Abstract
Major depressive disorder (MDD) is a severe disease of unknown pathogenesis that will affect ∼10% of people during their lifetime. Therapy for MDD requires prolonged treatment and often fails, predicating a need for novel treatment strategies. Here, we report increased ceramide levels in the blood plasma of MDD patients and in murine stress-induced models of MDD. These blood plasma ceramide levels correlated with the severity of MDD in human patients and were independent of age, sex, or body mass index. In addition, intravenous injection of anti-ceramide antibodies or neutral ceramidase rapidly abrogated stress-induced MDD, and intravenous injection of blood plasma from mice with MDD induced depression-like behavior in untreated mice, which was abrogated by ex vivo preincubation of the plasma with anti-ceramide antibodies or ceramidase. Mechanistically, we demonstrate that ceramide accumulated in endothelial cells of the hippocampus of stressed mice, evidenced by the quantitative measurement of ceramide in purified hippocampus endothelial cells. We found ceramide inhibited the activity of phospholipase D (PLD) in endothelial cells in vitro and in the hippocampus in vivo and thereby decreased phosphatidic acid in the hippocampus. Finally, we show intravenous injection of PLD or phosphatidic acid abrogated MDD, indicating the significance of this pathway in MDD pathogenesis. Our data indicate that ceramide controls PLD activity and phosphatidic acid formation in hippocampal endothelial cells and thereby mediates MDD. We propose that neutralization of plasma ceramide could represent a rapid-acting targeted treatment for MDD.
Keywords: major depression, ceramide, endothelial cells, PLD, phosphatidic acid, behavior, neurogenesis
Abbreviations: CNS, central nervous system; HAM-D, Hamilton Depression Rating Scale; MDD, major depressive disorder; PLD, phospholipase D; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling
Major depressive disorder (MDD) is a severe and chronic disease with a lifetime prevalence of more than 10% (1). The main symptom is a depressed mood, but the disease is also characterized by a loss of interest, anhedonia, fear, feelings of worthlessness, weight loss, insomnia, and concentration deficits (1). Because approximately 10% of MDD patients attempt suicide, MDD is also often a life-threatening or even fatal illness (1).
Although alterations of neurotransmitter functions and concentrations, synaptic changes mainly in the hippocampus, aberrant neuroplasticity, alterations in growth factors and their receptors, a reduced proliferation of hippocampus neurons, changes of the glucocorticoid system, immunological factors, or distinct dysfunctions of endothelial cells in the brain, to name a few hypotheses, are some of the most important mechanisms that have been implied in the pathogenesis of MDD (e.g., 1, 2, 3, 4, 5, 6, 7, 8), the pathogenesis of MDD still requires definition. It has been assumed that antidepressants interfere with the uptake of monoamines, resulting in the monoamine hypothesis of major depression and the actions of antidepressants (9). However, this hypothesis was revised because the effects of several antidepressants do not fit within it. For instance, the antidepressant tianeptine is a serotonin reuptake enhancer (10), and ketamine, which has been also introduced for treatment of MDD, targets N-methyl-D-aspartate receptors (11, 12, 13), but not the uptake of monoamine neurotransmitters. Furthermore, antidepressants directly target the synaptic uptake of monoamines and rapidly induce an increase in the concentrations of neurotransmitters in the synapse. This early drug-mediated event clearly contrasts with the delayed onset, i.e., usually 2 to 4 weeks, of the therapeutic effects of antidepressants. Thus, the molecular mechanisms of the pathogenesis and the treatment of MDD require definition.
MDD is mainly treated with classic antidepressants, sleep deprivation, electroconvulsive therapy, or ketamine (1), but the treatment of MDD requires improvement; classical antidepressants suffer from a delayed onset of several weeks of the clinical effects, which is a major clinical problem for the treatment of patients with MDD (14). In addition, only approximately 2/3 of the patients respond to classical antidepressants (14).
Ketamine acts very rapidly against MDD, but the drug causes serious adverse effects if used for longer than a few days or weeks (15). Likewise, electroconvulsive therapy is usually only applied to severe cases and for a limited time. Thus, there is still an urgent and unmet need for a rapid and reliable treatment of MDD.
It is very interesting to note that MDD is also associated with many somatic symptoms, such as an increased incidence of cardiovascular disease and osteoporosis, adrenocortical activation, increased oxidative stress, and increased plasma concentrations of proinflammatory cytokines and phospholipase A2, as well as dyslipoproteinemia (16, 17, 18). Therefore, MDD can be seen as a systemic disease and not just as a disease of the central nervous system (CNS). At present, it is unknown how the peripheral symptoms can be explained and how they are linked to the CNS symptoms of MDD.
Previous studies have shown that antidepressants function by targeting the acid sphingomyelinase in the hippocampus (19, 20) and thereby increasing sphingomyelin and decreasing ceramide levels. In addition, several studies have shown that ceramide levels are increased in the blood plasma of patients with MDD (21, 22, 23, 24) and also in brain tissue of mice with different models of MDD (25), raising the question whether ceramide might also play a role in the pathogenesis of MDD.
Here, we postulate a novel view of the pathogenesis and treatment of MDD. We suggest that MDD is initiated as a peripheral response to stress that evolves to manifest itself in the brain. We show an increase of ceramide levels in the blood plasma of humans with MDD and mice with stress-induced experimental MDD. Ceramide levels correlated with severity of MDD in human patients. Ceramide accumulated in endothelial cells of the hippocampus and inhibited phospholipase D (PLD), resulting in a decrease of phosphatidic acid, the product of PLD activity in the hippocampus. Intravenous injection of PLD or phosphatidic acid into depressed mice rapidly normalized behavior and neuronal proliferation indicating the significance of this novel pathway for MDD. This concept also allows for novel treatments of MDD; instead of targeting molecules in the brain, we demonstrate that neutralizing or consuming ceramide in the blood plasma by antibodies or ceramidases or restoration of PLD activity or its product phosphatidic acid restored normal functions in mice with MDD within as little as 24 h.
Results
Ceramide is increased in the blood plasma of patients with MDD and in mouse models of MDD
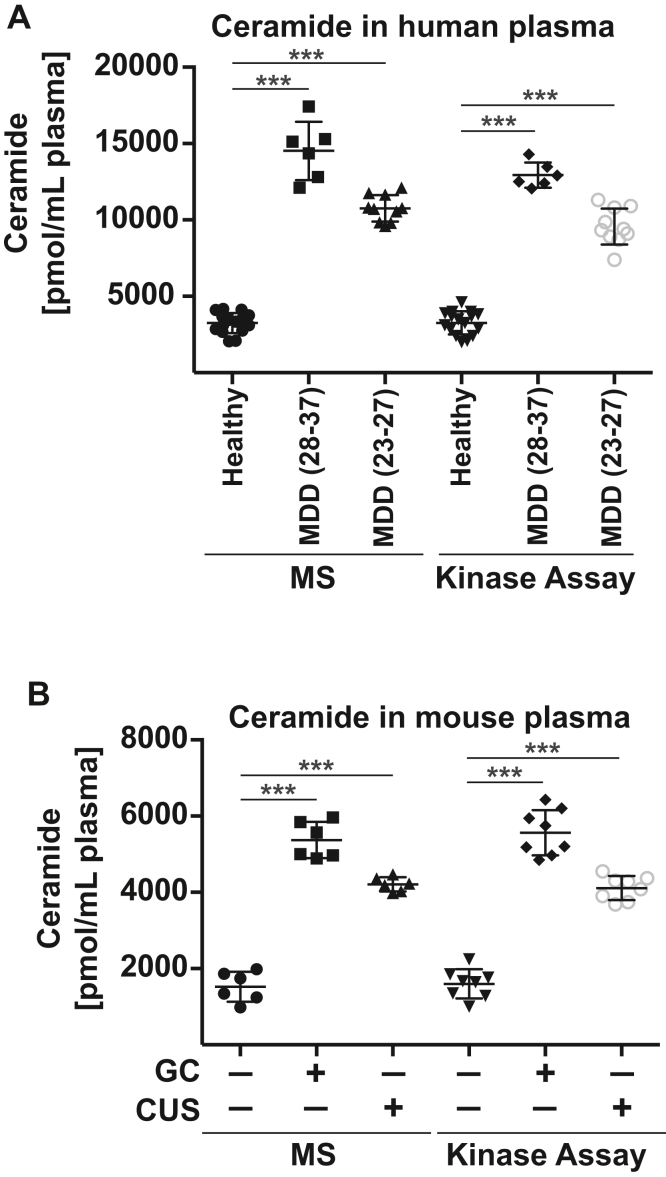
MDD is often caused by stress. We tested the hypothesis that exogenous stress induces the release of ceramide into the blood. To this end, we determined ceramide in the blood plasma of patients with MDD and that of healthy control subjects. In addition, we determined the concentration of ceramide in the blood plasma of mice that were exposed to glucocorticosterone-induced or chronic unpredictable environmental stress (i.e., a reversal of the light/dark cycle, 3 h of 45° tilting of the cage twice each week, shaking at 125 rpm for 45 min, food deprivation for 14 h, predator sounds for 15 min, or wet cages for 1 h with two forms of stress per day in a randomized, unpredictable order) or left unstressed. Patients suffered from moderately severe or very severe MDD, as determined by the Hamilton Depression Rating Scale (HAM-D) (Table 1). The results of these studies demonstrate that patients with MDD show a marked increase in ceramide concentrations in the plasma compared to healthy individuals (Fig. 1A). We confirmed the finding of an increase of ceramide in the blood plasma of patients with MDD in a second, independent set of patients and controls (Fig. S1). Ceramide in the blood plasma correlated with the HAM-D Rating scale, i.e., the severity of MDD (Table 1 and Fig. S2). Patients and controls did not significantly differ in sex, body mass index, and age (Table 1, Table S1 and Fig. S2).
Table 1.
Hamilton Depression Rating Scale scores (HAM-D), body mass index, age, and sex of patients with MDD and healthy controls
| Criteria | Healthy | MDD (23–27) | MDD (28–37) |
|---|---|---|---|
| HAM-D score (mean ± SD) | 0.3 ± 0.6 | 24.7 ± 1.6a | 32.3 ± 3.9a |
| Body mass index (mean ± SD) | 25.8 ± 3.3 | 24.5 ± 4.9 | 26.0 ± 2.3 |
| Age (mean ± SD) | 43.2 ± 11.8 | 44.2 ± 10.9 | 43.5 ± 10.0 |
| Sex | 8 female, 8 male | 3 female, 5 male | 5 female, 1 male |
The HAM-D rating scale scores, body mass index (BMI), age, and sex of the patients are given. Age and BMI did not significantly differ between patients and healthy controls and did not correlate with ceramide levels. Shown are the mean ± SD, each n = 16 for samples from healthy individuals and patients with MDD.
p < 0.001, ANOVA and post hoc Tukey test. Please note that we only included patients with very severe or moderately severe MDD, who were not previously treated for MDD.
Figure 1.
Ceramide levels in blood plasma are higher in stressed mice and humans with major depressive disorder than in control subjects. A, venous blood samples from patients with major depressive disorder (MDD) and from healthy control subjects were collected, and blood plasma was prepared. Ceramide concentrations (expressed as sum of C16 to C24 subspecies) were determined by mass spectrometry (MS) and ceramide kinase assays. Ceramide levels in the blood plasma of patients with MDD were higher than those in the plasma from control subjects and correlated with the severity of MDD as determined by the Hamilton Depression Rating Scale (HAM-D scores). The range of the HAM-D for the groups is given in parenthesis. B, wildtype mice were stressed with either glucocorticosterone (GC) or chronic unpredictable environmental stress (CUS) or were left untreated as controls (-). Ceramide accumulated in the blood plasma of wildtype mice after various forms of stress. Shown are the mean ± SD, each n = 16 for human samples from healthy individuals and patients with MDD. Please note that we only included patients with very or moderately severe MDD, who were not previously treated for MDD. For the mouse studies, mean ± SD of n=6 to 8 animals/group are displayed. ∗∗∗p < 0.001, ANOVA and post hoc Tukey test.
The significance of these findings in humans is strongly supported by the finding that glucocorticoid or chronic unpredictable environmental stress also induced a marked increase of ceramide levels in the blood plasma of wildtype mice (Fig. 1B).
We controlled for age and sex as calculated by ANCOVA using the SPSS28 software. Ceramide was used as dependent variable and age, sex, and the HAM-D score as independent variables. We grouped all patients with MDD in one group. The results show that age and sex do not have a statistically significant influence on ceramide levels, while the HAM-D score had a highly significant correlation with levels of ceramide (p < 0.001).
Neutralization of blood plasma ceramide rapidly abrogates symptoms of MDD
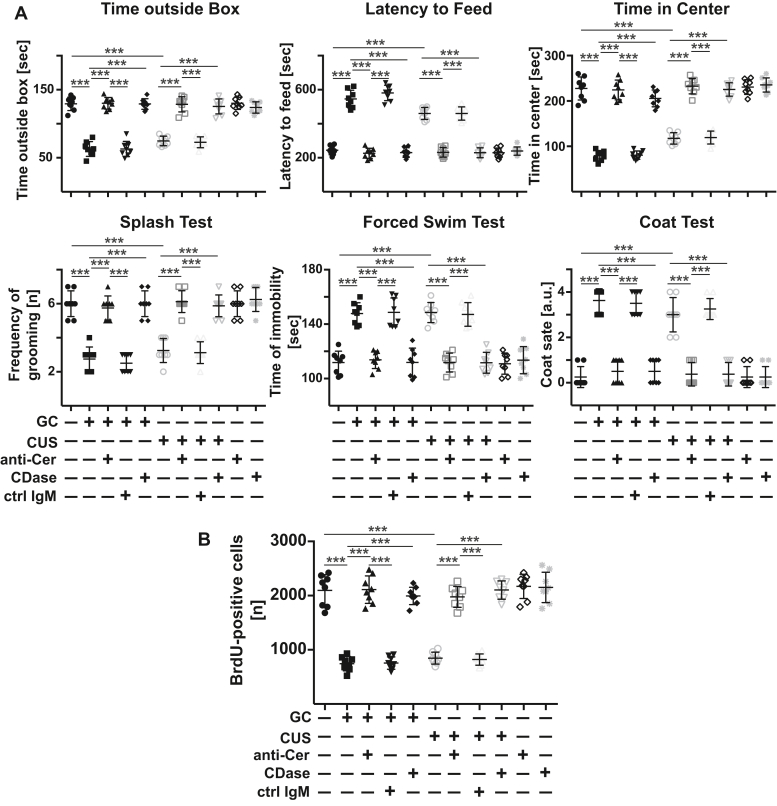
Next, we determined the biomedical importance of the increase of ceramide concentrations in the blood plasma. To this end, 24 h and 12 h before analysis, we injected mice that had already been exposed to glucocorticosterone or chronic unpredictable environmental stress for 6 days or left untreated with (i) three different anti-ceramide IgM and IgG antibodies to neutralize ceramide in the plasma or with (ii) recombinant neutral ceramidase to consume plasma ceramide. Control mice were injected with irrelevant IgM and IgG antibodies or PBS. We determined behavior and neuronal proliferation already 24 h after the first injection of anti-ceramide antibodies or ceramidase to test whether the neutralization of ceramide is able to revert functional changes in the brain that cause MDD. The pathogenetic role of neuronal proliferation in MDD is controversial, but it is clearly reduced in stress and is at least a good marker for MDD and the effects of antidepressants (7, 8, 26, 27). The results show that neutralizing or consuming ceramide with anti-ceramide antibodies or ceramidase abrogated depression-like behavior and normalized neurogenesis in stressed mice (Figs. 2, A and B, S3, S4). Injecting control IgM or IgG exerted no effect on stress-induced major depression (Figs. 2, A and B, S3, S4).
Figure 2.
Neutralization or consumption of ceramide in the blood plasma prevents major depressive disorder in mice. Wildtype mice were stressed for 6 days with either glucocorticosterone (GC) or chronic unpredictable environmental stress (CUS) or were left untreated. On day 5, anti-ceramide IgM antibodies clone S58-9 (anti-Cer), control immunoglobulin M (ctrl IgM), or recombinant ceramidase (CDase) were i.v. injected. Controls were not stressed and left untreated or received i.v. injections of anti-ceramide antibodies or ceramidase. A, behavioral changes were analyzed 24 h after treatment by the light/dark box test, the novelty-suppressed feeding test, the open-field arena test, the splash test, the forced swim test, and the coat status. The results indicate that neutralization or consumption of ceramide in the blood is sufficient to prevent stress-induced major depressive disorder. B, neurogenesis in the hippocampus 24 h after treatment was determined by staining for 5′-bromo-2′-deoxyuridine (BrdU). Shown are the mean ± SD from each eight mice. ∗∗∗p < 0.001, ANOVA and post hoc Tukey test.
These findings indicate that the release of ceramide into the blood upon the application of stress is crucial for the induction of MDD.
Blood plasma ceramide is sufficient to induce symptoms of MDD in mouse models
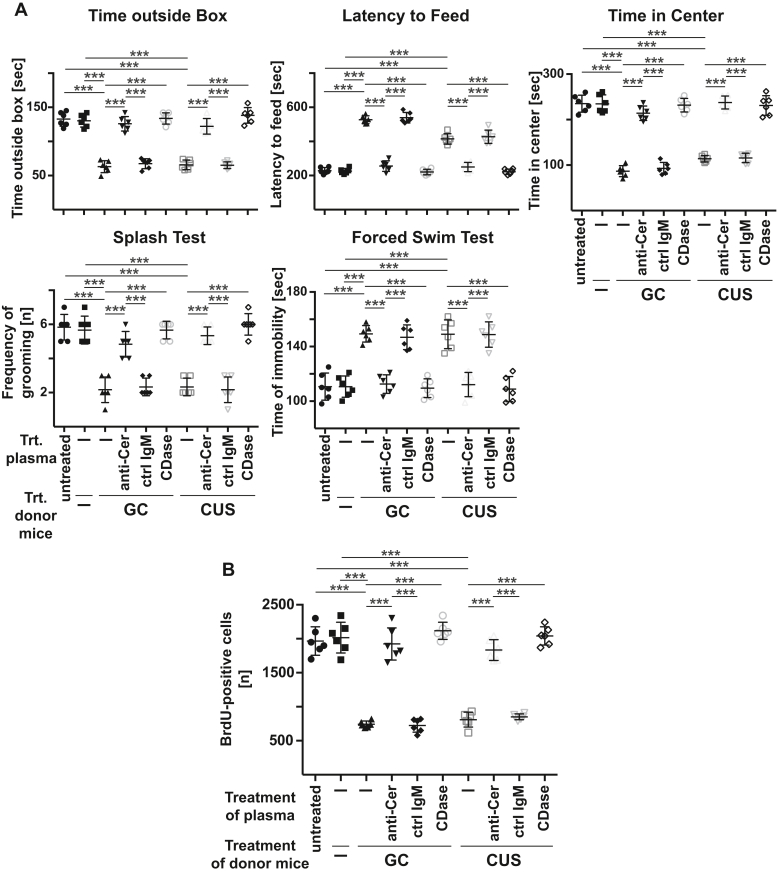
To further confirm this hypothesis, we obtained blood plasma from wildtype mice that had been exposed to glucocorticosterone or chronic unpredictable environmental stress or left untreated. We incubated the plasma with different anti-ceramide antibodies, clone S58-9 (IgM), clone 15B4 (IgM), or an IgG anti-ceramide antibody, neutral ceramidase, or control IgM or control IgG ex vivo or left it untreated. We then re-injected these samples into untreated (nonstressed) wildtype mice and determined neuronal proliferation and behavior 24 h later. The results show, first, that injection of blood plasma from stressed mice into nonstressed, healthy mice transferred the symptoms of MDD (Figs. 3, A and B, S5, S6). Second, in vitro incubation of plasma from stressed mice with anti-ceramide antibodies or ceramidase prior to re-injection into nonstressed mice prevented the development of MDD symptoms, whereas control IgM or control IgG exerted no effect (Figs. 3, A and B, S5, S6).
Figure 3.
Injection of blood plasma from stressed mice or patients with MDD induces symptoms of major depressive disorder, which is prevented by ex vivo neutralization or consumption of ceramide. A and B, wildtype mice were stressed with either glucocorticosterone (GC) or chronic unpredictable environmental stress (CUS) or were left untreated and blood plasma was obtained (donor mice). Blood plasma of the donor mice was treated in vitro with anti-ceramide IgM antibodies clone S58-9 (anti-Cer), control immunoglobulin M (IgM), or recombinant ceramidase (CDase) or left untreated. 125 μl of the blood plasma samples were injected intravenously (i.v.) into healthy wildtype mice and (A) behavioral changes and (B) neurogenesis in the hippocampus were determined 24 h later as readouts for MDD. Shown are the mean ± SD from each six animals analyzed per group. ∗∗∗p < 0.001, ANOVA and post hoc Tukey test.
To exclude the possibility that we were measuring the effects of corticosterone that might have remained in the plasma of these mice, we also injected untreated mice with a high dose of corticosterone (1 mg/kg) and determined the presence of biochemical and behavioral symptoms of major depression after 24 h. These studies indicate that treatment with glucocorticosterone (even at a very high dose) for 24 h is not sufficient to induce MDD (not shown).
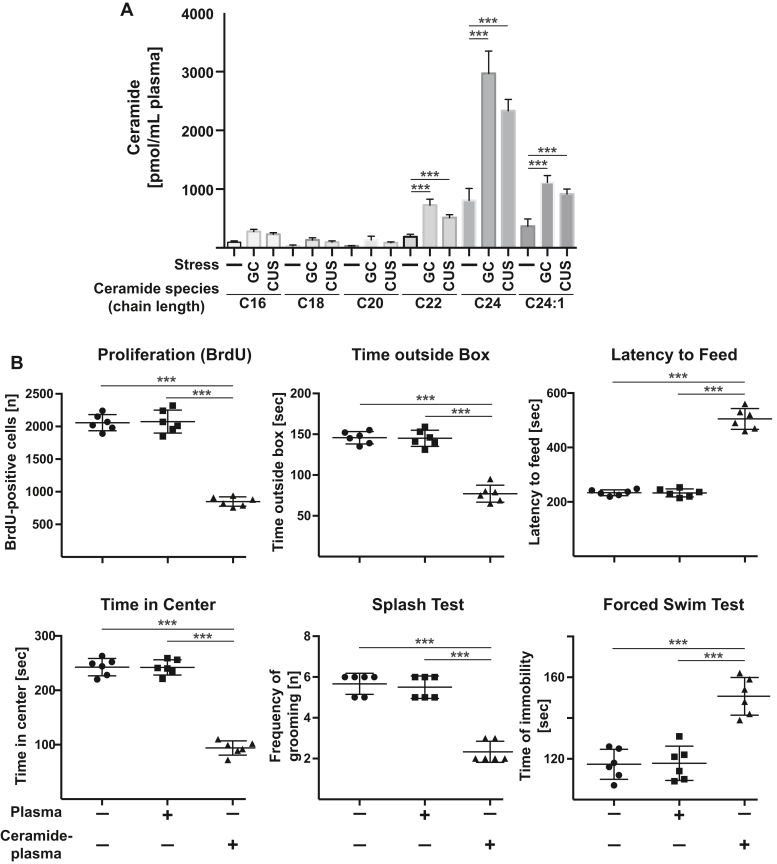
We then tested whether we could induce MDD in untreated mice by increasing plasma concentrations of ceramide. Thus, we first determined the different ceramide species in the blood plasma of mice after glucocorticoid stress. The results show a significant increase of C22, C24, and C24:1 ceramide (Fig. 4A). In total, we observed 5356 ± 477 pmol/ml ceramide in the plasma of stressed mice with a ratio of 5.2%: 2.4%: 2.5%: 13.6%: 55.5%: 20.8% of C16: C18: C20: C22: C24: C24:1 ceramides. Since the blood plasma volume of mice is approximately 1 ml, we loaded 200 μl blood plasma for 1 h in vitro with a 5-fold higher concentration of the different ceramides, i.e., 5 nmol ceramide total (i.e., 25 nmol/ml) in the ratio observed in the blood plasma of stressed mice. These 200 μl blood plasma were i.v. injected into healthy, nonstressed mice. We then determined biochemical and depression-like behavior. These experiments showed that loading the blood plasma with ceramide is sufficient to induce MDD-like behavior and a reduction of neuronal proliferation within 24 h (Fig. 4B).
Figure 4.
Intravenous injection of ceramide-loaded blood plasma induces symptoms of major depressive disorder. A, ceramide species in the blood plasma of mice that were left untreated or stressed with glucocorticosterone (GC) or chronic unpredictable stress (CUS) were determined by mass spectrometry. Shown are the mean ± SD of six independent samples (same as in Fig. 1), ANOVA followed by Sidak´s multiple comparison test. Compared were the corresponding values of ceramide species after glucocorticosterone or CUS with those of untreated animals. B, plasma was isolated from the blood of untreated wildtype mice, loaded with a total of 5 nmol ceramide at a ratio of 5.2% C16 : 2.4% C18 : 2.5% C20 : 13.6% C22 : 55.5% C24 : 20.8% C24:1 ceramides, and injected intravenously (i.v.) into wildtype mice. Neurogenesis and behavioral changes were measured as readout for the induction of MDD by i.v. ceramide loaded plasma. Presented are the mean ± SD from each six mice. ∗∗∗p < 0.001, ANOVA and post hoc Tukey test.
Ceramide accumulates in endothelial cells of the brain upon stress
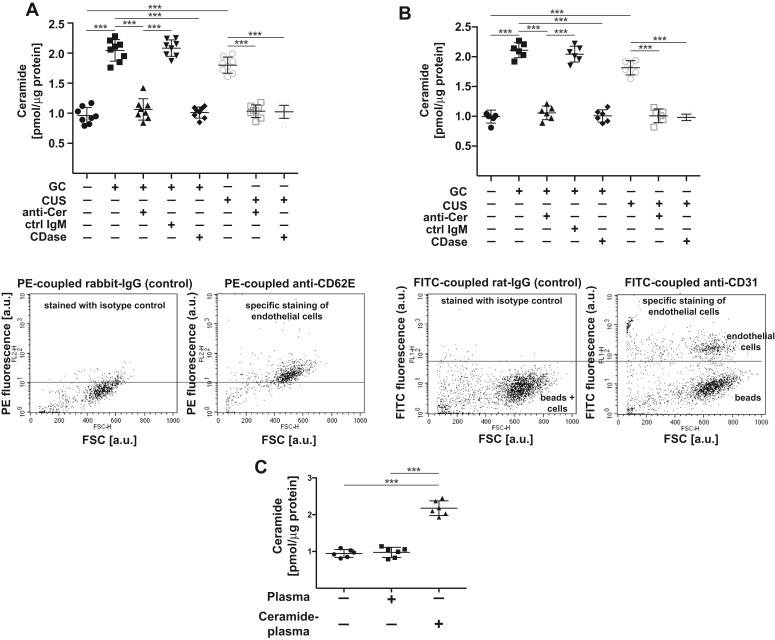
We have previously shown that stress does not result in a significant increase of ceramide in the whole hippocampus (19, 20), although this does not exclude accumulation of ceramide in a small population of cells, which could not be detected in lysates of the whole hippocampus. In accordance, injection of deuterated C16 ceramide (d18:1-d7/16:0) failed to reveal a significant accumulation of deuterated ceramide in the whole hippocampus (not shown) supporting the hypothesis that plasma ceramide acts in a specific and small population of cells in the hippocampus. Since ceramide is a very hydrophobic molecule, it seems to be unlikely that ceramide diffuses into deeper areas of the hippocampus. We therefore tested whether ceramide accumulates in hippocampus endothelial cells upon induction of stress in wildtype mice. To this end, we isolated and purified endothelial cells from the hippocampus of stressed and unstressed mice by two independent methods and determined ceramide concentrations in these cells. The results of both methods are very similar and demonstrate a marked increase of ceramide in endothelial cells of the hippocampus upon application of glucocorticosterone or chronic unpredictable environmental stress (Fig. 5, A and B). The accumulation of ceramide in hippocampus endothelial cells was prevented by i.v. injection of anti-ceramide antibodies or neutral ceramidase (Fig. 5, A and B). Staining and flow cytometry of isolated and purified cells with PE- or FITC-coupled anti-CD31 antibodies confirmed the purity of the endothelial preparations (Fig. 5, A and B). We also determined the concentration of ceramide in the hippocampus and in hippocampus endothelial cells after i.v. injection of blood plasma loaded with a total of 25 nmol/ml ceramide at the ratio of C16: C18: C20: C22: C24: C24:1 ceramides as described above. The results demonstrate an increase of ceramide in hippocampal endothelial cells after i.v. injection of ceramide-loaded plasma (Fig. 5C), while we did not observe a significant increase of ceramide in the total hippocampus.
Figure 5.
Stress-induced ceramide accumulates in hippocampal endothelial cells. A and B, endothelial cells from the hippocampus of stressed mice or unstressed control mice were isolated and purified using (A) anti-CD31 antibodies coupled to supramagnetic beads (Miltenyi) or (B) anti-CD62 E antibodies coupled to Dynabeads. Ceramide concentrations were determined biochemically in these cells. The results demonstrate a marked increase of ceramide in endothelial cells of the hippocampus upon application of glucocorticosterone or chronic unpredictable environmental stress. The accumulation of ceramide in hippocampus endothelial cells was prevented by i.v. injection of anti-ceramide antibodies or neutral ceramidase. Shown are the mean values ± SD from six to eight mice/group, ∗∗∗p < 0.001, ANOVA and post hoc Tukey test. Staining and flow cytometry of aliquots of isolated and purified cells with PE-coupled anti-CD62 E antibodies (A) or FITC-coupled anti-CD31 antibodies (B) confirmed the purity of the endothelial preparations used for ceramide measurements. In panel A, the flow cytometry studies show one population of cells that is positively stained for CD62 E indicating a very pure population of endothelial cells after sorting. Miltenyi beads are very small and not detected. In panel B, the lower population contains the large dynabeads and endothelial cells in the isotype control samples. Specific staining with FITC-anti-CD31 antibodies results in a shift of the endothelial cell population, while the large beads remain unstained. Displayed are typical results of the flow cytometry studies for the samples (n=6–8). Fluorescence and FSC are given in arbitrary units (a.u.). C, mice were injected with ceramide-loaded blood plasma (ceramide-plasma) in the ratio of ceramide-species as described above or injected with unloaded plasma or left untreated, endothelial cells from the hippocampus were isolated and purified using anti-CD31 antibodies coupled to supramagnetic beads (Miltenyi), and ceramide concentrations were determined biochemically in these cells. Shown are the mean ± SD, n = 6 mice, ∗∗∗p < 0.001, ANOVA and post hoc Tukey test.
Peripheral ceramide inhibits PLD and reduces phosphatidic acid concentrations in the hippocampus
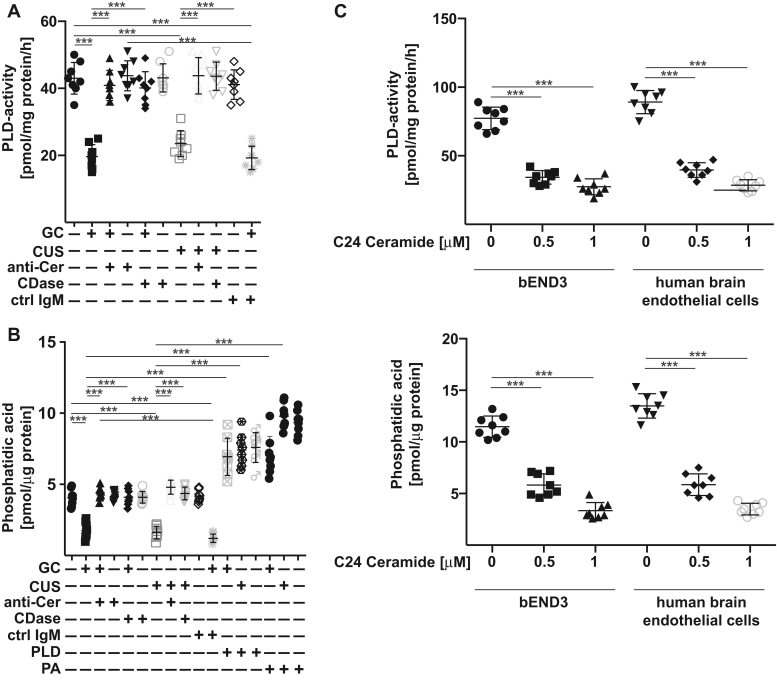
Since several previous studies implied an important role of endothelial cells in the hippocampus for MDD and, in particular, of endothelial PLD for the regulation of neurogenesis (6, 28), we tested whether ceramide might regulate PLD activity in the hippocampus. The results reveal that glucocorticosterone or chronic unpredictable stress reduced the activity of PLD in the hippocampus of mice (Fig. 6A). Treatment of the mice with anti-ceramide antibodies or ceramidase restored PLD-activity in the hippocampus within 24 h (Fig. 6A).
Figure 6.
Ceramide inhibits endothelial PLD and reduces phosphatidic acid in the hippocampus. A and B, wildtype mice were stressed for 6 days with either glucocorticosterone (GC) or chronic unpredictable environmental stress (CUS) or were left untreated. On day 5, anti-ceramide IgM antibodies clone S58-9 (anti-Cer), control immunoglobulin M (ctrl IgM), recombinant ceramidase (CDase), recombinant PLD, or phosphatidic acid (PA) were i.v. injected. The hippocampus was removed 24 h after treatments and PLD activity (A), and the concentration of phosphatidic acid (B) were measured in total lysates of the hippocampus. C, treatment of bEnd3 or human brain-derived microvascular cells with 0.5 μM or 1 μM C24 ceramide results in inhibition of PLD activity and reduces cellular phosphatidic acid concentrations. Shown are the mean ± SD from each eight animals/group (A and B) or eight independent experiments (C). ∗∗∗p < 0.001, ANOVA and post hoc Tukey test. PLD, phospholipase D.
PLD activity results in the formation of phosphatidic acid, and consistent with PLD inhibition in depressed mice, we observed a marked reduction of phosphatidic acid concentrations in the hippocampus of stressed mice (Fig. 6B). Intravenous injection of anti-ceramide antibodies or ceramidase or intravenous injection of PLD or phosphatidic acid normalized hippocampal phosphatidic acid concentrations in stressed mice within 24 h (Fig. 6B) indicating that the PLD—phosphatidic acid system is targeted by peripheral plasma ceramide.
Treatment of cultured mouse brain endothelial cells bEnd3 or human brain microvascular endothelial cells with C24 ceramide resulted in a reduction of PLD activity and phosphatidic acid levels in these cells (Fig. 6C) supporting the notion that endothelial cells are targets for plasma ceramide in MDD.
PLD and phosphatidic acid control neurogenesis and behavior in models of MDD
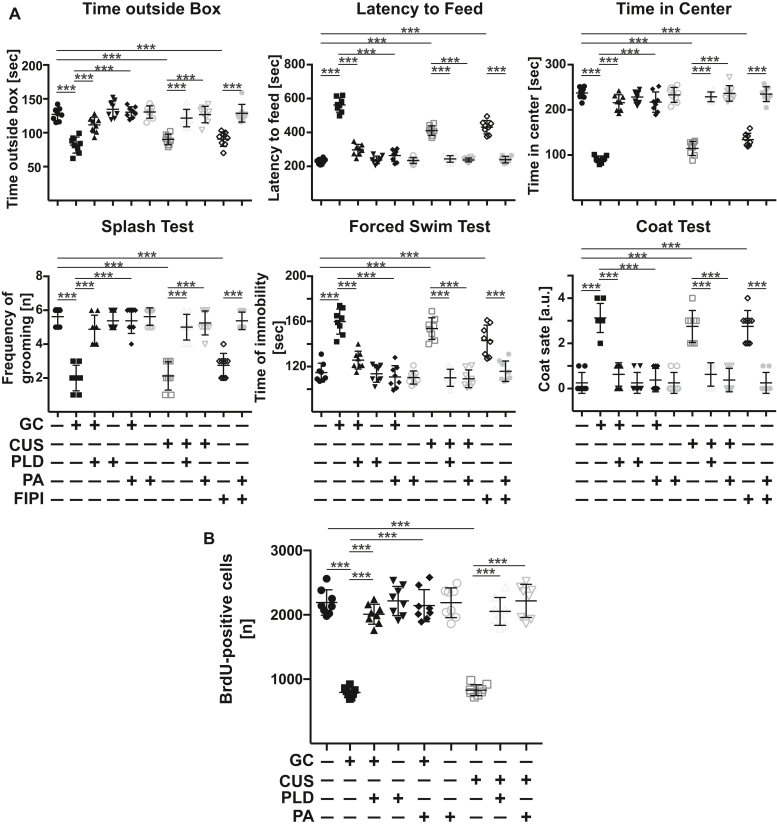
We next investigated the significance of PLD and phosphatidic acid for the pathogenesis of MDD. To this end, we injected recombinant PLD or phosphatidic acid intravenously into mice with MDD and determined behavior and neurogenesis. The results indicate that both injection of PLD as well as phosphatidic acid normalized depressed behavior and neurogenesis of stressed mice within 24 h (Fig. 7, A and B). Vice versa, intravenous injection of FIPI, an inhibitor of PLD, into healthy mice resulted in rapid development of depressed behavior (Fig. 7A).
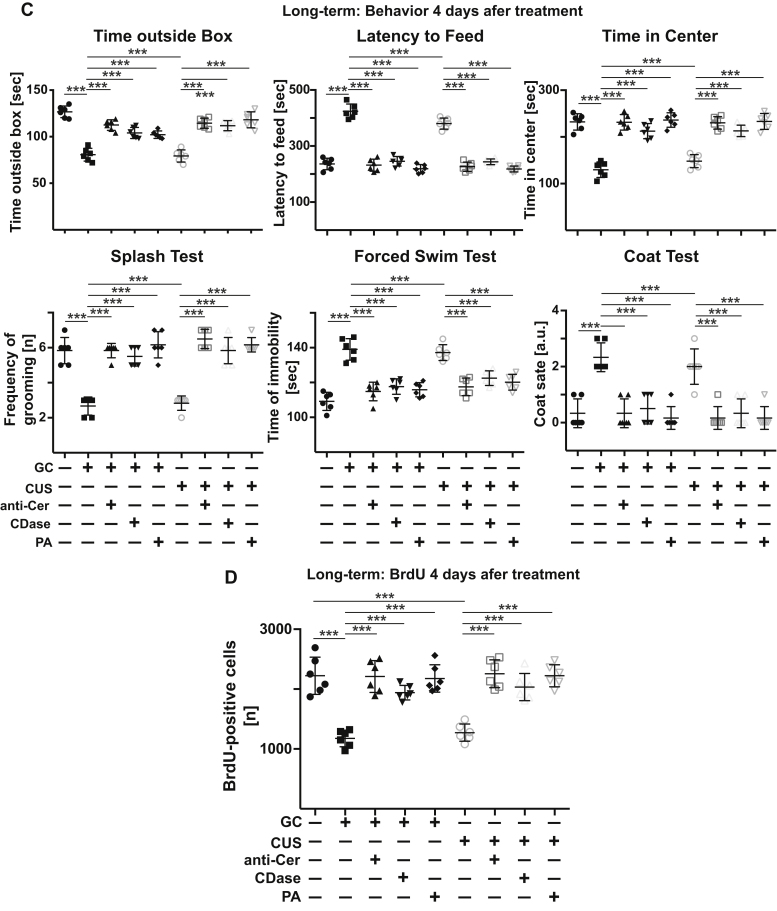
Figure 7.
Targeting PLD and phosphatidic acid allows control of and long-term recovery from major depression. A and B, stress-induced effects on behavior (A) and hippocampal neurogenesis (B) and are reversed by i.v. injection of PLD or phosphatidic acid (PA). Vice versa, inhibition of PLD upon i.v. injection of the PLD inhibitor FIPI into nonstressed mice is sufficient to induce behavioral changes of major depression in these mice. C and D, injection of anti-ceramide antibodies clone S58-9 (anti-Cer), ceramidase (CDase), or phosphatidic acid results in a long-term correction of major depression in stressed mice. Mice were stressed for a total of 14 days, treated at day 7 and 10, and behavior (C) as well as neurogenesis (D) were determined 4 days after the second treatment. Shown are the mean ± SD from each eight (A and B) and six (C and D) animals/group. ∗∗∗p < 0.001, ANOVA and post hoc Tukey test. PLD, phospholipase D.
Next, we determined whether injection of anti-ceramide, ceramidase, or phosphatidic acid also has a long-term effect on neurogenesis and behavior in stressed mice with MDD. Mice were stressed with glucocorticosterone or chronic unpredictable stress for a total of 14 days. At 7 and 10 days after initiation of stress, mice were i.v. injected with anti-ceramide antibodies, ceramidase, or phosphatidic acid. Stress was continued until day 14, behavior was measured, mice were sacrificed, and neurogenesis was determined. The results show that the effects of a treatment with anti-ceramide, ceramidase, or phosphatidic acid on behavior and neurogenesis are stable for at least 4 days and protect against a relapse of MDD (Fig. 7, C and D).
Finally, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays on histologies of the heart, lung, liver, and kidney as well as analysis of blood chemistry parameters revealed no side effects of the injection of anti-ceramide antibodies or phosphatidic acid (Table 2).
Table 2.
Blood chemistry and cell death in heart, lung, liver, and kidney after i.v. injection of anti-ceramide antibodies or phosphatidic acid
| Criteria | Untreated | Anti-ceramide-antibody i.v. | Phosphatidic acid i.v. |
|---|---|---|---|
| T-Pro | 5.6 ± 0.2 | 5.0 ± 0.3 | 4.9 ± 0.3 |
| Alb | 2.7 ± 0.05 | 2.5 ± 0.05 | 2.4 ± 0.1a |
| T-Bil | 0.25 ± 0.06 | 0.19 ± 0.03 | 0.23 ± 0.08 |
| GOT | 50.3 ± 16.7 | 39.3 ± 4.8 | 62 ± 36.6 |
| GPT | 20.8 ± 9.4 | 26.5 ± 18.0 | 45.5 ± 22.9 |
| LDH | 629 ± 289 | 493 ± 240 | 560 ± 87 |
| BUN | 26.0 ± 2.7 | 22.3 ± 1.0 | 19 + 15.8 |
| CPK | 958 ± 299 | 741 ± 101 | 766 ± 245 |
| γGT | under 10 IU/L | under 10 IU/L | under 10 IU/L |
| Crea | under 0.2 mg/dL | under 0.2 mg/dL | under 0.2 mg/dL |
| Amylase | 915 ± 189 | 814 ± 113 | 1031 ± 153 |
| Cell death heart | 2 ± 2 | 3 ± 2 | 1 ± 1 |
| Cell death lung | 3 ± 2 | 2 ± 2 | 4 ± 3 |
| Cell death liver | 2 ± 1 | 3 ± 2 | 2 ± 2 |
| Cell death kidney | 3 ± 2 | 4 ± 3 | 3 ± 3 |
Blood plasma was analyzed using a SpotChem EZ chemistry analyzer with the corresponding parameter strips or a colorimetric assay kit. Cell death in the tissues was determined by TUNEL assays and microscopic analysis. Shown are the mean ± SD of each four independent measurements.
Abbreviations: T-Pro, T-Troponin (cardiac Troponin); Alb, albumin, T-Bil, total bilirubin; GOT, glutamat-oxalat transaminase; GPT, glutamat-pyruvat transaminase; LDH, lactatedehydrogenase; BUN, blood urea nitrogen; CPK, creatine-phosphokinase; γGT, gamma-glutamyl-transferase; Crea, Creatinine.
p < 0.05, ANOVA and post hoc Tukey test.
Discussion
The present study provides evidence for a novel concept of the pathogenesis of MDD and suggests several novel potential treatment options. We demonstrate that stress causing MDD symptoms in mouse models results in an increase of ceramide in the blood plasma of mice. Most importantly, ceramide is also increased in the blood of patients with MDD. Injection of blood plasma from stressed mice or patients with MDD into untreated mice is sufficient to induce behavioral and biochemical changes indicating and typical for MDD. Neutralizing ceramide within the blood plasma by administering anti-ceramide antibodies or ceramidase prevents MDD in mice.
We show that peripheral ceramide accumulates in endothelial cells of the hippocampus, inhibits PLD in the hippocampus and thereby reduces the basal release of phosphatidic acid in the hippocampus. We indicate that ceramide in the blood plasma acts on endothelial PLD and thereby regulates the crosstalk between endothelial cells and neurons. This crosstalk seems to be mediated, at least in part, by phosphatidic acid. However, the exact mechanism how ceramide regulates PLD activity in endothelial cells is beyond the present manuscript. It should be indicated that the observed inhibition of PLD by ceramide is in accordance with several previous studies already demonstrating an inhibition of PLD by ceramide (for instance 29, 30, 31, 32, 33).
We restricted our analysis to a very well-defined human study group allowing us very defined biochemical studies that are not compromised by confounding variables. We believe that our study group is very well suited to define the role of blood plasma ceramide in the pathogenesis of MDD, while a separate study using a larger, less selected population of patients needs to be performed to test whether blood plasma ceramide can be also used as diagnostic marker for MDD.
Our studies are in accordance with previous studies that indicated a central role of endothelial cells in the pathogenesis of MDD (6, 34, 35, 36). Thus, it was demonstrated that chronic social stress as a mouse model of depression reduced expression of the endothelial cell tight junction protein claudin-5, altered blood–brain barrier permeability, and increased concentrations of the peripheral cytokine interleukin-6 in brain parenchyma (6). It is possible that ceramide, possibly via reduced activity of PLD and formation of phosphatidic acid, alters the blood–brain barrier and thereby determines major depression. Further studies also indicate endothelial dysfunction in patients with MDD; specifically, these studies demonstrated a decrease of endothelial progenitor cells in MDD patients (36). It is unknown whether ceramide has an impact onto the formation and/or integration of endothelial progenitor cells.
A recent manuscript demonstrated that blood plasma collected from volunteerly running mice contained higher concentration of clusterin than blood plasma from „resting“ mice (34). Clusterin reduced neuroinflammation in a mouse model of Alzheimer´s disease (34). Although these studies did not investigate MDD, they demonstrate that blood plasma factors have a marked impact on brain functions. Finally, we have previously shown that endothelial cell ceramide in the hippocampus determines proliferation of neuronal stem cells (35), consistent with the present studies.
Collectively, these studies indicate a central role of endothelial cells in the pathogenesis of MDD, although the molecular details are largely unknown. The present study provides important insights into the molecular changes of endothelial cells in the hippocampus in MDD and how these changes contribute to MDD.
We suggest that phosphatidic acid is one of the factors that mediates the crosstalk between endothelial cells and neurons and prevents MDD. It is unknown how phosphatidic acid prevents MDD. However, it has been previously shown that phosphatidic acid regulates mTOR signaling in mammalian cells (37). Signaling of mTOR has been also shown to regulate synapse formation upon treatment with ketamine (38). Thus, it is tempting to speculate that phosphatidic acid does not only act in endothelial cells but also in neurons and that mTOR integrates signaling of phosphatidic acid and ketamine to regulate neuronal functions and prevent MDD. Future studies are required to analyze the exact targets of phosphatidic acid in the hippocampus.
A recent study showed that another CNS symptom, anxiety in mice upon application of electronic foot shocks, is caused by mitochondrial fission in CD4+ T cells, which results in the synthesis of purines. Xanthine derived from CD4+ T cells binds to adenosine A1 receptors in the amygdala and thereby induces anxiety (39). This is another example of a CNS symptom, i.e., anxiety that is caused by the communication of peripheral cells with the CNS. However, the purine-mediated mechanism seems to be independent of the present results since purine was shown to play no role in stress responses to corticosterone (39).
Previous studies demonstrated that antidepressants target the acid sphingomyelinase in the hippocampus, in particular in hippocampus neurons (19, 20). Inhibition of the acid sphingomyelinase resulted in increased sphingomyelin levels, which stimulated protein phosphatase 2A via inhibition of sphingomyelin synthase and accumulation of ceramide in the endoplasmatic reticulum. Increased protein phosphatase 2A activity stimulated autophagy in neurons of depressed/stressed mice, which was markedly reduced in depressed/stressed mice, and thereby normalized behavior and neurogenesis upon treatment with antidepressants. It is important to note that these studies already revealed that stress did not change ceramide levels in the total hippocampus and that mice lacking the acid sphingomyelinase responded to stress with the development of MDD symptoms. Thus, these pharmacological and genetic studies demonstrated that the acid sphingomyelinase is central for the response to antidepressants. As mentioned, deficiency of the acid sphingomyelinase did not protect from induction of major depression upon stress, suggesting that in the stress-induced model of MDD, the acid sphingomyelinase does not play a role and that ceramide is generated independently of the acid sphingomyelinase in this model of MDD. On the other hand, overexpression of the acid sphingomyelinase has been shown to induce depressive-like symptoms in mice (40), suggesting that an overactivity of the enzyme with a reduction of sphingomyelin and an increase of ceramide triggers MDD. It is possible that different stimuli activate different pathways of ceramide generation and/or accumulation of ceramide in endothelial cells and/or neurons of the hippocampus and thereby induce MDD. In this model, the accumulation of ceramide in endothelial cells plays a key role in the pathogenesis of MDD, regardless which enzyme generates ceramide.
It is very important to note that treatment with anti-ceramide antibodies, ceramidase, PLD, or phosphatidic acid reverses the symptoms of MDD in mice as early as 24 h after administration. The action is remarkably fast and indicates that stress, via peripheral ceramide, induces functional changes in the brain that can be rapidly corrected, similar to the fast action of ketamine (11). In addition, treatment with anti-ceramide antibodies, ceramidase, or phosphatidic acid has a long-term effect on MDD-like behavior, and a single treatment prevents a relapse for at least 4 days even under continuous exposure to stress. The rapid beneficial effect of ceramide neutralization on MDD is in marked contrast to the delayed onset of action of conventional antidepressants, which is clinically a major problem in patients with MDD. We assume that neutralization/consumption of ceramide allows a recovery of neuronal functions that are stable for a longer time. Thus, clinically one or two treatments per week might be sufficient to obtain a fast-acting, safe, and therapeutic effect with anti-ceramide antibodies, ceramidase, or phosphatidic acid.
Our findings also explain many of the peripheral symptoms often associated with MDD: Ceramide has been shown to induce cardiovascular disease and to promote oxidative stress (41); it also exerts a proinflammatory effect and mediates the effects of proinflammatory cytokines (16, 17). Furthermore, phosphorylation of ceramide to ceramide 1-phosphate may be involved in the activation of phospholipase A2, an alteration also observed in MDD (42).
Our studies indicate that neutralization or consumption of blood plasma ceramide may serve as a novel, fast-acting targeted treatment option for MDD. Antibodies or enzyme therapies are widely used in medicine, and we assume that anti-ceramide antibodies or ceramidase exert no serious adverse effects, because ceramide is usually not present on the extracellular leaflet of the cell surface in healthy organisms. Likewise, application of phosphatidic acid or even PLD might also be novel approaches to treat MDD.
Experimental procedures
Ethics statement
The study was approved by the Ethics Committees of the Medical Faculty of the Friedrich-Alexander University Erlangen-Nürnberg (FAU, ID 148_13 B, 17.07.2013) (43) of the University of Duisburg-Essen (Nr. 16–7061-BO) and of the Nordwest-und Zentralschweiz (EKNZ; No. 2015–148).
The studies followed the Helsinki guidelines. All patients gave informed consent.
Human studies
In a first set of studies, we used 16 plasma samples from unmedicated patients with MDD and 16 samples from healthy controls. All patients in this study group showed severe depression and were not treated with any antidepressant for at least the last 2 weeks. It is very difficult to find such a severely affected patient group allowing studies that are not potentially compromised by previous recent treatment. In addition, patients were clinically extensively phenotyped. In detail, all participants underwent a multistep screening procedure to exclude severe physical and psychiatric morbidities, the use of corticosteroids or anti-inflammatory drugs in the past 7 days, in addition to excluding mediation of any antidepressant for at least the last 14 days. For diagnosis and exclusion of psychiatric comorbidities, we used the structured clinical interview from the DSM-IV (SKID-I) and quantified depression severity using the Beck Depression Inventory-II, the 17-item HAM-D, and the Montgomery-Åsberg Depression Rating Scale.
In a second set, we used plasma from eight patients with MDD and nine healthy control subjects. All patients were also extensively investigated with neuropsychological tests, in particular the Beck Depression Inventory-II, the HAM-D, and the Geriatric Depression Scale.
Depressed patients were recruited from inpatients and outpatients of the Departments of Psychiatry and Psychotherapy at the University Hospitals Erlangen, Essen, and Basel and further interested individuals in parallel to local citizens as healthy controls. Healthy individuals had no history of MDD. All participants provided written informed consent. Controls were matched in regard to age, sex, and body mass index. It is important to note that we only included patients that were without medication at the time point of inclusion. All blood samples were collected after overnight fasting in the morning for all individuals to minimize circadian effects. In the primary study group, blood was drawn into Lithium heparin vials (Sarstedt, 9 ml, #02.1065). The samples were centrifuged (10 min at 2000g at room temperature), and plasma aliquots of 1.8 ml were prepared in polypropylene screw cap vials (Kuhnle, 2 ml, vials 4–1563–00, lids 4–1540–00) and stored at −80 °C for later analysis.
In the second population (supporting information), blood was drawn into EDTA tubes (Sarstedt, 9 ml, #04.1951.100) and processed as above. 1.5 ml aliquots were frozen at −80 °C in sialynized Eppendorf tubes.
We also tested for binding of ceramide to the tubes by addition of 0.2 μCi [14C16]-ceramide (ARC0831, 55 mCi/mmol) to blood plasma that was obtained from healthy volunteers and prepared as above. The samples were stored for 4 or 8 weeks at −80 °C, thawed, aliquots of the plasma were removed, and the radioactivity was counted and compared to the original radioactivity. We observed a recovery of the total radioactivity of 95% indicating that ceramide did not bind to plastic, very likely because the affinity to plasma proteins is higher than to the plastic surface. In addition, 50 μl plasma aliquots were extracted in 150 μl H2O and 600 μl CHCl3:CH3OH:HCl (100:100:1, v/v/v). The lower phase was dried, samples were resuspended in CHCl3:CH3OH (1:1, v/v), and separated by thin-layer chromatography using CHCl3:CH3OH:ammoniumhydroxide (90:20:0.5, v/v/v) as the developing solvent. The plates were analyzed using a Fuji-Imager, and ceramide was quantified by comparison with standards. We did not detect any degradation of ceramide indicating that ceramide remained stable during storage at -80 oC and was not degraded.
In addition to screening for the presence of a major depressive episode using the structured clinical interview for DSM-IV (SKID-I), depression severity was quantified by the HAM-D (44).
Mice and treatments
All studies were performed in accordance with animal permissions of the State Agency for Nature, Environment and Consumer Protection (LANUV) NRW, Recklinghausen, Germany, # 81-02.04.2017.A084, # 81-02.04.2019.A211, # 81-02.04.2018.A413, and 81-02.2019.A003, and the local IACUC.
We used C57BL/6 mice wildtype mice on a C57BL/6 background at an age of 8 to 12 weeks. We used females in the present studies since they can be housed together and do not need to be isolated.
Glucocorticosterone (Sigma) was administered via the drinking water at 100 mg/L for 7 days. In the chronic unpredictable stress model, the mice were challenged for 7 days with unpredictable environmental stress, i.e., a reversal of the light/dark cycle, 3 h of 45° tilting of the cage twice each week, shaking at 125 rpm for 45 min, food deprivation for 14 h, predator sounds for 15 min, or wet cages for 1 h with two forms of stress per day in a randomized (unpredictable) order. Blood was collected from the heart into heparin-coated needles and tubes immediately after the mice were sacrificed by cervical dislocation. Blood plasma was centrifuged at 830g for 5 min at 4 °C in an Eppendorf centrifuge. The plasma was carefully removed and shock frozen in liquid nitrogen.
Bromodeoxyuridine (BrdU, 2 mg/25 g body weight) was intraperitoneally injected three times, once every 2 h, at a dose of 75 mg/kg, starting 16 h before the mice were sacrificed.
Anti-ceramide antibodies clone S58-9 (0.08 μg/g body weight; purified IgM anti-ceramide antibodies, Glycobiotech, #MAB0011), clone 15B4 (0.08 μg/g body weight; purified IgM anti-ceramide antibodies, Sigma, #C8104), a rabbit monoclonal anti-ceramide IgG (0.08 μg/g body weight; Antibody Research Corporation, #111583), control IgM or IgG (0.08 μg/g body weight, Dako), and recombinant neutral ceramidase (0.04 μg/g body weight; Asah2, R&D, 3000 pmol/min/μg) were intravenously injected for in vivo treatment of mice with stress-induced MDD.
Recombinant GPI-PLD protein (Abcam, #ab116799) was diluted in PBS and intravenously injected at 100 ng/g. Phosphatidic acid (Sigma, #1535722) was suspended at 1 mg/ml in PBS, sonicated to obtain a stable suspension, and intravenously injected at 4 μg/g body weight.
The PLD inhibitor FIPI (ChemCruz, # sc300694) was dissolved in DMSO and i.v. injected at 4 μg/g body weight.
The reagents were injected 24 h and 12 h prior to any biochemical or behavioral study. In the long-term studies, we injected anti-ceramide antibodies clone S58-9, neutral ceramidase or phosphatidic acid twice at 6 AM and 6 PM on day 7 and day 10 after initiation of the stress and then analyzed behavior and neurogenesis 4 days later.
To achieve in vitro neutralization or consumption of ceramide, we incubated 125 μl blood plasma with 0.5 μg purified IgM anti-ceramide antibodies clones S58-9 or 15B4 or IgG anti-ceramide antibodies, 0.5 μg control IgM or control IgG or 1 μg recombinant neutral ceramidase.
Measurement of ceramide by kinase assay
We added 20 μl of blood plasma to 180 μl H2O and extracted it in 600 μl CHCl3:CH3OH:1N HCl (100:100:1, v/v/v). The lower phase was collected, dried, resuspended in 20 μl of a detergent solution (7.5% [w/v] n-octyl glucopyranoside and 5 mM cardiolipin in 1 mM diethylenetriamine-pentaacetic acid), and micelles were obtained by bath sonication for 10 min. The kinase reaction was initiated by the addition of 70 μl of a reaction mixture containing 10 μl diacylglycerol kinase (GE Healthcare Europe), 0.1 M imidazole/HCl (pH 6.6), 0.2 mM diethylenetriamine-pentaacetic acid, 70 mM NaCl, 17 mM MgCl2, 1.4 mM ethylene glycol tetraacetic acid, 2 mM dithiothreitol, 1 μM adenosine triphosphate, and 5 μCi [32P]γATP. The kinase reaction was performed for 60 min at room temperature under shaking at 300 rpm. The reaction was terminated by the addition of 1 ml CHCl3:CH3OH:1N HCl (100:100:1, v/v/v), 170 μl buffered saline solution (135 mM NaCl, 1.5 mM CaCl2, 0.5 mM MgCl2, 5.6 mM glucose, and 10 mM Hepes; pH 7.2), and 30 μl of a 100 mM ethylenediaminetetraacetic acid solution. The samples were vortexed, phases were separated, and the lower phase was collected. The samples were then dried, separated on Silica G60 thin-layer chromatography plates with chloroform/acetone/methanol/acetic acid/H2O (50:20:15:10:5, v/v/v/v/v), and developed with a Fuji phosphorimager. Ceramide levels were determined by comparison with a standard curve; C16 to C24 ceramides were used as substrates.
Sphingolipid quantification by liquid chromatography tandem-mass spectrometry
Samples were subjected to lipid extraction with 1.5 ml methanol/chloroform (2:1, v/v) as described (22). The extraction solvent contained C17 ceramide (Cer17), C16-d31-sphingomyelin (d31-SM16), and d7-sphingosine (d7-Sph) (all Avanti Polar Lipids) as internal standards. In order to track deuterated sphingolipids after loading plasma and exosomes with d7-C16 ceramide (d18:1-d7/16:0), d7-Sph was replaced by C17 sphinganine (C17 dhSph) (Avanti Polar Lipids) as reference compound. Chromatographic separations were achieved on a 1260 Infinity HPLC (Agilent Technologies) equipped with a Poroshell 120 EC-C8 column (3.0 × 150 mm, 2.7 μm; Agilent Technologies). A mobile phase system consisting of water (solvent A) and acetonitrile/methanol (1:1, v/v; solvent B), both acidified with 0.1% formic acid, was used for gradient elution at an initial composition of 40:60 (A:B, v/v) and a flow rate of 0.5 ml/min. MS/MS analyses were carried out using a 6490 triple-quadrupole mass spectrometer (Agilent Technologies) operating in the positive electrospray ionization mode (ESI+). The following ion source parameters were set: sheath gas temperature, 375 °C; sheath gas flow, 12 L/min of nitrogen; nebulizer pressure, 30 psi; drying gas temperature, 200 °C; drying gas flow, 15 L/min of nitrogen; capillary voltage, 4000 V; nozzle voltage, 1500 V; iFunnel high pressure RF voltage, 150 V, and iFunnel low pressure RF voltage, 60 V. Sphingolipids and their deuterated analogs (m/z ratios given in parentheses) were analyzed by selected reaction monitoring using the following mass transitions: m/z 288.5 → 270.5 for C17 dhSph, m/z 300.3 (307.3) → 282.3 (289.3) for Sph, m/z 520.5 (527.6) → 264.3 (271.3) for Cer16, m/z 534.5 → 264.3 for Cer17, m/z 548.5 (555.6) → 264.3 (271.3) for Cer18, m/z 576.6 (583.6) → 264.3 (271.3) for Cer20, m/z 604.6 (611.6) → 264.3 (271.3) for Cer22, m/z 630.6 (637.7) → 264.3 (271.3) for Cer24:1, m/z 632.6 (639.7) → 264.3 (271.3) for Cer24, m/z 703.6 (710.6) → 184.1 for SM16, m/z 731.6 (738.7) → 184.1 for SM18, m/z 734.8 → 184.1 for d31-SM16, m/z 759.6 (766.7) → 184.1 for SM20, m/z 787.7 (794.7) → 184.1 for SM22, m/z 813.7 (820.7) → 184.1 for SM24:1 and m/z 815.7 (822.7) → 184.1 for SM24. Quantification was performed with MassHunter Software (Agilent Technologies). Determined sphingolipid amounts were normalized to the actual protein content or volume of the sample used for lipid extraction. Ceramide contents in plasma (murine and human) are expressed as sums of the individually determined subspecies C16, C18, C20, C22, C24, and C24:1 ceramide.
Loading of plasma with ceramide
Plasma (200 μl) was loaded with a total of 5 nmol of C16, C18, C20, C22, C24, and C24:1 ceramides at a ratio of 5.2%: 2.4%: 2.5%: 13.6%: 55.5%: 20.8%. Since the blood plasma volume of mice is approximately 1 ml, we loaded 200 μl blood plasma with a 5-fold concentration of the different ceramides than observed in blood plasma of stressed mice and injected these 200 μl intravenously. All ceramides were suspended in 0.9% NaCl and sonicated prior to use to get a homogenous suspension. Blood plasma was incubated with the ceramide mixture for 60 min at 37 °C, 125 rpm shaking in glass tubes, and then directly injected i.v. into healthy mice.
Immunohistochemical bromodeoxyuridine staining
For BrdU staining, mice were injected with BrdU 3-times, once every 2 h. Mice were euthanized and perfused via the left heart for 2 min with 0.9% NaCl followed by a perfusion with 4% paraformaldehyde buffered in PBS (pH 7.3) for 15 min. Brains were removed, fixed for an additional 36 h in 4% buffered paraformaldehyde in PBS, embedded in paraffin, serially sectioned, dewaxed, incubated for 30 min with pepsin paraffin-embedded sections were dewaxed, treated for 20 min with pepsin at 37 °C, washed, incubated for 2 h with 50% formamide in 300 mM NaCl and 30 mM sodium citrate (pH 7.0) at 65 °C, and washed twice in saline sodium citrate buffer. DNA was denatured for 30 min at 37 °C with 2 M HCl, washed, neutralized for 10 min with 0.1 M borate buffer (pH 8.5), washed, and blocked with 0.05% Tween 20 and 5% FCS in PBS (pH 7.4). The samples were then stained for 45 min at 22 °C with 5 μg/ml BrdU-specific antibodies (Roche, # 111703760001), washed, and stained with Cy3-coupled F(ab)2 anti-mouse IgG antibody fragments (Jackson ImmunoResearch).
All sections were analyzed with a LEICA TCS SL fluorescence confocal microscope. Every 10th section of serial sections of the hippocampus was counted by an investigator blinded to the nature of the samples. We counted each 10 sections/mouse, and the numbers were then extrapolated to the total number of proliferating cells by multiplication with 10.
Cultured endothelial cells
bEND3 cells (ATCC; LGC Standards) were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal calf serum, 10 mM penicillin/streptomycin, 1 mM sodium pyruvate, and 2 mM L-glutamine.
Primary human brain cortex microvascular endothelial cells, Passage 3, were from Cell Systems, were cultured according to the instructions of the vendor.
For treatment, human brain endothelial or bEND3 cells were incubated for 60 min to 4 h with 0.5 μM or 1 μM C24 ceramide (Avanti Polar Lipids/Merck, #860524) or left untreated. C24 ceramide was suspended in 0.9% NaCl and sonicated using a tip sonicator prior to addition to the cells. Samples were shock frozen, thawed, and the lysates were used to determine PLD activity and phosphatidic acid as described below.
Isolation of endothelial cells from the hippocampus
Mice were stressed or injected with ceramide-loaded blood plasma as above, sacrificed, the hippocampus removed, digested for 10 min in 0.1% dispase (R&D, #D4693), 0.005% liberase (Roche, #134262), and 0.01% DNase (Roche, #4536282001) in HEPES/saline (H/S; 132 mM NaCl, 20 mM HEPES [pH 7.4], 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2, 0.8 mM MgSO4) at 37 oC for 10 min, and cells were isolated by pipetting. Cells were then washed 3-times in ice-cold H/S, resuspended in H/S and incubated for 30 min with anti-CD31 antibodies coupled to magnetic beads (20 μl/sample, Miltenyi, #120–009–894) at 4 °C. Cells were then purified using Miltenyi LS columns (#130–042–401), and endothelial cells were magnetically retained. Columns were extensively washed with H/S, endothelial cells were eluted, pelleted by centrifugation at 425g for 7 min, and resuspended in H2O, and ceramide was biochemically quantified. As an independent approach, we incubated the cells with anti-CD62E antibodies (2 μg/sample, Abcam, #18981) for 30 min at 4 °C, followed by addition of anti-rabbit IgG Dynabeads (30 μl/sample, Thermofisher #M280) and further incubation for 30 min. Cells bound to the magnetic beads were isolated using a magnet and washed on the magnet, and ceramide was biochemically quantified as above.
As control, we stained the cells sorted with Miltenyi anti-CD31 beads with with PE-coupled anti-CD62 E antibodies (1:100) and the Dynabead-sorted cells with FITC-anti-CD31 antibodies (1:100, Becton Dickinson #550274) and analyzed the samples by flow cytometry using a BD FACS Calibur. Controls were stained with PE coupled rabbit IgG and FITC-coupled rat-IgG.
PLD activity
PLD activity was measured employing a commercial colorimetric activity assay (Abcam, #ab183306). The assay is based on the cleavage of phosphatidylcholine to choline depending on the activity of PLD. Choline is oxidized, and the intermediate reacts with a probe to a color detected at 570 nm in a plate reader. The assay was performed according to the instructions of the vendor. Briefly, hippocampus was removed, homogenized in assay buffer, incubated on ice for 15 min, insoluble material was removed by 5 min centrifugation at 14,000 rpm at 4 °C, and an aliquot of the lysate was mixed with the PLD substrate, the PLD probe, and the converter enzyme to generate the colored substrate. Each sample was measured against a background sample that contained the same amount of lysate and reagents with the omission of PLD substrate. Samples were analyzed at absorbance 570 nm in a microplate reader (Fluostar Omega, BMG Labtech) in kinetic mode every 2 min, and the enzyme activity was calculated and normalized for protein determined as described below.
To determine PLD activity in cultured or freshly isolated hippocampus endothelial cells, the medium was removed, the cells washed, homogenized, and the enzyme activity was measured.
Phosphatidic acid analysis
Phosphatidic acid was measured in hippocampus extracts and in cultured endothelial cells extracts using a commercial assay (PromoKine, #PK-CA577-K748) exactly following the protocol of the vendor. Hippocampus was removed, homogenized by tip sonication in 200 μl H2O, and extracted in 750 μl CHCl3:CH3OH:12N HCl (2:4:0.1, v:v:v) and each 250 μl CHCl3 and 1 M NaCl. Samples were vortexed, the organic phase was dried in a speed vac, samples were dissolved in 5% Triton X-100, and the reaction was started by addition of a phosphatidic acid converter that hydrolyzes phosphatidic acid to an intermediate that is converted to a fluorescence substrate in the presence of an enzyme mix. Samples were analyzed in a fluorescence reader at excitation/emission of 535/587 nm. The amount of phosphatidic acid was calculated by comparison to a standard curve.
Protein measurements
Protein was measured using the Bio-Rad Protein Assay Dye (#500006) from aliquots of the hippocampus homogenates or cell lysates and served to normalize the samples.
Clinical chemistry analysis
Blood plasma was analyzed using a SpotChem EZ chemistry analyzer with the corresponding parameter strips (Scil). When necessary, samples were diluted to enable measurement of small sample volumes or when samples exceeded the maximum detection limit of a given parameter. Creatinine levels were quantified using a colorimetric assay kit (Cayman).
TUNEL assays
Mice were sacrificed 12 h after the last application of anti-ceramide antibodies or phosphatidic acid. The heart, lung, liver, and kidney were removed, and frozen sections were obtained. Sections were air-dried for 5 min, fixed in ice-cold acetone for 5 min, and cell death was determined using TUNEL staining. To this end, sections were treated in 0.1 M sodium citrate (pH 6.0) in a microwave at 450 W for 5 min, washed twice in PBS, and incubated with each 5 μl TUNEL enzyme, 20 μl tetramethylrhodamine labeling, and 25 μl TUNEL dilution buffer (all from Roche) according to the vendor’s instructions (Roche). Samples were incubated for 60 min at 37 °C and washed three times in PBS. The samples were then incubated in PBS at 70 °C for 10 min to reduce background staining, washed once in PBS, and finally embedded in Mowiol (Sigma, Deisenhofen, Germany). Incubating the samples with DNAse served as a positive control for the TUNEL reaction. We then analyzed at least 500 cells in the tissues and determined the percentage of TUNEL-positive cells. The investigator was blinded to the identity of the samples. Samples were analyzed on a LEICA TCS SL microscope.
Behavioral studies
Behavioral testing was performed between 3:00 PM and 6:00 PM under diffuse indirect room light. All tests were performed on separate days. If appropriate, animals were tracked with a video camera (Noldus Systems). For the novelty-suppressed feeding test (latency to feed), mice were fasted for 24 h. We then recorded the time during which the mice explored a new environment before they began eating. For the light/dark box test, mice were placed in a dark and safe compartment that was connected via a 5-cm × 5-cm rounded-corner aperture to an illuminated, open, and thus aversive area. The time that the mouse spent in each of the separate compartments was measured. In the open-field arena test, the mice were released near the wall of a 50-cm x 50-cm white plastic cage with sidewalls 30 cm high. Animals were observed for 30 min, and the time during which the animal was more than 10 cm away from the wall was recorded. In the coat state test, the appearance of the coat (groomed versus unkempt coat) was scored on the head, neck, back, and ventrum with either a zero for normal status or a one for unkempt status. For the forced swim test, mice were placed in a cylinder filled with water (21–23 °C) for 15 min. After 24 h, the mice were again placed in a water-filled cylinder for 6 min, and the time of mobility or immobility during the last 4 min of the second trial was recorded. Mice were judged immobile when they moved only to keep their heads above water.
Quantification and statistical analysis
Data are expressed as arithmetic means ± SD. For the comparison of continuous variables from independent groups with one variable (treatment), we used one-way ANOVA followed by post-hoc Tukey test for all pairwise comparisons, applying the Bonferroni correction for multiple testing. The p values for the pairwise comparisons were calculated after Bonferroni correction. All values were normally distributed, and the variances were similar. For the analysis of groups with two variables (treatment and genotype), we used one-way ANOVA and post hoc Tukey test for multiple comparison. Statistical significance was set at a p value of 0.05 or lower (two-tailed). The sample size planning was based on the results of two-sided Wilcoxon–Mann–Whitney tests (free software: G∗Power, Version 3.1.7, University of Duesseldorf, Germany). Investigators were blinded to results of histologic analyses and to animal identity. Before the experiments, animals were randomly assigned to cages by a technician who was not involved in the experiments. Cages were randomly assigned to the various experimental groups.
Based on the results obtained with stressed mice, we calculated the number, i.e., the sample size, of patients required to determine whether ceramide is increased in the blood plasma of patients with major depressive disorder with a power of at least 0.95 and α of less than 0.05.
The ceramide kinase data of the mouse experiments were (mean ± SD):
untreated: 1600 ± 382.8 pmol/mL
treated with glucocorticosterone: 5565 ± 594.4 pmol/mL
treated with chronic unpredictable stress: 4113 ± 314.6 pmol/mL
n=8 for all samples
The mass spectrometry data of the mouse experiments were (mean ± SD):
untreated: 1523 ± 394.4 pmol/mL
treated with glucocorticosterone: 5372 ± 476.7 pmol/mL
treated with chronic unpredictable stress: 4210 ± 187.7 pmol/mL
n=6 for all samples
We then assumed that the standard deviation in human patients is 3-fold higher than in mice (ceramide kinase assay: 1148.4/1783.2/943.8 and mass spectrometry 1183.2/1430.1/563.1). Since our study included severely depressed patients, we assumed similar ceramide levels than in severely depressed mice.
Based on the mouse data and the assumption of higher standard deviation in humans, we calculated effect sizes and the required number of blood samples from healthy humans and individuals with major depression using the freely available program G∗Power 3.1.
The results indicate a required sample size of five when using the data obtained in the kinase assays untreated versus glucocorticosterone and a sample size of six using the data obtained in the kinase assays untreated versus chronic environmental stress.
For the corresponding mass spectrometry data, the sample sizes were each five.
Our studies included 16 healthy controls and 16 patients with severe MDD (i.e., 6 patients with a Hamilton Depression Score of 28–37 and 10 patients with a Hamilton Depression Score of 23–27), indicating a high statistical power (at least 0.95) of the present data at α of less than 0.05.
The second study population presented in the supplementary data included nine healthy controls and eight patients with severe MDD also providing high statistical power (at least 0.95) at α of less than 0.05.
We controlled for age and sex as calculated by ANCOVA using the SPSS28 software.
Data availability
All data are contained within the manuscript.
Supporting information
This article contains supporting information
Conflict of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This study was supported by DFG grants GU 335/29 to -3 and GU 335/32 to -2 to EG, KO 947/13 to -3 to JK and MU 2789/8 to -2 to CPM. CM is an associated fellow of the research training group 2162 funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 270949263/GRK2162.
We thank Bernd Lenz and Claudia von Zimmermann for the organization of the CeraBiDe study and recruitment of participants. Furthermore, we acknowledge the excellent support of Daniel Herrmann for the LC-MS analyses.
Author contributions
E. G., J. K., A. C., G. C. W., and C. M. conceptualization; E. G. and M. J. E. methodology; E. G. and M. J. E. supervision; E. G., B. W., S. K., M. S., F. S., B. K., and G. C. W. investigation; J. K., C. M., C. P. M., C. L., U. E. L., B. W. M., and N. S. resources; E. G., B. W., S. K., M. S., F. S., B. K., J. K., A. C., G. C. W., C. M., C. P. M., C. L., U. E. L., M. J. E., B. W. M., and N. S. writing-review and editing.
Funding and additional information
This study was supported by DFG grants GU 335/29-3 and GU 335/32-2 to E.G., KO 947/13-3 to J.K., and MU 2789/8-2 to C.P.M. C.M. is an associated fellow of the research training group 2162 funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—270949263/GRK2162.
Edited by Dennis Voelker
Supporting information
References
- 1.Belmaker R.H., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S., Yin Y., Du L. Blood-brain barrier dysfunction in the pathogenesis of major depressive disorder. Cell Mol. Neurobiol. 2021 doi: 10.1007/s10571-021-01153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filatova E.V., Shadrina M.I., Slominsky P.A. Major depression: one brain, one disease, one set of intertwined processes. Cells. 2021;10:1283. doi: 10.3390/cells10061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schloss P., Henn F.A. New insights into the mechanisms of antidepressant therapy. Pharmacol. Ther. 2004;102:47–60. doi: 10.1016/j.pharmthera.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Menard C., Pfau M.L., Hodes G.E., Kana V., Wang V.X., Bouchard S., et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017;20:1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 8.Malberg J.E., Eisch A.J., Nestler E.J., Duman R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willner P. Antidepressants and serotonergic neurotransmission: an integrative review. Psychopharmacology (Berl) 1985;85:387–404. doi: 10.1007/BF00429653. [DOI] [PubMed] [Google Scholar]
- 10.Brink C.B., Harvey B.H., Brand L. Tianeptine: a novel atypical antidepressant that may provide new insights into the biomolecular basis of depression. Recent Pat. CNS Drug Discov. 2006;1:29–41. doi: 10.2174/157488906775245327. [DOI] [PubMed] [Google Scholar]
- 11.Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 12.Moda-Sava R.N., Murdock M.H., Parekh P.K., Fetcho R.N., Huang B.S., Huynh T.N., et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:6436. doi: 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran D.V., Meyer J.P., Farber K.G., Chen X.R., Rosenthal B.D., Kellner C.H. Rapid response to electroconvulsive therapy: a case report and literature review. J. ECT. 2017;33:e20–21. doi: 10.1097/YCT.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 14.Frazer A., Benmansour S. Delayed pharmacological effects of antidepressants. Mol. Psychiatry. 2002;7:S23–S28. doi: 10.1038/sj.mp.4001015. [DOI] [PubMed] [Google Scholar]
- 15.Kryst J., Kawalec P., Pilc A. Efficacy and safety of intranasal esketamine for the treatment of major depressive disorder. Expert Opin. Pharmacother. 2020;21:9–20. doi: 10.1080/14656566.2019.1683161. [DOI] [PubMed] [Google Scholar]
- 16.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 18.Maes M., Smith R., Christophe A., Vandoolaeghe E., Van Gastel A., Neels H., et al. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta Psychiatr. Scand. 1997;95:212–221. doi: 10.1111/j.1600-0447.1997.tb09622.x. [DOI] [PubMed] [Google Scholar]
- 19.Gulbins E., Palmada M., Reichel M., Lüth A., Böhmer C., Amato D., et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat. Med. 2013;19:934–938. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- 20.Gulbins A., Schumacher F., Becker K.A., Wilker B., Soddemann M., Boldrin F., et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol. Psychiatry. 2018;23:2324–2346. doi: 10.1038/s41380-018-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira T.G., Chan R.B., Bravo F.V., Miranda A., Silva R.R., Zhou B., et al. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry. 2016;21:80–88. doi: 10.1038/mp.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracia-Garcia P., Rao V., Haughey N.J., Bandaru V.V., Smith G., Rosenberg P.B., et al. Elevated plasma ceramides in depression. J. Neuropsychiatry Clin. Neurosci. 2011;23:215–218. doi: 10.1176/appi.neuropsych.23.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunkhorst-Kanaan N., Klatt-Schreiner K., Hackel J., Schröter K., Trautmann S., Hahnefeld L., et al. Targeted lipidomics reveal derangement of ceramides in major depression and bipolar disorder. Metabolism. 2019;95:35–76. doi: 10.1016/j.metabol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Hong L., Hongmei W., Leijie X., Dandan Z., Peng L., Zhifei H., et al. Serum ceramide concentrations are associated with depression in patients after ischemic stroke-A two-center case-controlled study. Clin. Chim. Acta. 2021;518:110–115. doi: 10.1016/j.cca.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Demirkan A., Isaacs A., Ugocsai P., Liebisch G., Struchalin M., Rudan I., et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J. Psychiatr. Res. 2013;47:357–362. doi: 10.1016/j.jpsychires.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 26.David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I., et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner-Schmidt J.L., Duman R.S. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz A.M., Fan X., Bieri G., Smith L.K., Sanchez-Diaz C.I., Schroer A.B., et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venable M.E., Bielawska A., Obeid L.M. Ceramide inhibits phospholipase D in a cell-free system. J. Biol. Chem. 1996;271:24800–24805. doi: 10.1074/jbc.271.40.24800. [DOI] [PubMed] [Google Scholar]
- 30.Abousalham A., Liossis C., O'Brien L., Brindley D.N. Cell-permeable ceramides prevent the activation of phospholipase D by ADP-ribosylation factor and RhoA. J. Biol. Chem. 1997;272:1069–1075. doi: 10.1074/jbc.272.2.1069. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura S., Sakai H., Ohguchi K., Nakashima S., Banno Y., Nishimura Y., et al. Changes in the activity and mRNA levels of phospholipase D during ceramide-induced apoptosis in rat C6 glial cells. Neurochem. 1997;69:713–720. doi: 10.1046/j.1471-4159.1997.69020713.x. [DOI] [PubMed] [Google Scholar]
- 32.Singh I.N., Stromberg L.M., Bourgoin S.G., Sciorra V.A., Morris A.J., Brindley D.N. Ceramide inhibition of mammalian phospholipase D1 and D2 activities is antagonized by phosphatidylinositol 4,5-bisphosphate. Biochemistry. 2001;40:11227–11233. doi: 10.1021/bi010787l. [DOI] [PubMed] [Google Scholar]
- 33.Webb L.M., Arnholt A.T., Venable M.E. Phospholipase D modulation by ceramide in senescence. Mol. Cell Biochem. 2010;337:153–158. doi: 10.1007/s11010-009-0294-z. [DOI] [PubMed] [Google Scholar]
- 34.De Miguel Z., Khoury N., Betley M.J., Lehallier B., Willoughby D., Olsson N., et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021;600:494–499. doi: 10.1038/s41586-021-04183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulbins A., Grassmé H., Hoehn R., Wilker B., Soddemann M., Kohnen M., et al. Regulation of neuronal stem cell proliferation in the hippocampus by endothelial ceramide. Cell Physiol. Biochem. 2016;39:790–801. doi: 10.1159/000447789. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Vilchez I., Diaz-Ricart M., Navarro V., Torramade S., Zamorano-Leon J., Lopez-Farre A., et al. Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl. Psychiatry. 2016;6:e886. doi: 10.1038/tp.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2021;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 38.Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan K.Q., Li Y.Y., Wang H.L., Mao X.T., Guo J.X., Wang F., et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell. 2019;179:864–879. doi: 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Zoicas I., Schumacher F., Kleuser B., Reichel M., Gulbins E., Fejtova A., et al. The forebrain-specific overexpression of acid sphingomyelinase induces depressive-like symptoms in mice. Cells. 2020;9:1244. doi: 10.3390/cells9051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greaney J.L., Saunders E.F.H., Santhanam L., Alexander L.M. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ. Res. 2019;124:564–574. doi: 10.1161/CIRCRESAHA.118.313764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song C., Zhang X.Y., Manku M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J. Neurosci. 2009;29:14–22. doi: 10.1523/JNEUROSCI.3569-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mühle C., Wagner C.J., Färber K., Richter-Schmidinger T., Gulbins E., Lenz B., et al. Secretory acid sphingomyelinase in the serum of medicated patients predicts the prospective course of depression. J. Clin. Med. 2019;8:846. doi: 10.3390/jcm8060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.