Summary
Background
Early detection of asymptomatic incipient tuberculosis (TB) could improve clinical outcomes and reduce the spread of Mycobacterium tuberculosis (MTB) infection, particularly in HIV endemic settings. This study assessed TB disease activity over 5 years in people living with HIV co-infected with MTB using a surrogate biomarker.
Methods
Between Jan 1, 2013 and Aug 31, 2018, 2014 people living with HIV were screened annually for active TB using the Xpert MTB/RIF diagnostic assay in 11 clinics in Kenya, Tanzania, Uganda, and Nigeria. Longitudinal blood mononuclear cell samples from 46 selected patients with active and recurrent tuberculosis, latent infection, or incipient TB were further analysed for MTB-specific T-cell activation (defined by CD38 expression) as a well-defined surrogate marker for TB disease covering a total of 1758 person-months.
Findings
MTB-specific CD4 T-cell activation differentiated active, Xpert MTB/RIF positive TB from latent TB with a sensitivity and specificity of 86% and was reduced upon TB treatment initiation. Activated MTB-specific T cells were present in 63% and 23% of incipient TB cases 6 and 12 months before diagnosis of active disease, respectively. Transient increases of MTB-specific T cell activation were also observed in individuals with latent infection, while persistent activation was a hallmark of recurrent TB after the end of treatment.
Interpretation
In most cases, progression to active TB disease started 6–12 months before diagnosis by clinical symptoms and sputum occurrence of bacilli. Blood biomarkers could facilitate early detection of incipient TB, improve clinical outcomes, and reduce the transmission of MTB.
Funding
This work was supported by the President's Emergency Plan for AIDS Relief via a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174, W81XWH-18-2-0040] and by the Bundesministerium für Bildung und Forschung (BmBF) through funding of the Deutsches Zentrum für Infektionsforschung (DZIF, TTU-TB personalized medicine TTU 02_813).
Keywords: Incipient tuberculosis, Tuberculosis, Biomarker, HIV
Research in context.
Evidence before this study
Mycobacterium Tuberculosis (MTB)-specific T cell activation marker expression has repeatedly been shown to differentiate between different tuberculosis (TB) disease states with remarkable accuracy. TB disease is a major cause of morbidity and the single largest cause of mortality in people living with HIV, who may be disproportionately affected by subclinical TB. A search in Medline was performed using the search terms “Tuberculosis” OR “TB” OR “Mycobacterium tuberculosis” OR “MTB” AND “diagnosis” OR “diagnostic” OR “detect” OR “predict” OR “prognostic” AND “biomarker” OR “immune marker” OR “immunologic marker” OR “incipient tuberculosis” OR “subclinical TB”. The same parameters were used in a second search AND “HIV”. Several studies reported on assessment of MTB-specific CD4 T cell activation to distinguish between latent and active TB and to monitor the TB treatment response in adult and pediatric populations as well as in people living with HIV. Assessing whole blood RNA signatures for prediction of progression to active TB disease has been reported, but focused on individuals who were HIV-negative only.
Added value of this study
Our results show that within people living with HIV, MTB-specific T cell activation above the diagnostic threshold for active TB disease became detectable in the majority of incipient TB cases between 6–12 months before sputum-based diagnosis of TB. Further, persistent or recurring MTB-specific T cell activation after the end of treatment was detected in all recurrent TB cases.
Implications of all the available evidence
Our findings suggest that progression to active TB disease is often detectable at 6–12 months prior to the diagnosis of transmissible TB regardless of HIV infection. Early diagnosis of incipient TB before MTB bacilli are detectable in sputum could therefore help to improve short- and long-term clinical outcomes and intercept mycobacterial transmission and hence the spread of TB disease.
Alt-text: Unlabelled box
Introduction
One-quarter of the world's population is infected with Mycobacterium tuberculosis (MTB), leading to ten million active tuberculosis (aTB) cases and 1.5 million deaths every year.1 However, only 5% to 15% of infected individuals develop aTB, with the majority remaining in a state of clinical latency.2 Latent TB infection (LTBI) is commonly defined as the absence of aTB symptoms and microbiologic evidence of active infection in individuals with a MTB-specific T-cell response detected by interferon gamma (IFNγ) release assay (IGRA) or tuberculin skin test. This definition of LTBI therefore likely covers a range of infection and disease states including naturally cleared MTB infection, quiescent/dormant infection as well as incipient and subclinical aTB that may spontaneously resolve or progress to overt, transmittable disease.3,4
Because direct assessment of MTB activity during clinical latency is impossible in living patients, little is known about the dynamics of subclinical disease activity before progression to overt clinical aTB or subsequent spontaneous control by the immune system.5 Advanced imaging has been used to assess disease activity before and after progression to aTB.6, 7, 8, 9 In one study, roughly 30% of people living with HIV, who had clinically latent TB exhibited PET/CT patterns suggestive of subclinical aTB, and half of these progressed to clinical aTB within 6 months.6 Surprisingly, metabolically active TB lesions in the lung as well as MTB-derived nucleic acids in the sputum are also often detectable after end of TB treatment in patients who cured TB disease.7,10
Biomarkers provide a powerful tool for diagnosis of aTB and are increasingly considered for treatment monitoring.11, 12, 13, 14, 15, 16, 17 The activation status of MTB-specific CD4 T cells can discriminate between aTB, LTBI and cured TB with remarkable accuracy and has recently been commonly employed to assess disease activity in vivo in many human cohort studies.11,13,18, 19, 20, 21, 22 This approach also showed potential for early detection of progressive TB disease months before clinical diagnosis and sputum-culture negative TB disease in children.13,23 Here, we therefore used this surrogate biomarker to study TB disease activity in people living with HIV for up to 5 years.
Methods
Study participants and sample selection
The AFRICOS study cohort is a systematic longitudinal cohort study enrolling people living with HIV and HIV-uninfected adults at 11 clinics across 5 geographically distinct programs supported by the president's Emergency Plan for AIDS Relief (PEPFAR) in Kenya, Tanzania, Uganda, and Nigeria. HIV-infected study participants were invited to the study based on random selection from existing clinic patient lists or new enrollees to the clinic.24 The study was approved by the institutional review boards of the Walter Reed Army Institute of Research (WRAIR #1897/RV329), the Higher Degrees Research and Ethics Committee, (HS, 1175) Makerere University, Uganda and the Kenya Medical Research Institute (KEMRI SCC # 2371) and the Ethical Committee of the Ludwig-Maximilians-University Munich, Germany (LMU # 21-0351). All participants provided written informed consent.
At the baseline visit, screening for aTB was performed in all individuals by Xpert MTB/RIF on the Cepheid GeneXpert system.23,25 HIV history and symptoms suggestive of lung disease, including TB, were recorded using a detailed questionnaire at baseline and during 6-monthly follow-up visits. Rescreening by Xpert MTB/RIF was then performed annually for all study participants, regardless of aTB symptoms. For antiretroviral Naive individuals an Interferon gamma release assay (IGRA) using Quantiferon TB Gold (Cellestis) was performed at the baseline visit. Isoniazid preventive therapy (IPT) was not given regularly to IGRA positive individuals at the time of the study.
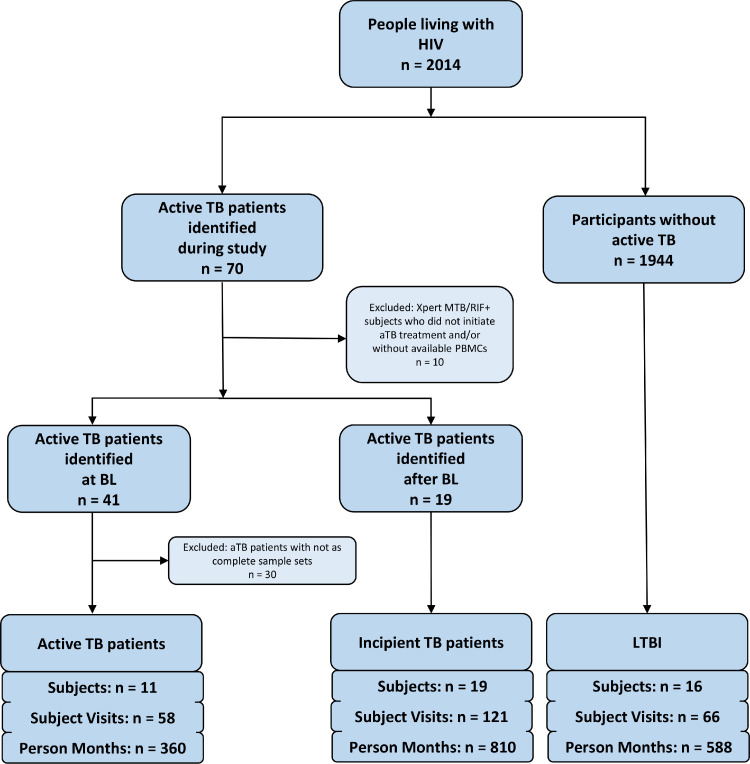
Figure 1 shows how AFRICOS study participants from two of the countries, Uganda and Kenya, were selected between Jan 1, 2013 and Aug 31, 2018, and provides the case definitions for active TB and incipient TB cases and LTBI used for the purpose of this study.
Figure 1.
Flowchart describing the selection of active TB cases, incipient TB cases and controls with latent TB included for analyses.
Cases of active TB were defined by a positive Xpert MTB/RIF test result at the study baseline and initiation of TB therapy. Incipient TB cases were identified based on absence of TB symptoms and a negative Xpert MTB/RIF test result at baseline with subsequent development of Xpert MTB/RIF-confirmed aTB and initiation of TB treatment. LTBI was defined by a detectable IFNγ response after in vitro stimulation with MTB antigens, continuous Xpert MTB/RIF negativity, no known history of aTB and absence of aTB symptoms throughout the study period. The number of participants, visits and total person months covered by the analyses is indicated for each group. Selection of participants to be included was based on completeness of available PBMC sample set. More details on the selection of PBMC samples for analyses are also provided in the supplementary material. Abbrevations: MTB=Mycobacterium tuberculosis, TB=tuberculosis, aTB=active tuberculosis, LTBI=latent tuberculosis infection, IFNγ=interferon gamma.
Classification of study participants
All participants with pulmonary aTB were defined and classified by a sputum-positive Xpert MTB/RIF result and subsequent initiation of TB therapy upon clinical diagnosis. Incipient TB cases were identified based on the absence of TB symptoms and a negative Xpert MTB/RIF result at baseline with subsequent development of Xpert MTB/RIF-confirmed aTB and initiation of TB treatment upon clinical diagnosis. LTBI was defined by continuous Xpert MTB/RIF negativity, absence of aTB symptoms, no known history of aTB and a detectable IFN-γ response after in vitro stimulation with MTB antigens. X-ray diagnostic was not available or not done according to the local screening and treatment algorithms.
If a participant was not able to generate sputum spontaneously at their visit, the study team was encouraged to follow-up with the participant to try and obtain the sputum sample at a subsequent time within the study visit window. Sputum samples were stored and tested using the Xpert MTB/RIF test in real time; samples that were not evaluated in real time were tested retrospectively.
Selection of PBMC samples for analyses
All available PBMC samples from incipient TB cases were subjected to intracellular staining analyses. Of note, for three participants no samples were available and two aTB cases were only identified after sample shipment took place. In addition, all available samples from 11 of 41 participants with aTB at baseline were selected on the basis of available samples from a maximum of timepoints and to include participants with Xpert MTB/RIF confirmed recurrent TB. 16 of 1944 participants, who 1) were continuously Xpert MTB/RIF negative, 2) had no documented aTB symptoms throughout the study, 3) without previous aTB diagnosis, 4) a positive MTB-specific Interferon-γ baseline Quantiferon results or other MTB-specific T cell responses and with 5) a most complete PBMC sample sets were selected for shipment to Germany. 14 of 16 tested participants had MTB-specific T cell responses and were included as LTBI cases in these analyses. As only ART Naive individuals received a baseline Quantiferon test, the status of “latency” could be defined only for ART Naive individuals and they have been chosen for analysis of LTBI preferentially Figure 2.
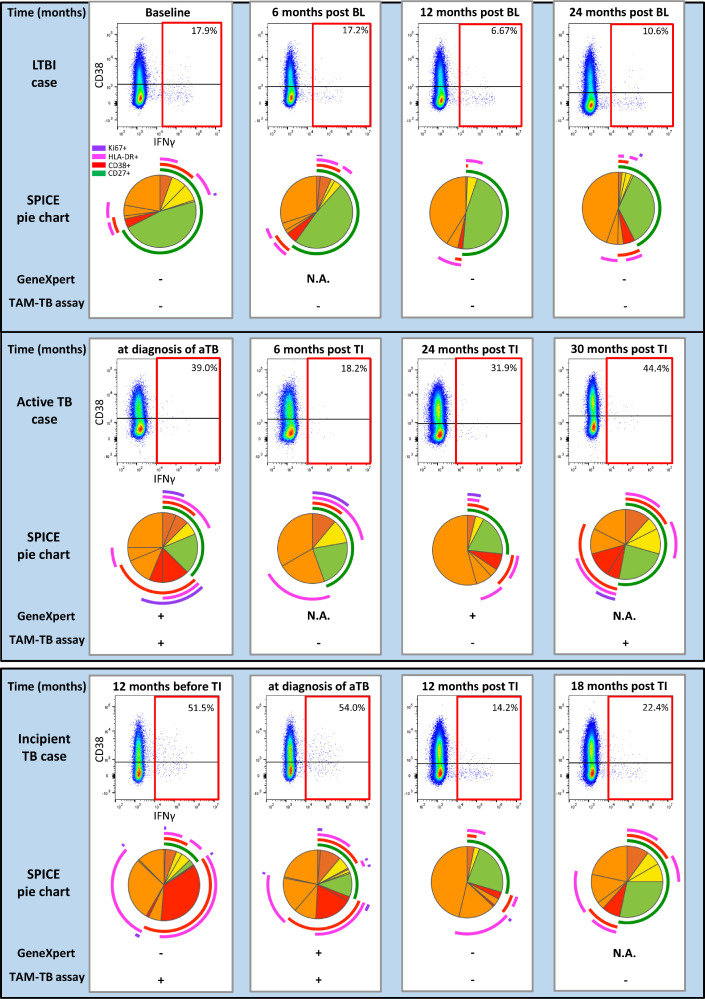
Figure 2.
Exemplary longitudinal phenotypic changes on MTB-specific CD4 T cells representative for PEOPLE LIVING WITH HIV with active TB, incipient TB and LTBI.
Flow cytometry dot plots for IFNγ and CD38 staining on CD4 T cells are shown for four different time points. IFNγ+ MTB-specific CD4 T cells are highlighted with a red gate. The SPICE pie chart slices show 16 different IFNγ+ MTB-specific cell subsets as defined by expression of Ki67 (purple archs) HLA-DR (pink archs), CD38 (red archs) and CD27 (green archs). The 16 individual pie chart slices therefore visualize all possible combinatorial expression patterns for the tested markers Ki67, CD38, HLA-DR and CD27. The green slices visualize the proportion of MTB-specific CD4 T cells expressing CD27, but no CD38, HLA-DR or Ki67. Red slices indicate expression of 2 or 3 activation markers without expression of CD27. All other marker combinations are orange. The time point indicated is provided in relation to the time point of diagnosis and treatment initiation for cases of active and incipient TB. The Xpert MTB/RIF test and TAM-TB assay results are indicated for each time point. MTB-specific T cells were analyzed after in vitro re-stimulation of PBMC with Purified Protein Derivative. NA: not applicable. Abbrevations: MTB=Mycobacterium tuberculosis, TB=tuberculosis, aTB=active tuberculosis, LTBI=latent tuberculosis infection, IFNγ=interferon gamma, HLA-DR=Human Leucocyte Antigen-DR, TAM-TB assay=T cell activation marker-Tuberculosis assay.
Flow cytometric analyses
32 ml of anticoagulated whole blood was collected from study participants in ACD tubes and processed according to standard procedures for FICOLL isolation of peripheral blood mononuclear cells (PBMC) and cryopreservation using standard methodology. Intracellular cytokine staining of peripheral blood mononuclear cells (PBMC) was performed to determine MTB-specific CD4 T-cell activation and maturation using an adapted protocol.19 Briefly, PBMCs were stimulated overnight with MTB-derived purified protein derivative (10 μg/ml), Staphylococcal enterotoxin B (0.6 μg/ml) as a positive control, or no stimulation as a negative control in the presence of Brefeldin A and the co-stimulatory antibodies anti-CD49d and anti-CD28. Cell surface staining was performed for 20 minutes with anti-CD38-BV785 (clone HIT2, Biolegend), anti-CD4-APC (clone 13B8.2, Beckmann Coulter), anti-CD27 ECD (clone 1A4CD27, Beckmann Coulter), and anti-HLA-DR APC-H7 (clone G46-6, Becton Dickinson), followed by fixation, permeabilization, and intracellular cytokine staining using anti-IFNγ FITC (clone B27, BD Pharmingen), anti-Ki67 BV421 (clone B56, BD Pharmingen), and anti-CD3 APC-A700 (clone UCHT1, Beckmann Coulter). Cells were acquired on a CytoFlex flow cytometer (Beckman Coulter). Positive MTB-specific CD4 T-cell responses were defined by a frequency of ≥0.03% of IFNγ+ CD4 T cells and ≥2-fold increase over the unstimulated control. Samples without an IFNγ response to the positive control antigen were excluded. Gating analysis was performed blinded to any TB diagnostic data using FlowJo_V10.6.2. Pestle and Spice 6.0 software was used to analyze combinatorial expression of four phenotypic markers on MTB-specific CD4 T cells.26
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 6 and CRAN R 3.6.2. The two-sided Mann-Whitney test for comparison between different groups was used, with a p-value significance cutoff of 0.05. To determine how well expression of CD38 on MTB-specific CD4 T cells would differentiate active, Xpert MTB/RIF positive aTB from LTBI in the current data study, we used ROC analysis and we determined that 20.25% CD38+ cells of all IFNγ+ MTB-specific CD4 T cells is the optimal cut-off to differentiate between aTB and LTBI, with a sensitivity of 86% and specificity of 86% (Figure 3D, supplementary table 2). Further, multivariable linear regression analysis was used to adjust for any potential confounders [group, site, sex, age, ART intake, CD4 count, and viral load] on the frequencies of CD38+ MTB-specific IFNγ+ CD4 T cells. Model selection was done using a combination of stepwise Akaike information criterion values and biological relevance.
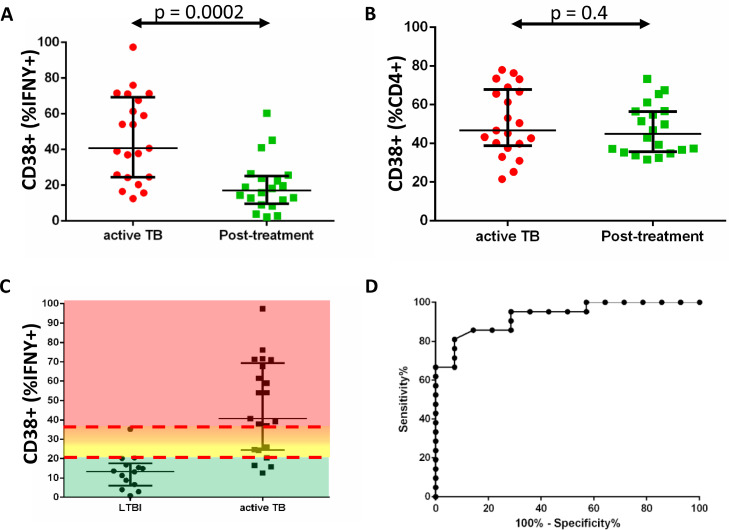
Figure 3.
Frequencies of CD38+ MTB-specific IFNγ+ CD4 T cells differentiate time points of patients with active TB disease (before starting versus after treatment) and LTBI.
The figure shows (A) the frequency of MTB-specific CD4 T cells that express CD38 (as %IFNγ+ cells) at time of aTB diagnosis (red dots, n=21), as compared to their frequencies after TB treatment (green squares, n = 20). The MTB-specific cell activation was measured by CD38 expression analyses on MTB-specific IFNγ+ cells and shows a significant decline after TB treatment, whereas the CD38 expression on total CD4+ T cells (B) was not influenced by TB treatment. The frequency of CD38+ MTB-specific CD4 T cells (as %IFNγ+ cells) is compared between participants with aTB and LTBI in (C). Color shading indicates the range of CD38+ cell frequencies (green<20.25%, yellow 20.25% to 27.25%, orange 27.25% to 35.20%, red >35.20%); The median and interquartile ranges are indicated. The separation of the distribution for each group was tested using the Mann-Whitney test. ROC analysis (D) was used to determine 20.25% CD38+ cells (% of IFNγ+ cells) as the optimal cut-off to differentiate between patients with aTB and the LTBI/healthy group with a sensitivity of 86% and “specificity” of 86%. A frequency of IFNγ+ cells above 35.20%, which corresponds to the highest frequency of CD38+ MTB-specific CD4 T cells detected in the LTBI/healthy group, defined patients with TB with 100% specificity. CD38 frequencies from 20.25% to 27.25% and from 27.25% to 35.20% were further delineated in this analysis. These cut-offs are the basis for the heat map in Figure 4. Abbrevations: MTB=Mycobacterium tuberculosis, TB=tuberculosis, aTB=active tuberculosis, LTBI=latent tuberculosis infection, IFNγ=interferon gamma
Role of the funding source
The funders had no role in study design, in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Results
Between January 2013 and August 2018, 2014 people living with HIV were recruited at the AFRICOS TB study sites in Kenya and Uganda (Figure 1). Thirteen percent of the individuals reported previous episodes of TB. During enrolment into the study at baseline 41 individuals were diagnosed as aTB and initiated antimycobacterial treatment. An additional 19 individuals were diagnosed with aTB during subsequent follow-up visits, who initiated anti-TB treatment, had available PBMC sample and were classified as incipient TB cases. In 1944 participants, Xpert MTB/RIF remained negative throughout the study. We then selected 46 patients, who were categorized into three groups for longitudinal profiling of MTB-specific CD4 T cells. Table 1 summarizes the basic clinical characteristics for these three groups. Details on selection and classification are provided in the supplementary appendix. Group 1 were incipient TB cases (n=19), comprising 137 participant visits and 810 person months. Group 2 were aTB cases diagnosed at baseline (n=11), comprising 58 participant visits and 360 person months; and Group 3 were LTBI positive (n=16), comprising 66 participant visits and 588 person months.
Table 1.
Summary of groups: Responders only.
| Incipient TB | Active TB at BL | LTBI | |
|---|---|---|---|
| All | 19 | 11 | 16 |
| Origin (UG/KE) | 2/17 | 7/4 | 10/6 |
| Sex (Male/Female) | 9/10 | 9/2 | 8/8 |
| Median Age at V1 (Range) | Median 39,5 Range 27 -66 |
Median 39 Range 22-58 |
Median 33 Range 22-63 |
| ART status at baseline | 14 on ART 5 ART Naive |
4 on ART 7 ART Naive |
5 on ART 11 ART Naive |
| ART status at TB dx | 19 on ART | 4 on ART 7 ART Naive |
NA |
| CD4 count at baseline Median/Range |
304 (11-539) |
389 (19-787) |
556 (68-1432) |
| CD4 count at TB dx Median/Range |
395 (91-650) |
389 (19-787) |
NA |
| Viral load at baseline Median/Range |
0 (0-102.555) |
80.582 (0-623.568) |
7.438 (0-672.120) |
| Viral load at TB dx Median/Range |
0 (0-85.053) |
80.582 (0-623.568) |
NA |
| Incident time to TB diagnosis Median/Range |
12 (0-30 month) |
NA | NA |
NA (not applicable).
Analysis of MTB-specific CD4 T-cell activation and maturation in people living with HIV
MTB-specific CD4 T-cell activation was characterized with the primary focus on the frequency of CD38+ cells. Representative flow cytometry dot plots and gating for longitudinal assessment of MTB-specific CD4 T cells are shown in supplementary figure 1. Figure 2 shows exemplary participant results for each of the groups. Participants of the LTBI group showed mostly low fractions of activated but high fractions of CD27only MTB-specific CD4 T cells - consistent with no or minimal TB disease throughout the studied period. In patients with TB, MTB-specific T cell activation was typically high at diagnosis and reduced upon TB treatment initiation. However, persistent or recurrent MTB-specific T cell activation after end of treatment was observed in a portion of these patients with TB, including all three participants with aTB (also see below). Incipient TB cases were defined by absence of aTB at enrolment, but often had high percentages of activated MTB-specific CD4 T cells before and at diagnosis of aTB.
Monitoring of CD38 expression on MTB-specific CD4 T cells as a surrogate biomarker of in vivo disease activity
Treatment specifically reduced percentages of activated, CD38+ cells for MTB-specific CD4 T cells (p<0.0002), but not for total CD4 T cells (p=0.4, Figure 3), similar to our observations in HIV negative patients with TB,19 CD38+ MTB-specific CD4 T cell frequencies after treatment were comparable to those observed in the LTBI group (Figure 3A,C). Using ROC analysis, we determined that 20.25% CD38+ cells of all IFNγ+ MTB-specific CD4 T cells is the optimal cut-off to differentiate between aTB and LTBI, with a sensitivity of 86% and specificity 86 % (Figure 3D, supplementary table 2). Multivariable regression analysis to adjust for potential confounders also showed that high frequency of activated CD38+ MTB-specific T cells correlated with active TB in this study (supplementary table 3). Of note, the terms “sensitivity” and “specificity” do not account for subclinical aTB, because no diagnostic reference standard exists for this disease category. CD38+ cell frequencies below 20.25% were defined as indicative of no (or minimal) TB disease activity. A frequency above 35.20% was used as the cutoff indicative for high TB disease activity, because it defined Xpert+ patients with TB with 100% specificity (Figure 3C). CD38+ cell frequency ranges from 20.25% to 27.25% and between 27.25% and 35.20% were used to indicate intermediate disease activity stages.
Longitudinal patterns of CD38 expression on MTB-specific CD4 T cells before, during and after diagnosis of aTB
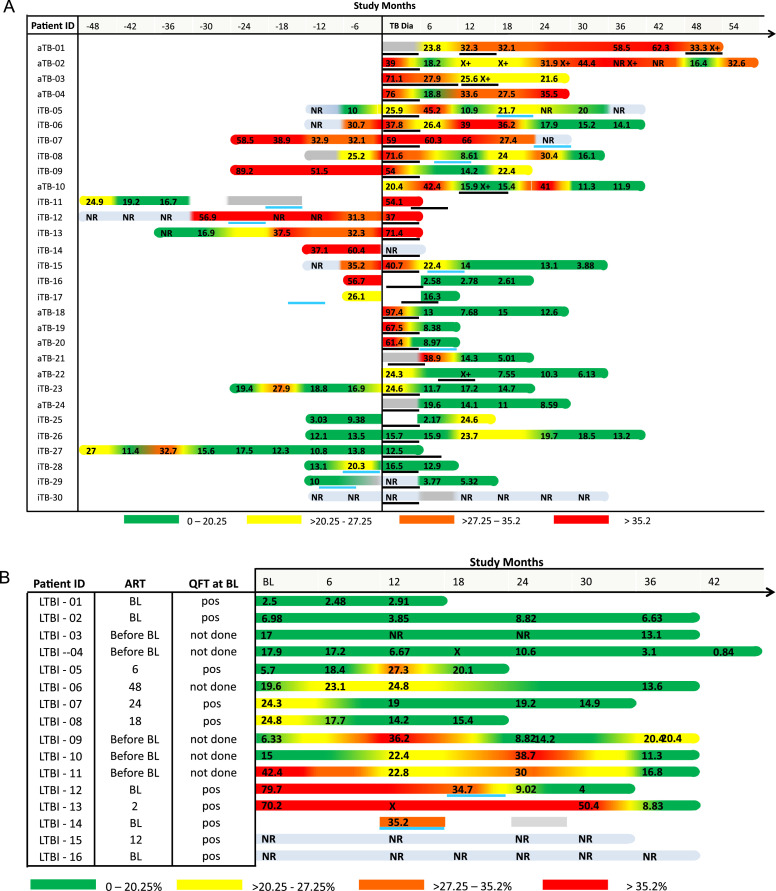
We analyzed MTB-specific CD4 T cell activation in 30 aTB cases during an average of seven visits from before and/or after TB diagnosis (Figure 4A). Eleven of those were diagnosed with aTB at enrollment and 19 study participants identified as incipient TB cases. PBMCs were available for 24 participants at the visit when aTB was confirmed and the TAM-TB assay was performed. At the time of diagnosis, 75% of aTB cases (18 of 24) had activated MTB-specific T cell frequencies above our CD38 threshold for aTB. 12.5% (3 of 24) had detectable MTB-specific CD4 T cells with low activation profile, and 12.5% (3 of 24) did not respond to MTB-specific stimulation. Importantly, we analyzed 52 visits from 6 to 48 months before diagnosis of aTB. At 6 months before diagnosis of aTB, 63% (10 of 16) of incipient TB cases had activated cells above the threshold, whereas 31% (5 of 16) were below the threshold, and one participant did not respond. At 12 months before aTB diagnosis, PBMC from 13 incipient TB cases were available; 23% (3 of 13) of these had CD38 expression levels above the threshold of 20.25%, whereas 46% (6 of 12) were below and 31% (4 of 12) showed no response. At 18 to 48 months prior to TB diagnosis, 26 study visits from seven incipient TB cases were analyzed. Three individuals had evidence of persistent MTB-specific T-cell activation long before diagnosis of aTB, whereas in the others a more mixed pattern was observed. One exemplary participant is iTB-13, who was enrolled 36 months before the TB diagnosis was made but showed no clinical symptoms. Thirty months before TB diagnosis, this participant showed signs of mycobacterial infection, with MTB-specific IFNγ-producing cells. However, since MTB-specific T cells were not activated, this finding suggests latent MTB infection. The first evidence of MTB-specific T-cell activation was obtained 18 months before aTB diagnosis and continued until the TB diagnosis was made and treatment started. The combinatorial analyses of CD38, HLA-DR, Ki67 and CD27 expression on MTB-specific CD4 T cells for individuals with incipient TB and those diagnosed at baseline is shown in supplementary figure 2A and B, respectively. Overall, the pattern of activation markers observed in the combinatorial analyses was consistent with the pattern observed for CD38 alone. In summary, our longitudinal biomarker analysis suggests an onset of TB disease activity at 6-12 months prior to diagnosis in the majority of incipient TB cases studied.
Figure 4.
Frequencies of CD38+ MTB-specific IFNγ+ CD4 T cells over time in AFRICOS study participants with LTBI, incipient TB and active TB.
The figure shows the frequencies of MTB-specific CD4 T cells that express CD38 (as % IFNg+ cells) over time using the indicated color code. (A) shows CD38+ cell frequencies from participants with active and incipient TB from time points before and after TB diagnosis (X axis, study months). A positive Xpert MTB/RIF test result is indicated by an “X+”, black lines show the initiation and duration of HZRE TB treatment for each patient with TB. A blue line indicates initiation and duration of INH preventive treatment. CD38+ cell frequencies from participants with LTBI are shown in (B) together with their ART, baseline QuantiFERON (QFT) data. (NR: no response). TB=tuberculosis. Abbrevations: MTB=Mycobacterium tuberculosis, TB=tuberculosis, aTB=active tuberculosis, LTBI=latent tuberculosis infection, IFNγ=interferon gamma, HZRE=isoniazid, pyrazinamide, rifampicin and ethambutol, INH=Isonazid.
MTB-specific T cell activation was assessed after treatment with Isoniazid, Rifampicin, Pyrazinamide and Ethambutol (HRZE) until 2.5 years after treatment. Two distinct longitudinal post-treatment patterns were observed. Reduction of T-cell activation was observed in 11 of 21 participants (with two or more post-treatment visits subjected to analyses). Ten of these were continuously Xpert MTB/RIF negative after treatment, whereas one was again positive at 3 months after treatment had ended. More persistent MTB-specific T cell activation was detected in eight study participants. Three of these had recurrent TB cases defined by disease symptoms, persistent Xpert MTB/RIF positivity and re-initiation of TB treatment at 7, 12 and 36 month after completing the first course of treatment (Figure 4A). Persistent or recurrent MTB-specific T-cell activation therefore was a hallmark of recurrent TB and treatment failure after treatment.
Longitudinal patterns of MTB-specific CD4 T cell activation during LTBI in people living with HIV
The frequency of activated CD38+ MTB-specific CD4 T cells in 16 participants with clinically latent TB is shown in Figure 4B for a time period of up to 42 months. The combinatorial analyses of activation and maturation markers in this group is shown in supplementary figure 2C. MTB-specific CD4 T cells were continuously detected in most of these participants. At baseline, 57% of responders (8 of 14) had a CD38 expression profile below the threshold of ≤20.25% MTB-specific IFNγ+ CD4 T cells, suggestive of no or minimal disease activity. However, 29% of responders (4 of 14, one was first assessed at month 12) were above this threshold, suggesting aTB disease activity. In individual LTBI-12, isoniazid preventive treatment (IPT) was temporally associated with reduction of MTB-specific CD38+ T cell activation from 34.7% to 9%. Overall, three distinct MTB-specific activation patterns were observed; a low activation profile throughout the study period in four participants (LTBI-1 to LTBI-4), a transiently activated profile during one of several visits in three participants (LTBI-5, LTBI-7 and LTBI-8) and more persistent or recurring activation during two or more study visits in six participants (LTBI-6, LTBI-9, LTBI-10, LTBI-11, LTBI-12, LTBI-13). Together, these data support the concept of recurring episodes of subclinical TB disease with spontaneous resolution in people living with HIV with LTBI.
Discussion
Our data suggest that by using a surrogate blood biomarker, incipient TB can be detected between 6 to 12 months before sputum-based TB diagnosis in many people living with HIV. Further, we provide evidence that episodes of subclinical aTB followed by spontaneous disease clearance may frequently occur in these people. HIV-associated systemic activation of CD4 T cells may contribute to the activation of MTB-specific T cells, particularly before the initiation of ART.27,28 However, our data argue against HIV being a major driver since TB treatment specifically reduced MTB-specific T cell activation - consistent with many previous studies.11,15,19 Hence, longitudinal changes in MTB-specific CD4 T-cell activation should primarily be driven by changes in vivo TB disease activity.
CD38 expression on MTB-specific CD4 T cells was chosen as the primary surrogate marker for TB disease activity because, among the four tested markers, CD38 expression using comparable cut-offs as in previous studies differentiated best between microbiologically confirmed aTB and LTBI or cured TB in previous studies.11,19 Our data show that this biomarker distinguishes well between LTBI and aTB in people living with HIV.
Twenty-three percent of the incipient TB cases had activated MTB-specific CD4 T cells at 12 months and 63% at 6 months prior to sputum-based diagnosis of aTB. This time interval is comparable to our own previous results in an acute HIV infected patient, in whom a phenotypic switch of MTB-specific CD4 T cells preceded the diagnosis of aTB by 9 months.23 Other studies in HIV-negative incipient TB cases, in whom RNA signatures were studied, suggested onset of incipient TB before microbiological TB confirmation in 25% to 50%, 70% and 83% of cases at 12 months, 6 months, and within 3 months of TB diagnosis.14,29,30 A similar time interval between MTB infection and overt aTB is also suggested upon low-dose exposure of “progressing” cynomolgus macaques, which mostly develop aTB by 4 to 5 months.31 Incipient disease activity therefore often appears to start several months before diagnosis of overt, symptomatic aTB in the majority of patients with TB regardless of HIV coinfection. This time interval provides a window of opportunity for early identification of aTB and therapeutic intervention to intercept further disease progression and transmission.
Three incipient TB cases showed no MTB-specific T-cell activation at the time of TB diagnosis. One of these patients had a history of TB treatment (iTB28) 4 years before and was later diagnosed with esophagus cancer, providing an alternative explanation and may therefore have been misclassified. It is well known that even after curative treatment of aTB, Xpert MTB/RIF can be positive even years later.32
While speculative, our data also suggest that subclinical aTB disease is present in roughly 30% of HIV-positive individuals with clinically latent TB. Five of 16 participants with LTBI at baseline and roughly a third of all study visits in the LTBI group had activated MTB-specific CD4 T cells at one or more visits. Based on the high specificity of the chosen approach, we argue that this pattern reflects transient flare ups of incipient or subclinical disease. Using PET-CT imaging, Esmael and colleagues also have shown that roughly a third of HIV-positive individuals with LTBI likely have subclinical aTB,6 consistent with our data. Notably, the sensitivity of Xpert MTB/RIF is approximately 85%, and misclassification of aTB cases into the LTBI group cannot be ruled out.33 In any case, our data argue that many people living with HIV resolve subclinical disease spontaneously, whereas others progress to overt, transmissible TB. In the pre-antibiotic era, spontaneous resolution of aTB disease was quite common with approximately 50% of cases being cured without receiving antibiotic treatment.4,34 Spontaneous resolution of subclinical disease may hence occur in people living with HIV more frequently than commonly anticipated.
Likewise, roughly half of patients showed patterns of transient or persistent MTB-specific T-cell activation after TB treatment, including all cases of recurrent TB. While the etiology of this specific activation remains speculative, subclinical disease may still frequently occur in these patients. Regardless of HIV status, this approach appears to identify cases of active TB disease and potentially allows these to be targeted before bacilli become detectable in the sputum by molecular diagnosis. Similarly, this assay could potentially support treatment monitoring for early identification and interception of recurring TB.
Our study had several limitations. We selected individuals with positive Xpert MTB/RIF who then initiated antimycobacterial treatment as “active TB cases”. In the resource constraint setting of the AFRICOS study, clinical information surrounding the diagnosis of aTB, such as radiology and MTB culture, were often not available. Using a sputum-based diagnostic tool, extra-pulmonary TB would have been missed. A positive Xpert MTB/RIF result could potentially be explained by a history of TB and consequently may have triggered TB therapy initiation in some patients with other reasons for clinical presentation. Another limitation is the small sample size in each group. The results are not generalizable as patients have been selected according to the mentioned criteria and not randomized. Our key finding that TB disease activity often becomes detectable by a blood-based surrogate biomarker long before sputum occurance of MTB bacilli therefore should be confirmed by additional studies. However, biomarker-based approaches, such as the TAM TB assay, may improve TB diagnosis specifically in resource-poor settings. Notably, flow cytometry-based determination of CD4 cell counts were available at all included study sites and analyses of MTB-specific T cells using simplified whole-blood protocols should be possible in such laboratories.
In summary, use of flow cytometry enables monitoring of specific surrogates of TB disease activity over long time periods in MTB-infected people living with HIV in resource-poor settings. The results suggest that, in many of these individuals, onset of aTB disease starts between 12 and 6 months before diagnosis of aTB. Thus, regardless of concomitant HIV infection, earlier identification of incipient aTB could improve short- and long-term clinical outcomes of infected patients and reduce the spread of the disease.
Contributors
IK, MH and CG participated in the design of the AFRICOS-TB study. MIMA and SH performed experiments, analyzed the data and created figures and tables. RL performed the computational analysis of flow cytometric data. IK and CG supervised the centralized experimental and analytical work. JA and CP conceptualized and acquired funding for the AFRICOS study, and contributed to the AFRICOS-TB sub study methodology, resources, supervision, and formal analysis of data. LAE contributed to study conceptualization, laboratory methodology, resources and supervision. AE contributed to data curation and formal analysis. AP assisted with project administration. HK, BM, PN, MS, and JK participated in collecting participant data in Uganda, oversaw participant visits, and participated in writing the manuscript and providing final approval. JM, VS, JO, and ER participated in collecting participant data in Kenya, oversaw participant visits, and participated in writing the manuscript and providing final approval. JK and ER performed laboratory Xpert MTB/RIF testing in Uganda and Kenya, respectively. All authors contributed to review and editing of the manuscript. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication. IK, MIMA, SH and CG have verified the underlying data.
Data sharing statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy protections but are available from the corresponding author on reasonable request. The Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF) and the Water Reed Army Institute of Research (WRAIR) are committed to safeguarding the privacy of research participants. Distribution of data will require compliance with all applicable regulatory and ethical processes, including establishment and approval of an appropriate data-sharing agreement. To request a minimal data set, please contact the data coordinating and analysis center (DCAC) at PubRequest@hivresearch.org and indicate the RV329 study along with the name of the manuscript.
Declaration of interests
We declare no competing interests.
Acknowledgments
The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25. Funding organizations had no role in the study design or data analyses. Editorial support was provided by Dr. Iain Patten and funded by in house funds from the Division of Infectious Diseases and Tropical Medicine, University Hospital of Munich.
The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
We thank the study participants, local implementing partners, and hospital leadership at Kayunga District Hospital, Kericho District Hospital, AC Litein Mission Hospital, Kapkatet District Hospital, Tenwek Mission Hospital, Kapsabet District Hospital, Nandi Hills District Hospital, Kisumu West District Hospital, Mbeya Zonal Referral Hospital, Mbeya Regional Referral Hospital, Defence Headquarters Medical Center, and the 68th Nigerian Army Reference Hospital.
Funding
This work was supported by the President's Emergency Plan for AIDS Relief via a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174, W81XWH-18-2-0040] and by the Bundesministerium für Bildung und Forschung (BmBF) through funding of the Deutsches Zentrum für Infektionsforschung (DZIF, TTU-TB personalized medicine TTU 02_813).
References
- 1.WHO. Health Topics - Tuberculosis. 09.06.2020 2020. https://www.who.int/health-topics/tuberculosis#tab=tab_1.
- 2.Organization. WH. Global Tuberculosis Report 2015. 2015. http://www.who.int/tb/publications/global_report/en/. Accessed 23 November 2015.
- 3.Barry CE, 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendall EA, Shrestha S, Dowdy DW. The Epidemiological Importance of Subclinical Tuberculosis. A Critical Reappraisal. Am J Respir Crit Care Med. 2021;203(2):168–174. doi: 10.1164/rccm.202006-2394PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drain PK, Bajema KL, Dowdy D, et al. Incipient and Subclinical Tuberculosis: a Clinical Review of Early Stages and Progression of Infection. Clin Microbiol Rev. 2018;31(4) doi: 10.1128/CMR.00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esmail H, Lai RP, Lesosky M, et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[(18)F]fluoro-D-glucose positron emission and computed tomography. Nature Medicine. 2016;22(10):1090–1093. doi: 10.1038/nm.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malherbe ST, Shenai S, Ronacher K, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nature Medicine. 2016;22(10):1094–1100. doi: 10.1038/nm.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghesani N, Patrawalla A, Lardizabal A, Salgame P, Fennelly KP. Increased cellular activity in thoracic lymph nodes in early human latent tuberculosis infection. Am J Respir Crit Care Med. 2014;189(6):748–750. doi: 10.1164/rccm.201311-1976LE. [DOI] [PubMed] [Google Scholar]
- 9.Priftakis D, Riaz S, Zumla A, Bomanji J. Towards more accurate (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG PET) imaging in active and latent tuberculosis. Int J Infect Dis. 2020;92S:S85–S90. doi: 10.1016/j.ijid.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Yu WY, Lu PX, Assadi M, et al. Updates on (18)F-FDG-PET/CT as a clinical tool for tuberculosis evaluation and therapeutic monitoring. Quant Imaging Med Surg. 2019;9(6):1132–1146. doi: 10.21037/qims.2019.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adekambi T, Ibegbu CC, Cagle S, et al. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Investig. 2015;125(5):1827–1838. doi: 10.1172/JCI77990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joosten SA, Goeman JJ, Sutherland JS, et al. Identification of biomarkers for tuberculosis disease using a novel dual-color RT-MLPA assay. Genes Immun. 2012;13(1):71–82. doi: 10.1038/gene.2011.64. [DOI] [PubMed] [Google Scholar]
- 13.Portevin D, Moukambi F, Clowes P, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect Dis. 2014;14(10):931–938. doi: 10.1016/S1473-3099(14)70884-9. [DOI] [PubMed] [Google Scholar]
- 14.Zak DE, Penn-Nicholson A, Scriba TJ, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387(10035):2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riou C, Du Bruyn E, Ruzive S, et al. Disease extent and anti-tubercular treatment response correlates with Mycobacterium tuberculosis-specific CD4 T-cell phenotype regardless of HIV-1 status. Clin Transl Immunology. 2020;9(9):e1176. doi: 10.1002/cti2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chegou NN, Sutherland JS, Malherbe S, et al. Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax. 2016;71(9):785–794. doi: 10.1136/thoraxjnl-2015-207999. [DOI] [PubMed] [Google Scholar]
- 17.Penn-Nicholson A, Mbandi SK, Thompson E, et al. RISK6, a 6-gene transcriptomic signature of TB disease risk, diagnosis and treatment response. Sci Rep. 2020;10(1):8629. doi: 10.1038/s41598-020-65043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streitz M, Tesfa L, Yildirim V, et al. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PloS One. 2007;2(1):e735. doi: 10.1371/journal.pone.0000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed MIM, Ntinginya NE, Kibiki G, et al. Phenotypic changes on mycobacterium tuberculosis-specific CD4 T cells as surrogate markers for tuberculosis Treatment Efficacy. Front Immunol. 2018;9:2247. doi: 10.3389/fimmu.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harari A, Rozot V, Bellutti Enders F, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17(3):372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riou C, Berkowitz N, Goliath R, Burgers WA, Wilkinson RJ. Analysis of the phenotype of mycobacterium tuberculosis-specific CD4+ T cells to discriminate latent from active tuberculosis in HIV-uninfected and HIV-infected individuals. Front Immunol. 2017;8:968. doi: 10.3389/fimmu.2017.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliday A, Whitworth H, Kottoor SH, et al. Stratification of latent mycobacterium tuberculosis infection by cellular immune Profiling. J Infect Dis. 2017;215(9):1480–1487. doi: 10.1093/infdis/jix107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuetz A, Haule A, Reither K, et al. Monitoring CD27 expression to evaluate mycobacterium tuberculosis activity in HIV-1 infected individuals In vivo. PloS One. 2011;6(11):e27284. doi: 10.1371/journal.pone.0027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahemana E, Esber A, Dear N, et al. Impact of age on CD4 recovery and viral suppression over time among adults living with HIV who initiated antiretroviral therapy in the African Cohort Study. AIDS Res Ther. 2020;17(1):66. doi: 10.1186/s12981-020-00323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geldmacher C, Schuetz A, Ngwenyama N, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198(11):1590–1598. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr (1988) 1993;6(8):904–912. [PubMed] [Google Scholar]
- 28.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta RK, Turner CT, Venturini C, et al. Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis. Lancet Respir Med. 2020;8(4):395–406. doi: 10.1016/S2213-2600(19)30282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roe J, Venturini C, Gupta RK, et al. Blood Transcriptomic stratification of short-term risk in contacts of tuberculosis. Clin Infect Dis. 2020;70(5):731–737. doi: 10.1093/cid/ciz252. [DOI] [PubMed] [Google Scholar]
- 31.Flynn JL, Gideon HP, Mattila JT, Lin PL. Immunology studies in non-human primate models of tuberculosis. Immunol Rev. 2015;264(1):60–73. doi: 10.1111/imr.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costantini L, Marando M, Gianella P. Long-Term GeneXpert positivity after treatment for pulmonary tuberculosis. Eur J Case Rep Intern Med. 2020;7(10) doi: 10.12890/2020_001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rachow A, Clowes P, Saathoff E, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clin Infect Dis. 2012;54(10):1388–1396. doi: 10.1093/cid/cis190. [DOI] [PubMed] [Google Scholar]
- 34.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PloS One. 2011;6(4):e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]