Summary
The cleavage and functionalization of carbon-carbon (C–C) bonds has emerged as a powerful tool for preparing value-added chemicals. In this protocol, we describe the preparation of amorphous manganese oxide and its application as a heterogeneous catalyst in the direct synthesis of amides via successive cleavage and amidation of C–C bonds in alcohols. Furthermore, we describe how a slight modification of reaction conditions allows for the cleavage and cyanation of alcohols to access sterically hindered nitriles.
For complete details on the use and execution of this protocol, please refer to He et al. (2022).
Subject areas: Chemistry, Material sciences
Graphical abstract

Highlights
-
•
Heterogeneous MnOx-catalyzed cleavage and amidation/cyanation of alcohols
-
•
Convenient preparation of MnOx in large scale
-
•
Recycling and reusing of MnOx
-
•
Wide substrate scope and gram-scale synthesis allowable
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The cleavage and functionalization of carbon-carbon (C–C) bonds has emerged as a powerful tool for preparing value-added chemicals. In this protocol, we describe the preparation of amorphous manganese oxide and its application as a heterogeneous catalyst in the direct synthesis of amides via successive cleavage and amidation of C–C bonds in alcohols. Furthermore, we describe how a slight modification of reaction conditions allows for the cleavage and cyanation of alcohols to access sterically hindered nitriles.
Before you begin
The successive cleavage and functionalization of C–C bonds in alcohols has recently emerged as a powerful tool for preparing value-added chemicals (Fernandes and Kumar, 2003; Rahimi et al., 2013; Li et al., 2015). However, the high dissociation energy of C–C bonds (76–77 kcal mol–1) hampers its cleavage (Shi et al., 2021). In the last few decades, several elegant strategies have been well established in homogeneous catalysis manner for the cleavage and functionalization of alcohols to one- or multiple-carbon shorter aldehydes (Liu et al., 2019a, 2019b), acids (Xu et al., 2014; Wang et al., 2017; Liu et al., 2019c), and esters (Liu et al., 2020). Despite impressive progress made in this area, a large excess of strong base, an over-reliance on stoichiometric and toxic oxidants, and/or high loading of precious metal catalysts still limit most of these reaction classes and leave room for further development in an eco-friendly manner. Heterogeneous catalysis possesses many advantages, including easy separation, efficient recycling, convenient large-scale production, and minimization of metal impurities in the final products, which are not exhibited by homogeneous catalysis. Manganese oxides, as heterogeneous catalysts, have been widely used in oxidation catalysis owing to their unique properties such as multiple oxidation states, high abundancy, and low toxicity (Chandra et al., 2020; Yamaguchi et al., 2012, 2013). As a continuation of our long-time interest in the development of the catalytic transformation of alcohols (Luo et al., 2019, 2020; Shang et al., 2016, 2018; Chen et al., 2014, 2016), we intend to explore value-added transformation of various alcohols via cleavage and functionalization of C–C bonds with the versatile manganese oxides as catalysts. Amides are a ubiquitous class of compounds present in organic chemistry, pharmaceutical industry, agrochemical, materials science, and polymers (Valeur and Bradley, 2009). Many efforts have been devoted to sustainable procedures for the synthesis of amides with cheap and industrially abundant alcohols (Gunanathan et al., 2007; Soulé et al., 2011; de Figueiredo et al., 2016; Piszel et al., 2019). However, poor chemoselectivity, low efficiency, and narrow substrate scope have impeded their widespread applications. Therefore, the current protocol describes the preparation of amorphous manganese oxides and their application as heterogeneous catalyst in the direct synthesis of amides via successive cleavage and amidation of C–C bonds in alcohols. Furthermore, we have shown that a slight modification of reaction conditions allows for the cleavage and cyanation of alcohols to access sterically hindered nitriles.
A wide range of primary alcohols, secondary alcohols and 1,2-diols can be applied to this catalysis system for the corresponding amides synthesis. Moreover, β-O-4 and β-1 lignin model compounds are also suitable to the method. Various meta-substituted aryl alcohols can be transformed into desired sterically hindered nitriles with a slight modification of reaction conditions. In this protocol, 2-phenylethanol, 1- phenylethanol, 1-phenyl-1,2-ethanediol and 1-(phenoxymethyl)benzenemethanol are chosen as model compounds for the amides synthesis and 1-(2-methoxyphenyl)ethanol is chosen for the nitriles synthesis. The gram-scale synthesis of amide and catalyst recycling and reusing experiment are also presented in detail.
Preparation of the reagents and equipment
A complete list of reagents and equipment can be found in the ‘‘key resources table”.
Preparation of manganese oxides (MnOx)
Timing: 30 h
In this procedure, the catalyst MnOx is prepared with KMnO4, Mn(NO3)2 and NH3·H2O within 30 h (Figures 1 and 2).
-
1.
Carefully weigh 10.16 g (64 mmol, 1.0 eq.) KMnO4 on analytical balance and dissolve it in 900 mL (14.1 mL/mmol) deionized water by stirring the mixture for 1 h in a 2 L beaker at 20°C–25°C to form a clear dark purple solution. Set the speed of stirring to ∼600 rpm (AS ONE RS-6DN magnetic stirrer, Figure 1B).
-
2.
Dilute 22.7 mL (34.82 g, 97 mmol, 1.536 g/mL, 1.5 eq. based on KMnO4) Mn(NO3)2 (50 wt% in H2O) with 100 mL (1.0 mL/mmol) H2O (Figure 1B), pour it into the KMnO4 solution and stir the mixture for 15 min. Keep the speed of stirring at ∼600 rpm.
-
3.
Dilute 122.2 mL (111.20 g, 1.63–1.83 mol, 0.910 g/mL, 25.5 eq. based on KMnO4) NH3·H2O (25–28 wt% in H2O) with 100 mL (61.3 mL/mol) H2O (Figure 1B), pour it into the solution and stir the mixture for 1 h. Keep the speed of stirring at ∼600 rpm. Please note the pH of solution after alkalizing is ∼10 (Figure 1C).
CRITICAL: The solution needs to be alkalized to pH 8.0–10.5 with NH3·H2O.
-
4.
Transfer the resultant solution into Teflon-sealed stainless-steel autoclaves (Figure 1D) and crystalize in a 120°C oven for 12 h (Figure 1E).
CRITICAL: Make sure the solution fills 0.6–0.8 of the volume of each Teflon-sealed stainless-steel autoclave.
Note: 100 mL and 300 mL autoclaves are used in this procedure. Based on our experience, the size of autoclave does not affect the preparation of manganese oxides (MnOx).
-
5.
After the autoclave being cooled down to 20°C–25°C, open the autoclave, decant the supernatant, and remove the remaining solution from the autoclave (Figure 2A).
-
6.
Wash the obtained dark brown solid with deionized water (100 mL∗3, 4.7 mL/mmol based on KMnO4) and methanol (100 mL∗2, 3.1 mL/mmol based on KMnO4).
Alternatives: Methanol used here can be replaced by ethanol.
-
7.
Dry the solid product in vacuo at 80°C for 12 h (Figure 2B). After cooling down, the obtained product is grinded into fine powder (Figure 2C). Troubleshooting 1.
Pause point: MnOx can be stored for months in glass vials with a lid at 20°C–25°C (Figure 2D). X-ray diffraction (XRD) pattern of MnOx is performed on a Bruker D8-Advance X-ray diffractometer (Bruker, Germany) using a Cu Kα radiation source. The scanning speed is 10° min−1, and the tube voltage and current are 40 kV and 40 mA, respectively (Figure 3A). N2 adsorption-desorption isotherm of MnOx is measured at 77K using an ASAP 2020 Plus Station and used to measure the Brunauer-Emmett-Teller (BET) specific surface area and porous property of the sample. Before the measurement, the sample is degassed at 473 K under vacuum for 10 h. The average pore diameter of the sample is calculated by the Barrett-Joyner-Halenda (BJH) absorption method (Figure 3B). Scanning electron microscope (SEM) images are conducted on a JSM-7800F Schottky field emission scanning electron microscope operating at an acceleration voltage of 20 kV (Figures 3C and 3D). For more characterization of MnOx, please refer to He et al. (2022).
Figure 1.
Photographs for the synthesis of MnOx
(A) Starting materials for MnOx preparation.
(B) Solutions of starting materials.
(C) pH measuring.
(D) Autoclaves that we used.
(E) Crystallization.
Figure 2.
Photographs for the post-treatment of MnOx preparation
(A) The MnOx mixture after cooling down.
(B) MnOx after drying.
(C) MnOx after grinding.
(D) MnOx stored in glass vials.
Figure 3.
Characterization of MnOx
(A) XRD pattern.
(B) N2 adsorption-desorption isotherm.
(C and D) SEM images.
Note: The scale for MnOx preparation in this procedure is 20 g and there is no obvious difference in catalytic activity with the published 10 g-scale MnOx preparation (He et al., 2022) based on our experience.
Equipment setup
Timing: 1 h
-
8.Setup of gas chromatography–mass spectrometry (GC-MS).
-
a.Perform GC-MS analyses on an Agilent 7890A gas chromatograph equipped with an Agilent 5675C mass-selective detector. A representative method for monitoring the reaction is as follows: set the oven temperature at 50°C, and then hold the temperature at 50°C for 5 min upon injection. Increase the temperature to 250°C for 13.33 min, and then hold the temperature at 250°C for 15 min (Table 1).
-
a.
-
9.Setup of gas chromatography (GC).
-
a.Perform GC analysis on an Agilent 7890A series GC system with a flame ionization detector (FID), column SE-30 30 m × 0.25 mm × 0.32 μm. A representative method for monitoring the reaction is as follows. Set the oven temperature at 100°C, and then hold the temperature at 100°C for 1 min before injection. Increase the temperature to 160°C for 6 min upon injection, and then to 200°C for 2 min. Hold the temperature at 200°C for another 15 min (Table 2).
-
a.
Table 1.
Setup of gas chromatography–mass spectrometry (GC-MS)
| Time (min) | Temperature (°C) | Temperature ramp (°C/min) |
|---|---|---|
| 0–5 | 50 | 0 |
| 5–18.33 | 250 | 15 |
| 18.33–33.33 | 250 | 0 |
Table 2.
Setup of gas chromatography (GC)
| Time (min) | Temperature (°C) | Temperature ramp (°C/min) |
|---|---|---|
| 0 | 100 | 0 |
| 0–6 | 160 | 10 |
| 6–8 | 200 | 20 |
| 8–23 | 200 | 0 |
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Potassium permanganate (KMnO4) (99.5%) | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) | CAS: 7722-64-7 |
| Manganese nitrate (Mn(NO3)2) (AR. 50 wt% in H2O) | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) | CAS: 10377-66-9 |
| Ammonium hydroxide (NH3·H2O) (AR. 25–28 wt% in H2O) | Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China) | CAS: 1336-21-6 |
| Methanol (AR) | Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China) | CAS: 67-56-1 |
| 2-Phenylethanol (GC. >99.0%) | Aladdin (Shanghai, China) | CAS: 60-12-8 |
| 1-Phenylethanol (97%) | Alfa Aesar | CAS: 98-85-1 |
| 1-Phenylethane-1,2-diol (98%) | Aladdin (Shanghai, China) | CAS: 93-56-1 |
| 1-(Phenoxymethyl)benzenemethanol | Synthesized in our lab | CAS: 4249-72-3 (Galkin et al., 2015) https://doi.org/10.1002/cssc.201500117 |
| 1-(2-Methoxyphenyl)ethanol (97.48%) | Bide Pharmatech Ltd. (Shanghai, China) | CAS: 13513-82-1 |
| 1,4-Dioxane (AR) | Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China) | CAS: 123-91-1 |
| Acetonitrile (AR) | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) | CAS: 75-05-8 |
| t-Amyl alcohol (98%) | Aladdin (Shanghai, China) | CAS: 75-85-4 |
| Naphthalene (99+%) | Alfa Aesar | CAS: 91-20-3 |
| Other | ||
| Ultrasonic cleaning machine | Scientz | SB-5200DT |
| Vacuum drying oven | Products in Shanghai Jinghong Laboratory Instrument Co., Ltd. | DZF-6020 |
| Magnetic stirrer, 0–1,500 rpm | AS ONE | RS-6DN |
| Digital stirring hot plates, 60–1,150 rpm, 5°C–200°C | Corning | PC-420D |
| 100 mL autoclave | Tengyu Laboratory Instrument | N/A |
| 120 mL autoclave with pressure gauge | Tengyu Laboratory Instrument | N/A |
| 160 mL autoclave with pressure gauge | Tengyu Laboratory Instrument | N/A |
| 300 mL autoclave | Tengyu Laboratory Instrument | N/A |
| CombiFlash NextGen 300+ flash column chromatography system | Teledyne ISCO | N/A |
| 4 g silica pre-packed flash column | Agela Technologies | CS140004-0 |
| 20 g silica pre-packed flash column | Agela Technologies | CS140020-0 |
| Centrifuge with 5 mL∗12 rotor, max. RCF [× g]: 15845, max. speed [r/min]:15000 | Hunan Xiangyi Laboratory Instrument Development Co., Ltd. | H1650 |
| Forced air oven | EYELA | WFO-520W |
| Rotary evaporator | EYELA | N-1300D-W |
| Aspirator | EYELA | A-1000S |
| Analytical balance | METTLER TOLEDO | ME204E |
| Pipette, 5–50 μL | DragonLab | TopPette 7010101006 |
| Pipette, 1,000–5,000 μL | DragonLab | TopPette 7010101017 |
| 5 mL glass vial | Synthware | T89140519 |
| 60 mL Buchner funnel | Synthware | F964019 |
| 400 MHz Bruker AVANCE III NMR spectrometer | Bruker | N/A |
| ASAP 2020 Plus Station | Micromeritics Instruments Corporation | N/A |
| Bruker D8-Advance X-ray diffractometer | Bruker | N/A |
| JSM-7800F Schottky field emission scanning electron microscope | JEOL | N/A |
| Gas chromatograph (GC) | Agilent Technologies | 7890A |
| Mass Spectrometry (MS) | Agilent Technologies | 5675C |
Materials and equipment
Reagents
Potassium permanganate (KMnO4).
Manganese nitrate (Mn(NO3)2).
Ammonium hydroxide (NH3·H2O).
2-Phenylethanol.
1-Phenylethanol.
1-Phenyl-1,2-ethanediol.
1-(Phenoxymethyl)benzenemethanol.
1-(2-Methoxyphenyl)ethanol.
t-Amyl alcohol.
Naphthalene.
Methanol.
Acetonitrile.
1,4-Dioxane.
Deionized water.
O2 (99.99%).
NH3 (99.9%).
Stock solution of KMnO4 solution
| Reagent | Final concentration | Amount |
|---|---|---|
| KMnO4 | 0.07 M | 10.16 g |
| Deionized water | n/a | 900 mL |
| Total | n/a | 900 mL |
Note: The KMnO4 solution can be stored at 20°C–25°C for one day.
Stock solution of Mn(NO3)2 solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Mn(NO3)2 (50 wt% in H2O) | 0.79 M | 22.7 mL |
| Deionized water | n/a | 100 mL |
| Total | n/a | 122.7 mL |
Note: The Mn(NO3)2 solution can be stored at 20°C–25°C for one day.
Stock solution of NH3·H2O solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NH3·H2O (25–28 wt% in H2O) | 7.34–8.25 M | 122.2 mL |
| Deionized water | n/a | 100 mL |
| Total | n/a | 222.2 mL |
Note: The NH3·H2O solution should be freshly prepared before use.
CRITICAL: Methanol, t-amyl alcohol, acetonitrile and 1,4-dioxane are hazardous chemicals because of their inflammability. NH3 has a distinct pungent smell and O2 can promote combustion. The NH3·H2O is extremely hazardous because it is corrosive and has a distinct pungent smell. One should wear face shield, antierode glove and protective apron and operate in a fume cupboard for safety. Due to the high pressure of autoclaves and the high temperature of hot plates during the reaction, all steps have to be operated with extra care.
Alternatives: The solvents (e.g., methanol, t-amyl alcohol, acetonitrile and 1,4-dioxane) and the substrates (e.g., potassium permanganate, manganese nitrate, ammonium hydroxide, 2-phenylethanol, 1-phenylethanol, 1-phenyl-1,2-ethanediol, and 1-(2-methoxyphenyl)ethanol ) applied in this protocol can be replaced by those with the same or higher purity from other commercial sources (e.g., TCI, Sigma-Aldrich, Alfa Aesar, Acros Organics, Oakwood Chemical and Fluorochem, etc.). The equipment applied in this protocol can be replaced by those with similar functions.
Step-by-step method details
Synthesis of benzamide from 2-phenylethanol (small scale)
Timing: 14 h
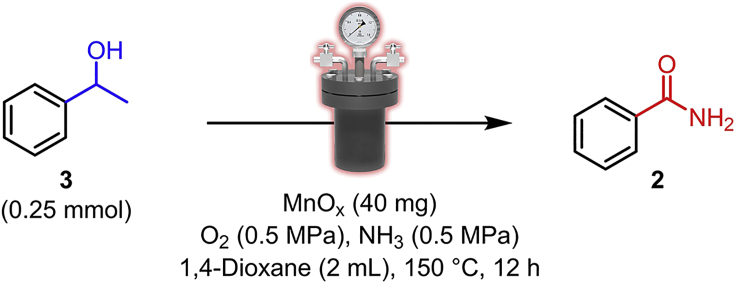
In this procedure, the direct synthesis of benzamide 2 via heterogeneous MnOx-catalyzed successive cleavage and amidation of C-C bonds in 2-phenylethanol 1 has been described (Scheme 1).
Note: This procedure can be also applied to other (hetero)aryl primary alcohols referring to He et al. (2022).
-
1.
Place a 10 mm long fusiform magnetic stir bar in a 5 mL glass vial (Figure 4A) and add 40.0 mg (160 mg/mmol based on 2-phenylethanol) MnOx catalyst (prepared in “Preparation of manganese oxides”) to the vial. Then add 2 mL (8.0 mL/mmol based on 2-phenylethanol) 1,4-dioxane to the vial.
Alternatives: 1,4-Dioxane used here can be replaced by t-amyl alcohol with slightly decreasing yield.
-
2.
Carefully measure out 29.9 μL (30.5 mg, 0.25 mmol, 1.020 g/mL, 1 eq.) of 2-phenylethanol with pipette (DragonLab TopPette, 5–50 μL) and add it into above-mentioned vial.
-
3.
Seal the prepared vial using a Teflon cap with hole and place it in a 160 mL stainless-steel autoclave with 1 cm deep metal sand bath (Figures 4B, 4C and 4F). Then seal the autoclave.
-
4.
Flush the autoclave with 0.5 MPa O2 3 times and then charge it with 0.5 MPa NH3 and 0.5 MPa O2 in sequence.
CRITICAL: Because the inevitable dissolving of NH3 in the solution can makes the pressure dropped before the reaction performed, to ensure a stable and precise initial pressure, charge the autoclave with 0.7 MPa NH3, keep 20 s and release to 0.5 MPa (the same below).
-
5.
Place the autoclave into 150°C oil bath and stir the reaction for 12 h. Set the speed of stirring to 600 rpm (Figures 4D and 4E). Troubleshooting 2.
CRITICAL: To make sure the autoclave heated evenly, a stainless-steel support and a magnetic stir bar are placed in the oil bath. The temperature of the oil bath should be verified by a temperature sensor to obtain the required reaction temperature (Figure 4D, the same below).
Note: While the reaction system is heated, a temperature gradient between the oil bath and the reaction vial inside the autoclave occurs. The monitoring of the temperature inside the autoclave shows that it takes ∼30 min to preheat the autoclave to the desired reaction temperature after placing the autoclave into 150°C oil bath. Please note the maximum pressure of the autoclave can reach ∼1.5 MPa while heating up the reaction system. Troubleshooting 3.
-
6.
After completion of the reaction, cool down the autoclave to 20°C–25°C. Discharge the remaining gas and remove the sample from the autoclave.
-
7.Quantify the sample by GC.
-
a.Carefully weigh 20.0 mg naphthalene (80 mg/mmol based on 2-phenylethanol) on analytical balance and add it to above-mentioned sample as an internal standard.
-
b.Dilute the mixture with 2 mL (8.0 mL/mmol based on 2-phenylethanol) methanol and stir it for 3 min to dissolve the naphthalene completely. Set the speed of stirring to 600 rpm.
-
c.Completely transfer the solution to a 5 mL centrifuge tube and centrifuge the solution for 3 min. Set the relative centrifugal force (RCF) of centrifugation to 10142 × g (H1650 centrifuge with 5 mL∗12 rotor from Hunan Xiangyi Laboratory Instrument Development Co., Ltd.).
-
d.Take 0.2 μL supernatant and use GC with the calibrated method of the product using the method described in “equipment setup”.
-
a.
-
8.Purify the crude product (Figure 5).
-
a.After quantifying the sample by GC, collect the supernatant obtained in step 7(c).
-
b.Add 3 mL (12.0 mL/mmol based on 2-phenylethanol) methanol to the above-mentioned centrifuge tube, sonicate the mixture for 5 min and centrifuge it at 10142 × g for 3 min. Then collect the supernatant.
-
c.Repeat step 8(b) 3 times.
-
d.Combine all the supernatant obtained and concentrate it under reduced pressure using a rotary evaporator (35°C, ∼15 mmHg, ∼10 min).
-
e.Completely transfer the crude product to an upper column containing 2 g (8.0 g/mmol based on 2-phenylethanol) silica using 1.5 mL (0.5 mL∗3, 6.0 mL/mmol, based on 2-phenylethanol) dichloromethane.
 CRITICAL: Strictly control the amount of dichloromethane. Excessive use of dichloromethane will affect the separation result (the same below).
CRITICAL: Strictly control the amount of dichloromethane. Excessive use of dichloromethane will affect the separation result (the same below). -
f.Purify the crude product by flash column chromatography (CombiFlash NextGen 300+ flash column chromatography system, 4 g silica pre-packed flash column from Agela Technologies) using a mixture of hexane and ethyl acetate as eluent (hexane/ethyl acetate = 80/20 progressively brought to hexane/ethyl acetate = 50/50, v/v, 13 mL/min, ∼30 min).Optional: The above-mentioned flash column chromatography system is only based on our experience. In principle, the flash column chromatography systems can be obtained from any reliable commercial sources and do not need to be limited to those listed in our method (the same below).
-
g.Collect the combined fractions containing pure product and concentrate under reduced pressure using a rotary evaporator (35°C, ∼15 mmHg) to yield the desired product.
-
a.
-
9.
Analyze the structure of the product by GC-MS and NMR spectral analysis.
Pause point: Control experiments on the loading of MnOx, reaction temperature and thickness of autoclave have been performed and reveal that these parameters have effects on the yields (Table 3).
Scheme 1.
Synthesis of benzamide from 2-phenylethanol under MnOx catalysis (He et al., 2022)
Figure 4.
Equipment and equipment setup for cleavage and amidation (or cyanation) of alcohols
(A) 5 mL glass vials, magnetic stir bars and Teflon caps with hole.
(B) Autoclave with pressure gauge.
(C) Prepared vials in metal sand bath.
(D) Oil bath with a stainless-steel support and a magnetic stir bar in it.
(E) The autoclave is placed in an oil bath.
(F) The size of the autoclave we used.
Figure 5.
CombiFlash NextGen 300+ flash column chromatography system
Yellow box: the size of pre-packed flash column and flow rate control. Green box: gradient control. Red box: detector control. Blue box: eluent control.
Table 3.
Control experiments of synthesis of benzamide from 2-phenylethanol
| Entry | Variation from standard conditions | Conversion of 1 (%) | Yields of 2 (%) |
|---|---|---|---|
| 1 | none | 100 | 93 |
| 2 | loading of MnOx: 30 mg | 100 | 90 |
| 3 | loading of MnOx: 50 mg | 100 | 85 |
| 4 | thickness of autoclave: 15 mm | 100 | 90 |
| 5 | thickness of autoclave: 10 mm | 100 | 90 |
| 6 | reaction temperature: 130°C | 88 | 51 |
Synthesis of benzamide from 2-phenylethanol in gram scale
Timing: 26 h
In this procedure, the direct synthesis of benzamide in gram-scale via heterogeneous MnOx-catalyzed successive cleavage and amidation of C-C bonds in 2-phenylethanol has been described.
-
10.
Place a 25 mm long fusiform magnetic stir bar in a 120 mL Teflon vessel-fitted stainless-steel autoclave (Figure 6A) and add 1.00 g (100 mg/mmol based on 2-phenylethanol) MnOx catalyst (prepared in “Preparation of manganese oxides”) to the autoclave. Then add 50 mL (5 mL/mmol based on 2-phenylethanol) t-amyl alcohol to the vial. Troubleshooting 4.
CRITICAL:t-Amyl alcohol used here is the critical solvent for achieving good reaction selectivity and yield.
-
11.
Carefully measure out 1.20 mL (1.22 g, 10 mmol, 1.020 g/mL, 1 eq.) of 2-phenylethanol with pipette (DragonLab TopPette, 1,000–5,000 μL) and add it into above-mentioned autoclave (Figure 6B). Then seal the autoclave.
-
12.
Flush the autoclave with 1.0 MPa O2 3 times and then charge it with 0.5 MPa NH3 and 1.0 MPa O2 in sequence.
-
13.
Place the autoclave into 160°C oil bath and stir the reaction for 24 h. Set the speed of stirring to 600 rpm.
Note: Please note the maximum pressure of the autoclave can reach ∼2.0 MPa while heating up the reaction system.
-
14.
After completion of the reaction, cool down the autoclave to 20°C–25°C. Discharge the remaining gas and remove the sample from the autoclave.
-
15.Quantify the sample by GC.
-
a.Carefully weigh 800 mg (80 mg/mmol based on 2-phenylethanol) naphthalene on analytical balance and add it to above-mentioned sample as an internal standard.
-
b.Dilute the mixture with 50 mL (5 mL/mmol based on 2-phenylethanol) methanol and stir it for 5 min to dissolve the naphthalene completely. Set the speed of stirring to 600 rpm.
-
c.Take an aliquot (3 mL) of this solution to a 5 mL centrifuge tube and centrifuge the solution for 3 min. Set the RCF of centrifugation to 10142 × g.
-
d.Take 0.2 μL supernatant and use GC with the calibrated method of the product using the method described in “equipment setup”.
-
a.
-
16.Purify the crude product (Figures 6C and 6D).
-
a.After quantifying the sample by GC, collect the mixture obtained in steps 15(b) and 15(c).
-
b.Separate the catalyst from the mixture using a Buchner funnel with double filter paper and rinse it with methanol (25 mL∗3, 7.5 mL/mmol based on 2-phenylethanol).
-
c.Concentrate the filtrate under reduced pressure using a rotary evaporator (35°C, ∼15 mmHg, ∼30 min).
-
d.Add 4 g (400 mg/mmol based on 2-phenylethanol) silica and 20 mL (2 mL/mmol based on 2-phenylethanol) dichloromethane to the crude product obtained, gently swirl the flask, and evaporate the solvent under reduced pressure using a rotary evaporator (35°C, ∼15 mmHg, ∼10 min).
-
e.Purify the crude product by flash column chromatography (CombiFlash NextGen 300+ flash chromatography system, 20 g silica pre-packed flash column from Agela Technologies) using a mixture of hexane and ethyl acetate as eluent (hexane/ethyl acetate = 80/20 progressively brought to hexane/ethyl acetate = 50/50, v/v, 40 mL/min, ∼30 min).
-
f.Collect the combined fractions containing pure product and concentrate under reduced pressure using a rotary evaporator (35°C, ∼15 mmHg) to yield the desired product.
-
a.
Figure 6.
Photographs for the gram-scale synthesis
(A) Autoclave with Teflon-vessel.
(B) Prepared Teflon-vessel in autoclave.
(C) Benzamide obtained in gram-scale synthesis.
(D) Flash column chromatography system settings.
Catalyst recycling and reusing experiment
Timing: 24 h
In this procedure, the synthesis of benzamide from 2-phenylethanol (steps 1–7 of “step-by-step method details”) is employed as model reaction for catalyst recycling and reusing experiment.
-
17.Catalyst recycling experiment.
-
a.After the reaction finished and GC quantification, separate the catalyst by decanting the supernatant.
-
b.Add 3.5 mL methanol to the centrifuge tube, sonicate the mixture for 5 min and centrifuge it at 10142 × g for 3 min. Then separate the catalyst by decanting the supernatant.
-
c.Repeat step 17(b) twice.
-
d.Dry the catalyst in vacuo at 80°C for 12 h.
-
a.
Pause point: The dried catalyst can be stored for months in glass vials with a lid at 20°C–25°C. Reuse it directly without further reactivation or purification for the next run.
-
18.Catalyst reusing experiment.
-
a.Place a 10 mm long fusiform magnetic stir bar in a 5 mL glass vial and completely transfer above recovered catalyst to the vial. Then add 2 mL 1,4-dioxane to the vial and sonicate the mixture for 5 min.
-
b.Implement steps 2–7 of “step-by-step method details” to proceed with catalyst reusing experiment and quantify the sample by GC.
-
a.
Pause point: Based on our experience, the amount of MnOx has an effect on yields and the inevitable loss of catalyst material during catalyst recycling experiment will cover up the real efficiency of MnOx in catalyst reusing experiment. To investigate the reusability of MnOx properly and accurately, the loss of catalyst material is offset by the recovered MnOx from the parallel experiments. According to the results, MnOx can be reused at least 5 times without obvious loss of activity (Table 4).
Table 4.
Recycling and reusing experiments of MnOx
| Cycles | MnOx (mg) | Recovered MnOx (mg) | Loss of MnOx (mg) | Yields of 2 (%) |
|---|---|---|---|---|
| 0 | 40.0 | 38.7 | 1.3 | 89 |
| 1 | 40.0 | 37.7 | 2.3 | 89 |
| 2 | 40.2 | 38.1 | 2.1 | 89 |
| 3 | 40.1 | 40.0 | 0.1 | 85 |
| 4 | 40.1 | 38.6 | 1.5 | 87 |
| 5 | 40.1 | 39.4 | 0.7 | 87 |
Synthesis of benzamide from 1-phenylethanol
Timing: 14 h
In this procedure, the direct synthesis of benzamide 2 via heterogeneous MnOx-catalyzed successive cleavage and amidation of C-C bonds in 1-phenylethanol 3 has been described (Scheme 2).
Note: This procedure can be also applied to other (hetero)aryl secondary alcohols referring to He et al. (2022).
-
19.
Place a 10 mm long fusiform magnetic stir bar in a 5 mL glass vial and add 40.0 mg (160 mg/mmol based on 1-phenylethanol) MnOx catalyst (prepared in “Preparation of manganese oxides”) to the vial. Then add 2 mL (8.0 mL/mmol based on 1-phenylethanol) 1,4-dioxane to the vial.
Alternatives: 1,4-Dioxane used here can be replaced by t-amyl alcohol with slightly decreasing yield.
-
20.
Carefully measure out 30.1 μL (30.5 mg, 0.25 mmol, 1.012 g/mL, 1 eq.) of 1-phenylethanol with pipette (DragonLab TopPette, 5–50 μL) and add it into above-mentioned vial.
-
21.
Seal the prepared vial using a Teflon cap with hole and place it in a 160 mL stainless-steel autoclave with 1 cm deep metal sand bath. Then seal the autoclave.
-
22.
Flush the autoclave with 0.5 MPa O2 3 times and then charge it with 0.5 MPa NH3 and 0.5 MPa O2 in sequence.
-
23.
Place the autoclave into 150°C oil bath and stir the reaction for 12 h. Set the speed of stirring to 600 rpm.
Note: Please note the maximum pressure of the autoclave can reach ∼1.5 MPa while heating up the reaction system.
-
24.
After completion of the reaction, cool down the autoclave to 20°C–25°C. Discharge the remaining gas and remove the sample from the autoclave.
-
25.Quantify the sample by GC.
-
a.Carefully weigh 20.0 mg (80 mg/mmol based on 1-phenylethanol) naphthalene on analytical balance and add it to above-mentioned sample as an internal standard.
-
b.Dilute the mixture with 2 mL (8.0 mL/mmol based on 1-phenylethanol) methanol and stir it for 3 min to dissolve the naphthalene completely. Set the speed of stirring to 600 rpm.
-
c.Completely transfer the solution to a 5 mL centrifuge tube and centrifuge the solution for 3 min. Set the RCF of centrifugation to 10142 × g.
-
d.Take 0.2 μL supernatant and use GC with the calibrated method of the product using the method described in “equipment setup”.
-
a.
Scheme 2.
Synthesis of benzamide from 1-phenylethanol under MnOx catalysis (He et al., 2022)
Synthesis of benzamide from 1-phenyl-1,2-ethanediol
Timing: 14 h
In this procedure, the direct synthesis of benzamide 2 via heterogeneous MnOx-catalyzed successive cleavage and amidation of C-C bonds in 1-phenyl-1,2-ethanediol 4 has been described (Scheme 3).
Note: This procedure can be also applied to other (hetero)aryl 1,2-diols referring to He et al. (2022).
-
26.
Place a 10 mm long fusiform magnetic stir bar in a 5 mL glass vial and add 40.0 mg (160 mg/mmol based on 1-phenyl-1,2-ethanediol) MnOx catalyst (prepared in “Preparation of manganese oxides”) to the vial.
-
27.
Carefully weigh 34.5 mg (0.25 mmol, 1 eq.) 1-phenyl-1,2-ethanediol on analytical balance and add it into above-mentioned vial. Then add 2 mL (8.0 mL/mmol based on 1-phenyl-1,2-ethanediol) 1,4-dioxane to the vial.
Alternatives: 1,4-Dioxane used here can be replaced by t-amyl alcohol with slightly decreasing yield.
-
28.
Seal the prepared vial using a Teflon cap with hole and place it in a 160 mL stainless-steel autoclave with 1 cm deep metal sand bath. Then seal the autoclave.
-
29.
Flush the autoclave with 0.5 MPa O2 3 times and then charge it with 0.5 MPa NH3 and 0.5 MPa O2 in sequence.
-
30.
Place the autoclave into 150°C oil bath and stir the reaction for 12 h. Set the speed of stirring to 600 rpm.
Note: Please note the maximum pressure of the autoclave can reach ∼1.5 MPa while heating up the reaction system.
-
31.
After completion of the reaction, cool down the autoclave to 20°C–25°C. Discharge the remaining gas and remove the sample from the autoclave.
-
32.Quantify the sample by GC.
-
a.Carefully weigh 20.0 mg (80 mg/mmol based on 1-phenyl-1,2-ethanediol) naphthalene on analytical balance and add it to above-mentioned sample as an internal standard.
-
b.Dilute the mixture with 2 mL (8.0 mL/mmol based on 1-phenyl-1,2-ethanediol) methanol and stir it for 3 min to dissolve the naphthalene completely. Set the speed of stirring to 600 rpm.
-
c.Completely transfer the solution to a 5 mL centrifuge tube and centrifuge the solution for 3 min. Set the RCF of centrifugation to 10142 × g.
-
d.Take 0.2 μL supernatant and use GC with the calibrated method of the product using the method described in “equipment setup”.
-
a.
Scheme 3.
Synthesis of benzamide from 1-phenyl-1,2-ethanediol under MnOx catalysis (He et al., 2022)
Synthesis of benzamide from 1-(phenoxymethyl)benzenemethanol
Timing: 26 h
In this procedure, the direct synthesis of benzamide 2 via heterogeneous MnOx-catalyzed successive cleavage and amidation of C-C bonds in 1-(phenoxymethyl)benzenemethanol 5 has been described (Scheme 4).
Note: This procedure can be also applied to other β-O-4 and β-1 lignin model compounds referring to He et al. (2022).
-
33.
Place a 10 mm long fusiform magnetic stir bar in a 5 mL glass vial and add 40.0 mg (160 mg/mmol based on 1-(phenoxymethyl)benzenemethanol) MnOx catalyst (prepared in “Preparation of manganese oxides”) to the vial.
-
34.
Carefully weigh 53.6 mg (0.25 mmol, 1 eq.) 1-(phenoxymethyl)benzenemethanol on analytical balance and add it into above-mentioned vial. Then add 2 mL (8.0 mL/mmol based on 1-(phenoxymethyl)benzenemethanol) t-amyl alcohol to the vial.
CRITICAL:t-Amyl alcohol used here is the critical solvent for achieving good reaction selectivity and yield.
-
35.
Seal the prepared vial using a Teflon cap with hole and place it in a 160 mL stainless-steel autoclave with 1 cm deep metal sand bath. Then seal the autoclave.
-
36.
Flush the autoclave with 0.5 MPa O2 3 times and then charge it with 0.7 MPa NH3 and 0.8 MPa O2 in sequence. Troubleshooting 5.
CRITICAL: Because the inevitable dissolving of NH3 in the solution can makes the pressure dropped before the reaction performed, to ensure a stable and precise initial pressure, charge the autoclave with 0.8 MPa NH3, keep 20 s and release to 0.7 MPa.
-
37.
Place the autoclave into 160°C oil bath and stir the reaction for 24 h. Set the speed of stirring to 600 rpm.
Note: Please note the maximum pressure of the autoclave can reach ∼2.0 MPa while heating up the reaction system.
-
38.
After completion of the reaction, cool down the autoclave to 20°C–25°C. Discharge the remaining gas and remove the sample from the autoclave.
-
39.Quantify the sample by GC.
-
a.Carefully weigh 20.0 mg (80 mg/mmol based on 1-(phenoxymethyl)benzenemethanol) naphthalene on analytical balance and add it to above-mentioned sample as an internal standard.
-
b.Dilute the mixture with 2 mL (8.0 mL/mmol based on 1-(phenoxymethyl)benzenemethanol) methanol and stir it for 3 min to dissolve the naphthalene completely. Set the speed of stirring to 600 rpm.
-
c.Completely transfer the solution to a 5 mL centrifuge tube and centrifuge the solution for 3 min. Set the RCF of centrifugation to 10142 × g.
-
d.Take 0.2 μL supernatant and use GC with the calibrated method of the product using the method described in “equipment setup”.
-
a.
Scheme 4.
Synthesis of benzamide from 1-(phenoxymethyl)benzenemethanol under MnOx catalysis (He et al., 2022)
Synthesis of 2-methoxybenzonitrile from 1-(2-methoxyphenyl)ethanol
Timing: 10 h
In this procedure, the direct synthesis of 2-methoxybenzonitrile 7 via heterogeneous MnOx-catalyzed successive cleavage and cyanation of C-C bonds in 1-(2-methoxyphenyl)ethanol 6 has been described (Scheme 5).
Note: This procedure can be also applied to other meta-substituted aryl alcohols referring to He et al. (2022).
-
40.
Place a 10 mm long fusiform magnetic stir bar in a 5 mL glass vial and add 40.0 mg (160 mg/mmol based on 1-(2-methoxyphenyl)ethanol) MnOx catalyst (prepared in “Preparation of manganese oxides”) to the vial.
-
41.
Carefully weigh 38.0 mg (0.25 mmol, 1 eq.) 1-(2-methoxyphenyl)ethanol on analytical balance and add it into above-mentioned vial. Then add 2 mL (8.0 mL/mmol based on 1-(2-methoxyphenyl)ethanol) acetonitrile to the vial.
CRITICAL: Acetonitrile used here is the critical solvent for achieving good reaction selectivity and yield.
-
42.
Seal the prepared vial using a Teflon cap with hole and place it in a 160 mL stainless-steel autoclave with 1 cm deep metal sand bath. Then seal the autoclave.
-
43.
Flush the autoclave with 0.5 MPa O2 3 times and then charge it with 0.5 MPa NH3 and 0.5 MPa O2 in sequence.
-
44.
Place the autoclave into 110°C oil bath and stir the reaction for 8 h. Set the speed of stirring to 600 rpm.
Note: Please note the maximum pressure of the autoclave can reach ∼1.3 MPa while heating up the reaction system.
-
45.
After completion of the reaction, cool down the autoclave to 20°C–25°C. Discharge the remaining gas and remove the sample from the autoclave.
-
46.To quantify the sample by GC.
-
a.Carefully weigh 20.0 mg (80 mg/mmol based on 1-(2-methoxyphenyl)ethanol) naphthalene on analytical balance and add it to above-mentioned sample as an internal standard.
-
b.Dilute the mixture with 2 mL (8.0 mL/mmol based on 1-(2-methoxyphenyl)ethanol) methanol and stir it for 3 min to dissolve the naphthalene completely. Set the speed of stirring to 600 rpm.
-
c.Completely transfer the solution to a 5 mL centrifuge tube and centrifuge the solution for 3 min. Set the RCF of centrifugation to 10142 × g.
-
d.Take 0.2 μL supernatant and use GC with the calibrated method of the product using the method described in “equipment setup”.
-
a.
-
47.To purify the crude product (Figure 7).
-
a.After quantifying the sample by GC, collect the supernatant obtained in step 46(c).
-
b.Add 3 mL (12.0 mL/mmol based on 1-(2-methoxyphenyl)ethanol) methanol to the above-mentioned centrifuge tube, sonicate the mixture for 5 min and centrifuge it at 10142 × g for 3 min. Then collect the supernatant.
-
c.Repeat step 47(b) 3 times.
-
d.Combine all the supernatant obtained and concentrate it under reduced pressure using a rotary evaporator (30°C, ∼15 mmHg, ∼10 min).
 CRITICAL: Because of the volatility of 2-methoxybenzonitrile, the temperature for concentration must be kept below 30°C.
CRITICAL: Because of the volatility of 2-methoxybenzonitrile, the temperature for concentration must be kept below 30°C. -
e.Completely transfer the crude product to an upper column containing 2 g (8.0 g/mmol based on 1-(2-methoxyphenyl)ethanol) silica using 1.5 mL (0.5 mL∗3, 6.0 mL/mmol based on 1-(2-methoxyphenyl)ethanol) dichloromethane.
-
f.Purify the crude product by flash column chromatography (CombiFlash NextGen 300+ flash chromatography system, 4 g silica pre-packed flash column from Agela Technologies) using a mixture of hexane and ethyl acetate as eluent (pure hexane progressively brought to hexane/ethyl acetate = 95/5, v/v, 13 mL/min, ∼30 min).
-
g.Collect the combined fractions containing pure product and concentrate under reduced pressure using a rotary evaporator (30°C, ∼15 mmHg) to yield the desired product.
-
a.
-
48.
Analyze the structure of the product by GC-MS and NMR spectral analysis.
Scheme 5.
Synthesis of 2-methoxybenzonitrile from 1-(2-methoxyphenyl)ethanol under MnOx catalysis (He et al., 2022)
Figure 7.
Flash column chromatography system settings for the synthesis of 2-methoxybenzonitrile
Expected outcomes
For the synthesis of benzamide from 2-phenylethanol (small scale), the yield of benzamide determined by GC is 93% and the isolated yield is 84%. For the synthesis of benzamide from 2-phenylethanol in gram scale, the yield of benzamide determined by GC is 85% and the isolated yield is 80%. For the synthesis of benzamide from 1-phenylethanol, the yield of benzamide determined by GC is 96%. For the synthesis of benzamide from 1-phenyl-1,2-ethanediol, the yield of benzamide determined by GC is 88%. For the synthesis of benzamide from 1-(phenoxymethyl)benzenemethanol, the yield of benzamide determined by GC is 70%. For the synthesis of 2-methoxybenzonitrile from 1-(2-methoxyphenyl)ethanol, the yield of 2-methoxybenzonitrile determined by GC is 90% and the isolated yield is 86%.
The analytical data of benzamide (white solid): 1H NMR (400 MHz, DMSO) δ 7.97 (s, 1H), 7.88 (d, J = 7.8 Hz, 2H), 7.51 (t, J = 7.2 Hz, 1H), 7.44 (t, J = 7.5 Hz, 2H), 7.36 (s, 1H); 13C NMR (101 MHz, DMSO) δ 168.36, 134.73, 131.67, 128.66, 127.91; GC-MS (EI) m/z [M]+ calculated for C7H7NO 121, found 121.137.
The analytical data of 2-methoxybenzonitrile (colorless liquid): 1H NMR (400 MHz, DMSO) δ 7.72 (d, J = 7.6 Hz, 1H), 7.70–7.64 (m, 1H), 7.25 (d, J = 8.5 Hz, 1H), 7.09 (t, J = 7.6 Hz, 1H), 3.91 (s, 3H); 13C NMR (101 MHz, DMSO) δ 161.31, 135.65, 134.13, 121.51, 116.92, 112.71, 100.74, 56.69; GC-MS (EI) m/z [M]+ calculated for C8H7NO 133, found 133.147.
Limitations
Although non-aromatic alcohol can undergo cleavage and amidation, a diminished level of efficiency will be observed in this case. Tertiary alcohols are not suitable for this protocol. For aryl nitriles syntheses, good reaction selectivity can be achieved only when meta-substituted aryl alcohols are employed as substrate.
Troubleshooting
Problem 1
Step 7 (before you begin): Potential effect of particle size on catalytic efficiency.
Potential solution
The particle size of the catalyst can affect the catalytic efficiency. After checking the characterization of the catalyst with Figure 3, to ensure the expected catalytic efficiency, the catalyst should be grinded more than 10 min and sieved with 80 mesh sieve.
Problem 2
Step 5 (step-by-step method details): Potential effects of autoclave thickness on the yield.
Potential solution
The thickness of the stainless-steel autoclave can affect thermal conductivity resulting in low yield. Slightly adjust the temperature and time of the reaction according to the thickness of autoclave to make sure the substrate transformed completely.
Problem 3
Step 5 (step-by-step method details): The pressure does not increase after heating up.
Potential solution
There may be problems with autoclave tightness or pressure gauge. Every time after charging the gas, check the tightness of autoclave by placing it underwater completely for 1 min. If there are bubbles, reseal the autoclave or replace it with another one. If there is no bubble, please replace the pressure gauge.
Problem 4
Step 10 (step-by-step method details): Potential effects of magnetic stir bar on the yield.
Potential solution
The size of magnetic stir bar has impact on the heat and mass transfer of the reaction. Make sure the magnetic stir bar is one-half to two-thirds the diameter of the Teflon vessel.
Problem 5
Step 36 (step-by-step method details): The autoclave cannot be pressurized to 0.7 MPa by NH3 after tightness and pressure gauge checking.
Potential solution
The room temperature has impact on the maximum pressure of NH3 in autoclave. For a stable and precise initial pressure, make sure the room temperature is 20°C–25°C.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wen Dai (daiwen@dicp.ac.cn).
Materials availability
All reagents generated in this study are available from the lead contact without restriction.
Acknowledgments
Financial support from the Dalian Institute of Chemical Physics.
Author contributions
W.D. designed, planned, and supervised the entire study. F.X. and H.L. wrote the manuscript and performed experiments and analysis. All the authors read and accepted the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Fukai Xie, Email: xiefukai02t6@dicp.ac.cn.
Hongliang Liang, Email: lianghl@dicp.ac.cn.
Wen Dai, Email: daiwen@dicp.ac.cn.
Data and code availability
The published article includes all datasets/code generated or analyzed during this study.
References
- Chandra P., Ghosh T., Choudhary N., Mohammad A., Mobin S.M. Recent advancement in oxidation or acceptorless dehydrogenation of alcohols to valorised products using manganese based catalysts. Coord. Chem. Rev. 2020;411:213241. doi: 10.1016/j.ccr.2020.213241. [DOI] [Google Scholar]
- Chen B., Li J., Dai W., Wang L., Gao S. Direct imine formation by oxidative coupling of alcohols and amines using supported manganese oxides under an air atmosphere. Green Chem. 2014;16:3328–3334. [Google Scholar]
- Chen B., Shang S., Wang L., Zhang Y., Gao S. Mesoporous carbon derived from vitamin B12: a high-performance bifunctional catalyst for imine formation. Chem. Commun. 2016;52:481–484. doi: 10.1039/c5cc06179b. [DOI] [PubMed] [Google Scholar]
- de Figueiredo R.M., Suppo J.S., Campagne J.M. Nonclassical routes for amide bond formation. Chem. Rev. 2016;116:12029–12122. doi: 10.1021/acs.chemrev.6b00237. [DOI] [PubMed] [Google Scholar]
- Fernandes R.A., Kumar P. PCC-mediated novel oxidation reactions of homobenzylic and homoallylic alcohols. Tetrahedron Lett. 2003;44:1275–1278. doi: 10.1016/s0040-4039(02)02784-3. [DOI] [Google Scholar]
- Galkin M.V., Dahlstrand C., Samec J.S.M. Mild and robust redox-neutral Pd/C-catalyzed lignol β-O-4' bond cleavage through a low-energy-barrier pathway. ChemSusChem. 2015;8:2187–2192. doi: 10.1002/cssc.201500117. [DOI] [PubMed] [Google Scholar]
- Gunanathan C., Ben-David Y., Milstein D. Direct synthesis of amides from alcohols and amines with liberation of H2. Science. 2007;317:790–792. doi: 10.1126/science.1145295. [DOI] [PubMed] [Google Scholar]
- He P., Chen B., Huang L., Liu X., Qin J., Zhang Z., Dai W. Heterogeneous manganese-oxide-catalyzed successive cleavage and functionalization of alcohols to access amides and nitriles. Chem. 2022 doi: 10.1016/j.chempr.2022.02.021. [DOI] [Google Scholar]
- Li C., Zhao X., Wang A., Huber G.W., Zhang T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015;115:11559–11624. doi: 10.1021/acs.chemrev.5b00155. [DOI] [PubMed] [Google Scholar]
- Liu J., Qiu X., Huang X., Luo X., Zhang C., Wei J., Pan J., Liang Y., Zhu Y., Qin Q., et al. From alkylarenes to anilines via site-directed carbon-carbon amination. Nat. Chem. 2019;11:71–77. doi: 10.1038/s41557-018-0156-y. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhang Z., Shen X., Liu H., Zhang P., Chen B., Han B. Stepwise degradation of hydroxyl compounds to aldehydes via successive C–C bond cleavage. Chem. Commun. 2019;55:925–928. doi: 10.1039/c8cc09504c. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhang Z., Song J., Liu S., Liu H., Han B. Nitrogen dioxide catalyzed aerobic oxidative cleavage of C(OH)–C bonds of secondary alcohols to produce acids. Angew. Chem. Int. Ed. 2019;58:17393–17398. doi: 10.1002/anie.201908788. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhang Z., Yan J., Liu S., Liu H., Liu Z., Wang W., He Z., Han B. Aerobic oxidative cleavage and esterification of C(OH)–C bonds. Chem. 2020;6:3288–3296. doi: 10.1016/j.chempr.2020.09.006. [DOI] [Google Scholar]
- Luo H., Wang L., Shang S., Li G., Lv Y., Gao S., Dai W. Cobalt nanoparticles catalyzed widely applicable successive C-C bond cleavage in alcohols to access esters. Angew. Chem. Int. Ed. 2020;59:19268–19274. doi: 10.1002/anie.202008261. [DOI] [PubMed] [Google Scholar]
- Luo H., Wang L., Shang S., Niu J., Gao S. Aerobic oxidative cleavage of 1, 2-diols catalyzed by atomic-scale cobalt-based heterogeneous catalyst. Commun. Chem. 2019;2:17. doi: 10.1038/s42004-019-0116-5. [DOI] [Google Scholar]
- Piszel P.E., Vasilopoulos A., Stahl S.S. Oxidative amide coupling from functionally diverse alcohols and amines using aerobic copper/nitroxyl catalysis. Angew. Chem. 2019;131:12339–12343. doi: 10.1002/ange.201906130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi A., Azarpira A., Kim H., Ralph J., Stahl S.S. Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 2013;135:6415–6418. doi: 10.1021/ja401793n. [DOI] [PubMed] [Google Scholar]
- Shang S., Chen P.-P., Wang L., Lv Y., Li W.-X., Gao S. Metal-free nitrogen and boron-codoped mesoporous carbons for primary amides synthesis from primary alcohols via direct oxidative dehydrogenation. ACS Catal. 2018;8:9936–9944. doi: 10.1021/acscatal.8b02889. [DOI] [Google Scholar]
- Shang S., Wang L., Dai W., Chen B., Lv Y., Gao S. High catalytic activity of mesoporous Co–N/C catalysts for aerobic oxidative synthesis of nitriles. Catal. Sci. Technol. 2016;6:5746–5753. doi: 10.1039/c6cy00195e. [DOI] [Google Scholar]
- Shi S.H., Liang Y., Jiao N. Electrochemical oxidation induced selective C–C bond cleavage. Chem. Rev. 2021;121:485–505. doi: 10.1021/acs.chemrev.0c00335. [DOI] [PubMed] [Google Scholar]
- Soulé J.F., Miyamura H., Kobayashi S. Powerful amide synthesis from alcohols and amines under aerobic conditions catalyzed by gold or gold/iron, -nickel or -cobalt nanoparticles. J. Am. Chem. Soc. 2011;133:18550–18553. doi: 10.1021/ja2080086. [DOI] [PubMed] [Google Scholar]
- Valeur E., Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009;38:606–631. doi: 10.1039/b701677h. [DOI] [PubMed] [Google Scholar]
- Wang M., Lu J., Li L., Li H., Liu H., Wang F. Oxidative C(OH)C bond cleavage of secondary alcohols to acids over a copper catalyst with molecular oxygen as the oxidant. J. Catal. 2017;348:160–167. doi: 10.1016/j.jcat.2017.02.017. [DOI] [Google Scholar]
- Xu S., Itto K., Satoh M., Arimoto H. Unexpected dehomologation of primary alcohols to one-carbon shorter carboxylic acids using o-iodoxybenzoic acid (IBX) Chem. Commun. 2014;50:2758–2761. doi: 10.1039/c3cc49160a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Kobayashi H., Wang Y., Oishi T., Ogasawara Y., Mizuno N. Green oxidative synthesis of primary amides from primary alcohols or aldehydes catalyzed by a cryptomelane-type manganese oxide based octahedral molecular sieve, OMS-2. Catal. Sci. Technol. 2013;3:318–327. doi: 10.1039/c2cy20178j. [DOI] [Google Scholar]
- Yamaguchi K., Kobayashi H., Oishi T., Mizuno N. Heterogeneously catalyzed synthesis of primary amides directly from primary alcohols and aqueous ammonia. Angew. Chem. Int. Ed. 2012;51:544–547. doi: 10.1002/anie.201107110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The published article includes all datasets/code generated or analyzed during this study.