Abstract
Introduction
Heritable lung cancer may occur in the context of germline TP53 mutations (Li-Fraumeni syndrome). Limited cases of intrafamily tumor genomic characteristics have been reported.
Main concerns, Important Clinical Findings, Primary Diagnoses, Interventions, Outcomes
A 40-year-old woman with no smoking history or known environmental exposure risk was incidentally found to have stage II (T2N1) NSCLC harboring an EGFR exon 19 p.Glu746_Ala750 deletion. Family history was notable for an identical twin sister with colorectal cancer (diagnosed at age 31 y) and a mother with stage I NSCLC harboring an EGFR exon 21 c.2573T>G (p.Leu858Arg) mutation (diagnosed at age 69 y). Genetic testing revealed a germline TP53 c.542G>A (p.Arg181His) mutation in the patient, her mother, and her sister, consistent with Li-Fraumeni syndrome. No germline EGFR mutations were detected.
Conclusion
Shared germline TP53 mutations may be associated with distinct NSCLC somatic EGFR mutations within families with Li-Fraumeni syndrome. Further understanding of the association between genetic cancer syndromes and lung cancer risk may improve early lung cancer detection in populations not otherwise meeting screening eligibility.
Keywords: Case report, Epidermal growth factor receptor, Genetics, Inherited, Lung cancer
Introduction
Lung cancer almost always occurs sporadically. More than 80% of cases are attributable to environmental exposure, most often tobacco but also radon, asbestos, and other carcinogens. Although the identification of somatic driver alterations in oncogenes such as EGFR or ALK provides a mechanistic description for lung cancer in individuals without obvious environmental causes, why some individuals develop these genomic events remains unknown. In rare cases, heritable lung cancer may occur in the context of a pathogenic germline variant.1,2 Here, we report lung cancer cases harboring distinct somatic EGFR variants occurring within family members found to have a germline TP53 mutation consistent with Li-Fraumeni syndrome.
Case Presentation
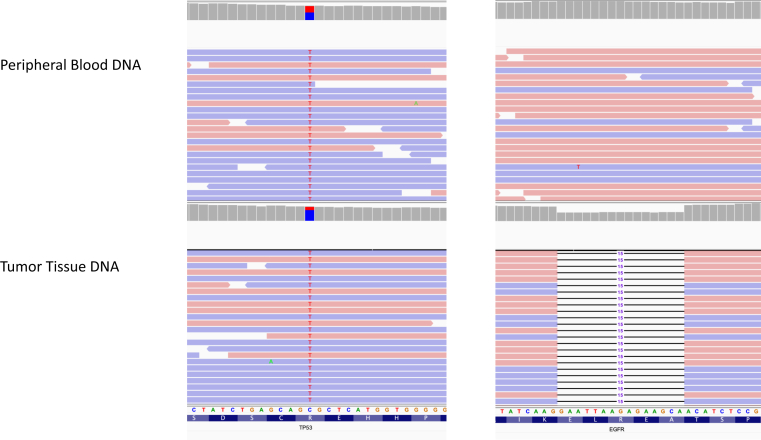
A 40-year-old woman with no smoking history, no concerning environmental exposures, and no cardiopulmonary symptoms underwent total body computed tomography (CT) as part of an initial annual executive medical assessment. These imaging studies revealed a right upper lobe mass. Result of a CT-guided biopsy revealed adenocarcinoma consistent with lung primary. No distant disease was found on brain magnetic resonance imaging (MRI) and positron emission tomography-CT. The patient underwent right upper lobectomy and regional lymph node dissection, with pathologic evaluation identifying a stage II (T2N1M0) lung adenocarcinoma harboring an EGFR exon 19 p.Glu746_Ala750 deletion (Fig. 1).
Figure 1.
TP53 and EGFR variants detected in the proband. The EGFR variant was only detected in the tumor tissue but not in the peripheral blood. The TP53 variant was detected in both tissue and peripheral blood, revealing germline inheritance. Images from Integrated Genomics Viewer software.
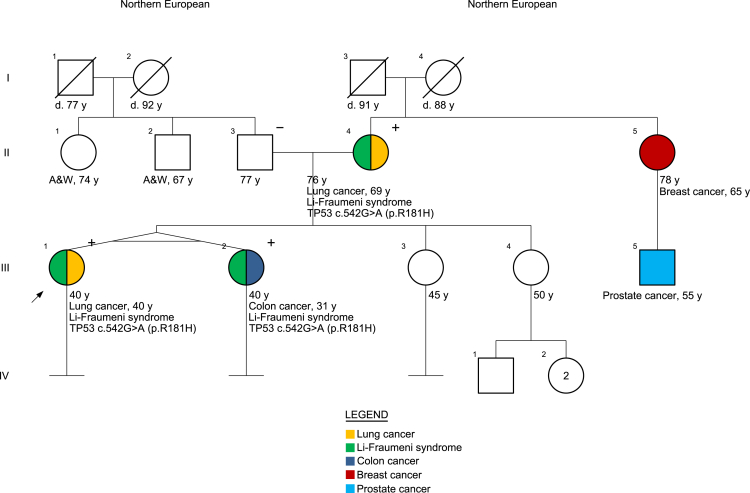
Review of the patient’s family history noted multiple relatives with cancer (Fig. 2). Specifically, her mother, who had a distant and minimal smoking history, had been diagnosed with having a stage 1B RUL NSCLC harboring an EGFR exon 21 c.2573T>G (p.Leu858Arg) mutation at age 69 years (Fig. 1). At age 31 years, an identical twin sister had been diagnosed with having invasive moderately differentiated adenocarcinoma of the sigmoid colon with well-differentiated neuroendocrine tumor features, KRAS wild type, NRAS wild type, and BRAF wild type, with immunohistochemistry for mismatch repair proteins intact. A maternal aunt had breast cancer diagnosed at age 65 years, and her son (first cousin to the proband) was diagnosed with having prostate cancer at age 55 years. On the basis of the occurrence of multiple cancers including young-onset cases, the patient and family members were referred for genetic counseling. Germline genetic testing by a 91-gene pan-cancer panel (see Supplementary Materials) in the proband revealed a heterozygous likely pathogenic germline TP53 variant c.542G>A (p.Arg181His), which was subsequently confirmed in her mother and her twin sister, consistent with Li-Fraumeni syndrome. No germline EGFR mutations were detected.
Figure 2.
Family pedigree. A&W, alive and well; d, died.
Because of concern for heightened leukemogenic potential of cytotoxic agents in the setting of a germline TP53 mutation,3 adjuvant chemotherapy was not recommended. Approximately 6 weeks after surgical resection, the patient started adjuvant osimertinib.
Furthermore, cancer screening was implemented for these family members on the basis of published TP53 variant guidelines. Specific testing—designed to limit radiation exposure given concerns for heightened carcinogenic potential—includes complete physical examination every 6 months, clinical breast examination every 6 months with breast MRI every 12 months, annual brain MRI, annual whole-body MRI, annual dermatologic examination, colonoscopy and upper endoscopy every 2 to 5 years, and ultrasonography of the abdomen and pelvis every 12 months.4
Discussion
Li-Fraumeni syndrome is an autosomal-dominant condition characterized by germline mutations of the TP53 tumor suppressor gene resulting in heightened cancer risk. Characteristic malignancies include sarcoma, leukemia, breast cancer, primary brain tumors, and adrenal cortical tumors. Lung cancer, in particular EGFR-mutant NSCLC, and colorectal cancers have also been reported.1,2 The present case supports a single prior report describing multiple lung cancers harboring different EGFR mutations within a single Li-Fraumeni family.2
The occurrence of two distinct somatic EGFR mutations within this genetic context further establishes the association of TP53 germline mutations with this genomic alteration.
Most EGFR-mutant NSCLC cases reported within Li-Fraumeni cohorts have occurred after earlier cancers, most often breast cancer.1,2 Such patterns have historically led to a two-hit hypothesis, with a germline mutation providing the first hit and additional environmental exposure (such as ultraviolet radiation exposure, radiation therapy, or chemotherapy administered for a prior cancer) providing the second hit. Nevertheless, in the present case, the NSCLC was the first (and to date only) cancer diagnosis for both the patient and her mother. Among Li-Fraumeni-associated breast cancer cases, HER2-positive cases seem over-represented, providing evidence that HER family protein kinases across tumor types may be particularly susceptible to TP53-related alterations.5
Conclusion
History of lung cancer in first-degree relatives seems to confer increased lung cancer risk, independent of age, sex, and smoking.6 Attention to familial lung cancer patterns may help identify individuals at heightened risk for lung cancer who do not otherwise meet age- or smoking-related eligibility for screening. It may also contribute to further understanding of the development of EGFR mutations and other genomic alterations in this disease.
CRediT Authorship Contribution Statement
David E. Gerber, Mitchell S. von Itzstein, Shelby Edmondson: Conceptualization.
Shelby Edmondson, Mitchell S. von Itzstein, Brian Reys, Melissa Mayer, Jeffrey Gagan, David E. Gerber: Investigation.
David E. Gerber, Mitchell S. von Itzstein, Melissa Mayer: Project administration.
Shelby Edmondson, David E. Gerber: Roles/Writing—original draft.
Shelby Edmondson, Mitchell S. von Itzstein, Brian Reys, Melissa Mayer, Jeffrey Gagan, David E. Gerber: Writing—review and editing.
Acknowledgments
The authors wish to acknowledge the patients and their families. All patients presented in the case provided informed consent to participate in this case report.
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Edmondson S, von Itzstein MS, Reys B, Mayer M, Gagan J, Gerber DE. Distinct NSCLC EGFR variants in a family with Li-Fraumeni syndrome: case report. JTO Clin Res Rep. 2022;3:100368.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100368.
Supplementary Data
References
- 1.Michalarea V., Calcasola M., Cane P., Tobal K., Izatt L., Spicer J. EGFR-mutated lung cancer in Li-Fraumeni syndrome. Lung Cancer. 2014;85:485–487. doi: 10.1016/j.lungcan.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Mezquita L., Jove M., Nadal E., et al. High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-Fraumeni syndrome. J Thorac Oncol. 2020;15:1232–1239. doi: 10.1016/j.jtho.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Valdez J.M., Nichols K.E., Kesserwan C. Li-Fraumeni syndrome: a paradigm for the understanding of hereditary cancer predisposition. Br J Haematol. 2017;176:539–552. doi: 10.1111/bjh.14461. [DOI] [PubMed] [Google Scholar]
- 4.Kratz C.P., Achatz M.I., Brugieres L., et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res. 2017;23:e38–e45. doi: 10.1158/1078-0432.CCR-17-0408. [DOI] [PubMed] [Google Scholar]
- 5.Gallardo-Alvarado L.N., Tusie-Luna M.T., Tussie-Luna M.I., et al. Prevalence of germline mutations in the TP53 gene in patients with early-onset breast cancer in the Mexican population. BMC Cancer. 2019;19:118. doi: 10.1186/s12885-019-5312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson S., Thorsteinsdottir U., Gudbjartsson D.F., et al. Familial risk of lung carcinoma in the Icelandic population. JAMA. 2004;292:2977–2983. doi: 10.1001/jama.292.24.2977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.