Abstract
Myotonic dystrophy-related Cdc42-binding kinase alpha (MRCKα) is an integral component of signaling pathways controlling vital cellular processes, including cytoskeletal reorganization, cell proliferation and cell survival. In this study, we investigated the physiological role of MRCKα in milk protein and fat production in dairy cows, which requires a dynamic and strict organization of the cytoskeletal network in bovine mammary epithelial cells (BMEC). Within a selection of 9 Holstein cows, we found that both mRNA and protein expression of MRCKα in the mammary gland were upregulated during lactation and correlated positively (r > 0.89) with the mRNA and protein levels of β-casein. Similar positive correlations (r > 0.79) were found in a primary culture of BMEC stimulated with prolactin for 24 h. In these cells, silencing of MRCKα decreased basal β-casein, sterol-regulatory element binding protein (SREBP)-1 and cyclin D1 protein level, phosphorylation of mTOR, triglyceride secretion, cell number and viability—while overexpression of MRCKα displayed the reversed effect. Notably, silencing of MRCKα completely prevented the stimulatory action of prolactin on the same parameters. These data demonstrate that MRCKα is a critical mediator of prolactin-induced lactogenesis via stimulation of the mTOR/SREBP1/cyclin D1 signaling pathway.
Keywords: Myotonic dystrophy-related Cdc42-binding kinase alpha, Bovine mammary gland, Prolactin, Mechanistic target of rapamycin, Sterol regulatory element-binding protein-1, Cyclin D1
1. Introduction
Lactogenesis is a complex process involving mammary gland cell proliferation and the reduction of the apoptotic process to evoke milk synthesis and secretion, which is under the strict control of lactogenic hormones such as prolactin (PRL) (Osorio et al., 2016). Prolactin is produced by the anterior pituitary gland and once secreted it can bind to PRL receptors in the mammary gland to exert multiple cellular effects, ranging from the stimulation of growth to the initiation of milk synthesis and the maintenance of lactation. It is known that PRL receptors couple via Janus kinase (JAK) to signal transducer and activation of transcription (STAT) proteins, in particular STAT5, which is required in almost all mammary responses by PRL (Teglund et al., 1998). However, the mechanisms that regulate PRL signaling at the membrane level are not completely understood. Much attention has focused on the role of the JAK2/STAT5 signaling pathway, but less attention has been paid to the link with the cytoskeleton, that also fulfills an essential role in transducing the signals between the PRL receptor and milk protein gene transcription (Zoubiane et al., 2004). Notably, the Ras homologous (Rho) GTPases Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division cycle 42 (Cdc42), 2 well-known molecular switches regulating actin dynamics and polarized morphogenesis in epithelial cells (Chauhan et al., 2011; Pichaud et al., 2019), have been implemented in the regulation of PRL-induced synthesis of milk protein in primary mouse mammary epithelial cells (Akhtar and Streuli, 2006).
Previous studies have implicated crosstalk between PRL receptors and the mechanistic target of rapamycin (mTOR) signaling cascade (Bishop et al., 2006). Apart from PRL, mTOR, a serine/threonine kinase, integrates signals from many kinds of intracellular or extracellular environmental changes in order to control vital cellular processes, including protein and fat synthesis, cell proliferation, and autophagy (Cho, 2011; Saxton and Sabatini, 2017; Wang et al., 2019). Known targets of mTOR are the sterol regulatory element-binding protein (SREBP)-1 (Porstmann et al., 2008), a pivotal transcription factor for milk fat synthesis (Li et al., 2019), and cyclin D1, a key regulator of G1-to-S phase progression of the cell cycle (Fu et al., 2004) in bovine mammary epithelial cells (BMEC) (Yang et al., 2006; Yuan et al., 2019). The potential mechanism through which PRL activates the mTOR pathway and its downstream targets related to milk synthesis is largely unknown.
The myotonic dystrophy-related Cdc42-binding kinase alpha (MRCKα, also known as Cdc42BPA) is a serine/threonine kinase of the superfamily of AGC kinases (Pearce et al., 2010). Evidence has been reported that this kinase controls cell morphology, division and cytoskeletal reorganization by increasing myosin II regulatory light chain phosphorylation (Leung et al., 1998). MRCKα contains multiple functional domains, including a Cdc42-and Rac-interactive binding domain. Indeed, MRCKα interacts with GTP-bound Cdc42 (Leung et al., 1998) and constitutively active Rac1 (Schwarz et al., 2012), suggesting that MRCKα serves as a downstream effector in the Rac1/Cdc42 signaling pathway. Although these findings point out that MRCKα is a pivotal kinase controlling cell homeostasis, its physiological role in mammary gland development and lactation is not known.
Therefore, we hypothesized that MRCKα might be a key player in milk synthesis and cell proliferation in BMEC. The current study investigated the regulation of MRCKα on milk synthesis and cell proliferation in BMEC, and explored whether MRCKα is necessary for PRL-stimulated milk synthesis.
2. Materials and methods
2.1. Animal ethics
All animal care and animal related experimental procedures were approved by the Animal Welfare and Ethical Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (No. IAS20180115).
2.2. Reagents and antibodies
Dulbecco's modified Eagle's high glucose and pyruvate medium (DMEM), Ham's F12 medium (F12), Opti-MEM I Reduced-Serum Medium (Opti-MEM), Lipofectamine 3000, phosphate buffered saline (PBS) and fetal calf serum were purchased from Thermo Fisher Scientific (Waltham, MA). Penicillin-streptomycin solution, bovine serum albumin (BSA) and DAPI (4′,6-diamidino-2-phenylindole, Dihydrochloride) Antifade Mounting Medium were obtained from Beyotime (Shanghai, China). Recombinant rat PRL was procured from Beyotime (Shanghai, China; purity > 98%). Rabbit polyclonal anti-human MRCKα antibody (ab96659; 1:1,000), rabbit polyclonal anti-human β-casein antibody (ab112595; 1:200), rabbit polyclonal anti-mouse SREBP1 antibody (ab28481; 1:1,000), rabbit monoclonal anti-mouse cyclin D1 antibody (ab40754; 1:1,000), mouse monoclonal anti-human cytokeratin 18 (CK18) antibody (ab7797; 1:1,000), mouse monoclonal anti-human β-actin antibody (ab8226; 1:1,000) and goat anti-rabbit Horse Radish Peroxidase (HRP)-conjugated IgG (ab205718; 1:5,000) were purchased from ABCAM (Cambridge, UK). Rabbit polyclonal anti-human phosphorylated Ser2448-mTOR antibody (YT2913; 1:1,000) and rabbit polyclonal anti-human mTOR antibody (YP1134; 1:1,000) were obtained from ImmunoWay Biotechnology Company (Plano, TX). Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (A0568; 1:200) was procured from Beyotime (Shanghai, China) and goat anti-mouse HRP-conjugated IgG (ZB-2305; 1:5,000) from ZSGB-BIO (Beijing, China).
2.3. Mammary gland tissue
For measuring expression levels in bovine udder, 9 healthy Holstein cows, having a shared evolutionary genetic background, were selected based on different lactation stages; 3 were in mid lactation (M) (second parity, 164 ± 19 days in milk [DIM], milk production 29.7 ± 5.2 kg/d), 3 in late lactation (L) (second parity, 230 ± 20 DIM, milk production 15.0 ± 5.0 kg/d), and 3 in the dry period (D) (30 ± 20 d post milk). Tissues of mid-lactation cows were collected by biopsy, while other tissues were sampled from the upper third of the posterior mammary gland after slaughter. Tissue samples were stored in liquid nitrogen pending analyses.
2.4. Cell culture
BMEC, obtained from mammary gland tissues of one euthanized Holstein cow in mid-lactation at a commercial abattoir (Harbin, China), were cultured as reported previously (Hu et al., 2009). Briefly, BMEC were routinely grown in complete medium (DMEM/F12 [1:1, vol:vol] supplemented with 10% heat-inactivated fetal bovine serum, penicillin [100 units/mL], and streptomycin [100 units/mL]) at 37 °C in a 5% CO2 humidified incubator. BMEC from 4 to 10 passages were stored in liquid nitrogen for further use. To study the effects of PRL stimulation, cells were thawed, acclimated for 1 passage, and seeded in 6-well plates at densities of 4 × 105/well in complete medium. When the cells were grown at 80% confluence, i.e., still in their log phase of growth, complete medium was replaced with Opti-MEM (in the absence of serum and antibiotics) for serum starvation and treated with or without 0.6 μg/mL PRL at 37 °C in a 5% CO2 humidified incubator. Cells were harvested 24 h after PRL stimulation for Western blot, quantitative real-time PCR (RT-qPCR) for cell number and viability analysis (see below).
2.5. Immunocytochemistry
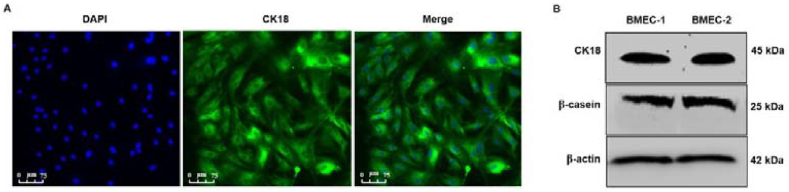
For immunocytochemical analysis, BMEC were seeded onto glass coverslips in 6-well plates at a density of 1 × 105/well, and allowed to attach for 24 h. After washing with PBS, cells were fixed in 4% (wt/vol) paraformaldehyde for 15 min on ice, then rinsed 3 times with TBS-T (20 mM Tris-base, 140 mM NaCl, 0.1% [vol/vol] Tween-20) and blocked with 5% (wt/vol) BSA in TBS-T for 1.5 h at 37 °C. Subsequently, cells were incubated with mouse monoclonal anti-CK18 antibody at 4 °C overnight, washed with PBS, incubated with FITC-conjugated goat anti-mouse IgG at 37 °C in the dark for 1 h, washed with TBS-T, followed by incubation with DAPI in the dark for 20 min. After mounting of the cells in Antifade Mounting Medium, images were obtained with a Leica TCS SP8 inverted confocal laser microscope and Leica software (Wetzlar, Germany).
2.6. Detection of triglycerides
The number of triglycerides (TG) secreted by BMEC, which were transfected with either MRCKα siRNA, scrambled siRNA, MRCKα-FLAG or empty vector pcDNA3.1 constructs in Opti-MEM (in the absence of serum and antibiotics) for 24 h, were measured with a TG Colorimetric Assay Kit (Applygen Technologies Inc, Beijing, China) according to the manufacturer's instructions. Optical density values were obtained at 490 nm via a Clinical Laboratory Enzyme Labeling Instrument (AMIS Medical, Nanjing, China) and the calculated TG content was normalized to cell number.
2.7. Cell number and viability
To determinate the cell number, aliquots (10 μL) of BMEC were mixed with 1 μL trypan blue dye, transferred to a cell counter plate, and imaged and counted with a Countess I Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA).
Cell viability measurement, based on the mitochondrial conversion of MTT to formazan, was conducted with the colorimetric Cell Proliferation and Cytotoxicity Assay Kit (Solarbio, Beijing, China). Optical densities were detected with an AM-M3000 microplate reader at 490 nm.
2.8. Western blot analysis
For immunoblot analysis, cell lysates were cleared by centrifugation and separated using the ExpressPlus precast PAGE Gel system (gradient gels ranging from 4% to 20%; GenScript, Piscataway, NJ). Separated proteins were blotted to nitrocellulose membranes (0.45 μm), probed with primary antibodies then with goat anti-rabbit or goat anti-mouse HRP-conjugated secondary antibodies and eventually developed by ECL Western Blotting Detection Reagents (Thermo Fisher Scientific, Waltham, MA). Densitometry analysis of the positive bands was performed using the Bio-Rad Gel Doc XR system and ImageJ Gel Analysis software tool (version 1.52a; https://imagej.nih.gov/ij/). All presented blots are representative of at least 3 separate experiments yielding similar results.
2.9. RT-qPCR
For RT-qPCR, total RNA from BMEC and mammary gland tissues was extracted with a RNeasy Mini Kit, according to the manufacturer's protocol (Qiagen, Dusseldorf, Germany). RNA concentrations were determined with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), ensuring that each value of OD260nm/280nm was between 1.8 and 2.0. Subsequently, total RNA (500 ng) was reverse transcribed into cDNA in a total volume of 10 μL with the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). Quantitative PCR was performed by amplifying cDNA for 40 cycles (95 °C for 5 s, 60 °C for 34 s, and 72 °C for 1 min) using the SYBR Premi EX Taq II (TaKaRa, Dalian, China) on an Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA), following 1 cycle of 30 s at 95 °C. The specificity of the reactions was confirmed by a subsequent melting curve analysis. Data analysis was performed with the 2−ΔΔCT method (Livak and Schmittgen, 2001), where ribosomal protein S9 (RPS9) was used as the internal reference, since geNorm algorithm (Vandesompele et al., 2002) identified RPS9 as the most stable reference gene among 4 others: ubiquitously expressed transcript protein (UXT), mitochondrial ribosome associated GTPase 1 (MTG1), S100 calcium-binding protein A10 (S100A10) and Glyceraldehyde 3-phosphate Dehydrogenase (GAPDH). The primer pairs used for qPCR are presented in Table 1.
Table 1.
Primers used for RT-qPCR.
| Gene | Genera | Accession number | Primer sequence (5′–3′) |
|---|---|---|---|
| MRCKα | Bos taurus | XM_024976363 | ATGAAAAGGATGCACGAGGGT |
| GGCATATCTGTTGCTCGGGT | |||
| CSN2 | Bos taurus | XM_010806178.2 | AGTGAGGAACAGCAGCAAACAG |
| AGCAGAGGCAGAGGAAGGTG | |||
| MTG1 | Bos taurus | NM_001025327.2 | GGGTACGTGCAGCACTACGG |
| CACCTTCACCTTCTGGGTCTTC | |||
| UXT | Bos taurus | NM_001037471.2 | TGGACCATCGTGACAAGGTA |
| TGAAGTGTCTGGGACCACTG | |||
| GAPDH | Bos taurus | NM_001034034.2 | TGGAAAGGCCATCACCATCT |
| CCCACTTGATGTTGGCAG | |||
| S100A10 | Bos taurus | NM_174650.1 | GCCAAGGTTTCAACAGACTTCAC |
| TGAGTACTCTCAGGTCTTCCTTTGTTAA | |||
| RPS9 | Bos taurus | NM_001101152.2 | ATCCCGTCCTTCATCGTGC |
| CCCTTCTTGGCGTTCTTCC |
MRCKα = myotonic dystrophy-related Cdc42-binding kinase alpha; CSN2 = β-casein; MTG1 = mitochondrial ribosome associated GTPase 1; UXT = ubiquitously expressed transcript protein; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; S100A10 = S100 calcium-binding protein A10; RPS9 = ribosomal protein S9.
2.10. Cloning and overexpression of bovine MRCKα
The open reading frame of bovine MRCKα mRNA (Bos taurus; XM_024976363), 4,917 bp in length, was generated by using appropriate primers and subcloning the amplified DNA between the Kpn-I and Xho-I site of the pcDNA3.1 (+) eukaryotic expression vector (Thermo Fisher Scientific, Waltham, MA) and was confirmed by DNA sequencing. A FLAG octapeptide-encoding DNA fragment was generated in frame at the C-terminal by removal of the stop codon of MRCKα.
BMEC were transfected with MRCKα-FLAG plasmid using Lipofectamine 3000 in the presence of Opti-MEM, according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA). Transfected cells, including their empty vector and non-transfected controls, were further cultured in Opti-MEM without serum and antibiotics at 37 ℃ for 24 h until analysis.
2.11. MRCKα silencing
Silencing of MRCKα was conducted by introducing small interfering RNA (MRCKα small interfering RNA; MRCKα siRNA) via transfection with Lipofectamine 3000 in the presence of Opti-MEM, according to the manufacturer's instruction (Thermo Fisher Scientific, Waltham, MA). The MRCKα siRNAs were synthesized by GenePhama (Suzhou, China). MRCKα siRNA were: 5′-GCAACUGAAGCUUAUGAAATT-3’ (sense); 5′- UUUCAUAAGCUUCAGUUGCTT-3’ (antisense). For mock transfection, scrambled siRNA (SC; GenePhama, Suzhou, China) was used. Cells were cultured in serum and antibiotics-free Opti-MEM at 37 °C for 24 h before further analysis.
2.12. Statistical analyses
Data are reported as means ± SEM of 3 independent experiments. Comparison of protein, mRNA, TG levels and cell number, and viability under 3 or more different treatments was attained by analysis of variance (ANOVA) followed by a Tukey's post-hoc test. In the case of 2 treatment groups, a pairwise T test was performed. Analyses were performed with SPSS Statistics 17 software (IBM, NY). Differences were considered statistically significant at P < 0.05 and as tendencies between 0.05 ≤ P < 0.10.
3. Results
3.1. MRCKα expression positively correlates with that of β-casein in dairy cow mammary gland
Based on a limited number of observations, protein expression levels of MRCKα and β-casein per unit mammary tissue of dairy cows were highest (P < 0.05; n = 3) during mid lactation, followed by late lactation, and lowest in the dry period (Fig. 1A and B). In the same tissues, a similar pattern was found for the mRNA abundance of MRCKα and β-casein (P < 0.05; n = 3) being highest in mid lactation, followed by late lactation, and lowest in the dry period (Fig. 1D). MRCKα was strongly positively correlated with β-casein at the level of protein (r = 0.89; P < 0.01) (Fig. 1C) and mRNA expression (r = 0.90; P < 0.01) (Fig. 1E), suggesting a physiological role of MRCKα in bovine milk protein synthesis.
Fig. 1.
Protein expression and mRNA abundance of MRCKα and β-casein in mammary gland tissues of dairy cows in mid lactation (Mid-L; M1 to 3, 3 different cows, 164 ± 19 days in milk [DIM]), late lactation (Late-L; L1 to 3, 3 different cows, 230 ± 20 DIM) and dry period (Dry; D1 to 3, 3 different cows, 30 ± 20 d post milk). (A) Western blot analysis of mammary tissue lysates stained for β-casein, MRCKα and β-actin. (B) Immunopositive bands were quantified by densitometry, normalized to β-actin and presented as protein expression levels relative to Dry. (C) The protein expression of β-casein and MRCKα is positively correlated in the bovine mammary gland. (D) Abundance of β-casein and MRCKα mRNA measured by RT-qPCR in the bovine mammary gland. (E) The mRNA expression of β-casein and MRCKα is positively correlated in the bovine mammary gland. Values in (B) and (D) are presented as mean ± SEM and means without a common letter differ, P < 0.05. MRCKα = myotonic dystrophy-related Cdc42-binding kinase alpha.
3.2. PRL stimulates both MRCKα and β-casein expression in BMEC
We further explored the possible role of MRCKα in milk production in a primary culture of BMEC. Our BMEC culture consisted of a homogenous population of cells that abundantly expressed the epithelial marker CK18 and intracellular β-casein (Fig. 2A and B). Upon PRL stimulation, both MRCKα and β-casein protein in BMEC were increased compared to the untreated control by 20.3% (P < 0.01; n = 3) and 30.2% (P < 0.05; n = 3), respectively (Fig. 3A and B). PRL also increased (P < 0.05; n = 3) both β-casein and MRCKα mRNA (Fig. 3D). In BMEC, intracellular MRCKα and β-casein protein tended (r = 0.79; P = 0.06) to be positively correlated with each other (Fig. 3C), and there was a highly significant positive correlation (r = 0.97; P < 0.01) between MRCKα and β-casein mRNA abundance in these cells (Fig. 3E).
Fig. 2.
Immunocytochemical characterization of the primary culture of bovine mammary epithelial cells (BMEC). (A) Isolated BMEC were cultured on coverslips for 24 h and analyzed by immunocytochemistry using anti-CK18 monoclonal antibody. Images show CK18 (green), nuclear DNA (DAPI, blue) and merged fluorescence confocal microscope emissions. (B) In parallel, cultured BMEC were lysed after 24 h and subjected to Western blot analysis using anti-CK18 monoclonal or β-casein polyclonal antibody. Equal amounts of total protein (25 μg) were loaded in each lane. BMEC-1 and BMEC-2 represent 2 independent batches of BMEC. DAPI, 4′,6-diamidino-2-phenylindole, a blue-fluorescent DNA stain. CK18 = cytokeratin 18.
Fig. 3.
Prolactin stimulates the expression of both β-casein and MRCKα. (A) Confluent bovine mammary epithelial cells (BMEC) were stimulated with prolactin (PRL, 0.6 μg/mL) or left untreated (Control) for 24 h, lysed and subjected to Western blot analysis using polyclonal antibodies against β-casein, MRCKα or β-actin. (B) Immunopositive bands were quantified by densitometry, normalized to β-actin and presented as protein expression levels relative to their corresponding control. (C) β-casein protein expression in BMEC tended to correlate positively with that of MRCKα. (D) Abundance of β-casein and MRCKα mRNA measured by RT-qPCR in BMEC upon stimulation with prolactin (PRL, 0.6 μg/mL) or vehicle (Control) for 24 h. (E) β-casein mRNA expression in BMEC correlated positively with that of MRCKα. Values are presented as mean ± SEM of 3 independent experiments. Means without a common letter differ, P < 0.05. MRCKα = myotonic dystrophy-related Cdc42-binding kinase alpha.
3.3. MRCKα positively regulates β-casein synthesis
To obtain evidence that MRCKα plays a regulatory role in β-casein production in BMEC, we choose to overexpress or silence MRCKα and measure β-casein production in these cells (Fig. 4A and D). Transfection of BMEC with MRCKα siRNA decreased (P < 0.05; n = 3) MRCKα protein by 60% (Fig. 4B), which was paralleled by a 50% reduction (P < 0.05; n = 3) of intracellular β-casein (Fig. 4C). Inversely, the 1.8-fold elevated MRCKα protein expression (Fig. 4E) in BMEC transfected with Flag-tagged MRCKα was accompanied with a 1.5-fold increase (P < 0.05; n = 3) in β-casein (Fig. 4F). These data confirm that MRCKα is a positive regulator of β-casein production in these cells.
Fig. 4.
MRCKα positively regulates the synthesis of β-casein in bovine mammary epithelial cells (BMEC). (A) Confluent BMEC were transfected with MRCKα siRNA (SI), scrambled siRNA (control, SC) or left untreated (control, Blank) for 24 h, lysed and subjected to Western blot analysis using polyclonal antibodies directed against MRCKα, β-casein and β-actin. Immunopositive bands were analyzed by densitometry. The resulted protein levels were normalized to β-actin and the average fold-change over Blank for MRCKα (B) and β-casein (C) protein expression are presented of 3 independent experiments. (D) Confluent BMEC were transfected with empty vector pcDNA3.1 (EV), MRCKα-FLAG (OE), or left untreated (control, Blank). After 24 h, cells were lysed and analyzed by immunoblotting using polyclonal antibodies against MRCKα and β-casein. Immunopositive bands were analyzed by densitometry. Protein levels were normalized to β-actin and the average fold-change over Blank for MRCKα (E) and β-casein (F) protein expression is presented of 3 independent experiments. Means without a common letter differ, P < 0.05. MRCKα = myotonic dystrophy-related Cdc42-binding kinase alpha.
3.4. MRCKα positively regulates TG synthesis
We next investigated if fat, another major constituent of milk, is also regulated by MRCKα. Silencing of MRCKα decreased (P < 0.05; n = 3) the concentration of TG accumulated in the culture medium by BMEC (Fig. 5A), whereas MRCKα overexpression had the opposite effect (P < 0.05; n = 3) (Fig. 5B), indicating that MRCKα is also a positive regulator of TG production in BMEC.
Fig. 5.
Production of triglycerides (TG) is dependent on MRCKα expression. (A) Confluent bovine mammary epithelial cells (BMEC) were transfected with MRCKα small interfering RNA (siRNA, SI), scrambled siRNA (control, SC) or left untreated (control, Blank) for 24 h. The content of TG accumulated in the medium was measured and presented relative to Blank of 3 independent experiments. (B) Confluent BMEC were transfected with MRCKα-FLAG (OE), empty vector pcDNA3.1 (EV) constructs or left untreated (control, Blank). After 24 h the content of TG accumulated in the medium was measured and presented relative to Blank of 3 independent experiments. The level of the Blank group was set at 1.0. Means without a common letter differ, P < 0.05.
3.5. MRCKα stimulates the mTOR/SREBP1/cyclin D1 signaling pathway
Since the above findings indicate that MRCKα regulates milk synthesis in BMEC, we further explored the effects of MRCKα on key signaling pathways in the process of bovine lactogenesis (Fig. 6A and E). The expression levels of several components of the mTOR pathway, including phosphorylation level at Ser2448 of mTOR, SREBP1 and cyclin D1, were markedly decreased (Fig. 6B, C and D; P < 0.05; n = 3) in MRCKα-silenced BMEC, and increased (Fig. 6F, G and H; P < 0.05; n = 3) in cells overexpressing MRCKα. These data indicate that MRCKα stimulates mTOR activity and at least 2 of its targets.
Fig. 6.
MRCKα positively regulates the phosphorylation of mTOR and protein levels of SREBP1 and cyclin D1. (A) Confluent bovine mammary epithelial cells (BMEC) were transfected with MRCKα siRNA (SI), scrambled siRNA (control, SC) or left untreated (control, Blank). After 24 h, cells were lysed and subjected to Western blot analysis of mTOR, p-mTOR, SREBP1, cyclin D1, MRCKα and β-actin. Densitometric analysis of the immunopositive bands generated ratios of p-mTOR/mTOR (B) and protein levels for SREBP1 (C) and cyclin D1 (D). (E) Confluent BMEC were transfected with MRCKα-FLAG (OE), empty vector pcDNA3.1 (EV) constructs or left untreated (control, Blank). After 24 h, cells were lysed and subjected to Western blot analysis of mTOR, p-mTOR, SREBP1, cyclin D1, MRCKα and β-actin. Densitometric analysis of the immunopositive bands generated ratio of p-mTOR/mTOR (F) and protein levels for SREBP1 (G) and cyclin D1 (H). Protein levels presented were first normalized to β-actin and then to its corresponding Blank (untreated control) group. Values are presented as mean ± SEM for 3 independent experiments. Means without a common letter differ, P < 0.05. MRCKα = myotonic dystrophy-related Cdc42-binding kinase alpha; SREBP1 = sterol regulatory element-binding protein 1; mTOR = mechanistic target of rapamycin.
3.6. MRCKα is a mediator of PRL-induced milk synthesis via the mTOR signaling pathway
To confirm that PRL-driven casein production is dependent on the expression of MRCKα, the expression of lactogenic genes upon PRL stimulation were measured in BMEC following siRNA mediated knockdown of MRCKα (Fig. 7A). Indeed, siRNA targeting MRCKα completely blocked (P < 0.05; n = 3) the stimulatory effect of PRL on both MRCKα protein expression (Fig. 7B) and β-casein production (Fig. 7C). Moreover, MRCKα silencing prevented (P < 0.05; n = 3) the increase in phosphorylation of mTOR (Fig. 7D) and protein expression of SREBP1 (Fig. 7E) in response to PRL. The latter effect suggests that MRCKα also affects PRL-induced TG secretion, which was in line with the observation that the amount of TG accumulation in the medium upon PRL stimulation was fully blocked (P < 0.05; n = 3) by MRCKα-silencing (Fig. 7F).
Fig. 7.
Prolactin promotes the synthesis of β-casein in bovine mammary epithelial cells (BMEC) through MRCKα. (A) BMEC were transfected with scrambled siRNA (control, SC) or MRCKα siRNA (SI) and stimulated with Blank (untreated control) or prolactin (PRL; 0.78 μg/mL) for 24 h. Thereafter, cells were lysed, and protein levels were detected by immunoblotting. (B to E) Densitometric analysis of the immunopositive bands generated for MRCKα, β-casein, the ratio of p-mTOR/mTOR, and SREBP1. (F) Amounts of triglycerides (TG) accumulated in the medium were detected using a TG detection kit. All protein levels were first normalized to β-actin and then to the SC + Blank group. Values are presented as mean ± SEM for 3 independent experiments. Means without a common letter differ, P < 0.05. MRCKα = myotonic dystrophy-related Cdc42-binding kinase alpha; SREBP1 = sterol regulatory element-binding protein 1; mTOR = mechanistic target of rapamycin.
3.7. MRCKα controls cell number and viability
PRL has been implicated in the proliferation of mammary tissue. Fig. 8A and B shows that, in control (SC) cells, PRL greatly enhanced (P < 0.05; n = 3) the expression of cyclin D1, which was abrogated when MRCKα was downregulated (P < 0.05; n = 3). Overexpression of MRCKα led to a 1.8-fold and 1.5-fold increase in cell number and cell viability (P < 0.05; n = 3), respectively, compared to the (empty vector) control group (data not shown). Furthermore, as expected, PRL increased (P < 0.05; n = 3) the cell number and cell viability of BMEC by approximately 20% and 30%, respectively in a period of 24 h after PRL stimulation, which was also blunted (P < 0.05; n = 3) upon MRCKα silencing (Fig. 8C and D). The concerted action of PRL on the expression of cyclin D, a kinase associated with cell cycle progression, and on total cell number, which were both dependent on MRCKα expression, indicates that PRL increases cell proliferation through the intermediator MRCKα; thereby increasing the capacity of milk production in the BMEC population.
Fig. 8.
Silencing of MRCKα prevents the stimulatory effect of prolactin on cyclin D1 expression, cell number and cell viability. (A) Bovine mammary epithelial cells (BMEC) were transfected with scrambled siRNA (control, SC) or MRCKα siRNA (SI) and stimulated with Blank (untreated control) or prolactin (PRL; 0.78 μg/mL) for 24 h. Cells were lysed and protein level of cyclin D1 was detected by immunoblotting. (B) Densitometric analysis of the immunopositive bands generated protein level for cyclin D1. Protein levels were first normalized to β-actin and then to the control SC + Blank group. Cell number (C) and cell viability (D) were calculated by a cell counter and 3-[4,5-dimethylthiazolyl-2]-2,5-diphenyltetrazolium bromide (MTT), respectively. Values are presented as mean ± SEM for 3 independent experiments. Means without a common letter differ, P < 0.05.
4. Discussion
Our data show that MRCKα is abundantly expressed in the mammary gland tissues of lactating dairy cows, displaying a positive correlation with β-casein expression at both mRNA and protein level. The BMEC model confirmed that MRCKα mRNA expression is positively related with β-casein mRNA expression and that their protein levels are numerically (P = 0.06) correlated. By gene knockdown and overexpression experiments, we demonstrated that PRL-stimulated β-casein and TG production is mediated by MRCKα via activation of the mTOR/SREBP1/cyclin D1 signaling pathway.
Expression of MRCKα was high in the mammary tissues of lactating dairy cows compared with dry cows, and greater in mid-lactation cows (29.7 kg milk/d) than in late lactation cows (15.0 kg milk/d). Strikingly, MRCKα expression responds positively to the application of PRL in BMEC, demonstrating that MRCKα is an inducible protein, and it is possible that it responds to other extracellular stimuli of milk synthesis as well. In breast cancer, the lactogenic hormone estradiol downregulates microRNA miR-515-5p (Pinho et al., 2013), thereby relieving the transcriptional repression on the MRCKα gene (Pardo et al., 2016). It would be interesting to investigate the possible role of miR-515-5p in the PRL-elevated MRCKα expression.
In the present study, we demonstrated that the production of β-casein and TG in response to PRL was completely blocked upon MRCKα silencing. This indicates that PRL requires MRCKα activity in the control of milk production. PRL binds to PRL receptors to initiate signaling to several downstream mediators, including JAKs, STATs, mitogen-activated protein kinases, PI3K, AKT, src kinases and Rho GTPases (Clevenger et al., 2003). We observed that, in BMEC, PRL stimulation and MRCKα overexpression increased the phosphorylation of mTOR on the Ser2448 residue, which is a well-known phosphorylation site of both AKT and mTOR downstream effector S6 kinase (Cheng et al., 2004; Chiang and Abraham, 2005). Notably, the stimulatory action of PRL on mTOR was abolished upon MRCKα silencing. Simultaneously, MRCKα silencing prevented the PRL-induced SREBP1 and cyclin D1 expression, which is sensible as mTOR activity controls SREBP1 and cyclin D1 expression (Averous et al., 2008; Norrmén et al., 2014). The block of PRL-stimulated SREBP1 synthesis in MRCKα-silenced BMEC is consistent with the paralleled abrogation of TG production. Interestingly, AKT has been implicated in enhancing the SREBP1 synthesis (Porstmann et al., 2005) and cyclin D1 stability (Diehl et al., 1998). Taken together, our data suggest that PRL utilizes the PI3K/AKT signaling pathway to activate the mTOR signaling pathway in a MRCKα-dependent mechanism.
Another aspect of lactogenesis, besides milk production, is the promotion of cell proliferation and reduction of apoptotic processes in mammary tissue. PRL increased cyclin D1 expression accompanied with an increase in cell number and cell viability of BMEC; proliferation-associated effects were abolished when MRCKα was downregulated. In a similar cell model, a positive association with cyclin D1 expression and transit of cell from the G0/G1 to G2/M phase of the cell cycle has been suggested (Liu et al., 2019; Yuan et al., 2019). MRCKα seems to play an essential role in PRL-stimulated cell proliferation, and indeed, a recent paper suggests a regulatory role of MRCKα in the proliferation of high-grade serous ovarian carcinoma cells (Kurimchak et al., 2020).
A remarkable observation for us was that silencing of MRCKα also led to an approximately 50% reduction of the basal protein expression levels of β-casein, SREBP1, cyclin D1 and phosphorylation of mTOR. Since mTOR is a nutrient-responsive kinase, integrating the input of many other external signals (Sengupta et al., 2010), this implies that other factors, e.g., nutrients present in the control (i.e., PRL-free) medium, positively acts on MRCKα to maintain a sustained activation of the mTOR/SREBP1/cyclin D1 pathway. This would place MRCKα as a pivotal component in PRL signaling, as well as in that of other lactogenic stimuli. Current studies in our laboratory are underway to examine this concept.
The present study provides evidence that the PRL-induced lactogenesis is mediated by MRCKα in BMEC. If MRCKα is activated upon PRL stimulation, what would be the molecular mechanisms of activation and the consequences for the cytoskeletal kinetics in BMEC? MRCKα has homologies with Rho-associated kinase, is a downstream effector protein of Cdc42 and Rac1 (Leung et al., 1998; Schwarz et al., 2012), and is shown to be involved in controlling cytoskeleton reorganization and cell migration. For example, it is involved in dendritogenesis, including filopodia formation (Chen et al., 1999; Nakamura et al., 2000; Wilkinson et al., 2005), and the idea that MRCKα expression enhances cell motility is supported by its correlation with metastasis in colon cancer (Hu et al., 2018). Furthermore, MRCKα may enhance the link between the cytoskeleton and membranes by phosphorylating ezrin-radixin-moesin proteins in dendrites (Nakamura et al., 2000). These findings thus suggest that MRCKα relays signals between the extracellular matrix (ECM)-cytoskeleton complexes and the PRL receptor signaling pathway.
It is unlikely that the cytoskeleton is directly involved in how MRCKα permits PRL-signaling, since previous work showed that 1) disruption of the actin skeleton by cytochalasin D had no effect on the PRL signaling cascade (Zoubiane et al., 2004), and 2) introducing dominant negative RhoA had no effect on PRL-induced β-casein synthesis (Akhtar and Streuli, 2006). Like all epithelial cells, BMEC interact with the basement membrane in vivo by a varied set of receptors, including dystroglycan and β1 integrin (Yurchenco and Patton, 2009). Elegant knockout experiments in mice demonstrated that loss of either dystroglycan or β1 integrin leads to aberrant tissue architecture which was accompanied with a loss in STAT5 signaling and β-casein production induced by PRL (Leonoudakis et al., 2010; Naylor et al., 2005). Others have shown that integrin-linked kinase (ILK) couples β1 integrin to Rac1, promoting PRL-induced STAT5 activation and, eventually, β-casein gene expression (Akhtar et al., 2009). PI3K has been identified as the essential mediator downstream of laminin stimulation, which facilitates Rac1 activation and sustained STAT5 tyrosine phosphorylation (Xu et al., 2010). Intriguingly, PRL treatment leads to an increase in activity of Cdc42, but not of Rac1, in mammary epithelial cells (Akhtar and Streuli, 2006). It is suggested that PI3K acts as an upstream activator of Rac1 and Cdc42, in growth factor-stimulated pathways in other models (Benard et al., 1999; Hawkins et al., 1995). It is conceivable that, in BMEC, PI3K responds to PRL to activate Cdc42, which in turn enhances MRCKα activity. Additionally, it is conceivable that Rac1, whose activity appeared to be regulated by proteins of the ECM (Akhtar and Streuli, 2006), also facilitates MRCKα activity to allow PRL-induced lactogenesis.
In summary, this study reveals a novel role for MRCKα as a mediator of the lactogenesis processes in BMEC through the activation of the mTOR/SREBP1/cyclin D1 signaling pathway. We suggest that MRCKα may function as an important hub in the crosstalk between PRL receptor signaling and ECM-cytoskeleton dynamics. This opens new avenues to explore the detailed mechanisms of milk synthesis.
Author contributions
Fang Wang: investigation, data curation, writing - original draft. Jürgen van Baal: conceptualization, methodology, writing - review & editing, supervision. Lu Ma: conceptualization, methodology. Xuejun Gao: conceptualization, methodology, writing - review & editing. Jan Dijkstra: conceptualization, writing - review & editing, supervision. Dengpan Bu: conceptualization, writing - review & editing, project administration, supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This study was supported by the Natural Science Foundation of China, China (31872383), the Key Research and Development Program of the Ningxia Hui Autonomous Region, China (2021BEF02018), the Scientific Research Project for Major Achievements of the Agricultural Science and Technology Innovation Program (ASTIP) of Chinese Academy of Agricultural Sciences, China (ASTIP-IAS07-1, CAAS-XTCX2016011-01), and International Atomic Energy Agency Technical Co-Operation and Assistance Programme, China (no. CPR5025).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Akhtar N., Streuli C.H. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N., Marlow R., Lambert E., Schatzmann F., Lowe E.T., Cheung J., et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Devel. 2009;136:1019–1027. doi: 10.1242/dev.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averous J., Fonseca B.D., Proud C.G. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncog. 2008;27:1106–1113. doi: 10.1038/sj.onc.1210715. [DOI] [PubMed] [Google Scholar]
- Benard V., Bohl B.P., Bokoch G.M. Characterization of Rac and Cdc42 activation in chemo attractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bishop J.D., Nien W.L., Dauphinee S.M., Too C.K. Prolactin activates mammalian target-of-rapamycin through phosphatidylinositol 3-kinase and stimulates phosphorylation of p70S6K and 4E-binding protein-1 in lymphoma cells. J Endocrinol. 2006;190:307–312. doi: 10.1677/joe.1.06368. [DOI] [PubMed] [Google Scholar]
- Chauhan B.K., Lou M., Zheng Y., Lang R.A. Balanced Rac1 and RhoA activities regulate cell shape and drive invagination morphogenesis in epithelia. P Natl Acad Sci USA. 2011;108:18289–18294. doi: 10.1073/pnas.1108993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.Q., Tan I., Leung T., Lim L. The myotonic dystrophy kinase-related Cdc42-binding kinase is involved in the regulation of neurite outgrowth in PC12 cells. J Biol Chem. 1999;274:19901–19905. doi: 10.1074/jbc.274.28.19901. [DOI] [PubMed] [Google Scholar]
- Cheng S.W., Fryer L.G., Carling D., Shepherd P.R. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- Chiang G.G., Abraham R.T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- Cho C.H. Frontier of epilepsy research - mTOR signaling pathway. Exp Mol Med. 2011;43:231–274. doi: 10.3858/emm.2011.43.5.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger C.V., Furth P.A., Hankinson S.E., Schuler L.A. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J.A., Cheng M., Roussel M.F., Sherr C.J. Glycogen synthase kinase-3 beta regulates cyclin D1 proteolysis and subcellular localization. Gene Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M., Wang C., Li Z., Sakamaki T., Pestell R.G. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Hawkins P.T., Eguinoa A., Qiu R.G., Stokoe D., Cooke F.T., Walters R., et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Hu H., Wang J., Bu D., Wei H., Zhou L., Li F., et al. In vitro culture and characterization of a mammary epithelial cell line from Chinese Holstein dairy cow. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.F., Xu W.W., Wang Y., Zheng C.C., Zhang W.X., Li B., et al. Comparative proteomics analysis identifies Cdc42-Cdc42BPA signaling as prognostic biomarker and therapeutic target for colon cancer invasion. J Proteome Res. 2018;17:265–275. doi: 10.1021/acs.jproteome.7b00550. [DOI] [PubMed] [Google Scholar]
- Kurimchak A.M., Herrera-Montavez C., Brown J., Johnson K.J., Sodi V., Srivastava N., et al. Functional proteomics interrogation of the kinome identifies MRCKA as a therapeutic target in high-grade serous ovarian carcinoma. Sci Signal. 2020;13 doi: 10.1126/scisignal.aax8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D., Singh M., Mohajer R., Mohajer P., Fata J.E., Campbell K.P., et al. Dystroglycan controls signaling of multiple hormones through modulation of STAT5 activity. J Cell Sci. 2010;123:3683–3692. doi: 10.1242/jcs.070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T., Chen X.Q., Tan I., Manser E., Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Bio. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Zhou C., Li X., Yu M., Li M., Gao X. CRTC2 is a key mediator of amino acid-induced milk fat synthesis in mammary epithelial cells. J Agric Food Chem. 2019;67:10513–10520. doi: 10.1021/acs.jafc.9b04648. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Zhen Z., Yu Y., Qiu Y., Xiang W. GRP78 regulates milk biosynthesis and the proliferation of bovine mammary epithelial cells through the mTOR signaling pathway. Cell Mol Biol Lett. 2019;24:57. doi: 10.1186/s11658-019-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Oshiro N., Fukata Y., Amano M., Fukata M., Kuroda S., et al. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Gene Cell. 2000;5:571–581. doi: 10.1046/j.1365-2443.2000.00348.x. [DOI] [PubMed] [Google Scholar]
- Naylor M.J., Li N., Cheung J., Lowe E.T., Lambert E., Marlow R., et al. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén C., Figlia G., Lebrun-Julien F., Pereira J.A., Trötzmüller M., Köfeler H.C., et al. mTORC1 controls PNS myelination along the mTORC1-RXRγ-SREBP-lipid biosynthesis axis in Schwann cells. Cell Rep. 2014;9:646–660. doi: 10.1016/j.celrep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Osorio J.S., Lohakare J., Bionaz M. Biosynthesis of milk fat, protein, and lactose: roles of transcriptional and posttranscriptional regulation. Physiol Genomics. 2016;48:231–256. doi: 10.1152/physiolgenomics.00016.2015. [DOI] [PubMed] [Google Scholar]
- Pardo O.E., Castellano L., Munro C.E., Hu Y., Mauri F., Krell J., et al. miR-515-5p controls cancer cell migration through MARK4 regulation. EMBO Rep. 2016;17:570–584. doi: 10.15252/embr.201540970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L.R., Komander D., Alessi D.R. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Bio. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Pichaud F., Walther R.F., Nunes de Almeida F. Regulation of Cdc42 and its effectors in epithelial morphogenesis. J Cell Sci. 2019;132:jcs217869. doi: 10.1242/jcs.217869. [DOI] [PubMed] [Google Scholar]
- Pinho F.G., Frampton A.E., Nunes J., Krell J., Alshaker H., Jacob J., et al. Down-regulation of microRNA-515-5p by the estrogen receptor modulates sphingosine kinase 1 and breast cancer cell proliferation. Cancer Res. 2013;73:5936–5948. doi: 10.1158/0008-5472.CAN-13-0158. [DOI] [PubMed] [Google Scholar]
- Porstmann T., Griffiths B., Chung Y.L., Delpuech O., Griffiths J.R., Downward J., et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- Porstmann T., Santos C.R., Griffiths B., Cully M., Wu M., Leevers S., et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Peterson T.R., Sabatini D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mole Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J., Proff J., Havemeier A., Ladwein M., Rottner K., Barlag B., et al. Serine-71 phosphorylation of Rac1 modulates downstream signaling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S., McKay C., Schuetz E., Van Deursen J.M., Stravopodis D., Wang D., et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Van Baal J., Ma L., Loor J.J., Wu Z.L., Dijkstra J., et al. Short communication: relationship between lysine/methionine ratios and glucose levels and their effects on casein synthesis via activation of the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J Dairy Sci. 2019;102:8127–8133. doi: 10.3168/jds.2018-15916. [DOI] [PubMed] [Google Scholar]
- Wilkinson S., Paterson H.F., Marshall C.J. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- Xu R., Spencer V.A., Groesser D.L., Bissell M.J. Laminin regulates PI3K basal localization and activation to sustain STAT5 activation. Cell Cycle. 2010;9:4315–4322. doi: 10.4161/cc.9.21.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Hitomi M., Stacey D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Zhang M., Ao J., Zhen Z., Gao X., Li M. NUCKS1 is a novel regulator of milk synthesis in and proliferation of mammary epithelial cells via the mTOR signaling pathway. J Cell Physiol. 2019;234:15825–15835. doi: 10.1002/jcp.28240. [DOI] [PubMed] [Google Scholar]
- Yurchenco P.D., Patton B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubiane G.S., Valentijn A., Lowe E.T., Akhtar N., Bagley S., Gilmore A.P., et al. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J Cell Sci. 2004;117:271–280. doi: 10.1242/jcs.00855. [DOI] [PubMed] [Google Scholar]