicated the presence of catechin in the core of niosomes. The niosomes thus serve as a potential delivery system to control leakage of catechin. The use of lauryl alcohol might also be responsible for lowering the rate of release by participating and stabilizing the bilayer vesicular system, through charges and hydrogen bonding and get loaded into the bilayer vesicles along with the non-ionic surfactant and bioactive (Bandyopadhyay & Neeta 2007).
In vitro antioxidant activity of catechins-loaded niosomes
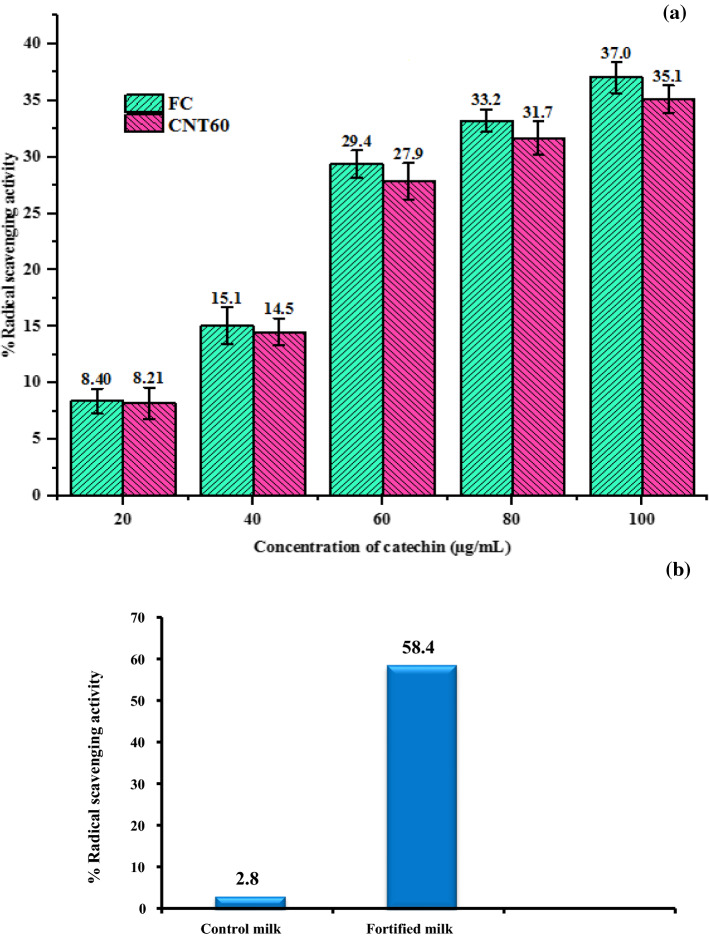
It may be observed from Fig. 4a the RSA of free and niosomal suspensions of catechins increased with increase in concentration. The RSA of free catechin and catechins in niosomes was 37.0 and 35.1% at 100 μg/mL concentration. It is evident that antioxidant property of catechins was retained and stable after nanoencapsulation. Peres et al. (2011) also observed that the EGCG encapsulated in carbohydrate nanomatrix was able to preserve its antioxidant property. Yadav et al. (2014) determined the antioxidant activity of catechins and epicatechins-loaded BSA nanoparticles by DPPH method, and reported that the functional activity of catechins and epicatechins in the nanoparticles was maintained.
Fig. 4.
Antioxidant activity of free catechin and catechin niosomes (a) control and fortified milk (b)
Fortification of milk with catechins loaded niosomes
The primary aim of this research was to fortify them into foods. Milk is one of the most convenient and popular foods for fortification as it offers better stability and bioavailability of added bioactive compounds. Pasteurized toned milk was fortified with catechin loaded niosomes which provides 12 mg of catechin per 240 mL of milk. There is no recommended dietary guideline for intake of catechins. The physico-chemical characteristics and sensory properties of milk fortified with catechins-loaded niosomes were evaluated (Table 2). It was observed that pH, titratable acidity and colour of fortified milk were shown no significant difference, while the viscosity was decreased significantly. However, the viscosity of fortified milk was in acceptable range. The sensory evaluation of control and fortified milk was carried out using 9-point hedonic scale. The overall acceptability of fortified milk was 8.13, which was on par with control. From organoleptic scores and colour data, it was observed that no change in colour and taste were perceived in fortified milk. Hence it could be concluded that fortification of milk with catechins-loaded niosomes did not alter the inherent and organoleptic properties of milk.
Table 2.
Physico-chemical and sensory properties of control and fortified milk
| Sample | Physico-chemical properties | Sensory attributes | ||||||
|---|---|---|---|---|---|---|---|---|
| pH | Titratable acidity (% lactic acid) | Viscosity (cP) | Colour (Whiteness index) | Colour and appearance | Flavour | Body and texture | Overall acceptability | |
| Control (milk) | 6.62 ± 0.03a | 0.15 ± 0.03a | 1.66 ± 0.01a | 82.83 ± 1.29a | 8.35 ± 0.51a | 8.25 ± 0.35a | 8.23 ± 0.42a | 8.27 ± 0.57a |
| Fortified milk | 6.66 ± 0.04a | 0.13 ± 0.01a | 1.57 ± 0.02b | 80.18 ± 1.02a | 8.23 ± 0.68a | 8.16 ± 0.25 a | 8.00 ± 0.15a | 8.13 ± 0.25a |
Values in columns with different superscripts with small letters (a and b) differ significantly (p < 0.05)
Release of catechins in fortified milk
Release pattern of catechins from fortified milk was studied under simulated gastrointestinal conditions at pH 1.2 and 7.4 for 10 h by dialysis method. It was observed that there was controlled and sustained release of catechin from fortified milk; only 5.26% of catechin was released in 2 h at simulated gastric pH 1.2. Later, the release study was continued in simulated intestinal condition at pH 7.4. About 8.26, 10.69, 12.28 and 15.88% catechin was released from milk fortified with catechin niosomes after 4, 6, 8 and 10 h, respectively, as depicted in Fig. 3b. The possible reason for more controlled and slow release of catechins that niosomes can protect the catechins by preventing their interaction with milk proteins and against harsh environmental gastric and intestinal condition, and facilitate their release in a controlled manner. Similar studies were reported by Rashidinejad et al. (2015) observed nanoencapsulation of catechins improved the recovery under simulated GI conditions when fortified into full fat cheese when compared with addition of free catechin.
Antioxidant property of fortified milk
The radical scavenging activity of control and fortified milk was illustrated in Fig. 4b. The antioxidant activity of milk increased by 20.86-fold by addition of catechin niosomes to milk. The reasons for higher antioxidant property in fortified milk might be due to the presence of catechins, which contain 5 to 8 hydroxyl groups that have more scavenging potential against DPPH free radicals. These results are well corroborated with Rashidinejad et al. (2016), the antioxidant activity of full fat cheese fortified with catechins or green tea extract nanoliposomes increased and higher activity was found in cheese fortified with nanoliposomes. Soliman et al. (2019) also reported Labneh cheese prepared with nanoencapsulated wheat germ oil showed higher antioxidant property and acceptable quality.
Conclusion
In the present study, the effect of different stabilizers such as lauryl alcohol or cetyl alcohol or cholesterol using Tween 60 in preparation of catechin loaded niosomes was optimized. The niosomes prepared with lauryl alcohol showed lower hydrodynamic diameter and high entrapment efficiency when compared with cetyl alcohol and cholesterol. The results of DLS, SEM and AFM showed similar particle size distribution. Compared with free catechin, the niosome samples observed relatively controlled and sustain release in simulated GI tract. The niosomal method preserved the antioxidant activity of catechin. The milk fortified with catechin loaded niosomes did not significantly affect the inherent sensory and physico-chemical properties of milk. Hence, it could be concluded from this study that niosomes are a novel nanoencapsulation technique to improve bioavailability of catechins and can be effectively used for food applications.
Acknowledgements
Authors are thankful to National Agricultural Science Fund (NASF), ICAR for providing financial support and providing instrumentation facilities vide Grant No.VI-D&P/54/2016-17/TDT (G). The authors also thank Director, ICAR-NDRI for providing necessary laboratory facilities for this study.
Abbreviations
- AOAC
Association of Official Analytical Collaboration
- C
Catechin
- DCP
Dihexadecyl phosphate
- DLS
Dynamic light scattering
- EC
Epicatechin
- ECG
Epicatechin-3-gallate
- EDS
Energy dispersion X-ray spectroscopy
- EE
Entrapment efficiency
- EGCG
Epigallocatechin gallate
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- NIS
Non-ionic surfactant
- PDI
Polydispersity index
- ROS
Reactive oxygen species
- RSA
Radical scavenging activity
- TFA
Trifluoracetic acid
- ZP
Zeta potential
Authors' contributions
SG conceptualized, collected methodology, carried out the experiment and wrote the manuscript; SNB conceptualized, supervised the work, reviewed and edited the manuscript; SM conceptualized, collected methodology and data curation; HAP conceptualized, validated the data and edited the manuscript; LNN and MEE provided resources and verified and validated data.
Funding
Authors are thankful to National Agricultural Science Fund (NASF), ICAR for providing financial support and providing instrumentation facilities vide Grant No. VI-D&P/54/2016–17/TDT (G).
Availability of data and material
The datasets generated and/or analysed during the current study are available from the first author.
Code availability
No code availability, the datasets used during the current study are available from the first and corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
The manuscript does contain any individual person’s data in any form (including any individual details, images or videos).
Ethics standards
Manuscript does not involve any biological studies, the use of any animal or human data or tissue “Not applicable”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad M, Mudgil P, Gani A, Hamed F, Masoodi FA, Maqsood S. Nano encapsulation of catechin in starch nanoparticles: characterization; release behavior and bioactivity retention during in-vitro digestion. Food Chem. 2018 doi: 10.1016/j.foodchem.2018.07.024. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of theAOAC. 21. Arlington: Association of Official Analytical Chemists; 2019. [Google Scholar]
- Bandyopadhyay P, Neeta NS. Evidence for vesicle formation from 1:1 nonionic surfactant span 60 and fatty alcohol mixtures in aqueous ethanol: Potential delivery vehicle composition. Colloids Surf, B. 2007;58(2):305–308. doi: 10.1016/j.colsurfb.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Bhardwaj P, Tripathi P, Gupta R, Pandey S. Niosomes: A review on niosomal research in the last decade. J Drug Deliv Sci Technol. 2020;56:101581. doi: 10.1016/j.jddst.2020.101581. [DOI] [Google Scholar]
- El-Sherbiny IM, El-Baz NM, Yacoub MH. Inhaled nano -and microparticles for drug delivery. Global Cardiol Sci Pract. 2015;2015(1):1–14. doi: 10.5339/gcsp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez G, Matos M, Barrero P, Pando D, Iglesias O, Pazos C. Iron-entrapped niosomes and their potential application for yogurt fortification. LWT. 2016;74:550–556. doi: 10.1016/j.lwt.2016.08.025. [DOI] [Google Scholar]
- Hu B, Ting Y, Huang Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (-)-epigallocatechin-3-gallate. J Agric Food Chem. 2013 doi: 10.1021/jf304821k. [DOI] [PubMed] [Google Scholar]
- Huai C, Shi L, Li N. Synthesis of a novel betaine-type asphalt emulsifier and its investigation by online FTIR spectrophotometry. Res Chem Intermed. 2013;39(2):597–614. doi: 10.1007/s11164-012-0582-1. [DOI] [Google Scholar]
- Khindri S, Aggarwal G, Hari Kumar SL (2015) Role of niosomes and proniosomes for enhancing bioavailability of drugs. J Drug Deliv Ther 5(1). 10.22270/jddt.v5i1.1043
- Li B, Du W, Jin J, Du Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J Agric Food Chem. 2012;60(13):3477–3484. doi: 10.1021/jf300307t. [DOI] [PubMed] [Google Scholar]
- Li D, Martini N, Wu Z, Wen J. Development of an isocratic HPLC method for catechin quantification and its application to formulation studies. Fitoterapia. 2012;83(7):1267–1274. doi: 10.1016/j.fitote.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Liang R, Chen L, Yokoyama W, Williams PA, Zhong F. Niosomes consisting of tween-60 and cholesterol improve the chemical stability and antioxidant activity of (-)-epigallocatechin gallate under intestinal tract conditions. J Agric Food Chem. 2016;64(48):9180–9188. doi: 10.1021/acs.jafc.6b04147. [DOI] [PubMed] [Google Scholar]
- Managuli RS, Raut SY, Reddy MS, Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expert Opin Drug Deliv. 2018;15(8):787–804. doi: 10.1080/17425247.2018.1503249. [DOI] [PubMed] [Google Scholar]
- Mazyed EA, Helal DA, Elkhoudary MM, Abd Elhameed AG, Yasser M. Formulation and optimization of nanospanlastics for improving the bioavailability of green tea epigallocatechin gallate. Pharmaceuticals. 2021;14(1):1–30. doi: 10.3390/ph14010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon SA, Lo TY, Barbhuiya SA, Xu W. Development of form-stable composite phase change material by incorporation of dodecyl alcohol into ground granulated blast furnace slag. Energy Build. 2013;62:360–367. doi: 10.1016/j.enbuild.2013.03.026. [DOI] [Google Scholar]
- Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MNV. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Peres I, Rocha S, Gomes J, Morais S, Pereira MC, Coelho M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr Polym. 2011;86(1):147–153. doi: 10.1016/j.carbpol.2011.04.029. [DOI] [Google Scholar]
- Pool H, Quintanar D, de D Figueroa J, Marinho Mano C, Bechara JEH, Godínez LA, Mendoza S (2012) Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Total phenolic content and antioxidant properties of hard low-fat cheese fortified with catechin as affected by in vitro gastrointestinal digestion. LWT-Food Sci Technol. 2015;62(1):393–399. doi: 10.1016/j.lwt.2014.12.058. [DOI] [Google Scholar]
- Rashidinejad A, Birch EJ, Everett DW. Effects of (+)-catechin on the composition, phenolic content and antioxidant activity of full-fat cheese during ripening and recovery of (+)-catechin after simulated in vitro digestion. Antioxidants (Basel, Switzerland) 2016;5(3):29. doi: 10.3390/antiox5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravalika V, Sailaja AK. Formulation and evaluation of etoricoxib niosomes by thin film hydration technique and ether injection method. Nano Biomed Eng. 2017;9(3):242–248. doi: 10.5101/nbe.v9i3.p242-248. [DOI] [Google Scholar]
- Rocha S, Generalov R, Pereira Mdo C, Peres I, Juzenas P, Coelho MAN. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine (London, England) 2011;6(1):79–87. doi: 10.2217/nnm.10.101. [DOI] [PubMed] [Google Scholar]
- Soliman TN, Farrag AF, Zahran HAH, El-Salam MEHA. Preparation and properties nano-encapsulated wheat germ oil and its use in the manufacture of functional labneh cheese. Pak J Biol Sci. 2019;22(7):318–326. doi: 10.3923/pjbs.2019.318.326. [DOI] [PubMed] [Google Scholar]
- Song Q, Li D, Zhou Y, Yang J, Yang W, Zhou G, Wen J. Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int J Nanomed. 2014;9:2157–2165. doi: 10.2147/IJN.S59331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankayala JS, Battula SN, Kandasamy R, Mariya GA, Franklin MEE, Pushpadass HA, Naik LN (2018) Surfactants and fatty alcohol based novel nanovesicles for resveratrol: Process optimization, characterization and evaluation of functional properties in RAW 264.7 macrophage cells. J Mol Liquids 261:387–396. 10.1016/j.molliq.2018.04.058
- Wasnik PG, Menon RR, Sivaram M, Nath BS, Balasubramanyam BV, Manjunatha M. Development of mathematical model for prediction of adulteration levels of cow ghee with vegetable fat using image analysis. J Food Sci Technol. 2019;56(4):2320–2325. doi: 10.1007/s13197-019-03677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Kumar D, Kumari A, Yadav SK. Encapsulation of catechin and epicatechin on BSA NPs improved their stability and antioxidant potential. EXCLI J. 2014;13:331. [PMC free article] [PubMed] [Google Scholar]
- Zaiter A, Becker L, Karam M-C, Dicko A. Effect of particle size on antioxidant activity and catechin content of green tea powders. J Food Sci Technol. 2016;53(4):2025–2032. doi: 10.1007/s13197-016-2201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Peng S, Liu W, Gan L, Liu W, Liang R, Liu C, Niu J, Cao Y, Liu Z. Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res Int. 2014;64:492–499. doi: 10.1016/j.foodres.2014.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the first author.
No code availability, the datasets used during the current study are available from the first and corresponding author on reasonable request.