Abstract
Aflatoxins are common food contaminants threating human and animal health. Aflatoxin B1 (AFB1) toxication can lead to important health issues. Recent studies have revealed the therapeutic effect of curcumin (Cur) and have drawn attention in the pharmaceutical industry. The therapeutic efficacy of Cur on AFB1-induced oxidative stress, pro-inflammatory response, and hepatorenal damage has not been adequately studied. This study was conducted to evaluate the protective efficacy of Cur on several lipid peroxidation and antioxidant defense system enzymes, some pro-inflammatory cytokines, and liver function tests in rats suffering from chronic aflatoxicosis induced by AFB1 administered for sixty days. Rats were divided into five groups; Control (K), Dimethyl sulfoxide (D), Curcumin (Cur; 300 mg/kg/day, orally), AFB1 (AF; 250 μg/kg/day, oral) and AFB1+ Curcumin (AF + Cur). Oxidative stress caused by AFB1 caused an increase in Malondialdehyde (MDA), a lipid peroxidation product, and a decrease in glutathione (GSH) and superoxide dismutase (SOD) activities. In addition, AFB1 led to increased levels of pro-inflammatory cytokines such as tumor necrosis factor-a (TNF-a), interleukin-1b (IL-1b), and interleukin-6 (IL-6). Liver function tests after chronic exposure to AFB1 showed that this toxic substance causes liver damage. Concomitant Cur administration normalized AFB1-induced oxidative damage, inflammatory response, and liver functions. This therapeutic effect of Cur on AFB1 was thought to be related to its antioxidant and anti-inflammatory activities. Our results suggest that CUR supplementation in food as it shows beneficial effects particularly on liver impairment exerted by AFB1.
Keywords: AFB1, Curcumin, Liver enzymes, Oxidative stress, Cytokines
Graphical abstract
AFB1; Curcumin; Liver enzymes; Oxidative stress; Cytokines.
1. Introduction
Mycotoxins are substances that cause significant health problems in both humans and animals by contaminating feed raw materials and foods in many countries of the world [1]. Among the hundreds of mycotoxins identified, aflatoxins (AFs) produced by Aspergillus flavus and Aspergillus parasiticus, which pose a significant public health problem, are considered critical food pollutants in hot and humid climates such as Central America, Asia, and Africa [2]. Aflatoxin B1 (AFB1), the most potent member of the AF family, is classified as a group 1 carcinogen by the International Agency for Research on Cancer (IARC) [3]. AFB1 also has mutagenic, genotoxic, teratogenic, immunotoxic, nephrotoxic effects [4, 5].
After AFs are taken into the body, 75% are kept in the feces, 15–20% in the urine, and 5–6% in the liver within the first 24 h, while the rest is excreted in milk as metabolites or unchanged [6, 7, 8]. AFs mainly target the liver, causing hepatocellular damage [9]. It was reported that AFB1 which is formed after being metabolized, causes damage to DNA and different cellular proteins through its toxic metabolites such as AFB1-8,9 epoxide (AFBO) [10, 11], and disrupts protein and lipid metabolism by stimulating the production of reactive oxygen species (ROS) [12]. It is unclear whether AFB1 directly stimulates lipid peroxidation through increased ROS synthesis or the increased sensitivity of tissue to peroxidation results from impaired antioxidant defense, but both mechanisms are probably involved [13] AFB1 is involved in antioxidant enzyme activity (GSH, SOD and CAT etc.) [14] decrease and in cell membrane damage by increasing the level of malondialdehyde (MDA), a product of lipid peroxidation [13].
It is unclear how aflatoxin and other mycotoxins affect immunomodulation. Mycotoxins are said to affect many different mechanisms and cause immunosuppression by inhibiting DNA, RNA, and protein synthesis [15, 16]. According to research, AFB1 modulates cytokine production to exert immunomodulatory effects [17]. Aflatoxin B1 may also be able to stop the activation of some kinases that play a role in the activation of genes that make cytokines [18, 19] (Rossano ve diğerleri, 1999; Liu ve diğerleri, 2002).
Physical, chemical, and biological means are used for the decontamination of feeds contaminated with AF. Sodium calcium aluminosilicate, activated charcoal, glucomannan, clay, zeolite, sodium bentonite, clinoptilolite, black cumin seed as an antioxidant, and amino acids containing thymoquinone, Vitamin A, C, E, and sulfhydryl groups (methionine, cysteine, N-acetyl cysteine) are frequently used to prevent aflatoxicosis [10, 20, 21].
Researchers noted that many extracts from herbs and plants can prevent AFB1 toxicity [22, 23]. Curcumin, Curcuma longa L., is a popular spice and food additive widely used in South Asian and Middle Eastern countries [24]. In recent studies, it was reported that curcumin targets over one signal molecule and has potent antioxidant and anti-inflammatory properties by showing activity at the cellular level [25]. Besides, curcumin has a protective effect against various neurological, cardiovascular, and metabolic diseases thanks to its antiviral, antiallergic, anticarcinogenic, antibacterial properties [26]. Besides all these effects, it is reported that curcumin provides efficient protection against the toxicity caused by AFB1, but further studies should be done on this subject [14].
The aflatoxicosis problem remains up to date worldwide. Since this toxic substance is consumed accidently, it is taken with food by humans and animals, causing many harmful effects. This study aimed to determine the protective effect of curcumin on the oxidant/antioxidant balance, liver functions, and pro-inflammatory response, which is impaired because of AFB1 exposure.
2. Materials and methods
2.1. Chemicals
AFB1 (≥99%) was supplied by Acros Organics [Gell, Belgium (Cat. No: 227340100)]. Curcumin (≥99%) was purchased by Sigma Aldrich [St. Louis, MO, USA (Cat. No: C1386)]. Malondialdehyde (MDA, Cat. No: E0156Ra), glutathione (GSH, Cat. No: E1101Ra), superoxide dismutase (SOD, Cat. No: E0168Ra) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Bioassay Technology Laboratory (Shangai, China). Tumor necrosis factor-alpha (TNF-α, Cat. No: KRC3011), interleukin-1 beta (IL1β, Cat. No: BMS630), interleukin-6 (IL-6, Cat. No: BMS625) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Thermo Fisher Scientific Inc. (USA). Alanine transaminase (ALT, Cat. No: DF143), aspartate transaminase (AST, Cat. No: DF41A), alkaline phosphatase (ALP, Cat. No: DC150), and gamma-glutamyl transferase (GGT, Cat. No: DF45A) kits were obtained from Siemens Medical System (Erlangen, Germany).
2.2. Animals
Thirty-eight male Wistar albino rats (34–36 g) were used in the investigation. Selcuk University Experimental Application and Research Center provided the animal material. Before beginning the study, the animals' general health state was examined, their body weights were determined, and they were separated into five groups based on their average body weight. The rats were kept in plastic rat cages throughout the study (60 days), with 12/12 day-night light cycles and a room temperature of 23±2 °C were housed ad libitum.
2.3. Experimental design
This study protocol was approved by Selcuk University Experimental Medicine Research and Application Center Ethics Committee (Report no. 2018-26). Rats were weighed before the start of the experiment. They were divided into five groups, with the mean body weight of each group being equal. Group I (Control (K), n = 6) animals were fed ad libitum; Group II (DMSO (D), n = 6) 1 ml 10% DMSO; Group III (Curcumin (Cur), n = 6) 300 mg/kg curcumin [27]; Group IV (AFB1 (AF), n = 10) 250 μg/kg AFB1 [28]; Group V (AF + Cur, n = 10) 250 μg/kg AFB1 + 300 mg/kg curcumin. The trial period was ended on the 60th day, and all applications were administered orally to the animals. AFB1 and curcumin were dissolved in 10% DMSO and made ready for use. At the end of the 60th day, sufficient blood was taken from the heart of all groups of animals under general anesthesia (Xylazine 10 mg/kg and Ketamine 5 mg/kg). The blood was collected in serum (BD Vacutainer SSTTM II Advance-367953) tubes and centrifuged at 4500 rpm for 10 min at +4 °C (Hettich Universal 32R). Serum samples were stored at -80 °C in Eppendorf tubes until analysis.
2.4. Analysis of lipid peroxidation and antioxidant enzyme activities
The targeted MDA, GSH, and SOD levels were measured using the ELISA method, using commercial kits, following the methods specified in the product catalog [29]. Spectrophotometric measurements were performed using ELx800 (Bio-Tek Instruments, Winooski, VT, USA) at a wavelength of 450 nm.
2.5. Analysis of pro-inflammatory parameters
In serum samples, pro-inflammatory cytokines were quantified using commercial ELISA kits. TNF-α, IL-6, IL-1β levels were measured with anti-rat ELISA kits according to the manufacturer's instructions, as mentioned above [30]. The concentration units were expressed as pg/mL.
2.6. Analysis of liver enzymes
ALT, AST, ALP, and GGT levels were measured in Siemens CentaurXP Immunoassay System following the package inserts using commercial kits from sera stored at -80οC until the time of analysis.
2.7. Data analysis
The SPSS 20.00 package program was used to conduct a statistical analysis of the data gathered at the study's end and determine the significance of the differences between groups. Visual and analytical methods were used to analyze variables for normal distribution. All variables were reported as mean and standard deviations. The groups were compared using a one-way ANOVA test. Following the determination of variance homogeneity, where the p-value was less than 0.05, pairwise post hoc comparisons (Tukey) were employed to test the significance of the groups, and Duncan's Multiple Range test was used in the analysis of variance.
3. Results
3.1. Evaluation of MDA, GSH, and SOD levels
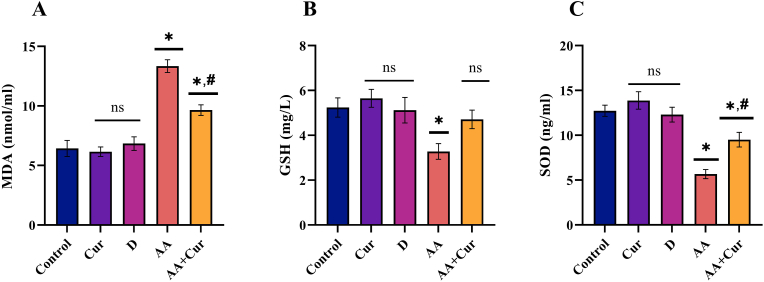
The results of the present study shows that the amount of MDA, which is the end product of lipid peroxidation, was significantly greater in the AF group than in the other four experimental groups (K, Cur, D, and AF + Cur) (p < 0.05). Following curcumin application, the MDA level obtained from the AF + Cur group was found to be statistically lower than that of the AF group, although it was statistically greater than the values obtained from the K and Cur groups (p < 0.05) (Figure 1).
Figure 1.
Effect of curcumin on serum MDA, GSH and SOD levels in rats administered orally Aflatoxin B1 (X ± SEM) (Statistically significant (p < 0,05) differences are indicated by ∗ when compared to the control group, and statistically significant differences (p < 0,05) are indicated by # when compared with the AA group).
GSH level was found to be significantly lower in the AF group compared to the Control group (p < 0.05). The AF + Cur group showed an increase in GSH level increased compared to the AF group and reached similar levels to the control, Cur, and D groups (p < 0.05) (Figure 1).
The AF group's serum SOD level was statistically lower than all other groups (p < 0.05). At the same time, the SOD level in the AF + Cur group was statistically lower than in the K group (p < 0.05), it was found to be statistically greater than in the AF (p < 0.05) (Figure 1).
3.2. Evaluation of pro-inflammatory cytokine levels
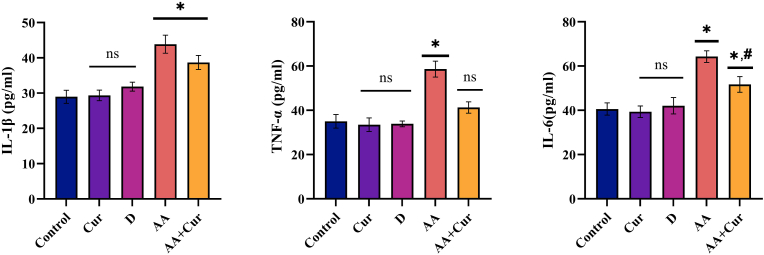
Results on cytokine level show that IL-1β levels were statistically greater in the AF and AF + Cur groups than in the K, Cur, and D groups (p < 0.05). Although it was not statistically significant, it was noted that the IL-1β level decreased in the AF + Cur group compared to the AF group (p > 0.05) (Figure 2).
Figure 2.
Effect of curcumin on serum pro-inflammatory cytokine levels in rats administered orally Aflatoxin B1 (X ± SEM) (Statistically significant (p < 0,05) differences are indicated by ∗ when compared to the control group, and statistically significant differences (p < 0,05) are indicated by # when compared with the AA group).
TNF-α levels in the AF group were statistically higher than in the other four experimental groups (p < 0.05) TNF-α levels were found to be statistically lower in the AF + Cur group than in the AF group (p < 0.05), and no significant difference was identified among the other three groups (K, Cur, D) (p > 0.05) (Figure 2).
In terms of IL-6, the value obtained in the AF group was significantly greater than in the other four groups (p < 0.05) IL-6 level of the AF + Cur group was statistically lower than that of the AF group (p < 0.05) (Figure 2).
3.3. Evaluation of liver functions
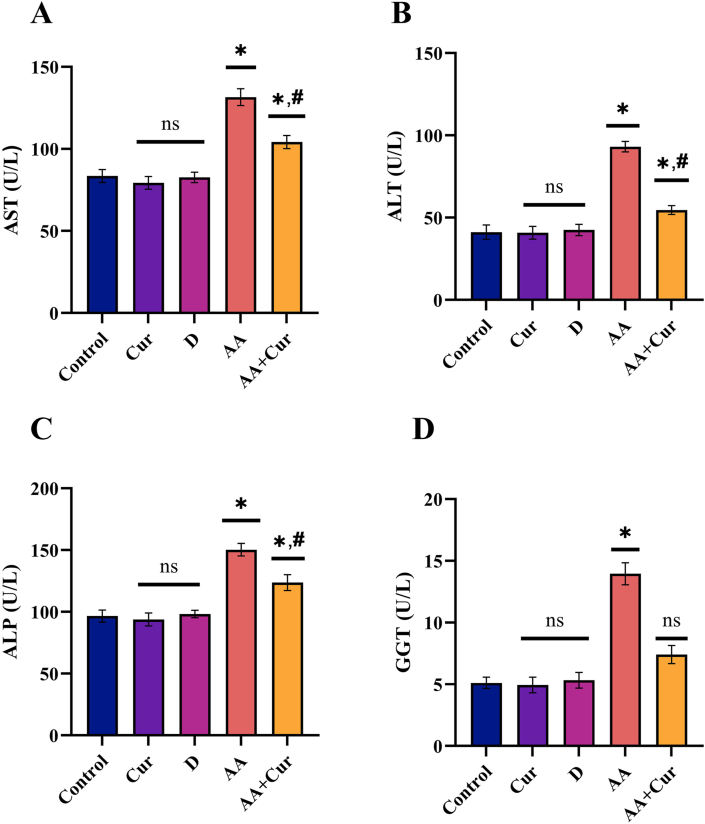
Serum AST, ALT, ALP, and GGT levels were significantly greater in the AF group than in the other groups (K, Cur, D, AF + Cur) (p < 0.05). Serum AST, ALT, ALP, and GGT levels were statistically lower in the AF + Cur group than in the AF group (p < 0.05). When the results from the Cur and DMSO groups were compared to the K group, there was no significant difference in enzyme levels (p > 0.05) (Figure 3).
Figure 3.
Effect of curcumin on serum liver enzyme levels in rats administered orally Aflatoxin B1 (X ± SEM) (Statistically significant (p < 0,05) differences are indicated by ∗ when compared to the control group, and statistically significant differences (p < 0,05) are indicated by # when compared with the AA group).
4. Discussion
It has been reported that aflatoxins cause inhibition of antioxidant enzymes and anti-inflammatory cytokines, lipid peroxidation, stimulation of proinflammatory cytokines and apoptosis of hepatocytes [31, 32]. It was reported that curcumin targets more than one signal molecule and has strong antioxidant and anti-inflammatory properties by showing activity at the cellular level [33, 34]. In the study investigating the protective effect of curcumin on aflatoxicosis the effects on liver function tests such as ALT, AST, ALP, and GGT, lipid peroxidation product such as MDA, antioxidant defense system members such as GSH and SOD, and pro-inflammatory cytokines such as TNF-a, IL-1β, and IL-6 were investigated.
AFB1 has been reported to induce DNA damage by oxidative stress, which occurs through the production of ROS, which indirectly attacks membrane phospholipids and releases different mutagenic aldehydes [2]. In this study, it is suggested that the increase in MDA level in the aflatoxicosis-induced group may be caused by the formation of free radicals that cannot be tolerated by the antioxidant defense system (Figure 1). The increase in MDA level can be considered as evidence of lipid peroxidation caused by the deficiency in the antioxidant defense system or the increase in free radicals [35]. The curative effect of curcumin against AFB1-induced lipid peroxidation was consistent with previous reports [36, 37]. Numerous studies have shown that curcumin is a potent scavenger of various reactive oxygen species, including hydroxyl radicals [38] and nitrogen dioxide radicals, and inhibits lipid peroxidation, which leads to cell damage and cell death in different animal models [39, 40, 41].
It is stated that antioxidant defense system members such as GSH and SOD play a significant role in the detoxification of reactive and toxic metabolites of AFs, and chronic exposure may cause a decrease in the level of these antioxidants [42, 43, 44]. In our study, it was determined that the decrease in GSH levels and in SOD activity have been improved by adding curcumin to the diet (Figure 1). There are antioxidant defense system members such as SOD, CAT, and GSH that suppress or prevent the formation of free radicals and reactive species in cells [45]. Based on the findings obtained from the present study, we concluded that curcumin can be used as a therapeutic agent to return the decreased GSH and SOD levels because of AFB1 exposure to physiological limits and to prevent the increase of lipid peroxidation products.
Inflammation and immune response are particularly important in the early response to tissue damage, and therefore a measurement of serum cytokines is used as a biomarker in routine toxicity studies [46, 47]. In parallel with other studies, in the current study, it was found that AFB1 increased IL-1β, TNF-α, and IL-6 levels [48, 49, 50] (Figure 2). This is considered as an indication that systemic inflammation is triggered [51]. It was determined that curcumin decreased TNF-α and IL-6 levels statistically significantly compared to the AF group. This decrease in IL-1B level in the AF group was not statistically significant (Figure 2). Curcumin has been reported to block pro-inflammatory cytokines through mitogen-interacting protein kinases (MAPK), signal transducer and activator of transcription 3 (STAT3), extracellular signal-regulated kinases (ERK), and NF-κB [52, 53, 54]. In our study, it was thought that the inhibition of these pathways activated by AFB1 by curcumin might have resulted in a decrease in the levels of these cytokines.
Increased serum ALT, AST, ALP and GGT activities are accepted as diagnostic indicators of liver injury. Elevated liver enzymes suggest hepatic parenchymal cell damage (ALT and AST) or degenerative changes in the bile duct (ALP). According to the findings we obtained as a result of the study, the increase in AST and ALT levels in the blood due to the loss of structural integrity of hepatocytes in the AF group indicates that a certain liver damage has occurred. Because AST and ALT, which are normally found in the cytoplasm of hepatocytes, are released into the circulatory system only when the structural integrity of the liver is affected [55]. AFB1 leads to disruption of membrane permeability and mitochondrial membrane in hepatocytes, resulting in increased levels of ALT, AST and GGT. Besides, the increase in ALP level caused by AFB1 also shows that biliary excretion is inhibited (Wang et al 2019). Abdel-Wahhab et al. (2016), report that ALT and AST levels are higher in AF group than in the control group and that curcumin treatment reduces the levels of these enzymes compared to the AFB1 group [56]. El-Agamy (2010) and El-Bahr (2015) reported that ALT, AST, and GGT levels increased significantly in rats with experimental aflatoxicosis, and that oral curcumin treatment applied to these animals resulted in significant improvement in the levels of these enzymes, similar to the current study [14, 57]. Curcumin is thought to protect the liver against oxidative damage caused by AFB1 thanks to its ability to scavenge reactive oxygen species by suppressing oxidative enzymes, normalizing antioxidant levels, and chelating metal ions [57].
5. Conclusion
AFB1 administered orally to rats had negative effects on liver functions, pro-inflammatory cytokines, and antioxidants. In our study, oral curcumin supplementation showed a hepatoprotective effect against AFB1 by supporting the antioxidant system and regulating liver functions. Curcumin supplementation also played a role in providing immunomodulation by improving pro-inflammatory cytokine levels. The development of new methods and strategies that increase the bioavailability of curcumin may provide further protection against liver damage caused by aflatoxin. Still, further research on the physiological, cellular, and molecular mechanisms involved in liver protection is needed to suggest it as a potential therapeutic agent against oxidative damage-induced liver injury because of exposure to aflatoxin.
Declarations
Author contribution statement
Durmus Hatipoglu & Ercan Keskin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Selcuk University Scientific Research Projects Coordination Office (Project No: 18102035).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The study was generated from some part of the first author's Ph.D. thesis entitled "The Effect of Curcumin on Some Liver Enzymes, Cytokines, and Renal Functions in Rats Given Orally Aflatoxin B1.”
References
- 1.Wu F. Global impacts of aflatoxin in maize: trade and human health. World Mycotoxin J. 2015;8:137–142. [Google Scholar]
- 2.Benkerroum N. Chronic and acute toxicities of aflatoxins: mechanisms of action. Int. J. Environ. Res. Publ. Health. 2020;17:423. doi: 10.3390/ijerph17020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC . 2012. A Review of Human Carcinogens. F. Chemical Agents and Related Occupations: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar P., Mahato D.K., Kamle M., Mohanta T.K., Kang S.G. Aflatoxins: a global concern for food safety, human health and their management. Front. Microbiol. 2017;7:2170. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samah Abou Asa E., Nahass W.A.M., Nour El-Houda Y. Aflatoxin induced renal toxicity in Albino rats and the ameliorative effect of green tea aqueous extract: histological, Morphometric and Immunohisto-chemical Study. J. Exp. Appl. Animal Sci. 2018;2:273–285. [Google Scholar]
- 6.Jager A.V., Ramalho F.S., Zambelli L.N., Oliveira C.A.F. Biomarkers of aflatoxin exposure and its relationship with the hepatocellular carcinoma. Aflatoxins Biochem. Mol. Biol. 2011:107–126. [Google Scholar]
- 7.Vidyasagar T., Vyjayanthi V., Sujatiia N., Rao B.S., Bhat R.V. Quantitation of aflatoxin B1-N7-guanine adduct in urine by enzyme-linked immunosorbent assay coupled with immunoaffinity chromatography. J. AOAC Int. 1997;80:1013–1022. [PubMed] [Google Scholar]
- 8.Mykkänen H., Zhu H., Salminen E., Juvonen R.O., Ling W., Ma J., Polychronaki N., Kemiläinen H., Mykkänen O., Salminen S., El-Nezami H. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB-N7-guanine) in young Chinese males. Int. J. Cancer. 2005;115:879–884. doi: 10.1002/ijc.20951. [DOI] [PubMed] [Google Scholar]
- 9.Ortatatli M., Oğuz H., Hatipoğlu F., Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005;78:61–68. doi: 10.1016/j.rvsc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Ates M.B., Ortatatli M. The effects of Nigella sativa seeds and thymoquinone on aflatoxin phase-2 detoxification through glutathione and glutathione-S-transferase alpha-3, and the relationship between aflatoxin B1-DNA adducts in broilers. Toxicon. 2021;193:86–92. doi: 10.1016/j.toxicon.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Mungamuri S.K., Mavuduru V.A. Role of epigenetic alterations in aflatoxin-induced hepatocellular carcinoma. Liver Cancer Int. 2020;1:41–50. [Google Scholar]
- 12.de Freitas Souza C., Baldissera M.D., Baldisserotto B., Petrolli T.G., da Glória E.M., Zanette R.A., Da Silva A.S. Dietary vegetable choline improves hepatic health of Nile tilapia (Oreochromis niloticus) fed aflatoxin-contaminated diet. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;227 doi: 10.1016/j.cbpc.2019.108614. [DOI] [PubMed] [Google Scholar]
- 13.Marin D.E., Taranu I. Overview on aflatoxins and oxidative stress. Toxin Rev. 2012;31:32–43. [Google Scholar]
- 14.El-Bahr S.M. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin B1. Phytother Res. 2015;29:134–140. doi: 10.1002/ptr.5239. [DOI] [PubMed] [Google Scholar]
- 15.Corrier D. Mycotoxicosis: mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 1991;30:73–87. doi: 10.1016/0165-2427(91)90010-a. [DOI] [PubMed] [Google Scholar]
- 16.Berek L., Petri I.B., Mesterhazy A., Téren J., Molnár J. Effects of mycotoxins on human immune functions in vitro. Toxicol. Vitro. 2001;15:25–30. doi: 10.1016/s0887-2333(00)00055-2. [DOI] [PubMed] [Google Scholar]
- 17.Bruneau J.C., Stack E., O’Kennedy R., Loscher C.E. Aflatoxins B1, B2 and G1 modulate cytokine secretion and cell surface marker expression in J774A. 1 murine macrophages. Toxicol. Vitro. 2012;26:686–693. doi: 10.1016/j.tiv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Rossano F., De Luna L.O., Buommino E., Cusumano V., Losi E., Catania M.R. Secondary metabolites of Aspergillus exert immunobiological effects on human monocytes. Res. Microbiol. 1999;150:13–19. doi: 10.1016/s0923-2508(99)80042-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X., Gan F., Hou L., Liu Z., Su J., Lin Z., Le G., Huang K. Aflatoxin B1 induces immunotoxicity through the DNA methyltransferase-mediated JAK2/STAT3 pathway in 3D4/21 cells. J. Agric. Food Chem. 2019;67:3772–3780. doi: 10.1021/acs.jafc.8b07309. [DOI] [PubMed] [Google Scholar]
- 20.Dönmez N., Dönmez H., Keskin E., Kısadere I. Effects of aflatoxin on some haematological parameters and protective effectiveness of esterified glucomannan in Merino rams. Sci. World J. 2012:2012. doi: 10.1100/2012/342468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oğuz H., Kurtoğlu F., Kurtoğlu V., Bırdane Y. Evaluation of biochemical characters of broiler chickens during dietary aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2002;73:101–103. doi: 10.1016/s0034-5288(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 22.Ates M.B., Ortatatli M. Phase-1 bioactivation mechanisms of aflatoxin through AhR, CAR and PXR nuclear receptors and the interactions with Nigella sativa seeds and thymoquinone in broilers. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111774. [DOI] [PubMed] [Google Scholar]
- 23.Li S., Liu R., Wei G., Guo G., Yu H., Zhang Y., Ishfaq M., Fazilani S.A., Zhang X. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111725. [DOI] [PubMed] [Google Scholar]
- 24.Nair K.P. Springer; 2019. Turmeric (Curcuma Longa L.) and Ginger (Zingiber Officinale Rosc.)-World's Invaluable Medicinal Spices: the Agronomy and Economy of Turmeric and Ginger. [Google Scholar]
- 25.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewlings S.J., Kalman D.S. Curcumin: a review of its effects on human health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menozzi A., Pozzoli C., Poli E., Martelli M., Martelli L., Zullian C., Bertini S. Effects of oral curcumin on indomethacin-induced small intestinal damage in the rat. Drug Discov. Ther. 2009;3 [PubMed] [Google Scholar]
- 28.Tang L., Guan H., Ding X., Wang J.-S. Modulation of aflatoxin toxicity and biomarkers by lycopene in F344 rats. Toxicol. Appl. Pharmacol. 2007;219:10–17. doi: 10.1016/j.taap.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q., Su Y.-W., Xian C.J. Determining oxidative damage by lipid peroxidation assay in rat serum. Bio Protocol. 2019;9:e3263. doi: 10.21769/BioProtoc.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousuf S., Atif F., Sayeed I., Wang J., Stein D.G. Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: immunomodulation and neuroprotection by progesterone. Neuroscience. 2013;239:92–102. doi: 10.1016/j.neuroscience.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarru L.P., Settivari R.S., Gowda N.K.S., Antoniou E., Ledoux D.R., Rottinghaus G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poultry Sci. 2009;88:2620–2627. doi: 10.3382/ps.2009-00204. [DOI] [PubMed] [Google Scholar]
- 32.Huang L., Zhao Z., Duan C., Wang C., Zhao Y., Yang G., Gao L., Niu C., Xu J., Li S. Lactobacillus plantarum C88 protects against aflatoxin B1-induced liver injury in mice via inhibition of NF-κB–mediated inflammatory responses and excessive apoptosis. BMC Microbiol. 2019;19:1–9. doi: 10.1186/s12866-019-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards R.L., Luis P.B., Varuzza P.V., Joseph A.I., Presley S.H., Chaturvedi R., Schneider C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017;292:21243–21252. doi: 10.1074/jbc.RA117.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priyadarsini K.I., Maity D.K., Naik G., Kumar M.S., Unnikrishnan M., Satav J., Mohan H. Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003;35:475–484. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 35.Choi K.-C., Chung W.-T., Kwon J.-K., Yu J.-Y., Jang Y.-S., Park S.-M., Lee S.-Y., Lee J.-C. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food Chem. Toxicol. 2010;48:2747–2753. doi: 10.1016/j.fct.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Soliman G., Hashem A., Arafa M. Vol. 80. The Medical Journal of Cairo University; 2012. Protective Effect of Curcuma Longa or Nigella Sativa on Aflatoxin B1-Induced Hepato-Toxicity in Rats in Relation to Food Safety on Public Health. [Google Scholar]
- 37.Wang X.-h., Li W., Wang X.-h., Han M.-y., Muhammad I., Zhang X.-y., Sun X.-q., Cui X.-x. Water-soluble substances of wheat: a potential preventer of aflatoxin B1-induced liver damage in broilers. Poultry Sci. 2019;98:136–149. doi: 10.3382/ps/pey358. [DOI] [PubMed] [Google Scholar]
- 38.Aslanturk A., Uzunhisarcikli M. Protective potential of curcumin or taurine on nephrotoxicity caused by bisphenol A. Environ. Sci. Pollut. Control Ser. 2020;27:23994–24003. doi: 10.1007/s11356-020-08716-1. [DOI] [PubMed] [Google Scholar]
- 39.Jangra A., Kwatra M., Singh T., Pant R., Kushwah P., Sharma Y., Saroha B., Datusalia A.K., Bezbaruah B.K. Piperine augments the protective effect of curcumin against lipopolysaccharide-induced neurobehavioral and neurochemical deficits in mice. Inflammation. 2016;39:1025–1038. doi: 10.1007/s10753-016-0332-4. [DOI] [PubMed] [Google Scholar]
- 40.Agnihotri N., Mishra P. Scavenging mechanism of curcumin toward the hydroxyl radical: a theoretical study of reactions producing ferulic acid and vanillin. J. Phys. Chem. 2011;115:14221–14232. doi: 10.1021/jp209318f. [DOI] [PubMed] [Google Scholar]
- 41.Wei Q.-Y., Chen W.-F., Zhou B., Yang L., Liu Z.-L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta Gen. Subj. 2006;1760:70–77. doi: 10.1016/j.bbagen.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Abbès S., Ben Salah-Abbes J., Jebali R., Younes R.B., Oueslati R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: possible protective role using lactic acid bacteria. J. Immunot. 2016;13:46–54. doi: 10.3109/1547691X.2014.997905. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Wahhab M.A., Ahmed H., Hagazi H.,M.M. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J. Appl. Toxicol.: Int. J. 2006;26:229–238. doi: 10.1002/jat.1127. [DOI] [PubMed] [Google Scholar]
- 44.Mary V.S., Theumer M.G., Arias S.L., Rubinstein H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology. 2012;302:299–307. doi: 10.1016/j.tox.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Ighodaro O., Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54:287–293. [Google Scholar]
- 46.Kerns W., Schwartz L., Blanchard K., Burchiel S., Essayan D., Fung E., Johnson R., Lawton M., Louden C., MacGregor J. Drug-induced vascular injury--a quest for biomarkers. Toxicol. Appl. Pharmacol. 2005;203:62–87. doi: 10.1016/j.taap.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Lacour S., Gautier J.-C., Pallardy M., Roberts R. Cytokines as potential biomarkers of liver toxicity. Cancer Biomarkers. 2005;1:29–39. doi: 10.3233/cbm-2005-1105. [DOI] [PubMed] [Google Scholar]
- 48.Akinrinmade F.J., Akinrinde A.S., Amid A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Res. 2016;32:53–60. doi: 10.1007/s12550-016-0239-9. [DOI] [PubMed] [Google Scholar]
- 49.Chen J., Chen K., Yuan S., Peng X., Fang J., Wang F., Cui H., Chen Z., Yuan J., Geng Y. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicol. Ind. Health. 2016;32:278–284. doi: 10.1177/0748233713500819. [DOI] [PubMed] [Google Scholar]
- 50.Pauletto M., Giantin M., Tolosi R., Bassan I., Barbarossa A., Zaghini A., Dacasto M. Curcumin mitigates AFB1-induced hepatic toxicity by triggering cattle antioxidant and anti-inflammatory pathways: a whole transcriptomic in vitro study. Antioxidants. 2020;9:1059. doi: 10.3390/antiox9111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safieh-Garabedian B., Poole S., Allchorne A., Winter J., Woolf C.J. Contribution of interleukin-1β to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao X., Kuo J., Jiang H., Deeb D., Liu Y., Divine G., Chapman R.A., Dulchavsky S.A., Gautam S.C. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem. Pharmacol. 2004;68:51–61. doi: 10.1016/j.bcp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Panahi Y., Fazlolahzadeh O., Atkin S.L., Majeed M., Butler A.E., Johnston T.P., Sahebkar A. Evidence of curcumin and curcumin analogue effects in skin diseases: a narrative review. J. Cell. Physiol. 2019;234:1165–1178. doi: 10.1002/jcp.27096. [DOI] [PubMed] [Google Scholar]
- 54.Park J., Ayyappan V., Bae E.-K., Lee C., Kim B.-S., Kim B.K., Lee Y.-Y., Ahn K.-S., Yoon S.-S. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol. Oncol. 2008;2:317–326. doi: 10.1016/j.molonc.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salem R., El-Habashi N., Fadl S.E., Sakr O.A., Elbialy Z.I. Effect of probiotic supplement on aflatoxicosis and gene expression in the liver of broiler chicken. Environ. Toxicol. Pharmacol. 2018;60:118–127. doi: 10.1016/j.etap.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Abdel-Wahhab M.A., Salman A.S., Ibrahim M.I.M., El-Kady A.A., Abdel-Aziem S.H., Hassan N.S., Waly A.I. Curcumin nanoparticles loaded hydrogels protects against aflatoxin B1-induced genotoxicity in rat liver. Food Chem. Toxicol. 2016;94:159–171. doi: 10.1016/j.fct.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 57.El-Agamy D.S. Comparative effects of curcumin and resveratrol on aflatoxin B 1-induced liver injury in rats. Arch. Toxicol. 2010;84:389–396. doi: 10.1007/s00204-010-0511-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.