Summary
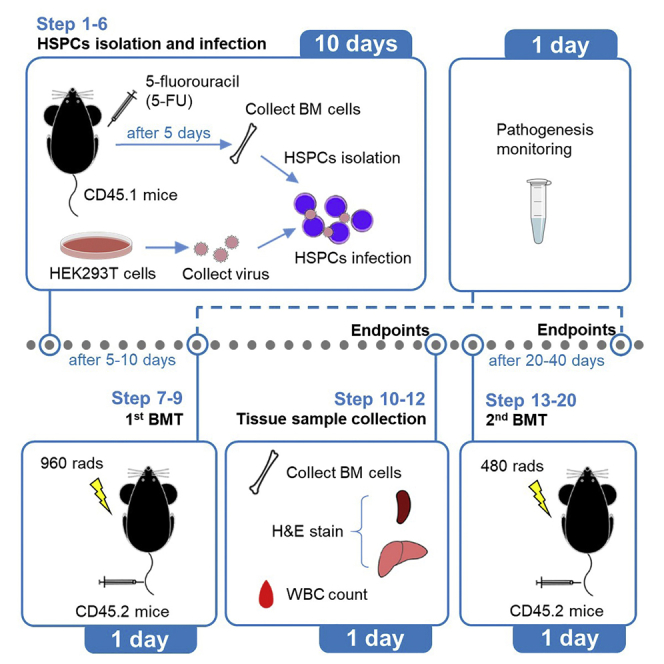
Acute myeloid leukemia (AML) is one of the most common and fatal forms of hematopoietic malignancies. Here, we describe a mouse MLL-AF9_AML model to investigate AML. We have optimized the protocols for retrovirus infection, bone marrow transplantation (BMT), and leukemia monitoring to create a stable mouse model. In particular, we have used two rounds of BMT to enhance stability and efficiency. This model can be used to conduct drug administration and/or other interventions easily.
For complete details on the use and execution of this protocol, please refer to Zhao et al. (2022).
Subject areas: Cancer, Cell isolation, Cell separation/fractionation, Cell-based Assays
Graphical abstract
Highlights
-
•
Optimized BMT accelerates full blast leukemia in recipient mice
-
•
Step by step details of two rounds of BMT and tissue sample collection
-
•
Protocols for monitoring AML pathogenesis after each BMT
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Acute myeloid leukemia (AML) is one of the most common and fatal forms of hematopoietic malignancies. Here, we describe a mouse MLL-AF9_AML model to investigate AML. We have optimized the protocols for retrovirus infection, bone marrow transplantation (BMT), and leukemia monitoring to create a stable mouse model. In particular, we have used two rounds of BMT to enhance stability and efficiency. This model can be used to conduct drug administration and/or other interventions easily.
Before you begin
The steps below describe how to create an AML mouse model, i.e., the MLL-AF9_AML, for therapeutic investigation purposes. Stable MLL-AF9 expression in primary donor cells is achieved through retroviral infection. Two times of BMT help accelerate the pathogenesis of full blast leukemia in recipient mice. This protocol also fits for the modeling of other AMLs that can be induced by single gene mutation or gene fusion, such as the MLL-AF10_AML, the MLL-ENL_AML, and the AML-ETO9a_AML, etc.
Preparation of animal
4-6 week-old B6.SJL (CD45.1) mice will be used as primary BMT donor mice, and should receive 150 mg/kg 5-Fluorouracil (5-FU) injection five days prior to the start retrovirus infection of HEK293T cells. All animals should be housed and maintained with a 12-h light/dark cycle at 23 ± 2°C.
Note: The volume of injection is 40–50 μL per adult mouse, with the maximum not exceeding 100 μL.
Preparation of target plasmids
The MSCV-neo-MLL-AF9 and retroviral package plasmid were kind gifts from Dr. Jianjun Chen of Beckman Research Institute of City of Hope.
Institutional permissions
All experiments should be carried out according to the ethical standards of animal care agencies. Randomization, allocation concealment and blind outcome assessment should be conducted throughout all the related experiments. Sample animals studied in this protocol were housed and maintained at the Laboratory Animal Center of Zhejiang University (Hangzhou, China), and experiments were carried out based on the approval of the animal care agency of Zhejiang University.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Mo Ly-6G (Gr-1) Monoclonal Antibody (RB6-8C5), PE-Cyanine5 (1:100) | eBioscience | 48-5931-82 |
| CD11b Monoclonal Antibody (M1/70), Super Bright 600 (1:100) | eBioscience | 63-0112-82 |
| Anti-Mo CD45.1, PE-Cyanine5.5 (1:100) | eBioscience | 45-0453-82 |
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant mouse IL-3 | PeproTech | 213-13 |
| Recombinant human IL-6 | PeproTech | 200-06 |
| Recombinant mouse SCF | PeproTech | 250-03 |
| 2-Mercaptoethanol (BME) | Macklin | M6230 |
| 5-Fluorouracil (5-FU) | Selleck | S1209 |
| Geneticin sulfate (G418) | TargetMol | T6512 |
| Lipofectamine™ 2000 | Thermo Fisher Scientific | 11668019 |
| Opti-MEM | Gibco | 31985062 |
| RPMI-1640 | Gibco | C11875500CP |
| DMEM (high glucose) | Gibco | C11995500BT |
| FBS | Gibco | 10099141C |
| 1 × PBS (pH7.4) | Solarbio | P1010 |
| Penicillin-Streptomycin Liquid | Solarbio | P1400 |
| BSA | Solarbio | A8020 |
| HEPES | Solarbio | H8090 |
| Sodium pyruvate | Sigma-Aldrich | P5280 |
| Polybrene | Sigma-Aldrich | H9268 |
| Ammonium chloride | Sinopharm | 10009617 |
| Potassium bicarbonate | Sinopharm | 20030218 |
| EDTA | Sinopharm | 10009617 |
| Anhydrous ethanol | Sinopharm | 10009259 |
| Human TruStain FcXTM | BioLegend | 422302 |
| Staining buffer | BD Biosciences | 554657 |
| IC Fixation Buffer | eBioscience | 00-8222-49 |
| Critical commercial assays | ||
| Lineage Cell Depletion Kit, mouse | Miltenyi Biotec | 130-090-858 |
| Experimental models: Cell liness | ||
| HEK293T cells | ATCC | CRL-3216 |
| Experimental models: Organisms/Strains | ||
| B6.SJL (CD45.1) mouse | A gift from Dr. Fudi Wang | N/A |
| C57BL/6 mouse | Beijing Vital Laboratory Animal Technology | 213 |
| Recombinant DNA | ||
| MSCV-neo-MLL-AF9 | A gift from Dr. Jianjun Chen | N/A |
| pCL-ECO | A gift from Dr. Jianjun Chen | N/A |
| Softwareand Algorithms | ||
| BD FACSDiva Software | BD Biosciences | https://www.bdbiosciences.com/zh-cn/products/software/instrument-software/bd-facsdiva-software |
| GraphPad Prism 8.0 software | GraphPad Software, Inc. | http://www.graphpad.com/scientificsoftware/prism/ |
| Other | ||
| 60 mm dish | NEST | 705001 |
| 6-Well plate | NEST | 703001 |
| 15 mL centrifuge tube | NEST | 601052 |
| 50 mL centrifuge tube | NEST | 602052 |
| 1 mL Syringe (with 27G 0.5 inch Needle) | BD Biosciences | 309623 |
| 3 mL Syringe (with 25G 1 inch Needle) | BD Biosciences | 309582 |
| 0.22 μm filter | Millipore | SLGPR33RB |
| 40 μm cell strainer | BD Falcon | 352340 |
| MS Columns | Miltenyi Biotec | 130-042-201 |
| 0.5 mL MiniCollect tube K3E K3EDTA | Greiner Bio-One | 450530 |
| Cell counting plate | Countstar | 12-0005-50 |
| Sterile scalpel blade | JZ Surgical Instruments | J0B080 23# |
| X-ray irradiator | Rad Source | RS2000Pro |
| Refrigerated centrifuge | Thermo Fisher Scientific | ST16R |
| Countstar Automated Cell Counter | Countstar | IC 1000 |
| Mouse tail vein injection device | KEW Basis | KW-XXY |
| Hematology analyzer | URIT, China | URIT-2900Vet Plus |
| Flow cell sorter | BD Biosciences | BD FACSAria III |
| OctoMACS Separator | Miltenyi Biotec | 130-042-109 |
| MACS MultiStand | Miltenyi Biotec | 130-042-303 |
| Lamp | OPPLE | E27 |
Note: Antibodies with other fluorescent labels can also be used. Other flow cytometry systems equipped with the requested laser channels would fit for the experimental purpose as well. Other cell counter equipment, e.g., the Countess (Thermo Fisher Scientific), can also be used.
Materials and equipment
0.4% trypan blue solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Trypan blue | 0.4% | 40 mg |
| 1 × PBS | n/a | 10 mL |
| Total | n/a | 10 mL |
Note: This solution should be used after filtering with 0.22 μm filter and can be stored at 24°C for long.
HEK293T medium
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 10% | 1 mL |
| Penicillin-Streptomycin Liquid | 1% | 500 μL |
| DMEM (high glucose) | n/a | 48.5 mL |
| Total | n/a | 50 mL |
Note: This medium can be stored at 4°C for 1 month.
BM washing medium
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 2% | 1 mL |
| RPMI-1640 | n/a | 49 mL |
| Total | n/a | 50 mL |
Note: This medium can be stored at 4°C for 1 month.
ACK lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NH4Cl | 155 mM | 8.29 g |
| KHCO3 | 10 mM | 1.0 g |
| EDTA | 0.1 mM | 0.2 mL (500 mM) |
| Total | n/a | 1 L |
Note: This medium should be used after filtering with 0.22 μm filter and can be stored at 4°C for 1 month.
Progenitor cell culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Recombinant mouse IL-3 | 10 ng/mL | 100 ng |
| Recombinant human IL-6 | 10 ng/mL | 100 ng |
| Recombinant mouse SCF | 100 ng/mL | 1 μg |
| BME | 55 mM | 0.55 mM |
| Penicillin-Streptomycin Liquid | 1% | 100 μL |
| HEPES | 1% | 100 μL |
| FBS | 10% | 1 mL |
| RPMI-1640 | n/a | 9 mL |
| Total | n/a | 10 mL |
Note: This medium should be made freshly on the day of use and kept storage at 4°C.
MCs buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| BSA | 0.5% | 8.29 g |
| EDTA | 2 mM | 29.2 mg |
| 1 × PBS | n/a | 50 mL |
| Total | n/a | 50 mL |
Note: This buffer should be used after filtering with 0.22 μm filter and can be stored at 4°C for 1 month.
Frozen stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 20% | 200 μL |
| DMSO | 10% | 100 μL |
| RPMI-1640 | n/a | 700 μL |
| Total | n/a | 1 mL |
Note: This solution should be made freshly on the day of use.
Step-by-step method details
Retroviral particle production and mouse hematopoietic stem/progenitor cell (mHSPC) spinoculation
Timing: 10 day
The aim of this session is to produce retroviral particles encoding MLL-AF9 oncogenic gene fusion, and infect mHSPCs isolated from CD45.1+ donor mice with this retrovirus.
-
1.Day 0: Seed HEK293T cells in 60 mm culture dishes at 0.5 × 106 cells per dish in 5 mL HEK293T medium, and culture the cells in 37°C, 5% CO2 incubator.
-
a.Cells are counted as follows: Add 20 μL cell suspension into a 1.5 mL tube. Mix well with 20 μL 0.4% trypan blue solution.
-
b.Take out 20 μL of the mixture and add the mixture into the cell counting plate. Place the cell counting plate into the Countstar Automated cell counter and count the cell number.
-
a.
Note: 1 × 106 HEK293T cells will be needed for virus producing purpose for 1 × 106 bone marrow (BM) progenitor cells of one infection group.
-
2.Day 1: Transfect HEK293T cells.
-
a.Change the medium of HEK293T cells with 4 mL fresh HEK293T medium before transfection.
-
b.Prepare two 1.5 mL centrifuge tubes, label the tubes with Tube 1 and Tube 2, and then add 250 μL Opti-MEM per tube.
-
c.Add plasmid and Lipofectamine™ 2000 into each tube according to the list below:Transfection system
NO. Reagent Amount Tube 1 MSCV-neo-MLL-AF9 plasmid 1.8 μg
pCL-ECO plasmid 1.2 μg Tube 2 Lipo2000 10 μL -
d.Mix each tube gently and incubate for 5 min at 24°C.
-
e.Combine tube 2 with tube 1, mix slightly and incubate the mixture for 20 min at 24°C.
-
f.Add the transfection mixture into HEK293T cells and culture the cells at a 37°C, 5% CO2 incubator.Note: To obtain higher transfection efficiency, the ideal density of HEK293T cells is of 50%–60% confluence and well adherent.
-
a.
-
3.Day 2: Insolate BM progenitor cells.
-
a.Change medium of transfected HEK293T cells with 4 mL fresh HEK293T medium and 1.0 mM sodium pyruvate early in the morning.
-
b.Euthanize donor mice. Disinfect the mice with 70% ethanol. Dissect the tibia and the femur of both rear arms, remove the muscles, adipose and disconnect the joints as much as possible (Figure 1).
-
c.Put the bones into a 60 mm tissue dish containing 4 mL fresh BM washing medium. Flush BM cells into a new 60 mm tissue dish with 3 mL BM washing medium by use of a 3 mL syringe with 25 G 1 1/2 inch needle (Figures 2A and 2B).
-
d.Eliminate cell debris and clumps by filtering the cell suspension with a 40 μm cell strainer, and collect the flow-through into a 50 mL centrifuge tube (Figures 2C and 2D). Wash the cell strainer with 1 mL BM washing medium.
-
e.Spin down at 400 g for 8 min at 4°C.
-
f.Aspirate the supernatant and resuspend the cell pellets with 20 mL ACK lysis buffer at 4°C for 8 min to remove the red blood cells lysis.
-
g.Spin down at 400 g for 8 min at 4°C. Discard the supernatant and add 5 mL MCs buffer to resuspend the cell pellets.
-
h.Count cell numbers.
-
i.Centrifuge cells at 400 g for 8 min. Aspirate the supernatant.
-
j.Use Lineage Cell Depletion Kit for magnetic labeling according to the manufacturer’s protocol. Add 40 μL MCs buffer per 107 total cells to resuspend the cell pellets.
-
k.Add 10 μL Biotin-Antibody Cocktail per 107 cells and mix well. Incubate at 4°C for 10 min.
-
l.Add 30 μL MCs buffer and 20 μL Anti-Biotin MicroBeads per 107 total cells and mix well. Incubate at 4°C for 15 min.
-
m.Add 1 mL MCs buffer per 107 total cells to wash the cells. Spin down at 300 g for 10 min.
-
n.Aspirate the supernatant and add 500 μL MCs buffer per 5 × 107 total cells to resuspend the cell pellets.
-
o.Place the separation column in a MACS separator device. Rinse the separation column with 500 μL MCs buffer. Transfer the cell suspension to separation column and collect the pass-through.
-
p.Wash the separation column with 500 μL MCs buffer for three times.
-
q.Count the enriched progenitor cells. Centrifuge cells at 400 g for 8 min and discard the supernatant. Resuspend the cell pellets at a density of 1–5 × 106 cells/mL with progenitor cell culture medium. Culture the cells at 37°C, 5% CO2 incubator.
-
a.
Note: According to the instruction, the maximum volume of a MS column is 500 μL. According to our experiences, it is expected that one 5-FU injected healthy donor yields 2.5–3.5 × 106 BM cells. Other groups also reported the expected yields were around 3.04 ± 0.79×106 four days after 150 mg/kg 5-FU treatment. (Shaikh et al., 2016).
-
4.Day 3: Infect BM progenitor cells.
-
a.Pre-warm the centrifuge at 30°C.
 CRITICAL: It is important to keep the temperature consistently at 30°C to ensure the transfection efficiency and cell survival.
CRITICAL: It is important to keep the temperature consistently at 30°C to ensure the transfection efficiency and cell survival. -
b.Collect the retrovirus and filter the virus medium with a 22 μm cell strainer into a 15 mL centrifuge tube. Change medium as the same as Day 2 for HEK293T cells.
-
c.Add the 3.2 mL filtered retrovirus into one well of 6 well plates. Add 800 μL cell suspension into the virus soup.
-
d.Add 4 μg/mL polybrene into the wells.
-
e.Centrifuge cells at 550 g, 30°C for 3 h.
-
f.Incubate the cells at 37°C, 5% CO2 incubator for 1 h.
-
g.Collect the BM cells into a 15 mL centrifuge tube and centrifuge at 400 g for 8 min.
-
h.Aspirate the supernatant and resuspend the cell pellets with 4 mL progenitor cell culture medium.
-
a.
-
5.
Day 4: Repeat the procedure as Day 3 to infect BM progenitor cells again.
-
6.
From Day 5–10: Add 50 mg/mL G418 into each well of the cell culture plate.
CRITICAL: Take care of progenitor cells; record cell numbers and viability every other day if necessary and keep cell density at 1–5× 106 cells/ml.
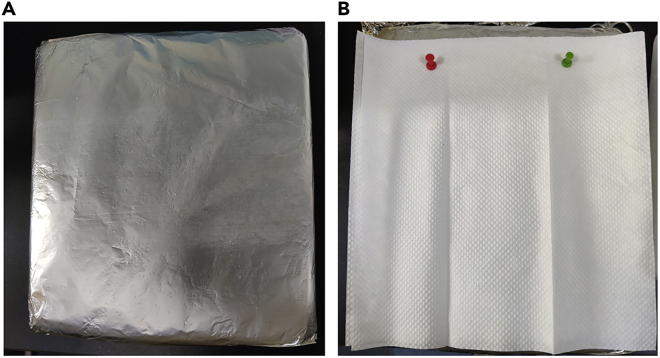
Figure 1.
Preparation of an instant mouse fixation and operation board
(A) Wrap the foam board with aluminum foil.
(B) Cover the aluminum foil with 3 layers of paper towels. And then euthanized mouse can be fixed on top of the board for further operations.
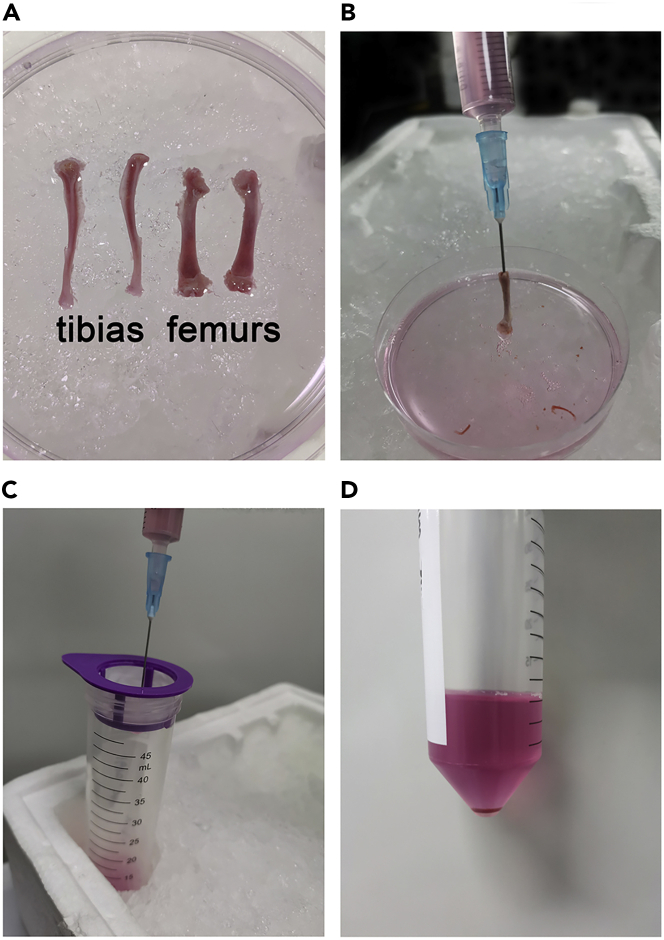
Figure 2.
Harvest the bone marrow (BM) cells
(A) Dissect the tibias and femurs into a 60 mm tissue culture dish with 4 mL fresh BM washing buffer.
(B) Flush the BM cells.
(C and D) Filter the cell suspension through a 40 μm cell strainer.
1st BMT
Timing: 1 day
In this session, transfected primary donor cells are transplanted into primary recipient mice to trigger primary AML.
-
7.
Irradiate C57BL/6 (CD45.2) recipient mice at 960 rads in the morning.
-
8.Prepare donor cells for BM transplantation.
-
a.Sacrifice one CD45.2 mouse (not irradiated) as helper and collect its BM cells according to the above procedure.
-
b.Harvest transfected BM progenitor cells and filter the cell suspension with a 40 μm cell strainer into a 50 mL centrifuge tube to remove cell clumps.
-
c.Count cell number and take out appropriate cells as BMT donor cells. Keep these cells in another 50 mL centrifuge tube.
-
d.Add 1 × 106 helper cells per recipient mouse into collection tube containing donor cells.
-
e.Centrifuge the cells mixture at 400 g for 8 min.
-
f.Remove the supernatant and resuspend the cell pellets with 1 × PBS.
-
g.Centrifuge cells at 400 g for 8 min.
-
h.Discard the supernatant. Add appropriate amount of 1 × PBS (100 μL per recipient mouse) into the cell pellets.
-
a.
-
9.Transplant donor cells into recipient mice via tail vein injection.
-
a.Pre-warm the recipient mice adequately with a warm lamp, for about 10–15 min, to dilate blood vessels prior to injection.
-
b.Use a 1 mL syringe and a 25 G 1 inch needle to mix the cell.
-
c.Change the needle with a 27 G 0.5 inch one and inject 100 μL of the cell suspension per mouse through tail veins.
-
d.Feed recipient mice with antibiotic-containing water.
-
a.
Note: Pay attention to the health condition of the irradiated mice. If necessary, give some auxiliary care, e.g., water gel, soft food, etc., in the first one or two weeks after BMT.
Tissue sample collection from leukemia mice
Timing: 1 day
In this session, BM cells from the primary leukemic recipient mice are collected.
-
10.
Harvest the BM cells and spleen according to the procedure 3b-i when the recipient mice are meeting the endpoints.
Optional: Harvest the blood, liver and spleen according to your experimental purposes (Figure 3).
-
11.
Prepare 0.1–0.2 × 106 1st BMT recipient mice’ BM cells as donor cells for each 2nd recipient mouse in 2nd BMT.
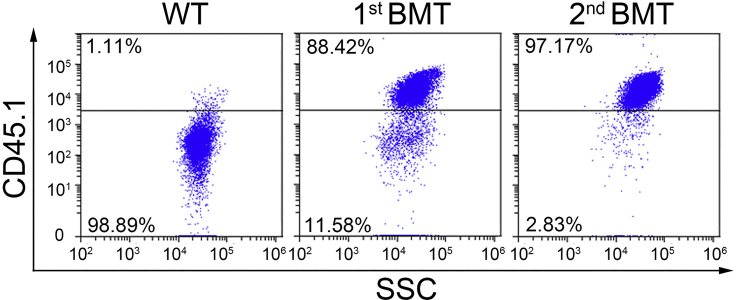
Note: Since engraftment (i.e., CD45.1+ population) of 1st BMT recipients at the end point is usually above 85% (Figure 4), these BM cells (i.e., 2nd BMT donor cells) are considered to be leukemic cells with malignant expansion, and can be used as 2nd BMT donor cells directly. If the primary recipients’ final engraftment is below 85%, purify the leukemic cells through flow cytometry isolation (see step 20 for details).
-
12.
Freeze remaining cells in liquid nitrogen with frozen stock solution.
Pause point: Cells can be stored in liquid nitrogen for several months.
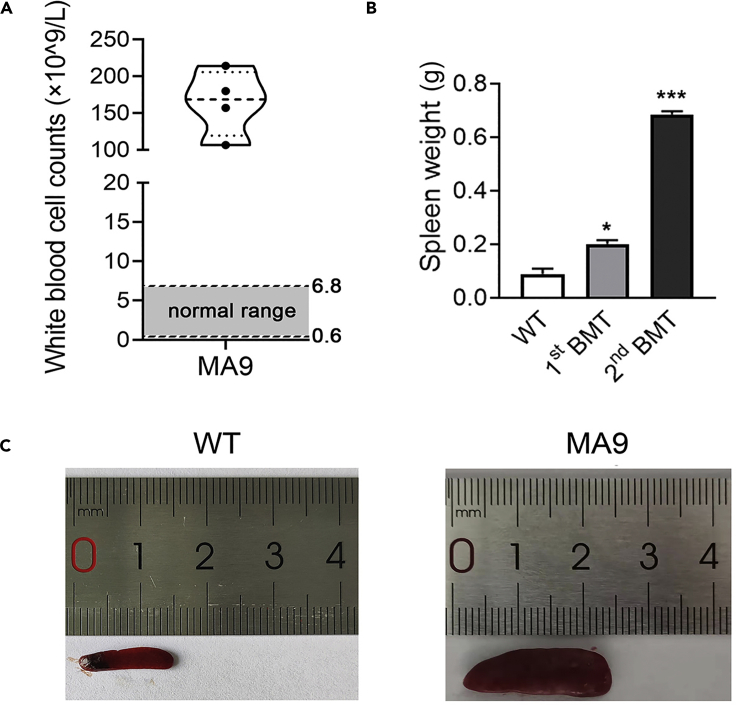
Figure 3.
WBC counts and spleen weights of non-treated C57BL/6 mice, and leukemic recipient mice after 1st BMT or 2nd BMT
(A) WBC counts of MLL-AF9_AML mice at the endpoints of 2nd BMT.
(B) Spleen weights of non-treated C57BL/6 mice, and leukemic recipient mice after 1st BMT or 2nd BMT.
(C) Spleen images of a non-treated C57BL/6 mouse and a MLL-AF9_AML mouse. Mean ± SEM, ∗∗∗p<0.001, two-tailed t-test.
Figure 4.
Flow analysis showing the engraftment of CD45.1+ cells in BM of non-treated C57BL/6 mice, 1st BMT or 2nd BMT recipient mice at the endpoints
2nd BMT
Timing: 20–40 days
In this session, AML cells collected from the primary leukemic recipients are transplanted as donor cells into secondary recipient mice to trigger 2nd leukemia.
-
13.
Collect the BM cells from primary BMT leukemic mice according to the procedure shown above when the mice reach the endpoints. Freeze 5 × 106 cells per tube in liquid nitrogen with frozen stock solution.
-
14.
Thaw one vial of primary BMT leukemic mice BM cells one day before 2nd transplantation as the 2nd BMT donor cells.
-
15.
Irradiate C57BL/6 (CD45.2) mice as 2nd BMT recipients at 480 rads in the morning.
-
16.
Prepare 2nd BMT donor cells (0.1–0.2 × 106 cells per mouse) according to the procedure 8 b-c and e-h.
-
17.
Inject donor cells into tail veins according to the procedure 9.
-
18.Monitor the engraftment of donor cells in recipient mice, and monitor leukemia pathogenesis by collecting peripheral blood (PB) and measuring the percentage of CD45.1+ cell via flow cytometry.
-
a.Restrict a mouse manually or using a mouse restainer for blood collection.
-
b.Aseptically prepare the tail with 70% ethanol.
-
c.Nick the tail with a sterile scalpel blade on a lateral side.
-
d.Collect 50 μL blood in a 0.5 mL MiniCollect tube.
-
e.Press the tail to stop bleeding and return the mouse into the cage.
-
f.Use 1 mL ACK lysis buffer to lyse the red blood cells and move the lysis in to a new 1.5 mL tube. Incubate at 4°C for 8 min.
-
g.Spin down at 400 g for 8 min at 4°C and remove the lysis.
-
h.Use 1 mL ACK lysis buffer to resuspend cell pellets. Repeat the incubation and centrifugation.
-
i.Use 1 mL ice-cold 1 × PBS to resuspend cell pellets and spin down at 400 g for 8 min at 4°C. Remove the supernatant.
-
j.Sample preparation and detection for flow cytometry according to the steps 20b–g.
-
a.
Pause point: Fixed flow cytometry samples can be stored in 4°C for 2–3 days before testing.
Optional: Drug administration or other manipulation can be carried out in this period.
Pathological sample collection and pathogenesis monitoring
Timing: 1 day
In this session, AML pathogenesis after each BMT is monitored through flow cytometry analysis.
-
19.
Collect PB from heart into 0.5 mL MiniCollect tube by a 1 mL syringe with 27 G 0.5 inch needle and test WBC (Figure 3A). Harvest the BM cells and weigh the liver and spleen of leukemia mice (Figures 3B and 3C).
Optional: Collect liver and spleen tissues, perform paraffin embedding, slide cutting and H&E staining, to investigate leukemic cell infiltration condition. Observe cell morphology with cytospin specimens of BM cells.
-
20.Detecting leukemic cell engraftment and differentiation through BM cells and flow cytometry analysis.
-
a.Collect 1 × 106 leukemia mice BM cells and wash with ice cold 1 × PBS.
-
b.Add 30 μL BD staining buffer and 0.3 μL Human TruStain FcXTM to resuspend the cell pellets.
-
c.Blend the cells by flicking and stay at 24°C for 10 min.
-
d.For the leukemic cell engraftment test, add 0.5 μL anti-mouse CD45.1- PE-Cyanine5.5 into TruStain FcXTM blocking cells. Stain cell samples with anti-mouse CD11b-Super Bright 600 and anti-mouse Gr-1- PE-Cyanine5 antibodies for cell differentiation test. Keep a blank control without adding any antibodies or simple IgG.
-
e.Incubate at 4°C for 25 min. Avoid light.
-
f.Add 1 mL ice cold 1 × PBS to wash away excess antibodies. Centrifuge cells at 400 g for 8 min.
-
g.Discard the supernatant and resuspend the cell pellets with 400 μL IC Fixation Buffer and then load for flow cytometry analysis on BD FACSAria III.
-
a.
Pause point: Fixed flow cytometry samples can be stored in 4°C for 2–3 days before testing.
Expected outcomes
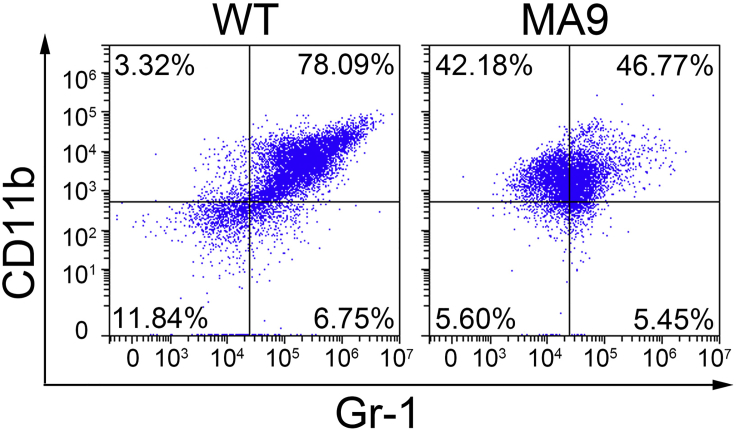
This protocol describes a mouse model of acute myeloid leukemia for therapeutic investigation purposes. Drug administrations or other inventions can be conducted when the engraftments reach 15%–20%, i.e., the onset of leukemia. The engraftment can be tested by collecting PB and measuring the percentage of CD45.1+ cell via flow cytometry every 10 days post BMT. Usually it takes 60–90 days for the primary BMT recipient mice and 25–40 days for the secondary BMT recipient mice to develop full blast leukemia. Significant differences can be found in spleen size between non-treated C57BL/6 mice, 1st BMT leukemic recipients and 2nd BMT leukemic recipients, indicating the infiltration of AML cells into spleen of the recipient mice (Figure 3). The engraftment of MLL-AF9 leukemic cells (CD45.1+) can be observed through flow cytometry analysis in 1st BMT leukemic recipients and 2nd BMT leukemic recipients (Figure 4). The proportion of Gr1+/CD11b+ cells was remarkably reduced in BM cells of MLL-AF9 leukemic recipient mice as compared with BM cells of non-treated C57BL/6 mice, indicating the increase of immature neutrophilic cell proportion in AML recipient mice (Figure 5).
Figure 5.
Flow analysis showing cell lineages of BM cells of MLL-AF9_AML mice and C57BL/6 controls
Limitations
This protocol provides a complete method to establish the MLL-AF9_AML disease model through two rounds of BMTs (Jiang et al., 2012, 2016, 2017). Because of the lethal dosage of irradiation, especially in the primary BMTs, it is inevitable that a small proportion (around 10–20%) of the recipient mice might turn weak or even die after exposure. The other limitation is that it takes relatively long for the primary BMT recipients to develop leukemia. For example, it takes up to 9 months for the AML-ETO9a primary recipients to reach the onset of leukemia.
Troubleshooting
Problem 1
The yield of HSPCs is not enough (step 3 of step-by-step method details).
Potential solution
After injecting with 150 mg/kg 5-FU, the 6-week-old mouse should yield at least 3.5 × 106 BM cells. To avoid low yield of HSPCs, we suggest: (1) Make sure that the dose of 5-FU is 150 mg/kg per adult mouse of body weight; (2) Ensure that all BM cell collection processes are carried out on ice, and shorten the whole collection procedure as much as possible; (3) The capacity of MS column is 1 × 10⁷ magnetically labeled cells from up to 2 × 10⁸ total cells according to the manufacturer’s protocol. An excessive count of total cells could clog the column, so that the cell fluid won’t flow through smoothly. For this issue, we recommend to use more than one MS column and combine the pass-through, or use a LS column, whose capacity is 10 times larger than the MS column.
Problem 2
The virus titer is not high enough (step 5 of step-by-step method details).
Potential solution
Given the extremely high expansion capability of MLL-AF9-AML cells, the workable virus titer range is quite broad. In order to obtain optimal retrovirus production, make sure the HEK293T cells are in good condition. All the plasmids used for transfection should be of proper purity and concentration, and endotoxin free.
Problem 3
Low cell viability of HSPCs after spinoculation and G418 selection (step 9 of step-by-step method details).
Potential solution
The centrifuge should be preheated before spinoculation, and ensure the temperature stay stable during the 3 h’ spinning. It is not recommended to change culture medium immediately after spinoculation. The infected HSPCs should be incubated at a 37°C, 5% CO2 incubator for 1 h before changing medium. Cell viability should be monitored and cell density should be adjusted from time to time after two times of spinoculation as well as the G418 selection period.
Problem 4
The recipient mice die after irradiation and BMT (steps 10–12 of step-by-step method details).
Potential solution
It is highly recommended to give supportive care to the recipient mice in the first one or two weeks after irradiation and BMTs. Antibiotics can be added into drinking water to prevent infection. Soft food and water gel can be used. Donor cells for BMT should be sieved with a 40 μm cell strainer to remove large cell clumps prior to cell counting, and thus to prevent blood vessels from being clogged after tail vein injection. All the surgical instrument should be sterilized at 103.4k Pa steam pressure (121.3°C) for 20 min in advance. The injection process should be quick and skilled, and bleeding should be stopped in time after surgery.
Problem 5
The engraftment of transplanted cells is low or slow (steps 19 and 20 of step-by-step method details).
Potential solution
The BM CD45.1+ cell proportion of a primary AML recipient mouse is usually above 85%, and these cells are with high malignant expansion potency. Obvious engraftment (i.e., CD45.1+>10%) of these donor cells can be often detected 20 days post 1st BMT or 10 days post 2nd BMT through tail vein blood collection and flow cytometry analysis, as shown in steps 19 and 20. The speed of engraftment is closely related with the malignant potency of the donor cells, as well as the condition of the recipient mice. It is recommended to use cells with high viability and malignant expansion potency as donor cells. MTT assays can be done prior to BMT for the purpose of selecting optimal donor cells. Different kinds of recipient mice have slightly different responses to irradiation. The irradiation dose can be adjusted among 800–960 rads for 1st BMT, and 400–500 rads for 2nd BMT, with one dosage or two split dosages. In some cases, BM suppressive drugs, e.g., Busulfan, can be an alternative choice.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xi Jiang (xjiang@zju.edu.cn).
Materials availability
No new reagent or materials were generated in this study.
Acknowledgments
We would like to thank the Core Facilities, Zhejiang University School of Medicine for the technical support. The National Natural Science Foundation of China (Grant Nos. 31900426 and 81970144) (to X.J.) supported this work.
Author contributions
J.L., H.Z., L.Y., and X.J. analyzed the data and wrote the paper.
Declaration of interests
The authors declare no conflicts of interest.
Data and code availability
No new datasets or code was generated in this study.
References
- Jiang X., Bugno J., Hu C., Yang Y., Herold T., Qi J., Chen P., Gurbuxani S., Arnovitz S., Strong J., et al. Eradication of acute myeloid leukemia with FLT3 ligand-targeted miR-150 nanoparticles. Cancer Res. 2016;76:4470–4480. doi: 10.1158/0008-5472.can-15-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Hu C., Ferchen K., Nie J., Cui X., Chen C.H., Cheng L., Zuo Z., Seibel W., He C., et al. Targeted inhibition of STAT/TET1 axis as a therapeutic strategy for acute myeloid leukemia. Nat. Commun. 2017;8:2099. doi: 10.1038/s41467-017-02290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Huang H., Li Z., Li Y., Wang X., Gurbuxani S., Chen P., He C., You D., Zhang S., et al. Blockade of miR-150 maturation by MLL-fusion/MYC/LIN-28 is required for MLL-associated leukemia. Cancer Cell. 2012;22:524–535. doi: 10.1016/j.ccr.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh A., Bhartiya D., Kapoor S., Nimkar H. Delineating the effects of 5-fluorouracil and follicle-stimulating hormone on mouse bone marrow stem/progenitor cells. Stem Cell Res. Ther. 2016;7:59. doi: 10.1186/s13287-016-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Lu J., Yan T., Han F., Sun J., Yin X., Cheng L., Shen C., Wunderlich M., Yun W., et al. Opioid receptor signaling suppresses leukemia through both catalytic and non-catalytic functions of TET2. Cell Rep. 2022;38:110253. doi: 10.1016/j.celrep.2021.110253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new datasets or code was generated in this study.