Abstract
Bone injury repair has always been a tricky problem in clinic, the recent emergence of bone tissue engineering provides a new direction for the repair of bone injury. However, some bone tissue processes fail to achieve satisfactory results mainly due to insufficient vascularization or cellular immune rejection. Exosomes with the ability of vesicle-mediated intercellular signal transmission have gained worldwide attention and can achieve cell-free therapy. Exosomes are small vesicles that are secreted by cells, which contain genetic material, lipids, proteins and other substances. It has been found to play the function of material exchange between cells. It is widely used in bone tissue engineering to achieve cell-free therapy because it not only does not produce some immune rejection like cells, but also can play a cell-like function. Exosomes from different sources can bind to scaffolds in various ways and affect osteoblast, angioblast, and macrophage polarization in vivo to promote bone regeneration. This article reviews the recent research progress of exosome-loaded tissue engineering, focusing on the mechanism of exosomes from different sources and the application of exosome-loaded scaffolds in promoting bone regeneration. Finally, the existing deficiencies and challenges, future development directions and prospects are summarized.
Keywords: Exosomes, Biomaterials, Bone tissue engineering, Osteoinduction, Biocompatibility
Graphical abstract
1. Introduction
Bone defects caused by various events are one of the most common diseases treated in orthopedic surgery. With the aging population and the increase in traffic accidents, the incidence of bone defects has increased continuously worldwide. It is shown that 5%–10% of fracture patients suffer from union or nonunion. Risk factors, including advanced age, diabetes, smoking, and local infection, may inhibit the healing of bone defects [1,2]. Bone defects and subsequent nonunion not only bring tremendous mental and physical pain to patients but also lead to a long-term treatment process associated with a substantial economic burden [3]
Bone has the ability to regenerate. Bone regeneration is a complex process that requires the coordinated participation of multiple cells. The healing of bone regeneration is very slow. It takes about 12 weeks for a common fracture to develop into a mature callus, while it takes a year or two for the fracture to complete its final bone plasticity. However, for some large bone defects, the amount of bone regeneration required exceeds the body's ability to heal naturally [4,5]. Current clinical treatments for serious fractures or large bone defects are primarily based on autologous bone grafts, allogeneic grafts, xenogeneic bone grafts, and artificial bone grafts. However, bone grafts also have coexistent problems, such as infection, graft-versus-host disease (GVHD), and insufficient bone source [6,7]. Artificial bone is constructed and cultivated through bone tissue engineering, which can be easily prepared according to different requirements on a large scale and is available at a low price, and is therefore considered the ideal treatment for bone defects. With the rapid development of bone tissue engineering in recent years, new research directions for therapies of fractures and bone defects have been explored [8]. However, common bone tissue engineering cannot provide sufficient blood supply in the initial stage after implantation, and blood vessels would take several weeks to grow into the center of the biomaterials [9]. Insufficient vascularization and following nutrient limitation may contribute to cell death in tissue engineering [10].
Research on exosomes that can be used for cell-free therapy has gradually received increased attention [11]. Generally, exosomes are generated from the endosomal compartment, which is formed by the budding multivesicular body (MVB) membrane. The endosomal membrane invaginates to form intraluminal vesicles (ILVs), and then the ILVs fuse with the cell membrane and are released outside the cell to form exosomes. Most cells in our body can produce exosomes, such as mesenchymal stem cells (MSCs), dendritic cells, epithelial cells, adipocytes, and B cells [12]. And the exosomes are also widely found in body fluids such as blood, urine, and saliva [13]. Recent research on exosomes has mainly focused on their ability to transmit signals through vesicle-mediated transport among cells. Exosomes serve as a “messenger” that can be attached to bone tissue engineering scaffolds and exhibit various advantages. They are widely distributed and rich in proteins, lipids, and genetic materials. In addition, they avoid the risks of poor survival rate, strong immune rejection, and high mutation tumorigenicity caused by direct use of cells [14]. Attaching exosomes to bone tissue engineering biomaterials can not only provide a stable platform for exosomes but also avoid many problems caused by the cells.
In this review, we provide a comprehensive overview of the advantages of exosome-mediated bone tissue regeneration by discussing the main components of exosomes and the role of exosomes in repairing bone and cartilage damage. Then, for the application of exosomes-loaded biomaterials, we discuss the mechanism of exosomes from different sources and the application of exosome-loaded scaffolds in promoting bone regeneration. Finally, based on the isolation and purification and attachment methods of exosomes, we present an outlook for the future development of exosome-loaded materials It is hoped that this article can provide a convenient reference for future exosome research and help the development of exosome-mediated bone tissue regeneration (Fig. 1).
Fig. 1.
Exosomes and bone regeneration. The main components of exosomes and the role of exosomes in repairing bone and cartilage damage. (mRNA: messenger RNA, miRNA: microRNA, lncRNA: long noncoding RNA, SM: sphingomyelin, PA: phosphatidic acid).
2. Exosomes
In 1983, Pan et al. found exosomes in reticulocytes, but exosomes were initially regarded as products of cell metabolism and had no effect on other adjacent cells [15]. However, Ratajczak et al. discovered for the first time that exosomes could transfer the RNA contained in them to other cells and tissues. In subsequent studies, exosomes mediated the transfer of mRNAs and miRNAs and regulated the function and activity of target cells, which has raised the study of exosomes to a new level [16,17]. With further study, the role of exosomes in the immune response and antigen presentation has been gradually found [18,19]. Exosomes play an important role in the occurrence, diagnosis, and treatment of diseases because they can mediate intercellular communication and have biological effects similar to those of their source cells. For example, nicotine-treated macrophages can release exosomal miR-21-3p and aggravate atherosclerosis by increasing vascular smooth muscle cell migration and proliferation by acting on phosphatases and tonic homologues [20]. miR-499, miR-133 and other exosomal miRNAs associated with cardiovascular disease are up-regulated in patients with acute myocardial infarction and heart failure. The exosomal proteins CD151 and miR-638 can also be used to help diagnose lung and colorectal cancers [21]. The role of exosomes in orthopedic treatment is what we are concerned about. In the following, we will introduce in more detail how exosomes promote bone regeneration and cartilage regeneration.
In this section, we focus on the important parts of exosomes and the functions of each part. The main content of this review is about exosomes promoting bone regeneration. Therefore, we outline the process and influencing factors of bone regeneration, and finally summarize in detail how exosomes repair bone defects.
2.1. The structure and composition of exosomes
The size, density, and shape of exosomes are different due to the content and state of their contents. Generally, exosomes are single-membrane vesicles with a diameter of 30–200 nm, and their density is 1.13 (B cell-derived exosomes) - 1.19 (intestinal-cell-derived exosomes) g/ml [22,23]. The exosomes produced by artificial drying show typical cup-like or biconcave shapes, while they appear spheroid in solution [24].
The composition of exosomes is very complex. There are nearly 4400 proteins, 194 lipids, 1639 mRNAs, and 764 miRNAs contained in exosomes from different resources, which also illustrates the diversity of their functions [25].The complex compositions in exosomes determine their functions. Exosomes exert physiological activities by loading proteins. Tetraspanins (CD9, CD63, CD81, CD82) are related to cell penetration, invasion, and fusion events. Heat shock proteins (HSP70, HSP90) are involved in antigen binding and presentation. MVB formation proteins are involved in exosome release (Alix, TSG101). Annexins and Rab are responsible for membrane transport and fusion (annexins and Rab) [26]. Some proteins (Alix, flotillin, and TSG101) participate in exosome biogenesis, and some proteins (TSG101, HSP70, CD81, and CD63) are specifically enriched in exosomes and can be regarded as exosomal markers [25]. RNA, as a hot spot in the study of exosomes, plays an important role in intercellular communication. MicroRNAs (miRNAs) are the most abundant among various RNAs (mRNAs, miRNAs, lncRNAs, and circRNAs), accounting for over 42.32% of all raw reads and 76.20% of all mappable reads. It plays an important role in tumorigenesis, chromosomal abnormalities, protein phosphorylation, RNA splicing, angiogenesis, and other biological functions [18,27]. As a template, mRNA plays a crucial role in protein production. Some mRNAs are unique in exosomes, and the mRNA in exosomes can be translated into receptor cells and modify the protein expression programs of recipient cells [28,29]. Studies have shown that lncRNAs also play a role in the occurrence, development, and adhesion of tumors, especially in liver cancer. LncRNAs in exosomes released by hepatoma cells can be transported to other cells and regulate cell functions. Exosomes released by CD90+ cancer cells are rich in lncRNA H19, which can regulate endothelial cells, promote the angiogenesis phenotype and intercellular adhesion, and promote cancer cell metastasis [30,31]. Some studies have shown that compared with normal people, 67 circRNAs are absent and 257 new circRNAs appear in patients with colon cancer, and the circRNAs in exosomes can be used as tumor markers because of their relatively stable closed-loop structure [32]. In addition, the lipids in exosomes, such as sphingomyelin (SM), phosphatidic acid, and cholesterol, can enhance the stability and structural rigidity of exosomes. Moreover, the lipids in exosomes also have biological activities. Studies have shown that the unsaturated 22:6 fatty acid DHA from exosomes can inhibit the activity of cholesterol epoxide hydrolase by acting on antioestrogen binding sites [33,34].
2.2. Bone regeneration process and influencing factors
Bone can be regenerated in two different ways: intramembranous ossification or endochondral ossification. Flat bones are mainly formed through intramembranous ossification [35]. In areas with rich blood supply and nutrients, MSCs promote osteoblast differentiation and inhibit the chondrogenesis of mesenchymal progenitor cells through the Wnt signaling pathway in the presence of the transcription factors Runx2 and Osterix (Osx/Sp7). Osteogenitor cells differentiated from MSCs enlarge to form osteoblasts, which secrete osteoids to encapsulate osteoblasts to form osteocytes, and bone matrix is formed by calcification of osteoid. These early bone tissues become the ossification center, a trabecular bone, cancellous bone and periosteum were gradually formed on the basics of ossification center. Vertebrae and long bones are mainly formed by endochondral ossification. Briefly, the mesenchyme first condenses to form a growth plate as a template, and after the proliferation of chondrocytes, a series of matrices rich in proteoglycans, type IIa collagen, and aggrecan are produced by the resting cells on the growth plate, which makes chondrocytes stop proliferating and start to hypertrophy. Then the expression of type IIa collagen is downregulated by hypertrophic chondrocytes. Meanwhile, they synthesize type X collagen, mineralize the matrix, and promote vascularization as well as the differentiation and migration of osteoblasts. After the apoptosis of hypertrophic chondrocytes and the infiltration of osteoblasts and vessels, the ossification begins [36]. Endothelial cells also play a role in bone regeneration by stimulating the maturation and activity of osteoblasts. In addition, the success of angiogenesis also determines the success or failure of bone regeneration. Some bone regeneration failures are caused by damage to new blood vessels or the absence of new blood vessels. VEGF-mediated capillary invasion can regulate growth plate morphogenesis and trigger cartilage remodeling. VEGF is an important signal for remodeling and is an important coordinator of chondrocyte death, chondrocyte function, extracellular matrix (ECM) remodeling, angiogenesis, and bone formation in the growth plate [37].
The process of bone regeneration is so complicated that it requires the participation of various cells and precise regulation. Communication through the secretion of cytokines has a short half-life and cannot be transmitted over long distances. The unique function of exosomes in cell-to-cell communication and genetic material transfer is gradually gaining attention in bone defect repair.
2.3. Exosomes promote bone tissue regeneration
2.3.1. Exosomes promote osteogenesis
Bone marrow mesenchymal stem cells (BMSCs) derived exosomes can promote bone regeneration by regulating osteogenic differentiation and gene expression [38,39]. Zhao et al. found that MSC-derived exosomes can shorten the cell cycle and increase the expression of related proteins via MAPK signaling pathway and GLUT3, thereby promoting the proliferation of osteoblasts [40]. Exosomes can also affect the remodeling and regeneration of bone tissue through the transmission of miRNAs. Cui et al. found that exosomes secreted by mineralizing osteoblasts (MOB) are able to raise 91 miRNAs in bone marrow stromal cells (ST2), of which four highly expressed miRNAs can be detected in exosomes. The upregulated miRNAs tend to activate the Wnt signaling pathway by inhibiting Axin1 expression and increasing β-catenin expression, and promote the differentiation of ST2 into osteoblasts by increasing the expression of Runx2 and ALP [41].
Narayanan et al. confirmed that exosomes could be endocytosed by MSCs, and the endocytosed exosomes could increase the expression of bone morphogenetic protein 9 (BMP9) and transforming growth factor β1 (TGFβ1) in 2D cultures, which are good inducers of MSC osteogenic differentiation. While in 3D cultures, both regular and osteogenic exosomes could upregulate Runx2 and Osterix, which are significant transcription factors for the induction of osteogenic differentiation and osteogenesis [42]. Xu et al. probed BMSCs-derived exosomal miRNAs that 9 exosomal miRNAs were upregulated, and 4 miRNAs were downregulated [43]. Most upregulated miRNAs have a positive effect on osteogenesis. MiRNA let-7 can promote osteogenesis by regulating high mobility group AT-hook 2 (HMGA2), miRNA-199b can promote osteogenic differentiation by regulating Runx2, miRNA-218 promotes the osteogenic differentiation of human adipose-derived stem cells (hASCs) through the Wnt/β-catenin signaling pathway, and miRNA-135b can regulate mineralization in the osteogenic differentiation of human unrestricted somatic stem cells [[44], [45], [46]]. Regarding the downregulated miRNAs, a study showed that miRNA-221 could downregulate the osteogenic differentiation of stem cells [47].
Bone matrix mineralization refers to the process in which inorganic salts such as calcium and phosphorus are deposited in bone tissue. This process is related to osteoblasts and plays an important role in bone regeneration. A study found that compared with the control group, scaffolds containing exosomes had an increased presence of calcium and phosphorus, indicating that osteogenic exosomes can induce matrix mineralization of human bone marrow mesenchymal stem cells (HMSCs) [42]. Tom et al. found that preosteoblast MC3T3-E1 cells treated with C2C12 exosomes showed increased ALP activity and matrix mineralization, which could promote osteoblast differentiation [48]. In addition, exosomes from human induced pluripotent stem cells (hiPSC-MSCs) upregulated the gene expression of MSCs derived from ovariectomized (OVX) rats, and the results of alizarin red S staining showed that compared to the control group, the deposition of minerals in the cells treated with exosomes significantly increased [49].
2.3.2. Exosomes can promote cartilage regeneration
Cartilage is a highly differentiated connective tissue composed of chondrocytes and intercellular matrix. Characterized by a lack of vascular and neural innervation, it hardly has the ability of self-healing. However, cartilage damage and defects caused by OA and sports injury are prevalent in the clinic [50]. At present, the clinical treatments of cartilage damage and defects include conservative treatment and surgical treatment. Conservative treatment includes rest, cold compress, weight loss, and the usage of NSAIDs, amino acid glucose, hormones, calcium, or vitamins. Surgical treatment includes joint replacement and autologous cartilage transplantation. However, the therapeutic effect of these methods cannot essentially regenerate cartilage. The emergence of tissue engineering has brought new therapeutic methods for cartilage regeneration by combining chondrocytes or stem cells as “seed cells” with scaffolds. However, these two kinds of seed cells have the disadvantages of long treatment periods and contamination. The most crucial factor is that the differentiation of chondrocytes and stem cells cannot be regulated, which may lead to dedifferentiation of chondrocytes or hypertrophy and calcification in stem cell differentiation [51,52].
Using exosomes for cartilage regeneration can avoid these disadvantages caused by cells. As a messenger, exosomes are rich in genetic materials and proteins, which can induce the expression of the chondrocyte markers (type II collagen and proteoglycan), increase the expression of anabolic markers (ACAN, COL1, and COL2B), and reduce catabolic markers (MMP-1, ADAMPS5) and inflammatory markers (iNOS). Exosomes containing miR-146a can also promote M2 macrophage polarization, reduce inflammatory factor activation, and promote cartilage growth [53,54]. It was shown that exosomes could accomplish rapid cell proliferation and infiltration during cartilage regeneration through CD73-mediated adenosine activation of AKT and ERK signaling and the enhanced histone H3 acetylation. In addition, exosomes can also affect the autophagy of chondrocytes by promoting the polarization of M2 macrophages, inhibiting the expression of inflammatory factors IL-1β and TNF-α, significantly increasing the autophagy level of chondrocytes, thereby inducing a regenerative phenotype of chondrocytes, promoting chondrocyte proliferation and enhancing matrix synthesis by inhibiting the mTOR signaling pathway [55,56]. The Wnt/β-catenin pathway also plays a key role in cartilage regeneration. MiR-8485 contained in the exosome regulates GSK3-β expression and phosphorylation to activate the Wnt/β-catenin pathway. The mRNA and protein levels of COL2A1, ACAN, SOX9, and MATN3 were increased by downregulating GSK3-β and targeting DACT1 in BMSCs. Overexpression of miR-8485 also increased proteoglycan production in BMSCs [57].
2.3.3. Exosomes can promote angiogenesis
Blood vessels can transport nutrients and metabolic wastes, which are important for bone regeneration. For bone defects with poor vascularization, delayed union or nonunion is prone to occur [58]. Xie et al. found that compared with the untreated control, the experimental group of human umbilical vein endothelial cells (HUVECs) in the presence of exosomes grew faster and was proportional to the concentration of exosomes [59]. Liu et al. found that exosomes derived from MSCs can promote the proliferation, migration, and the tube-forming ability of endothelial cells by activating the PI3K signaling pathway, thereby increasing the microvessel density of the femoral head [60]. In addition, another study found that exosomes derived from umbilical cord mesenchymal stem cells (uMSCs) can increase the expression of vascular endothelial growth factor (VEGF) and hypoxia inducible factor-1α (HIF-1α), thereby promoting bone regeneration and angiogenesis in rats with femoral fractures, and HIF-1α plays an important role in this process [61,62].
2.3.4. Exosomes can regulate macrophage polarization
Bone immunity is a hot topic in recent research. It aims to study the basic interaction between the immune system and bone, mainly involving the relationships between osteoblasts and osteoclasts, lymphocytes and osteoclasts, osteoblasts and hematopoietic cells. The discovery of multiple immune functions of RANK/RANKL pathway further promotes the progress of bone immunology [63]. As an important effector of the innate immune system, macrophages play a key role in host defense and inflammation. Macrophages can be activated and polarized into two main types: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages. The first step of bone tissue repair is to initiate a cascade of inflammatory responses. In the early stage of inflammation, the pro-inflammatory factors produced by M1 macrophages destroy the tissue. Then M2 macrophages inhibit the inflammatory response, remove the damaged tissue fragments, and restore homeostasis. Benign inflammation can initiate healing, while excessive inflammation hinders healing. Therefore, macrophage polarization plays an important role in the process of bone healing [64].
Studies have shown that M2 macrophage polarization can promote the osteogenic differentiation of MSCs and induce the formation of osteoblasts [65,66]. M1 macrophages induce bone destruction and form fiber wrapping instead of new bone formation. However, bone tissue engineering scaffolds usually induce M1 polarization, which leads to an inflammatory foreign body reaction, forms granuloma, wraps the scaffolds with fibers, and finally leads to implantation failure [67,68]. Exosomes carry a large number of genetic materials and proteins. Some genetic materials can promote the M2 polarization of macrophages by downregulating the expression of PRKCCD and PTEN, promoting the expression of p-AKT, and activating the PI3K/AKT signaling pathway [69,70]. Proteins carried in exosomes can also regulate the polarization of macrophages. Yuki Nakao et al. found that TNF-α-stimulated exosomes could induce polarization of M2 macrophages by promoting CD73 expression [71]. Combining exosomes with bone tissue engineering scaffolds can not only play the osteogenic role of exosomes but also inhibit the excessive inflammatory response caused by scaffold implantation and promote bone healing.
2.4. Summary
In recent years, research on exosomes has shown explosive growth. As a medium of communication among cells, the lipids, proteins, and genetic materials contained in exosomes have great potential in the diagnosis and treatment of diseases. However, due to the limitation of production technology, the extraction efficiency of exosomes is low. There is still a long way to go from laboratory research to clinical application because of its deficient standards of preparation, testing, and analysis. However, it is pleasing to see that many studies are working to address these problems so that they can be better served clinically [72,73].
3. The applications of exosomes-loaded biomaterials for bone tissue engineering
Currently, tissue engineering can be applied to various clinical aspects, such as wound healing, corneal regeneration, bone repair, heart valve repair, and liver and kidney diseases [[74], [75], [76], [77]]. Compared with traditional clinical treatments, bone tissue engineering has the advantages of a shorter growth cycle, a sufficient supply of raw materials, and a low probability of immune rejection and infection in promoting bone repair [78]. The construction of bone tissue engineering mainly consists of seed cells, scaffold materials, and growth factors. Since cell adhesion, growth, and proliferation depend on the scaffold, the selection of scaffold material plays an important role in the construction of bone tissue engineering [79]. Therefore, scaffold materials used in bone tissue engineering should meet the following requirements: (1) good biocompatibility and biodegradability; (2) favorable plasticity; (3) a three-dimensional porous structure that can support seed cells; (4) a good material-cell interface; and (5) a stable structure [80,81].
Types of materials used for bone tissue engineering, sources of exosomes, and ways of attaching exosomes to bone tissue engineering. (hBMSCs: human bone marrow mesenchymal stem cells, SHED: stem cells from human exfoliated deciduous teeth, hUCMSCs: human umbilical cord mesenchymal stem cells, rBMSCs: rat bone marrow stromal cells, hGMSCs: human gingival mesenchymal stem cells, hDPSCs: human dental pulp stem cells, hUMSCs: human umbilical cord mesenchymal stem cells, SMSCs: synovial mesenchymal stem cells, ADSCs: adipose-derived stem cells).
Exosomes regulate the proliferation and activity of osteoblasts and promote angiogenesis, osteogenic differentiation, and cell matrix mineralization, thus promoting bone tissue regeneration. These processes are mainly achieved by the proteins, lipids, and genetic materials inside the exosomes. Attaching exosomes to bone tissue engineering materials not only provides a favorable environment for the function of exosomes but also compensates for the shortcomings of bone tissue engineering materials, such as slow angiogenesis and intercellular communication barriers. Common exosomes used for bone tissue engineering include BMSCs, human-induced pluripotent stem cell-derived mesenchymal stem cells (hiPS-MSCs), human adipose mesenchymal stem cells (hAD-MSCs), and other sources of exosomes. These exosomes are combined with scaffolds from different sources by dropwise, co-incubation, lyophilization or other methods. Beside traditional scaffolds, bone tissue engineering also involves various fillers, hydrogels, nanotubes, etc (see Table 1). In the following section, we introduce various examples of exosomes used in bone tissue engineering in recent years (Fig. 2).
Table 1.
Exosomes-loaded biomaterials for bone tissue engineering applications.
| Scaffold materials | Materials | Exosome source | Combination mode | References |
|---|---|---|---|---|
| Inorganic materials | Metal | Schwann cells, hBMSCs, hADMSCs | Filling, incubate | [[82], [83], [84], [85], [86]] |
| β-TCP | hiPS-MSCs, SHED, hiPS-MSCs | Drip and lyophilize, incubate, adding dropwise | [49,87,88] | |
| HAP | hBMSCs, hUCMSCs | Inject, mix, load and cure | [89] | |
| BAG | rBMSCs | Drip and lyophilize | [90] | |
| Organic materials | ECM | BMSCs | Inject | [91] |
| Liposomes | CXCR4+ NIH-3T3 | Exosome-guided bone targeted delivery of Antagomir-188 | [92] | |
| PCL | hBMSC, ATDC5 | CP05 modification and Incubated with PCL | [93,94] | |
| PLA | hGMSCs, hAD-MSCs | Incubate, fix and dehydrate, drip and incubate | [95,96] | |
| PLGA | hAD-MSCs, SHED | Soak and incubate | [97,98] | |
| PEEK | BMSCs | Drip and incubate | [99] | |
| PLLA | hDPSCs | Physically attache | [100] | |
| Hydrogels | BMSCs, hUMSCs, hiPS-MSCs, SMSCs, SHED, ADSCs | Inject, dissolve and mix, dynamic projection stereolithography, irradiate | [61,[101], [102], [103], [104], [105], [106], [107], [108], [109], [110]] |
Fig. 2.
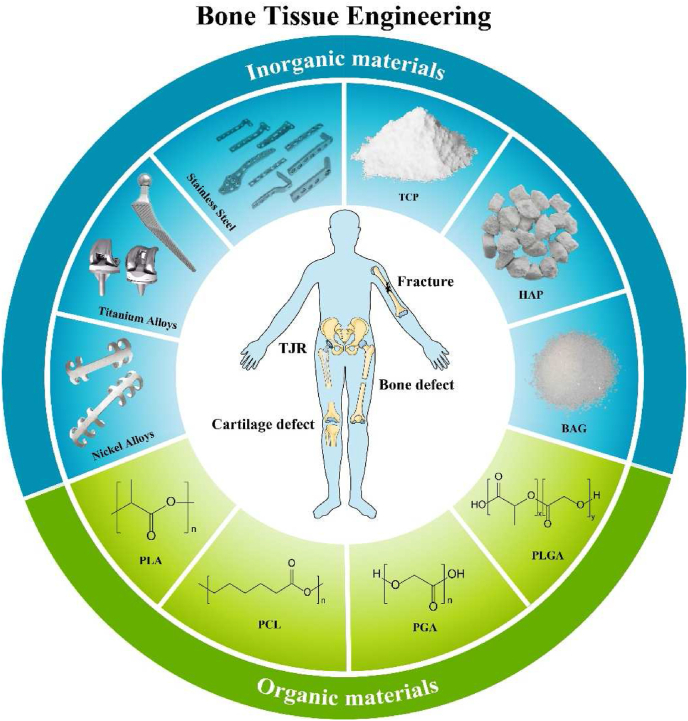
Bone tissue engineering materials. The materials commonly used in bone tissue engineering can be generally divided into metal materials, organic materials, and inorganic materials. Different materials have their own characteristics and shortcomings. (TJR: total joint replacement, TCP: tricalcium phosphate, HAP: hydroxyapatite, BAG: bioactive glass, PLGA: poly(lactic-co-glycolic acid), PGA: polyglycolic acid, PCL: polycaprolactone, PLA: polylactic acid).
3.1. Exosomes-loaded inorganic materials for bone tissue engineering
Inorganic materials commonly used for bone tissue engineering are metal materials, tricalcium phosphate (TCP), hydroxyapatite (HAP), and bioactive glass (BAG), which have good toughness and biocompatibility as well as different characteristics.
3.1.1. Metal materials
Metal materials are the most widely used materials in engineering and are commonly used for cardiovascular stents and bone implantations clinically. The excellent mechanical strength and load-bearing capacity of metal materials make them particularly suitable for orthopedic implantations, such as the internal plate fixation, artificial femoral heads used in total hip arthroplasty, and artificial joints used in total knee arthroplasty. Currently, commonly used metal materials include platinum, stainless steel, titanium alloys, nickel alloys, magnesium alloys, cobalt-based alloys, etc. Metal materials possess the advantages of good biocompatibility, mild stretchability, easy production and processing, corrosion resistance, and low modulus [[111], [112], [113]]. However, the poor absorbability of metal materials sometimes requires surgically removing them after bone healing. In addition, metal materials can be affected by wear and stress shielding effects, resulting in harmful substances and reduced bone density. The metal scaffolds can only provide a stable platform, but it is difficult to attach other osteoinductive materials to their surface [114]. Titanium alloys have become the most commonly used metal materials in orthopedics because of their excellent biocompatibility, high friction coefficient, high porosity and corrosion resistance [[115], [116], [117]]. Not only that, the micro/nano-textured layered titanium morphology can also promote the secretion of exosomes, which in turn promotes bone regeneration [82]. Recent studies have gradually focused on the development of porous, 3D printed and nanoscale metal materials and the modification of metal scaffolds. The research on the modification of metal scaffolds by exosomes has achieved striking results. The excellent porosity of the 3D printed titanium alloy scaffolds is not only conducive to the attachment of exosomes, but also to the migration and proliferation of cells. 3D printed titanium alloy scaffolds modified with exosomes derived from human mesenchymal stem cells can upregulate osteogenic miRNAs and promote osteogenesis through PI3K/Akt and MAPK pathways [83]. Besides, attaching exosomes derived from Schwann cells to porous Ti6Al4V scaffolds could promote osteogenesis. Porous Ti6Al4V can reduce the elastic modulus of the material to make it closer to natural bone tissue, with a closer connection between the scaffold and the new bone and a better bone integration, which proves that Ti6Al4V scaffolds with exosomes can promote the regeneration and repair of bone tissues better than traditional scaffolds [84]. The genetic material transfer function of exosomes plays a key role in the osteogenesis process. Similarly, a study applied bone morphology protein (BMP2) to stimulate macrophages and encapsulated the extracted exosomes in titanium nanotubes. With the advancement of materials science, nanotechnology is increasingly being used in tissue engineering with its unique advantages in drug delivery [118,119]. It was found that titanium nanotube-containing exosomes could stimulate the bone activity of BMSCs, while only BMP2/macrophage-derived exosomes could enhance bone differentiation. The macrophages stimulated by BMP2 showed different miRNA profiles with increased expression of miR-530, chr9_22,532, and chr16_34,840. In addition, titanium nanotube-containing exosomes were found to stimulate autophagy activation of BMSCs, thereby regulating MSC differentiation and self-renewal, showing a critical role in MSC bone differentiation. The modification of metal scaffolds by exosomes provides a new direction for the development of bone tissue engineering materials for bone repair (Fig. 3A and B) [85].
Fig. 3.
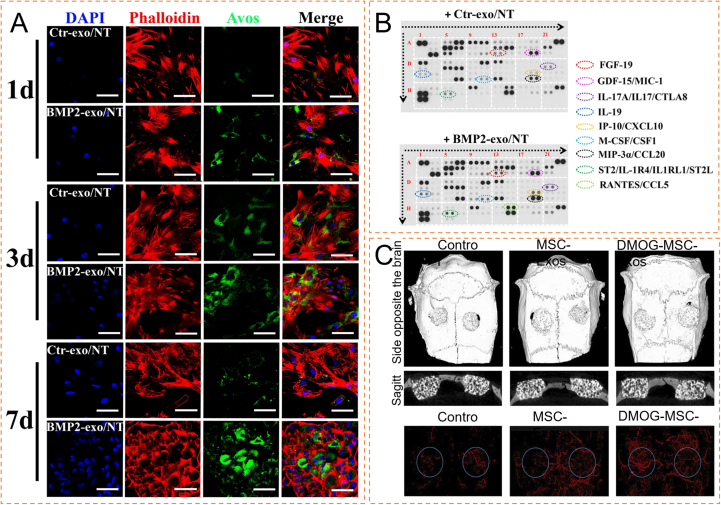
Application of exosome-loaded inorganic materials in bone tissue engineering (A) Autophagic activity of hBMSCs stimulated by experimental group or control group. Representative confocal microscopic images of hBMSCs stimulated with experimental group or control group encapsulated nanotubes for 1, 3 and 7 days under osteogenic medium. (B) hBMSCs were incubated with experimental group encapsulated-nanotubes or control group encapsulated nanotubes under osteogenic differentiation for 4 days. The conditioned medium collected was measured using proteome profiling human XL cytokine array (Reproduced with permission of Ref. [85]). (C) Three-dimensional reconstruction and sagittal images showed different reparative effects of HA, exosomes derived from MSCs (MSC-Exos), and DMOG-stimulated MSCs (DMOG-MSC-Exos), and new blood vessels in calvarial defects are shown in three-dimensional reconstruction images (Reproduced with permission of Ref. [89]).
In recent years, metal-organic frameworks (MOFs) have gradually become a hot spot in materials research. MOFs are organic-inorganic hybrid materials with intramolecular pores formed by organic ligands and metal ions or clusters, and their advantages are structural and functional diversity, porosity and large specific surface area. Kang et al. used the features that exosomes could promote bone regeneration and angiogenesis, Mg2+ could promote osteogenic differentiation and calcium deposition, gallic acid could be anti-oxidant and anti-inflammatory, to form PLGA/Exo-Mg-GA MOF composite scaffolds. The researchers found that the composite scaffold could not only stably release exosomes and magnesium ions, accelerate the adhesion and proliferation of cells, but also exert anti-inflammatory and osteogenic effects, but the mechanisms of anti-inflammatory and osteogenic were not explained in the experiment [86].
3.1.2. β-TCP
Tricalcium phosphate includes α-tricalcium phosphate (α-TCP) and β-tricalcium phosphate (β-TCP). β-TCP is similar in composition to the human autologous bone, with advantages of abundant source, high safety, easy storage, being sterile and degradable, and the ability to release calcium ions and sulfates in vivo to maintain the balance between material degradation and osteogenesis and promote bone regeneration [[120], [121], [122]]. Horch et al. demonstrated the remarkable degradability and osteoconductivity of TCP in a clinical trial for the treatment of 156 cases of mandibular defects [123]. β-TCP hardly degrades in the non-weight-bearing area, while the degradation rate mediated by body fluids is very fast in the weight-bearing area. Inoculating osteoblasts on β-TCP scaffolds can slow the degradation rate [124], but β-TCP is unable to promote bone repair. Therefore, a classical porous β-TCP scaffold was modified with exosomes derived from human-induced pluripotent stem cell-derived mesenchymal stem cells (hiPS-MSC-Exos) by Zhang et al. [87] Compared with the pure β-TCP scaffold, the combined exosome/β-TCP scaffold increased the expression of the PI3K/Akt signaling pathway and showed higher osteogenic activity. The implantation of exosome-loaded scaffolds not only upregulated the mRNA and protein expression of bone-related genes but also promoted osteogenesis by enhancing angiogenesis. Qi et al. dropped exosomes onto a porous β-TCP scaffold and combined them through freeze-drying. The in vivo experiments showed that the hiPSC-MSC-Exos+β-TCP scaffolds could promote bone regeneration and vascular regeneration of osteoporotic rat skull defect models in a concentration-dependent manner [49]. Another study combining β-TCP with exosomes secreted by stem cells from human exfoliated deciduous teeth (SHED) also demonstrated that exosomes/β-TCP enhanced the expression of angiogenesis-related genes, osteogenesis-related genes, and phosphorylated (p)-AMPK and promoted periodontal bone regeneration and vascular regeneration [88].
3.1.3. Hydroxyapatite (HAP)
HAP is the main component of human bone tissue, with great biocompatibility and osteogenic capacity [125]. After being implanted in the body, HAP will not induce toxicity, inflammatory reactions, or foreign body reactions, and will exist as the stable calcium phosphate (CaP) in human body fluids at neutral or alkaline pH. HAP also releases calcium, phosphorus, and other elements that facilitate tissue repair in vivo [126,127]. In addition, HAP particles can stimulate macrophages to secrete angiogenic and osteogenic growth factors to promote bone regeneration [128]. Morishita et al. applied a BMSCs-filled HAP scaffold in the bone cavity after the curettage of bone tumors. No obvious adverse reactions were observed after surgery, the implant and the bone were integrated tightly three months post-surgery, and they completely fused one year post-surgery [129].
HAP materials also have some properties that hinder their applications. Despite their large hardness, they are brittle and possess poor fracture toughness. Besides, HAP materials have a long degradation time and poor cell adhesion [[130], [131], [132]]. However, attaching exosomes extracted from HMSCs to a classic HAP scaffold stimulated by low-density dimethyl oxychloroglycine (DMOG) can activate the AKT/mTOR pathway, promote the regeneration of HUVECs, and enhance the osteogenic capacity of HA scaffolds further (Fig. 3C) [89].
3.1.4. Bioactive glass (BAG)
BAG has been known as the only artificial biological material that can be bonded to bone and soft tissues so far. After implanted in the body, BAG can form HAP on the glass surface through a series of reactions and then firmly bond with bone tissue. The transformation ability is also an indicator of its biological activity. In addition, BAG has potent osteoconductive and osteogenic properties, and can stimulate bone regeneration and bone repair through degradation products [133].
Common BAG materials include silicate, borate and phosphate. In the early 1970s, the first generation of silicate-based BAG was developed by Hench et al. but it tended to precipitate to form crystals, could not be completely converted into HA and possessed a slow degradation rate in vivo [134]. Borate BAG is highly reactive and can be almost completely converted into HA. However, borate BAG will produce the cytotoxic (BO3)3- dissolving in the body [135,136]. Phosphate BAG has a rapid degradation rate that can be controlled by adding metal oxides such as TiO2 and MnO2, but it is not widely used since phosphate BAG has poor chemical durability and its degradation products Na+ and (PO4)2- will cause a high pH environment which inhibits cell proliferation [[137], [138], [139]].
With the integration and development of material science and molecular biology, novel BAG materials are being modified in bone tissue engineering, including those modified with exosomes. Liu et al. found that miRNAs (let-7a-5p, let-7c-5p, miR-328a-5p, and miR-31a-5p) in BMSC–OI–exos could regulate the competitive balance of Bmpr2/Acvr2b toward Bmpr-initiated Smad1/5/9 phosphorylation to achieve the osteogenic activity. The BMSC–OI–exos was freeze-dried, filled on the layered mesoporous bioactive glass (MBG) scaffold, implanted in a rat skull defect model, and the material was found to induce bone formation. Although the freeze-drying process caused broken exosomes and a slight decrease in ALP activity, there was no significant difference overall compared with other loading methods. In addition, the high specific surface area and microporosity of the BAG scaffold also protect and facilitate the exosome attachment. These findings prove the reliability of freeze-drying attachment and provide a new idea for the attachment method (Fig. 4) [90].
Fig. 4.
Application of exosome-loaded MBG in bone tissue engineering (A) An overview of the study. (B):(i) Principal component analysis (PCA) of miRNAs of MSC-exos from different sources and conditions. (ii) Heatmaps of the differentially expressed miRNAs of BMSC-derived exosomes before and after osteoinductive treatment. (iii) Heatmaps of the differentially expressed exosomal miRNAs of exosomes under different interventions. (C) Histological evaluation of the bone sections 12 weeks post-implantation stained with VG (undecalcified), Masson's trichrome and HE (decalcified). (M: material; nb: new bone.) (Reproduced with permission of Ref. [90]).
3.2. Exosomes-loaded organic materials for bone tissue engineering
Organic materials used in bone tissue engineering include natural polymer materials and synthetic polymer materials. Common natural polymer materials include extracellular matrix and liposomes, and common synthetic polymer materials include polycaprolactone (PCL), polylactic acid (PLA), polyglycolic acid (PGA), and their copolymer poly (lactic-co-glycolic acid) (PLGA). The following will review the characteristics of different materials and how they exert osteogenic effects when loaded with exosomes.
3.2.1. Natural polymer materials
ECM, synthesized by various cells, is a complex network of macromolecules surrounding cells. Its main components include collagen, elastin, and proteoglycans. ECM not only protects cells and provides a good cellular microenvironment but also regulates cell functions by communicating with endogenous cells [140]. ECM can also be used as a material for bone tissue engineering. The tissue-derived ECM can maintain its properties in the organism that are beneficial to vascular regeneration [141]. However, there are still challenges in retaining these properties. Besides, the method to form and maintain the structural gradient of ECM in vivo remains to be further explored [142].
The artificially prepared ECMs are derived from organisms and can provide a good microenvironment for cell growth and proliferation with favorable biocompatibility. Scaffolds prepared by injecting exosomes into the artificial ECM can promote cartilage regeneration by inhibiting inflammation and enhancing the regeneration of osteochondral ECM. However, the concentration of exosomes at the defect site is uncontrollable with this injection method, indicating its limited application for large bone defects (Fig. 5) [91].
Fig. 5.
Application of exosome-loaded ECM in bone tissue engineering (A) Schematic illustration of the whole study. (B) (i): Micro-MRI findings. The red arrowheads show the locations of the defects. (ii): Micro-CT findings, the red rectangles show the subchondral bone at the defect sites. (C) (iii): Macroscopic results for new cartilage. The red circles show the locations of the defects. Scale bar = 5 mm. (iv): ICRS cartilage repair macroscopic score. (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005, ∗∗∗∗P < 0.001). (D) (v): Macroscopic view of the ACECM scaffold. (vi) SEM results of the cross-section of the ACECM scaffold. (vii): SEM results for a longitudinal section of the ACECM scaffold. (viii): SEM results for BMSCs planted on the ACECM scaffold for 4 days (the red arrowhead indicates the clustered cells). (ix–xi) Confocal laser observation of live/dead-stained BMSCs planted on the ACECM scaffold for 1, 4, and 7 days (green indicates live cells, and red indicates dead cells) (Reproduced with permission of Ref. [91]).
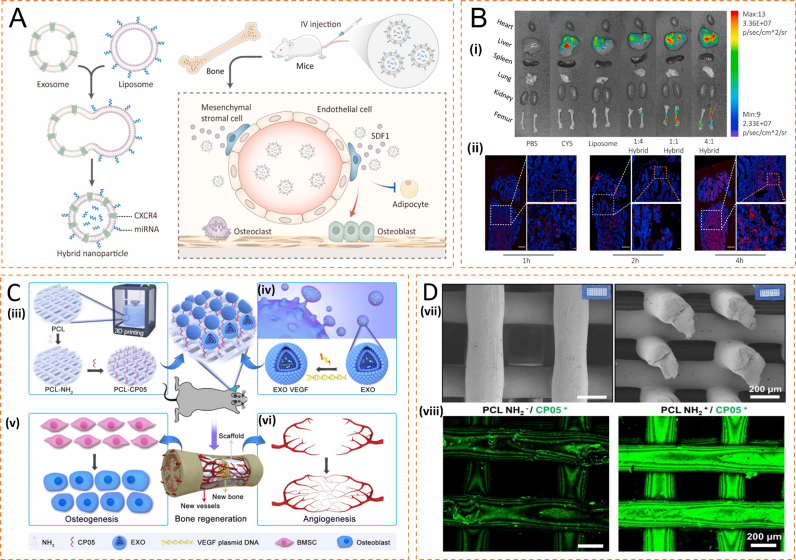
Liposomes are synthetic microvesicles with phospholipid bilayers resembling cell membranes. With the hydrophobic head end and the hydrophilic tail end of phospholipids, it is possible to carry hydrophilic drugs in liposomes and hydrophobic drugs in bilayers [143]. As a carrier, liposomes not only passively target the tumor area through enhanced permeability and retention effects but also show controlled drug release to reduce side effects and enhance drug stability [144]. Exosomes are unable to exert sustained functions due to their short half-life. The combination of exosomes and liposomes can complement each other. In the elderly, miR-188 in BMSCs increases, and miR-188 induces adipogenesis of BMSCs to inhibit osteogenesis, while antagomir-188 inhibits the adipogenesis of BMSCs and promotes osteogenesis. Hu et al. exploited the high-level stromal cell-derived factor 1 (SDF1) of bone marrow to recruit CXCR4+ cells homing. They combined exosomes with CXCR4 on their surfaces and liposomes loaded with antagomir-188 to fabricate the hybrid nanoparticles (NPs). In this study, the targeted aggregation of CXCR4+ exosomes was demonstrated for the first time through in vivo tracing experiments. The exosomes-loaded hybrid NPs accumulated in the bone marrow, inhibited the adipogenic differentiation of MSCs, and promoted their osteogenic differentiation. Unfortunately, the sustained release of exosomes has not been verified to accumulate in the bone marrow in this study (Fig. 6A and B) [92].
Fig. 6.
Application of exosome-loaded liposomes and PCL scaffolds in bone tissue engineering (A) Schematic illustration of exosome-guided miRNA blocking. (B) (i) Biophotonic images of the organ distribution 4 h after intravenous injection of PBS, Cy5, Cy5 labeled liposomes and Cy5-labeled hybrid NPs, with various exosome-liposome ratios. (ii) Representative fluorescence microscopic images of the femur 1, 2, and 4 h after injection of Cy5-labeled CXCR4+ hybrid NPs (Reproduced with permission of Ref. [92]). (C) General idea of engineered exosome enhanced therapies on osteogenesis and angiogenesis. (iii) 3D-printed porous PCL scaffolds were modified with 1,6-hexanediamine to generate the amino group on PCL scaffolds that were subsequently modified with the exosomal anchor peptide CP05. (iv) Engineered exosomes were fabricated by encapsulating the VEGF plasmid DNA into ATDC5-derived exosomes. The well-designed bone scaffolds were constructed by combining the engineered exosomes with the CP05 modified 3D-printed scaffolds, and eventually implanted into a rat radial defect model to promote osteogenesis (v) and angiogenesis (vi). (D) Modification of 3D-printed PCL scaffolds. (vii) 3D-printed PCL scaffolds exhibited a highly intercommunicating porous morphology by SEM observation (Left image for the top view and Right image for the side view). (viii) The graft efficiency of the anchor peptide CP05 was obviously higher in PCL NH2+ than that in PCL NH2-. The CP05 was conjugated with Alexa Fluor 488 to present green fluorescence (Reproduced with permission of Ref. [93]).
3.2.2. Synthetic polymers materials
As a commonly used biodegradable polyester polymer material, PCL is widely used in various tissue engineering applications, such as heart tissue engineering, vascular tissue engineering, skin tissue engineering, nerve tissue engineering and other fields, especially in bone tissue engineering [[145], [146], [147], [148]]. Approved by FDA, PCL is generally used as a scaffold material for bone tissue engineering, with good biocompatibility, plasticity, toughness, and controllable degradation rate. However, PCL itself has low mechanical strength and cannot bear weight, showing no bioactive or osteoconductive properties. Besides, the low surface energy and the lack of surface signals on pure PCL make it difficult for cell attachments [[149], [150], [151]].
Porous PCL scaffolds have been widely used in bone tissue engineering, and the development of 3D technology gradually facilitates the design and preparation of these porous materials. Zha et al. encapsulated the VEGF plasmid in ATDC5-derived exosomes and planted the exosomes in a 3D-printed PCL scaffold to prevent the unbalanced concentration distribution and the embolism risk caused by intravenous injection (Fig. 6C and D). In addition, they modified the PCL scaffold with the anchoring peptide CP05 of exosomes to improve the efficiency of implantation and implanted the scaffolds in rat radius defect models. The exosomes showed a strong osteogenic ability, VEGF plasmids displayed a potent angiogenic ability, and the PCL scaffold that combines the exosomes and VEGF plasmids can promote osteogenesis and angiogenesis both [93]. The PCL material itself can also be modified to optimize material properties. For example, modification of PCL scaffolds with low concentrations of silver ions not only inhibits the inflammatory process but also promotes the osteogenic differentiation of BMSCs. In addition, PCL scaffolds modified with silver ions and exosomes derived from MSCs can increase the expression levels of osteogenic genes (LP, OPN, Col-I, Runx2) and promote the osteogenic differentiation of hBMSCs [94].
The FDA-approved PLA is also widely used as an important medical material. In orthopedics, PLA is used in absorbable screws, meniscus repair, and bone tissue engineering [[152], [153], [154]].As aliphatic polyester, PLA can be transformed into lactic acid (LA) through hydrolysis and enzymatic hydrolysis in vivo, and its crystallinity determines its degradation rate. PLA has remarkable advantages of good biocompatibility and mechanical strength, low immunogenicity, and easy materials access. However, PLA also has some limitations, such as complicated processing. Besides, as a hydrophobic aliphatic polyester, the hydrophobicity, low degradation rate, and low impact toughness of PLA also limit the application [155,156]. However, attaching exosomes to PLA scaffolds directly may lead to low attachment efficiency, so both the scaffolds and the surface of exosomes can be modified to enhance their adhesion. A “proton sponge” polyethyleneimine (PEI) was used to improve the adhesion of exosomes derived from human gingival mesenchymal stem cells (HGMSCs) and was applied to modify 3D printed porous PLA scaffolds, and the modified scaffold induced the expression of 31 osteogenesis-related genes and showed better osteogenesis in rat skull injuries [95]. Composite materials are gradually becoming the mainstream in which different materials complement each other. Maria et al. developed a novel mineral-doped PLA porous scaffold by thermally induced phase separation of PLA, CaSi, and dicalcium phosphate dihydrate (DCPD) and dropping exosomes from human adipose mesenchymal stem cells (hAD-MSCs) onto the scaffold surface. They cultured the scaffold in a culture medium to better fuse the exosomes with the new scaffold. In vitro experiments showed that mineral-doped scaffolds could form a microenvironment facilitating bone growth. The addition of exosomes further increased the expression of osteogenic marker genes. Maria et al. proved that mineral filling is beneficial to osteogenesis, which provides a new direction for optimizing bone tissue engineering scaffolds. Unfortunately, the mineral-doped PLA scaffolds were cultured in simulated body fluids in vitro, but in vivo experiments have not been conducted to prove their efficacy [96].
Similar to PLA, PGA is also commonly used in tissue engineering and has excellent biocompatibility and biodegradability. PGA can be rapidly degraded into non-toxic carbon dioxide and water in the body. The degradation rate and mechanical properties of PGA can be controlled by changing the ratio of LA and glycolic acid. Besides, PGA has strong plasticity and can adjust its properties as needed. However, its poor load-bearing stability makes it difficult to bear weight, the degradation rate of PGA is too fast, and the acidic decomposition products can cause severe inflammation [[157], [158], [159]]. PLGA is formed by random polymerization of LA and glycolic acid. PLGA50:50, which consists of 50% LA and 50% glycolic acid, is the most commonly used type, and different ratios of LA and glycolic acid will lead to different properties. Generally, the drug release and degradation rate of PLGA at a high LA proportion are relatively slow, except that the degradation rate of PLGA50:50 is the fastest [160,161]. Despite its excellent biocompatibility, PLGA has poor load-bearing capacity and osteoconductivity and cannot promote cell adhesion and proliferation. Therefore, other materials, such as chitosan, HAP, and BAG are usually used in combination with PLGA [[162], [163], [164]]. Other modifications were also utilized to enhance the adhesion between scaffolds and exosomes. A study combined exosomes from hASCs with polydopamine-coating PLGA scaffolds by soaking. In mouse skull defect models, the exosome-composite scaffold showed a stronger bone repair ability than simple PLGA scaffolds. In this study, exosomes collected within two days were suggested for scaffold implantation, consistent with the clinical requirements for the timely treatment of bone defects. In addition, it was speculated that the osteogenic ability of the extracted exosomes might diminish with the loss of MSC cellular characteristics, which has not been validated yet. Moreover, the attachment efficiency by soaking and the sustained release of exosomes from the scaffolds are not ideal. Although the modification of polydopamine in this study could achieve a certain sustained release, it was shown that only approximately 30% of the exosomes remained on the scaffold after 8 days, which is not conducive to long-term bone repair (Fig. 7) [97]. To enhance the sustained-release effect, W. Benton Swanson et al. used double emulsion technology to encapsulate exosomes in PLGA-PEG-PLGA polymer microspheres and implanted them into PLLA nanoporous scaffolds. They found that after a sudden release on the first day, the release curve of encapsulated exosomes for the next 10 weeks was almost linear, indicating the encapsulation method as an effective way to release exosomes, thereby prolonging osteogenesis [100]. Compared with lyophilization, surface adsorption using an affinity coating can protect exosomes, therefore, researchers developed an exosome-affinity biomimetic polydopamine coating applied on injectable PLGA microsphere scaffolds and using SHED to induce exosomes in hypoxic culture to attach to the scaffold surface. The microsphere scaffolds not only prolonged the low retention and stability of exosomes in vivo, but also exhibited strong osteogenic and angiogenic abilities [98].
Fig. 7.
Surface characterization of engineered PLGA substrates (A) Scanning electron microscopy of PLGA scaffolds (PLGA), PLGA scaffolds coated with polydopamine coating (PLGA/pDA), and PLGA scaffolds coated with polydopamine and exosomes (PLGA/pDA-Exo). (B) Distribution of PKH-26 labeled exosomes on the PLGA-only scaffold (middle) and PLGA/pDA scaffold (right), with PKH-26-stained scaffold as control (left). (C) In vitro exosome release kinetics in saline from exosomes-loaded PLGA by physical absorption (PLGA/Exo) and PLGA/pDA-Exo scaffolds. (D) Exosomes increased bone formation in critical-sized mouse calvarial defects. Mice were treated with PLGA scaffolds (PLGA), PLGA scaffolds with polydopamine coating (PLGA/pDA), or PLGA scaffolds coated with polydopamine and exosomes (PLGA/pDA-Exo). (i) Micro-CT images of bone formation in each group after 6 weeks. (ii) Quantitative comparison of new bone volume among the different groups. ∗∗p < 0.01 compared with groups without exosomes. Histological assessment of bone formation in each group: (iii) HE staining. (iv) Masson staining. The collagen in the bone matrix was stained blue-green. The purple inclusions indicated by the white arrows were the remaining PLGA material. HB, host bone. Immunohistochemical staining for the osteogenic markers (v) RUNX2 and (vi) osteocalcin (OCN). Dark-brown granules indicating positive staining are marked by red arrows. The black arrows marked the newly formed tissue and white arrows indicated the area where the remaining PLGA material was located (Reproduced with permission of Ref. [97]).
Polyetheretherketone (PEEK) is an aromatic polymer material that is chemically stable and maintains its stability at high temperatures. In addition, PEEK as an implant is noncytotoxic and highly biocompatible. Compared with Ti, osteocytes show stronger survival and proliferation capabilities on the surface of PEEK [165]. However, PEEK has poor osseointegration properties and biological activity after being implanted in the body, and the addition of exosomes can complement the deficiency. Exo-SPEEK can regulate the M2 polarization of macrophages via the NF-κB pathway and has the characteristics of immunomodulation and direct osteogenesis. To promote the binding of exosomes and PEEK, PEEK was treated with concentrated sulfuric acid to form a porous structure on its surface that facilitated osseointegration and a tannic acid layer on the surface of sulfonated PEEK (SPEEK) by soaking. When dropping the exosomes-containing solution on TA-Speek, the polyphenol groups in tannic acid bind with exosomes, preventing them from being released rapidly. In vivo experiments showed that Exo-TA-SPEEK could promote osteogenesis and strengthen the osseointegration between the implant and the new bone in the body (Fig. 8) [99].
Fig. 8.
Flow chart of encapsulation of exosomes by double emulsion technology and application of exosome-loaded PLGA scaffolds in bone tissue engineering (A) Development of a dual flow-focusing junction microfluidic device to facilitate exosome encapsulation. The device was designed to facilitate the formation of a water/oil/water double emulsion (i) via two flow focusing junctions (ii) where immiscible solvents contacted and form droplets which later become particles (iii). CAD drawings of the device were used to simulate fluid flow using COMSOL, demonstrating the formation of droplets at both junctions (iv, v). Droplet size was controlled by the relative flow rates of two immiscible solvents when they contacted at the flow focusing junction (vi). PDMS devices were interfaced with pressure-driven pumps and droplet formation was visualized under a light microscope (vii, viii). Representative images shown. Scale = 100 μm (Reproduced with permission of Ref. [100]). (B) Characteristics of different samples. (ix) Illustration of surface modification of PEEK. Fe3+ acts as an ionic cross-linker that can interact with up to three 3,4-dihydroxy-l-phenylalanine (DOPA) catechol functionalities to promote TA cross-linking. BMSC-derived Exos were reversibly bound to TA-SPEEK via hydrogen bond formation between phosphate groups in Exos phospholipid and polyphenol groups in the TA molecule. (x) FE-SEM images of PEEK, SPEEK, TA-SPEEK and Exo-TA-SPEEK. Scale bar represents 500 nm. (C) In vitro RAW264.7 cells polarization. (xi) IBa-1 and Arg-1 immunofluorescent staining of RAW264.7 cells on each sample surface are shown three days following culture. IBa-1 was stained green, Arg-1 was stained red and nuclei was stained blue. Scale bar represents 50 μm. (xii) IBa-1 and iNOS immunofluorescent staining of RAW264.7 cells on each sample surface three days after culture. IBa-1 was stained green, iNOS was stained red and nuclei was stained blue. Scale bar represents 50 μm (Reproduced with permission of Ref. [99]).
3.2.3. Exosomes-loaded hydrogels for bone tissue engineering
Hydrogels are hydrophilic gels with cross-linked networks, including natural hydrogels, synthetic hydrogels, and biohybrid hydrogels. Natural hydrogels have different types of polymers, such as collagen, hyaluronic acid (HA), fibrin, alginate, agarose, and chitosan [166]. Due to the high plasticity, hydrogels can change their molecular structures and be synthesized into temperature-responsive or pH-responsive hydrogels or molecularly imprinted hydrogels for molecular recognition. Its plasticity, porosity, and biocompatibility have led to its widespread use in treatment and diagnosis, especially in tissue engineering, controlled drug delivery, and bionanotechnology applications [167]. Exosomes attached to bone tissue engineering scaffolds can promote the repair and regeneration of bone tissue. However, one of the prominent shortcomings of exosomes is their short half-life, which is 2–4 min in plasma. Liu et al. found that encapsulating exosomes in hydrogels could protect exosomes and ensure their local concentration to prolong their half-life and allow them to exert biological functions for several weeks [168]. Encapsulation of exosomes from mir-375 overexpressed hAD-MSCs in hydrogels resulted in slower and more controlled release of exosomes in vivo, and the encapsulated exosomes could also inhibit IGFBP3 expression to promote osteogenesis [101]. Studies have shown that exosomes can regulate angiogenesis via the miR-21/NOTCH1/DLL4 signaling axis. Researchers have encapsulated exosomes from human umbilical cord mesenchymal stem cells (hucMSCs) in HA hydrogels and filled the hydrogels with nanohydroxyapatite/poly-ε-caprolactone (nHP) scaffolds to fabricate EXOs/Gel/nHP composite materials. The composite materials were then placed in an artificial skull defect model. Through imaging examination and histological analysis of the defect site in the 4th and 8th weeks, Zhang et al. found significantly more new bones and collagen ingrowth in the composite material group than in the controls. In addition, it was found through high-throughput sequencing that miR-21 is the most abundant miRNA in exosomes derived from hucMSCs, and angiogenesis and bone regeneration can be promoted in vitro and in vivo by regulating miR-21 expressions [102]. Exosomes encapsulated in hydrogels can also enhance the expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) to promote fracture healing and angiogenesis in a rat model of femoral neck fracture [61]. Similarly, exosomal miR-451a also plays an important role in promoting bone regeneration. The exosomes from adipose-derived mesenchymal stem cells (ADSC-Exos) were mixed in injectable gelatine nanoparticles hydrogels, and in vivo experiments demonstrated that miR-451a can regulate macrophage M1 to M2 phenotype polarization to promote bone healing [103].
However, as a bone tissue engineering material, hydrogels have poor strength and load-bearing properties. HAP, the material resembling human autologous bone, can be combined with hydrogels to complement the shortcomings. Yang et al. combined exosomes with an injectable HAP embedded in an in situ cross-linked hyaluronic acid-alginate (HA-ALG) hydrogel system. After the combination, the elastic modulus of the combined composite hydrogel increased, and the maximum stress increased more than two folds. Besides, the water absorption capacity decreased, making the hydrogel resemble a solid material. In addition, the hydrolysis rate becomes slower, so the composite hydrogel has a certain load-bearing capacity and can be used as a bone tissue engineering material in vivo for a prolonged time. Under the scanning electron microscope (SEM), a three-dimensional porous structure with a rough outer surface of the composite hydrogel was observed, which facilitated the attachment of exosomes. After injection into a rat skull injury model, Yang et al. found more newly formed bone-like tissue and blood vessels in the hydrogel-exo group [104].
Traditional hydrogels are more prone to damage when subjected to external forces, which hinders the biological functions of hydrogels. Zhang et al. prepared hydrogels with self-repair ability by cross-linking chitosan with small-molecule dialdehydes. The self-repair hydrogel could repair itself rapidly without specific stimulation after external force damage [169]. Wang et al. placed the CHA/SF/GCS/DF-PEG hydrogel with hucMSC-derived exosomes (hydrogel-exosomes groups) into the artificial femoral condyle defect incision. The CT, X-ray, histological and immunohistochemical analysis showed that compared with the obvious bone defect area in the control group at 30 and 60 days, the hydrogel and the tissue around the defect were already tightly connected in the hydrogel and hydrogel-exosomes groups, with a tighter connection in the hydrogel-exosomes group. After 90 days of implantation, the bone defect in the control group was still visible, while the bone defect was healed in the hydrogel and hydrogel-exosomes groups, and many new blood vessels were formed in the new bone tissue in the hydrogel-exosomes group [105].
Cartilage can hardly regenerate due to the lack of blood vessels and nerves. Although stem cells can differentiate into cartilage, their differentiation is not easy to regulate, and unexpected conditions such as hypertrophy and ossification may occur. Stem cells mainly function through the secretion of extracellular vesicles. Therefore, Liu et al. developed a light-induced subamine cross-linked hydrogel as an exocytological tissue patch for exosomes. This tissue patch can slowly release exosomes, promote the repair and growth of cartilage, and most importantly, connect with the damaged cartilage seamlessly. The seamless connection is essential for the repair and regeneration of bone or cartilage as in vitro prefabricated tissue patches were unable to closely connect to defective cartilage tissue, resulting in obvious gaps between the new tissue and natural tissue and failed healing. In contrast, the EHG tissue patch in situ formed in the experimental group could tightly and stably bond with the natural cartilage matrix, presenting a seamless interface under SEM [106]. However, conventional hydrogels are not easy to be attached in wet surface, especially on the surface of cartilage. The emergence of mussel materials provides a solution to this problem. Mussel foot protein can provide specific catechol groups to give mussels strong adhesion at wet interfaces, while chondroitin sulfate (CS), a glycosaminoglycan, can promote cartilage formation, the hydrogel to encapsulate exosomes was made using a crosslinked network of alginate-dopamine, chondroitin sulfate, and regenerated silk fibroin (AD/CS/RSF), CS has strong bonding strength to the wet surface, after implantation in vivo, it can promote the migration, retention and differentiation of BMSCs and realize cartilage regeneration [107]. Chen et al. fabricated a 3D-printed ECM/GelMA/exosome scaffold with SLA technology and found that the scaffolds could regulate the mitochondrial function to reduce the degeneration and damage to chondrocytes. In addition, the gelatin scaffold also promoted the chondrocyte migration and the transformation of macrophages towards the M2 type in synovial joints to accelerate osteochondral healing (Fig. 9) [108].
Fig. 9.
Exosome-loaded hydrogels for cartilage regeneration (A) Schematic illustration of the one-step operation system for facilitating osteochondral defect regeneration. (i) Stereolithography-based ECM/GelMA/exosome bioprinting and osteochondral defect implantation. (ii) Migration of chondrocytes to the defect regions. (iii) Controlled administration of exosomes by the 3D-printed scaffolds. (iv) Enhanced chondrocyte mitochondrial biogenesis by the scaffolds. (B) Cartilage ECM/GelMA bioink preparation. (v) Images of the hydrogel before and after cross-linking. (vi) SEM images of the hydrogel with 1–3 wt % ECM in different magnifications (scale bar = 100 μm or 50 μm). (vii) 2D and 3D AFM images of the hydrogel with different ECM concentrations. (viii) μCT reconstruction imaging of repaired knees at 6 and 12 weeks after surgery in various groups. (C) Differentially expressed proteins in normal and OA chondrocytes. (ix) Heat map of 289 downregulated and 191 upregulated proteins in OA samples compared with normal samples. (x-xii) GO classification of differentially expressed proteins (Reproduced with permission of Ref. [108]).
In the above, we reviewed the ECM as a scaffold to carry exosomes for osteogenesis. The hydrogel can also play a similar role to the ECM, because the hydrogel has similar properties to the ECM. Therefore, a study incorporated aldehyde-functionalized chondroitin sulfate (OCS) into hydrogels, and together with exosomes, which also promoted cartilage regeneration, formed GMOCS-Exos hydrogels. GMOCS-Exos hydrogel not only sustained the release of exosomes for 14 days, but also promoted cartilage regeneration by promoting the polarization of RAW cells from M1 to M2 through the NF-κB pathway and attenuating IL-1 damage to chondrocytes [170].
The gradual loss of cartilage cells causes joint cartilage damage and osteoarthritis (OA), in which reactive oxygen species (ROS) are the leading cause. Studies have shown that sleep can prevent the development of OA because melatonin (MT) secreted during sleep, as the body's ideal free radical scavenger, can protect cartilage cells and prevent the occurrence of osteoarthritis [171]. Tao et al. found that the expression of circRNA3503 increased significantly after the use of MT, which could slow the OA development by acting as a miRNA sponge for hsa-miR-181c-3p and hsa-let-7b-3p to slow the degradation of the cartilage ECM. Thus, Tao et al. isolated circRNA3503-loaded sEVs from SMSCs and used PDLLA-PEG-PDLLA triblock copolymer gels as carriers of exosomes. In vivo and in vitro experiments demonstrated that PDLLA-PEG-PLLAD gel containing circRNA3503-OE-sEVs could prevent the development of OA, providing a new research direction for OA treatment (Fig. 10) [109].
Fig. 10.
Exosome-loaded hydrogels for OA treatment (A) As indicated at day 21, the pellets with different treatments were stained with Safranin O, Alcian blue and Toluidine blue. Experiments were repeated at least three times, and representative results are shown. Error bars show standard deviation. (B) Schematic diagram of the approach for combining sEVs derived from SMSCs (SMSC-sEVs) and sleep-related circRNA3503 used in this study. (C) Schematic diagram of the mechanism of sEVs derived from SMSCs with circRNA3503 overexpression (circRNA3503-OE-sEVs). (D) Schematic diagram summarising the mechanism by which circRNA3503-OE-sEVs prevent OA progression (Reproduced with permission of Ref. [109]).
At present, the pore size of common hydrogels is mostly nanoscale. However, a study shows that after the nanoporous hydrogel is implanted into the body, it may hinder the migration of cells and the generation of new tissues, and reduce the survival rate of cells, which undoubtedly prevents the “seed cells” from fully functioning. Therefore, large-pore hydrogels came into being. Ma et al. used the method of double-crosslinking microfibers (μ-fibers) to prepare sodium alginate (SA) and hyaluronic acid (HA) μ-fibers, and then used ultraviolet (UV) light to cross-link the free HA macrofibers. Molecular chain to form large-pore hydrogels blocks with fixed structures. KGN, which can promote cartilage differentiation and cartilage regeneration, is encapsulated in PLGA microspheres, and encapsulated in large-pore hydrogel together with exosomes, in vivo, exosomes and KGN are sequentially released to regulate inflammatory responses and promote MSCs migration and chondrocyte differentiation [110].
4. Conclusion and further perspectives
The process of natural bone growth is slow, which makes bone healing difficult, while bone grafts, the most commonly used clinical treatment, are faced with problems such as limited sources, high cost, and immune rejection. Tissue engineering brings new potentials for the rapid recovery of bone defects, with MSCs as the most commonly used “seed cells.” However, stem cells are inevitably limited due to immune rejection, uncontrollable differentiation processes, and the death of attached cells during differentiation. Therefore, why should the “seed cell” be a real cell?
In cell-free therapy, exosomes have been a hot topic in recent years, especially in bone tissue engineering. Exosomes rely on the proteins, lipids, and RNA contained in their vesicles to regulate the proliferation and activity of osteoblasts and simultaneously promote angiogenesis, osteogenic differentiation, and cell matrix mineralization to enhance bone tissue regeneration. Attaching exosomes to bone tissue engineering materials not only provides a favorable environment for exosome function but also compensates for the disadvantages of bone tissue engineering materials, such as slow angiogenesis and intercellular communication barriers. However, exosomes themselves have the problem of a short half-life and a fast metabolism when injected into the body, so the scaffold has to both “attach” and “retain” the exosomes. This review also discussed the various methods, including encapsulating exosomes to enhance the binding between exosomes and scaffolds and inducing the directional aggregation of exosomes in bone, which all aimed to achieve the sustained release of exosomes and prolong their function time. Recently, the emergence of composite materials and 3D printing has provided various options for the attachment of exosomes, but there are still some potential problems. For example, it is still difficult to regulate the exosome release by scaffolds. Although a slow release is possible, a stable release has not been achieved, and the most suitable release rate for bone growth has not been explored yet. In addition, the degradation rate of scaffolds is difficult to match the bone regeneration cycle. Bone defects may not be healed completely with scaffolds of a fast degradation rate; otherwise, the slow-degraded residual scaffold will affect bone regeneration.
Exosomes also have some shortcomings, mainly because of their unclear function mechanism and especially their isolation and purification technology that needs to be improved. A general manufacturing practice standard for exosomes has not been established, and stable characterization is still lacking for exosome isolation. Currently, ultrafiltration is the most commonly used method for isolating exosomes, which is easy to operate and screen based on the size. However, this method also has problems with membrane blockage and vesicle interception. Although these problems can be solved by tangential flow filtration, the efficiency of tangential flow filtration is too low to meet the large consumption. Size-exclusion chromatography is a potential production method with less damage to exosomes, which screens exosomes by gravity.
Although there is still a long way to go in exosome studies, research on exosomes has been increasing in recent years, especially on the combination of exosomes and tissue engineering, which provides a new direction for bone injuries that are difficult to repair. In the future, we hope to see the improved production efficiency and quality of exosomes and that their function mechanism can be gradually revealed. Based on the functional characteristics, the matched scaffold can stably release exosomes at the most appropriate rate, and the exosomes can maximize their function.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (21875092, 82072425, 82072498, 82074473, 81873991 and 81873990), the Young Medical Talents of Jiangsu Province (QNRC2016751), the “Jiangsu Specially-Appointed Professor” Program, the Natural Science Foundation of Jiangsu Province (BK20200198, BE2021650 and BK20220059), and Special Project of Diagnosis and Treatment Technology for Key Clinical Diseases in Suzhou (LCZX202003, LCZX201824), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Contributor Information
Jiaxiang Bai, Email: jxbai1995@163.com.
Guoqing Pan, Email: panguoqing@ujs.edu.cn.
Dechun Geng, Email: szgengdc@suda.edu.cn.
References
- 1.Kostenuik P., Mirza F.M. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J. Orthop. Res. 2017;35(2):213–223. doi: 10.1002/jor.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z.Y., Zhou J., Liu C., Zhang J.M., Shibata Y., Kong N., Corbo C., Harris M.B., Tao W. Emerging biomimetic nanotechnology in orthopedic diseases: progress, challenges, and opportunities. Trends Chem. 2022;4(5):420–436. [Google Scholar]
- 3.Schmal H., Brix M., Bue M., Ekman A., Ferreira N., Gottlieb H., Kold S., Taylor A., Toft Tengberg P., Ban I., Danish S. Orthopaedic trauma, nonunion - consensus from the 4th annual meeting of the Danish orthopaedic trauma society. EFORT Open Rev. 2020;5(1):46–57. doi: 10.1302/2058-5241.5.190037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J., Zhang Z., Joseph J., Zhang X., Ferdows B.E., Patel D.N., Chen W., Banfi G., Molinaro R., Cosco D., Kong N., Joshi N., Farokhzad O.C., Corbo C., Tao W. Biomaterials and nanomedicine for bone regeneration: progress and future prospects. Explorations. 2021;1(2) doi: 10.1002/EXP.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J., Byun H., Madhurakkat Perikamana S.K., Lee S., Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthc. Mater. 2019;8(4) doi: 10.1002/adhm.201801106. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz C.E., Martha J.F., Kowalski P., Wang D.A., Bode R., Li L., Kim D.H. Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual. Life Outcome. 2009;7:49. doi: 10.1186/1477-7525-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Griensven M. Preclinical testing of drug delivery systems to bone. Adv. Drug Deliv. Rev. 2015;94:151–164. doi: 10.1016/j.addr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Zhang X., Zhou J., Zhang L., Xue J., Tao W. Non-Invasive thermal therapy for tissue engineering and regenerative medicine. Small. 2022 doi: 10.1002/smll.202107705. [DOI] [PubMed] [Google Scholar]
- 9.Pelissier P., Villars F., Mathoulin-Pelissier S., Bareille R., Lafage-Proust M.H., Vilamitjana-Amedee J. Influences of vascularization and osteogenic cells on heterotopic bone formation within a madreporic ceramic in rats. Plast. Reconstr. Surg. 2003;111(6):1932–1941. doi: 10.1097/01.PRS.0000055044.14093.EA. [DOI] [PubMed] [Google Scholar]
- 10.Rouwkema J., Khademhosseini A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 2016;34(9):733–745. doi: 10.1016/j.tibtech.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Phinney D.G., Pittenger M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cell. 2017;35(4):851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 12.Minciacchi V.R., Freeman M.R., Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider A., Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352(1):33–47. doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggenhofer E., Luk F., Dahlke M.H., Hoogduijn M.J. The life and fate of mesenchymal stem cells. Front. Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 16.Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 17.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 18.Mittelbrunn M., Gutierrez-Vazquez C., Villarroya-Beltri C., Gonzalez S., Sanchez-Cabo F., Gonzalez M.A., Bernad A., Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greening D.W., Gopal S.K., Xu R., Simpson R.J., Chen W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J., Liu B., Wang Z., Wang D., Ni H., Zhang L., Wang Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9(23):6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Dev. Reprod. Biol. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobrie A., Colombo M., Raposo G., Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 23.Zakharova L., Svetlova M., Fomina A.F. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 2007;212(1):174–181. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- 24.Yellon D.M., Davidson S.M. Exosomes: nanoparticles involved in cardioprotection? Circ. Res. 2014;114(2):325–332. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Waldenstrom A., Ronquist G. Role of exosomes in myocardial remodeling. Circ. Res. 2014;114(2):315–324. doi: 10.1161/CIRCRESAHA.114.300584. [DOI] [PubMed] [Google Scholar]
- 28.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 30.Kogure T., Yan I.K., Lin W.L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4(7–8):261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M., De Leo G., Alessandro R. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedjouar B., de Medina P., Oulad-Abdelghani M., Payre B., Silvente-Poirot S., Favre G., Faye J.C., Poirot M. Molecular characterization of the microsomal tamoxifen binding site. J. Biol. Chem. 2004;279(32):34048–34061. doi: 10.1074/jbc.M405230200. [DOI] [PubMed] [Google Scholar]
- 34.de Medina P., Paillasse M.R., Segala G., Khallouki F., Brillouet S., Dalenc F., Courbon F., Record M., Poirot M., Silvente-Poirot S. Importance of cholesterol and oxysterols metabolism in the pharmacology of tamoxifen and other AEBS ligands. Chem. Phys. Lipids. 2011;164(6):432–437. doi: 10.1016/j.chemphyslip.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Bradley E.W., Carpio L.R., van Wijnen A.J., McGee-Lawrence M.E., Westendorf J.J. Histone deacetylases in bone development and skeletal disorders. Physiol. Rev. 2015;95(4):1359–1381. doi: 10.1152/physrev.00004.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin Y., Sun R., Wu C., Wang L., Zhang C. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int. J. Mol. Sci. 2016;17(5) doi: 10.3390/ijms17050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 38.Qin Y., Wang L., Gao Z., Chen G., Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016;6 doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z., Chen Y., Dunstan C., Roohani-Esfahani S., Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. 2017;23(21–22):1212–1220. doi: 10.1089/ten.tea.2016.0548. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P., Xiao L., Peng J., Qian Y.Q., Huang C.C. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22(12):3962–3970. doi: 10.26355/eurrev_201806_15280. [DOI] [PubMed] [Google Scholar]
- 41.Cui Y., Luan J., Li H., Zhou X., Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–192. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 42.Narayanan R., Huang C.C., Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J.F., Yang G.H., Pan X.H., Zhang S.J., Zhao C., Qiu B.S., Gu H.F., Hong J.F., Cao L., Chen Y., Xia B., Bi Q., Wang Y.P. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei J., Li H., Wang S., Li T., Fan J., Liang X., Li J., Han Q., Zhu L., Fan L., Zhao R.C. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cell. Dev. 2014;23(13):1452–1463. doi: 10.1089/scd.2013.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W.B., Zhong W.J., Wang L. A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation. Bone. 2014;58:59–66. doi: 10.1016/j.bone.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Schaap-Oziemlak A.M., Raymakers R.A., Bergevoet S.M., Gilissen C., Jansen B.J., Adema G.J., Kögler G., le Sage C., Agami R., van der Reijden B.A., Jansen J.H. MicroRNA hsa-miR-135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cell. Dev. 2010;19(6):877–885. doi: 10.1089/scd.2009.0112. [DOI] [PubMed] [Google Scholar]
- 47.Bakhshandeh B., Hafizi M., Ghaemi N., Soleimani M. Down-regulation of miRNA-221 triggers osteogenic differentiation in human stem cells. Biotechnol. Lett. 2012;34(8):1579–1587. doi: 10.1007/s10529-012-0934-3. [DOI] [PubMed] [Google Scholar]
- 48.Xu Q., Cui Y., Luan J., Zhou X., Li H., Han J. Exosomes from C2C12 myoblasts enhance osteogenic differentiation of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem. Biophys. Res. Commun. 2018;498(1):32–37. doi: 10.1016/j.bbrc.2018.02.144. [DOI] [PubMed] [Google Scholar]
- 49.Qi X., Zhang J., Yuan H., Xu Z., Li Q., Niu X., Hu B., Wang Y., Li X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016;12(7):836–849. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Sullivan J., D'Arcy S., Barry F.P., Murphy J.M., Coleman C.M. Mesenchymal chondroprogenitor cell origin and therapeutic potential. Stem Cell Res. Ther. 2011;2(1):8. doi: 10.1186/scrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou J., Bai B., Yao Y. Progress of co-culture systems in cartilage regeneration. Expet Opin. Biol. Ther. 2018;18(11):1151–1158. doi: 10.1080/14712598.2018.1533116. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L., He A., Yin Z., Yu Z., Luo X., Liu W., Zhang W., Cao Y., Liu Y., Zhou G. Regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials. 2014;35(18):4878–4887. doi: 10.1016/j.biomaterials.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 53.Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., Zhang J., Ding J., Chen Y., Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J., Kuang L., Chen C., Yang J., Zeng W.N., Li T., Chen H., Huang S., Fu Z., Li J., Liu R., Ni Z., Chen L., Yang L. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Mao G., Hu S., Zhang Z., Wu P., Zhao X., Lin R., Liao W., Kang Y. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell Mol. Med. 2018;22(11):5354–5366. doi: 10.1111/jcmm.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z., Wang Y., Xiang S., Zheng Z., Bian Y., Feng B., Weng X. Chondrocytes-derived exosomal miR-8485 regulated the Wnt/beta-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem. Biophys. Res. Commun. 2020;523(2):506–513. doi: 10.1016/j.bbrc.2019.12.065. [DOI] [PubMed] [Google Scholar]
- 58.Yan Y., Chen H., Zhang H., Guo C., Yang K., Chen K., Cheng R., Qian N., Sandler N., Zhang Y.S., Shen H., Qi J., Cui W., Deng L. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190–191:97–110. doi: 10.1016/j.biomaterials.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Xie H., Wang Z., Zhang L., Lei Q., Zhao A., Wang H., Li Q., Cao Y., Jie Zhang W., Chen Z. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci. Rep. 2017;7 doi: 10.1038/srep45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Li Q., Niu X., Hu B., Chen S., Song W., Ding J., Zhang C., Wang Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017;13(2):232–244. doi: 10.7150/ijbs.16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Hao Z., Wang P., Xia Y., Wu J., Xia D., Fang S., Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2) doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H., Jia P., Kang H., Zhang H., Liu Y., Yang P., Yan Y., Zuo G., Guo L., Jiang M., Qi J., Liu Y., Cui W., Santos H.A., Deng L. Upregulating hif-1α by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv. Healthc. Mater. 2016;5(8):907–918. doi: 10.1002/adhm.201501018. [DOI] [PubMed] [Google Scholar]
- 63.Walsh M.C., Takegahara N., Kim H., Choi Y. Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat. Rev. Rheumatol. 2018;14(3):146–156. doi: 10.1038/nrrheum.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai J., Wang H., Chen H., Ge G., Wang M., Gao A., Tong L., Xu Y., Yang H., Pan G., Chu P.K., Geng D. Biomimetic osteogenic peptide with mussel adhesion and osteoimmunomodulatory functions to ameliorate interfacial osseointegration under chronic inflammation. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120197. [DOI] [PubMed] [Google Scholar]
- 65.Jin S.S., He D.Q., Luo D., Wang Y., Yu M., Guan B., Fu Y., Li Z.X., Zhang T., Zhou Y.H., Wang C.Y., Liu Y. A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano. 2019;13(6):6581–6595. doi: 10.1021/acsnano.9b00489. [DOI] [PubMed] [Google Scholar]
- 66.Horwood N.J. Macrophage polarization and bone formation: a review. Clin. Rev. Allergy Immunol. 2016;51(1):79–86. doi: 10.1007/s12016-015-8519-2. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z., Yuen J., Crawford R., Chang J., Wu C., Xiao Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated beta-tricalcium phosphate. Biomaterials. 2015;61:126–138. doi: 10.1016/j.biomaterials.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 68.Gu Q., Yang H., Shi Q. Macrophages and bone inflammation. J. Orthop. Translat. 2017;10:86–93. doi: 10.1016/j.jot.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W., Yu M., Chen F., Wang L., Ye C., Chen Q., Zhu Q., Xie D., Shao M., Yang L. A novel delivery nanobiotechnology: engineered miR-181b exosomes improved osteointegration by regulating macrophage polarization. J. Nanobiotechnol. 2021;19(1):269. doi: 10.1186/s12951-021-01015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao S., Mi Y., Guan B., Zheng B., Wei P., Gu Y., Zhang Z., Cai S., Xu Y., Li X., He X., Zhong X., Li G., Chen Z., Li D. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020;13(1):156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakao Y., Fukuda T., Zhang Q., Sanui T., Shinjo T., Kou X., Chen C., Liu D., Watanabe Y., Hayashi C., Yamato H., Yotsumoto K., Tanaka U., Taketomi T., Uchiumi T., Le A.D., Shi S., Nishimura F. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–324. doi: 10.1016/j.actbio.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan J., Lee C.S., Kim S., Chen C., Aghaloo T., Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. 2020;14(9):11973–11984. doi: 10.1021/acsnano.0c05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeCastro J., Littig J., Chou P.P., Mack-Onyeike J., Srinivasan A., Conboy M.J., Conboy I.M., Aran K. The microfluidic toolbox for analyzing exosome biomarkers of aging. Molecules. 2021;26(3) doi: 10.3390/molecules26030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445(7130):874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 75.Germain L., Carrier P., Auger F.A., Salesse C., Guérin S.L. Can we produce a human corneal equivalent by tissue engineering? Prog. Retin. Eye Res. 2000;19(5):497–527. doi: 10.1016/s1350-9462(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 76.Roseti L., Parisi V., Petretta M., Cavallo C., Desando G., Bartolotti I., Grigolo B. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;78:1246–1262. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 77.Sacks M.S., Schoen F.J., Mayer J.E. Bioengineering challenges for heart valve tissue engineering. Annu. Rev. Biomed. Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 78.Ouyang J., Xie A., Zhou J., Liu R., Wang L., Liu H., Kong N., Tao W. Minimally invasive nanomedicine: nanotechnology in photo-/ultrasound-/radiation-/magnetism-mediated therapy and imaging. Chem. Soc. Rev. 2022;51(12):4996–5041. doi: 10.1039/d1cs01148k. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X., Li L., Ouyang J., Zhang L., Xue J., Zhang H., Tao W. Electroactive electrospun nanofibers for tissue engineering. Nano Today. 2021;39 [Google Scholar]
- 80.Hosseini V., Maroufi N.F., Saghati S., Asadi N., Darabi M., Ahmad S.N.S., Hosseinkhani H., Rahbarghazi R. Current progress in hepatic tissue regeneration by tissue engineering. J. Transl. Med. 2019;17(1):383. doi: 10.1186/s12967-019-02137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amiel G.E., Yoo J.J., Atala A. Renal therapy using tissue-engineered constructs and gene delivery. World J. Urol. 2000;18(1):71–79. doi: 10.1007/s003450050013. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z., Xu R., Yang Y., Liang C., Yu X., Liu Y., Wang T., Yu Y., Deng F. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnol. 2021;19(1):78. doi: 10.1186/s12951-021-00826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhai M., Zhu Y., Yang M., Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv. Sci. (Weinh) 2020;7(19) doi: 10.1002/advs.202001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Z., Pu P., Su Z., Zhang X., Nie L., Chang Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020;531(4):559–565. doi: 10.1016/j.bbrc.2020.07.094. [DOI] [PubMed] [Google Scholar]
- 85.Wei F., Li M., Crawford R., Zhou Y., Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480–492. doi: 10.1016/j.actbio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Kang Y., Xu C., Meng L., Dong X., Qi M., Jiang D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022;18:26–41. doi: 10.1016/j.bioactmat.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J., Liu X., Li H., Chen C., Hu B., Niu X., Li Q., Zhao B., Xie Z., Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016;7(1):136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J., Chen L., Wang R., Song Z., Shen Z., Zhao Y., Huang S., Lin Z. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater. Sci. Eng. 2019;5(7):3561–3571. doi: 10.1021/acsbiomaterials.9b00607. [DOI] [PubMed] [Google Scholar]
- 89.Liang B., Liang J.M., Ding J.N., Xu J., Xu J.G., Chai Y.M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019;10(1):335. doi: 10.1186/s13287-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu A., Lin D., Zhao H., Chen L., Cai B., Lin K., Shen S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120718. [DOI] [PubMed] [Google Scholar]
- 91.Jiang S., Tian G., Yang Z., Gao X., Wang F., Li J., Tian Z., Huang B., Wei F., Sang X., Shao L., Zhou J., Wang Z., Liu S., Sui X., Guo Q., Guo W., Li X. Enhancement of acellular cartilage matrix scaffold by Wharton's jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021;6(9):2711–2728. doi: 10.1016/j.bioactmat.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu Y., Li X., Zhang Q., Gu Z., Luo Y., Guo J., Wang X., Jing Y., Chen X., Su J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact. Mater. 2021;6(9):2905–2913. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zha Y., Li Y., Lin T., Chen J., Zhang S., Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11(1):397–409. doi: 10.7150/thno.50741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu H., Zhang Y., Xiong S., Zhou Y., Xiao L., Ma Y., Xiao Y., Wang X. Modulatory role of silver nanoparticles and mesenchymal stem cell-derived exosome-modified barrier membrane on macrophages and osteogenesis. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.699802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diomede F., Gugliandolo A., Cardelli P., Merciaro I., Ettorre V., Traini T., Bedini R., Scionti D., Bramanti A., Nanci A., Caputi S., Fontana A., Mazzon E., Trubiani O. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res. Ther. 2018;9(1):104. doi: 10.1186/s13287-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gandolfi M.G., Gardin C., Zamparini F., Ferroni L., Esposti M.D., Parchi G., Ercan B., Manzoli L., Fava F., Fabbri P., Prati C., Zavan B. Mineral-doped poly(L-lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem cells. Nanomaterials (Basel) 2020;10(3) doi: 10.3390/nano10030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li W., Liu Y., Zhang P., Tang Y., Zhou M., Jiang W., Zhang X., Wu G., Zhou Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces. 2018;10(6):5240–5254. doi: 10.1021/acsami.7b17620. [DOI] [PubMed] [Google Scholar]
- 98.Gao Y., Yuan Z., Yuan X., Wan Z., Yu Y., Zhan Q., Zhao Y., Han J., Huang J., Xiong C., Cai Q. Bioinspired porous microspheres for sustained hypoxic exosomes release and vascularized bone regeneration. Bioact. Mater. 2022;14:377–388. doi: 10.1016/j.bioactmat.2022.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan L., Guan P., Xiao C., Wen H., Wang Q., Liu C., Luo Y., Ma L., Tan G., Yu P., Zhou L., Ning C. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact. Mater. 2021;6(9):2754–2766. doi: 10.1016/j.bioactmat.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swanson W.B., Zhang Z., Xiu K., Gong T., Eberle M., Wang Z., Ma P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020;118:215–232. doi: 10.1016/j.actbio.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen S., Tang Y., Liu Y., Zhang P., Lv L., Zhang X., Jia L., Zhou Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5) doi: 10.1111/cpr.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y., Xie Y., Hao Z., Zhou P., Wang P., Fang S., Li L., Xu S., Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl. Mater. Interfaces. 2021;13(16):18472–18487. doi: 10.1021/acsami.0c22671. [DOI] [PubMed] [Google Scholar]
- 103.Li R., Li D., Wang H., Chen K., Wang S., Xu J., Ji P. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res. Ther. 2022;13(1):149. doi: 10.1186/s13287-022-02823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang S., Zhu B., Yin P., Zhao L., Wang Y., Fu Z., Dang R., Xu J., Zhang J., Wen N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater. Sci. Eng. 2020;6(3):1590–1602. doi: 10.1021/acsbiomaterials.9b01363. [DOI] [PubMed] [Google Scholar]
- 105.Wang L., Wang J., Zhou X., Sun J., Zhu B., Duan C., Chen P., Guo X., Zhang T., Guo H. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.564731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y., Bao C., Xie Z., Lin Q., Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–4438. doi: 10.1039/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 107.Zhang F.X., Liu P., Ding W., Meng Q.B., Su D.H., Zhang Q.C., Lian R.X., Yu B.Q., Zhao M.D., Dong J., Li Y.L., Jiang L.B. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121169. [DOI] [PubMed] [Google Scholar]
- 108.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., Ning L., Shi Y., Fang C., Fan S., Lin X. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tao S.C., Huang J.Y., Gao Y., Li Z.X., Wei Z.Y., Dawes H., Guo S.C. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021;6(12):4455–4469. doi: 10.1016/j.bioactmat.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma Z., Song W., He D., Zhang X., He Y., Li H. Smart μ-fiber hydrogels with macro-porous structure for sequentially promoting multiple phases of articular cartilage regeneration. Adv. Funct. Mater. 2022;32(22) [Google Scholar]
- 111.Kapanen A., Ryhanen J., Danilov A., Tuukkanen J. Effect of nickel-titanium shape memory metal alloy on bone formation. Biomaterials. 2001;22(18):2475–2480. doi: 10.1016/s0142-9612(00)00435-x. [DOI] [PubMed] [Google Scholar]
- 112.Long M., Rack H.J. Titanium alloys in total joint replacement--a materials science perspective. Biomaterials. 1998;19(18):1621–1639. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 113.Yazdimamaghani M., Razavi M., Vashaee D., Moharamzadeh K., Boccaccini A.R., Tayebi L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;71:1253–1266. doi: 10.1016/j.msec.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 114.Liao C.Z., Li K., Wong H.M., Tong W.Y., Yeung K.W., Tjong S.C. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33(3):1380–1388. doi: 10.1016/j.msec.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 115.Chang Y.S., Oka M., Kobayashi M., Gu H.O., Li Z.L., Nakamura T., Ikada Y. Significance of interstitial bone ingrowth under load-bearing conditions: a comparison between solid and porous implant materials. Biomaterials. 1996;17(11):1141–1148. doi: 10.1016/0142-9612(96)85917-5. [DOI] [PubMed] [Google Scholar]
- 116.Hu N., Wu Y., Xie L., Yusuf S.M., Gao N., Starink M.J., Tong L., Chu P.K., Wang H. Enhanced interfacial adhesion and osseointegration of anodic TiO(2) nanotube arrays on ultra-fine-grained titanium and underlying mechanisms. Acta Biomater. 2020;106:360–375. doi: 10.1016/j.actbio.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Mei S., Wang H., Wang W., Tong L., Pan H., Ruan C., Ma Q., Liu M., Yang H., Zhang L., Cheng Y., Zhang Y., Zhao L., Chu P.K. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35(14):4255–4265. doi: 10.1016/j.biomaterials.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 118.Liu C., Shin J., Son S., Choe Y., Farokhzad N., Tang Z., Xiao Y., Kong N., Xie T., Kim J.S., Tao W. Pnictogens in medicinal chemistry: evolution from erstwhile drugs to emerging layered photonic nanomedicine. Chem. Soc. Rev. 2021;50(4):2260–2279. doi: 10.1039/d0cs01175d. [DOI] [PubMed] [Google Scholar]
- 119.Zhang X., Koo S., Kim J.H., Huang X., Kong N., Zhang L., Zhou J., Xue J., Harris M.B., Tao W., Kim J.S. Nanoscale materials-based platforms for the treatment of bone-related diseases. Matter. 2021;4(9):2727–2764. [Google Scholar]
- 120.Okuda T., Ioku K., Yonezawa I., Minagi H., Kawachi G., Gonda Y., Murayama H., Shibata Y., Minami S., Kamihira S., Kurosawa H., Ikeda T. The effect of the microstructure of beta-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials. 2007;28(16):2612–2621. doi: 10.1016/j.biomaterials.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 121.Wang J., Chen W., Li Y., Fan S., Weng J., Zhang X. Biological evaluation of biphasic calcium phosphate ceramic vertebral laminae. Biomaterials. 1998;19(15):1387–1392. doi: 10.1016/s0142-9612(98)00014-3. [DOI] [PubMed] [Google Scholar]
- 122.Le Nihouannen D., Duval L., Lecomte A., Julien M., Guicheux J., Daculsi G., Layrolle P. Interactions of total bone marrow cells with increasing quantities of macroporous calcium phosphate ceramic granules. J. Mater. Sci. Mater. Med. 2007;18(10):1983–1990. doi: 10.1007/s10856-007-3098-2. [DOI] [PubMed] [Google Scholar]
- 123.Horch H.H., Sader R., Pautke C., Neff A., Deppe H., Kolk A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral Maxillofac. Surg. 2006;35(8):708–713. doi: 10.1016/j.ijom.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 124.Handschel J., Wiesmann H.P., Stratmann U., Kleinheinz J., Meyer U., Joos U. TCP is hardly resorbed and not osteoconductive in a non-loading calvarial model. Biomaterials. 2002;23(7):1689–1695. doi: 10.1016/s0142-9612(01)00296-4. [DOI] [PubMed] [Google Scholar]
- 125.Fu X., Li Y., Huang T., Yu Z., Ma K., Yang M., Liu Q., Pan H., Wang H., Wang J., Guan M. Runx2/Osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Adv. Sci. (Weinh) 2018;5(4) doi: 10.1002/advs.201700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen L., McCrate J.M., Lee J.C., Li H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology. 2011;22(10) doi: 10.1088/0957-4484/22/10/105708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar Saini R., Prasad Bagri L., Bajpai A.K. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf. B Biointerfaces. 2019;177:211–218. doi: 10.1016/j.colsurfb.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 128.Ji X., Yuan X., Ma L., Bi B., Zhu H., Lei Z., Liu W., Pu H., Jiang J., Jiang X., Zhang Y., Xiao J. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(epsilon-caprolactone)/nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics. 2020;10(2):725–740. doi: 10.7150/thno.39167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morishita T., Honoki K., Ohgushi H., Kotobuki N., Matsushima A., Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients' mesenchymal stem cells. Artif. Organs. 2006;30(2):115–118. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 130.Liao J., Li Y., Li H., Liu J., Xie Y., Wang J., Zhang Y. Preparation, bioactivity and mechanism of nano-hydroxyapatite/sodium alginate/chitosan bone repair material. J. Appl. Biomater. Funct. Mater. 2018;16(1):28–35. doi: 10.5301/jabfm.5000372. [DOI] [PubMed] [Google Scholar]
- 131.Zhou K., Yu P., Shi X., Ling T., Zeng W., Chen A., Yang W., Zhou Z. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano. 2019;13(8):9595–9606. doi: 10.1021/acsnano.9b04723. [DOI] [PubMed] [Google Scholar]
- 132.Ducheyne P., Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20(23–24):2287–2303. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 133.Montazerian M., Dutra Zanotto E. History and trends of bioactive glass-ceramics. J. Biomed. Mater. Res. 2016;104(5):1231–1249. doi: 10.1002/jbm.a.35639. [DOI] [PubMed] [Google Scholar]
- 134.Fu Q., Rahaman M.N., Fu H., Liu X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. 2010;95(1):164–171. doi: 10.1002/jbm.a.32824. [DOI] [PubMed] [Google Scholar]
- 135.Brown R.F., Rahaman M.N., Dwilewicz A.B., Huang W., Day D.E., Li Y., Bal B.S. Effect of borate glass composition on its conversion to hydroxyapatite and on the proliferation of MC3T3-E1 cells. J. Biomed. Mater. Res. 2009;88(2):392–400. doi: 10.1002/jbm.a.31679. [DOI] [PubMed] [Google Scholar]
- 136.Huang W., Day D.E., Kittiratanapiboon K., Rahaman M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006;17(7):583–596. doi: 10.1007/s10856-006-9220-z. [DOI] [PubMed] [Google Scholar]
- 137.Delahaye F., Montagne L., Palavit G., Baillif P., Touray J.C. Dissolution of (50-x)Na2O-xCaO-50P(2)O(5) metaphosphate glasses in different saline solutions: mechanism and kinetic control. Glass Sci. Technol.-Glastechnische Berichte. 1999;72(5):161–166. [Google Scholar]
- 138.Abou Neel E.A., Chrzanowski W., Pickup D.M., O'Dell L.A., Mordan N.J., Newport R.J., Smith M.E., Knowles J.C. Structure and properties of strontium-doped phosphate-based glasses. J. R. Soc. Interface. 2009;6(34):435–446. doi: 10.1098/rsif.2008.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salih V., Franks K., James M., Hastings G.W., Knowles J.C., Olsen I. Development of soluble glasses for biomedical use Part II: the biological response of human osteoblast cell lines to phosphate-based soluble glasses. J. Mater. Sci. Mater. Med. 2000;11(10):615–620. doi: 10.1023/a:1008901612674. [DOI] [PubMed] [Google Scholar]
- 140.da Silva R.P., Serrano S.H. Electrochemical oxidation of biological molecules at carbon paste electrodes pre-treated in guanine solutions. J. Pharm. Biomed. Anal. 2003;33(4):735–744. doi: 10.1016/s0731-7085(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 141.Zhu M., Li W., Dong X., Yuan X., Midgley A.C., Chang H., Wang Y., Wang H., Wang K., Ma P.X., Wang H., Kong D. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019;10(1):4620. doi: 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Font Tellado S., Balmayor E.R., Van Griensven M. Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015;94:126–140. doi: 10.1016/j.addr.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Gabizon A., Shiota R., Papahadjopoulos D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J. Natl. Cancer Inst. 1989;81(19):1484–1488. doi: 10.1093/jnci/81.19.1484. [DOI] [PubMed] [Google Scholar]
- 144.Cheng R., Liu L., Xiang Y., Lu Y., Deng L., Zhang H., Santos H.A., Cui W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119706. [DOI] [PubMed] [Google Scholar]
- 145.Perumal G., Sivakumar P.M., Nandkumar A.M., Doble M. Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;109 doi: 10.1016/j.msec.2019.110527. [DOI] [PubMed] [Google Scholar]
- 146.Constantinides C., Basnett P., Lukasiewicz B., Carnicer R., Swider E., Majid Q.A., Srinivas M., Carr C.A., Roy I. Vivo tracking and (1)H/(19)F magnetic resonance imaging of biodegradable polyhydroxyalkanoate/polycaprolactone blend scaffolds seeded with labeled cardiac stem cells. ACS Appl. Mater. Interfaces. 2018;10(30):25056–25068. doi: 10.1021/acsami.8b06096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fu W., Liu Z., Feng B., Hu R., He X., Wang H., Yin M., Huang H., Zhang H., Wang W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014;9:2335–2344. doi: 10.2147/IJN.S61375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shafei S., Foroughi J., Stevens L., Wong C.S., Zabihi O., Naebe M. Electroactive nanostructured scaffold produced by controlled deposition of PPy on electrospun PCL fibres. Res. Chem. Intermed. 2017;43(2):1235–1251. [Google Scholar]
- 149.Hassanajili S., Karami-Pour A., Oryan A., Talaei-Khozani T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104 doi: 10.1016/j.msec.2019.109960. [DOI] [PubMed] [Google Scholar]
- 150.Xue R., Qian Y., Li L., Yao G., Yang L., Sun Y. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017;8(1):148. doi: 10.1186/s13287-017-0588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dorozhkin S.V. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015;6(3):708–832. doi: 10.3390/jfb6030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Larsen M.W., Pietrzak W.S., DeLee J.C. Fixation of osteochondritis dissecans lesions using poly(l-lactic acid)/poly(glycolic acid) copolymer bioabsorbable screws. Am. J. Sports Med. 2005;33(1):68–76. doi: 10.1177/0363546504265927. [DOI] [PubMed] [Google Scholar]
- 153.Athanasiou K.A., Agrawal C.M., Barber F.A., Burkhart S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14(7):726–737. doi: 10.1016/s0749-8063(98)70099-4. [DOI] [PubMed] [Google Scholar]
- 154.Baptista R., Guedes M. Morphological and mechanical characterization of 3D printed PLA scaffolds with controlled porosity for trabecular bone tissue replacement. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;118 doi: 10.1016/j.msec.2020.111528. [DOI] [PubMed] [Google Scholar]
- 155.Singhvi M.S., Zinjarde S.S., Gokhale D.V. Polylactic acid: synthesis and biomedical applications. J. Appl. Microbiol. 2019;127(6):1612–1626. doi: 10.1111/jam.14290. [DOI] [PubMed] [Google Scholar]
- 156.Tyler B., Gullotti D., Mangraviti A., Utsuki T., Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016;107:163–175. doi: 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 157.Mooney D.J., Mazzoni C.L., Breuer C., McNamara K., Hern D., Vacanti J.P., Langer R. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials. 1996;17(2):115–124. doi: 10.1016/0142-9612(96)85756-5. [DOI] [PubMed] [Google Scholar]
- 158.Martin C., Winet H., Bao J.Y. Acidity near eroding polylactide-polyglycolide in vitro and in vivo in rabbit tibial bone chambers. Biomaterials. 1996;17(24):2373–2380. doi: 10.1016/s0142-9612(96)00075-0. [DOI] [PubMed] [Google Scholar]
- 159.Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 160.Felix Lanao R.P., Jonker A.M., Wolke J.G., Jansen J.A., van Hest J.C., Leeuwenburgh S.C. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. B Rev. 2013;19(4):380–390. doi: 10.1089/ten.teb.2012.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gentile P., Chiono V., Carmagnola I., Hatton P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014;15(3):3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu Y.C., Shaw S.Y., Lin H.R., Lee T.M., Yang C.Y. Bone tissue engineering evaluation based on rat calvaria stromal cells cultured on modified PLGA scaffolds. Biomaterials. 2006;27(6):896–904. doi: 10.1016/j.biomaterials.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Thomson R.C., Yaszemski M.J., Powers J.M., Mikos A.G. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials. 1998;19(21):1935–1943. doi: 10.1016/s0142-9612(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 164.Wu C., Ramaswamy Y., Zhu Y., Zheng R., Appleyard R., Howard A., Zreiqat H. The effect of mesoporous bioactive glass on the physiochemical, biological and drug-release properties of poly(DL-lactide-co-glycolide) films. Biomaterials. 2009;30(12):2199–2208. doi: 10.1016/j.biomaterials.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 165.Gu X., Sun X., Sun Y., Wang J., Liu Y., Yu K., Wang Y., Zhou Y. Bioinspired modifications of PEEK implants for bone tissue engineering. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.631616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem. Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 167.Peppas N., Hilt J., Khademhosseini A., Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 2006;18(11):1345–1360. [Google Scholar]
- 168.Liu B., Lee B.W., Nakanishi K., Villasante A., Williamson R., Metz J., Kim J., Kanai M., Bi L., Brown K., Di Paolo G., Homma S., Sims P.A., Topkara V.K., Vunjak-Novakovic G. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2(5):293–303. doi: 10.1038/s41551-018-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Y., Tao L., Li S., Wei Y. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules. 2011;12(8):2894–2901. doi: 10.1021/bm200423f. [DOI] [PubMed] [Google Scholar]
- 170.Guan P., Liu C., Xie D., Mao S., Ji Y., Lin Y., Chen Z., Wang Q., Fan L., Sun Y. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property Facilitates/promotes growth plate injury repair. Bioact. Mater. 2022;10:145–158. doi: 10.1016/j.bioactmat.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hosseinzadeh A., Kamrava S.K., Joghataei M.T., Darabi R., Shakeri-Zadeh A., Shahriari M., Reiter R.J., Ghaznavi H., Mehrzadi S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal Res. 2016;61(4):411–425. doi: 10.1111/jpi.12362. [DOI] [PubMed] [Google Scholar]