Abstract
Psittacosis is a infectious disease caused by Chlamydia psittaci (C. psittaci), which presents as pneumonia in humans. The diagnosis of psittacosis is challenging, however, Metagenomic next-generation sequencing (MNGS) is very efficient. Herein we documented the clinical characteristics of two patients with severe C. psittaci pneumonia who were admitted to the Intensive Care Unit. C. psittaci nucleic acid sequences were detected by MNGS of bronchoalveolar lavage fluid from both patients. Doxycycline was administered and the treatment was effective. Implementation of MNGS is helpful for the early identification of pathogens, shortening the diagnosis and treatment time, and improving the prognosis of patients.
Keywords: Chlamydia psittaci, Community-acquired pneumonia, Metagenomic next-generation sequencing
1. Introduction
The incidence of Chlamydia psittaci (C. Psittaci) pneumonia is low, accounting for only 1% of community-acquired pneumonia (CAP) [1]. Due to the lack of specificity of clinical manifestations, rapid progression of the disease, and the low specificity and sensitivity of traditional detection modalities, the diagnosis of psittacosis is challenging. Metagenomic next-generation sequencing (MNGS) of bronchoalveolar lavage fluid (BALF) can rapidly and with a high sensitivity detect C. psittaci, which is much higher than the pathogen detection rate of traditional methods. In this paper, we retrospectively analyzed two cases of severe C. psittaci pneumonia admitted to the Department of Intensive Care Unit of the Second Affiliated Hospital of Fujian Medical University. The cases were analyzed in order to improve clinicians' understanding of the clinical characteristics, diagnosis and treatment methods of severe C. psittaci pneumonia, and to explore the application value of MNGS in the diagnosis of psittacosis.
2. Case presentation
Case 1
A 49-year-old man was admitted with a history of fever which peaked at 39.8 °C and dyspnea for 5 days. At the onset of symptoms, he visited the local hospital, his partial pressure of arterial oxygen was 42 mmHg, a chest computed tomography (CT) revealed bilateral diffuse infiltrates, and a small amount of pleural effusion. He was treated with antibiotics (details unknown) for 5 days, his fever decreased, but his dyspnea was progressively aggravated. He was then transferred to our hospital for further treatment. He has no medical history of disease. Upon arrival he had a fever of 37.5 °C, and a respiratory rate of 53 breathes/min. A physical examination revealed a number of moist rales in auscultation. The patient was in severe respiratory failure with mask oxygen inhalation, the oxygenation index was 171 mmHg. Laboratory test showed 13.3 × 10^9/L white blood cell count, 95.7%neutrophils, 2.2%lymphocytes, C-reactive protein (CRP) 94.77mg/L, and procalcitonin (PCT) 8.628ng/ml (Table 1).
Empirical treatment with imipenem-Cilastatin (1g intravenous (IV). drops (gtt) 8 hourly) in combination with linezolid (600mg, IV.gtt, 12 hourly) for the infection, methylprednisolone (40mg, IVgtt, 12 hourly) for anti-inflammation, and oseltamivir (150mg, per os (po), q12h) for anti-virus. Other measures include supportive and symptomatic treatment. Three hours after admission, the patient presented with intensified dyspnea with an accelerated respiratory rate of 64 breaths/minute, and an oxygen saturation (SPO2) level of 97%. Bedside endotracheal intubation and invasive ventilator-assisted ventilation were immediately performed (Bilevle positive airway pressure (BIPAP), parameters positive-end-expiratory pressure (PEEP) 10 cmH2O, and fraction of inspired oxygen (FiO2 70%)), and his respiratory rate decreased to 30 breaths/minute and SPO2 99–100%. On the 5th day of admission, the patient's fever subsided to normal ranges.
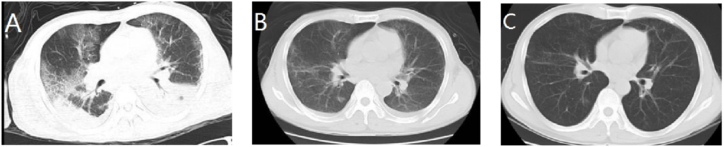
Bronchoalveolar lavage was conducted on day 3 after admission, and BALF was sent for testing using MNGS. C. psittaci (nucleic acid sequence was 289) was still being detected by MNGS of BALF on the 7th day of admission, so treated the patient with doxycycline (100mg, po, daily (qd)) for anti-chlamydia, subsequently we discontinued the methylprednisolone and empirical antiviral treatment. The patient was extubated successfully after his respiratory condition stabilized, and he was switched to nasal high flow oxygen therapy (FiO2 40%). On the 11th day of admission, a reexamination of chest CT showed that exudation and pleural effusion had reduced. On the 16th of admission, achromobacter xylosoxidans was detected in the final sputum culture report, so imipenem-cilastatin and linezolid were replaced with piperacillin-tazobactam (4.5mg, IV gtt, 8hourly) for anti-Gram-negative bacilli. However, his blood culture detected no pathogens throughout his admission. On the 20th day of admission, the patient was discharged since his condition had significantly improved. Oral doxycycline therapy was continued after discharge. One week later, the patient returned to the hospital for a reexamination of chest CT, which showed that the lesions in both lower lungs had significantly decreased since the initial CT (Fig. 1).
Case 2
A 50-year-old man was admitted with a cough for more than 10 days and a fever for 2 days. More than 10 days prior to admission, the patient had a non-irritating cough with a small amount of white sticky sputum. The patient visited the local hospital and received treatment with antibiotics (details unknown). His condition gradually deteriorated, he developing a fever which peaked at 38.5 °C, accompanied by transient disturbance of consciousness. The patient was transfered to our hospital for further diagnosis and treatment. His history revealed that he engaged in dust work for more than 30 years, and had has been smoking for more than 30 years (20 cigarettes/day). His fever at the time of arrival at the hospital was 38.6 °C. A physical examination revealed moist rales in auscultation. The patient was in respiratory failure under oxygen inhalation, the oxygenation index was 209 mmHg. White blood cell count was largely normal: total count 8.18 × 10^9/L, 84.9% neutrophils, 10.7% lymphocytes, CRP was 121.6mg/L, and PCT was 1.876ng/ml. CT manifestations included multiple patchy-like shadows especially in the right upper lobe, bronchial inflation sign, bilateral hilar, and mediastinal lymphadenopathy with calcification.
Empirical treatment with imipenem-cilastatin (1g, IVI gtt, 8hourly) for anti-infection and other measures include supportive and symptomatic treatment. Three hours after admission, the patient had progressed to having dyspnea with a SPO2 level of 93%, therefore, we replaced the oxygen inhalation method with nasal high-flow oxygen therapy (FiO2 55%). On the 3rd day of admission, the patient's symptoms had not significantly improved, therefore, we treated the patient with moxifloxacin (0.4g, po, daily) for the -infection and peramivir (100ml, IV gtt, daily) for antiviral. On the 5 ofadmission, the patient went into anaphylactic shock, therfore, moxifloxacin was replaced with linezolid (0.6g, IV gtt, 12hourly) for the infection. On the 7th day of admission, the patient's fever subsided. A reexamination chest CT indicated that the patchy-like shadows were smaller and had reduced from the previous CT, and the pleural effusion was almost completely absorbed. We then discontinued treatment of imipenem-cilastatin and switched to piperacillin-tazobactam (4.5mg, IV gtt, 8hourly). Throughout the patients' admission, no pathogen was detected in any of the 5 sputum cultures and 4 blood cultures that were collected.
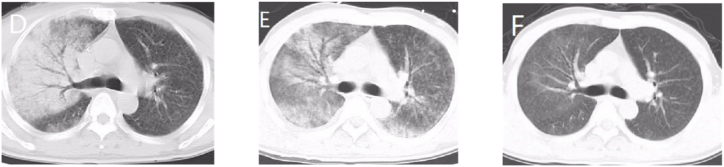
Bronchoalveolar lavage was conducted on day 9 of admission, and BALF was sent for testing using MNGS. On the 10th day of admission, C. psittaci (nucleic acid sequence was 8) was detected by MNGS of BALF, so we treated the patient with doxycycline (100mg, po, 12hourly) for anti-chlamydia, and discontinued the use of linezolid. The patient was discharged on the 14th day of admission since his condition had signficantly improved, he was discharged with oral doxycycline treatment. One week later, the patient returned to the hospital for a reexamination chest CT, which showed that the inflammatory lesions in both lungs were significantly reduced (Fig. 2).
Table 1.
Clinical characteristics of the two cases.
| Case 1 | Case 2 | |
|---|---|---|
| Sex | Male | Male |
| Age | 49 years old | 50 years old |
| Underlying disease | None | Silicosis |
| Personal History | History of contact with poultry | History of contact with poultry |
| Alcohol History | Smoking history | |
| Clinical manifestation | Fever (39.8 °C) Progressive dyspnea One bloody stools |
Fever (38.5 °C) Progressive dyspnea Cough with a small amount of white sticky sputum Transient disturbance of consciousness |
| Physical examination | Plenty moist rale in auscultation | Moist rale in auscultation |
| Arterial blood gas analysis | PH 7.55, PaCO2 29.1 mmHg, PaO2 60 mmHg, oxygenation index 171 mmHg | PH 7.5, PaCO2 31.4 mmHg, PaO2 73 mmHg, oxygenation index 209 mmHg; |
| Routine blood tests | WBC 13.3 × 10^9/L, NE%95.7%, LY% 2.2% | WBC 8.18 × 10^9/L, NE% 84.9%, LY% 10.7% |
| CRP | 94.77mg/L | 121.6mg/L |
| PCT | 8.628ng/ml | 1.876ng/ml |
| Biochemical indicators | Elevated liver enzymes Hypoproteinemia Hypokalemia and hyponatremia. |
Hypoproteinemia Hypokalemia and hyponatremia. |
| Sputum culture | Achromobacter xylosoxidans | no pathogen detected |
| Blood culture | no pathogen detected | no pathogen detected |
| Other serological test for pathogensa | Negative | Negative |
| CT | Bilateral diffuse infiltrates and a small amount of pleural effusion | Multiple patchy-like shadows and a small amount of pleural effusion |
| Respiratory support | Invasive mechanical ventilation | Mechanical ventilation |
| Main diagnosis | 1. Severe pneumonia 2. ARDS 3. Hepatic insufficiency |
1. Severe pneumonia 2. Silicosis |
WBC white blood cell, NE Neutrophil, LN Lymphocyte, CRP C-reactive protein, PCT Procalcitonin, CT computed tomography.
Other serological test for pathogens include serum-specific IgM antibodies to nine respiratory pathogens (include legionella pneumophila, mycoplasma pneumoniae, coxiella burnetii, chlamydia pneumoniae, adenovirus, respiratory syncytial virus, type A and type B influenza virus, parainfluenza virus), and RNA real-time fluorescent constant temperature amplification detection (include mycoplasma pneumoniae, enterovirus, enterovirus 71, coxsackievirus A16, and universal influenza A virus).
Fig. 1.
Chest CT of case 1: The initial CT scan (day 5) shows multiple exudations with bilateral lower lobe consolidation and pleural effusion (A). Chest CT (day 12) shows that exudation, lower lobe atelectasis, and pleural effusion decreased more than before (B). On follow up (1 week after discharge), the lesions in both lower lungs were significantly reduced and more localized, no significant effusion was observed (C).
Fig. 2.
Chest CT of case 2: The initial CT scan (day 1) shows multiple patchy-like shadows, especially in the right upper lobe and bronchial inflation (D). CT scan (day 7) shows that the patchy-like shadows were smaller and reduced than previouslye, and the pleural effusion was almost completely absorbed (E). On follow up (1 week after discharge), the patchy-like shadows almost disappeared and no obvious effusion was observed (F).
3. Discussion
Psittacosis is a zoonotic disease caused by C. psittaci which is a Gram-negative intracellular parasitic bacterium. C. psittaci was first isolated from parrots, with birds being the main host. Pigeons, geese, chickens, ducks, and other poultry are alsosources of infection. Human infections are mainly caused by pathogenic birds and their pollutants, of which aerosols formed by inhalation of sick birds and poultry excreta through the respiratory tract are mainly infected [1]. Both patients in this report had a history of poultry exposure.
The incubation period of psittacosis is usually 5–14 days. The general symptoms of infection are quite similar to those of upper respiratory tract infection. However, most patients have pneumonia, manifested as high fever, dyspnea, headache, myalgia, cough, and pulmonary infiltrative lesions. In addition to pneumonia and pulmonary complications, the infected patients have secondary systemic damage [2], such as endocarditis, hepatitis, encephalitis, etc. Psittacosis can affect multiple organs throughout the body. KnittlerMR and SachseK [3] reported that C. psittaci is more pathogenic and multiplies faster than other chlamydiae, therefore, C. psittaci causes a more severe inflammatory response and causes high mortality. Severe cases can rapidly progress to severe pneumonia with symptoms such as persistent high fever and dyspnea. Both patients in this report had progressive dyspnea, they both required admission to ICU and mechanical ventilation, and the patient in case 1 had caused the acute respiratory distress syndrome (ARDS). The changes in oxygenation index in the blood gas analysis of the two patients after admission were continuously monitored (Table 2), which indicated that both patients had severe respiratory failure.
Table 2.
Changes of arterial blood gas analysis results in the two patients.
| Date |
Case 1 |
Case 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PH | PaCO2 | PaO2 | FiO2 | PaO2/FiO2 | PH | PaCO2 | PaO2 | FiO2 | PaO2/FiO2 | |
| Day 1 | 7.54 | 35 | 215 | 100 | 215 | 7.51 | 27.4 | 82.7 | 35 | 236 |
| Day 2 | 7.51 | 37 | 89 | 70 | 127 | 7.29 | 44.5 | 111 | 35 | 317 |
| Day 3 | 7.48 | 39 | 139 | 65 | 215 | 7.48 | 29.3 | 97 | 35 | 277 |
| Day 4 | 7.50 | 41 | 152 | 55 | 276 | 7.47 | 29 | 53.7 | 35 | 153 |
| Day 5 | 7.48 | 45 | 123 | 45 | 273 | 7.46 | 25.5 | 57.0 | 45 | 190 |
| Day 6 | 7.46 | 48 | 109 | 40 | 272 | – | – | – | 45 | – |
| Day 7 | 7.52 | 43 | 67 | 40 | 168 | – | – | – | 40 | – |
| Day 8 | 7.53 | 40 | 65 | 40 | 163 | – | – | – | 35 | – |
| Day 9 | 7.50 | 38 | 90 | 35 | 257 | – | – | – | 35 | – |
| Day 10 | 7.47 | 38 | 69 | 35 | 197 | – | – | – | – | – |
It has been reported that most patients with C. psittaci pneumonia have normal or slightly elevated inflammation indexes such as white blood cell count, CRP, PCT, and ESR etc [4]. If C. psittaci pneumonia is combined with systemic damage, patients may experience liver damage with elevated liver enzymes, hyponatremia, and mild impairment of renal function [5]. In this report, changes in laboratory results were generally consistent with previous studies. It has also been reported that chest imaging of C. psittaci pneumonia has a few distinct characteristics and usually shows varying degrees of exudation and consolidation [6]. The most common of these characteristics include patchy ground-glass opacities and large confluent consolidations, generally distributed along the lung segments, starting in the upper lobe and continuing to develop into the lobe, eventually with predominant involvement of the lower lobe, occasionally accompanied by pleural effusion [7]. In this case, both patient's pulmonary infiltrative changes and a small amount of pleural effusion were consistent with previous reports, which indicated severe inflammation of the lungs, but these features are not sufficient to distinguish from other types of CAP.
In addition to the clinical manifestations of patients, a positive diagnosis of C. psittaci requires at least one of the following laboratory test [8]: (1) positive respiratory tract pathogen culture; (2) double serum antibodies in the acute and convalescent phases detected by complement fixation method showing a 4-fold or more increase; (3) IgM antibody titer detected by microimmunofluorescence method ≥1:16. However, serological tests are not suitable for the diagnosis of acute diseases because they required sera from both the acute and convalescent phases. In addition, Cps-inde184-PCR technology [9] was reported that ti is a specific and fastest detection testing for C. psittaci, while it is currently only carried out in the laboratory and cannot be carried out in most hospitals. Notably, the sensitivity of PCR rapidly decreases with the progression of the disease.
MNGS is an accurate detection method with a detection rate of nearly 89%, which is much higher than the traditional pathogen detection rate (25.73%) (P < 0.001) [10]. MNGS is based on high-throughput sequencing technology, analyzing the microbial nucleic acid (DNA and/or RNA) sequence in the sample [11], and identifying the pathogenic microorganism by comparing it with the nucleic acid sequence of the existing microorganism in the database. Theoretically, all pathogens in clinical practice can be detected, MNGS is especially suitable for rare and atypical complex infections. In addition, MNGS is less affected by the previous use of antibiotics [12]. MNGS can overcome the limitations of current diagnostic techniques, allow hypothesis-free, non-cultured pathogen detection directly from clinical specimens, and can obtain results within 48–72 hours.
When compared with MNGS, traditional sputum and blood pathogen culture is time-consuming and has a low detection rate [13]. C. psittaci is a strict intracellular bacteria that must be isolated in tissue culture or embryonated chicken eggs and it requires specific procedures for sample collection and transportation and needs to be sent to specialized laboratory facilities.
In most reports, where repeated sputum and blood cultures were not able to detect chlamydia, all patients were definitively diagnosed by MNGS [14]. For critically ill patients, it should be recommended that MNGS of BALF be the first choice to ensure timely and effective treatment [4].
The limitation of MNGS is that there is a lack of recognized interpretation criteria for MNGS sequence results [15]. The specific nucleic acid sequence for C. psittaci detected at the genus/species level in case 1 was 289 while in case 2 it was 8. Comparing the two sequences is not suitable since the assay was conducted by different companies. We speculate that the reason for the relatively low nucleic acid sequence of C. psittaci in case 2 is related to the treatment course of empirical antibiotic received by the patient before sample collection. It may also reflects that pathogen nucleic acid is degraded to some extent during the collection and transportation of samples. It should also be noted that the detected microbial nucleic acid sequence is only used as one of the diagnostic criteria, and clinicians must also take the clinical condition of the patient into consideration when making a diagnosis.
At present, tetracycline antibiotics are the first choice for the treatment of C. psittaci pneumonia [8], doxycycline or minocycline are the preferred choices. The second choice is macrolides drugs, however, fluoroquinolones drugs are also effective. Doxycycline takes effect rapidly within 24–48 hours. It is recommended that the course of treatment for critical patients is 2–3 weeks, otherwise, insufficient course can easily lead to recurrence [6]. Patients with life-threatening serious infections may require combination therapy with tetracyclines, macrolides, and quinolones.
Empirical anti-infection treatment with broad-spectrum antibiotics can be selected as first line treatment. Herein, case 2 used moxifloxacin, but it was discontinued due to the patient's allergic reaction. Although the symptoms tend to be relieved after empirical and symptomatic treatment, the definitive diagnosis still relies on MNGS of BALF.
4. Conclusion
-
•
It is recommended that MNGS should be performed as early as possible for patients with severe pneumonia, presenting with atypical pathogens and accompanied by high fevers, coughing, and large lung shadows.
-
•
MNGS greatly shortens the diagnosis and treatment time of the disease, and it has significant diagnostic value in patients with severe C. psittaci pneumonia.
-
•
The interpretation criteria of MNGS needs to be further studied in order to better guide clinical diagnosis and treatment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Contributor Information
Chunhua Luo, Email: luoch@fjmu.edu.cn.
Xuri Sun, Email: xuri_sun@fjmu.edu.cn.
References
- 1.Hogerwerf L., De Gier B., Baan B., et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 2017;145(15):3096–3105. doi: 10.1017/S0950268817002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojeda Rodriguez J.A., Modi P., Brady M.F. StatPearls [Internet] StatPearls Publishing; Treasure Island (Florida), PMID: 2021. Psittacosis pneumonia, 2021. [M] [PubMed] [Google Scholar]
- 3.Knittler M.R., Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog. Dis. 2015;73:1–15. doi: 10.1093/femspd/ftu007. [DOI] [PubMed] [Google Scholar]
- 4.Wu H.H., Feng L.F., Fang S.Y. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm. Med. 2021;21:300. doi: 10.1186/s12890-021-01673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rane V., Khailin K., Williams J., et al. Underdiagnosis of Chlamydia trachomatis and Chlamydia psittaci revealed by introduction of respiratory multiplex PCR assay with Chlamydiaceae family primers. Diagn. Microbiol. Infect. Dis. 2018;90:163–166. doi: 10.1016/j.diagmicrobio.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., Chen J., Shi X., et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect. Dis. 2021;21:621. doi: 10.1186/s12879-021-06205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutts I.I., Mackenzie S., White R.J. Clinical and radiographic features of psittacosis infection. Thorax. 1985;40:530–532. doi: 10.1136/thx.40.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balsamo G., Maxted A.M., Midla J.W., et al. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis) J. Avian Med. Surg. 2017;31:262–282. doi: 10.1647/217-265. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Angen Ø., Johannesen T.B., Petersen R.F., et al. Development of a species-specific real-time PCR test for Chlamydia psittaci and its employment in the investigation of zoonotic transmission from racing pigeons in Denmark. Diagn. Microbiol. Infect. Dis. Prepublish. 2021 doi: 10.1016/J.DIAGMICROBIO.2021.115341. [DOI] [PubMed] [Google Scholar]
- 10.Huang J., Jiang E., Yang D.L., et al. Metagenomic Next-Generation Sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect. Drug Resist. 2020;13:567–576. doi: 10.2147/IDR.S235182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simner P.J., Miller S., Carroll K.C. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin. Infect. Dis. 2018;66:778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao Q., Ma Y., Wang Q., et al. Microbiological diagnostic performance of metagenomic Next-generation sequencing when applied to clinical practice. Clin. Infect. Dis. 2018;67 doi: 10.1093/cid/ciy693. (suppl_2):S231–S240. [DOI] [PubMed] [Google Scholar]
- 13.Balsamo G., Maxted A.M., Midla J.W., et al. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J. Avian Med. Surg. 2017;31(3):262–282. doi: 10.1647/217-265. Sep. [DOI] [PubMed] [Google Scholar]
- 14.Teng X.Q., Gong W.C., Qi T.T., et al. Clinical analysis of metagenomic next-generation sequencing confirmed Chlamydia psittaci pneumonia: a case series and literature review. Infect. Drug Resist. 2021;14:1481–1492. doi: 10.2147/IDR.S305790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieuwenhuizen A.A., Dijkstra F., Notermans D.W., van der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect. Dis. 2018;18:442. doi: 10.1186/s12879-018-3317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]