Highlights
-
•
Nomograms constructed by overall survival and cancer-specific survival can predict more accurately than AJCC stage system for GCLM patients.
-
•
The study includes the prognostic factor as many as possible and evaluated all of them in the cohort.
-
•
In our cohort, surgery is a beneficial factor associated with survival.
Keywords: Overall survival, Nomogram, Gastric cancer, Liver metastases
Abbreviations: AUC, Area under the curve; CSS, Cancer-specific survival; DCA, Decision curve analysis; GC, Gastric cancer; GCLM, Gastric cancer and liver metastasis; IDI, Integrated differentiation improvement; LR, Liver resection; NRI, Net reclassification improvement; OS, Overall survival; ROC, Receiver operating characteristic; TG, Total gastrectomy; SEER, Surveillance, Epidemiology, and End Results Program
Abstract
Background
Gastric cancer is heterogeneous and aggressive, especially with liver metastasis. This study aims to develop two nomograms to predict the overall survival (OS) and cancer-specific survival (CSS) of gastric cancer with liver metastasis (GCLM) patients.
Methods
From January 2000 to December 2018, a total of 1936 GCLM patients were selected from the Surveillance, Epidemiology, and End Results Program (SEER) database. They were further divided into a training cohort and a validation cohort, with the OS and CSS serving as the study's endpoints. The correlation analyses were used to determine the relationship between the variables. The univariate and multivariate Cox analyses were used to confirm the independent prognostic factors. To discriminate and calibrate the nomogram, calibration curves and the area under the time-dependent receiver operating characteristic curve (time-dependent AUC) were used. DCA curves were used to examine the accuracy and clinical benefits. The clinical utility of the nomogram and the AJCC Stage System was compared using net reclassification improvement (NRI) and integrated differentiation improvement (IDI) (IDI). Finally, the nomogram and the AJCC Stage System risk stratifications were compared.
Results
There was no collinearity among the variables that were screened. The results of multivariate Cox regression analysis showed that six variables (bone metastasis, lung metastasis, surgery, chemotherapy, grade, age) and five variables (lung metastasis, surgery, chemotherapy, grade, N stage) were identified to establish the nomogram for OS and CSS, respectively. The calibration curves, time-dependent AUC curves, and DCA revealed that both nomograms had pleasant predictive power. Furthermore, NRI and IDI confirmed that the nomogram outperformed the AJCC Stage System.
Conclusion
Both nomograms had satisfactory accuracy and were validated to assist clinicians in evaluating the prognosis of GCLM patients.
Introduction
Gastric cancer (GC) is a common clinical malignant tumor of the digestive tract that is responsible for the fourth and fifth leading causes of cancer-related deaths in men and women, respectively [1]. In 2020, more than one million (1,089,103) new cases of gastric cancer were diagnosed worldwide, with an estimated 760,000 deaths [1]. There are several non-surgical therapies available today, including chemotherapy, radiotherapy, and immunotherapy. Furthermore, more researchers are focusing on tumor immunology and tumor-associated immune cells [2]. The use of a combination of Trastuzumab and chemotherapy in gastric adenocarcinoma patients with overexpressed human epidermal growth factor receptor 2 (HER2) has been a huge success and is considered a first-line treatment currently [3]. Moreover, his approach has made a significant improvement even as third-line therapy in advanced gastric cancer patients [4]. However, radical surgical resection is the primary treatment for localized GC [5]. Despite the development in therapeutic technology, recurrence rates in advanced cases remain high (40–80%) [6]. According to reports, approximately 35–40% of gastric cancer patients developed synchronous metastasis, with the vast majority of patients presenting with hepatic metastasis. Furthermore, after performing a curative surgical resection, more than 30% of GC patients developed metachronous liver metastasis [7,8]. With a 5-year survival rate of only 10%, liver metastasis from gastric cancer (GCLM) is an indicator of poor prognosis [9]. Currently, a lack of effective treatment modalities can improve overall survival [10].
The prognosis of GCLM varies considerably such that personalized prediction of GCLM has become the focus of various studies, including those of the American Joint Committee on Cancer (AJCC) gastric cancer staging system, which has confirmed the importance and practicability for the evaluation of the prognosis [11]. However, it is difficult to obtain satisfactory prediction outcomes with the TNM staging. More predictors and classification of continuous variables should be considered to improve the accuracy of prognosis.
The nomogram has been demonstrated to enhance predictive accuracy and widely used in oncology in recent years [12,13]. The nomogram model is simple, intuitive, and practical for visualizing the linear prognosis and quantifying individual patient survival in order to guide clinical decision-making and emphasize personalized medicine [14]. In this study, we aimed to develop a more detailed nomogram to predict the prognosis of GCLM patients using a large GCLM dataset from the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and methods
Data source and inclusion criteria
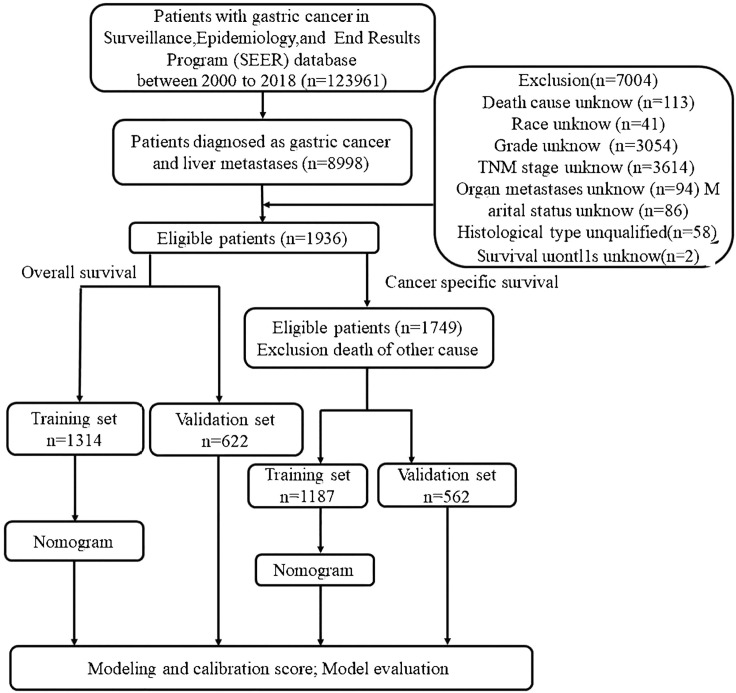
The data for this study were abstracted using the SEER*Stat software version 8.3.9. The SEER database is a multi-center and multi-population registry funded by the National Cancer Institute that is not subject to medical ethics review and does not require informed consent. The data used for this study was extracted from the SEER Research Plus (with additional treatment fields) 18 Registry from 2000 to 2018, and it was subjected to strict inclusion and exclusion criteria, which are listed below. The inclusion criteria: (1) patients diagnosed with gastric cancers (Site recode of ICD-O-3/WHO2008:C160-C169);(2) liver metastases (SEER Combined Mets at DX-liver); (3) basic demographic variables, including age, race and gender; (4) complete survival data and follow-up data; (5) tumor characteristics, including histological information and type, TNM stage; and (6) therapeutic measures that whether received surgery, chemotherapy and radiotherapy. Individuals with gastric cancer who lacked information or a pathological diagnosis were excluded. Fig. 1 contains additional information. Furthermore, this work satisfies all STROCSS criteria [15].
Fig. 1.
Flow diagram illustrating recruitment of patients.
Cohort definition and clinicopathological factors
We divided the data set into a 7:3 training cohort and validation cohorts using the R package (CreateDataPartition). The training set was used to create the model, while the validation set was used to optimize the model parameters and perform the evaluation. For the following variables, fifteen clinicopathological factors were extracted from the SEER database: age (<65 and ≥65 years), sex (female and male), race (White, Black, Asian or Pacific islander and American Indian/Alaska Native), primary site (cardia, fundus, body, gastric antrum, lesser, greater, other), histologic type (adenocarcinoma, signet ring cell, special type), grade (grade I, grade II, grade III, and grade IV), T stage (T0, T1, T2, T3, and T4), N stage (N0, N1, N2 and N3), bone metastasis (yes or no), brain metastasis (yes or no), lung metastasis(yes or no), radiotherapy (yes or no), chemotherapy (yes or no), surgery (yes or no), marital status (yes or no). The collected follow-up data included overall survival (OS) and cancer-specific survival (CSS), which were considered endpoint times. We performed univariate Cox regression analysis on all fifteen prognostic factors and obtained independent prognostic factors through multivariate Cox regression analysis based on univariate Cox regression analysis (P < 0.05).
Statistical analysis
R software was used for all statistical analyses (version 4.1.0). We established three models: the Cox-AJCC model, the multi-factor Cox model and the competitive risk model. First, simple data processing was performed, converting raw data into factors for subsequent analysis. Pearson correlation was used to assess the existence of correlation among the variables in the correlation analyses. After randomly dividing the data into training and validation cohorts in a 7:3 ratio, univariate Cox analysis was used to screen independent variables based on P values (P < 0.1). To compare the significant factors and estimate the hazard ratios (HRs) and 95% confidence intervals following the standard of P < 0.05, the multivariate Cox regression analysis was performed in variables that exhibit differences in univariate Cox regression analysis. After that, using Cox regression, which is based on the training cohort, to analyze the nomogram that was created to predict the 1-year, 3-year, and 4-year OS and CSS rates. With the establishment of these models, we use the net reclassification index (NRI) and integrated discrimination improvement (IDI) methods to evaluate the clinical benefits and utility of the Cox-AJCC model and the multivariate Cox model, to select the best predictive model. There are two mutually complementary validation methods, but the NRI, which is primarily used to compare the prediction ability of the old model with the new one, only considers the improvement when a specific cutoff point is set, whereas the IDI, which is primarily used to investigate the overall improvement of the model, inspects the overall improved performance of the model [16]. In the case of CSS, the Fine-Gray proportional hazards model was used to create the competing risk nomogram.
The discriminative power of the nomograms was evaluated using the area under the curve (AUC) values, which reflect the overall estimation value for all thresholds [17]. Lastly, the performance of our nomograms was investigated in the test and validation cohort in terms of the Calibration Curve, Receiver operating characteristic analysis (ROC), and Decision curve analysis (DCA).
Results
Flowchart
The flow diagram is displayed in Fig. 1.
Demographic and clinical features
There were 1936 patients in the study for OS analysis, with 1398 men and 538 women in this study. In addition, the patients were randomly assigned to one of two cohorts: training (n = 1314) and validation (n = 522). We described the demographic and clinical characteristics of GCLM patients. When first diagnosed, the majority of GCLM patients (55.84%) are poorly differentiated (Grade III) and over 65 years old. T1 (39.20%), T4 (31.15%), T3 (23.81%), T2 (5.68%), and T0 were the classifications (0.15%). More than half of the patients were male (72.21%) and white (71.69%), and the majority (84.61%) did not have surgery or radiotherapy (81.46%). Adenocarcinoma was diagnosed in 83.11% of the patients, and chemotherapy was used as their treatment (60.73%). Table 1 lists the demographic and clinical characteristics of patients in the OS group in detail.
Table 1.
Demographic and clinical characteristics of patients with GCLM in OS group.
| Characteristics | All samples N Percentage (%) |
Training N Percentage (%) |
Validation N Percentage (%) |
P | |||
|---|---|---|---|---|---|---|---|
| Age(years) | |||||||
| <65 years | 855 | 44.16% | 584 | 44.44% | 271 | 43.57% | 0.7172 |
| >65 years | 1081 | 55.84% | 730 | 55.56% | 351 | 56.43% | |
| Sex | |||||||
| female | 538 | 27.79% | 379 | 28.84% | 159 | 25.56% | 0.1324 |
| male | 1398 | 72.21% | 935 | 71.16% | 463 | 74.44% | |
| Race | |||||||
| American Indian/Alaska Native | 16 | 0.83% | 10 | 0.76% | 6 | 0.96% | 0.6237 |
| Asian/Pacific Islander | 209 | 10.80% | 143 | 10.88% | 66 | 10.61% | |
| Black | 323 | 16.68% | 216 | 16.44% | 107 | 17.20% | |
| White | 1388 | 71.69% | 945 | 71.92% | 443 | 71.22% | |
| Primary Site | |||||||
| Cardia | 802 | 41.43% | 551 | 41.93% | 251 | 40.35% | 0.4580 |
| Pylorus | 95 | 4.91% | 62 | 4.72% | 33 | 5.31% | |
| Body | 169 | 8.73% | 118 | 8.98% | 51 | 8.20% | |
| Antrum | 308 | 15.91% | 208 | 15.83% | 100 | 16.08% | |
| Fundus | 28 | 1.44% | 19 | 1.45% | 9 | 1.45% | |
| Lesser curve | 119 | 6.14% | 80 | 6.09% | 39 | 6.27% | |
| Greater curve | 67 | 3.46% | 46 | 3.50% | 21 | 3.38% | |
| Other | 348 | 17.98% | 230 | 17.50% | 118 | 18.97% | |
| Histologic type | |||||||
| Adenocarcinoma | 1609 | 83.11% | 1089 | 82.88% | 520 | 83.60% | 0.6867 |
| Signet ring cell | 137 | 7.08% | 95 | 7.23% | 42 | 6.75% | |
| Special type | 190 | 9.81% | 130 | 9.89% | 60 | 9.65% | |
| Grade | |||||||
| I | 57 | 2.94% | 41 | 3.12% | 16 | 2.57% | 0.4192 |
| II | 621 | 32.08% | 416 | 31.66% | 205 | 32.96% | |
| III | 1214 | 62.71% | 834 | 63.47% | 380 | 61.09% | |
| IV | 44 | 2.27% | 23 | 1.75% | 21 | 3.38% | |
| T Stage | |||||||
| T0 | 3 | 0.15% | 2 | 0.15% | 1 | 0.16% | 0.7041 |
| T1 | 759 | 39.20% | 520 | 39.57% | 239 | 38.49% | |
| T2 | 110 | 5.68% | 71 | 5.40% | 39 | 6.28% | |
| T3 | 461 | 23.81% | 311 | 23.67% | 150 | 24.15% | |
| T4 | 603 | 31.15% | 410 | 31.20% | 193 | 31.08% | |
| N Stage | |||||||
| N0 | 756 | 39.05% | 516 | 39.27% | 240 | 38.59% | 0.7374 |
| N1 | 864 | 44.63% | 583 | 44.37% | 281 | 45.18% | |
| N2 | 158 | 8.16% | 108 | 8.22% | 50 | 8.04% | |
| N3 | 158 | 8.16% | 107 | 8.14% | 51 | 8.20% | |
| Radiotherapy | |||||||
| Yes | 359 | 18.54% | 235 | 17.88% | 124 | 19.94% | 0.2781 |
| No | 1577 | 81.46% | 1079 | 82.12% | 498 | 80.06% | |
| Chemotherapy | |||||||
| Yes | 1171 | 60.73% | 803 | 59.61% | 368 | 63.35% | 0.4131 |
| No | 765 | 39.27% | 511 | 40.39% | 254 | 36.65% | |
| Surgery | |||||||
| Yes | 298 | 15.39% | 199 | 15.14% | 99 | 15.92% | 0.6604 |
| No | 1638 | 84.61% | 1115 | 84.86% | 523 | 84.08% | |
| Bone Metastasis | |||||||
| Yes | 157 | 8.85% | 110 | 8.37% | 62 | 9.97% | 0.2490 |
| No | 1618 | 91.15% | 1204 | 91.63% | 560 | 90.03% | |
| Brain Metastasis | |||||||
| Yes | 26 | 1.34% | 14 | 1.07% | 12 | 1.93% | 0.1231 |
| No | 1910 | 98.66% | 1300 | 98.93% | 610 | 98.07% | |
| Lung Metastasis | |||||||
| Yes | 315 | 16.27% | 209 | 15.91% | 106 | 17.04% | 0.5271 |
| No | 1621 | 83.73% | 1105 | 84.09% | 516 | 82.96% | |
| Marital Status | |||||||
| Yes | 1191 | 61.52% | 820 | 62.40% | 371 | 59.65% | 0.2441 |
| No | 745 | 38.48% | 494 | 37.60% | 251 | 40.35% | |
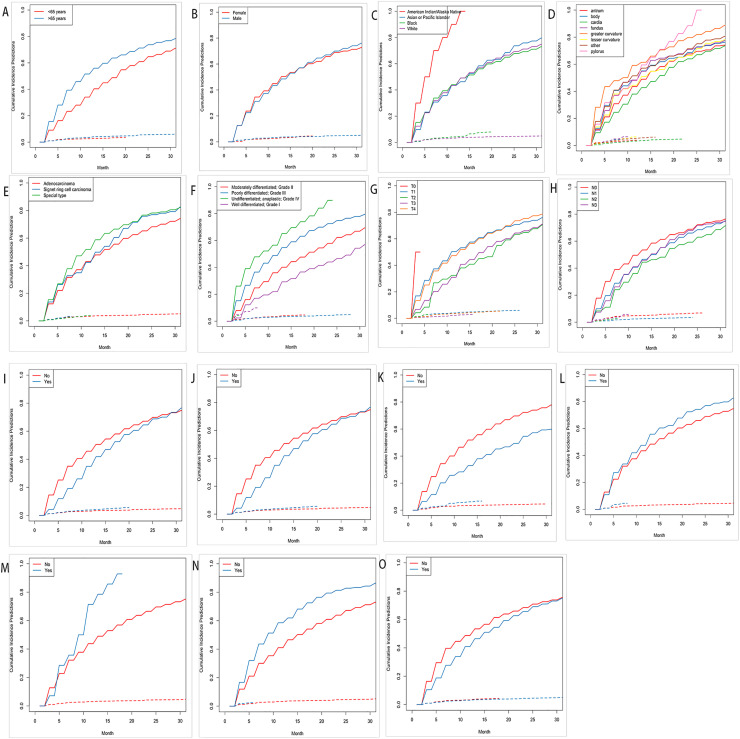
A total of 1749 patients were included in the CSS analysis, with 1187 patients in the training cohort and the remaining 562 patients in the validation cohort. There were 72.78% male patients and 27.22% female patients among these 1749 patients. The majority of the patients (71.93%) were white. 1086 patients (62.09%) were married, and less than 15% of patients underwent surgery as part of their treatment. Table 2 shows the baseline clinical-pathological characteristics of patients in the CSS group. The Cumulative Incidence Function (CIF) subgroup assessment data reported that high CSS occurred primarily in GCLM patients aged 65 years (Fig. 2A), American Indian/Alaska native (Fig. 2C), advanced grade (Fig. 2F), along with brain metastasis and lung metastasis (Fig. 2M,N), as well as patients who did not undergo chemotherapy (Fig. 2I) or radiotherapy (Fig. 2N,J).
Table 2.
Demographic and clinical characteristics of patients with GCLM in CSS group.
| Characteristics | All samples N Percentage (%) |
Training N Percentage (%) |
Validation N Percentage (%) |
P | |||
|---|---|---|---|---|---|---|---|
| Age(years) | |||||||
| <65 | 773 | 44.20% | 523 | 44.06% | 250 | 44.48% | 0.8678 |
| >65 | 976 | 55.80% | 664 | 55.94% | 312 | 55.52% | |
| Sex | |||||||
| female | 476 | 27.22% | 337 | 28.39% | 139 | 24.73% | 0.1085 |
| male | 1273 | 72.78% | 850 | 71.61% | 423 | 75.27% | |
| Race | |||||||
| American Indian/Alaska Native | 16 | 0.91% | 10 | 0.84% | 6 | 1.07% | 0.9427 |
| Asian/Pacific Islander | 194 | 11.09% | 134 | 11.29% | 60 | 10.68% | |
| Black | 281 | 16.07% | 189 | 15.92% | 92 | 16.37% | |
| White | 1258 | 71.93% | 854 | 71.95% | 404 | 71.89% | |
| Primary Site | |||||||
| Cardia | 741 | 42.37% | 507 | 42.71% | 234 | 41.64% | 0.9731 |
| Pylorus | 88 | 5.03% | 58 | 4.89% | 30 | 5.34% | |
| Body | 153 | 8.75% | 107 | 9.01% | 46 | 8.19% | |
| Antrum | 265 | 15.15% | 182 | 15.33% | 83 | 14.77% | |
| Fundus | 26 | 1.49% | 17 | 1.43% | 9 | 1.60% | |
| Lesser curve | 104 | 5.95% | 70 | 5.90% | 34 | 6.05% | |
| Greater curve | 60 | 3.43% | 42 | 3.54% | 18 | 3.20% | |
| Other | 312 | 17.84% | 204 | 17.19% | 108 | 19.22% | |
| Histologic type | |||||||
| Adenocarcinoma | 1452 | 83.02% | 983 | 82.81% | 469 | 83.45% | 0.9448 |
| Signet ring cell | 127 | 7.26% | 87 | 7.33% | 40 | 7.12% | |
| Special type | 170 | 9.72% | 117 | 9.86% | 53 | 9.43% | |
| Grade | |||||||
| I | 43 | 2.46% | 31 | 2.61% | 12 | 2.14% | 0.0936 |
| II | 553 | 31.62% | 374 | 31.51% | 179 | 31.85% | |
| III | 1113 | 63.64% | 762 | 64.20% | 351 | 62.46% | |
| IV | 40 | 2.29% | 20 | 1.68% | 20 | 3.56% | |
| T Stage | |||||||
| T0 | 2 | 0.11% | 1 | 0.08% | 1 | 0.18% | 0.9488 |
| T1 | 688 | 39.34% | 472 | 39.76% | 216 | 38.43% | |
| T2 | 99 | 5.66% | 65 | 5.48% | 34 | 6.05% | |
| T3 | 418 | 23.90% | 283 | 23.84% | 135 | 24.02% | |
| T4 | 542 | 30.99% | 366 | 30.83% | 176 | 31.32% | |
| N Stage | |||||||
| N0 | 674 | 38.54% | 459 | 38.67% | 215 | 38.26% | 0.9483 |
| N1 | 788 | 45.05% | 537 | 45.24% | 251 | 44.66% | |
| N2 | 144 | 8.23% | 97 | 8.17% | 47 | 8.36% | |
| N3 | 143 | 8.18% | 94 | 7.92% | 49 | 8.72% | |
| Radiotherapy | |||||||
| Yes | 325 | 18.58% | 215 | 18.11% | 110 | 19.57% | 0.4635 |
| No | 1424 | 81.42% | 972 | 81.89% | 452 | 80.43% | |
| Chemotherapy | |||||||
| Yes | 1061 | 60.66% | 727 | 61.25% | 334 | 59.43% | 0.4678 |
| No | 688 | 39.34% | 460 | 38.75% | 228 | 40.57% | |
| Surgery | |||||||
| Yes | 240 | 13.72% | 161 | 13.56% | 79 | 14.06% | 0.7795 |
| No | 1509 | 86.28% | 1026 | 86.44% | 483 | 85.94% | |
| Bone Metastasis | |||||||
| Yes | 157 | 8.98% | 103 | 8.68% | 54 | 9.61% | 0.5246 |
| No | 1592 | 91.02% | 1084 | 91.32% | 508 | 90.39% | |
| Brain Metastasis | |||||||
| Yes | 24 | 1.37% | 13 | 1.10% | 11 | 1.96% | 0.1478 |
| No | 1725 | 98.63% | 1174 | 98.90% | 551 | 98.04% | |
| Lung Metastasis | |||||||
| Yes | 297 | 16.98% | 199 | 16.76% | 98 | 17.44% | 0.7264 |
| No | 1452 | 83.02% | 988 | 83.24% | 464 | 82.56% | |
| Marital Status | |||||||
| Yes | 1086 | 62.09% | 746 | 62.85% | 340 | 60.50% | 0.3443 |
| No | 663 | 37.91% | 441 | 37.15% | 222 | 39.50% | |
Fig. 2.
Cumulative incidence predictions of CSS in gastric cancer with liver metastasis. (A) Age (B) Sex (C) Race (D) Primary Site (E) Histological type (F) Grade (G) T Stage (H) N Stage (I) Radiotherapy (J) Chemotherapy (K) Surgery (L) Bone Metastasis (M) Brain Metastasis (N) Lung Metastasis (O) Marital Status.
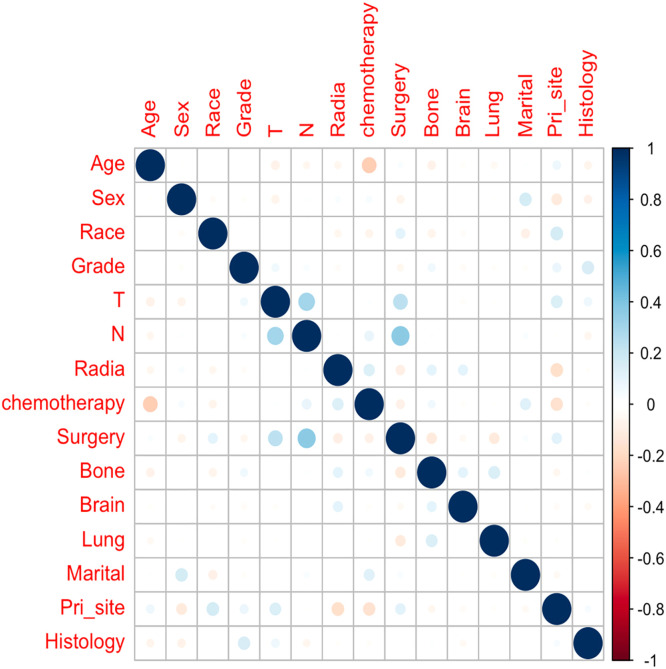
Correlations among variables
Before we performed the Cox regression analysis, Spearman's correlation was used to ensure that there was no collinearity existed between screened variables. The results of correlation analyses are presented in Fig. 3.
Fig. 3.
The results of correlation analysis between all included variables.
Nomogram variable screening
According to the Cox regression results, the model containing age, grade, surgery, chemotherapy, lung metastasis and bone metastasis had minimal P value in the training cohort. In the univariate regression analysis, nine variables (age, grade, T stage, N stage, chemotherapy, surgery, bone metastasis, lung metastasis and primary site) were significantly associated with OS. Multivariate Cox regression analysis showed that age (>65 years old) (P = 0.003, hazard ratios (HR) = 1.194, 95% confidence interval (CI) = 1.061–1.343), grade III (P < 0.001, HR = 1.456, 95% CI = 1.287–1.648) and chemotherapy (P < 0.001, HR = 0.268, 95% CI = 0.235–0.305) were independent prognostic factors in patients with GCLM. More details are presented in Table 3.
Table 3.
Univariate and multivariate Cox proportional hazards regression analysis of patients with GCLM in the OS group.
| Variables Univariate Cox regression analysis Multivariate Cox regression analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||||
| age | |||||||||||
| <65 | 1 (reference) | 1 (reference) | |||||||||
| >65 | 1.359 | 1.215–1.521 | 0.000 | 1.194 | 1.061–1.343 | 0.003 | |||||
| Sex | |||||||||||
| female | 1 (reference) | ||||||||||
| male | 1.124 | 0.994–1.272 | 0.062 | ||||||||
| Race | |||||||||||
| American Indian/Alaska Native | 1 (reference) | ||||||||||
| Asian/Pacific Islander | 0.572 | 0.301–1.087 | 0.088 | ||||||||
| Black | 0.592 | 0.314–1.118 | 0.106 | ||||||||
| White | 0.605 | 0.324–1.129 | 0.114 | ||||||||
| Grade | |||||||||||
| I | 0.682 | 0.483–0.965 | 0.030 | 0.566 | 0.399–0.805 | 0.002 | |||||
| II | 1(reference) | 1 (reference) | |||||||||
| III | 1.382 | 1.225–1.561 | 0.000 | 1.456 | 1.287–1.648 | 0.000 | |||||
| IV | 0.683 | 0.483–0.965 | 0.331 | 1.416 | 0.906–2.211 | 0.127 | |||||
| T Stage | |||||||||||
| T0 | 1(reference) | 1(reference) | |||||||||
| T1 | 4.165 | 0.585–29.65 | 0.154 | 4.816 | 0.671–34.577 | 0.118 | |||||
| T2 | 2.921 | 0.405–21.06 | 0.288 | 4.142 | 0.569–30.161 | 0.161 | |||||
| T3 | 3.052 | 0.428–21.76 | 0.266 | 4.502 | 0.625–32.459 | 0.135 | |||||
| T4 | 3.960 | 0.556–28.21 | 0.170 | 5.146 | 0.714–37.076 | 0.104 | |||||
| N Stage | |||||||||||
| N0 | 1 (reference) | 1(reference) | |||||||||
| N1 | 0.865 | 0.767–0.977 | 0.019 | 0.910 | 0.802–1.033 | 0.146 | |||||
| N2 | 0.714 | 0.576–0.886 | 0.002 | 0.845 | 0.672–1.063 | 0.151 | |||||
| N3 | 0.836 | 0.674–1.036 | 0.102 | 1.188 | 0.940–1.501 | 0.150 | |||||
| Radiation | |||||||||||
| Yes | 0.919 | 0.796–1.061 | 0.252 | ||||||||
| No | 1 (reference) | ||||||||||
| Chemotherapy | |||||||||||
| Yes | 0.329 | 0.293–0.370 | 0.000 | 0.268 | 0.235–0.305 | 0.000 | |||||
| No | 1 (reference) | 1 (reference) | |||||||||
| Surgery | |||||||||||
| Yes | 0.583 | 0.496–0.685 | 0.000 | 0.476 | 0.394–0.574 | 0.000 | |||||
| No | 1 (reference) | 1 (reference) | |||||||||
| Bone Metastasis | |||||||||||
| Yes | 1.250 | 1.026–1.524 | 0.027 | 1.256 | 1.025–1.540 | 0.028 | |||||
| No | 1 (reference) | 1 (reference) | |||||||||
| Brain Metastasis | |||||||||||
| Yes | 1.368 | 0.808–2.318 | 0.244 | ||||||||
| No | 1 (reference) | ||||||||||
| Lung Metastasis | |||||||||||
| Yes | 1.526 | 1.312–1.775 | 0.000 | 1.511 | 1.293–1.766 | 0.000 | |||||
| No | 1 (reference) | 1 (reference) | |||||||||
| Marital | |||||||||||
| Yes | 0.965 | 0.860–1.082 | 0.540 | ||||||||
| No | 1 (reference) | ||||||||||
| Primary site | |||||||||||
| Cardia | 1.050 | 0.889–1.240 | 0.564 | 1.096 | 0.920–1.306 | 0.304 | |||||
| Pylorus | 0.982 | 0.598–1.614 | 0.943 | 0.886 | 0.537–1.462 | 0.637 | |||||
| Body | 1.141 | 0.904–1.441 | 0.266 | 1.044 | 0.825–1.322 | 0.719 | |||||
| Antrum | 1 (reference) | 1(reference) | |||||||||
| Fundus | 1.240 | 0.930–1.653 | 0.142 | 1.079 | 0.806–1.445 | 0.610 | |||||
| Lesser curve | 1.043 | 0.798–1.363 | 0.00475 | 0.958 | 0.732–1.254 | 0.754 | |||||
| Greater curve | 1.414 | 1.015–1.969 | 0.040 | 1.211 | 0.864–1.696 | 0.267 | |||||
| other | 1.306 | 1.075–1.587 | 0.007 | 1.108 | 0.910–1.350 | 0.308 | |||||
| Histologic type | |||||||||||
| Adenocarcinoma | 1 (reference) | ||||||||||
| Signet ring cell | 1.152 | 0.928–1.429 | 0.200 | ||||||||
| Other | 1.057 | 0.876–1.275 | 0.566 | ||||||||
For the grouping status of CSS, the detailed information of patients with GCLM in the CSS group is shown in Table 4. Univariate Cox regression analysis demonstrated that sex, race, grade, N stage, chemotherapy, surgery, lung metastasis and bone metastasis were CSS-related prognostic factors. Then the multivariate Cox regression analysis was carried out to screen the independent prognostic factors in patients with GCLM. These results were tabulated in Table 4.
Table 4.
Results of univariate and multivariate analyses by Fine-Gray proportional subdistribution hazards model.
| Variables | Univariate Cox regression analysis analysis | Multivariate Cox regression | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||||||||||||||
| age | |||||||||||||||||||||
| <65 | 1 (reference) | ||||||||||||||||||||
| >65 | 1.048 | 0.927–1.184 | 0.454 | ||||||||||||||||||
| Sex | |||||||||||||||||||||
| female | 1 (reference) | 1(reference) | |||||||||||||||||||
| male | 1.157 | 1.005–1.332 | 0.043 | 1.147 | 0.980–1.343 | 0.088 | |||||||||||||||
| Race | |||||||||||||||||||||
| American Indian/Alaska Native | 1 (reference) | 1(reference) | |||||||||||||||||||
| Asian/Pacific Islander | 0.610 | 0.246–1.513 | 0.286 | 0.892 | 0.370–2.147 | 0.798 | |||||||||||||||
| Black | 0.391 | 0.159–0.959 | 0.004 | 0.626 | 0.264–1.486 | 0.288 | |||||||||||||||
| White | 0.502 | 0.207–1.222 | 0.129 | 0.702 | 0.300–1.642 | 0.414 | |||||||||||||||
| Grade | |||||||||||||||||||||
| I | 0.540 | 0.390–0.748 | 0.000 | 0.453 | 0.327–0.630 | 0.000 | |||||||||||||||
| II | 1 (reference) | 1 (reference) | |||||||||||||||||||
| III | 1.214 | 1.064–1.385 | 0.0000 | 1.181 | 1.026–1.359 | 0.020 | |||||||||||||||
| IV | 0.893 | 0.512–1.557 | 0.0441 | 0.940 | 0.532–1.660 | 0.830 | |||||||||||||||
| T Stage | |||||||||||||||||||||
| T0 | 1 (reference) | ||||||||||||||||||||
| T1 | 2.864 | 0.197–41.73 | 0.441 | ||||||||||||||||||
| T2 | 2.902 | 0.197–42.66 | 0.437 | ||||||||||||||||||
| T3 | 2.884 | 0.198–42.04 | 0.438 | ||||||||||||||||||
| T4 | 2.991 | 0.205–43.64 | 0.423 | ||||||||||||||||||
| N Stage | |||||||||||||||||||||
| N0 | 1 (reference) | 1 (reference) | |||||||||||||||||||
| N1 | 1.215 | 1.062–1.391 | 0.005 | 1.240 | 1.070–1.438 | 0.004 | |||||||||||||||
| N2 | 1.006 | 0.795–1.273 | 0.961 | 1.214 | 0.946–1.558 | 0.128 | |||||||||||||||
| N3 | 1.104 | 0.866–1.403 | 0.423 | 1.526 | 1.171–1.989 | 0.002 | |||||||||||||||
| Radiation | |||||||||||||||||||||
| Yes | 0.974 | 0.844–1.124 | 0.716 | ||||||||||||||||||
| No | 1 (reference) | ||||||||||||||||||||
| Chemotherapy | |||||||||||||||||||||
| Yes | 0.578 | 0.490–0.681 | 0.000 | 0.459 | 0.384–0.548 | 0.000 | |||||||||||||||
| No | 1 (reference) | 1 (reference) | |||||||||||||||||||
| Surgery | |||||||||||||||||||||
| Yes | 0.581 | 0.486–0.693 | 0.000 | 0.516 | 0.420–0.635 | 0.000 | |||||||||||||||
| No | 1 (reference) | 1 (reference) | |||||||||||||||||||
| Bone Metastasis | |||||||||||||||||||||
| Yes | 1.315 | 1.078–1.603 | 0.007 | 1.062 | 0.848–1.330 | 0.599 | |||||||||||||||
| No | 1 (reference) | 1(reference) | |||||||||||||||||||
| Brain Metastasis | |||||||||||||||||||||
| Yes | 1.163 | 0.673–2.010 | 0.588 | ||||||||||||||||||
| No | 1 (reference) | ||||||||||||||||||||
| Lung Metastasis | |||||||||||||||||||||
| Yes | 1.717 | 1.444–2.041 | 0.000 | 1.583 | 1.315–1.906 | 0.000 | |||||||||||||||
| No | 1 (reference) | 1 (reference) | |||||||||||||||||||
| Marital | |||||||||||||||||||||
| Yes | 1.008 | 0.882–1.151 | 0.908 | ||||||||||||||||||
| No | 1 (reference) | ||||||||||||||||||||
| Primary site | |||||||||||||||||||||
| Cardia | 0.991 | 0.726–1.277 | 0.927 | ||||||||||||||||||
| Pylorus | 0.832 | 0.489–1.417 | 0.498 | ||||||||||||||||||
| Body | 0.963 | 0.726–1.277 | 0.795 | ||||||||||||||||||
| Antrum | 1 (reference) | ||||||||||||||||||||
| Fundus | 1.117 | 0.813–1.534 | 0.496 | ||||||||||||||||||
| Lesser curve | 0.864 | 0.638–1.169 | 0.342 | ||||||||||||||||||
| Greater curve | 1.138 | 0.720–1.799 | 0.580 | ||||||||||||||||||
| other | 1.052 | 0.823–1.343 | 0.687 | ||||||||||||||||||
| Histologic type | |||||||||||||||||||||
| Adenocarcinoma | 1 (reference) | ||||||||||||||||||||
| Signet ring cell | 1.351 | 1.073–1.702 | 0.011 | ||||||||||||||||||
| Special type | 1.047 | 0.824–1.331 | 0.708 | ||||||||||||||||||
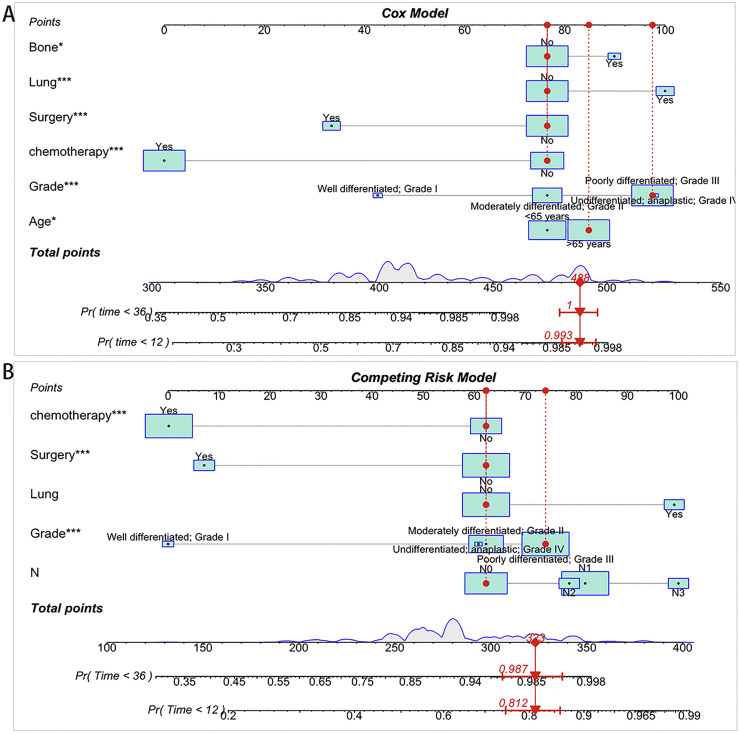
Nomogram construction and validation
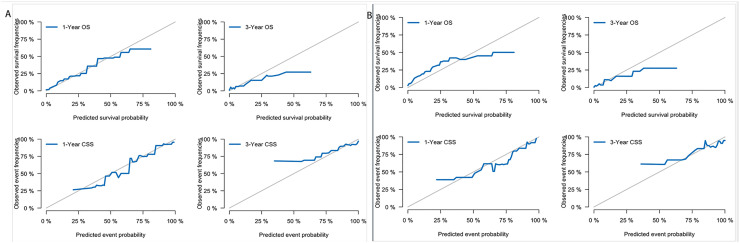
We constructed two nomograms for GCLM patients according to the variables screen. Fig. 4A shows an example of using the nomogram to predict the overall survival probability of a given patient. And Fig. 4B shows a competing event nomogram to assess the 1- and 3-year chances of CSS by incorporating those independent prognostic predictors. The likelihood that other causes contributed to or directly caused the death of gastric cancer was assessed via this model by computing the total score by each of the measured individual variables. And the calibration curves of these nomograms showed optimal consistency of the actual likelihood with the nomogram-forecasted likelihoods in both the training and validation cohort (Fig. 5).
Fig. 4.
Constructed nomograms for prognostic prediction of overall survival and cancer-specific survival. (A) Nomogram for overall survival in GCLM patients. (B) Nomogram for cancer-specific survival in GCLM patients.
Fig. 5.
Calibration curves. (A) 1-year and 3-year likelihoods of OS and CSS in the training dataset. (B) 1-year and 3-year likelihoods of OS and CSS in the validation dataset.
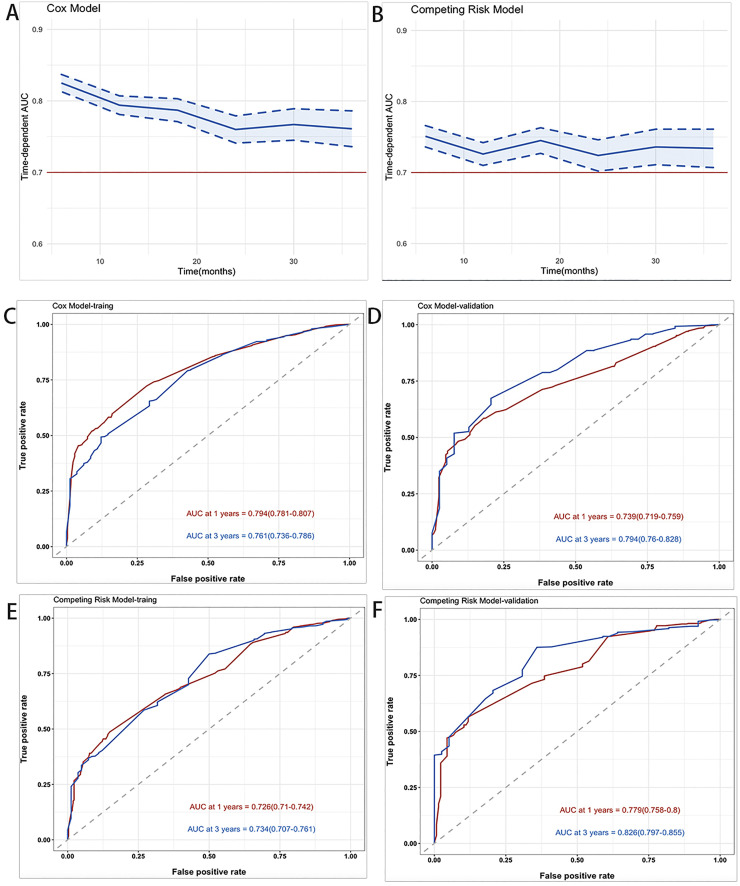
Fig. 6A,B depict the AUC in time-dependent AUC curves in the Cox model and the Competing risk model, respectively. It was greater than 0.7 for the prediction of OS and CSS within three years, indicating that the nomogram had good discrimination. In the training cohort, the AUCs of the Cox model for forecasting 1 and 3 years were 0.794 and 0.761, respectively (Fig. 6C). The AUCs at 1 and 3 years in the validation cohort were 0.739 and 0.794, respectively (Fig. 6D). The results showed that the AUCs of the competing risk model was 0.726 at one year and 0.734 at three years, while in the verification group, the AUC was 0.779 at one year and 0.826 at three years (Fig. 6E,F).
Fig. 6.
Time‐dependent AUC and receiver operating characteristic (ROC) curves of OS and CSS. (A,B) Time‐dependent AUC of using the nomogram to OS and CSS probability within 3 years in the training cohort and validation cohort. The blue line represents AUC = 0.7, which is considered ideal. And the shading area between blue dotted curves represents 95% credible intervals. (C,D) ROC curves corresponding to 1-year and 3-year OS in the training and validation cohort, respectively. (E,F) ROC curves corresponding to 1-year and 3-year CSS in the training and validation cohort, respectively.
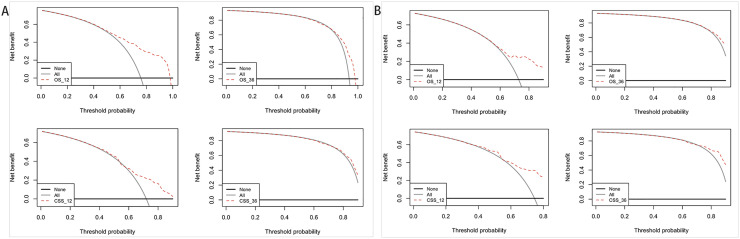
The decision curve analysis was performed on the training and verification cohort, and the results are shown in Fig. 7. Besides that, the plots for 1-year and 3-year survival showed good net benefits, indicating the nomogram's superior prediction accuracy.
Fig. 7.
Decision curve analysis of the nomogram in the estimation of OS and CSS of patients with GCLM. (A) Training cohort. (B) Validation cohort.
Clinical value comparison between nomograms and AJCC stage system
To further estimate the clinical usefulness of nomograms in this study, we used NRI, IDI and C-Index to compare the accuracy between the nomogram and the AJCC stage system. While using the nomogram in the training cohort, the C‐index was 0.7403 (95% CI = 0.7259–0.7546) in the nomogram and 0.5724 (95% CI = 0.5536–0.5912) in the AJCC Stage system, the NRI for the 1‐ and 3‐year OS were 0.7870 (95% CI = 0.6564–0.9210) and 0.6991 (95% CI = 0.4523–0.8901), and the IDI values for 1‐ and 3‐year OS were 0.184 (95% CI = 0.156–0.212, P < 0.001) and 0.095 (95% CI = 0.067–0.132, P < 0.001) (Table 5). These results were verified in the validation cohort (Table 5), indicating that the nomogram predicted prognosis with greater accuracy than the AJCC Stage System.
Table 5.
Comparison of different models for estimating the overall survival of GCLM patients.
| Training cohort | Validation cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Estimate | 95%CI | P value | Estimate | 95%CI | P value | |||||
| NRI (vs. AJCC stage System) | |||||||||||
| For 1-year OS | 0.7870 | 0.6564–0.9210 | 0.6826 | 0.4294–0.8421 | |||||||
| For 3-year OS | 0.6991 | 0.4523–0.8901 | 0.5485 | 0.2511–0.9997 | |||||||
| IDI (vs. AJCC stage System) | |||||||||||
| For 1-year OS | 0.184 | 0.156–0.212 | <0.0001 | 0.156 | 0.116–0.195 | <0.0001 | |||||
| For 3-year OS | 0.095 | 0.067–0.132 | <0.0001 | 0.108 | 0.061–0.169 | <0.0001 | |||||
| C-Index | |||||||||||
| The nomogram (OS) | 0.7403 | 0.7259–0.7546 | 0.6953 | 0.6728–0.7179 | |||||||
| AJCC Stage System | 0.5724 | 0.5536–0.5912 | 0.5495 | 0.5220–0.5769 | |||||||
Risk stratification for gastric cancer patients with liver metastasis
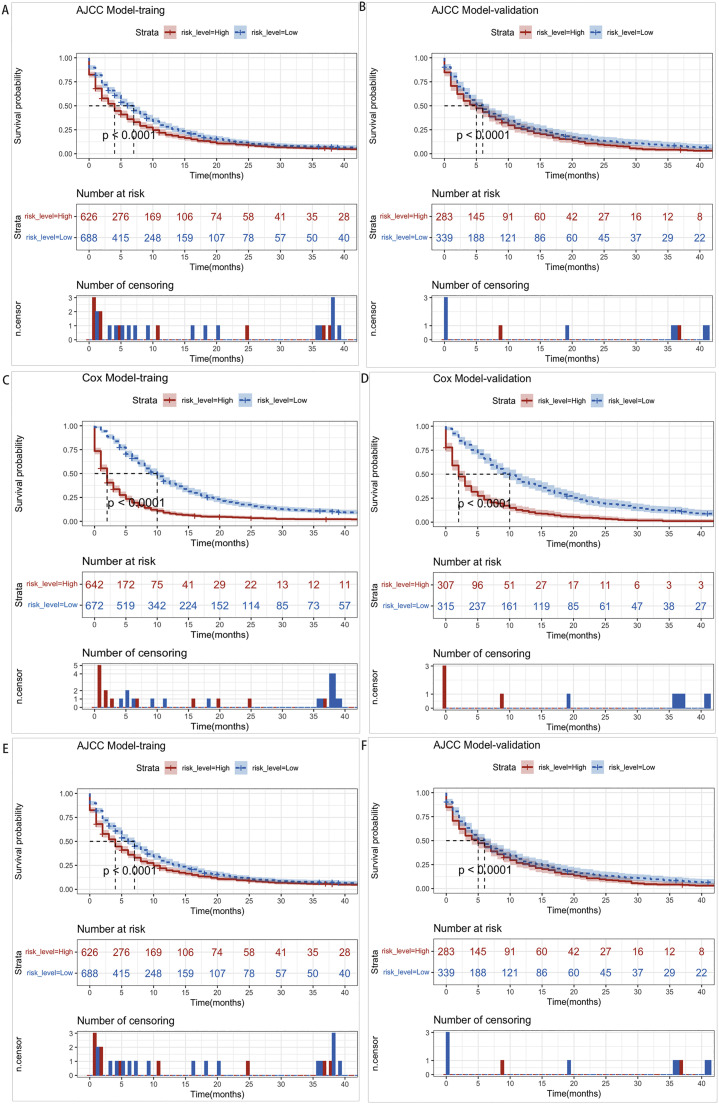
We calculated total scores based on the nomogram for risk stratification. The patient was therefore classified into two risk groups (low-risk and high-risk) based on the median risk score. The Kaplan-Meier survival analysis revealed that the low-risk group had a better OS and CSS than the high-risk group (Fig. 8C–F). Meanwhile, the nomogram demonstrated excellent discrimination between two risk groups, whereas the AJCC Stage System had limited ability to distinguish between these two groups (Fig. 8A,B). Both results were confirmed in the validation cohort.
Fig. 8.
Kaplan–Meier OS and CSS curves of GCLM patients with different risks stratified by the nomogram. (A,B) GCLM patients in the training and validation cohort at different stages are classified according to the AJCC staging system. (C,D) GCLM patients in the training and validation cohort at different stages are classified according to the cox model nomogram. (E,F) GCLM patients in the training and validation cohort at different stages are classified according to the competing risk model nomogram.
Discussion
Gastric cancer is one of the most common gastrointestinal tract malignant tumors with a low early diagnosis rate, low surgical resection rate, and high recurrence [18]. A significant proportion of gastric cancer patients had distant metastasis and the most frequent sites of gastric cancer are liver, peritoneal and distant lymph nodes [19]. These patients usually have a poor prognosis partially owing to the lack of effective treatment. In our study, we constructed two nomograms to predict the prognosis of GCLM patients. And the validation of these nomograms showed that they had good discriminative performance and calibration. In addition, the risk stratification also showed a favorable ability to categorize GCLM patients into high- and low-risk groups with significant differences.
Previous research suggests that some factors, such as differentiation, clinical phenotype, and adjuvant therapies, may potentially affect the survival of patients with GCLM. As a result, we included as many of these factors as possible in the Cox and competing risk model. According to Hu et al, differentiation degree was related to OS, and poorly differentiated adenocarcinoma was confirmed as a high-risk factor for GCLM [20]. Similar results were observed in our study, with GCLM patients with poor differentiation (Grade III) receiving the highest score. Different standards exist in the clinical phenotype of gastric cancer, and the WHO classification is now used globally. Although common types, such as papillary carcinoma and Signet ring cell carcinoma, are easily agreed upon, the specialized types remain contentious [21]. Cox regression analysis revealed no correlation between the prognosis and pathological types of GCLM patients, according to Li et al. [22]. In contrast, Daniela et al found pretty similar survival values for patients with various types of adenocarcinomas, but carcinomas with "signet-ring" cells proved to be extremely aggressive, which was consistent with our findings [23]. TIn randomized controlled trials, the clinical benefit of chemotherapy in patients with advanced gastric cancer has been demonstrated in randomized controlled trials [24], [25], [26]. Chemotherapy is currently the mainstay of therapy for GCLM patients, expected to alleviate disease-related symptoms and prolong survival [27].
Another unexpected factor that deserves special mention is surgery. The surgical treatment of patients with distant metastases has remained contentious in recent years. Systemic chemotherapy is currently the standard management for GCLM, but it does not produce satisfactory results [28]. For patients with incurable factors such as unresectable liver metastasis, the Japanese Guidelines (5th edition) strongly recommend not to perform gastrectomy as a reduction surgery. However, surgery such as hepatectomy is recommended in patients with limited metastasis where there is no other incurable factor [27]. A retrospective study reported by Sheraz R Marker et al, revealed hepatectomy for synchronous gastric cancer liver metastases may improve may carry survival benefits in certain patients [29]. On the contrary, the European Society for Medical Oncology (ESMO) reported that metastasis resection does not benefit patients with metastatic disease [30]. The clinical trial (REGATTA) identified that gastrectomy does not improve survival in patients with limited metastasis [31]. However, the guidelines also stated that after palliative chemotherapy, the possibility of surgery should be reconsidered. According to Al-Batran SE et al, gastric cancer patients with limited metastases who received neoadjuvant chemotherapy and underwent surgery had a favorable survival [32]. The purpose of this study was to determine whether surgery was a significant factor in survival in GCLM. Based on the univariate and multivariate Cox regression results (P < 0.001), we concluded that surgery affects the survival of patients with GCLM and was mentioned as a possible factor in the nomogram's establishment.
We identified that the surgery improved patients' survival based on the prediction model. Even though, various factors such as histology, depth of invasion, lymphatic or venous invasion, number and size of liver metastases, surgical options such as LR only, TG+LR, or TG only, and so on may be associated with the outcomes of undergoing surgery for patients with GCLM [27]. To the best of our knowledge, no other studies have used the SEER database to determine that surgery is an independent prognostic factor that has a positive impact on the survival of GCLM patients. Our research does, however, have some limitations. This study was not a randomized controlled trial, and selection bias in the cohort was unavoidable. Additionally, due to the small number of patient's limitations and incomplete information, some important indicators, such as the size and number of liver metastases, cannot be evaluated. As a result, more in-depth and comprehensive studies may be required.
The analysis of GCLM in the current study provided an opportunity to reconsider some factors that could be incorporated into the prognostic nomogram. Our nomograms incorporate more factors, such as demographic characteristics than the traditional AJCC staging system, and they are more accurate in predicting patient outcomes and assisting clinical practice. The AJCC Stage System has traditionally been the first choice for predicting the prognosis of gastric cancer patients. There are several nomograms for GC that have been shown to have clinical utility. Memorial Sloan-Kettering Cancer Center's Dikken JL et al established a nomogram to predict the survival of gastric cancer patients after an R0 resection [33]. Furthermore, the collagen nomogram and radionics nomogram make significant advances in the diagnosis and evaluation of recurrence and metastasis [34,35]. With today's technology, precision medicine is becoming more feasible. With the rapid progress of genomics, metabolomics, and radiomics, multi-omics analysis is becoming ever more popular. In the future, the physician will collect as much information about patients as possible during their visits, resulting in an exhaustive analysis of the various dimensions. We believe that these studies will provide a firm foundation for personalized treatment to improve GC patient life expectancy.
Despite the fact that the nomogram performed well, the current study had some weaknesses. The SEER database information, such as surgical methods and specific chemotherapy regimens, is insufficient. These factors may have an impact on the accuracy of those patients' prognoses. Further to that, it is unknown whether the patients have synchronous or metachronous liver metastases, and the medical management for the two groups of patients differs. What is more, a large proportion of gastric cancer patients have a signet-ring cancer phenotype, which is more likely to metastasize to the ovaries and peritoneum [36]. The database, however, only contains information on four types of metastases: liver, lung, bone, and brain. Our findings were derived from a cohort of Americans. As a result, a larger-sample multicenter study should be conducted to determine whether our study results are more broadly applicable.
Conclusion
We created two prognostic nomograms and a risk stratification system using the SEER database. It also laid the foundation for precision therapy and tailor-made treatment in GCLM patients. The optimal surgical procedure and conditions for GCLM should be studied further.
CRediT authorship contribution statement
Zhongyi Dong: Conceptualization, Methodology, Software, Writing – original draft. Yeqian Zhang: Data curation. Haigang Geng: Software, Writing – original draft. Bo Ni: Visualization. Xiang Xia: Investigation. Chunchao Zhu: Supervision. Jiahua Liu: Validation, Writing – review & editing. Zizhen Zhang: Writing – review & editing.
Declaration of Competing Interest
All authors declare that they have no competing interests.
Acknowledgments
Acknowledgments
We kindly thank all the staff of National Cancer Institute for their efforts toward the SEER program.
Funding
The study was sponsored by The National Natural Science Foundation of China (Grant Nos. 81972206 and 82173215)
Availability of data and materials
All data generated during this study are included in this published article. Further inquiry data can be obtained by the corresponding author.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101480.
Contributor Information
Jiahua Liu, Email: liujiahua@renji.com.
Zizhen Zhang, Email: zhangzizhen@renji.com.
Appendix. Supplementary materials
Reference
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rihawi K., Ricci A.D., Rizzo A., Brocchi S., Marasco G., Pastore L.V., et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: novel translational implications. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22083805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci A.D., Rizzo A., Rojas Llimpe F.L., Di Fabio F., De Biase D., Rihawi K. Novel HER2-directed treatments in advanced gastric carcinoma: AnotHER paradigm shift? Cancers. 2021;13(7) doi: 10.3390/cancers13071664. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzo A., Mollica V., Ricci A.D., Maggio I., Massucci M., Rojas Limpe F.L., et al. Third- and later-line treatment in advanced or metastatic gastric cancer: a systematic review and meta-analysis. Future Oncol. 2020;16(2):4409–4418. doi: 10.2217/fon-2019-0429. [DOI] [PubMed] [Google Scholar]
- 5.Xu X., Yang X., Xing C., Zhang S., miRNA CJ. The nemesis of gastric cancer (review) Oncol. Lett. 2013;6(3):631–641. doi: 10.3892/ol.2013.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang Y.J., Kim Y.W., Yang H.K., Chung H.C., Park Y.K., Lee K.H., et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2012;379(9813):315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 7.Kerkar S.P., Kemp C.D., Avital I. Liver resections in metastatic gastric cancer. HPB. 2010;12(9):589–596. doi: 10.1111/j.1477-2574.2010.00224.x. (Oxford) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riihimaki M., Hemminki A., Sundquist K., Sundquist J., Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7(32):52307–52316. doi: 10.18632/oncotarget.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baek H.U., Kim S.B., Cho E.H., Jin S.H., Yu H.J., Lee J.I., et al. Hepatic resection for hepatic metastases from gastric adenocarcinoma. J. Gastric Cancer. 2013;13(2):86–92. doi: 10.5230/jgc.2013.13.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto Y., Sano T., Shimada K., Esaki M., Saka M., Fukagawa T., et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J. Surg. Oncol. 2007;95(7):534–539. doi: 10.1002/jso.20739. [DOI] [PubMed] [Google Scholar]
- 11.Lu J., Dai Y., Xie J.W., Wang J.B., Lin J.X., Chen Q.Y., et al. Combination of lymphovascular invasion and the AJCC TNM staging system improves prediction of prognosis in N0 stage gastric cancer: results from a high-volume institution. BMC Cancer. 2019;19(1):216. doi: 10.1186/s12885-019-5416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balachandran V.P., Gonen M., Smith J.J., DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong J., Zheng J., Cai J., Wu S., Diao X., Xie W., et al. A nomogram for individualized estimation of survival among adult patients with adrenocortical carcinoma after surgery: a retrospective analysis and multicenter validation study. Cancer Commun. 2019;39(1):80. doi: 10.1186/s40880-019-0426-0. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narita Y., Kadowaki S., Oze I., Kito Y., Kawakami T., Machida N., et al. Establishment and validation of prognostic nomograms in first-line metastatic gastric cancer patients. J. Gastrointest. Oncol. 2018;9(1):52–63. doi: 10.21037/jgo.2017.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., et al. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Shi R., Zhang T., Sun H., Hu F. Establishment of clinical prediction model based on the study of risk factors of stroke in patients with type 2 diabetes mellitus. Front. Endocrinol. 2020;11:559. doi: 10.3389/fendo.2020.00559. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Steenbeek C.D., van Maaren M.C., Siesling S., Witteveen A., Verbeek X., Koffijberg H. Facilitating validation of prediction models: a comparison of manual and semi-automated validation using registry-based data of breast cancer patients in the Netherlands. BMC Med. Res. Methodol. 2019;19(1):117. doi: 10.1186/s12874-019-0761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L., Li S.H., Wu Y., Xin L. Establishment of a prognostic model of four genes in gastric cancer based on multiple data sets. Cancer Med. 2021;10(10):3309–3322. doi: 10.1002/cam4.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrelli F., Coinu A., Cabiddu M., Ghilardi M., Borgonovo K., Lonati V., et al. Hepatic resection for gastric cancer liver metastases: a systematic review and meta-analysis. J. Surg. Oncol. 2015;111(8):1021–1027. doi: 10.1002/jso.23920. [DOI] [PubMed] [Google Scholar]
- 20.Hu X. Risk factors and prognosis of liver metastasis from gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17(2):108–111. [PubMed] [Google Scholar]
- 21.Lochhead P., El-Omar EM. Gastric cancer. Br. Med. Bull. 2008;85:87–100. doi: 10.1093/bmb/ldn007. [DOI] [PubMed] [Google Scholar]
- 22.Li Z., Fan B., Shan F., Tang L., Bu Z., Wu A., et al. Gastrectomy in comprehensive treatment of advanced gastric cancer with synchronous liver metastasis: a prospectively comparative study. World J. Surg. Oncol. 2015;13:212. doi: 10.1186/s12957-015-0627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazar D., Taban S., Sporea I., Dema A., Cornianu M., Lazar E., et al. Gastric cancer: correlation between clinicopathological factors and survival of patients. II. Rom. J. Morphol. Embryol. 2009;50(2):185–194. [PubMed] [Google Scholar]
- 24.Murad A.M., Santiago F.F., Petroianu A., Rocha P.R., Rodrigues M.A., Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72(1):37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Glimelius B., Hoffman K., Haglund U., Nyren O., Sjoden PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann. Oncol. 1994;5(2):189–190. doi: 10.1093/oxfordjournals.annonc.a058778. [DOI] [PubMed] [Google Scholar]
- 26.Pyrhonen S., Kuitunen T., Nyandoto P., Kouri M. Randomized comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br. J. Cancer. 1995;71(3):587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Japanese Gastric Cancer A Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24(1):1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka K., Kinoshita T., Moehler M., Mauer M., Shitara K., Wagner A.D., et al. Current management of liver metastases from gastric cancer: what is common practice? New challenge of EORTC and JCOG. Gastric Cancer. 2017;20(5):904–912. doi: 10.1007/s10120-017-0696-7. [DOI] [PubMed] [Google Scholar]
- 29.Markar S.R., Mackenzie H., Mikhail S., Mughal M., Preston S.R., Maynard N.D., et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer. 2017;20(2):379–386. doi: 10.1007/s10120-016-0604-6. [DOI] [PubMed] [Google Scholar]
- 30.Smyth E.C., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D., et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 31.Fujitani K., Yang H.K., Mizusawa J., Kim Y.W., Terashima M., Han S.U., et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomized controlled trial. Lancet Oncol. 2016;17(3):309–318. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 32.Al-Batran S.E., Homann N., Pauligk C., Illerhaus G., Martens U.M., Stoehlmacher J., et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 Trial. JAMA Oncol. 2017;3(9):1237–1244. doi: 10.1001/jamaoncol.2017.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dikken J.L., Baser R.E., Gonen M., Kattan M.W., Shah M.A., Verheij M., et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann. Surg. Oncol. 2013;20(5):1623–1630. doi: 10.1245/s10434-012-2723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen D., Liu Z., Liu W., Fu M., Jiang W., Xu S., et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat. Commun. 2021;12(1):179. doi: 10.1038/s41467-020-20429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang W., Zhou K., Jiang Y., Chen C., Yuan Q., Han Z., et al. Radiomics nomogram for prediction of peritoneal metastasis in patients with gastric cancer. Front. Oncol. 2020;10:1416. doi: 10.3389/fonc.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon K.J., Shim K.N., Song E.M., Choi J.Y., Kim S.E., Jung H.K., et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17(1):43–53. doi: 10.1007/s10120-013-0234-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this published article. Further inquiry data can be obtained by the corresponding author.