Abstract
The aim of our study was to test the hypothesis that administration of Regenerating islet-derived protein 3α (Reg3α), a protein described as having protective effects against oxidative stress and anti-inflammatory activity, could participate in the control of glucose homeostasis and potentially be a new target of interest in the treatment of type 2 diabetes. To that end the recombinant human Reg3α protein was administered for one month in insulin-resistant mice fed high fat diet. We performed glucose and insulin tolerance tests, assayed circulating chemokines in plasma and measured glucose uptake in insulin sensitive tissues. We evidenced an increase in insulin sensitivity during an oral glucose tolerance test in ALF-5755 treated mice vs controls and decreased the pro-inflammatory cytokine C-X-C Motif Chemokine Ligand 5 (CXCL5). We also demonstrated an increase in glucose uptake in skeletal muscle. Finally, correlation studies using human and mouse muscle biopsies showed negative correlation between intramuscular Reg3α mRNA expression (or its murine isoform Reg3γ) and insulin resistance. Thus, we have established the proof of concept that Reg3α could be a novel molecule of interest in the treatment of T2D by increasing insulin sensitivity via a skeletal muscle effect.
Keywords: Insulin resistance, Glucose uptake, Skeletal muscles, Type 2 diabetes
Insulin resistance; Glucose uptake; Skeletal muscles; Type 2 diabetes
1. Introduction
The regenerating islet-derived protein 3α (Reg3α, Reg3A), also called hepatocarcinoma-intestinal-pancreas/pancreatitis-associated protein (HIP/PAP) is a 16 KDa type C lectin protein with a single carbohydrate binding domain [1]. Reg3α is mainly expressed throughout the small intestine and modulates host defense process via bactericidal activity [2]. Reg3α is also secreted and carries out its biological activities in an autocrine and paracrine manner [1]; it could also act in an endocrine manner since it is also detected in the bloodstream. This protein has been shown to drive tissue repair and regeneration as well as protection against oxidative stress and cell death in the liver, pancreas and intestine, it also promotes motor neuron axonal survival and guidance [3] and Schwann cell proliferation [4, 5, 6]. It shows anti-inflammatory activities [7] and yields antibacterial activities in the digestive tract [8, 9]. The protein shows an intrinsic ROS scavenger activity targeted on the extra-cellular matrix in the context of inflammation; in turn, several intra-cellular signaling pathways such as JAK/STAT3 and PI3/Akt, have been shown to be activated by Reg3α [10, 11].
All this data makes it a promising molecule in a therapeutic perspective and led to produce a human recombinant protein Reg3α (rcReg3α, also named ALF-5755). A murine model with induction of acute liver failure was the first model evidencing the efficacy of ALF-5755 treatment [11]. In this study ALF-5755 treatment protected in vitro primary hepatocytes against multiple cell death signals and prevented in vivo death of the mice upon treatment with Fas agonists; this activity was correlated with the scavenger ROS activity and the prevention of extra- and intra-cellular oxidative stress. ALF-5755 was then used in a phase 1 then 2a clinical trial in patients with acute liver failure and led to a significant although moderate clinical benefit in a per-protocol analysis of patients with hepatitis B virus or auto-immune acute or chronic hepatitis [12]. Due to its antioxidant and anti-inflammatory effects, ALF-5755 could also play an important therapeutic role in other pathological situations including metabolic disorders like insulin resistance (IR) and type 2 diabetes (T2D), two conditions characterized by chronic multi-tissular low grade inflammation and oxidative stress, hepatic and pancreatic β cells dysfunctions [13].
In this study the effect of treatment with ALF-5755 was addressed in a model of C57Bl6/J mice made insulin resistant and prediabetic when fed with high fat diet (HFD). We have evidenced that treatment with ALF-5755 decreased IR and increased glucose uptake in skeletal muscles compared to control group treated with vehicle. In addition, correlation studies allowed us to demonstrate that Reg3γ mRNA expression in skeletal muscles was negatively associated with IR in mice fed with a HFD. In Humans Reg3α mRNA expression in skeletal muscles was also negatively correlated with HOMA-IR2 and intramyocellular-triglycerides content. Altogether our data highlight a new role for Reg3α as a regulator of insulin-sensitivity through an effect on skeletal muscle glucose uptake.
2. Materials and methods
2.1. Recombinant human Reg3α protein (rcREG3α)
The rcREG3α protein (ALF-5755) has been synthetized by adding one amino-terminal methionine to the sequence of the secreted (i.e., lacking the 26–amino acid signal sequence) form of the human endogenous REG3α (HIP/PAP) (NP_620355). It was produced in Escherichia coli, purified to ≥99% and released in batches in compliance with the clinical grade manufacturing process by PX'Therapeutics (Grenoble, France) which permitted to achieve a phase 1 then a phase 2a in patients with acute liver disease [12].
2.2. Animal studies
All procedures were carried out in accordance with the ethical standards of French and European regulations (European Communities Council Directive, 86/609/EEC). The number of mice complied with institutional ethical rules and was consistent with common practice in the fields of metabolism studies. Procedures were approved by the ethics committee of the University of Paris and by the French Ministry of Research (approval #8728-20 16082615248655 v3). Four weeks old C57BL/6J male mice were used. High fat diet (HF260) composition was as follows: 5505 kcal/kg; 60% fat, 27% carbohydrates, SAFE, Augy, France). After 8 weeks on the diet, the mice were briefly anesthetized with isoflurane to implant a filled minipump on the right back for ALF-5755 administration. An osmotic minipump (Alzet model 2004) was implanted subcutaneously in 13-week-old mice delivering 50 μg/day of ALF-5755 or placebo (NaCl 0.9%) for 4 weeks.
2.3. Oral glucose tolerance test
Oral glucose tolerance test (OGTT) was performed in overnight fasted mice at day 28. They received a glucose solution at 2 g/kg body weight via oral gavage (glucose 30% CMD Lavoisier). Tail blood was sampled at time 0, 15, 30, 60, 90 and 120 min. Glucose levels were immediately measured with a glucometer (Glucofix mio, Menarini Diagnostics). Blood was centrifuged and plasma was collected and stored at −80 °C for further measurement of hipemia (t0) and insulinemia (t0, t15, t30 and t60).
2.4. Glucose uptake in specific tissues during OGTT
[1-14C]2-DG at 50 μCi was injected intraperitoneally concomitantly to the glucose gavage [14]. Specific activity was measured in plasma samples collected during the glucose tolerance test. At the end of the experiment the following tissues were collected and weighted to measure 2DG content and calculate glucose uptake: liver, skeletal muscle (extensor digitorum longus, EDL; soleus, SOL), subcutaneous white adipose tissue (WATsc) and visceral adipose tissue (WATv), cardiac atria and ventricles (see calculations below).
2.5. Measurements and calculations
2.5.1. Insulin sensitivity index (ISI)
ISI was calculated during OGTT by application in rodents of the Matsuda’s method widely used in humans, [15]. ISI was calculated as 10000/(√[G 0 × I 0 × G mean × I mean]), where the suffix mean indicates the average value of glucose and insulin concentration measurements during the whole length of the test.
2.5.2. Assessment of glucose uptake in insulin target tissues
During OGTT, 10 μL of blood contained in a heparinized capillary were collected at time 0, 15, 30, 45 and 60 min and directly added to a 1.5 mL tube containing 50 μL ZnSO4 (0.3 N), followed by 50 μL Ba(OH)2 (0.3 N) to precipitate proteins [14]. At completion of the experiment the samples were centrifuged at 12,000 g for 3 min and 50 μL of supernatant were move to a counting vial and evaporated in a hood. Dry pellet was resuspended in 500 μL water before cpm assessment. Selected tissues were processed as follows: each tissue was transferred to a tube containing 0.5 mL NaOH (1 M), shaken on a thermo-mixer (60 °C, 950–1000 rpm) for 1h, followed by addition of 0.5 mL of HCl (1 M). Two-hundred μL of the homogenate were transferred to tubes containing 0.5 mL ZnSO4 (0.3 N), to which 0.5 mL Ba(OH)2 (0.3 N) were then added. 200 μL of the homogenate were transferred to tubes containing 1 mL perchloric acid (6%). After vortexing and centrifuging samples for 2 min at 13000 g, 400 μL of supernatants were transferred to counting vials and radioactivity was determined. The first series with ZnSO4 and Ba(OH)2 allowed for determining Xcpm (2-DG). The second series with PCA allowed for determining Ycpm (2-DG plus 2-deoxyglucose 6 phosphate, 2-DG6P). Ycpm-Xcpm = 2-DG6P synthesis reflecting cellular 2-DG uptake within the tissue. In vivo glucose uptake (μg.g of tissue −1.min−1) for each tissue was calculated on the basis of the accumulation of 2DG-6-phosphate in the respective tissue and the disappearance rate of 2DG from plasma as described [16].
2.5.3. Measurement of circulating chemokines
On the last day of the infusion period and before the glucose tolerance test, a blood sample was taken from the tail vessels and the plasma collected for measurement of circulating cytokine concentrations. Briefly, plasma samples were screened using mouse Cytokine/Chemokine Magnetic Bead multiplex assay. The following 32 analytes classically involved in inflammation or immune system were dosed: G-CSF, GM-CSF, IFNγ, IL1α, IL1β, IL2, IL3, IL4, IL5, IL6, IL7, IL9, IL10, IL12 (p40), IL12 (p70), IL13, IL15, IL17, IP10, KC, LIF, LIX/CXCL5, MCP1, MCSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNFα, VEGF, Eotaxin/CCL11 (MCYTOMAG-70K, Millipore, Rabalot, France). All reagents and samples were prepared, and assays performed according to the manufacturer’s instructions provided by the kit. Luminex xMAP was used as the method of detection. To decrease the percentage of out-of-range values, results were analyzed based on the median fluorescence intensities (MFI) as previously described [17]. Mann-Whitney test was performed for each analyte.
2.6. Correlation studies in muscles
2.6.1. Human studies
The Malmö Men (MM) cohort is a subset of 203 non-obese Swedish men from the Malmö Prospective Project (MPP) that were asked to participate in an training intervention [18, 19]. The MPP was initiated in 1974 as an intervention project to prevent Type 2 diabetes in men born between 1926 and 1935. Upon inclusion in the MPP, all participants in MM had normal glucose tolerance, but at the baseline screening visit for the MM intervention, some of them had developed impaired glucose tolerance or Type 2 diabetes. Information from the MM baseline pre-intervention screening visit from individuals without Type 2 diabetes was used for work presented in this manuscript. Briefly, muscle samples were obtained at the time of diagnosis (before treatment with any hypoglycemic medication). Percutaneous muscle biopsy samples (20–50 mg) were taken from the vastus lateralis muscle under local anesthesia (1% lidocaine) after a 2h hyperinsulinemic-euglycemic clamp using a Bergström needle. The cohort and microarray experimental details have been described earlier in [18].
2.6.2. Mice studies
Correlations between the mRNA expression Reg3γ, the murine isoform of Reg3α, in mouse skeletal muscle and metabolic phenotypes were carried out on the basis of the database developed within the framework of the European programmes IMI Rhapsody and according to a previously described methodology [20, 21, 22]. Briefly, male mice from the three commonly used non-diabetic mouse strains C57BL6/J, DBA2/J and BalbC were fed a high fat or regular chow diet for one month as previously described [22]. Skeletal muscles were collected and phenotypic measurements (basal glycemia and insulinemia, oral GTT, 2g/kg, ITT, 0.75U/kg) were performed at 2 days, 10 days and 30 days to assess diabetes progression. Insulin Resistance (IR) was defined as the area under the curve of the glycemia during ITT. RNA-Seq was performed from skeletal muscles at each time-point and integrated with the phenotypic data in a network-based analysis [20, 21, 22]. Briefly, weighted gene co-expression network analysis (WGCNA) was performed on normalized RNASeq data and gene expression modules to phenotypic trait correlations were calculated using the Spearman method [23], as it has been described in Sánchez-Archidona et al [22].
3. Results
3.1. Animal studies
We first observed the effect of treatment on basal blood glucose (i.e. 5h fasted) and insulin (Figure 1A and B, respectively) and no effect were observed in both parameters compared to placebo. We then studied glucose homeostasis during an oral glucose challenge (Figure 1C–G). The evolution of blood glucose in response to glucose overload was similar in both groups with however a tendency to a systematically lower blood glucose level from 30 min onwards in the ALF-5755 group compared to the placebo group (1C). On the other hand, the increase in insulin levels observed during OGTT was lower in the ALF-5755 group as compared to the control group, especially at time 15 min where the difference was significant (1D). Similarly, insulin AUC during OGTT was significantly decreased in ALF-5755 treated group compared to control (1E). This strongly suggested that ALF therapy reduced IR, and this was confirmed by the calculation of the ISI (1F). Finally, the plasma ALF-5755 (Hipemia) concentration was inversely correlated to the plasma insulin concentration at time 15 min (1G).
Figure 1.
ALF-5755 decreases insulin resistance. 5h-fasted glycaemia (A) and insulinemia (B) measured after 24 days of continuous treatment (Unpaired t-test, At(11) = 1,5, p = 0,17, (B) t(11) = 2,19, p = 0,051). Overnight-fasted glycemia (mg/dL) during OGTT (C) measured at 27 days of treatment before glucose bolus (T0) and at 15, 30, 60, 90, 120 min post 2g/kg of oral glucose (two-ways ANOVA, treatment effect F(1,11) = 3,9, p = 0,07). Decreased insulinemia (D) during OGTT measured before oral glucose (T0) and at 15, 30 and 60 min following oral glucose in ALF-5755 groups vs placebo (two-ways ANOVA, treatment effect F(1,11) = 5,9, ∗p = 0,03, Sidak’s multiple test 15 min ∗∗p = 0,0036). (E) Area under curve (AUC) of insulinemia during OGTT calculated during the whole test (0–60 min, unpaired t-test t(11) = 2,5, ∗p = 0,03), between 0 and 15 min (unpaired t-test, t(11) = 2,17, p = 0,053) or between 15 and 60 min (unpaired t-test, t(11) = 2,6, ∗p = 0,024). (F) Insulin sensitivity index (ISIMatsuda) following Matsuda’s method (unpaired t-test, t(11) = 2,6, ∗p = 0,026). (G) Negative correlation between insulin concentration (ng/mg) at 15 min after oral glucose and ALF-5755 plasma concentration (hipemia) (r(pearson) = −0,79, ∗p = 0,019). Placebo (n = 7), ALF-5755 (n = 6). Error bars indicate SEM.
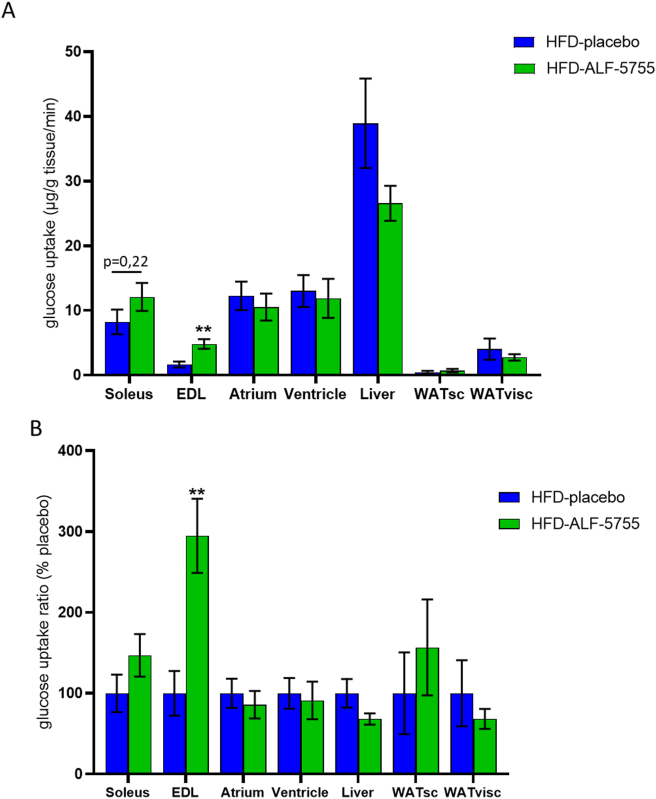
At this time, it was important to identify the tissue(s) targeted by ALF-5755 and responsible for the increase in insulin sensitivity. Glucose uptake by the main insulin target tissues was measured and evidenced a significant increase in glucose uptake specifically in EDL, a glycolytic skeletal muscle (Figure 2A and B). A similar, but non-significant trend was also observed in soleus muscle. No effect of ALF-5755 treatment on glucose uptake was observed in the liver nor in white adipose tissues (either subcutaneous or visceral).
Figure 2.
ALF-5755 promotes skeletal muscle glucose uptake. Glucose uptake in μg/g/min (A) and as a ratio in percentage of placebo values (B) in skeletal muscle (Soleus, EDL: unpaired t-test, t(8) = 3,6, ∗∗p = 0,0067), heart muscle (atrium, ventricle), liver, subcutaneous (WATsc) and visceral (WATvisc) adipose tissue. (B) glucose uptake ratio (%) in skeletal muscle (Soleus, EDL: unpaired t-test, t(8) = 3,6, ∗∗p = 0,0067), heart muscle (atrium, ventricle), liver, subcutaneous (WATsc) and visceral (WATvisc) adipose tissue of ALF-5755 groups compared to placebo. Placebo (n = 6), ALF-5755 (n = 6). Error bars indicate SEM.
Regarding measurement of circulating chemokines, 28 analytes were detected among 32. Of these 28the proinflammatory cytokine CXCL5, was significantly decreased by treatment with ALF-5755 (Figure 3A). Four other analytes showed a tendency to be differentially expressed following treatment with ALF-5755 including IFNγ (3B-E).
Figure 3.
ALF-5755 decreases plasma CXCL5 concentration. Plasma concentration of CXCL5 (A), IL-1a (B), eotaxin (C), IFNγ (D), and IL-13 in HFD fed mice after 28 days of treatment with placebo or ALF-5755. Luminex xMAP was used as the method of detection. To decrease the percentage of out-of-range values, results were analyzed based on the median fluorescence intensities (MFI) as described [17]. Placebo (n = 7), ALF-5755 (n = 8). Mann-Whitney test was performed for each analyte.
3.2. Correlation studies in rodents and humans
Since the increase in insulin sensitivity demonstrated during ALF-5755 administration was predominantly explained by an increase in glucose uptake in skeletal muscle, we investigated whether there was a correlation between intramuscular expression of Reg3 (both γ and α) and glucose homeostasis parameters in skeletal muscle biopsies from mice (Table 1) and human subjects (Table 2).
Table 1.
Correlations between mRNA expression of Reg3γ in skeletal muscles and metabolic parameters in mice fed HFD (n = 12 per mouse strain).
| R (Spearman) | P value | |
|---|---|---|
| Basal Glycemia | 0,414 | 0,0002 |
| Basal Insulinemia | −0,496 | 0,036 |
| Insulin resistance | −0,321 | 0,005 |
| OGTT (AUC) | −0,238 | 0,340 |
| Insulin during OGTT | −0,128 | 0,609 |
Bold means significative correlation between mRNA expression of Reg3 and measured parameter.
Table 2.
Correlations between Reg3α mRNA level in skeletal muscle and several key components of glucose metabolism in healthy subjects (n = 28): IMCL: intramyocellular triglycerides, basal glucose and insulin levels, HOMA2-IR, and waist to hip ratio..
| R (Spearman) | P value | |
|---|---|---|
| IMCL Triglycerides | −0,548 | 0,005 |
| Basal Glycemia | −0,455 | 0,020 |
| Basal Insulinemia | −0,497 | 0,010 |
| HOMA2-IR | −0,423 | 0,031 |
| Waist-Hip Ratio | −0,429 | 0,032 |
We observed in mice that Reg3γ expression was similar in soleus muscle of mice fed with HF diet compared to regular chow diet (14.41 ± 3,12 vs. 14.25 ± 1,94 A.U., ns), however we put in evidence that Reg3γ expression positively correlated with basal blood glucose and negatively with both insulinemia and IR (Table 1). IR was defined as the area under the curve of the glycemia during ITT. In humans, intramuscular expression of Reg3α was negatively correlated with intramyocellular triglycerides, basal glucose and insulin levels, HOMA-IR, and waist-hip ratio, all features of insulin resistance (Table 2). Of note, Reg3α expression in human muscles of patients with impaired glucose tolerance was slightly lower than in normo-tolerant patients: 264 ± 19 vs. 327 ± 26, A.U. p = 0.08).
4. Discussion
The aim of our study was to test the hypothesis that a treatment with the Reg3α protein could improve insulin efficacy in mice made glucose intolerant and insulin resistant upon HFD. This hypothesis was based on previously published data showing an antioxidant and anti-inflammatory effect of Reg3α, two parameters that are involved in the development of IR. We have shown here for the first time that treatment with the human Reg3α decreased IR in this rodent model. Furthermore, we have shown that this effect was due at least in part to an increase in glucose uptake by muscle tissue (significantly in glycolytic muscle and with a non-significant trend in oxidative muscle). Finally, we have also demonstrated a correlation between the expression of muscle Reg3γ in mice and Reg3α in humans and the improvement of main parameters of glucose homeostasis, namely insulinemia and basal glycemia, HOMA-IR and intramyocellular triglyceride content.
Such specific increase in muscle glucose transport is of particular interest because muscles are the main responsible for increased glucose uptake during meals. Indeed, in healthy humans, 70–80% of glucose uptake occurs in skeletal muscle in the postprandial state [24, 25]. In contrast, in T2D subjects, the onset of insulin action is delayed and the ability of insulin to maximally stimulate glucose uptake is markedly blunted. DeFronzo et al have highlighted that insulin-stimulated leg muscle glucose uptake is reduced by about 50% in T2D [24]. In addition, quantification of leg muscle glucose uptake in T2D with positron emission tomography has evidenced severe muscle IR [26]. This lack of muscle glucose uptake in T2D partly accounts for the more marked postprandial hyperglycemia in these patients and a slower return of glucose levels to basal value. Moreover, studies of genetically predisposed individuals have indicated that insulin resistance in the muscle is the primary or initiating defect leading to the ultimate development of T2D [27]. Thus, skeletal muscle is a main target to fight against development of insulin-resistance in prediabetic state or during early stage of T2D [28]. In addition to these interventional data in animals, the analysis of the correlation data that we have carried out in both mice and humans were also extremely interesting and brings new elements of interest in the use of Reg3α for the therapeutic management of IR in overweight or obese subjects with or without T2D. Indeed, we have demonstrated a negative correlation between basal insulinemia and IR in mice and humans. Of note the positive correlation between basal glycemia and Reg3γ mRNA expression in mouse muscles may suggest a different regulation compared to Reg3α in Human skeletal muscles and may highlight an adaptation to increased glycemia in the same way that glucose induces an increase in insulin synthesis and secretion [29]. Moreover, the inverse correlation between Reg3α expression in skeletal muscle and intramyocellular triglyceride content in healthy humans is also a very interesting finding; indeed excessive intramyocellular lipid deposition and associated reduced muscle contractile activity are characteristics of degenerative muscular diseases and muscle disorders not only in T2D [30, 31] but also in systemic diseases [32]. Thus, it has been demonstrated that insulin resistance promotes metabolic and functional breakdowns of skeletal muscle which may manifest clinically even in young and middle-aged adults [33]. At the opposite, muscle fat accumulation and muscular IR were positively correlated with higher fasting plasma glucose and lower glucose tolerance in humans [34]. Thus, Reg3α treatment could prevent these defects by decreasing intramyocellular triglycerides content and concomitantly improving glucose uptake. It should also be noted that in our study the increase in muscle glucose uptake was greater in glycolytic (EDL) than in oxidative muscle (soleus). This could be due to methodology we used but it is interesting to keep in mind that glycolytic muscles are more susceptible to age-related muscular dystrophy [35, 36]; thus increased glucose uptake in glycolytic muscles may also contribute to partly protect against muscular dysfunction during progression of T2D [37]. Similarly, glucose uptake in glycolytic muscles is less insulin-dependent with a higher fraction of contraction-induced glucose uptake compared to oxidative muscles [38]. Thus, Reg3α could have a potentiating effect on insulin by increasing its action in glycolytic muscles.
Several studies have shown an effect of Reg3α on hepatocyte regeneration and viability; yet we did not observe any effect on glucose uptake by the liver in our glucose tolerance test experiments upon Reg3α administration; this suggests that at least in these conditions the liver is probably not a major site of action for Reg3α. This is consistent with the fact that basal glycemia depends mainly on hepatic glucose production whereas decreased glycemia in response to a meal (here mimicked by OGTT) is mainly due to increased glucose uptake by muscles.
The lack of increased uptake of glucose in adipose tissue during Reg3α administration is also of great interest as it may prevent significant weight gain in the long term; indeed an increase in glucose uptake by adipose tissue might promote a rapid return of glycemia to its basal value but the long-term effect might be deleterious since the increased glucose uptake could actually drive lipogenesis and triglyceride storage and ultimately body weight gain as observed during therapy with pioglitazone [39]. In fact, data obtained from our interventional study in mice should be compared with those obtained in humans regarding the inverse correlation between the expression of Reg3α in skeletal muscle and the waist to hip ratio; this suggests a decrease in adiposity in those subjects with high expression of Reg3α in muscle, possibly due to a metabolic shift and a preferential use of glucose by muscles rather than adipose tissue as observed in mice treated with Reg3α. Thus, chronic treatment with Reg3α in the context of T2D may reduce or limit weight gain by reorienting the metabolism of glucose to the muscles with a beneficial impact on the overall energy homeostasis. In agreement with this result, it is interesting to note the tendency for plasma eotaxin to decrease in animals treated with ALF-5755 vs placebo. Indeed eotaxin, produced in particular by the vascular stroma fraction of adipose tissue, has been shown to be positively associated with obesity in humans whereas diet-induced weight loss led to its reduction [40]. Thus, our result may reflect a decrease in adiposity and adipose tissue inflammation.
Accordingly, the measurement of plasma cytokines concentration shows that treatment with ALF-5755 significantly reduces the level of the proinflammatory chemokine CXCL5. We previously evidenced that Reg3A controlled inflammation in mice with colitis [41]. It has been shown that plasma CXCL5 elevation is an inducer of insulin resistance and T2D [42, 43, 44, 45]. In fact, Chavey et al evidenced that CXCL5 inhibits insulin signaling [43]. Treatment of obese, insulin-resistant mice with anti-CXCL5 neutralizing antibodies improved insulin-sensitivity [43]. Thus, the decrease of CXCL5 could also partly explain the beneficial impact of ALF-5755 on insulin sensitivity.
In conclusion, our data show for the first time to the best of our knowledge that Reg3α regulates skeletal muscle insulin sensitivity not only by its expression in this tissue but also during its administration in an insulin resistant animal model. Reduction of muscular insulin resistance during treatment with Reg3α is linked to increased glucose uptake by skeletal muscle. Our study also reveals a new mode of action of Reg3α related to the decrease of plasma CXCL5 that could explain, at least in part, the increase in insulin sensitivity. Thus, pharmacological improvement of insulin resistance in muscle driven by Reg3α may be a promising additional metabolic benefit when associated with other hypoglycemic agents which do not target directly skeletal muscle.
Declarations
Author contribution statement
Aurélie Le Lay, Erwann Philippe, Fanny Roth, Ana Rodriguez Sanchez-Archidona, Florence Mehl, Jessica Denom, Rashmi Prasad, Olof Asplund, Ola Hansson, Mark Ibberson: Performed the experiments; Analyzed and interpreted the data.
Fabrizio Andreelli, Lyse Santoro, Paul Amouyal, Gilles Amouyal, Christian Brechot, Laure Jamot: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Céline Cruciani-Guglielmacci, Christophe Magnan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by a collaboration contract between Université Paris Cité -Erganeo and The Healthy Aging Company (grant number #Q82).
Data availability statement
Data associated with this study has been deposited at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164672.
Declaration of interest’s statement
The authors declare the following conflict of interests: Lyse Santoro is CEO of The Healthy Aging Company (THAC). Laure Jamot and Fanny Roth are employees of THAC. Gilles Amouyal, Paul Amouyal, Christian Bréchot and Christophe Magnan are co-founders of THAC.
Additional information
No additional information is available for this paper.
References
- 1.Chen Z., Downing S., Tzanakakis E.S. Four decades after the discovery of regenerating islet-derived (Reg) proteins: current understanding and challenges. Front. Cell Dev. Biol. 2019;7:235. doi: 10.3389/fcell.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin J.H., Seeley R.J. Reg3 proteins as gut hormones? Endocrinology. 2019;160(6):1506–1514. doi: 10.1210/en.2019-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimune H., Vasseur S., Wiese S., Birling M.-C., Holtmann B., Sendtner M., et al. Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat. Cell Biol. 2000;2(12):906–914. doi: 10.1038/35046558. [DOI] [PubMed] [Google Scholar]
- 4.Lieu H.-T., Batteux F., Simon M.-T., Cortes A., Nicco C., Zavala F., et al. HIP/PAP accelerates liver regeneration and protects against acetaminophen injury in mice. Hepatology. 2005;42(3):618–626. doi: 10.1002/hep.20845. [DOI] [PubMed] [Google Scholar]
- 5.Lieu H.-T., Simon M.-T., Nguyen-Khoa T., Kebede M., Cortes A., Tebar L., et al. Reg2 inactivation increases sensitivity to Fas hepatotoxicity and delays liver regeneration post-hepatectomy in mice. Hepatology. 2006;44(6):1452–1464. doi: 10.1002/hep.21434. [DOI] [PubMed] [Google Scholar]
- 6.Simon M.-T., Pauloin A., Normand G., Lieu H.-T., Mouly H., Pivert G., et al. HIP/PAP stimulates liver regeneration after partial hepatectomy and combines mitogenic and anti-apoptotic functions through the PKA signaling pathway. Faseb. J. 2003;17(11):1441–1450. doi: 10.1096/fj.02-1013com. [DOI] [PubMed] [Google Scholar]
- 7.Gironella M., Folch-Puy E., LeGoffic A., Garcia S., Christa L., Smith A., et al. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56(8):1091–1097. doi: 10.1136/gut.2006.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cash H.L., Whitham C.V., Behrendt C.L., Hooper L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., et al. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malka D., Vasseur S., Bödeker H., Ortiz E.M., Dusetti N.J., Verrando P., et al. Tumor necrosis factor α triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology. 2000;119(3):816–828. doi: 10.1053/gast.2000.16491. [DOI] [PubMed] [Google Scholar]
- 11.Moniaux N., Song H., Darnaud M., Garbin K., Gigou M., Mitchell C., et al. Human hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein cures fas-induced acute liver failure in mice by attenuating free-radical damage in injured livers. Hepatology. 2011;53(2):618–627. doi: 10.1002/hep.24087. [DOI] [PubMed] [Google Scholar]
- 12.Nalpas B., Ichaï P., Jamot L., Carbonell N., Rudler M., Mathurin P., et al. A proof of concept, phase II randomized European trial, on the efficacy of ALF-5755, a novel extracellular matrix-targeted antioxidant in patients with acute liver diseases. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105(2):141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bravo-San Pedro J.M., Sica V., Martins I., Pol J., Loos F., Maiuri M.C., et al. Acyl-CoA-binding protein is a lipogenic factor that triggers food intake and obesity. Cell Metabol. 2019;30(4):754–767. doi: 10.1016/j.cmet.2019.07.010. e9. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.K., Gimeno R.E., Higashimori T., Kim H.-J., Choi H., Punreddy S., et al. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J. Clin. Invest. 2004;113(5):756–763. doi: 10.1172/JCI18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breen E.J., Polaskova V., Khan A. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine. 2015;71(2):188–198. doi: 10.1016/j.cyto.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Mootha V.K., Lindgren C.M., Eriksson K.-F., Subramanian A., Sihag S., Lehar J., et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 19.Tripathy D., Eriksson K.F., Orho-Melander M., Fredriksson J., Ahlqvist G., Groop L. Parallel manifestation of insulin resistance and beta cell decompensation is compatible with a common defect in Type 2 diabetes. Diabetologia. 2004;47(5):782–793. doi: 10.1007/s00125-004-1393-8. [DOI] [PubMed] [Google Scholar]
- 20.Cruciani-Guglielmacci C., Bellini L., Denom J., Oshima M., Fernandez N., Normandie-Levi P., et al. Molecular phenotyping of multiple mouse strains under metabolic challenge uncovers a role for Elovl2 in glucose-induced insulin secretion. Mol. Metabol. 2017;6(4):340–351. doi: 10.1016/j.molmet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellini L., Campana M., Rouch C., Chacinska M., Bugliani M., Meneyrol K., et al. Protective role of the ELOVL2/docosahexaenoic acid axis in glucolipotoxicity-induced apoptosis in rodent beta cells and human islets. Diabetologia. 2018;61(8):1780–1793. doi: 10.1007/s00125-018-4629-8. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Archidona A.R., Cruciani-Guglielmacci C., Roujeau C., Wigger L., Lallement J., Denom J., et al. Plasma triacylglycerols are biomarkers of β-cell function in mice and humans. Mol. Metabol. 2021;54 doi: 10.1016/j.molmet.2021.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9(1):559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFronzo R.A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J.P. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 25.Meng Z.-X., Gong J., Chen Z., Sun J., Xiao Y., Wang L., et al. Glucose sensing by skeletal myocytes couples nutrient signaling to systemic homeostasis. Mol. Cell. 2017;66(3):332–344. doi: 10.1016/j.molcel.2017.04.007. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utriainen T., Takala T., Luotolahti M., Rönnemaa T., Laine H., Ruotsalainen U., et al. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia. 1998;41(5):555–559. doi: 10.1007/s001250050946. [DOI] [PubMed] [Google Scholar]
- 27.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(suppl_2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng S., Huang P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res. Ther. 2019;10(1):103. doi: 10.1186/s13287-019-1186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrannini E., Pilo A. Pattern of insulin delivery after intravenous glucose injection in man and its relation to plasma glucose disappearance. J. Clin. Invest. 1979;64(1):243–254. doi: 10.1172/JCI109445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.W., Goodpaster B.H., Lee J.S., Kuller L.H., Boudreau R., de Rekeneire N., et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh H.M., Elgzyri T., Alibegovic A., Hiscock N., Ekström O., Eriksson K.-F., et al. Relationship between insulin sensitivity and gene expression in human skeletal muscle. BMC Endocr. Disord. 2021;21(1):32. doi: 10.1186/s12902-021-00687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delmonico M.J., Harris T.B., Visser M., Park S.W., Conroy M.B., Velasquez-Mieyer P., et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodpaster B.H., Leland Thaete F., Simoneau J.-A., Kelley D.E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 34.Prior S.J., Joseph L.J., Brandauer J., Katzel L.I., Hagberg J.M., Ryan A.S. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J. Clin. Endocrinol. Metabol. 2007;92(3):880–886. doi: 10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]
- 35.Nilwik R., Snijders T., Leenders M., Groen B.B.L., van Kranenburg J., Verdijk L.B., et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013;48(5):492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Capel F., Buffière C., Patureau Mirand P., Mosoni L. Differential variation of mitochondrial H2O2 release during aging in oxidative and glycolytic muscles in rats. Mech. Ageing Dev. 2004;125(5):367–373. doi: 10.1016/j.mad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Jaiswal N., Gavin M.G., Quinn W.J., Luongo T.S., Gelfer R.G., Baur J.A., et al. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metabol. 2019;28:1–13. doi: 10.1016/j.molmet.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson B., Chan P., Lam C.W.K. An overview of alogliptin + pioglitazone for the treatment of type 2 diabetes. Expet Opin. Pharmacother. 2021:1–14. doi: 10.1080/14656566.2021.1985465. [DOI] [PubMed] [Google Scholar]
- 40.Vasudevan A.R., Wu H., Xydakis A.M., Jones P.H., Smith E.O., Sweeney J.F., et al. Eotaxin and obesity. J. Clin. Endocrinol. Metabol. 2006;91(1):256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 41.Darnaud M., Dos Santos A., Gonzalez P., Augui S., Lacoste C., Desterke C., et al. Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology. 2018;154(4):1009–1023. doi: 10.1053/j.gastro.2017.11.003. e14. [DOI] [PubMed] [Google Scholar]
- 42.Chavey C., Fajas L. CXCL5 drives obesity to diabetes, and further. Aging. 2009;1(7):674–677. doi: 10.18632/aging.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavey C., Lazennec G., Lagarrigue S., Clapé C., Iankova I., Teyssier J., et al. CXC Ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metabol. 2009;9(4):339–349. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunemaker C.S., Chung H.G., Verrilli G.M., Corbin K.L., Upadhye A., Sharma P.R. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J. Endocrinol. 2014;222(2):267–276. doi: 10.1530/JOE-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tejera-Segura B., López-Mejías R., de Vera-González A., Delgado-González A., González-Gay M.A., Ferraz-Amaro I. Implication of CXCL5 (epithelial neutrophil-activating peptide 78) in the development of insulin resistance in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2019;37(3):373–379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164672.