Abstract
Context
Torin2 has various pharmacological properties. However, its anti-inflammatory activity has not been reported.
Objective
This study focused on the potential anti-inflammatory properties of Torin2 in lipopolysaccharide (LPS)-evoked RAW264.7 murine macrophages. The study aimed to shed light on the molecular mechanisms that ameliorate these effects.
Methods
Torin2 was applied to 100 ng/mL lipopolysaccharide-induced RAW 264.7 macrophages in vitro. Nitric oxide (NO) levels were detected using the Griess reagent kit. Prostaglandin E2 (PGE2), pro-inflammatory cytokines interleukin (IL)-1β, interleukin (IL)-6, and tumor necrosis factor in the supernatant fraction were determined using enzyme-linked immunosorbent assay (ELISA). Gene expression of pro-inflammatory cytokines, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) were tested using real-time quantitative polymerase chain reaction (qPCR). Cyclooxygenase-2 and inducible nitric oxide synthase proteins, phosphorylation of mitogen-activated protein kinase (MAPK) subgroups, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38, I-kappa-B-alpha (IκBα), and nuclear factor-kappa-B (NF-κB), and activation in extracts were detected via western blotting. Nuclear factor-kappa-B/p65 nuclear translocation was tested using an immunofluorescence assay.
Results
The results demonstrated that pre-treatment with Torin2 profoundly attenuated the lipopolysaccharide-stimulated levels of nitric oxide and prostaglandin E2, pro-inflammatory cytokines, messenger ribonucleic acid (mRNA), and protein expression of cyclooxygenase-2 and inducible nitric oxide synthase. Collectively, Torin2 pre-treatment notably weakened lipopolysaccharide-induced damage by reducing the phosphorylation of nuclear factor-kappa-B, p38, c-Jun N-terminal kinase, extracellular signal-regulated kinase proteins, and nuclear factor-kappa-B/p65 nuclear translocation.
Conclusion
Numerous pieces of evidence indicated that Torin2 reversed inflammatory activation by regulating nuclear factor-kappa-B and mitogen-activated protein kinase signaling pathways and provided a tentative potential candidate for preventing and treating inflammatory diseases.
Keywords: Torin2, RAW264.7 murine macrophages, Lipopolysaccharide, Anti-inflammation, Mechanisms
Torin2; RAW264.7 murine macrophages; Lipopolysaccharide; Anti-inflammation; Mechanisms.
1. Introduction
Inflammation is not only the most primitive self-protective response in innate and adaptive immune systems to various stimuli but it is also a common pathophysiological occurrence in a variety of diseases, such as cancer, diabetes, Alzheimer's disease, and coronavirus disease 2019 (COVID-19) (Beinke et al., 2020; Zhang et al., 2020). Following pathogen invasion, chemical irritation, tissue damage, or exposure to endotoxins, such as lipopolysaccharide (LPS), chronic inflammation enhances the complex interaction of the immune system to scavenge lesions (Xu et al., 2020). LPS, as a fundamental part of the outer membrane of gram-negative bacteria, has a striking function in negotiating inflammation, and it is widely used to develop inflammation-related models of macrophages in vitro (Kim et al., 2019; Chen et al., 2021). Lymphocytes, dendritic cells, granulocytes, and macrophages are prominent in innate and adaptive immune regulation (Ginhoux and Jung 2014). In addition, studies have reported that RAW264.7 murine macrophages were routinely considered as a suitable anti-inflammatory macrophage model in vitro (Chu et al., 2016; Tian et al., 2019; Lee et al., 2020). Hence, the LPS-induced inflammation activation model could estimate whether a substance possesses anti-inflammatory properties.
When RAW264.7 murine macrophages were activated by LPS, toll-like receptor-4 (TLR-4) was stimulated to recognize and bind to LPS, subsequently resulting in the activation of massive intracellular signaling pathways containing nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinases (MAPKs). This ultimately facilitates the production of substantial inflammatory signaling molecules (nitric oxide [NO], prostaglandin E2 [PGE2], tumor necrosis factor-α [TNF-α], interleukin-β [IL-β], interleukin-6 [IL-6], cyclooxygenase-2 [COX-2], and induced nitrogen monoxide synthase [iNOS]), triggering a cytokine storm (Byun et al., 2013). MAPKs are the principal signaling pathways in primary signaling molecules (p38, extracellular regulated protein kinases [ERK], and c-Jun N-terminal kinase [JNK]). They act on inflammation-related diseases, including Alzheimer's disease, rheumatoid arthritis, and autoimmune diseases (Park et al., 2018; Yu et al., 2020). Furthermore, Tak and Firestein et al. previously deciphered that NF-κB, as a transcription factor, possessed primary effects on triggering gene and protein expression in immune response and cell activity (Tak and Firestein 2001). Lee et al. and Komatsu et al. revealed that the activation of NF-κB prompted translocation to the nucleus, where it elevated the transcription of target genes (TNF-α, IL-β, IL-6, COX-2, iNOS) (Lee et al., 2014; Komatsu et al., 2017). Consequently, any substance that suppresses the activity of the NF-κB pathway and signaling cascades ameliorated by MAPKs could be considered a therapeutic agent against inflammatory mediators.
Zhang et al. reported that the activation of TLR4 can induce mitochondrial dysfunction, oxidative stress, apoptosis, DNA damage, and inflammatory responses by mediating the mammalian target of the rapamycin (mTOR) pathway in autoimmune, infectious, and other diseases (Zhang et al., 2022). TLR4 is essential for LPS recognition and subsequent immune responses against gram-negative bacteria. The formation of the LPS-TLR4 complex causes the recruitment of the intracellular adaptor protein MyD88 (myeloid differentiation primary response gene 88) and leads to the formation of the TAK1/TAB1/TAB2 complex. Activation of MAPKs (p38 kinase, JNK, and ERK) induces the phosphorylation of mTOR and activation of the canonical NF-κB pathway (Zhou et al., 2018). Therefore, mTOR inhibitors may be potential compounds with anti-inflammatory effects.
Torin2 is a promising and novel second-generation ATP-competitive mTOR inhibitor with dual criteria for suppressing mTORC1 and mTORC2 (Liu et al., 2013). Luo et al. reported that Torin2 notably weakened proliferation and migration in lung, epithelial ovarian, thyroid, and liver cancers by attenuating DNA damage responses (Luo et al., 2018). Song et al. verified that Torin2 promotes autophagy and partially restores lipid droplet accumulation induced by bisphenol A (Song et al., 2019). Collectively, Udayakumar et al. demonstrated that Torin2 intensified irradiation-induced cancer cell residual chromosomal aberrations at metaphase or even death (Udayakumar et al., 2016). Nevertheless, to date, there is no scientific evidence for the immune regulation of Torin2. Existing evidence prompted us to establish an LPS-stimulated RAW264.7 murine macrophages model to elucidate the anti-inflammatory effects of Torin2.
2. Material and methods
2.1. Drugs and chemicals
Torin2 (CAS No: 1223001-51-1, Lot No: S281704) was obtained from Selleck, and LPS (CAS No. L2630-10MG, Lot No: 099M4002V) was purchased from Sigma (St. Louis, MO, USA). Dulbecco's modified Eagle's minimum essential medium (DMEM) was purchased from Hyclone (Logan, UT, USA). Fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Gaithersburg, MD, USA). Cell Counting Kit-8 (CCK-8), NO product kit (Griess reagent), 4% paraformaldehyde, phosphate-buffered saline (PBS), cell lysis buffer for western blotting and immunoprecipitation (IP), and enhanced bicinchoninic acid protein assay (BCA Protein Assay) kit were obtained from Beyotime (Shanghai, China). Primers (forward and reverse; IL-1β, IL-6, TNF-α, iNOS, COX-2, and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were purchased from Sangon Biotech (Shanghai, China). TRIzolTM was purchased from Life Technologies (Carlsbad, California, USA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, IL-6, and TNF-α were purchased from Xin Bosheng, Shanghai, China. PrimeScriptTM reverse transcription (RT) reagent kit and SYBR Premix Ex TaqTM were purchased from Takara (Shiga, Japan). An ELISA kit for PGE2 was purchased from BioLegend (San Diego, CA, USA). The phosphatase inhibitor cocktail and antibodies (COX-2, p65, p-p65, I-kappa-B-alpha (IκBα), ERK, p-ERK, JNK, p-JNK, p38, p-p38, GAPDH, and AlexaFluor488) were obtained from Cell Signaling Technology (Danvers, MA, USA), and antibodies (iNOS, p-IκBα) were purchased from Abcam (Cambridge, London, UK). Triton X-100 was purchased from SolarBio (Beijing, China).
2.2. Cell lines and cell culture
RAW264.7 murine macrophages was provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in DMEM containing 10% FBS, penicillin (100 μg/mL), and streptomycin (100 μg/mL) in a humidified incubator with 5% CO2 at 37 °C. Cells were washed with PBS and passaged to a new plate for culturing when they reached 90%–95% confluence. In all experiments, the cells were cultured in triplicate and allowed to acclimate before treatment.
2.3. Determination of cell viability
To analyze the cytotoxicity of Torin2, cell viability was measured using a CCK-8 assay. The RAW264.7 murine macrophages were left to acclimate in 96-well plates, treated with different concentrations of Torin2 (10, 25, 50, and 100 nM) for another 24 h and 48 h. Next, 10 μL of CCK-8 was added to each well and then incubated for 1 h in an incubator. The absorbance of each well was measured at a wavelength of 450 nm using a microplate reader (Molecular Devices, San Jose, CA, USA). The ratio of the absorbance of each treatment group to that of the control group was calculated, and was used as the relative cell viability value.
2.4. NO production measurement
According to the results of cell viability, RAW264.7 murine macrophages were divided into four groups: control group, model group (100 ng/mL LPS), low Torin2 + LPS group (15 nM Torin2 + 100 ng/mL LPS), and high Torin2 + LPS group (25 nM Torin2 + 100 ng/mL LPS). The RAW264.7 murine macrophages were seeded into 96-well plates and acclimated for 6 h. The cells were treated with different concentrations (15 and 25 nM) of Torin2 for 3 h, and then incubated with LPS (100 ng/mL) for another 24 h. The NO level in the culture supernatant was evaluated according to the manufacturer's instructions using the Griess reagent kit. The concentration of NO was calculated using a sodium nitrite standard curve.
2.5. ELISA determination of PGE2
According to the manufacturer's instructions, the level of secreted PGE2 in the culture supernatant was determined using an ELISA kit. The absorbance was measured at 450 nm, and the level of PGE2 was calculated according to the standard curve.
2.6. Measurement of IL-6, TNF-α, and IL-1β secretion
RAW264.7 murine macrophages were seeded in 12-well plates. Following overnight incubation, they were incubated with Torin2 (15 nM and 25 nM) for 6 h and stimulated by LPS (100 ng/mL) for another 12 h. ELISA determined the concentration of secreted IL-6, IL-1β, and TNF-α in the supernatant according to the manufacturer's protocol and calculated using the standard curve.
2.7. Ribonucleic acid (RNA) extraction and quantificational real-time PCR (qRT-PCR)
To assess IL-1β, IL-6, and TNF-α expression, total RNA was isolated from RAW264.7 murine macrophages using TRIzol reagent according to the manufacturer's instructions. RT reactions were performed using 5×reverse transcriptase to synthesize complementary deoxyribonucleic acid (cDNA) from RNA, and qRT-PCR was performed on a CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA) with SYBR Green, following the manufacturer's protocol.
The mRNA expression profiles were normalized to that of GAPDH, and the fold increase of each gene was calculated using the 2−ΔΔCT method. The primers used were: GAPDH forward 5′-ACCCAGAAGACTGTGGATGG-3′ and reverse 5′-CACATTGGGGGTAGGAACAC-3′; iNOS: forward 5′- CTCACTGGGACAGCACAGAA-3′, reverse 5′-GCTTGTCTCTGGGTCCTCTG-3′; COX-2: forward 5′-ACTTGCGTTGATGGTGGCTGTCTT-3′, reverse 5′-CTGTATCCCGCCCTGCTGGTG-3′; IL-1β: forward 5′-CAGGCAGGCAGTATCACTCA-3′, reverse 5′-AGCTCATATGGGTCCGACAG-3′; IL-6: forward 5′-AGTTGCCTTCTTGGGACTGA-3′, reverse 5′-CAGAATTGCCATTGCACAAC-3′; TNF-α: forward 5′-TCTCATCAGTTCTATGGCCC-3′, reverse 5′-GGGAGTAGACAAGGTACAAC-3′.
2.8. Western blot analysis
After specific treatments, RAW264.7 cells were lysed in cell lysis buffer containing 1% protease and phosphatase inhibitors for western blotting and IP. The cell lysates were centrifuged at 12,000 × g at 4 °C for 10 min to collect the total protein. The protein concentration was detected using an enhanced BCA Protein Assay Kit according to the manufacturer's recommendations. Equal amounts of protein in each lane were electrophoresed using 10% sodium dodecyl-sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane by electroblotting. The membrane was blocked with a blocking solution (5% skim milk) and incubated with primary antibodies against COX-2, iNOS, p65, p-p65, ERK, p-ERK, JNK, p-JNK, p38, and p-p38 at 4 °C. Following overnight incubation, the membranes were incubated with the appropriate secondary antibodies for 2 h. Protein bands were visualized using ECL western blotting substrate (Pierce; Thermo Scientific) and quantified using ImageJ software.
2.9. NF-κB/p65 nuclear translocation immunofluorescence assay
RAW264.7 murine macrophages were cultured in a glass chamber, pretreated with Torin2 for 6 h, and then stimulated with 100 ng/mL LPS for 30 min. The cells were removed by washing with PBS three times and fixed in freshly prepared 4% paraformaldehyde for 15 min. The fixed cells were washed three times with PBS and permeabilized in 0.5% Triton X-100 for 15 min. Permeabilized cells were washed thrice with PBS. After incubation with the p65 antibody and secondary AlexaFluor488 antibody, DAPI was used to stain the nuclei at 37 °C for 30 min in the dark. Images were obtained using a luminescence microscope (Nikon, Tokyo, Japan).
2.10. Statistical analysis
All values are presented as the mean ± S.E.M (standard error of the mean) deviation of three independent experiments. Statistical analyses were performed using GraphPad Prism software version 7.00 software and a one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of Torin2 on RAW264.7 murine macrophages viability
After administration of different concentrations of Torin2 for 24 h, cell viability at concentrations of 10, 25, and 50 nM exhibited no difference compared with that of the control group. Conversely, Torin2 (100 nM) dramatically attenuated cell activity in contrast to that of the control group (Figure 1B). After 48 h of culture, Torin2 (50 nM and 100 nM) markedly decreased cell activity compared to that of the control group (Figure 1C). Hence, the results demonstrated that Torin2 at concentrations of 10–25 nM was not cytotoxic to RAW264.7 murine macrophages, and subsequent studies were performed using 15 nM and 25 nM Torin2.
Figure 1.
Structural components and effect of Torin2 on viability in RAW264.7 murine macrophages. (A) The structure of Torin2. (B and C) Results of the CCK-8 assay in RAW264.7 murine macrophages of Torin2 for 24 h and 48 h respectively (n = 3). The values are expressed as means ± S.E.M, ∗∗p < 0.01 vs. the control. CCK-8, Cell Counting Kit-8.
3.2. Torin2 inhibited LPS-induced NO and PGE2 production in RAW264.7 murine macrophages
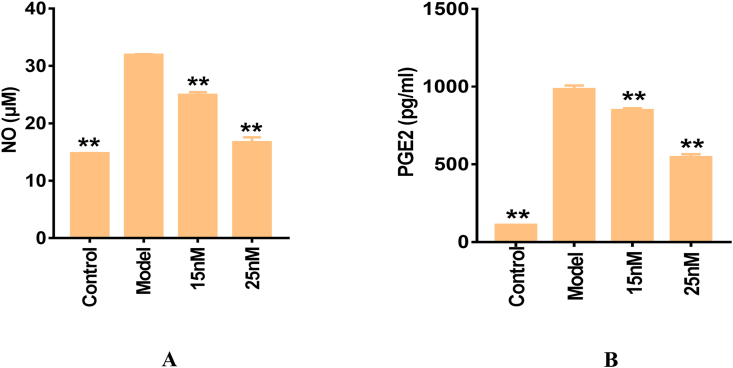
As shown in Figure 2A-B, the concentrations of NO and PGE2 produced by RAW264.7 murine macrophages were notably upregulated via LPS stimulation. The levels of NO and PGE2 were markedly elevated by approximately 2- and 10-fold, respectively, in contrast to those in the control group. Nevertheless, pre-treatment with Torin2 dramatically inhibited LPS-induced NO and PGE2 production in a dose-dependent manner. The above evidence indicates that Torin2 attenuates LPS-induced inflammation by reducing the production of NO and PGE2.
Figure 2.
Effect of Torin2 on NO and PGE2 productions in LPS-induced (100 ng/mL) RAW 264.7 murine macrophages respectively. The level of NO (A) and PGE2 (B) in culture supernatant, as measured by Griess reagent and ELISA respectively (n = 3). The values are expressed as means ± S.E.M, ∗∗p < 0.01 vs. the model. NO, nitric oxide; PGE2, prostaglandin E2; LPS, lipopolysaccharide; ELISA, enzyme-linked immunosorbent assay.
3.3. Torin2 decreased LPS-induced pro-inflammation cytokines level and mRNA expression levels in RAW264.7 murine macrophages
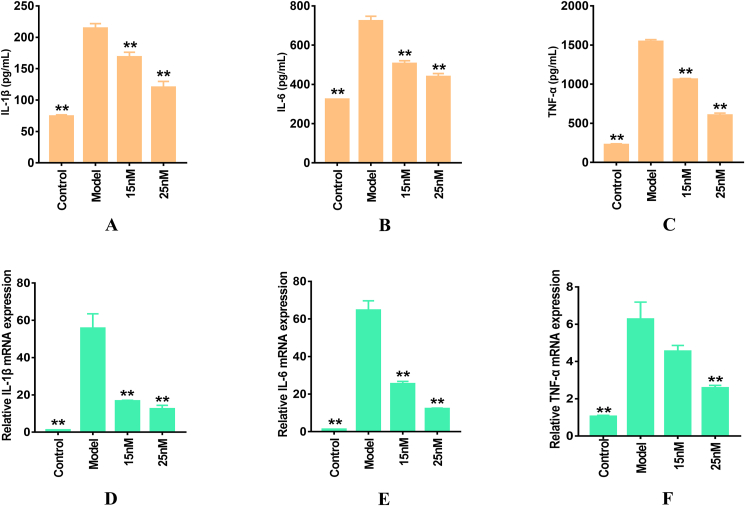
Figure 3A-C shows that the secretory levels of TNF-α, IL-β, and IL-6 were markedly elevated in LPS-induced RAW264.7 murine macrophages compared with those in the control group. Following Torin2 pre-treatment, the pro-inflammatory cytokine levels were dramatically diminished in a dose-dependent manner. Simultaneously, TNF-α, IL-β, and IL-6 mRNA expression levels were notably elevated by LPS stimulation and markedly weakened by Torin2 pre-administration in LPS-induced RAW264.7 murine macrophages (Figure 3D-F). These data showed that Torin2 reduced the production and transcription of TNF-α, IL-β, and IL-6 cytokines in LPS-induced RAW264.7 murine macrophages.
Figure 3.
Effect of Torin2 on the productions and mRNA expressions of IL-1β, IL-6, TNF-α in LPS-induced (100 ng/mL) RAW264.7 murine macrophages respectively. (A–C) The level IL-1β, IL-6, TNF-α in culture supernatant, as detected by ELISA respectively (n = 3). (D–F) mRNA relative expression of IL-1β, IL-6, TNF-α, as measured by qRT-PCR respectively (n = 3). The values are expressed as means ± S.E.M, ∗∗p < 0.01 vs. the model. IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; qRT-PCR, quantificational Real-Time PCR.
3.4. Torin2 suppressed LPS-induced COX-2 and iNOS mRNA and proteins expression levels in RAW264.7 murine macrophages
Figure 4A-B reveals that iNOS and COX-2 mRNA expression levels were profoundly upregulated by LPS stimulation compared with those of the control group. Conversely, Torin2 dramatically reduced iNOS and COX-2 mRNA expression in LPS-stimulated RAW264.7 murine macrophages. Additionally, the result of western blotting demonstrated that Torin2 dose-dependently markedly weakened the COX-2 and iNOS proteins expression levels in LPS-stimulated RAW264.7 cells (Figure 4C-D). Therefore, the above results validated that Torin2 possesses anti-inflammatory activity via the downregulation of COX, iNOS mRNA levels, and protein expression.
Figure 4.
Effect of Torin2 on the mRNA and protein expressions of COX-2 and iNOS respectively. (A–B) mRNA relative expression level of COX-2 and iNOS, as measured by qRT-PCR respectively (n = 3). (C–D) Protein level of COX-2 and iNOS, as detected as Western blot respectively (n = 3). The values are expressed as means ± S.E.M, ∗p < 0.05, ∗∗p < 0.01 vs. the model. COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; qRT-PCR, quantitative real-time PCR.
3.5. Torin2 down-regulated LPS-induced NF-κB signal pathway and nuclear translocation in RAW264.7 murine macrophages
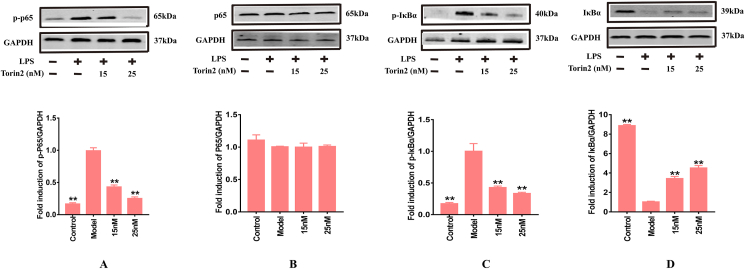
As previous research indicated, in the un-stimulated cells, NF-κB dimers are bound to IκBα and IκBβ as an inactive complex in the cytoplasm (Tak and Firestein, 2001). LPS stimulation induced the degradation of IκBα and activation of NF-κB and p- IκBα in RAW264.7 (Tian et al., 2019). Since IκBα ubiquitination and degradation are the key steps in NF-κB activation, the effect of Torin2 on IκBα degradation was investigated. As shown in Figure 5A-D, the levels of p-p65/GAPDH and p-IκBα/GAPDH phosphorylation proteins were markedly upregulated by LPS stimulation. Conversely, IκBα/GAPDH protein levels were down-regulated in LPS-induced cells. We further investigated whether Torin2 could dampen NF-κB p65 nuclear translocation in LPS-stimulated RAW264.7 murine macrophages. As shown in Figure 6, the nuclear translocation of p65 was increased by LPS stimulation. However, following pre-treatment with Torin2, the p65 nuclear translocation was profoundly decreased compared with that of the model group. Overall, this experimental work documented that Torin2 inhibited LPS-stimulated p65 nuclear translocation and the NF-κB signaling pathway.
Figure 5.
Torin2 inhibited related proteins expressions of NF-κB signaling pathways in LPS-induced (100 ng/mL) RAW 264.7 murine macrophages respectively. (A–D) Protein level of p-p65/GAPDH, p65/GAPDH, p- IκBα/GAPDH and IκBα/GAPDH in RAW264.7 murine macrophages, as detected by Western blot respectively (n = 3). The values are expressed as means ± S.E.M, ∗∗p < 0.01 vs. the model. NF-κB, nuclear factor kappa-B; IκBα, inhibitor kappa B alpha.
Figure 6.
Effect of Torin2 on the LPS-stimulated (100 ng/mL) p65 unclear translocation. The level of p65 unclear translocation in RAW264.7 murine macrophages, as measured by immunofluorescence staining (n = 3).
3.6. Torin2 down-regulated LPS-induced MAPKs pathways in RAW264.7 murine macrophages
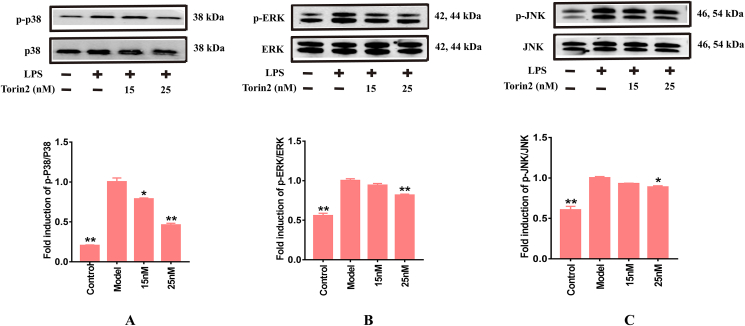
The results shown in Figure 7A-C validate that the ratio of p-ERK/ERK, p-JNK/JNK, and p-p38/p38 in LPS-induced RAW264.7 murine macrophages were notably elevated and could be reversed by pre-administration of Torin2 in a concentration-dependent manner. These results imply that Torin2 might exert anti-inflammatory effect by retarding the stimulation of NF-κB and MAPKs signaling pathways induced by LPS treatment.
Figure 7.
Torin2 inhibited related proteins expression of MAPKs signaling pathway in LPS-induced (100 ng/mL) RAW264.7 murine macrophages respectively. (A–C) Protein level of p-p38/p38, p-ERK/ERK, p-JNK/JNK in RAW264.7 murine macrophages, as detected by Western blot respectively (n = 3). The values are expressed as means ± S.E.M, ∗p < 0.05, ∗∗p < 0.01 vs. the model. The values are expressed as means ± S.E.M, ∗p < 0.05, ∗∗p < 0.01 vs. the model. MAPKs, mitogen-activated protein kinases; ERK, extracellular regulated protein kinases; JNK, c-Jun N-terminal kinase.
4. Discussion
Torin2, a new dual criteria mTOR inhibitor, suppresses the dual criteria of mTORC1 and mTORC2 (Liu et al., 2013). Previous studies have mainly focused on the pharmacological activities of Torin2 in enhancing autophagy, weakening DNA damage, and inhibiting cancer cell proliferation and migration (Hussain et al., 2015; Sadowski et al., 2015; Wang et al., 2015). However, whether Torin2 possesses an anti-inflammatory function remains unclear. Hou et al. and Wu et al. reported that structures with double bonds and hydroxyl groups might inhibit NO, TNF-α, and IL-6 in LPS-induced RAW264.7 murine macrophages (Olatunji et al., 2018). The existing evidence prompted us to investigate whether Torin2 could attenuate LPS-induced inflammation.
NO, produced from l-arginine by three NOS enzymes (endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS), and iNOS), is involved in many biological processes, including neurotransmission, wound healing, apoptosis, and inflammation (Eiserich et al., 1998). Lee et al. previously reported that low levels of NO are produced by eNOS and nNOS, whereas iNOS enzymes produce extensive amounts of NO in organisms (bacteria, fungi, and mammals) (Lee et al., 2018). PGE2, a pivotal lipid, is produced by the reaction catalyzed by COX-2, and it may contribute to the stimulation of inflammation (Jiang et al., 2017). Hence, we estimated the levels of NO and PGE2, and the mRNA and protein expression levels of COX-2 and iNOS to investigate the anti-inflammatory effect of Torin2. The existing data revealed that nanomolar concentrations of Torin2 pre-treatment dramatically diminished the production of NO and PGE2. It downregulated COX-2 and iNOS mRNA levels, and iNOS protein expression, which sheds light on the dramatic curative effect of Torin2.
Another important moderator, a pro-inflammatory cytokine secreted by stimulated macrophages, plays a crucial role in initiating and propagating inflammatory pathogenesis in LPS-evoked inflammation (Lee et al., 2016). Accumulating studies have demonstrated that over-production of TNF-α, IL-β, and IL-6 cytokines further affects other inflammatory mediators, stimulating systemic inflammatory responses and even organ damage (Kwon et al., 2008; Lee et al., 2018). Yang et al. reported that TNF-α, as a signal protein, regulates the immune response and amplifies the inflammatory response (Yang et al., 2013). Kwon et al. demonstrated that IL-6, secreted by macrophages and other cell types, is notably elevated in response to acute inflammatory responses (Kwon et al., 2008). Additionally, Samad et al. revealed that IL-β, a crucial mediator of the inflammatory response, influences vast cellular activities, including cell proliferation, differentiation, and apoptosis (Samad et al., 2001). Hence, to assess the regulation of Torin2, we further measured the concentrations and mRNA expression of TNF-α, IL-β, and IL-6. As expected, the results revealed that LPS treatment induced marked pro-inflammatory cytokine (TNF-α, IL-β, and IL-6) secretion and transcription, which Torin2 notably attenuated. As discussed above, the results demonstrated that Torin2 inhibited the production and transcription of TNF-α, IL-β, and IL-6 and might weaken the systemic inflammatory response to scavenge the lesions induced by LPS stimulation.
Accumulating evidence has confirmed that NF-κB regulates the expression of genes encoding pro-inflammatory inducible enzymes (iNOS and COX-2) and the production of pro-inflammatory cytokines, including TNF-α, IL-β, and IL-6 (Li et al., 2019). Park et al. previously reported that NF-κB forms a complex with IκB kinase in unstimulated cells (Park et al., 2016). Lee et al. revealed that activated NF-κB, released from the inhibition mediated by IκB, translocated to the nucleus to bind target DNAs, and controlled the transcription of numerous inflammatory genes (Lee et al., 2012). As NF-κB regulates the expression of these pro-inflammatory mediators, the present study subsequently detected protein expression in the NF-κB signaling pathway. LPS treatment induced the translocation of NF-κB p65 from the cytoplasm to the nucleus in RAW264.7, which was inhibited by Torin2 pre-treatment. Therefore, the above experimental data illustrated that Torin2 might retard LPS-evoked NF-κB signal pathway stimulation and restore lesions in LPS-induced RAW264.7 murine macrophages.
Oeckinghaus et al. indicated that MAPKs are involved in the TLR4-mediated signaling pathway, resulting in the stimulation of NF-κB and activator protein-1 (AP-1) in LPS-induced RAW264.7 murine macrophages (Oeckinghaus et al., 2011). MAPKs, including p38, ERK, and JNK signaling molecules, are pivotal pathways in the immune system that regulate cell functions, including cellular proliferation, differentiation, apoptosis, gene expression, inflammation, and nuclear signaling (Shin et al., 2015). Shine et al. previously illustrated that growth factors and mitogenic activation trigger ERKs signaling pathway stimulation. In contrast, stress-evoked signals and pro-inflammatory cytokines facilitate the phosphorylation of p38 and JNK proteins expression (Shin et al., 2015). Hence, any substance that inhibits the stimulation of NF-κB pathways and signaling cascades ameliorated by MAPKs can serve as a therapeutic agent against inflammatory damage. As expected, phosphorylation of JNK, ERK, and p38 proteins was markedly elevated, and it was reversed by pre-treatment with Torin2 in a dose-dependent manner. These results indicate that Torin2 might inhibit LPS-evoked inflammatory responses via suppressing the phosphorylation of JNK, ERK, and p38 proteins and the regulation of MAPKs signaling pathways.
5. Conclusion
Our experimental data demonstrated the anti-inflammatory response of Torin2 in LPS-stimulated RAW264.7 murine macrophages through the inhibition of NF-κB and MAPKs signaling pathways, suppression of iNOS and COX-2 transcription and protein expression, decrease in the production and transcription of pro-inflammatory cytokines (TNF-α, IL-β, and IL-6), and downregulation of NO and PGE2 levels. Hence, existing evidence indicates that Torin2 may be a potential new therapeutic drug for treating inflammatory diseases. However, further research is required to investigate the effective anti-inflammatory functions in human macrophages and clinical trials are needed to explore additional pharmacological activities of Torin2.
Declarations
Author contribution statement
Zhenzhen Zhang and Zhiyong Chu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Shanshan Wei: Performed the experiments; Wrote the paper.
Ting Wang, Qi Cao and Xue Chen: Analyzed and interpreted the data.
Funding statement
Prof Zhenzhen Zhang was supported by The Guidelines for the Technical Standard Program of the Science and Technology Innovation Action Plan [16DZ0501400].
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Zhiyong Chu, Email: zhiyongchuleader@163.com.
Zhenzhen Zhang, Email: zz_jane@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Beinke C., Scherthan H., Port M., Popp T., Hermann C., Eder S. Triterpenoid CDDO-Me induces ROS generation and up-regulates cellular levels of antioxidative enzymes without induction of DSBs in human peripheral blood mononuclear cells. Radiat. Environ. Biophys. 2020;59(3):461–472. doi: 10.1007/s00411-020-00847-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun E.B., Sung N.Y., Byun E.H., Song D.S., Kim J.K., Park J.H., Song B.S., Park S.H., Lee J.W., Byun M.W., Kim J.H. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int. Immunopharm. 2013;15(2):450–456. doi: 10.1016/j.intimp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Chen G., Zhou Y., Zhang W.D., Qin Y., Wei B., Sun Y.A., Chen Y. Methyl-β-cyclodextrin suppresses the monocyte-endothelial adhesion triggered by lipopolysaccharide (LPS) or oxidized low-density lipoprotein (oxLDL) Pharm. Biol. 2021;59(1):1036–1044. doi: 10.1080/13880209.2021.1953540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H.Q., Tang Q.Q., Huang H.P., H W.D., W X.T. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016;41:159–166. doi: 10.1016/j.etap.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Eiserich J.P., Hristova M., Cross C.E., Jones A.D., Freeman B.A., Halliwell B., Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014;14(6):392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- Hussain A.R., Al-Romaizan M., Ahmed M., Thangavel S., Al-Dayel F., Beg S., Uddin S., Siraj A.K., Al-Kuraya K.S. Dual targeting of mTOR activity with Torin2 potentiates anticancer effects of cisplatin in epithelial ovarian cancer. Mol. Med. 2015;21(1):466–478. doi: 10.2119/molmed.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Guan H.N., Liu D.Y., Wu X., Fan M.C., Han J.C. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017;8(3):1313–1322. doi: 10.1039/c6fo01873d. [DOI] [PubMed] [Google Scholar]
- Kim H.G., Yang W.S., Hong Y.H., Kweon D.H., Lee J., Kim S., Cho J.Y. Anti-inflammatory functions of the CDC25 phosphatase inhibitor BN82002 via targeting AKT2. Biochem. Pharmacol. 2019;164:216–227. doi: 10.1016/j.bcp.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Komatsu W., Itoh K., Akutsu S., Kishi H., Ohhira S. Nasunin inhibits the lipopolysaccharide-induced pro-inflammatory mediator production in RAW264 mouse macrophages by suppressing ROS-mediated activation of PI3 K/Akt/NF-κB and p38 signaling pathways. Biosci. Biotechnol. Biochem. 2017;81:1956–1966. doi: 10.1080/09168451.2017.1362973. [DOI] [PubMed] [Google Scholar]
- Kwon H.S., Park J.H., Kim D.H., Kim Y.H., Park J.H., Shin H.K., Kim J.K. Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J. Mol. Med. 2008;86(11):1287–1295. doi: 10.1007/s00109-008-0395-2. [DOI] [PubMed] [Google Scholar]
- Lee H.N., Shin S.A., Choo G.S., Kim H.J., Park Y.S., Kim B.S., Kim S.K., Cho S.D., Nam J.S., Choi C.S., Che J.H., Park B.K., Jung J.Y. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018;41:888–898. doi: 10.3892/ijmm.2017.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Ha S.J., Lee H.J., Kim M.J., Kim J.H., Kim Y.T., Song K.M., Kim Y.J., Kim H.K., Jung S.K. Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways. Food Funct. 2016;7(7):3263–3272. doi: 10.1039/c6fo00540c. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Kwon M.S., Choi J.W., Shin T., No H.K., Choi J.S., Byun D.S., Kim J.I., Kim H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012;60(36):9120–9129. doi: 10.1021/jf3022018. [DOI] [PubMed] [Google Scholar]
- Lee S.U., Ahn K.S., Sung M.H., Park J.W., Ryu H.W., Lee H.J., Hong S.T., Oh S.R. Indacaterol inhibits tumor cell invasiveness and MMP-9 expression by suppressing IKK/NF-κB activation. Mol. Cell. 2014;37(8):585–591. doi: 10.14348/molcells.2014.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Cho S.S., Bae C.S., Bae M.S., Park D.H. Socheongryongtang suppresses COPD-related changes in the pulmonary system through both cytokines and chemokines in a LPS COPD model. Pharm. Biol. 2020;58(1):538–544. doi: 10.1080/13880209.2020.1770808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Song X.W., Su G.F., Wang Y.L., Wang Z.Y., Qing S.R., Jia J.Y., Wang Y., Huang L.Z., Zheng K., Wang Y.F. AT-533, a Hsp90 inhibitor, attenuates HSV-1-induced inflammation. Biochem. Pharmacol. 2019;166:82–92. doi: 10.1016/j.bcp.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Liu Q.S., Xu C.X., Kirubakaran S., Zhang X., Hur W., Liu Y., Kwiatkowski N.P., Wang J., Westover K.D., Gao P., Ercan D., Niepel M., Thoreen C.C., Kang S.A., Patricelli M.P., Wang Y., Tupper T., Altabef A., Kawamura H., Held K.D., Chou D.M., Elledge S.J., Janne P.A., Wong K.K., Sabatini D.M., Gray N.S. Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res. 2013;73(8):2574–2586. doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Pi G.C., Xiao H., Ye Y.F., Li Q., Zhao L.H., Huang H., Luo H., Zhang Q., Wang D., Wang G. Torin2 enhances the radiosensitivity of MCF-7 breast cancer cells by downregulating the mTOR signaling pathway and ATM phosphorylation. Mol. Med. Rep. 2018;17(1):366–373. doi: 10.3892/mmr.2017.7848. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- Olatunji O.J., Tang J., Tola A., Auberon F., Oluwaniyi O., Ouyang Z. The genus Cordyceps: an extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2018;129:293–316. doi: 10.1016/j.fitote.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Park E., Kim Y., Kim H., Chang K.C. Luteolin activates ERK1/2- and Ca-dependent HO-1 induction that reduces LPS-induced HMGB1, iNOS/NO, and COX-2 expression in RAW264.7 cells and mitigates acute lung injury of endotoxin mice. Inflamm. Res. 2018;67(5):445–453. doi: 10.1007/s00011-018-1137-8. [DOI] [PubMed] [Google Scholar]
- Park J.W., Kwon O.K., Yuniato P., Marwoto B., Lee J., Oh S.R., Kim J.H., Ahn K.S. Amelioration of an LPS-induced inflammatory response using a methanolic extract of Lagerstroemia ovalifolia to suppress the activation of NF-κB in RAW264.7 macrophages. Int. J. Mol. Med. 2016;38(2):482–490. doi: 10.3892/ijmm.2016.2646. [DOI] [PubMed] [Google Scholar]
- Sadowski S.M., Boufraqech M., Zhang L., Mehta A., Kapur P., Zhang Y., Li Z., Shen M., Kebebew E. Torin2 targets dysregulated pathways in anaplastic thyroid cancer and inhibits tumor growth and metastasis. Oncotarget. 2015;6(20):18038–18049. doi: 10.18632/oncotarget.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad T.A., Moore K.A., Sapirstein A., Billet S., Allchorne A., Poole S., Bonventre J.V., Woolf C.J. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Shin I.S., Shin N.R., Park J.W., Jeon C.M., Hong J.M., Kwon O.K., Kim J.S., Lee I.C., Kim J.C., Oh S.R., Ahn K.S. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J. Pineal Res. 2015;58(1):50–60. doi: 10.1111/jpi.12192. [DOI] [PubMed] [Google Scholar]
- Song D., Chen Y., Wang B.R., Lie D.D., Xu C., Huang H., Huang S.F., Liu R. Bisphenol A inhibits autophagosome-lysosome fusion and lipid droplet degradation. Ecotoxicol. Environ. Saf. 2019;183 doi: 10.1016/j.ecoenv.2019.109492. 109492.1-109492.7. [DOI] [PubMed] [Google Scholar]
- Tak P.P., Firestein G.S. NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C.L., Chen X.Y., Chang Y., Wang R.X., Ning J., Cui C.C., Liu M.C. The regulatory effect of flavonoids extracted from leaves on gene expression in LPS-induced ALI mice via the NF-κB and MAPK signaling pathways. Pharm. Biol. 2019;57(1):514–518. doi: 10.1080/13880209.2019.1648523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayakumar D., Pandita R.K., Horikoshi N., Liu Y., Liu Q., Wong K.K., Hunt C.R., Gray N.S., Minna J.D., Pandita T.K., Westover K.D. Torin2 suppresses ionizing radiation-induced DNA damage repair. Radiat. Res. 2016;185(5):527–538. doi: 10.1667/RR14373.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.R., Wang X.J., Su Z.J., Fei H.J., Liu X.Y., Pan Q.X. The novel mTOR inhibitor Torin-2 induces autophagy and downregulates the expression of UHRF1 to suppress hepatocarcinoma cell growth. Oncol. Rep. 2015;34(4):1708–1716. doi: 10.3892/or.2015.4146. [DOI] [PubMed] [Google Scholar]
- Xu J.W., Zhou L., Sun L.L., Wang Z., Wang Y., Wang Y.H., He X.J. ent3α-Angeloyloxy--kaur-16-en-19-oic acid isolated from L. Alleviates xylene-induced mouse ear edema and inhibits NF-κB and MAPK pathway in LPS-stimulated macrophages. J. Nat. Prod. 2020;83(12):3726–3735. doi: 10.1021/acs.jnatprod.0c00990. [DOI] [PubMed] [Google Scholar]
- Yang G., Ham I., Choi H.Y. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem. Toxicol. 2013;58:124–132. doi: 10.1016/j.fct.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Yu C., Fu J., Guo L., Lian L., Yu D.J. UPLC-MS-based serum metabolomics reveals protective effect of Ganoderma lucidum polysaccharide on ionizing radiation injury. J. Ethnopharmacol. 2020;258:112814. doi: 10.1016/j.jep.2020.112814. [DOI] [PubMed] [Google Scholar]
- Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., He F.Y., Zhou L.F., Shi M., Li F.M., Jia H.G. Activation of TLR4 induces inflammatory muscle injury via mTOR and NF-kB pathways in experimental autoimmune myositis mice. Biochem. Biophys. Res. Commun. 2022;603:29–34. doi: 10.1016/j.bbrc.2022.03.004. [DOI] [PubMed] [Google Scholar]
- Zhou M.X., Xu W.M., Wang J.Z., Yan J.K., Shi Y.Y., Zhang C., Ge W.S., Wu J., Du P., Chen Y.W. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–360. doi: 10.1016/j.ebiom.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.