Abstract
Enhanced biological phosphorus removal at wastewater treatment plants that use anaerobic digesters for sludge treatment have historically encountered phosphate precipitation problems in the form of struvite. Literature on struvite is thin which is surprising given it can foul/block the sludge return lines and associated pumps and valves, causing significant operational problems. This study has evaluated if a typical large wastewater treatment plant can overcome this problem by adopting circular economy thinking. The struvite profile based on the supersaturation ratio of (Mg:NH4:PO42−), pH and temperature demonstrates the potential operational hotspots that can present uncontrolled struvite formation. Based on current struvite monitoring technologies and a cost-benefit analysis, the controlled struvite recovery via an Ostara crystallization reactor has been demonstrated to be economically viable with a pay-back period of less than a decade. An integrated evaluation illustrates the positive environmental impact arising from the utilisation of the recovered product. Economic viability and payback periods will vary according to circumstances, but we recommend that WWTP operators globally consider fitting a crystallisation reactor to appropriate plants, The outcomes and recommendation from this study are particularly timely given the global fertiliser shortage (2022) that is driving up food prices and reducing crop sizes.
Keywords: Wastewater treatment, Phosphorus, Struvite, Cost-benefit analysis, Circular economy
Graphical abstract
Highlights
-
•
Biological P removal at wastewater treatment plants causes precipitation problems.
-
•
We evaluated if problem can be overcome by adopting circular economy thinking.
-
•
Mass balance estimates & struvite precipitation profiles used to identify hotspots.
-
•
Struvite recovery via a crystallization reactor demonstrated as economically viable.
-
•
Struvite recovery from wastewater treatment is viable circular economy opportunity.
Wastewater treatment; Phosphorus; Struvite; Cost-benefit analysis; Circular economy.
1. Introduction
Wastewater treatment plants (WWTPs) incorporating secondary treatment and anaerobic sludge digestion facilities have historically encountered phosphate precipitates in the form of struvite (Barr and Münch, 2001; Jaffer et al., 2002). These agglomerates can foul and block the sludge return lines and associated pumps and valves (Ohlinger et al., 1999; Jaffer et al., 2002; Mudragada et al., 2014). Uncontrolled struvite precipitation increases pumping and maintenance costs, as well as reducing the overall capacity of the plant piping system via hydraulic loss capacity. This also lowers the biological treatment efficiency (Battistoni et al., 1997; Barr and Münch, 2001; Doyle and Parsons, 2002; Jaffer et al., 2002; Mudragada et al., 2014).
However, it is possible that controlled production and recovery of struvite could promote circular economy (CE) opportunities for the wastewater treatment industry (Barr and Münch, 2001; Jaffer et al., 2002; Molinos-Senante et al., 2011; Siciliano et al., 2020), especially in coastal areas (Roberts et al., 2021). The precipitated struvite can be recovered and sold as fertiliser, reducing resource depletion by replacing fossil-fuel based P (Jaffer et al., 2002; Zhang and Mo, 2013). Mavhungu et al. (2021) report that struvite precipitation could act as a fast, efficient, and environmentally friendly pre-treatment step to remove P and reduce N from wastewater. However, a potential concern is that the environmental impacts of struvite recovery via the use of additional chemicals and energy are not offset by its benefits (Sena et al., 2021). Nevertheless, removal of struvite can significantly decrease the operational and maintenance costs of WWTPs, promoting the optimisation of operational and environmental performance (Jaffer et al., 2002; Pastor et al., 2008).
Within WWTPs, enhanced biological phosphorus removal (EBPR) processes produce a P-rich activated sludge, causing struvite deposit problems in anaerobic sludge digestion (Barr and Münch, 2001). Generally, struvite (MgNH4PO4) is a white crystalline mineral compound that forms under conditions of elemental supersaturation within liquids (Barr and Münch, 2001). It can be found in WWTP systems when Mg2+, NH4+ and PO43− (magnesium, ammonium and phosphate – MAP) concentrations exceed solubility levels, supersaturation occurs and minerals combine and precipitate into solid form (Wu and Bishop, 2004).
WWTP design generally incorporates flow and partial pressure reduction through pump impellers and pipe bends, forcing the removal of dissolved CO2 and increasing the pH of the liquor (Battistoni et al., 1997; Fattah et al., 2010; Xavier et al., 2014). High pH levels (∼7.5–10) are essential for the struvite precipitation (Ohlinger et al., 1999; Barr and Münch, 2001; Wu and Bishop, 2004). Reduction of the partial pressure of reject waters from 0.5 atm to 0.05 atm within the WWTP contributes to the release of dissolved CO2 and thus increases pH levels from 7.0 to 8.0, increasing the chance of struvite precipitation (Fattah et al., 2010).
The most common P removal technologies include biological P removal, crystallization, chemical precipitation via ferric chloride, tertiary filtration and ion exchange (Morse et al., 1998). Crystallization of struvite is probably the most sustainable solution due to the minimal waste production that needs to be managed (Marti et al., 2008).
The primary aim of this study was to determine and evaluate the economic and environmental impacts arising from the uncontrolled struvite formation within a typical large WWTP. For this aim, the objectives were to:
-
i.
Establish a mass balance for the nutrients present in the system. This is to enable the specific locations within the operational system that present higher possibility for struvite precipitation to be identified (Jaffer et al., 2002; Marti et al., 2008).
-
ii.
Create a profile for struvite that incorporates the potential operational hotspots for uncontrolled struvite precipitation and other factors that can affect struvite formation.
-
iii.
Identify the operational and maintenance costs and the environmental impacts arising from struvite precipitation at the WWTP.
The secondary aim of this study was select the most appropriate monitoring pathway for the uncontrolled struvite precipitation based on the characteristics of the studied WWTP. For this aim, the objectives were to:
-
i.
Identify and evaluate current technologies regarding nutrient removal and/or controlled struvite formation.
-
ii.
Evaluate the economic costs and benefits of the controlled struvite formation.
-
iii.
Develop an integrated cost benefit analysis for the proposed struvite monitoring technology.
We critically discuss findings in the context of enabling wastewater treatment processes to recover more value from wastewater (resources), reducing treatment and maintenance costs and enabling circular economy (CE) thinking to be operationalized.
2. Methodology
2.1. WWTP characteristics
Budds Farm wastewater treatment plant (BF-WWTP), owned by Southern Water (SW) in England, treats the domestic wastewater of Portsmouth, Havant, Hayling Island, Cosham, Paulsgrove, Waterlooville, Horndean and Hambledon. It has a general capacity of 109,000 m3 of wastewater per day. Before its establishment, untreated wastewater arising from Hampshire was discharging to Langstone Harbour causing significant environmental impact at local and national level (Smith et al., 1999; Doyle and Parsons, 2002). Indeed, SW has admitted deliberately and illegally dumping untreated wastewater into this harbour, resulting in a huge fine (BBC News, 2021a) and damaging publicity (BBC News, 2021b).
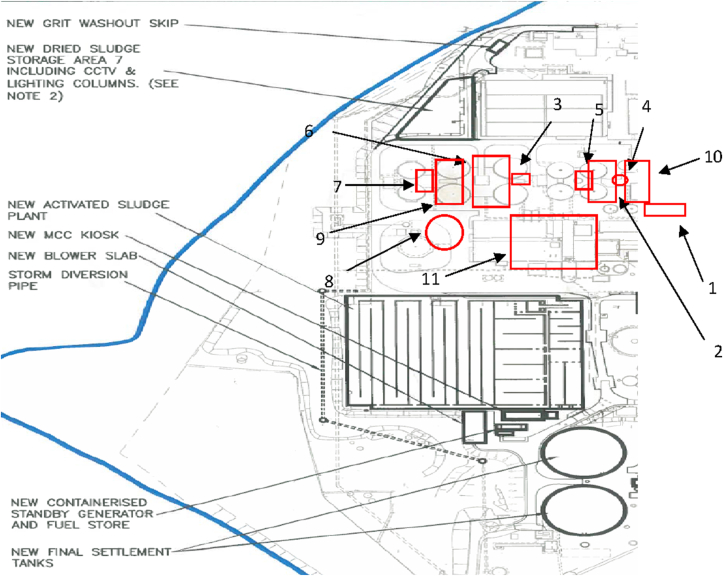
BF-WWTP is responsible for a population equivalent (PE) of approximately 410,000. It presents an outfall of an average 2000 L/s and it operates for 24/7 for 365 days per year. It has one of the biggest BNR facilities in UK with 3 main operational units. BF-WWTP handles indigenous and imported sludge with its anaerobic digesters to treat 800 m3 of activated sludge on 14 day cycle. The site is under tight nutrient consents, restricting discharges into the harbour to 10 mg/L total nitrogen or less. Figure 1 illustrates the main operational units associated with the wastewater treatment process within the BF-WWTP.
Figure 1.
Part of Budds Farm operational diagram. 1) Screened sludge storage tank, 2) Thickened sludge storage tank, 3) SAS storage tank – Surplus Activated Sludge (SAS), 4) Blended cake, 5) Digester feed, 6) Digester recirculation pump, 7) PDST (digested sludge) – Post digested sludge treatment, 8) Centrifuge feed, 9) SAS filtrate, 10) Primary thickening filtrate, 11) Centrate SAS after thickened (figure provided by Southern Water).
2.2. Sampling and analysis
Operational units and locations (hot spots) within WWTPs that have historically presented P precipitation in the form of struvite are summarised in Table 1 (Le Corre et al., 2009). A sampling strategy was created to determine concentrations for the three struvite ions (Mg:NH4:PO42−) or MAP. External factors such temperature (T) and pH were determined to evaluate struvite behaviour within the system (Bouropoulos and Koutsoukos, 2000; Doyle and Parsons, 2002; Jaffer et al., 2002; Fattah, 2012).
Table 1.
Hot spots of struvite precipitation and their operational impact (adapted from Le Corre et al., 2009).
| Operational hotspots | Effects | Type of plant | References |
|---|---|---|---|
| Pipes carrying supernatants of AD. | 2.5 cm accumulation. | WWTP (USA) | Rawn et al. (1937) |
| Activated sludge process - pump onto separating screen. | Diameter reduction from 310 mm to 150 mm. | Hyperion WWTP (USA) | Borgerding (1972) |
| Anaerobic supernatant: pump impellers, pipes, etc. | – | Livestock WWTP (USA) | Booram et al. (1975) |
| Outfall pipelines, waste pumps, pipelines. | Accumulation: from 5.88 up to 14.44 mm in aerators; from 8 up to 28 mm in pipes carrying digester effluents. | Pig waste treatment plant (Singapore) | Mohajit et al. (1989) |
| Pipes from sludge supernatant system. | Accumulation along 5.6 km of pipes. | WWTP (USA) | Ohlinger et al. (1999) |
| Precipitation in pipelines - sludge holding tank to the centrifuges. | Pipes bore reduction from 100 to 50 mm. | WWTP (UK) | Williams (1999) |
| Pipes of centrate liquors. | Pipe diameter reduction from 150 to 60 mm in 12 weeks. | Sludge treatment plant (UK) | Doyle and Parsons (2002) |
| Pipes of anaerobic supernatants. | Pipe diameter reduction. | Pilot fluidised bed reactor plant, WWTP (Italy) | Battistoni et al. (2005) |
| Streaming pipes. | Two-month build up in a rubber lined 90° elbow. | WWTP (USA) | Nethling and Benisch (2004) |
Wastewater samples were professionally collected by an operational engineer employed at BF-WWTP. The samples were processed and analysed on-site at BF-WWTP’s engineering laboratory. All samples were filtered via Advantec Grade No.1 filter paper or cellulose acetate syringe filter. Because of the high density of the sludge samples, filtration was necessary in order to remove coarse particles and prepare samples for qualitative analysis. Temperature (T) and pH were measured potentiometrically in the undiluted liquid wastewater sample after filtration and in less than one hour from sample collection. The DO700 analyser provided automatic measurements for dissolved oxygen, T, pH, conductivity and total dissolved solids (TDS) (Pass, Extech DO700 Portable Dissolved Oxygen Meter).
Ammonia concentrations were determined via a QUANTOFIX test kit that provides a detection range between 10 and 400 mg/L NH4+ (Macherey-Nagel, Quantofix test strips). Phosphate concentrations were determined via a QUANTOFIX test kit that only detected ortho-phosphate. This method provides a detection range between 3 and 100 mg/L PO43− (Macherey-Nagel, Quantofix test strips). Magnesium concentration was determined via a viscolar ECO total hardness test kit that provides a detection range between 10-100 mg/L CaO (Macherey-Nagel, VISOCOLOR ® ECO Hardness, total). Dilutions of 1:4 or 1:10 (based upon previous experience) of wastewater samples to double-distilled deionised water were required based on the colour indication of the test kits.
2.3. Creation of the struvite precipitation profile within BF-WWTP
Struvite precipitation generally correlates with the degree of supersaturation (Ohlinger et al., 1999; Bouropoulos and Koutsoukos, 2000; Kofina and Koutsoukos, 2005), magnesium to phosphorus molar ratio (Mg:P) (Barr and Münch, 2001), pH (Barr and Münch, 2001; Doyle and Parsons, 2002), temperature (Doyle and Parsons, 2002), crystal retention time, recycle ratio (Ohlinger et al., 1999; Bouropoulos and Koutsoukos, 2000), turbulence and mixing (Ohlinger et al., 1999; Bhuiyan, 2007). Eq. (1) summarises the kinetics of struvite formation:
| (1) |
The struvite profile (behaviour within the WWTP system) arose from the concentrations of the main chemical components of MAP and the struvite supersaturation rate (SSR) for each sampling point in correlation with pH and T. This correlation indicated which locations present higher potential for uncontrolled struvite precipitation (Doyle and Parsons, 2002; Le Corre et al., 2009; Fattah et al., 2010; Fattah, 2012). In general, uncontrolled formation can occur when the concentrations of Mg2+, NH4+ and PO43− exceed the Ksp of struvite and precipitates in a 1:1:1 molar ratio following Eq. (1) (Le Corre et al., 2009). If the general equation of soluble salt in water is assumed then Eq. (2) follows:
| AaBb(s) → aAz++bBz− | (2) |
and the constant solubility product Ksp can be expressed as:
| Ksp = [Az+]a.[Bz−]b | (3) |
where:
[Az+] and [Bz−] are the total concentrations of ions in solution
z+ and z− are the valencies of the considered ions.
By applying Eq. (3) on Equation 1, Equation 4 is produced (Michałowski and Pietrzyk, 2006):
| (4) |
Based on Eq. (4), if the product of the concentration of Mg2+, NH4+, and PO43− is greater than the value of Ksp, then the solution is supersaturated in terms of struvite and precipitation potentially will occur (Bhuiyan, 2007; Le Corre et al., 2009). This can also be described as the supersaturation ratio (SSR) and can be expressed as Eq. (5) (Le Corre et al., 2009):
| (5) |
2.4. Cost benefit analysis of P recovery via struvite precipitation
An economic feasibility study of P recovery was established using a cost-benefit analysis (CBA). The net profit (NP) is the difference between benefits and costs (i.e. NP = IB + EB where net profit [NP = total income - total costs]; internal benefit [IB = internal income - internal costs]; and external benefit [EB = positive impact – negative impact]). Through a CBA, a project is only economically feasible only if NP > 0 (Molinos-Senante et al., 2011).
The term internal income includes the sale of recovered P and treated water, as well as savings from reduced WWTP operating costs arising from chemical utilisation for the chemical precipitation of phosphorus; reduced sludge generation and associated management cost and reduced pipe and tube cleaning due to less uncontrolled struvite scaling (Molinos-Senante et al., 2011).
Internal costs include investment cost, civil works (equipment, machinery, and auxiliary facilities); operating and maintenance costs (reagents for chemical precipitation and pH value maintenance); and financial costs (Molinos-Senante et al., 2011).
Investment costs depend largely on the size of the WWTP (Molinos-Senante et al., 2011). Operating and maintenance cost proposed values depend on the concentration of P in the waste streams and the reagents used to operate the plant. The cost depends on the people equivalent covered from the plant and the operational streams the P is be extracted. Thus, considering these incomes and costs, the internal benefit can be expressed as Eq. (6) (Molinos-Senante et al., 2011):
| IB = ΣTt=0 [(ASR × SPP) + (ASR × CSM) + (ARP × CR) + (APR × CCD) – (IC + OMC + FC)] | (6) |
where:
IB = internal benefit ($)
ASR = annual volume of struvite recovered (kg)
SPP = present selling price of struvite ($/kg)
ASR = annual volume of sludge generation reduction (kg)
CSM = cost of sludge management ($/kg)
ARR = annual volume reduction of reagents (kg)
CR = cost of reagents ($/kg)
APR = annual volume reduction of uncontrolled struvite precipitation (kg)
CCD = cost of cleaning struvite deposit ($/kg)
IC = investment cost ($)
OMC = operational and maintenance cost ($)
FC = financial costs ($)
External benefits (EB) refer to any positive or negative impact that derives from a proposal and effects on people without economic value. P recovery from waste streams is regarded as having positive EB, such as an increase in the availability of a non-renewable resource and important environmental benefits, because if phosphorus discharge is prevented, then its level in water bodies is reduced and, consequently, there are fewer eutrophication problems. The external benefits can be expressed as Eq. (7) (Molinos-Senante et al., 2011):
| EB = ΣTt=0 (PI – NI) | (7) |
where:
PI = positive impact ($)
NI = negative impact ($)
In general, IB can be estimated directly as monetary units while the EB is difficult to estimate as marketable price. In order to give a comparable value for the EB, the term shadow price may be used (Molinos-Senante et al., 2011). Given the volatility of markets during the COVID19 pandemic, pre-COVID monetary values have been used to establish order-of-magnitude indicative costs/prices.
2.5. Integrated evaluation of chosen struvite monitoring technology
Except for the financial elements, the integrated evaluation (IE) took into consideration the environmental (shadow indicator) and social impacts that a proposal can cause at a national and international level. The CBA determined if a technology can be viable from an economic perspective. The IE demonstrated the sustainable trends of the modern industrial models and aimed to present a holistic approach regarding the P monitoring pathway (Molinos-Senante et al., 2011; Bird, 2015).
3. Results
3.1. Operational diagram and struvite precipitation
Based on the BF-WWTP operational diagram as well as the locations shown in Table 1, the sampling map was established in consultation with the BF-WWTP management team. Figure 2 illustrates the sludge treatment process within the plant focusing on the areas that present higher possibility for uncontrolled struvite precipitation.
Figure 2.
Budds Farm WWTP sludge treatment process and sampling map. This diagram demonstrates the operational hotspots regarding the uncontrolled struvite formation.
Table 2 presents data arising from the determination of the main factors that affect the struvite formation at the different operational hot-spots.
Table 2.
Data arising from the samples analysis of the most potential locations regarding the struvite formation within the operational system of Budds Farm WWTP. The locations that present the symbol ∗ or ∗∗ indicate locations with the same sludge characteristics due to operational design. In addition, the brackets indicate the dilution of wastewater samples: double-distilled deionised water in order to reach the detection limits.
| Hot-spots of struvite precipitation | pH | T (oC) | PO43−(mg/L) | NH4+ (mg/L) | Mg2+ (mg/L) |
|---|---|---|---|---|---|
| Screened sludge storage tank | 6.5 | 20.8 | 100 (1:4) | 400 | 390 |
| Thickened sludge storage tank∗ | 5.4 | 20.8 | 100 (1:4) | 100 | 390 |
| SAS storage tank | 7.2 | 21.1 | 100 | 10 | 350 |
| Blended cake | 6.4 | 22.0 | 100 | 100 | 190 |
| Digester feed∗ | 5.9 | 20.8 | 100 | 100 | 390 |
| Digester recirculation pump | 8.4 | 21.2 | 40 | 100 | 150 |
| PDST (digested sludge) ∗∗ | 8.4 | 20.8 | 200 (1:4) | 200 (1:4) | 190 |
| Centrifuge feed∗∗ | 8.4 | 20.8 | 200 | 200 | 190 |
| SAS filtrate | 7.1 | 20.3 | 100 (1:4) | 10 | 380 (1:4) |
| Primary thickening filtrate | 5.6 | 20.7 | 200 (1:4) | 50 | 340 |
| Centrate | 8.1 | 21.5 | 40 | 200 (1:4) | 230 |
| SAS after thickened | 7.0 | 20.5 | 100 | 10 | 260 |
Several struvite Ksp values have been proposed in the literature. The most widely accepted value was found to be 10−12.6 (Snoeyink and Jenkins, 1980; Michałowski and Pietrzyk, 2006). At full-scale wastewater treatment processes, the Ksp value was determined as 10−13.26 (Ohlinger et al., 1999), thus struvite estimated to be less soluble than presented to be based on other researches. Table 3 presents the concentrations of the tested locations of BF-WWTP in M (mol/L) and the SSR.
Table 3.
Concentrations of struvite ions in mol/L and the respective SSR.
| Hot-spots of struvite precipitation | PO43−(mol/L) | NH4+ (mol/L) | Mg2+ (mol/L) | Ksp | SSR |
|---|---|---|---|---|---|
| Screened sludge storage tank | 0.0010 | 0.0200 | 0.0163 | 3.26 × 10−7 | >1 |
| Thickened sludge storage tank∗ | 0.0010 | 0.0050 | 0.0163 | 8.15 × 10−8 | >1 |
| SAS storage tank | 0.0010 | 0.0005 | 0.0146 | 7.3 × 10−9 | >1 |
| Blended cake | 0.0010 | 0.0050 | 0.0079 | 3.95 × 10−8 | >1 |
| Digester feed∗ | 0.0010 | 0.0050 | 0.0163 | 8.15 × 10−8 | >1 |
| Digester recirculation pump | 0.0004 | 0.0050 | 0.0063 | 1.26 × 10−8 | >1 |
| PDST (digested sludge)∗∗ | 0.0021 | 0.0100 | 0.0079 | 1.659 × 10−7 | >1 |
| Centrifuge feed∗∗ | 0.0021 | 0.0100 | 0.0079 | 1.659 × 10−7 | >1 |
| SAS filtrate | 0.0010 | 0.0005 | 0.0158 | 7.9 × 10−9 | >1 |
| Primary thickening filtrate | 0.0021 | 0.0027 | 0.0142 | 8.0514 × 10−8 | >1 |
| Centrate | 0.0004 | 0.0100 | 0.0096 | 3.84 × 10−8 | >1 |
| SAS after thickened | 0.0010 | 0.0005 | 0.0110 | 5.5 × 10−9 | >1 |
The SSR for the selected locations indicates that the influent that reaches BF-WWTP presents extremely high concentrations of the three ions of MAP. Thus there is high possibility of uncontrolled struvite formation in the majority of the operational system; this is regularly observed on-site. However, a positive SSR does not ensure that struvite is necessarily formed (Fattah, 2012). This is mainly because the kinetics of P precipitation (how fast P is precipitated) and the competing reactions must be taken into consideration. Furthermore, ions such as Ca2+, K+, CO32−, etc., can influence the saturation of struvite by reacting with Mg2+, PO43−, and NH4+. It is therefore necessary to evaluate the availability of free ions (i.e. the ionic activity) for a given pH in order to accurately estimate the precipitation rates (Snoeyink and Jenkins, 1980; Ohlinger et al., 1999; Barr and Münch, 2001). Figure 3 presents the variation of pH in the different operational hot spots at BF-WWTP.
Figure 3.
Operational pH variations for the proposed hot spots. The red lines indicate the thresholds for struvite precipitation. Values lower than 7 and higher than 10.7 present high solubility of struvite and thus less danger for operational problems arising from scaling.
Struvite can potentially be formed at locations that present positive SSR and a pH range between 7 and 10.7 (Tables 2 and 3, Figure 3) (Snoeyink and Jenkins, 1980; Ohlinger et al., 1999; Barr and Münch, 2001). In general, the digester recirculation pumps, PDST, centrifuge feed, centrate and SAS storage tank demonstrate suitable conditions for P precipitation in the form of struvite. The correlation between Ksp and pH indicates that struvite solubility decreases with increasing pH which in turn leads to an increase in the precipitation potential of a solution (Booram et al., 1975; Ohlinger et al., 1999; Musvoto et al., 1999b; Doyle and Parsons, 2002).
Generally, in digestion of BNR sludge, the stabilising counter ions of polyphosphate produce Mg in a molar ration of ∼0.3 mol Mg/mol P. Thus, it can be assumed that all the removed Mg potentially can be precipitated as struvite (Doyle and Parsons, 2002). Moreover, in the presence of high influent P concentrations (>200 mg/L) struvite crystals may demonstrate a growth rate up to 0.177 mm/d (Abe, 1995). If it is assumed that BF-WWTP uses 3.94 in (100 mm) sludge lines, then based on these struvite growth rates, an operational hot spot in the optimum pH range (>7) can potentially present a full blockage in less than (100/0.177 mm/d = 564.97/365 days/year =) 1.55 years (Doyle and Parsons, 2002).
BNR sludge contains a higher concentration of Mg than activated sludge, thus more struvite can be formed (Table 3). Mg is taken up with polyphosphate formation, at a molar ratio of 0.34 mol Mg/mole of P and the amount of Mg released during polyphosphate hydrolysis is reported as 0.25 g of Mg per g of P (Wild et al., 1996).
Figure 4 demonstrates the T variations between the different hot-spots; Ksp is linearly correlated with T. A low T reduces the solubility of MgNH4PO4 causing uncontrolled struvite formation. Specifically, reduction of T from one operational hotspot to another in combination with an optimum pH range is a significant indicator for potential struvite formation (Doyle and Parsons, 2002).
Figure 4.
Temperature variations for the different operational hot-spots. T reduction is an indicator for uncontrolled struvite formation.
The T presents significant fluctuations between the different operational hotspots. If all the data provided are taken into account then the locations with the higher possibility to present uncontrolled struvite formation are: digester feed, digester recirculation pump and PDST. More specifically, it seems that anaerobic digester facilities and associated pumps are the most energetic hot spots mainly because they meet all the criteria regarding struvite uncontrolled formation.
3.2. Current and upcoming phosphorus removal techniques
There are a wide range of technologies to remove and recover P from wastewater, including chemical precipitation, biological phosphorus removal, crystallisation, novel chemical precipitation approaches. Phosphorus in wastewater represents a significant renewable resource and there is no environmental or technical reason why phosphorus cannot be recycled (Morse et al., 1998; De-Bashan and Bashan, 2004; Xie et al., 2016). Morse et al. (1998) summarises applied technologies focusing on the main inputs, auxiliary inputs, main output as well as the form that the P is recovered, industrial and agriculture value, associated advantages and limitations.
It was reported that chemical precipitation can achieve an average of 0.6 mg/L of total P in the effluent with an average Alum dose of 45 g/L (Patoczka, 2005). In real operational conditions, crystallization via chemical precipitation can remove 45 mg/L of P to 6 mg/L of P within a pH of 8.7. BNR can reduce mean influent total P concentration from 6 mg/L to 1.5 mg/L. Egle et al. (2016) categorised the most industrial applied and robust technologies in three categories; aqueous phase (digester supernatant, dissolved P in anaerobically digested sludge and effluent), sewage sludge and sewage sludge ash.
4. Discussion
4.1. Decision-making for struvite monitoring
Based on the finding regarding the availability of the main ions that constitute struvite as well as the external factors that can affect the uncontrolled formation, large plants such as BF-WWTP demonstrate a high potential for the application of an economically-viable struvite controlled-recovery technology. Within a typical operational system, locations such as digester feed, digester recirculation pump, PDST and centrate are likely to be highly suitable for struvite formation and hence extraction.
In general, monitoring technologies target: the disruption of solubility or alteration of the growth mechanisms of crystals or promotion of controlled struvite formation for recovery (Barr and Münch, 2001; Le Corre et al., 2009; Fattah, 2012; Egle et al., 2016). Since struvite precipitation is mainly based upon the equilibria of the main chemical components, most previous case studies tried to overcome this problem by either forming other phosphate based salts or by reducing the pH under the optimum formation range (Egle et al., 2016). This is mainly because once one of the P, Mg or NH3 ions are removed, this automatically leads inhibition of struvite formation (Le Corre et al., 2009).
Acid washing with mainly CH3COOH or H2SO4 has been used by many WWTPs in order to overcome blockage problems within their operating systems (Williams, 1999; Doyle and Parsons, 2002). The use of FeCl3 is the most dominant option for struvite inhibition. However, it can produce large quantities of sludge mainly because of the poor molar removal of P per mole of Fe added. A ratio between 0.38 and 0.48 molar removal of phosphate per mole Fe added has been reported (Mamais et al., 1994). Na2HPO4 has also been described as a struvite inhibitor.
The REM-NUT®, Ostara®, PRISA, Gifhorn, Stuttgart and LOPROX technologies produce struvite as final product with P content in the range of 10–12%. This final product typically presents similar plant uptake efficiency as commercial fertilizers in acidic soils and partially in alkaline soils (Kratz et al., 2010). In general, except from MgNH4PO4.6H2O (the most dominant form), P can be precipitated as magnesium hydrogen phosphate trihydrate or newberyite (MgHPO4·3H2O) at pH < 6 or trimagnesium phosphate (Mg3(PO4)2 · 22H2O/Mg3(PO4)2·8H2O) at pH between 6 - 9 (Musvoto et al., 1999b; Michałowski and Pietrzyk, 2006).
The REM-NUT technology is the most expensive (2017) with >€42 per kg P produced at full operational scale (pre-COVID value), because of the tremendous requirements for resins and chemicals (Egle et al., 2016). Ostara®, DHV®, PRISA and P-RoC®, present an annual cost of approximately €6–10 per kg P produced or €0.8–2 per PE per year (for BFWWTP = 0.8 + 2 = €2.8/PE/2 = €1.4/PE ∗ 410,000 PE/year = €574,000/year). The cost of 1 kg of P recovered via wet-chemical processes is €9–16 (Gifhorn and Stuttgart processes). The high requirements regarding chemicals, including acids, caustics and precipitation agents set the recovery costs at relatively high levels. The cost of wet-oxidation processes, such as Aqua Reci®, PHOXNAN and MEPHREC®, is approximately between €23–27 per kg Prec, which makes them economically unattractive (Egle et al., 2016).
DHV Crystalactor® is not economically viable, even with maximum revenues, maximum savings and an up-scaling of the plant to 500,000 PE due to the high operational resources demand (Egle et al., 2016). High annual costs have also been reported for the Aqua Reci® and the MEPHREC® processes.
A P controlled crystallization process appears to be a suitable method to recover P in the form of struvite (Battistoni et al., 1997, 2000, 2005; Barr and Münch, 2001; Doyle and Parsons, 2002; Pastor et al., 2008). Thus an Ostara fluidised bed crystallisation reactor seems to be a very promising option regarding the sustainable management of the struvite precipitation problem (Merlo, 2011; Bird, 2015; Egle et al., 2016).
4.2. P recovery and recycling through struvite crystallization
The operational design of BF-WWTP is likely to enhance struvite formation at specific operational hotspots when the optimum conditions are met. Consequently, a controlled struvite recovery can potentially be viable. Controlled struvite precipitation process via crystallisation can recover struvite at rates of 80–90% from reject waters (Shu et al., 2006; Xavier et al., 2014). This system can reduce ammonia concentrations by 29% (Shu et al., 2006; Xavier et al., 2014).
A struvite crystallizer reactor incorporates reverse gravitational flows of reject waters from anaerobic digesters and solids dewatering facilities (Britton et al., 2005; Fattah et al., 2010; Cullen et al., 2013). Struvite crystals are separated by density and size as result of the upward flow through increasingly larger reactor chambers. Spherical pellets or beads (known as prills) remain in the upper chamber until enough minerals accumulate, increasing the size and density while the smaller particles drop down into the smaller reactor chamber in the lower section. Each reactor zone has a reduced liquid retention time. The larger diameter area at the top of the reactor has the smallest struvite particles with higher retention time to allow for crystal growth (Cullen et al., 2013). The largest prills present in the lowest and smallest reactor chamber are harvested, dried and bagged.
Precipitation of struvite requires a molar ratio of 1(Mg2+):1(NH4+):1(PO43−) (De-Bashan and Bashan, 2004; Le Corre et al., 2009). It has been estimated that 95% of P can be precipitated as struvite from the centrifuge supernatant, by the addition of 1.05–1.3 or 1:1 molar ratio of Mg to P, i.e. a Mg dose of about 210 mg/L (if P concentrations 200 mg/L be assumed) (Fujimoto et al., 1991).
Supernatants and reject water pH levels are often unsuitable for struvite formation. Thus, pH levels must be regulated. Sodium hydroxide is mainly used for pH regulation within a crystallizer reactor (Jaffer et al., 2002; Britton et al., 2005; Xavier et al., 2014). In general, recovery cannot be viable in mainstream activated sludge, trickling filter and anaerobic processes mainly because of low of P and NH4 concentrations (Williams, 1999). However, based on the results arising from this study, plants such as BF-WWTP produce high concentrations of MgNH4PO4 at a variety of operational locations. Anaerobic digestion supernatants as well as centrate from sludge filter can be potential locations, because of the high decomposition of microbial mass due the microbial bio-activities and the release of optimum P and NH4 concentrations (Bhuiyan, 2007).
4.3. Cost benefit analysis
For the CBA of a WWTP the following (pre-COVID) data are considered (Molinos-Senante et al., 2010, 2011):
For a given data set of 22 WWTP with treated water volume between 1,000,000 and 8,000,000 m3/year the average operational cost is as follow: energy = 0.0392 €/m3, staff = 0.0712 €/m3, regents = 0.0301 €/m3, waste management = ∼0.0342 €/m3, maintenance = 0.0453 €/m3 (Molinos-Senante et al., 2010, Molinos-Senante et al., 2011). Based on these data, the overall (pre-COVID) operating cost of WWTPs are in the range 0.1158 €/m3 and 0.7491 €/m3. Based on the treating capacity the weighted average is approximately 0.2200 €/m3. Considering that Budds Farm WWTP presents treating capacity (109,000 m3/day ∗ 365 days/year = 39,785,000 m3/year = 4.97 times higher than the proposed, then the overall operational cost can be estimated approximately as (4.97 ∗ 0.22 €/m3 =) 1.09 €/m3. Because of the incorporation of BNR in the BFWWTP operational system, the annual cost for chemical struvite precipitation is estimated as (1.09 €/m3 ∗ 109,000 m3/day ∗ 365 days/year =) 43,365,650 €/year.
Environmental benefits reflect the value of the environmental damage resulting from the uncontrolled management and dumping of the undesirable outputs (known as shadow price). By taking into account the volume of pollutants removed in the treatment process (kg/year), the volume of treated wastewater (m3/year), and the prices obtained for each pollutant, the overall environmental benefit resulting from wastewater treatment is calculated between 0.0099 €/m3 and 1.0039 €/m3 (Molinos-Senante et al., 2010, 2011). Based on the treating capacity of the WWTPs that have be examined the weighted average is 0.3609 €/m3. For Budds Farm WWTP this value can be expressed as (0.3609 €/m3 ∗ 4.97 =) 1.79 €/m3 ∗ 109,000 m3/day ∗ 365 days/year = 71,215,150 €/year.
The price of treated water is estimated to be 0.345 €/m3 (Molinos-Senante et al., 2010). If the WWTP proceeds to sale of the treated water, then assuming 50% and 100% of the treated water is sold, the mean net profit is increased by ∼55% (= 0.3138 €/m3) and 71% (= 0.4863 €/m3), respectively (Molinos-Senante et al., 2010). If the 50% water sale scenario is applied to BF-WWTP, the (pre-COVID) income is estimated as (0.3138 €/m3 ∗ 109,000 m3/day ∗ 365 days/year =) 12.484.533 €/year.
Regarding a full-scale pilot plant with treating capacity 400 m3/d of homogeneous liquors, the NaOH addition costs range from 0.0014 to 0.51 €/m3, which can be responsible for the 97% of the chemical expenses (Barr and Münch, 2001; Jaffer et al., 2002; Battistoni et al., 2005). For BF-WWTP this estimation can be calculated as (0.51 + 0.014 = 0.5114/2 = 0.2557∗ 109,000 m3/day = 27,871 €/day. An alternative to NaOH addition was examined by Battistoni et al. (2005) with use of air stripping to adjust the pH of struvite precipitation. The addition of Mg(OH)2, which is cheaper than MgCl2 and simultaneously helps to increase the pH, is a possible option (Barr and Münch, 2001). A further potential option is the incorporation of sea water as an alternative source of magnesium. This proposal can reach 95% P removal compared to 97% removal with MgCl2. BF-WWTP could utilise saline water from the nearby Langstone Harbour and because of its location the transportation cost associated will be minimal. Furthermore, Battistoni et al. (2005) indicated that the cost associated with struvite recovery can be reduced from 0.28€ to 0.19€ per m3 (0.28–0.19 = 0.09 ∗100 = 9%). For BF-WWTW this reduction can be estimated as (1.09 €/m3 ∗ 0.09 = 0.0981 €/m3, 1.09 €/m3 - 0.0981 €/m3 = 0.99 €/m3 ∗ 109,000 m3/day ∗ 365 days/year =) 39,462,741.2 €/year by using sand as auto-nucleation media.
The feasibility of controlled recovery of struvite via crystallisation is heavily dependent on the profits generated from struvite sales as fertilizer. The revenue produced from the struvite sales is difficult to estimate because of the differences in regional demand and rates of production (Le Corre et al., 2009). It should be noted that currently (2022) there are concerns about the increasing costs of energy used for ammonia production for fertiliser and the associated greenhouse gas emissions; the process of making ammonia is not a “green” process as it is most commonly made from methane, water and air, using steam methane reforming and the Haber process. In Japan, struvite was historically sold as fertiliser for agricultural purposes for approximately 250 €/t. For a process treating 400 m3/d of centrate liquors incorporating an activated sludge handling system and a BNR, the potential revenue regarding the struvite recovery and sale has historically been estimated as ∼25,000 €/month (= 300,000 €/year) with a 90% P removal (Barr and Münch, 2001; Jaffer et al., 2002). It reported that 1 kg of struvite can be recovered from 100 m3. Based on the Budds Farm outfall [(2000 L/s ∗1 kg)/100,000 L = 0.01 kg/s ∗3600 s/h ∗ 24 h/day ∗365 day/year = ] 315 tonnes/year can be produced (Barr and Münch, 2001). Based on historical data, the average marketable price of struvite is estimated as 200–1,885 $/tonne. Assuming an average price of 1042.5 $/tonne (Barr and Münch, 2001; Doyle and Parsons, 2002) then an annual income of (1042.5 $/tonne ∗ 315 tonnes/year =) $328,387/year can be estimated. The income from this activity will cover only ∼33% of the chemical cost requirements (amount of NaOH and Mg needed for 90% P recovery). For BF-WWTP this is (109,000/400 = 272.05∗25,000 = 6,812,500 €/year) with a chemical cost 20,643,939.39 €/year; thus based on historical data and assuming all values change over time at the same rate, the proposal is financially unviable (−20,643,939.39 €/year +6,812,500 €/year = −13,831,439.39) (Nethling and Benisch, 2004; Le Corre et al., 2009).
Currently the fertiliser market is dominated by phosphate rock, with 2020 price ∼65 €/t (i.e. much cheaper than the assumption used). Hence rock P fertiliser is by far more economic. However, if the sludge handling optimisation is taken into consideration, the reduction of sludge disposal can significantly impact the feasibility of controlled struvite recovery. The recovery of phosphorus can reduce the volume of sludge generated by 49%. For WWTPs treating 100 m3/d, 1,000 m3/d, and 55,000 m3/d of wastewaters, the sludge disposal cost reduction would be significant (Shu et al., 2006). For BF-WWTP, overall cost of cake disposal pre-COVID was ∼ €880 k with a total sludge production of 48,000 tonnes. Based on historical data, an annual saving of (374€/d ∗ 2 = 748 €/d ∗ 365 days/year =) 273,020 €/year could be achieved.
An Ostara reactor presents an annual operational cost of 574,000 €/year (Egle et al., 2016). In general, the cost arising from struvite precipitation problems within a medium size WWTP (25 MGD = 25 × 106 gallon/day ∗ 4.54 L/gallon = 113,500,000 L/day) has historically exceeded US $100 000 or 83,366.68 €/year. By taking into account the savings arising from the reduction of operational and maintenance cost due of struvite scaling, including chemical addition for chemical struvite precipitation, manpower, and maintenance costs, the overall saving could range from 1,470€ to 7,350€ per 4,540 m3 depending on the size of the treatment plant (Nethling and Benisch, 2004; Le Corre et al., 2009). If a mean price is assumed then the cost saving is estimated as 0.97 €/m3 of waste water. For Budds Farm WWTP cost saving can be estimated as (0.97 €/m3∗109,000 m3/day ∗ 365 days/year =) 38,591,450 €/year.
The investment cost for a controlled crystallisation reactor depends on the size of the treatment facilities. Investment cost for the recovery of P from the effluent and sludge can be 3,732,549 € and 1,417,739 € respectively, for a population of 100,000. BF-WWTP covers a total of 410,000 PE thus, investment cost can be up to 15,303,450.9 € and 5,812,729.9 € respectively (mean price = 10,558,089.95 €) (Montag et al., 2009).
For the determination of the environmental benefits, according to the results that Molinos-Senante et al. (2011) presented, the average value of the P shadow price is approximately −42.74 €/kg (meaning that for every kg of phosphorus that is not dumped into the environment, the damage prevented, or the environmental benefit generated equals €42.74). Base on the data set tested, the weighted average shadow price, depending on the volume of treated wastewater and it is approximately 0.218 €/m3. If it is considered that BF-WWTP presents an outfall of an average 2000 L/s and it operates for 24/7 for 365 days per year, then the shadow price arising from its treating capacity is (0.218 €/m3 ∗ 2000 L/s ∗3600 s/min ∗60 min/h ∗ 24 h/day ∗ 365 days/year =) 824,981,760,000 €/year.
Table 4 summarises the integrated evaluation of the 3 proposed technologies by taking into account all the financial end environmental associated aspects.
Table 4.
Costs, benefits, and feasibility estimations for struvite chemical precipitation, utilisation of saline water and sand as auto-nucleation media in combination with chemical precipitation and Ostara controlled struvite recovery reactor.
| Mean (€ year/L) | |
|---|---|
| Chemical precipitation of struvite | |
| General WWTP operation and maintenance cost including chemical requirements. | 43,365,650 |
| Chemical cost requirements. | 20,643,939 |
| Water sale. | 12,484,533 |
| Struvite sale. | 6,812,500 |
| Savings regarding struvite precipitation. | 83,367 |
| Environmental benefits. |
71,215,150 |
|
Chemical precipitation in combination with saline water and sand usage | |
| General WWTP operation and maintenance. | 39,462,741.2 |
| Struvite sale. | 6,812,500 |
| Water sale. | 12,484,533 |
| Savings regarding struvite precipitation. | 83,367 |
| Environmental benefits. |
>71,215,150 |
|
Ostara crystallization reactor | |
| General WWTP operation and maintenance cost. | 22,721,711 |
| Reactor operational and maintenance cost. | 574,000 |
| Investment cost | 10,558,090 |
| Water sale. | 12,484,533 |
| Struvite sale. | 6,812,500 |
| Sludge disposal savings | 273,020 |
| Savings regarding struvite precipitation. | 83,367 |
| Saving in comparison with chemical precipitation of struvite | 38,591,450 |
| Environmental benefits | 824,981,760,00 |
Based on the data extracted from the CBA, struvite recovery via chemical precipitation is not viable. This also applies for chemical precipitation in combination with saline water for Mg requirements and sand as auto-nucleation media. However, a crystallisation reactor is potentially more attractive. Considering a WWTP with treatment capacity 410,000 PE that recovers P in the form of struvite from digested sludge and its wastewater volume is 110,000 m3/day with a phosphorus concentration in the influent of 200 mg/L, this proposal is potentially economically viable with pay-back period of less than 10 years (assuming interest rate 6% and discount rate 3.5%).
However, when the average value of the EB is taken into account, phosphorus recovery is economically feasible even for the chemical struvite precipitation. Note that for the estimation of EB, only environmental benefits have been estimated, and not the increase in resource availability; if this impact was incorporated into the feasibility analysis, the results would be even more favourable.
5. Conclusions
Uncontrolled struvite formation is a significant operational problem that many WWTPs, especially those incorporating secondary treatment and anaerobic sludge digestion facilities, are required to overcome. Although uncontrolled formation of struvite can be a nuisance, controlled production and recovery of struvite can be beneficial for WWTPs. This study has demonstrated for the first time that struvite recovery from wastewater treatment is an economically viable circular economy opportunity.
Plants such as BF-WWTP provide an example that could potentially deliver a sustainable solution to this problem around the world due to its particular design and operational characteristics. Operational hotspots such as digester recirculation pumps, PDST, centrifuge feed, centrate and SAS storage tank demonstrate suitable conditions for P precipitation in the form of struvite. These locations present a positive SSR, optimum pH ran between 7 and 10.7 and a significant T variation indicating, based on the literature, potential struvite precipitation.
Controlled crystallisation process is one of the most promising P recovery technologies in the form of struvite. Using a fluidised bed reactor, this technology discharges the WWTP operational system from Mg:NH4:PO42− by creating optimum formation conditions. A crystallisation reactor seems to be the most robust technology for this process. Considering the great environmental and recovery performance of this proposal, a CBA based on the characteristics of BF-WWTP indicates that the pay-back period would be less than 10 years, especially given the likely increasing future energy costs and greenhouse gas emissions associated with fertiliser production. Of course, economic viability and payback periods will vary according to circumstances, but we recommend that WWTP operators globally consider fitting a crystallisation reactor to appropriate plants, The outcomes and recommendation from this study are particularly timely given the global fertiliser shortage (2022) that is driving up food prices and reducing crop sizes.
Declarations
Author contribution statement
Panayiotis Achilleos: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ian D. Williams: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Keiron Roberts: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abe S. Phosphate Removal from Dewatering Filtrate by MAP Process at Seibu Treatment Plant in Fukuoka City. Sew works Jpn. 1995:59–64. [Google Scholar]
- Barr K., Münch E. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res. 2001;35(1):151–159. doi: 10.1016/s0043-1354(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Battistoni P., Boccadoro R., Fatone F., Pavan P. Auto-nucleation and crystal growth of struvite in a demonstrative fluidised bed reactor (FBR) Environ. Technol. 2005;26:975–982. doi: 10.1080/09593332608618486. [DOI] [PubMed] [Google Scholar]
- Battistoni P., Fava G., Pavan P., Musacco A., Cecchi F. Phosphate removal in anaerobic liquors by struvite crystallization without addition of chemicals: preliminary results. Water Res. 1997;31(11):2925–2929. [Google Scholar]
- Battistoni P., Pavan P., Prisciandaro M., Cecchi F. Struvite crystallization: a feasible and reliable way to fix phosphorus in anaerobic supernatants. Water Res. 2000;34:3033–3041. [Google Scholar]
- BBC News . 2021. Southern Water fined record £90m for dumping raw sewage.https://www.bbc.co.uk/news/uk-england-kent-57777935 Available at: [Google Scholar]
- BBC News . 2021. Drone captures sewage pumped into sea for days.https://www.bbc.co.uk/news/av/uk-59050129 Available at: [Google Scholar]
- Bhuiyan M.I.,H. UENSCO-IHE Institute for Water Education, Faculty of Civil Engineering, University of British Columbia; 2007. Investigation into Struvite Solubility, Growth and Dissolution Kinetics in the Context of Phosphorus Recovery from Wastewater. MSc thesis. [Google Scholar]
- Bird Amanda R. 2015. Evaluation of the Feasibility of Struvite Precipitation from Domestic Wastewater as an Alternative Phosphorus Fertilizer Resource.https://repository.usfca.edu/capstone/141/ Master's Projects. Paper 141. Available at: (Last accessed 16 January 2022) [Google Scholar]
- Booram C.V., Smith R.J., Hazen T.E. Crystalline phosphate precipitation from anaerobic animal waste treatment lagoon liquors. Trans Am Soc Agric Eng. 1975;18:340–343. [Google Scholar]
- Bouropoulos N., Koutsoukos P. Spontaneous precipitation of struvite from aqueous solutions. J. Cryst. Growth. 2000;213(3-4):381–388. [Google Scholar]
- Britton A., Koch F.A., Adnan A., Oldham W.K., Mavinic D.S. Pilot-scale struvite recovery from anaerobic digester supernatant at an enhanced biological phosphorus removal wastewater treatment plant. J. Environ. Eng. Sci. 2005;4:265–277. [Google Scholar]
- Cullen N., Baur R., Schauer P. Three years of operation of North America’s first nutrient recovery facility. Water Sci. Technol. 2013;68:763–768. doi: 10.2166/wst.2013.260. [DOI] [PubMed] [Google Scholar]
- De-Bashan L., Bashan Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003) Water Res. 2004;38(19):4222–4246. doi: 10.1016/j.watres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Doyle J., Parsons S. Struvite formation, control and recovery. Water Res. 2002;36(16):3925–3940. doi: 10.1016/s0043-1354(02)00126-4. [DOI] [PubMed] [Google Scholar]
- Egle L., Rechberger H., Krampe J., Zessner M. Phosphorus recovery from municipal wastewater: an integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016;571:522–542. doi: 10.1016/j.scitotenv.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Fattah K. Assessing struvite formation potential at wastewater treatment plants. Int. J. Environ. Sci. Dev. 2012:548–552. [Google Scholar]
- Fattah K., Zhang Y., Mavinic D., Koch F. Use of carbon dioxide stripping for struvite crystallization to save caustic dosage: performance at pilot scale operation. Can. J. Civ. Eng. 2010;37(9):1271–1275. [Google Scholar]
- Fujimoto N., Mizuochi T., Togami Y. Phosphorus fixation in the sludge treatment system of a biological phosphorus removal process. Water Sci. Technol. 1991;23:635–640. [Google Scholar]
- Jaffer Y., Clark T., Pearce P., Parsons S. Potential phosphorus recovery by struvite formation. Water Res. 2002;36(7):1834–1842. doi: 10.1016/s0043-1354(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Kofina A., Koutsoukos P. Spontaneous precipitation of struvite from synthetic wastewater solutions. Cryst. Growth Des. 2005;5(2):489–496. [Google Scholar]
- Kratz S., Haneklaus S., Schnug E. Chemical solubility and agricultural performance of P-containing recycling fertilizers. Agric. For. Res. 2010;4(60):227–240. [Google Scholar]
- Le Corre K., Valsami-Jones E., Hobbs P., Parsons S. Phosphorus recovery from wastewater by struvite crystallization: a review. Crit. Rev. Environ. Sci. Technol. 2009;39(6):433–477. [Google Scholar]
- Macherey-Nagel, Quantofix Test Strips Ammonium, Phosphate. http://www.mn-net.com/tabid/4928/Default.aspx (Date of last access 22/09/2017)
- Macherey-Nagel VISOCOLOR®ECOHardness (Total) http://www.mn-net.com/StartpageWaterAnalysisTesting/VISOCOLOR/VisocolorECO/VISOCOLORECOtotalhardness/tabid/4794/language/en-US/Default.aspx (Date of last access 22/09/2017)
- Mavhungu A., Foteinis S., Mbaya R., Masindi V., Kortidis I., Mpenyana-Monyatsi L., Chatzisymeon E. Environmental sustainability of municipal wastewater treatment through struvite precipitation: influence of operational parameters. J. Clean. Prod. 2021;285 2021. [Google Scholar]
- Mamais D., Pitt P., Cheng Y., Loiacono J., Jenkins D. Determination of ferric chloride dose to control struvite precipitation in anaerobic sludge digesters. Water Environ. Res. 1994;66(7):912–918. [Google Scholar]
- Marti N., Bouzas A., Seco A., Ferrer J. Struvite precipitation assessment in anaerobic digestion processes. Chem. Eng. J. 2008;141(1-3):67–74. [Google Scholar]
- Merlo R. Technical Memorandum 4.6, Brown and Caldwell, Project No: 136242-003, California. 2011. Struvite formation and control evaluation, fog evaluation, digester rehabilitation and gas line replacement. [Google Scholar]
- Michałowski T., Pietrzyk A. A thermodynamic study of struvite & water system. Talanta. 2006;68(3):594–601. doi: 10.1016/j.talanta.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Molinos-Senante M., Hernández-Sancho F., Sala-Garrido R. Economic feasibility study for wastewater treatment: a cost–benefit analysis. Sci. Total Environ. 2010;408(20):4396–4402. doi: 10.1016/j.scitotenv.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Molinos-Senante M., Hernández-Sancho F., Sala-Garrido R., Garrido-Baserba M. Economic feasibility study for phosphorus recovery processes. Ambio. 2011;40(4):408–416. doi: 10.1007/s13280-010-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D., Gethke K., Pinnekamp J. In: International Conference on Nutrient Recovery from Wastewater Streams. Ashley K., Mavinic D., Koch F., editors. IWA Publishing; London, UK: 2009. Different strategies for recovering phosphorus: technologies and costs. [Google Scholar]
- Morse G., Brett S., Guy J., Lester J. Review: phosphorus removal and recovery technologies. Sci. Total Environ. 1998;212(1):69–81. [Google Scholar]
- Mudragada R., Kundral S., Coro E., Moncholi M., Laha S., Tansel B. Phosphorous removal during sludge dewatering to prevent struvite formation in sludge digesters by full scale evaluation. J. Water Proc. Eng. 2014;2:37–42. [Google Scholar]
- Musvoto E.V., Wentzel M.C., Ekama G.A. Integrated chemical–physical process modelling II. Development of a kinetic based model for weak acid/base systems. Water Res. 1999;34:1868–1880. 2000. [Google Scholar]
- Nethling J.B., Benisch M. Struvite control through process and facility design as well as operation strategy. Water Sci Technology. 2004;49(2):191–199. [PubMed] [Google Scholar]
- Ohlinger K., Young T., Schroeder E. Predicting struvite formation in digestion. Water Res. 1999;32(12):3607–3614. [Google Scholar]
- Pass Extech DO700 Portable Dissolved Oxygen Meter. https://www.tester.co.uk/extech-do700-portable-dissolved-oxygen-meter (Date of last access 22/09/2017)
- Pastor L., Marti N., Bouzas A., Seco A. Sewage sludge management for phosphorus recovery as struvite in EBPR wastewater treatment plants. Bioresour. Technol. 2008;99(11):4817–4824. doi: 10.1016/j.biortech.2007.09.054. [DOI] [PubMed] [Google Scholar]
- Patoczka J. The New Jersey Dept. of Environmental Industrial Water World; 2005. Achieving 0.1 Mg/L Phosphorous Limit with Two-Staged Chemical Addition. [Google Scholar]
- Roberts T., Williams I., Preston J., Clarke N., Odum M., O’Gorman S. A virtuous circle? Increasing local benefits from ports by adopting circular economy principles. Sustainability. 2021;13:7079. [Google Scholar]
- Sena M., Seib M., Noguera D.R., Hicks A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 2021;280(Part 1) 2021. [Google Scholar]
- Shu L., Schneider P., Jegatheesan V., Johnson J. An economic evaluation of phosphorus recovery as struvite from digester supernatant. Bioresour. Technol. 2006;97(17):2211–2216. doi: 10.1016/j.biortech.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Siciliano A., Limonti C., Curcio G.M., Molinari R. Advances in struvite precipitation technologies for nutrients removal and recovery from aqueous waste and wastewater. Sustainability. 2020;12:7538. [Google Scholar]
- Smith V., Tilman G., Nekola J. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999;100(1-3):179–196. doi: 10.1016/s0269-7491(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Snoeyink V., Jenkins D. John Wiley & Sons; New York, NY: 1980. Water Chemistry. [Google Scholar]
- Williams S. Struvite precipitation in the sludge stream at slough wastewater treatment plant and opportunities for phosphorus recovery. Environ. Technol. 1999;20(7):743–747. [Google Scholar]
- Wu Q., Bishop P. Enhancing struvite crystallization from anaerobic supernatant. J. Environ. Eng. Sci. 2004;3(1):21–29. [Google Scholar]
- Xavier L., Cammarota M., Yokoyama L., Volschan I. Study of the recovery of phosphorus from struvite precipitation in supernatant line from anaerobic digesters of sludge. Water Sci. Technol. 2014;69(7):1546. doi: 10.2166/wst.2014.033. [DOI] [PubMed] [Google Scholar]
- Xie M., Shon H., Gray S., Elimelech M. Membrane-based processes for wastewater nutrient recovery: technology, challenges, and future direction. Water Res. 2016;89:210–221. doi: 10.1016/j.watres.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Mo W. Energy–nutrients–water nexus: integrated resource recovery in municipal wastewater treatment plants. J. Environ. Manag. 2013;127:255–267. doi: 10.1016/j.jenvman.2013.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.