Abstract
Planar chirality is one of the most fascinating expressions of chirality, which is exploited by nature to lock three‐dimensional chiral conformations and, more recently, by chemists to create new chiral reagents, catalysts, and functional organic materials. Nevertheless, the shortage of procedures able to induce and secure asymmetry during the generation of these unique chiral entities has dissuaded chemists from exploiting their structural properties. This Minireview intends to illustrate the limited but remarkable catalytic methods that have been reported for the production of planar chirality in strained molecules and serve as a source of inspiration for the development of new unconventional procedures, which are expected to appear in the near future.
Keywords: asymmetric catalysis, atropisomerism, cyclophanes, macrocyclization, planar chirality
Although the benefits of molecules exhibiting planar chirality have recently been recognized in different areas of chemistry, the shortage of available catalytic methods for generating planar chirality has precluded their use. This Minireview aims to highlight the infancy of the area and serve as a source of inspiration for the design of unconventional methods for the generation of three‐dimensional complexity from accessible simple/linear molecules.
1. Introduction
Molecules exhibiting planar chirality are a paradigm of how three‐dimensional complexity produces chirality/optical activity and affects, as a consequence, molecular recognition. The introduction of a plane of chirality, as an element of chirality (stereogenic unit) to specify and explain the occurrence of chiral molecules devoid of chirality centers, goes back to 1956. [1] The first definition by Cahn, Ingold, and Prelog that “a chiral plane is caused if a plane of symmetry is destroyed in such a way that chirality arises only by the difference of both sides of the plane” had to be further elaborated to embrace additional types of chiral compounds unknown at that time. [2] Cyclophanes, rigid cycloalkenes, certain bridged annulenes, and metallocenes are prototypes of planar‐chiral structures: molecules that provoke exceptional interest from synthetic, structural, biological, and chiroptical points of view. Strained cyclophane natural products comprise a fascinating class of molecules with outstanding biological activities; [3] Galeon, a diaryl heptanoid isolated from Myrica gale L., contains a plane of chirality as the only element of asymmetry. [4] The main planes of the two ether‐linked aryl rings are close to 90° to each other and they are held together by a seven‐membered carbon chain (Figure 1 a). “Small” artificial cyclophanes behave extraordinarily well as ligands in catalytic systems, [5] for example, phanephos in Ru‐catalyzed asymmetric hydrogenations (Figure 1 b), [6] as co‐enzyme models and compact chemical miniatures of holoenzymes (Figure 1 c), [7] and as efficient catalysts in metal‐free transformations. [8] In addition, the chemical robustness of cyclophanes and cycloparaphenylenes, in their macrocyclic version, along with their capacity for long‐distance electronic communication, [9] make them suitable vehicles for host–guest chemistry, [10] for the production of planar‐chiral entities for material science, and for use as emitters of circularly polarized light (CPL; Figure 1 d).[ 11 , 12 ]
Figure 1.
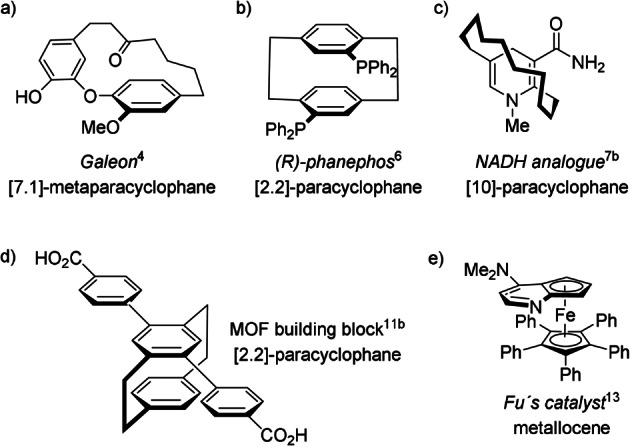
Selected examples of natural and artificial molecules exhibiting planar chirality only.
On the other hand, chiral planar structures of the metallocene family display high asymmetric induction abilities when acting as ligands or catalysts. For example, the planar‐chiral DMAP analogue shown in Figure 1 e serves as an effective catalyst for the kinetic resolution of racemic secondary alcohols. [13] Given that the huge success of chiral metallocenes, epitomized by disubstituted ferrocenes, has been the subject of numerous and recent reviews, [14] procedures for the generation of chiral metallocene and metal‐arene backbones and their applications have been considered beyond the scope of this Minireview. [15]
The design of new chiral reagents, catalysts, biologically active compounds, and functional organic materials benefits from the structural properties of these unique chiral planar entities. Nevertheless, their poor synthetic accessibility as optically pure materials has dramatically hampered the field. Herein, we seek to cover the limited, but remarkable, catalytic and enantioselective methods reported for the production of strained molecules with planar chirality according to their catalytic mode. The aim of this Minireview is to highlight the infancy of the area and serve as a source of inspiration for the design of new unconventional procedures devoted to conquering three‐dimensional complexity from accessible simple/linear molecules.
2. Background
Interest in strained compounds with planar chirality has been mainly centered around the cyclophane family (Figure 2). [16] The name, originally coined for molecules with two para‐phenylene rings held face to face by an aliphatic chain ([n.n]cyclophanes), [17] soon included structures with solely an aromatic moiety ([n]cyclophanes) and a cross‐linked side chain, [18] usually called an ansa chain. “Small” cyclophanes with short chains constitute a model for studies on fundamental aspects of strain and aromaticity. The tension imparted on the whole system generates distortion from aromatic planarity and provokes unusual reactivity behaviors. [19] In addition, the restricted rotation of the aromatic ring may generate planar chirality. The configurational stability of this stereogenic unit is hard to predict [20] and relies upon several factors, such as the length and constitution of the chain and the size of the substituents in the aromatic moiety. In contrast to chiral metallocenes and metal arenes, only one substituent is required to produce planar chirality. Chiral cyclophane structures typically belong to the para‐ and metacyclophane families, although planar chirality has also been reported for certain cyclic olefin based orthocyclophanes (Figure 2). [21]
Figure 2.
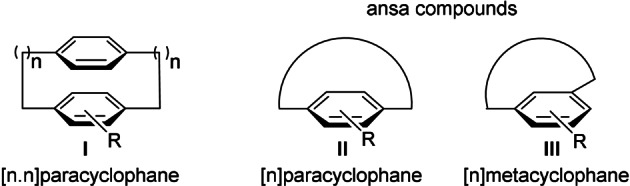
Frequent cyclophane prototypes with planar chirality.
Planar medium‐sized (E)‐cycloalkenes may also exhibit planar chirality. (E)‐Cyclooctene was the first chiral olefin synthesized, [22] but the strong dependence of the stereochemical stability on the ring size of the (E)‐cycloalkene has precluded their occurrence.
The main limiting factor for the development of this area of research is the shortage of general and efficient synthetic routes to enantiopure cyclophanes. During the last 20 years, intensive research has been carried out on chiral [2.2]cyclophane‐based backbones, generally constructed from 4‐monosubstituted [2.2]paracyclophanes. Nevertheless, their production as optically pure entities still relies on optical resolutions of racemic compounds by stoichiometric amounts of chiral reagents or chromatographic techniques.[ 5b , 8 ] In addition, their regioselective functionalization, which governs distinct features of planar chirality, represents an extra challenge, an area in which synthetic progress has recently been made. [23]
Chiral skeletons based on [n]cyclophanes, that is, chiral ansa compounds, include a vast pool of natural and artificial molecules that possess assorted structural characteristics. The macrocyclization of appropriately substituted aromatic compounds leading to ansa chains containing carbon–heteroatom or carbon–carbon bonds is usually the method of choice to accomplish their synthesis. High dilution conditions and/or conformational control elements are normally required to facilitate ring closure, regardless of the method selected to effect the macrocyclization step. [24] The racemic formation of [n]cyclophanes by constructing the aromatic ring from acyclic precursors through [2+2+2] cycloadditions [25] or from sequential Diels–Alder/retro‐Diels–Alder reactions [26] are also appealing.[ 27 , 28 ] After their synthesis, routine chemical and chromatographic resolutions are usually employed to produce them in an enantiomerically pure form. The use of asymmetric methods based on chiral reagents or chiral substrate control approaches has been comparatively less explored. [29] Interestingly, Kanomata et al. achieved the asymmetric synthesis of nicotinamide‐based [10]paracyclophanes by the spontaneous dynamic kinetic resolution of diastereomeric mixtures through crystallization. [30] It is also worth mentioning that in 2009 Suzuki and co‐workers succeeded in the asymmetric synthesis of enantiomerically pure C 2‐symmetric [10]‐ and [12]paracyclophanes by ring‐closing metathesis of chiral tetraenes, followed by subsequent desulfurization and hydrogenation (Scheme 1). [31] The hydrogen bonds established between the sulfinyl oxygen atoms and the phenol hydroxy groups in enantiomerically pure tetraenes 1 and 2 are able to induce poorly favored local conformations and enable the stereoselective formation of the corresponding ansa chain in intermediates 3 and 4. The asymmetric procedure is also compatible with 1,5‐ and 2,6‐naphthalene cores to produce [10]‐ and [12]paracyclophanes. More recently, Ohmori and co‐workers confirmed that the presence of only one chiral sulfinyl group suffices for the stereocontrolled synthesis of such C 2‐symmetric paracyclophanes. [32]
Scheme 1.
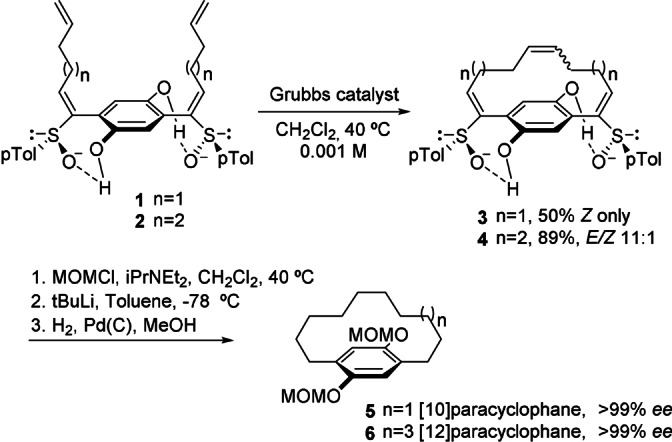
Stereoselective ring‐closing metathesis towards [10]‐ and [12]paracyclophanes.
During the last few years, asymmetric methods have been overtaken by efficient catalytic procedures for the production of chirality. Nevertheless, as is reflected in the following section, the potential of asymmetric catalysis in this particular field is far from being reached.
3. Asymmetric Catalysis
Asymmetric catalysis is widely recognized as the ideal strategy for the production of enantiomerically enriched compounds. [33] In recent decades, the organic community has made great efforts to develop new efficient asymmetric procedures, mainly devoted to produce molecules with central chirality and, more recently, axial chirality. [34] Meanwhile, planar chirality remains in its infancy and the exploitation of catalysis to induce asymmetry during the formation of planar‐chiral compounds is very uncommon. One major obstacle is to secure stable planar chirality or, in other words, the narrow line between resolvable and nonresolvable structures makes it hard to predict when planar chirality will arise. A second crucial hurdle is the assembly of the extremely rigid molecules used as starting materials, regardless of the method employed.
Catalytic routes for the synthesis of optically enriched molecules exhibiting planar chirality are mainly devoted to the production of planar‐chiral cyclophanes, with rare exceptions involving rigid cycloalkenes. In Scheme 2, these catalytic routes are exemplified for [n]cyclophanes, although they are also suitable for [n.n]cyclophanes and (E)‐cycloalkenes (with the exception of route d for the latter case):
Scheme 2.
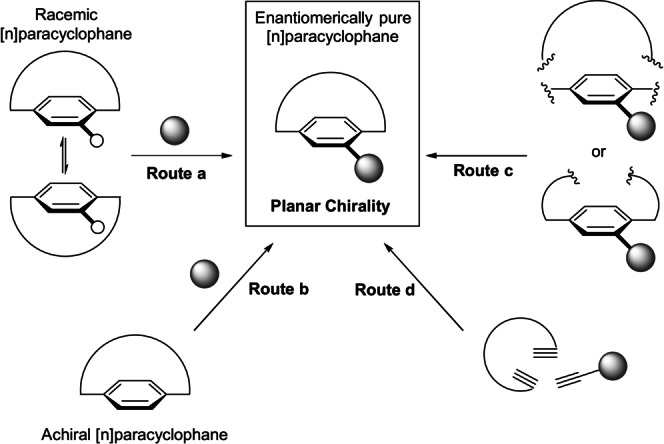
Catalytic enantioselective routes for the synthesis of planar‐chiral cyclophanes.
Route a: Kinetic dynamic resolution.
Route b: Desymmetrization and enantioselective substitutions of the aromatic ring.
Route c: Enantioselective construction of the ansa chain.
Route d: Enantioselective construction of the aromatic ring.
In the following sections, methods belonging to the routes described in Scheme 2 are presented according to the catalytic mode employed, namely chiral metal catalysis, organocatalysis, and biocatalysis.
3.1. Metal‐Catalyzed Methods
3.1.1. Kinetic Dynamic Resolutions and Desymmetrization
As mentioned above, cyclophanes are aromatic compounds that possess aliphatic chains between two non‐adjacent positions of one or two aromatic rings. When the chain(s) is (are) sufficiently long, interconversion between the enantiomers of chiral cyclophanes occurs readily, even at room temperature. Stable planar chirality may be generated by the introduction of larger and rigid moieties that prevent interconversion from occurring in a type of dynamic kinetic resolution (Scheme 2, route a). Shibata and co‐workers achieved the first catalytic construction of chiral cyclophanes by this approach through a double Pd‐catalyzed Sonogashira coupling (Scheme 3). [35] The starting achiral diiodoparacyclophane 7 was converted, in the presence of a catalyst generated in situ from PdCl2(CH3CN)2 and (R,Rp)‐Taniaphos, into differently substituted chiral [7.7]paracyclophanes 8 with significant levels of enantioinduction.
Scheme 3.
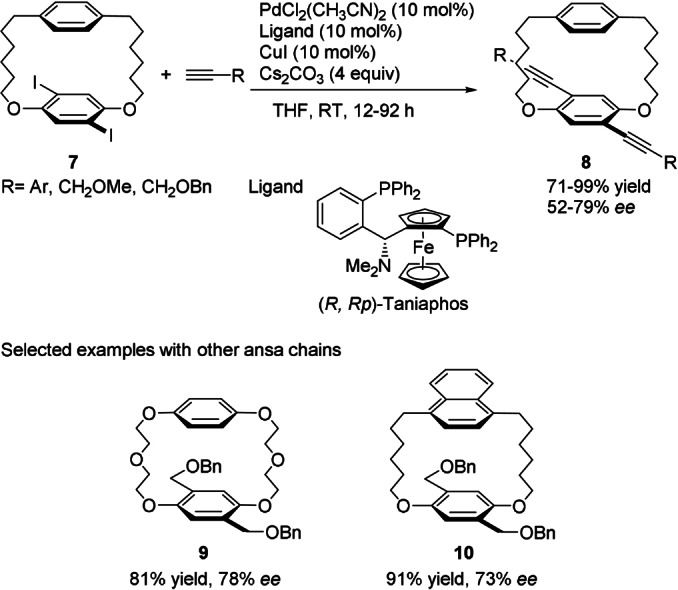
Construction of chiral [7.7]paracyclophanes through catalytic asymmetric Sonagashira coupling of racemic diiodoparacyclophanes.
Shibata and co‐workers were also able to produce planar chirality through the catalytic asymmetric ortho‐litiation and dilithiation of achiral 1,n‐dioxa[n]paracyclophanes with directing groups at the 1‐ and n‐positions of the ansa chain (Scheme 4).[ 36 , 37 ] The use of substochiometric amounts of (−)‐sparteine—to generate the chiral lithium reagent—and the subsequent quenching of the aryllithium compounds with iodine afforded the corresponding dioxa[n]paracylophanes 13 (n=10) and 14 (n=11) in good yields and enantioselectivities. A second lithiation provided diiododioxa[n]paracyclophanes 15 (n=10) and 16 (n=11) with slightly higher enantiomeric excess, probably because kinetic resolution occurred at the second lithiation step. Other electrophiles, such as MeI, DMF, benzophenone, and PPh2Cl, were also explored, but stoichiometric amounts of (−)‐sparteine were required to avoid sluggish metalations.
Scheme 4.
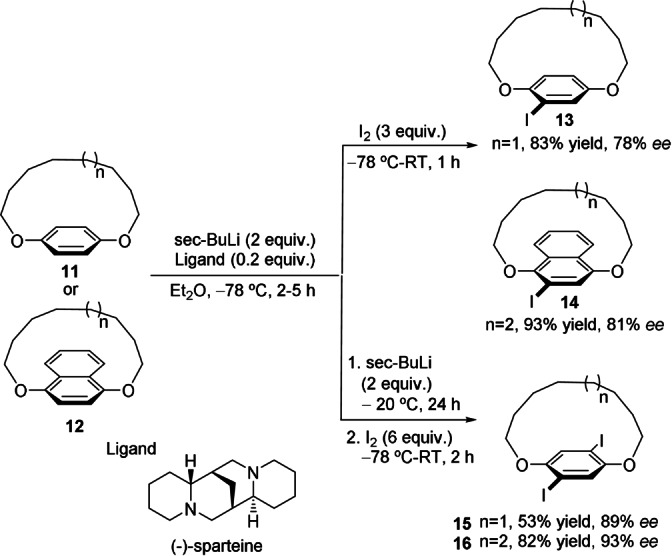
Catalytic enantioselective lithiation and dilithiation of dioxa[10]paracyclophanes and dioxa[11]paracyclophanes.
Benedetti, Micouin, and co‐workers recently documented the first desymmetrization of the centrosymmetric pseudo‐para‐diformyl[2.2]paracyclophane 17 by asymmetric transfer hydrogenation (ATH), [38] a catalytic method that falls into route b of Scheme 2. RuCl(p‐cymene)‐[(R,R)‐Ts‐DPEN] was confirmed to be the best catalyst to promote the desymmetrization to produce enantiomerically pure [2.2]paracyclophane 18 in high yield (Scheme 5). Interestingly, the reaction was run on a 1 g scale and both enantiomers of 18 were easily accessible by switching from the (R,R)‐ to the (S,S)‐Ru catalyst.
Scheme 5.

Gram‐scale synthesis of enantipure [2.2]paracyclophane 18 by catalytic ATH desymmetrization. DPEN: 1,2‐diphenyl‐1,2‐ethylenediamine.
3.1.2. Construction of the Ansa Chain
The first asymmetric synthesis of chiral cyclophanes through the catalytic enantioselective construction of the ansa chain (Scheme 2, route c) was described by Tanaka et al. in 2007 (Scheme 6). [39] The cationic rhodium(I)/(S)‐binaphane complex 20 promoted the reaction of dithiols 21 and disubstituted 1,4‐bis(bromomethyl)benzenes 22, thereby leading to dithioparacyclophanes 23 and 24 in low to moderate yields and enantioselectivities. Afterwards, the same group showed that a less‐expensive cationic palladium(II)/(R)‐binap complex was able to promote the above transformations with similar levels of reactivity and selectivity. [40]
Scheme 6.
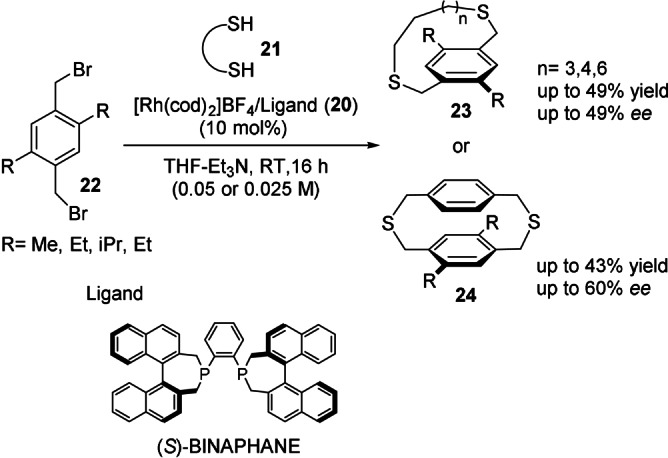
Rhodium‐catalyzed synthesis of enantioenriched dithia[n]paracyclophanes (n=9, 10, 12) and dithia[3.3]paracyclophanes.
In the context of the synthesis of natural products, Quamar Salih and Beaudry employed an enantioselective Ullman ether coupling from intermediate 25 to prepare diaryl ether heptanoids with planar chirality (Scheme 7). [41] The combined use of N‐methylproline and K3PO4 in the presence of CuI enabled the synthesis of enantioenriched cyclophane‐based diaryl ether 26, the direct precursor of diaryl ether heptanoid natural products such as (+)‐galleon and (+)‐pterocarine. Interestingly, racemization of the chiral [7.1]cyclophane 26 does not occur at the temperature of the Ullman coupling. The application of the Ullman reaction conditions to the required intermediate 29 led, after subsequent transformations, to the synthesis of (−)‐myricatomentogenin (30) and (−)‐jugcanthanin (31) with comparable levels of selectivity.
Scheme 7.
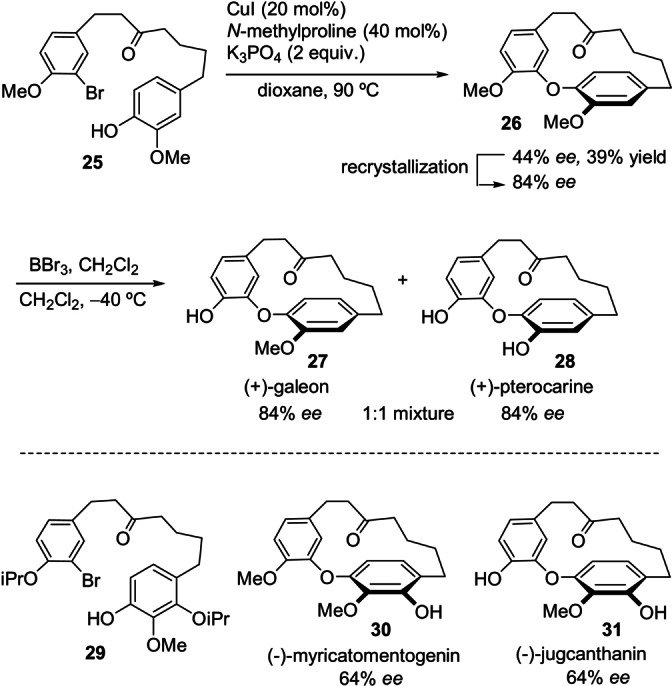
Synthesis of enantioenriched natural diaryl ether heptanoids by enantioselective Ullman ether coupling.
Palladium‐catalyzed asymmetric allylic substitutions (AAS) have traditionally been employed to generate central chirality through the stereoselective nucleophilic substitution of planar‐chiral π‐allyl‐Pd complexes. Tomooka and co‐workers applied this method for the first time to produce planar‐chiral nine‐membered cyclic amides. [42] The intramolecular Pd‐catalyzed AAS reaction of the achiral linear E‐alkene precursor, represented as 32 in Scheme 8, in the presence of the DACH‐phenyl Trost ligand produced the corresponding chiral cyclic amides of type 33 with moderate to excellent enantioselectivity. Theoretical studies corroborated that the enantiotopic faces of the π‐allyl‐Pd complex intermediate derived from the E‐alkene precursor were discriminated by the steric effect of the R1 group during the cyclization.
Scheme 8.
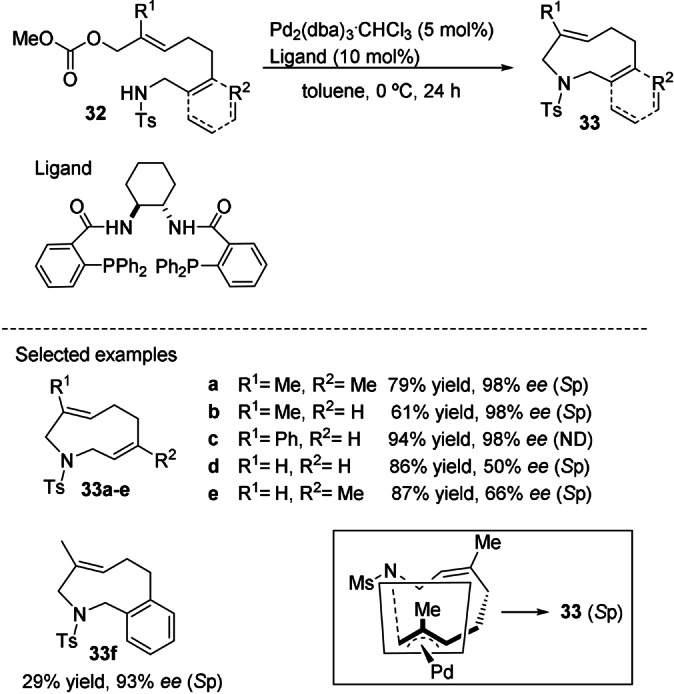
Pd‐catalyzed enantioselective synthesis of planar‐chiral cyclic amides and the transition‐state model that explains the stereoselectivity. Yields determined by 1H NMR spectroscopy. ND: absolute configuration not determined.
With the exception of the enantioselective Pd‐catalyzed AAS reaction, which produces chiral nine‐membered cycloalkenes, and the asymmetric transfer hydrogenation of pseudo‐para‐diformyl[2.2]paracyclophane, the above‐described metal‐catalyzed methods give access exclusively to medium‐strained paracyclophanes with heteroatom‐containing ansa chains.
3.1.3. Aromatic Ring Construction
The construction of the aromatic ring by alkyne cyclotrimerization (Scheme 2, route d) was actually the first successful approach towards the catalytic synthesis of enantiomerically enriched [n]cyclophanes. Tanaka et al. and others[ 43 , 44 ] reported a series of transition‐metal‐catalyzed inter‐/intramolecular [2+2+2] cycloadditions with more or less success. In 2007, the Tanaka group achieved the first successful catalytic enantioselective synthesis of planar‐chiral [7]–[10]metacyclophanes by means of an intramolecular alkyne cyclotrimerization catalyzed by the cationic rhodium(I)/(R)‐H8‐binap complex (Scheme 9 a). [45] The use of linear triynes 34 bearing substituents at the two alkyne termini impeded ring flipping, thus illustrating the importance of the ansa chain design. Despite metacyclophanes 35 being obtained as the minor isomers in low yields, the enantiomeric excesses were remarkably high for all examples (88–98 % ee). The preferential formation of intermediate IV could explain the enantioselectivity observed as a result of the high reactivity of the 1,6‐diyne moiety with the rhodium(I) complex, the coordination of the methoxy group, and the steric factors between the ansa chain and the PPh2 groups in the ligand (Scheme 9 b).
Scheme 9.
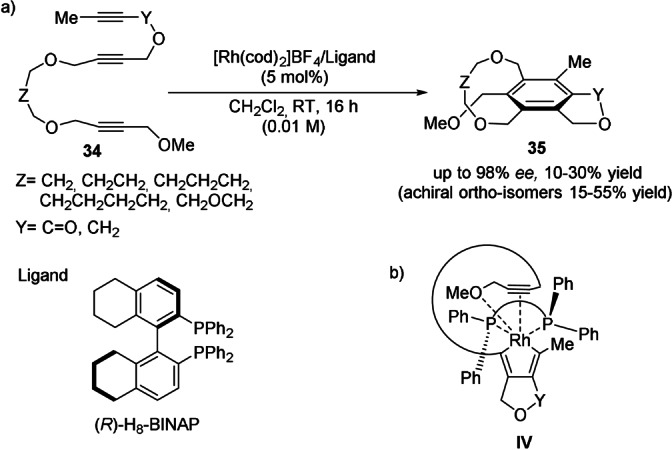
a) Enantioselective synthesis of chiral [7]–[10]metacyclophanes through rhodium‐catalyzed alkyne cyclotrimerization. b) Proposed reaction intermediate.
In 2009, Shibata et al. used an analogous approach for the intramolecular cyclotrimerization of nitrogen‐branched triynes of type 36 to produce chiral tripodal cage compounds 37. The [2+2+2] cycloaddition promoted by a cationic rhodium(I)/(S,S)‐Me‐DUPHOS complex afforded [8]‐, [9]‐, [10]‐, [11]‐, and [15]‐cyclophanes in good yield and excellent enantiomeric excesses (Scheme 10). [46]
Scheme 10.
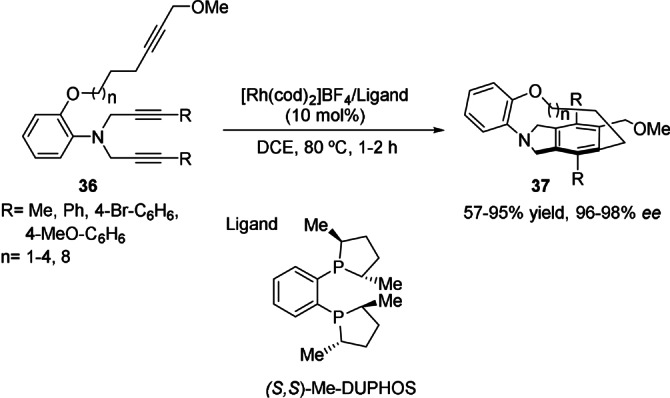
Enantioselective synthesis of chiral tripodal cage compounds 37 by rhodium‐catalyzed cyclotrimerization of nitrogen‐branched triynes. DCE: 1,2‐dichloroethane.
A few years later, Tanaka and co‐workers demonstrated that cationic rhodium(I)chiral bisphosphine complexes catalyzed the intermolecular [2+2+2] cycloaddition reactions of cyclic diynes, of type 38, with monoynes to afford planar‐chiral carba‐[10]–[12]paracyclophanes of type 39 in good yields and with moderate to excellent enantioselectivities (Scheme 11). [47]
Scheme 11.
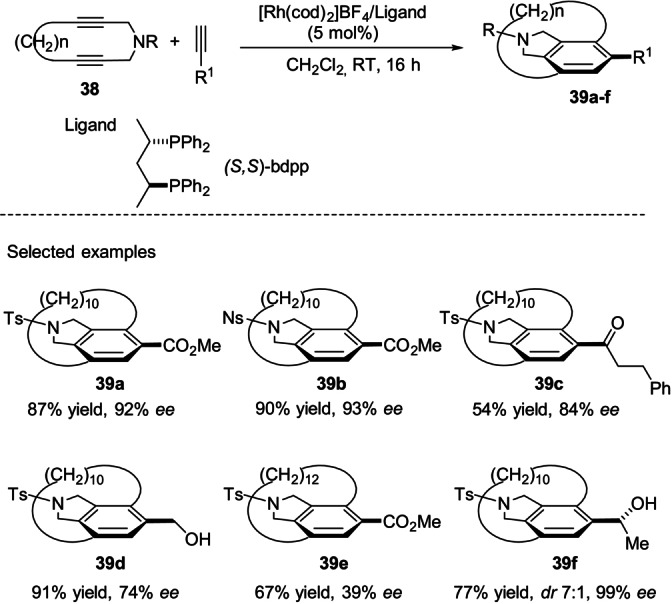
Enantioselective synthesis of [10]‐ and [12]paracyclophanes by rhodium‐catalyzed [2+2+2]cycloaddition of cyclic diynes with terminal monoynes.
As mentioned in the introduction, interest in the design of new reaction patterns and applications of the cyclophanes as functional materials is rapidly pushing the field. Very recently, the Tanaka group applied the successful rhodium‐catalyzed [2+2+2] intramolecular cycloaddition of enediynes 40 for the first regio‐ and enantioselective construction of planar‐chiral bent cyclophane structures based on polycyclic aromatic hydrocarbons (PAHs; Scheme 12). [48] The 9‐fluororenol‐based cyclophanes 42, obtained with excellent enantiomeric excess after stepwise oxidative transformations of intermediates 41, exhibited fluorescence quantum yields that were significantly higher than those of the acyclic analogues.
Scheme 12.
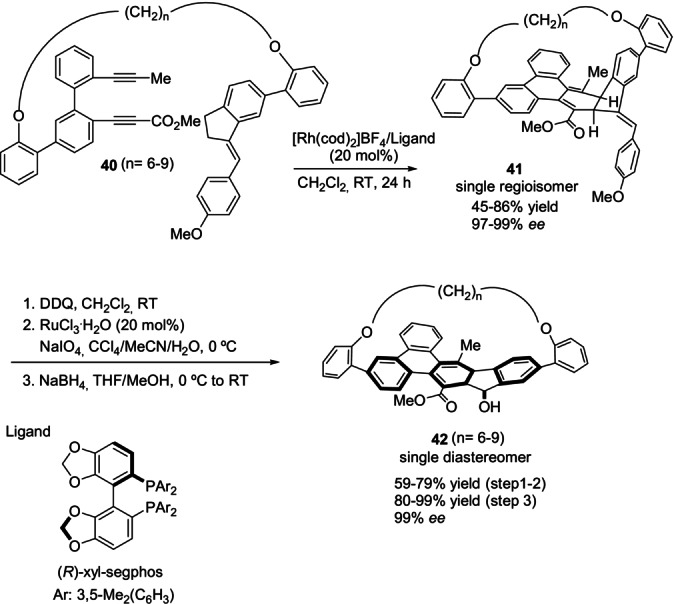
Selected examples of the enantioselective synthesis of PAH‐based planar chiral bent cyclophanes. DDQ: 2,3‐dichloro‐5,6‐dicyano‐1,4‐benzoquinone.
Concurrently, Tanaka and co‐workers also achieved the synthesis of planar‐chiral zigzag‐type [8]‐ and [12]cyclophenylene belts in good yield and high enantiomeric excess by the rhodium‐catalyzed sequential intramolecular cyclotrimerization of the corresponding cyclic polyynes. [49] Macroetherification of 43 and 44 produced cyclic dodecayne 45 and pentadecayne 46 in yields of 9 % and 10 %, respectively (Scheme 13). The intramolecular sequential quadruple cyclotrimerization of 45, using a cationic rhodium catalyst, produced the planar‐chiral zigzag‐type [8]cyclophenylene belt 47 in good yield and excellent enantioselectivity. Although the zigzag‐type vector for 47 is achiral, the asymmetric substitution in the skeleton (R=nBu) produces planar chirality (Scheme 13, bottom). The sequential sextuple cyclotrimerization of 46, under the same reaction conditions, also produced the corresponding [12]cyclophenylene belt with similar levels of selectivity. By using this strategy, Tanaka and co‐workers have recently succeeded in synthesizing cycloparaphenylene rings with excellent diastereo‐ and enantiocontrol. [50]
Scheme 13.
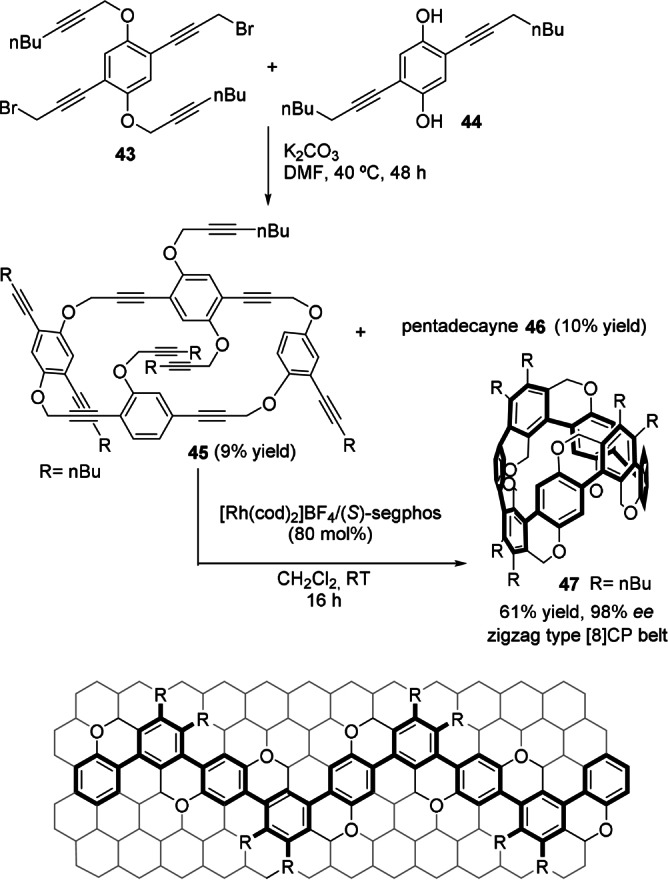
Enantioselective synthesis of planar‐chiral zigzag‐type [8]cyclophenylene belt 47. Segphos: 5,5′‐bis(diphenylphosphino)‐4,4′‐bi‐1,3‐benzodioxole.
3.2. Organocatalytic Methods
Organocatalysis is one of the most reliable asymmetric methods to produce enantiomerically pure compounds. [51] During the last decade, the blossoming of organocatalytic methods—based on unconventional and nontraditional polar mechanisms—has enabled the construction of molecules and structural scaffolds that, previously, were rarely thought to be substrates for organocatalysis. Nevertheless, this discipline lies far behind in offering reliable methods for conquering planar chirality. The scarcity of procedures (shown below) clearly exemplifies the immaturity of the field. [52]
Interestingly, one of the first catalytic approaches to induce planar chirality was effected by using planar‐chiral organic molecules as sensitizers in photochemical reactions. Kanomata, Inonue, and co‐workers detected planar to planar‐chirality transfer in the photoisomerization of cyclooctenes. For example, the photoisomerization of (1Z,5Z)‐cycloocta‐1,5‐diene sensitized by [10]paracyclophane 48 afforded the (1E,5Z)‐steroisomer upon irradiation. The enantiomeric excess gradually increased as the irradiation temperature was decreased, which enabled the (Rp)‐(1E,5Z) isomer to be produced with 87 % ee at −140 °C (Scheme 14). [53]
Scheme 14.
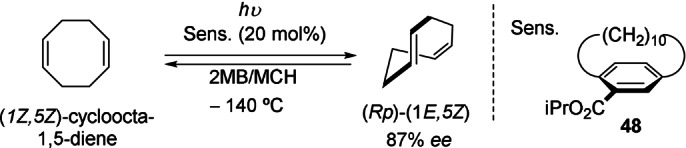
Planar to planar chirality transfer in the photoisomerization of cyclooctenes. 2MB: 2‐methylbutane; MCH: methylcyclohexane.
Prior to the synthesis of planar‐chiral nine‐membered cyclic amides by enantioselective Pd‐catalyzed AAS reactions (Scheme 8), Tomooka et al. tried to address the enantioselective preparation of planar‐chiral organonitrogen cycles from achiral linear precursors under organocatalytic conditions (Scheme 15). [54] The chiral linear amino halide 49 smoothly produced the corresponding planar‐chiral cyclic amide 50 under chiral phase‐transfer conditions. As representative results, the chiral salts 51 and 52 derived from the cinchona alkaloid imparted moderate levels of asymmetric induction, regardless of their chemical efficiency. Other structurally related chiral promoters provided similar levels of enantioselectivity, even when added in stoichiometric amounts. Modifications to the anion part instead had a positive impact on the stereoselectivity of the reaction, although stoichiometric amounts of promoter were finally required to obtain highly enantioenriched chiral nine‐membered cyclic amides; [55] for example, the lithium salt of sugar‐derived 53 produced the chiral nine‐membered cyclic amide Sp‐ 50 with 93 % ee.
Scheme 15.
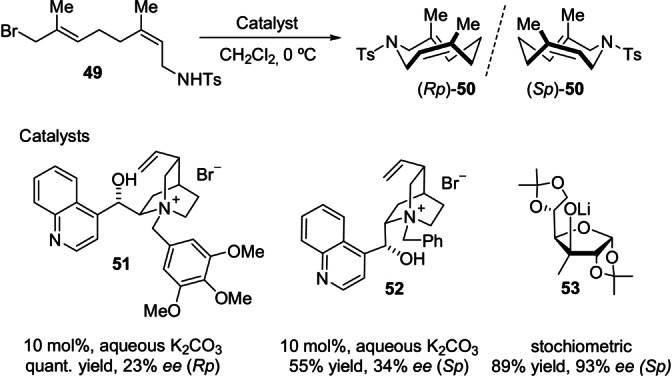
Enantioselective synthesis of chiral cyclic amide 50.
In contrast, catalytic chiral phase‐transfer conditions were suitable for SNAr cyclizations for the synthesis of [1,7]metaparacyclophanes. Cai and co‐workers reported that achiral linear diarylheptanoids of type 54 undergo cyclization to generate oxa[1,7]metaparacyclophanes 56 in the presence of chiral salts derived from cinchona alkaloids (Scheme 16). [56] Optimal reaction conditions, in which catalyst 55 was employed, gave rise, in good yields and enantioselectivities, to metaparacyclophanes 56 that were differently decorated in the ansa chain. Highly enantioenriched 56 a was elaborated into a direct precursor of (−)‐galleon and (−)‐pterocarine.
Scheme 16.
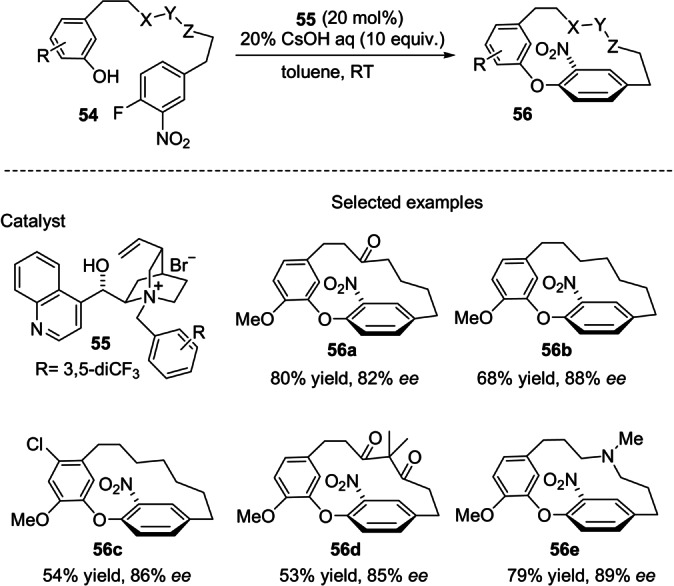
Asymmetric PTC‐catalyzed synthesis of diaryl ether cyclophane skeletons.
3.3. Biocatalytic Methods
The demands of modern synthesis require the continued development of novel and more efficient catalytic procedures. To this end, biocatalytic methods are becoming powerful tools, especially when it comes to transformations that require high chemo‐, regio‐, or stereoselectivity. [57] Only a couple of biocatalytic methods have recently appeared for the synthesis of molecules exhibiting planar chirality, but they perfectly exemplify the new opportunities offered by planar chirality.
Collins and co‐workers have reported the atroposelective biocatalytic synthesis of planar‐chiral macrocycles from common and simple building blocks (Scheme 17). [58] The authors were able to extend the well‐known ability of hydrolases to impart high asymmetric induction in reactions with stereogenic carbon atoms to reactions using stereogenic planes. The proper combination of benzylic‐substituted diols 57 and diacids 58 as aliphatic linkers modulates the Candida Antarctica lipase B (CALB) action to discriminate between diverse conformations while ensuring the configurational stability of the generated planar artificial chiral macrocycles 59. The active site of the enzyme tolerates several substitutions, both at the aromatic ring in 57 and the ansa chain in 58, thereby producing [12]‐, [13]‐, [14]‐, and [15]paracyclophanes such as 59 a–e generally in good yields and high enantioselectivities. Furthermore, halogen‐containing macrocycles of type 59 a, are suitable for producing complex diversity through cross‐coupling methods, as illustrated by compound 60.
Scheme 17.
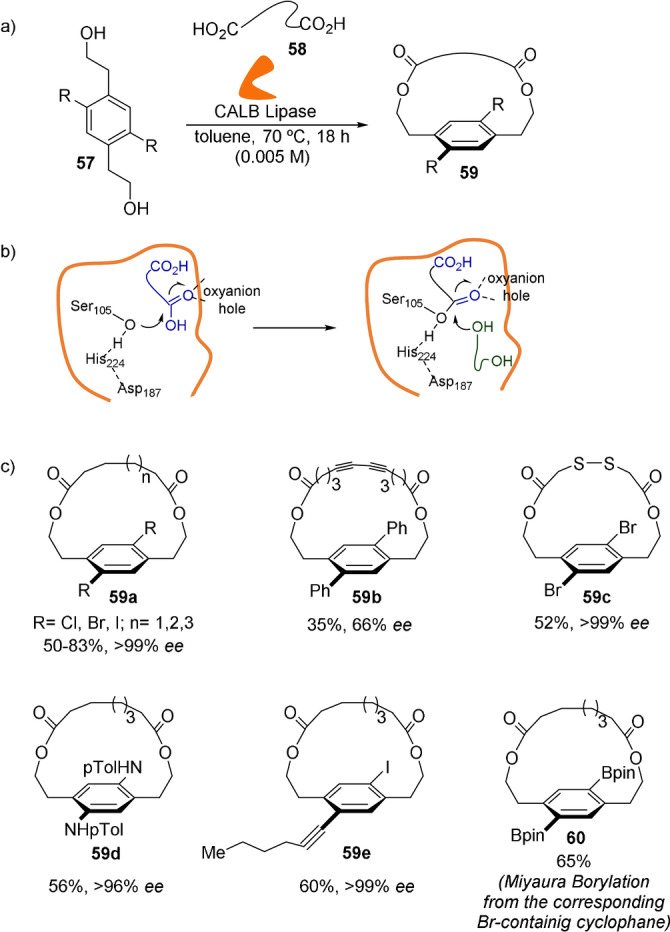
a) Reaction conditions for the biocatalytic synthesis of chiral [12]‐, [13]‐, [14]‐, and [15]paracyclophanes. b) Mechanism of the acylation reaction promoted by the Ser‐His‐Asp catalytic triad and the oxyanion hole. c) Selected examples of the atroposelective macrocyclization.
Almost simultaneously, Morinaka and co‐workers identified a series of post‐translational modifying enzymes from bacteria that promote the macrocyclizations of linear peptides through C−C bond formation. [59] In particular, an uncharacterized subfamily of radical S‐adenosylmethionine (rSAM) maturases enables the abstraction of hydrogen at inert sp3‐hybridized carbon atoms and their subsequent fusion to aromatic rings, at three residue motifs, to produce strained cyclophane macrocycles such as 61 and 62 (Figure 3). The formation of single or multiple cyclophanes can occur with different substituted templates. The macrocycles display restricted rotation and planar chirality when the chiral plane is the substituted indole. The three‐residue cyclophanes define a new family of cyclic ribosomally synthesized and post‐translationally modified (RiPP) peptides, which has been named “triceptide” (three‐residues in a cyclophane peptide).
Figure 3.
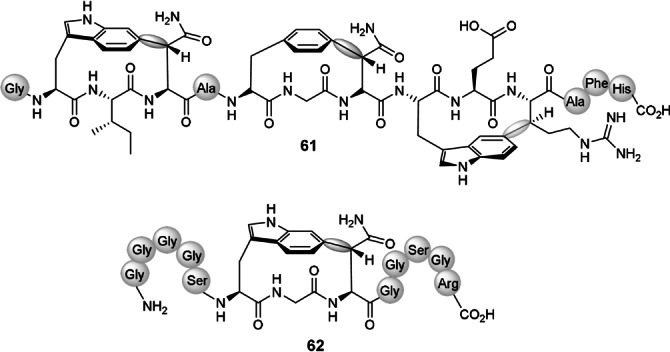
Planar‐chiral “triceptide” 61 and planar‐chiral cyclophane 62 post‐translationally produced in bacteria. The C−C bonds forming the cyclophanes are marked in gray.
It might appear that these two new examples address and solve specific issues for the production of enantiomerically pure unnatural molecules and peptides exhibiting planar chirality, but they actually offer a whole lot more. The macrocyclizations shown exemplify how the partial domestication of biological systems imparts reactivity and asymmetric induction through the seco acid strategy and the functionalization of chemically inert C−H bonds, methods that have no success in metal‐ and organocatalyzed transformations.
4. Conclusions
Molecules exhibiting planar chirality were initially treated as a chemical curiosity and strategies for their asymmetric synthesis were rare. Nowadays, their occurrence in biologically active molecules and the discovery of their optoelectronic properties, along with the development of efficient and high‐yielding synthetic methods to generate chiral derivatives, are extending the field. Despite recent developments gradually enabling their preparation through catalytic procedures involving a variety of reaction mechanisms, the area is in the early stages of its development. Catalytic routes are mainly based on metals and devoted to the production of planar‐chiral [n]paracyclophanes. In this regard, the underlying limitations are the difficulty to produce [n]paracyclophanes with greater strain and the lack of procedures for the production of full‐carbon ansa chains. The catalytic synthesis of [n]metacyclophanes and [n.n]paracyclophanes is far behind, with the case of the valuable chiral [2,2]paracyclophanes being remarkably striking. On the other hand, organocatalytic asymmetric methods are completely undeveloped, whereas the biocatalytic processes are remarkable; although very limited, they highlight that some of the most important challenges that the organic community need to face nowadays are “simplicity” and “diversity”: Simplicity in the sense of conquering three‐dimensional complexity using a pool of accessible linear molecules or fragments and diversity in the sense of identifying new product families to define distinct regions in sequence‐function space. In the same way that medicinal chemistry is escaping from the flatland towards molecules with a higher fraction of sp3‐hybridized centers, molecules exhibiting planar chirality would enlarge the pool of entities capable of accessing greater chemical space.[ 60 , 61 ]
The examples presented in this Minireview are expected to serve as a source of inspiration and challenge the design of unconventional and ambitious procedures (a mine for catalysis) towards the production of new molecules exhibiting planar chirality (a mine for structure discovery) with potential applications in catalysis, medicinal chemistry, and material science
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Rosa López obtained her PhD in 1994 at the Universidad Autónoma de Madrid under the guidance of Professor Alfonso Fernández‐Mayoralas. After postdoctoral research with Prof. Robert R. Rando (Harvard University) and Prof. Gregory C. Fu (MIT), she joined the Department of Organic Chemistry at San Sebastián in 1998. She was promoted to Associate Professor in 2012.

Biographical Information
Claudio Palomo studied Chemistry at the Instituto Químico de Sarria, in Barcelona, where he received his Chemical Engineering Degree in 1975. He obtained his Licenciatura in Chemistry in 1979 at the University of Barcelona and his PhD in 1983 at the University of the Basque Country under the supervision of Prof. R. Mestres. In 1989 he was promoted to Professor and in 1991 he joined Prof. H. Rapoport at the University of California at Berkeley as a visiting professor.

Acknowledgements
We thank the University of the Basque Country UPV/EHU (UFIQOSYC 11/22), the Basque Government (grant IT‐1236‐19) and Ministerio de Ciencia e Innovation (grant PID2019‐109633GB‐C21), Spain, for their continuous financial support.
R. López, C. Palomo, Angew. Chem. Int. Ed. 2022, 61, e202113504; Angew. Chem. 2022, 134, e202113504.
Contributor Information
Dr. Rosa López, Email: rosa.lopezalvarez@ehu.es.
Prof. Dr. Claudio Palomo, Email: claudio.palomo@ehu.es.
References
- 1. Cahn R. S., Ingold C. K., Prelog V., Experientia 1956, 12, 81–94. [Google Scholar]
- 2.
- 2a. Lemière G. L., Alderweireldt F. C., J. Org. Chem. 1980, 45, 4175–4179; [Google Scholar]
- 2b. Compendium of Chemichal Terminology “The Gold Book” (Eds.: McNaught A. D., Wilkinson A.), Blackwell Scientific Publications, Oxford, 1997; [Google Scholar]
- 2c. Eliel E. L., Wilen S. H. in Stereochemistry of Organic Compounds, Wiley, Hoboken, 1994. [Google Scholar]
- 3.For a review, see Gulder T., Baran P. S., Nat. Prod. Rep. 2012, 29, 899–934. [DOI] [PubMed] [Google Scholar]
- 4. Malterud K. E., Anthonsen T., Hjortås J., Tetrahedron Lett. 1976, 17, 3069–3072. [Google Scholar]
- 5.For leading references, see
- 5a. Gibson S. E., Knight J. D., Org. Biomol. Chem. 2003, 1, 1256–1269; [DOI] [PubMed] [Google Scholar]
- 5b. Hassan Z., Spuling E., Knoll D. M., Lahann J., Bräse S., Chem. Soc. Rev. 2018, 47, 6947–6963; [DOI] [PubMed] [Google Scholar]
- 5c. Bräse S. in Planar Chiral Ligands based on [2.2]Paracyclophanes. Asymmetric Synthesis: The Essentials (Eds.: Christmann M., Bräse S.), Wiley-VCH, Weinheim, 2006. [Google Scholar]
- 6. Pye P. J., Rossen K., Reamer R. A., Tsou N. N., Volante R. P., Reider P. J., J. Am. Chem. Soc. 1997, 119, 6207–6208. [Google Scholar]
- 7.For pioneering works, see
- 7a. Kanomata N., Nakata T., Angew. Chem. Int. Ed. Engl. 1997, 36, 1207–1211; [Google Scholar]; Angew. Chem. 1997, 109, 1263–1266; [Google Scholar]
- 7b. Kanomata N., Nakata T., J. Am. Chem. Soc. 2000, 122, 4563–4568; Recent works: [Google Scholar]
- 7c. Zhou Z.-H., Ding Y.-X., Wu B., Zhu Y.-G., Chem. Sci. 2020, 11, 10220–10224; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7d. Zhu Z.-H., Ding Y.-X., Zhou Y.-G., Tetrahedron 2021, 83, 131968. [Google Scholar]
- 8. Felder S., Wu S., Brom J., Micouin L., Benedetti E., Chirality 2021, 33, 506–527. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Morisaki Y., Chujo Y., Angew. Chem. Int. Ed. 2006, 45, 6430–6437; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 6580–6587; [Google Scholar]
- 9b. Elacqua E., MacGillivray L. R., Eur. J. Org. Chem. 2010, 6883–6894. [Google Scholar]
- 10.
- 10a. Gavin J. A., Deng N., Alcalá M., Mallouk E., Chem. Mater. 1998, 10, 1937–1944; [Google Scholar]
- 10b. Ramaiah D., Neelakandan P. P., Nair A. K., Avirah R. R., Chem. Soc. Rev. 2010, 39, 4158–4168; [DOI] [PubMed] [Google Scholar]
- 10c. Liu Z., Zhang H., Han J., Org. Biomol. Chem. 2021, 19, 3287–3302. [DOI] [PubMed] [Google Scholar]
- 11.For selected references, see
- 11a. Cragg P. J., Sharma K., Chem. Soc. Rev. 2012, 41, 597–607; [DOI] [PubMed] [Google Scholar]
- 11b. Cakici M., Gu Z.-G., Nieger M., Bürck J., Heinke L., Bräse S., Chem. Commun. 2015, 51, 4796–4798; [DOI] [PubMed] [Google Scholar]
- 11c.Ref. [5b];
- 11d. Sasai Y., Inoue R., Morisaki Y., Bull. Chem. Soc. Jpn. 2020, 93, 1193–1199; [Google Scholar]
- 11e. Chen J.-F., Ding J.-D., Wei T.-B., Chem. Commun. 2021, 57, 9029–9039; [DOI] [PubMed] [Google Scholar]
- 11f. Morisaki Y., in Circularly Polarized Luminescence of Isolated Small Organic Molecules, (Ed: Mori T.), Springer, Singapore, 2020, pp. 31–52. [Google Scholar]
- 12.Chiral inversion in configurationally labile planar-chiral structures, promoted by external stimuli, is also of high interest for its applications in material science and nanotechnology. For selected references, see
- 12a. Wang X., Jia F., Yang L.-P., Zhou H., Jang W., Chem. Soc. Rev. 2020, 49, 4176–4188; [DOI] [PubMed] [Google Scholar]
- 12b. Liu M., Plum E., Li H., Duan S., Li S., Xu Q., Zhang X., Zhang C., Zou C., Jin B., Han J., Zhang W., Adv. Opt. Mater. 2020, 8, 2000247; [Google Scholar]
- 12c. Fa S., Adachi K., Nagata Y., Egami K., Kato K., Ogoshi T., Chem. Sci. 2021, 12, 3483–3488; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12d. Chen Y., Fu L., Sun B., Qian C., Pangannaya S., Zhu H., Ma J., Jiang J., Ni Z., Wang R., Lu X., Wang L., Chem. Eur. J. 2021, 27, 5890–5896; [DOI] [PubMed] [Google Scholar]
- 12e. Yao J., Wu W., Xiao C., Su D., Zhong Z., Mori T., Yang C., Nat. Commun. 2021, 12, 2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruble J. C., Latham H. A., Fu G. C., J. Am. Chem. Soc. 1997, 119, 1492–1493. [Google Scholar]
- 14.
- 14a. Atkinson R. C. J., Gibson V. C., Long N. J., Chem. Soc. Rev. 2004, 33, 313–328; [DOI] [PubMed] [Google Scholar]
- 14b. Gómez Arrayás R., Adrio J., Carretero J. C., Angew. Chem. Int. Ed. 2006, 45, 7674–7715; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 7836–7878; [Google Scholar]
- 14c. Fu G. C., Acc. Chem. Res. 2006, 39, 853–860; [DOI] [PubMed] [Google Scholar]
- 14d. Miyake Y., Nishibayashi Y., Uemura S., Synlett 2008, 1747–1758; [Google Scholar]
- 14e. Noël T., Van der Eycken J., Green Process Synth. 2013, 2, 297–309; [Google Scholar]
- 14f. Arae S., Ogasawara M., Tetrahedron Lett. 2015, 56, 1751–1761; [Google Scholar]
- 14g. Butt N. A., Liu D., Zhang W., Synlett 2014, 25, 615–630; [Google Scholar]; Yoshida K., Yasue R., Chem. Eur. J. 2018, 24, 18575–18586; [DOI] [PubMed] [Google Scholar]
- 14h. Liu C.-X., Gu Q., You S.-L., Trends Chem. 2020, 737–749; [Google Scholar]
- 14i. Ogasawara M., Chem. Rec. 2021, 21, 3509–3519; [DOI] [PubMed] [Google Scholar]
- 14j. Malinakova H. C., Arkivoc 2021, 157–184; [Google Scholar]
- 14k. Chiral Ferrocenes in Asymmetric Catalysis: Synthesis and Applications, (Eds.: Dai L.-X., Hou X.-L.), Wiley-VCH, Weinheim, 2010; [Google Scholar]
- 14l. David O. R. P. in Planar Chiral Catalysts, Comprehensive Enantioselective Organocatalysis, (Ed.: Dalko P. I.), Wiley-VCH, Weinheim, 2013, pp. 195–219. [Google Scholar]
- 15.On some occasions, the term “metallocene chirality” is preferred to describe unsymmetrically disubstituted (chiral)metallocenes and arenes:
- 15a. Kalchhauser H., Schlögl K., Weissensteiner W., Werner A., J. Chem. Soc. Perkin Trans. 1 1983, 1723–1729; [Google Scholar]
- 15b. Schlögl K., Werner A., Widhalm M., J. Chem. Soc. Perkin Trans. 1 1983, 1731–1735. [Google Scholar]
- 16.
- 16a. Smith B. H., Bridged Aromatic Compounds, Academic Press, New York, 1964; [Google Scholar]
- 16b. Keehn P. M., Rosenfeld S. M. in Cyclophanes, Academic Press, New York, 1983; [Google Scholar]
- 16c. Diederich F. in Cyclophanes, Royal Society of Chemistry, Cambridge, 1991; [Google Scholar]
- 16d. Vögtle F. in Cyclophane Chemistry, Wiley-VCH, Weinheim, 1993; [Google Scholar]
- 16e. Gleiter R., Hopf H. in Modern Cyclophane Chemistry, Wiley-VCH, Weinheim, 2004; for a special issue on cyclophane chemistry, see [Google Scholar]
- 16f. Isr. J. Chem. 2012, 52, 1–192;
- 16g. Kotha S., Shirbhate M. E., Waghule G. T., Beilstein J. Org. Chem. 2015, 11, 1274–1331; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16h. Liu Z., Nalluri S. K. M., Stoddart J. F., Chem. Soc. Rev. 2017, 46, 2459–2478. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Cram D. J., Steinberg H., J. Am. Chem. Soc. 1951, 73, 5691–5704; [Google Scholar]
- 17b. Cram D. J., Cram J. M., Acc. Chem. Res. 1971, 4, 204–213. [Google Scholar]
- 18.
- 18a. Vögtle F., Tetrahedron Lett. 1969, 10, 3193–3196; [Google Scholar]
- 18b. Vögtle F., Neumann P., Tetrahedron Lett. 1969, 10, 5329–5334. [Google Scholar]
- 19.
- 19a. Noble K.-L., Hopf H., M. Jones, Jr. , Kammula S. L., Angew. Chem. Int. Ed. Engl. 1978, 17, 602–602; [Google Scholar]; Angew. Chem. 1978, 90, 629–630; [Google Scholar]
- 19b. Murad A. F., Kleinschroth J., Hopf H., Angew. Chem. Int. Ed. Engl. 1980, 19, 389–390; [Google Scholar]; Angew. Chem. 1980, 92, 388–389; [Google Scholar]
- 19c. Kane V. V., De Wolf W. H., Bickelhaupt F., Tetrahedron 1994, 50, 4575–4622; [Google Scholar]
- 19d. Henseler D., Hohlneicher G., J. Phys. Chem. A 1998, 102, 10828–10833; [Google Scholar]
- 19e. Smith P. J., Liebman J. F. in Strained Aromatic Molecules Strained Hydrocarbons: Beyond the van't Hoff and Le Bel Hypothesis, (Ed: Dodziuk H.) Wiley-VCH, Weinheim, 2009, pp. 147. [Google Scholar]
- 20.For selected references, see
- 20a. Lüttringhaus A., Gralheer H., Liebigs Ann. 1942, 550, 67–98; [Google Scholar]
- 20b. Hochmuth D. H., Konig W. A., Liebigs Ann. 1996, 947–951; [Google Scholar]
- 20c. Igawa K., Asano S., Yoshida Y., Kawasaki Y., Tomooka K., J. Org. Chem. 2021, 86, 9651–9657. [DOI] [PubMed] [Google Scholar]
- 21.
- 21a. Tomooka K., Iso C., Uehara K., Suzuki M., Nishikawa-Shimono R., Igawa K., Angew. Chem. Int. Ed. 2012, 51, 10355–10358; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 10501–10504. [Google Scholar]
- 22.
- 22a. Cope A. C., Howell C. F., Knowles A., J. Am. Chem. Soc. 1962, 84, 3190–3191; [Google Scholar]
- 22b. Cope A. C., Ganellin C. R., Johnson H. W., J. Am. Chem. Soc. 1962, 84, 3191–3192; [Google Scholar]
- 22c. Cope A. C., Ganellin C. R., Johnson H. W., Van Auken T. V., Winkler H. J. S., J. Am. Chem. Soc. 1963, 85, 3276–3279; [Google Scholar]
- 22d. Marshall J. A., Acc. Chem. Res. 1980, 13, 213–218. [Google Scholar]
- 23. Hassan Z., Spuling E., Knoll D. M., Bräse S., Angew. Chem. Int. Ed. 2020, 59, 2156–2170; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 2176–2190. [Google Scholar]
- 24.
- 24a. Blankenstein J., Zhu J., Eur. J. Org. Chem. 2005, 1949–1964; [Google Scholar]
- 24b. Parenty A., Moreau X., Niel G., Campagne J.-M., Chem. Rev. 2013, 113, PR1–PR40; [DOI] [PubMed] [Google Scholar]
- 24c. Li Y., Yin X., Dai M., Nat. Prod. Rep. 2017, 34, 1185–1192; [DOI] [PubMed] [Google Scholar]
- 24d. Zheng K., Hong R., Nat. Prod. Rep. 2019, 36, 1546–1575; [DOI] [PubMed] [Google Scholar]
- 24e. Saridakis I., Kaiser D., Maulide N., ACS Cent. Sci. 2020, 6, 1869–1889; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24f. Santandrea J., Bédard A.-C., de Léséleuc M., Raymond M., Collins S. K. in Practical Medicinal Chemistry with Macrocycles: Design, Synthesis and Case Studies, (Ed: Marsault E., Peterson M. L.), Wiley, Hoboken, 2017, pp. 307–337. [Google Scholar]
- 25.For pioneering references, see: Rh-catalyzed:
- 25a. Kinoshita H., Shinokubo H., Oshima K., J. Am. Chem. Soc. 2003, 125, 7784–7785; [DOI] [PubMed] [Google Scholar]
- 25b. Tanaka K., Toyoda K., Wada A., Shirasaka K., Hirano M., Chem. Eur. J. 2005, 11, 1145–1156; [DOI] [PubMed] [Google Scholar]
- 25c. Tanaka K., Sagae H., Toyoda K., Naguchi K., Eur. J. Org. Chem. 2006, 3575–3581; Co-catalyzed: [Google Scholar]
- 25d. Moretto A. F., Zhang H.-C., Maryanoff B. E., J. Am. Chem. Soc. 2001, 123, 3157–3158; [Google Scholar]
- 25e. Boñaga L. V. R., Zhang H.-C., Moretto A. F., Ye H., Gauthier D. A., Li J., Leo G. C., Maryanoff B. E., J. Am. Chem. Soc. 2005, 127, 3473–3485. [DOI] [PubMed] [Google Scholar]
- 26.For a representative example, see Krieger J.-P., Ricci G., Lesuisse D., Meyer C., Cossy J., Angew. Chem. Int. Ed. 2014, 53, 8705–8708; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 8849–8852. [Google Scholar]
- 27.For other representative approaches, see: Pd-catalyzed benzannulation:
- 27a. Saito S., Salter M. M., Gevorgyan V., Tsuboya N., Tando K., Yamamoto Y., J. Am. Chem. Soc. 1996, 118, 3970–3971; benzannulation of Fischer carbene complexes: [Google Scholar]
- 27b. Wang H., Wulff W. D., Rheingold A. L., J. Am. Chem. Soc. 2000, 122, 9862–9863. [Google Scholar]
- 28.An original procedure for stereospecific ring expansion from orthocyclophanes with central chirality to metacyclophanes with planar chirality has also been documented: Ishida N., Sawano S., Murakami M., Nat. Commun. 2014, 5, 3111. [DOI] [PubMed] [Google Scholar]
- 29.For selected examples, see: asymmetric Diels–Alder-retro Diels–Alder reactions of steroids:
- 29a. Schomburg D., Thielmann M., Winterfeldt E., Tetrahedron Lett. 1985, 26, 1705–1706; [Google Scholar]
- 29b. Bäurle S., Blume T., Mengel A., Parchmann C., Skuballa W., Bäsler S., Schäfer M., Sülzle D., Wrona-Metzinger H.-P., Angew. Chem. Int. Ed. 2003, 42, 3961–3964; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 4091–4094; [Google Scholar]
- 29c. Kumar N., Kiuchi M., Tallarico J. A., Schreiber S. L., Org. Lett. 2005, 7, 2535–2538; [DOI] [PubMed] [Google Scholar]
- 29d. Zhao P., Beaudry C. M., Angew. Chem. Int. Ed. 2014, 53, 10500–10503; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 10668–10671; asymmetric cycloetherifications: [Google Scholar]
- 29e. Nicolaou K. C., Boddy C. N. C., J. Am. Chem. Soc. 2002, 124, 10451–10455; [DOI] [PubMed] [Google Scholar]
- 29f. Islas-González G., Bois-Choussy M., Zhu J., Org. Biomol. Chem. 2003, 1, 30–32; [DOI] [PubMed] [Google Scholar]
- 29g. Takiguchi H., Ohmori K., Suzuki K., Angew. Chem. Int. Ed. 2013, 52, 10472–10476; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 10666–10670; asymmetric ring-closing metathesis: [Google Scholar]
- 29h. Yang L., Song L., Huang C., Huang M., Liu B., Org. Chem. Front. 2016, 3, 319–323; asymmetric N-acylation: [Google Scholar]
- 29i. Tian C., Fielden S. D. P., Pérez-Saavedra B., Vitorica-Yrezabal I. J., Leigh D. A., J. Am. Chem. Soc. 2020, 142, 9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.
- 30a. Kanomata N., Ochiai Y., Tetrahedron Lett. 2001, 42, 1045–1048. See also: [Google Scholar]
- 30b. Ueda T., Kanomata N., Machida H., Org. Lett. 2005, 7, 2365–2368; [DOI] [PubMed] [Google Scholar]
- 30c. Kanomata N., Oikawa J., Tetrahedron Lett. 2003, 44, 3625–3628. [Google Scholar]
- 31. Mori K., Ohmori K., Suzuki K., Angew. Chem. Int. Ed. 2009, 48, 5638–5641; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 5748–5751. [Google Scholar]
- 32. Jung S., Kitajima Y., Ueda Y., Suziki K., Ohmori K., Synlett 2016, 27, 1521–1526. [Google Scholar]
- 33.
- 33a. Comprehensive Asymmetric Catalysis, Vol. I–III (Eds.: N Jacobsen E., Pfaltz A., Yamamoto H.), Springer-Verlag, Heidelberg, 1999; [Google Scholar]
- 33b. Catalytic Asymmetric Synthesis (Ed.: Ojima I.), Wiley, Chichester, 2000; [Google Scholar]
- 33c.Special issue in Acc. Chem. Res. (Eds.: S. E. Denmark, E. N. Jacobsen), 2000, 323–439.
- 34. Atropoisomerism and Axial Chirality, (Ed.J. M. Lassaletta ;), WSPC, 2019. [Google Scholar]
- 35. Kanda K., Koike T., Endo K., Shibata T., Chem. Commun. 2009, 1870–1872. [DOI] [PubMed] [Google Scholar]
- 36. Kanda K., Endo K., Shibata T., Org. Lett. 2010, 12, 1980–1983. [DOI] [PubMed] [Google Scholar]
- 37. Kanda K., Hamanaka R., Endo K., Shibata T., Tetrahedron 2012, 68, 1407–1416. [Google Scholar]
- 38.
- 38a. Delcourt M.-L., Felder S., Benedetti E., Micouin L., ACS Catal. 2018, 8, 6612–6616; [Google Scholar]
- 38b.for a review on desymmetrisation of centrosymmetric molecules, see Anstiss M., Holland J. M., Nelson A., Titchmarsh J. R., Synlett 2003, 8, 1213–1220. [Google Scholar]
- 39. Tanaka K., Hori T., Osaka T., Noguchi K., Hirano M., Org. Lett. 2007, 9, 4881–4884. [DOI] [PubMed] [Google Scholar]
- 40. Hori T., Shibata Y., Tanaka K., Tetrahedron: Asymmetry 2010, 21, 1303–1306. [Google Scholar]
- 41. Quamar Salih M., Beaudry C. M., Org. Lett. 2013, 15, 4540–4543. [DOI] [PubMed] [Google Scholar]
- 42. Igawa K., Ichikawa N., Ano Y., Katamoda K., Ito M., Akiyama T., Tomooka K., J. Am. Chem. Soc. 2015, 137, 7294–7297. [DOI] [PubMed] [Google Scholar]
- 43.See Ref. [25].
- 44.For a review, see Tanaka K., Bull. Chem. Soc. Jpn. 2018, 91, 187–194. [Google Scholar]
- 45. Tanaka K., Sagae H., Toyoda K., Noguchi K., Hirano M., J. Am. Chem. Soc. 2007, 129, 1522–1523. [DOI] [PubMed] [Google Scholar]
- 46. Shibata T., Uchiyama T., Endo K., Org. Lett. 2009, 11, 3906–3908. [DOI] [PubMed] [Google Scholar]
- 47. Araki T., Noguchi K., Tanaka K., Angew. Chem. Int. Ed. 2013, 52, 5617–5621; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 5727–5731. [Google Scholar]
- 48. Aida Y., Nogami J., Sugiyama H., Uekusa H., Tanaka K., Chem. Eur. J. 2020, 26, 12579–12588. [DOI] [PubMed] [Google Scholar]
- 49. Nogami J., Tanaka Y., Sugiyama H., Uekusa H., Muranaka A., Uchiyama M., Tanaka K., J. Am. Chem. Soc. 2020, 142, 9834–9842. [DOI] [PubMed] [Google Scholar]
- 50. Nogami J., Nagashima Y., Miyamoto K., Muranaka A., Uchiyama M., Tanaka K., Chem. Sci. 2021, 12, 7858–7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.
- 51a. Pellissier H. in Recent Developments in Asymmetric Organocatalysis, ACS Publishing, Cambridge, 2010; [Google Scholar]
- 51b. List B., Maruoka M. in Sience of Synthesis: Asymmetric Organocatalysis 1: Lewis base and acid catalysts, Thieme, Stuttgart, 2012; [Google Scholar]
- 51c. List B., Maruoka K. in Sience of Synthesis: Asymmetric Organocatalysis 2: Brønsted base and acid catalysts, and additional topics, Thieme, Stuttgart, 2012. [Google Scholar]
- 52.For recent reviews on organocatalyzed procedures for the synthesis of axially, planar, and helical chiral compounds, see [DOI] [PubMed]
- 52a. Bencivenni G., Synlett 2015, 26, 1915–1922; [Google Scholar]
- 52b. Shirakawa S., Liu S., Kaneko S., Chem. Asian J. 2016, 11, 330–341. [DOI] [PubMed] [Google Scholar]
- 53. Maeda R., Wada T., Mori T., Kono S., Kanomata N., Inonue Y., J. Am. Chem. Soc. 2011, 133, 10379–10381. [DOI] [PubMed] [Google Scholar]
- 54. Tomooka K., Uehara K., Nishikawa R., Suzuki M., Igawa K., J. Am. Chem. Soc. 2010, 132, 9232–9233. [DOI] [PubMed] [Google Scholar]
- 55.For the enantioselective generation of planar chirality using stoichiometric amounts of organocatalysts, see also
- 55a.Ref. [29d];
- 55b. Zhao P., Beaudry C. M., Synlett 2015, 26, 1923–1929. [Google Scholar]
- 56. Ding Q., Wang Q., He H., Cai Q., Org. Lett. 2017, 19, 1804–1807. [DOI] [PubMed] [Google Scholar]
- 57.For an inspiring review on this topic, see Winkler C. K., Schrittwieser J. H., Kroutil W., ACS Cent. Sci. 2021, 7, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gagnon C., Godin E., Minozzi C., Sosoe J., Pochet C., Collins S. K., Science 2020, 367, 917–921. [DOI] [PubMed] [Google Scholar]
- 59. Nguyen T. Q. N., Tooh Y. W., Sugiyama R., Nguyen T. P. D., Purushothaman M., Leow L. C., Hanif K., Yong R. H. S., Agatha I., Winerdy F. R., Gugger M., Phan A. T., Morinaka B. I., Nat. Chem. 2020, 12, 1042–1053. [DOI] [PubMed] [Google Scholar]
- 60. Scannell J. W., Bosley J., PLoS One 2016, 11, e0147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown R. W., Future Med. Chem. 2021, 13, 211–224. [DOI] [PubMed] [Google Scholar]


