Abstract
In this work the different varieties of oats were subjected to three pre-treatments such as germination, sand roasting and γ-irradiation. The pretreated oat grains were evaluated for phenolic content, flavonoid content and antioxidant activity. RP-HPLC displayed that the amount of ferulic acid, chlorogenic acid, kaempferol, ellagic acid and epicatechin in native, roasted and γ-irradiated oats varied in the range of 2.51–3.23, 0.97–1.89, 4.35–5.33, 1.56–2.197 and 3.387–10.8 µg/100 g, respectively. Total phenolic content (TPC) expressed as µg GAE/g and µg FAE/g and total flavonoid content (TFC) expressed as µg rutin equivalent/g was found highest in germinated samples. Study reported increase in antioxidant activity in the following order; γ-irradiation > germination > roasting > native. It was concluded that the different pretreatments enhanced antioxidant properties of the oat grains therefore can be efficiently utilized as food or functional ingredient in various food systems.
Keywords: RP-HPLC, Germination, Antioxidant properties
Introduction
Oats is a nutritionally interesting value added food with good sources of protein, starch, dietary fiber, micronutrients and bioactive compounds. They are lipid-rich and contain significant quantities of polyphenolic compounds such as phenolic acids, flavonoids, isoflavones and lignans (Lin and Lai 2006). In oats phenolic compounds are present in three forms: as soluble free acids, soluble conjugates and insoluble bound acids. Various studies have demonstrated that consuming products which are oat based shows many health benefits such as decreasing blood cholesterol levels, managing diabetes and weight management through prolonged satiety, etc. (Shah et al. 2016a). Many studies have reported that phenolic compounds in oats are the important natural antioxidants (Tong et al. 2014). Moreover, phenolic compounds in oats have many advantages such as anti-ageing, anti-inflammatory, and antiproliferative activities. However, these phenolic compounds are concentrated in the outer layer of the kernel in the bran fraction and are mostly bound with the polysaccharides like arabinoxylans, cellulose, hemicellulose and even lipids, thereby limiting their bioavailability (Rasane et al. 2015; Beia et al. 2020). All these challenges are foremost for the development of an alternative method to overcome the limitation and increase the bioavailability of polyphenols. A recent literature survey suggests that different pretreatments such as germination, sand roasting and γ-irradiation ensure bioavailability of polyphenols which have therapeutic role against diseases can be used as food and functional ingredient in different food systems. Among the various pre-treatments germination (Chinma et al. 2015), γ-irradiation (Wani et al. 2015), roasting (Gujral et al. 2011), and fermentation (Beia et al. 2020) etc., have been employed to different cereal grains so as to increase its phenolic content. As oat grain is rich in polyphenols with proven biological activity such as lowering blood cholesterol, regulating blood glucose levels, and decreasing the risk of colorectal cancer. Hence, the main purpose of this work was to promote the pretreatment (γ-irradiation, roasting and germination) of oats for enhancing its nutraceutical value before its utilization as food or functional ingredient in various food systems. Also, it is hypothesized that pretreatments will increase the bioavailability of polyphenols. Additionally, we expect that this study will pave ways to provide a novel approach to obtain the polyphenols from oat grain which can find its use in food and pharmaceutical sectors.
Materials and methods
Materials
Oat varieties-Sabzaar, SKO20 and SKO90 were procured from SKAUST-K, J&K, India.
Pretreatment of oat grain
Oat grains were subjected to three different pretreatments; germination, sand roasting and γ-irradiation.
Germination
The germination of oat grain was done according to the method of Jodee et al. (2019). Oat grains (10% moisture d.b) were dispersed in 2% solution of sodium hypochlorite for 15 min at room temperature. Grains were washed with tap water for 20 min to remove any residue present. Oat seeds were soaked in distilled water for 24 h in dark at 16οC. After that grains were spread and covered with wet ordinary filter paper and incubated for 48 h in dark at 16οC. Meanwhile the paper was sprinkled with water to keep it wet. After germination, the seeds were dried at 45οC for 12 h in a hot air oven.
Sand roasting
For roasting hulled oat varieties (200 g each) were conditioned to a moisture content of 10% so as to maintain uniformity during roasting process. Sand roasting was performed by pouring hulled grains onto the sand maintained at 200@@οC, stirred well during roasting and allowed to roast for 400 s.
Gamma-irradiation
Gamma-irradiation of oat seeds was done following the method of Shah et al. (2016a, b, c). Oat seeds (30 g/bag) at a moisture content of 10.5% were packed in double layered polyethylene bags and irradiated at room temperature (20 ± 2 °C) in a cobalt-60 (60-Co) source irradiator. Doses of 5, 10, 15 and 20 kGy at the dose rate of 2 kGy h−1 were used. Irradiation was performed at Bhabha Atomic Research Center (BARC), Srinagar, J &K, India.
Sample preparation for antioxidant activity
Oats grains were ground and sieved using 60-mesh screen. Oat flour was defatted first with petroleum ether and then extraction with methanol in a Soxhlet apparatus. The extraction was carried for 30 min at 50 °C. The extract obtained was concentrated in rotary evaporator (Equitron) and then re-dissolved again in methanol to make a final concentration of 100 µg/mL (Shah et al. 2016a, b, c).
Determination and quantification of phenolic compounds using RP-HPLC
RP-HPLC system was used to quantify the phenolic acids following the method of Proestos et al. (2005), Shah et al. (2019). Dried extract of the sample were solubilized using 2 mL of methanol (HPLC grade) in rotary evaporator. Filtered solution was injected into the column (C18, 100 mm × 4.6 mm) and flow rate of 1 mL/min at 35 °C was maintained. Compounds were identified and quantified based on retention time and absorbance spectra of standard and the calibration curves. Values were presented as milligram of phenolic acid per kilogram of dry sample (mg Phenolic acid/kg).
Total phenolic content (TPC)
TPC of oat samples was determined following the method of Gani et al. (2021). The sample extract (0.5 mL) was mixed with 2.5 mL of 10% Folin-Ciocalteu’s reagent dissolved in water (1:10 dilution). After 4 min, 2.0 mL of sodium carbonate solution (7.5%) was added. Blank was prepared, by adding 0.5 mL methanol, 2.5 mL of Folin-Ciocalteu’s reagent and 2.0 mL of sodium carbonate. The sample and blank was thereafter incubated at 45 °C for 2 h. The absorbance was determined using spectrophotometer at 760 nm. TPC of samples were expressed as µg of gallic acid/ferulic acid equivalents per gram of oat sample from gallic acid and ferulic acid standard curve (50–700 µg/mL−1). The equation of the gallic acid and ferulic acid calibration curve was y = 0.001x − 0.036 and y = 0.001x + 0.03 and the correlation coefficients were R2 = 0.97 and R2 = 0.99, respectively.
Total flavonoid content (TFC)
TFC was calculated using the method described by Shen et al. (2009). The solution contained 1 mL of sample solution and 1 ml of 2% AlCl3 solution dissolved in methanol. The solution was incubated for an hour at room temperature and absorbance was taken at 415 nm. TFC was expressed as μg rutin equivalent g−1 sample dry weight (5–200 µg/mL−1). The standard equation of the rutin calibration curve was y = 0.003x + 0.145 and the correlation coefficient was R2 = 0.99.
Antioxidant activity
DPPH (1, 1-dihpenyl-2-picrylhydrazyl) radical scavenging activity
The scavenging activity against DPPH was measured according to the method used by Ashraf et al. (2021). The reaction mixture consists of 1 mL of 10 mg% of methanolic DPPH solution and 1 mL of the methanolic extract. The volume was made up to 3 mL by methanol and incubated in dark for 30 min. Absorbance of sample and α-tocopherol standard curve was read at 517 nm (5–20 µg/mL−1).
Reducing power
The reducing power was determined by the method described by Ashraf et al. (2020). The 1 ml extract was mixed with 0.2 M sodium phosphate buffer (pH 6.6), and 1% (w/v) aqueous potassium ferricyanide followed by incubation for 20 min at 50 °C. Trichloroacetic acid (10% w/v) was added to the mixture, centrifuged at 3000 rpm for 10 min. The supernatant was diluted with deionized water and 0.1% (w/v) ferric chloride was added. The absorbance of sample and BHT (Butylated hydroxy toluene) standard curve was measured at 700 nm (20–100 µg/mL−1).
Metal chelating (Fe2+) activity (MCA)
MCA was determined using the protocol described by Ashraf et al. (2020). Methanolic extract, ferrous chloride and ferrozine were mixed in the ratio of 4:2:1 respectively and volume was made to 3 mL using methanol. The mixture was vortexed and incubated for 10 min at room temperature. The absorbance of mixture and citric acid standard curve was measured at 562 nm (100–1000 µg/mL−1).
Inhibition of lipid peroxidation activity
The antioxidant activity of the oat extracts were determined by thiocyanate method with minor modifications Osawa and Namiki (1981). Different oat samples (1 mL) were added to1 mL of linoleic acid (0.1 g in 100 mL of pure ethanol), 0.2 mL of H2O2 (30 mM), 0.2 mL of ascorbic acid (100 mM) and 0.2 mL of ferric nitrate (20 mM). Then the mixture was incubated at 37 °C in water bath for 1 h.The reaction was stopped by adding 1.0 mL of TCA (Trichloroacetic acid, 10% w/v) and TBA (Thiobarbituric acid, 1% w/v). All the tubes were placed in a boiling water bath for 20 min and then centrifuged at 5000 rpm for 10 min. The amount of malonaldehyde formed was determined by measuring the absorbance of the supernatant and the standard BHT at 535 nm (10–100 µg/mL−1).
Statistical analysis
Mean values, standard deviation, two way analysis of variance (ANOVA) were computed using a commercial statistical package SPSS (IBM statistics 22) and data were compared using Duncan’s multiple range tests at 5% significance level.
Results and discussion
Composition and quantification of phenolic compounds in oats
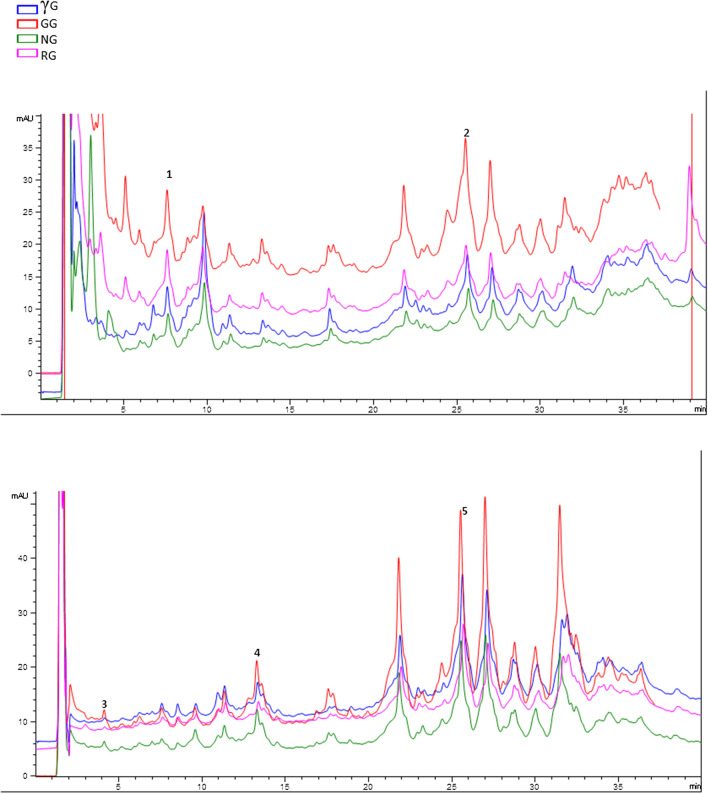
The phenolic acids present in native and treated oat flour were ferulic acid, chlorogenic acid, kaempferol, ellagic acid and epicatechin and are shown in a reference figure (Fig. 1) and Table 1. The germinated oat flour had the highest concentrations of these compounds as 6.919, 1.89, 3.66, 4.232 and 11.3 µg/100 g for kaempferol, chlorogenic acid, ellagic acid, ferulic acid, and epicatechin, respectively. Duenas et al. (2009), Jodee et al. (2019) also reported increased concentration of phenolics on germination of lupin seeds. The amount of ferulic acid, chlorogenic acid, kaempferol, ellagic acid and epicatechin in native, roasted and γ-irradiated oats varied from 2.51–3.23 µg/100 g, 0.97–1.89 µg/100 g, 4.35–5.33 µg/100 g, 1.56–2.197 µg/100 g, and 3.387–10.8 µg/100 g, respectively. However, our results are not in complete consistence with the reports from other studies on compositions of oat phenolics with and without any treatment. These variations may arise due to differences in samples, genetic factors, environmental factors and extraction method used.
Fig. 1.
RP-HPLC chromatograms of phenolics in oats subjected to different pretreatments. Whereas γG γ-irradiated grains, GG germinated grains, NG native grains, RG roasted grains, (1) epicatechin, (2) kaempferol, (3) ellagic acid, (4) chlorogenic acid, (5) ferulic acid
Table 1.
Composition and quantification of phenolic compounds in native and treated oat flour
| Compound | Untreated | Germinated | Sand roasted | Irradiated |
|---|---|---|---|---|
| Epicatechin | 3.387 ± 0.06a | 11.3 ± 0.26b | 9.91 ± 0.02c | 9.98 ± 0.1c |
| Kaempferol | 4.35 ± 0.12a | 6.919 ± 0.16b | 4.44 ± 0.03a | 4.93 ± 0.03a |
| Ellagic acid | 1.56 ± 0.09a | 3.66 ± 0.06b | 1.96 ± 0.05c | 2.19 ± 0.02d |
| Chlorogenic acid | 0.97 ± 0.09a | 1.89 ± 0.02b | ND | ND |
| Ferulic acid | 2.51 ± 0.01a | 4.23 ± 0.05b | 1.969 ± 0.2c | 3.23 ± 0.01d |
Values with the different superscripts in a row are significantly different (P < 0.05)
Results are mean ± SD
Results are expressed as µg /100 g of sample
ND not detected
Total phenolic content
Total phenolic content of native and treated oat flour expressed as µg equivalent gallic acid and ferulic acid is shown in (Table 2). The phenolic content of native oat flour varied significantly within the varieties which ranged from 752.27–795.37 µg GAE/g and 681.3–724.77 µg FAE/g (P < 0.05). After pretreatment, the phenolic content was increased in germinated oat flour samples in the range of 800.31–826.22 µg GAE/g and 734.08–757.38 µg FAE/g. The results are in agreement with the previous studies where the total phenolic content has been found to enhance during germination (Khattak et al. 2007; Fernandez-orozco et al. 2006; Kiran et al. 2013). Phytochemical modification during germination is considered as a natural phenomenon of plants. During germination, an increased enzyme activity including the avenanthramide synthesising enzyme induces the synthesis of phenolics, and in particular, the avenanthramides, which are the group of phenolics present exclusively in oats (Skoglund 2008). Roasting (sand and microwave) on the other side resulted in a significant decrease of phenolics. Sand roasting resulted in a more steep decrease of free phenolics in the range of 307.09–317.34 µg GAE/g and 243.75–265.34 µg FAE/g. The decreased phenolic content of roasted samples may be attributed to the degradation of phenolic compounds at the elevated temperatures. Similar findings were also reported by Paras and Gujral (2011) and Sasipriya and Siddhuraju (2012). Irradiation at lower doses was found to decrease the phenolics whereas a significant increase was observed at higher dose. These results have similarities with the findings of Shah et al. (2015). Irradiation at 15 kGy significantly reduced the phenolic acid contents in the range of 607.67–633.56 µg GAE/g and 539.07–567.12 µg FAE/g whereas irradiation at 20 kGy achieved the highest total phenolic acid content (783.27–806.67 µg GAE/g and 687.67–731.20 µg FAE/g). The total phenolic content of the irradiated samples depends on the balance between the disintegration of free phenolics by gamma irradiation mostly at lower doses, and the release of some bound phenolics like ferulic acid due to partial breaking of ester linkages which contribute to increased phenolic content at higher dose (Shao et al. 2013; Shah et al.2019).
Table 2.
Total phenolic content of native and treated of oat flour
| Treatments | Varieties | ||
|---|---|---|---|
| Sabzaar | SKO20 | SKO90 | |
| Gallic acid equivalent (µgGAE/g) | |||
| Untreated | 795.37 ± 0.46fr | 752.27 ± 0.38fp | 764.33 ± 0.49fq |
| Germination | 826.22 ± 4.27hq | 800.31 ± 0.50hp | 802.3 ± 3.15hp |
| Sand roasted | 317.34 ± 1.71aq | 307.09 ± 0.08ap | 316.82 ± 1.12aq |
| Irradiation dose | |||
| 5 kGy | 518.21 ± 0.68cq | 521.26 ± 0.28cr | 485.89 ± 1.01cp |
| 10 kGy | 578.67 ± 0.27dr | 522.74 ± 1.29dp | 553.96 ± 2.60dq |
| 15 kGy | 607.67 ± 1.93ep | 631.41 ± 0.80eq | 633.56 ± 2.11eq |
| 20 kGy | 806.67 ± 0.67gr | 783.27 ± 1.52gp | 789.66 ± 2.61gq |
| Ferulic acid equivalent (µgFAE/g) | |||
| Untreated | 724.77 ± 0.72fr | 681.3 ± 0.34fp | 693.29 ± 0.27fq |
| Germination | 757.38 ± 0.63h | 734.08 ± 0.06hp | 735.08 ± 0.07hq |
| Sand roasted | 265.34 ± 2.94ar | 243.75 ± 2.58ap | 252.97 ± 2.58aq |
| Irradiation dose | |||
| 5 kGy | 452.16 ± 0.96cq | 455.49 ± 0.57cr | 419.45 ± 0.67cp |
| 10 kGy | 512.67 ± 1.09dr | 457.41 ± 0.71dp | 489.02 ± 0.90dq |
| 15 kGy | 539.07 ± 0.06ep | 565.04 ± 0.12eq | 567.12 ± 0.015er |
| 20 kGy | 731.20 ± 1.05gr | 687.67 ± 1.28gp | 698.22 ± 0.82gq |
Results are mean ± SD
Values with the different superscripts in a row and column indicate significant difference (P < 0.05)
Total phenolic content are expressed as µg gallic acid/ ferulic acid equivalent/g
Total flavonoid content
Flavonoids are characterized by the presence of benzo-y-pyrone structure which has broad human health promoting effect (Wu et al. 2004). These properties mainly depend on the structure of the flavonoids and the substitution pattern of –OH group (Verma et al. 2016). The total flavonoid content of the native and treated samples was evaluated using rutin equivalent (µg rutin E/g) and is displayed in Table 3. The total flavonoid content ranged from 201.58–244.92 µg rutin equivalent/g varied significantly among the native varieties. It was observed that germination caused a significant increase in flavonoid content in the range of 240.16–271.18 µg rutin equivalent/g. The secondary plant metabolites i.e., phenolic acids and flavonoids are synthesized through different pathways including shikimate and phenylpropanoid pathway from glucose as a precursor (Lopez-Martinez et al. 2017). Phenylalanine ammonialyase (PAL) is considered as the main enzyme that is involved in the synthesis of phenolic compounds, and their transformation into flavonoids. During germination, it was reported that the activity of PAL gets increased (Tang and Zhao 1998). Similar increase in total flavonoid content during germination was also reported in buckwheat (Zhang et al. 2015). Irradiation at 5 kGy abruptly decreased the flavonoid content in all the oat varieties (48.37, 49.4, and 51.26 for Sabzaar, SKO20, and SKO90 µg rutin equivalent/g, respectively). However, the increased irradiation doses of 10, 15 and 20 kGy resulted in a slight non–significant decrease in the flavonoid content when compared to 5 kGy. The results are in agreement with the studies of Hussain et al. (2013) who reported a similar decrease of flavonoid content in dried apricots (Prunus armeniaca L.) revealing that these compounds may be sensitive to ionizing radiations. Sand roasting also resulted in decrease in total flavonoid content (89.73–98.48 µg rutin equivalent/g). The flavonoid compounds are heat sensitive substances, therefore, heat exposure during roasting could be a possible reason for their decrease (Vandana et al. 2014; Kawaljit et al. 2017; Zhang et al. 2010; Zhu et al. 2010).
Table 3.
Total flavonoid content of native and treated oat flour
| Treatments | Varieties | ||
|---|---|---|---|
| Sabzaar | SKO20 | SKO90 | |
| Untreated | 201.58 ± 2.33dp | 244.92 ± 1.46er | 221.06 ± 1.46eq |
| Germination | 240.16 ± 2.61ep | 245.48 ± 0.46fq | 271.18 ± 2.01fr |
| Sand roasted | 89.73 ± 0.57bp | 96.98 ± 0.38eq | 98.48 ± 1.08cr |
| Irradiation dose | |||
| 5 kGy | 48.37 ± 0.45ap | 49.48 ± 0.36bq | 51.26 ± 0.16br |
| 10 kGy | 45.47 ± 0.40ap | 45.48 ± 0.36ap | 45.26 ± 0.16ap |
| 15 kGy | 44.64 ± 0.02ap | 44.64 ± 0.00ap | 46.62 ± 0.05aq |
| 20 kGy | 44.61 ± 0.00ap | 44.58 ± 0.01ap | 46.62 ± 0.05aq |
Results are mean ± SD
Values with the different superscripts in a row and column indicate significant difference (P < 0.05)
Flavonoid content is expressed as µg rutin equivalent/g
Antioxidant activity
The effect of germination, irradiation and roasting on DPPH radical scavenging activity is displayed in Table 4 expressed as µg α-tocopherol equivalent/g. DPPH scavenging activity varied significantly (p < 0.05) among different varieties with Sabzaar (9.24 µg α-tocopherol equivalent/g) showing higher values than SKO20 (8.28 µg α-tocopherol equivalent/g) and SKO90 (7.33 µg α-tocopherol equivalent/g). Shah et al. (2016a) reported a significant positive correlation (p < 0.05) between the total phenolics (GAE/FAE) and the DPPH radical scavenging activity (R = 0.669; 0.671) for different varieties of whole oat flour. The germinated, irradiated and sand roasted samples had higher DPPH scavenging activity than their native counterpart samples in the range of 8.89–11.87, 13.62–17.62, 8.72–11.35 and 8.04–10.72 µg α-tocopherol equivalent/g, respectively. The increased antioxidant activity of the oat varieties after germination and irradiation is mainly attributed to the enhanced extractability of antioxidants from the tissues and enhanced enzyme activity e.g. phenylalanine ammonialyase and peroxidase, resulting in the formation of compounds that acts as electron donors, thereby stabilizing the free radicals and blocking the radical chain reaction (Alothman et al. 2009; Hardy et al. 2012; Uchegh 2015). The enhanced DPPH activity in roasted samples might be a result of the formation of Maillards reaction products such as HMF (5, Hydroxymethyl-2-furfuraldehyde) which renders high antioxidant activity (Paras and Gujral 2011).
Table 4.
DPPH activity and reducing power of native and treated oat flour
| Treatments | Varieties | ||
|---|---|---|---|
| Sabzaar | SKO20 | SKO90 | |
| DPPH (µg α-tocopherol equivalent/g) | |||
| Untreated | 9.24 ± 0.13ar | 8.28 ± 0.28aq | 7.33 ± 0.37ap |
| Germination | 11.87 ± 0.23cr | 10.66 ± 0.34cq | 8.89 ± 0.09cp |
| Sand roasted | 10.72 ± 0.46br | 9.9 ± 0.01bq | 8.04 ± 0.04bp |
| Irradiation dose | |||
| 5 kGy | 13.62 ± 0.41dp | 13.97 ± 0.10dp | 14.08 ± 0.06dp |
| 10 kGy | 14.38 ± 0.08eq | 14.04 ± 0.04dp | 14.36 ± 0.00eq |
| 15 kGy | 15.60 ± 0.10fr | 15.2 ± 0.01ep | 15.46 ± 0.03fq |
| 20 kGy | 17.62 ± 0.11gr | 17.25 ± 0.00fq | 17.02 ± 0.00gp |
| Reducing power (µg BHT equivalent/g) | |||
| Untreated | 12.07 ± 0.06bp | 12.60 ± 0.00bq | 13.07 ± 0.06ar |
| Germination | 22.32 ± 0.01cp | 25.64 ± 0.01cq | 30.31 ± 0.01dr |
| Sand roasted | 21.57 ± 0.09dp | 21.66 ± 0.038dq | 28.05 ± 0.04cr |
| Irradiation dose | |||
| 5 kGy | 61.21 ± 0.18ep | 62.05 ± 0.04eq | 68.64 ± 0.01er |
| 10 kGy | 68.07 ± 0.06fp | 71.04 ± 0.04fq | 76.07 ± 0.06fr |
| 15 kGy | 77.21 ± 0.39gp | 84.04 ± 0.04gq | 91.01 ± 0.01gr |
| 20 kGy | 101.06 ± 0.06hp | 89.35 ± 0.47hq | 104.25 ± 0.13h |
Results are mean ± SD
Values with the different superscripts in a row and column indicate significant difference (P < 0.05)
The effect of different pretreatments on the reducing power of the three oat varieties is represented in Table 4. The reducing power of the germinated samples (22.32–30.31 µg BHT equivalent/g) was significantly (p < 0.05) greater than that of untreated samples (12.07–13.07 µg BHT equivalent/g). These findings are in complete agreement with the previous work done by Lopez-Amoros et al. (2006), Mohd Esa et al. (2013). Higher reducing power after germination is possibly due to increased bioactive substances in germinated oat flours as revealed by high phenolic and flavonoid contents of germinated oat flours (Chinma et al. 2015). The enhanced reducing activity of the roasted samples might be attributed to the reductone formation due to the thermolysis of Amadori products at the early stage of Maillard reaction which can easily donate electrons. Irradiation on the other hand resulted in abrupt rise of reducing power which increased with increasing irradiation doses (61.21–104.2 µg BHT equivalent/g). γ-irradiation leads to breakdown of the glycosidic bonds of polyphenol and results in phenols with low molecular weight, high solubility and extractability which in turn contributes to the increased reducing power (Ahn et al. 2004).
The metal chelating activity of native and pretreated three varieties of oat flour is presented in Table 5. Metal chelating activity of flour changed significantly (p < 0.05) with variety and Sabzaar showed the lowest value (0.361 µg citric acid equivalent/g) while as SKO90 showed the highest value (0.377 µg citric acid equivalent/g). The difference in the antioxidant activity among oat flour is due to the difference in flavonoid and phenolic content (Chlopicka et al. 2012). Germination, sand roasting and irradiation resulted in significant increase in metal chelating activity (0.368–0.384, 0.365–0.379, 0.364–0.379, and 0.365–0.386 µg citric acid equivalent/g, respectively) when compared to their untreated counterparts. Germination results in the production of new compounds like alanine and γ-aminobutyrate and synthesis of bioactive compounds viz ascorbic acid and tocopherol which contributes to the enhanced metal chelating activity of germinated oat flour (Tian et al. 2004). The enhanced metal chelating activity in roasted samples might be attributed to alteration in phenolic structure with increased activity and/or formation of heat induced compounds like melanoidins which could act as antioxidants (Woffenden et al. 2002; Randhir et al. 2008). Similarly in case of γ-irradiated oat flour, irradiation results in exposure of functional groups like –OH, COOH and C=O which have a tendency to form complexes with Fe2+ and Cu2+.
Table 5.
Metal chelating and lipid peroxidation inhibition activity of native and treated oat flour
| Treatments | Varieties | ||
|---|---|---|---|
| Sabzaar | SKO20 | SKO90 | |
| Metal chelating (µg citric acid equivalent/g) | |||
| Untreated | 0.361 ± 0.01ap | 0.368 ± 0.00aq | 0.377 ± 0.00ar |
| Germination | 0.368 ± 0.0abcp | 0.378 ± 0.00bq | 0.384 ± 0.00bcr |
| Sand roasted | 0.364 ± 0.00abp | 0.369 ± 0.00aq | 0.379 ± 0.00abr |
| Irradiation dose | |||
| 5 kGy | 0.365 ± 0.00abp | 0.368 ± 0.00ap | 0.378 ± 0.00aq |
| 10 kGy | 0.366 ± 0.00abp | 0.368 ± 0.00aq | 0.379 ± 0.00abr |
| 15 kGy | 0.372 ± 0.00bcp | 0.374 ± 0.00bq | 0.379 ± 0.00abr |
| 20 kGy | 0.377 ± 0.00dp | 0.378 ± 0.00bq | 0.386 ± 0.00cr |
| Lipid peroxidation (µg BHT equivalent/g) | |||
| Untreated | 30.63 ± 0.35ap | 30.55 ± 0.08ap | 34.80 ± 0.02aq |
| Germination | 60.60 ± 0.32fp | 63.24 ± 0.14fq | 64.27 ± 0.14fr |
| Sand roasted | 46.25 ± 0.07cp | 52.32 ± 0.14dq | 60.11 ± 0.10dr |
| Irradiation dose | |||
| 5 kGy | 36.57 ± 0.30bq | 30.86 ± 0.10bp | 38.1 ± 0.18br |
| 10 kGy | 50.00 ± 0.01dr | 44.35 ± 0.38cq | 57.89 ± 0.11cp |
| 15 kGy | 82.58 ± 0.07gq | 75.07 ± 0.56gp | 82.27 ± 0.49gq |
| 20 kGy | 94.49 ± 0.34hq | 94.10 ± 0.09hp | 98.07 ± 0.06h |
Results are mean ± SD
Values with the different superscripts in a row and column indicate significant difference (P < 0.05)
The lipid peroxidation inhibition values of native and pretreated oat varieties are shown in Table 5 and expressed as µg BHT equivalent/g. The lipid peroxidation inhibition activity of the native oat flour was observed in the range of 30.55–34.80 µg BHT equivalent/g and varied significantly (p < 0.05) among the varieties. When compared to native, lipid peroxidation inhibition values of pretreated samples were significantly (p < 0.05) higher. Germination, sand roasting and irradiation have lipid peroxidation inhibition values in the range of 60.60–64.27, 54.37–62.82, 46.25–60.11, and 30.86–98.07 µg citric acid equivalent/g, respectively. Higher concentration of bioactive compounds in germinated flour, maillard reaction products in roasted flour, and deglycosylation of polyphenols due to irradiation, which makes them more bioactive are the possible reasons for increased inhibition of lipid peroxidation (Marathe et al. 2016; Sasipriya and Siddhuraju 2012).
Conclusion
This work was conducted to evaluate the effect of different pretreatment on the antioxidant properties of oats. Experimental studies revealed that phenolic content and flavonoid content increased in germinated samples in comparison to microwave and sand roasting. Germination for up to 48 h significantly increased kaempferol, chlorogenic acid, ellagic acid, ferulic acid, and epicatechin in oats compared to roasting and γ-irradiation. It was concluded that, among the various pretreatments, germination can be used for increasing the antioxidant potential of the formulations of functional foods for the management of various free radical mediated chronic diseases like obesity, diabetes, and cardiovascular diseases.
Acknowledgements
We would like to thank Prof. F.A. Masoodi, Dept. of Food Science & Technology, University of Kashmir for his kind guidance and support.
Author’s contributions
Dr. Asima Shah: Methodology, Investigation, Writing manuscript. Prof. Farooq Ahmad Masoodi: Supervision. Dr. Adil Gani: Project administration. Zanoor ul ashraf and Bilal ashwar: Formal analysis and paper editing.
Funding
NA.
Declarations
Conflict of interest
The authors do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Asima Shah, Email: Shahasima.au2@gmail.com.
F. A. Masoodi, Email: Masoodi_fa@yahoo.co.in
Adil Gani, Email: Adil.gani@gmail.com.
Zanoor ul Ashraf, Email: Zanoorashraf640@gmail.com.
Bilal Ahmad Ashwar, Email: Ashwar20@gmail.com.
References
- Ahn HJ, Kim JH, Jo C, Kim MJ, Byun MW. Comparison of irradiated phytic acid and other antioxidants for antioxidant activity. Food Chem. 2004;88:173–178. doi: 10.1016/j.foodchem.2004.02.001. [DOI] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Ashraf ZU, Shah A, Gani A, Masoodi FA, Noor N. Effect of nano-reduction on properties of β-glucan and its use as encapsulating agent for release of α-tocopherol. Bioact Carbohydr Diet Fibre. 2020;6:100230. doi: 10.1016/j.bcdf.2020.100230. [DOI] [Google Scholar]
- Ashraf ZU, Shah A, Gani A, Gani A, Masoodi FA, Noor N. Nanoreduction as a technology to exploit β-Glucan from cereal and fungal sources for enhancing its nutraceutical potential. Carbohydr Polym. 2021;66:258117664. doi: 10.1016/j.carbpol.2021.117664. [DOI] [PubMed] [Google Scholar]
- Beia Q, Wuc Z, Chena G. ssDynamic changes in the phenolic composition and antioxidant activity of oats during simultaneous hydrolysis and fermentation. Food Chem. 2020;305:125–269. doi: 10.1016/j.foodchem.2019.125269. [DOI] [PubMed] [Google Scholar]
- Chinma CE, Anuonye JC, Simon OC, Ohiare RO, Danbaba N. Effect of germination on the physicochemical and antioxidant characteristics of rice flour from three rice varieties from Nigeria. Food Chem. 2015;185:454–458. doi: 10.1016/j.foodchem.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Chlopicka J, Pasko P, Gorinstein S, Jedryas A, Zagrodzki P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci Technol. 2012;46:548–555. doi: 10.1016/j.lwt.2011.11.009. [DOI] [Google Scholar]
- Duenas M, Hernandez T, Estrella I, Fernandez D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.) Food Chem. 2009;117:599–607. doi: 10.1016/j.foodchem.2009.04.051. [DOI] [Google Scholar]
- Fernandez-Orozco R, Piskula MK, Zielinski H, Kozlowska H, Frias J, Valverde VC. Germination as a process to improve the antioxidant capacity of Lupinus angustifolius L. var. Zapaton. Eur Food Res technol. 2006;223:495–502. doi: 10.1007/s00217-005-0229-1. [DOI] [Google Scholar]
- Gani A, Jan R, Ashwar BA, Ashraf ZA, Shah A, Gani A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT. 2021;142:111035. doi: 10.1016/j.lwt.2021.111035. [DOI] [Google Scholar]
- Gujral HS, Sharma P, Rachna S. Effect of sand roasting on beta glucan extractability, physicochemical and antioxidant properties of oats. LWT Food Sci Technol. 2011;44:2223–2230. doi: 10.1016/j.lwt.2011.06.001. [DOI] [Google Scholar]
- Hardy M, Borzouei A, Rajabifar S, Ziaie F, Shafiei S. Effect of gamma irradiation on antioxidant activity of Ergosan. Iran J Radiat Res. 2012;4:245–249. [Google Scholar]
- Hussain PR, Chatterjee S, Variyar PS, Sharma A, Dar MA, Wani AM. Bioactive compounds and antioxidant activity of gamma irradiated sun References Page 244 dried apricots (Prunus armeniaca L.) J Food Compos Anal. 2013;30:59–66. doi: 10.1016/j.jfca.2013.02.001. [DOI] [Google Scholar]
- Jodee JD, Fang JY, Feng CH. Enhancement of γ-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound. Food Chem. 2019;283:239–247. doi: 10.1016/j.foodchem.2018.12.136. [DOI] [PubMed] [Google Scholar]
- Kawaljit SS, Poonam G, Maninder K, Sneh P. Effect of toasting on physical, functional and antioxidant properties of flour from oat (Avena sativa L.) cultivars. J Saudi Soc Agric. 2017;16:197–203. [Google Scholar]
- Khattak AB, Zeb A, Bibi N, Khalil SA, Khattak MS. Influence of germination techniques on phytic acid and polyphenols content of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2007;104:1074–1079. doi: 10.1016/j.foodchem.2007.01.022. [DOI] [Google Scholar]
- Kiran CR, Rao DB, Sirisha N, Rao TR. Assessment of phytochemicals and antioxidant activities of raw and germinating Ceiba pentandra (kapok) seeds. J Biomed Sci. 2013;27:1–6. doi: 10.7555/JBR.27.20120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Lai HM. Bioactive compounds in legumes and their germinated products. J Agric Food Chem. 2006;54:3807–3814. doi: 10.1021/jf060002o. [DOI] [PubMed] [Google Scholar]
- Lopez-Amoros ML, Hernandez T, Estrella I. Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Compost Anal. 2006;19(4):277–283. doi: 10.1016/j.jfca.2004.06.012. [DOI] [Google Scholar]
- Lopez-Martinez LX, Leyva-Lopez N, Gutierrez-Grijalva NP, Heredia JB. Effect of cooking and germination on bioactive compounds in pulses and their health benefits. J Funct. 2017;6:66. [Google Scholar]
- Marathe SA, Deshpande R, Khamesra A, Ibrahim G, Jamdar SN. Effect of radiation processing on nutritional, functional, sensory and antioxidant properties of red kidney beans. Radiat Phys Chem. 2016;125:1–8. doi: 10.1016/j.radphyschem.2016.03.002. [DOI] [Google Scholar]
- Mohd Esa N, A bdul Kadir KK, Amom Z, Azlan A (2013) Antioxidant activity of white rice, brown rice and germinated brown rice (in vivo and in vitro) and the effects on lipid peroxidation and liver enzymes in hyperlipidaemic rabbits. Food Chem 141(2):1306–1312 [DOI] [PubMed]
- Osawa T, Namiki M. A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric Biol Chem. 1981;45:735–739. [Google Scholar]
- Paras S, Gujral HS. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Int Food Res J. 2011;44:235–240. doi: 10.1016/j.foodres.2010.10.030. [DOI] [Google Scholar]
- Proestos C, Chorianopoulos N, Nychas G-JE, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005;53(4):1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain and sprouts and seedling. Innov Food Sci Emerg. 2008;9:355–364. doi: 10.1016/j.ifset.2007.10.004. [DOI] [Google Scholar]
- Rasane P, Jha A, Sabikhi L, Kumar A, Unnikrishnan VS. Nutritional advntages of oats and opportunities for its processing as value added foods—a review Int. J Food Sci. 2015;52:662–675. doi: 10.1007/s13197-013-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasipriya G, Siddhuraju P. Effect of different processing methods on antioxidant activity of underutilized legumes, Entada scandens seed kernel and Canavalia gladiata seeds. Food Chem Toxicol. 2012;50:2864–2872. doi: 10.1016/j.fct.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Shah A, Gani A, Masoodi FA, Ashwar BA. Effect of γ-irradiation on antioxidant and antiproliferative properties of oat β-glucan. Radiat Phys Chem. 2015;117:120–127. doi: 10.1016/j.radphyschem.2015.06.022. [DOI] [Google Scholar]
- Shah A, Masoodi FA, Gani A, Ashwar BA. In-vitro digestibility, rheology, structure, and functionality of RS3 from oat starch. Food Chem. 2016;212:749–758. doi: 10.1016/j.foodchem.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Shah A, Masoodi FA, Gani A, Ashwar BA. Newly released oat varieties of himalayan region—techno-functional, rheological, and nutraceutical properties of flour. LWT Food Sci Technol. 2016;70:111–118. doi: 10.1016/j.lwt.2016.02.033. [DOI] [Google Scholar]
- Shah A, Masoodi FA, Gani A, Ashwar BA. Effect of γ-irradiation on antioxidant and antiproliferative properties of oat β-glucan. Radiat Phys Chem. 2016;117:120–127. doi: 10.1016/j.radphyschem.2015.06.022. [DOI] [Google Scholar]
- Shah A, Masoodi FA, Gani A, Ashwar BA. Water extractable pentosans-quantification of ferulic acid using RP-HPLC, techno-rheological and antioxidant properties. Int J Biol Macromol. 2019;133:365–371. doi: 10.1016/j.ijbiomac.2019.04.112. [DOI] [PubMed] [Google Scholar]
- Shao Y, Tang F, Xu F, Wangb Y, Bao J. Effects of g-irradiation on phenolics content, antioxidant activity and physicochemical properties of whole grain rice. Radiat Phys Chem. 2013;85:227–233. doi: 10.1016/j.radphyschem.2013.01.022. [DOI] [Google Scholar]
- Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci. 2009;49:106–111. doi: 10.1016/j.jcs.2008.07.010. [DOI] [Google Scholar]
- Skoglund M (2008) Phenolic compounds in oat: Effects of steeping, germination and related enzymes. Swedish University of Agricultural Sciences, PhD dissertation
- Tang Y, Zhao G. The relationship between phenylalanine deaminase activities and xanthoketone contents in buckwheat. J Mianyang Coll Econ Technol. 1998;15(1):9–12. [Google Scholar]
- Tian S, Nakamura K, Kayahara H. Analysis of phenolic compounds in white rice, brown roce and germinated brown rice. J Agric Food Chem. 2004;52(15):4808–4813. doi: 10.1021/jf049446f. [DOI] [PubMed] [Google Scholar]
- Tong LT, Liu L, Zhongy K, Wang Y, Guo L, Zhou S. Effects of cultivar on phenolic content and antioxidant activity of Naked Oat in China. J Integr Agric. 2014;13:1809–1816. doi: 10.1016/S2095-3119(13)60626-7. [DOI] [Google Scholar]
- Uchegh NN (2015) Effect of germination on proximate, available phenol and flavonoid content and antioxidant activities of African Yam bean. In: International conference on chemical food and environmental engineering, Dubai (UAE)
- Vandana M, Neelam N, Vinita P. Effect of processing methods on the nutraceutical and antioxidant properties of red rice (Oryza nivara) Int J Food Sci Nutr. 2014;13(4):98–103. [Google Scholar]
- Verma M, Sharma P, Gour V, Kothari SL. Moisture mediated effects of γ-irradiation on antioxidant properties of mung bean (Vigna radiate L.) cultivars. Innov Food Sci Emerg. 2016;34:59–67. doi: 10.1016/j.ifset.2016.01.012. [DOI] [Google Scholar]
- Wani ID, Wani AA, Gani A, Muzaffar S, Gul MK, Masoodi FA, Wani TA. Effect of gamma-irradiation on physico-chemical and functional properties of arrowhead (Sagittaria sagittifolia L.) tuber flour. Food Biosci. 2015;11:23–32. doi: 10.1016/j.fbio.2015.04.003. [DOI] [Google Scholar]
- Woffenden HM, Ames JM, Chandra S, Anese M, Nicoli C. Effect of kilining on the antioxidant and pro-oxidant activities on pale malt. J Agric Food Chem. 2002;50:4925–4933. doi: 10.1021/jf020312g. [DOI] [PubMed] [Google Scholar]
- Wu XL, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen H, Li J, Pei Y, Liang Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing. LWT Food Sci technoL. 2010;43:181–185. doi: 10.1016/j.lwt.2009.06.020. [DOI] [Google Scholar]
- Zhang G, Xu Z, Gao Y, Huang X, Zou Y, Yang T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. J Food Sci. 2015;80:H1111–H1119. doi: 10.1111/1750-3841.12830. [DOI] [PubMed] [Google Scholar]
- Zhu F, Cai YZ, Bao J, Corke H. Effect of γ-irradiation on phenolic compounds in rice grain. Food Chem. 2010;120:74–77. doi: 10.1016/j.foodchem.2009.09.072. [DOI] [Google Scholar]